Abstract
Protein O-fucosyltransferase 1 (Pofut1) transfers fucose to serine or threonine on proteins, including Notch receptors, that contain EGF repeats with a particular consensus sequence. Here we demonstrate that agrin is O-fucosylated in a Pofut1-dependent manner, and that this glycosylation can regulate agrin function. Fucosylation of recombinant C45 agrin, both active (neural, z8) and inactive (muscle, z0) splice forms, was eliminated when agrin was overexpressed in Pofut1-deficient cells or by mutation of a consensus site for Pofut1 fucosylation (serine 1726 in the EGF4 domain). Loss of O-fucosylation caused a gain of function for muscle agrin such that it stimulated AChR clustering and MuSK phosphorylation in cultured myotubes at levels normally only found with the neural splice form. Deletion of Pofut1 in cultured primary myotubes and in adult skeletal muscle increased AChR aggregation. In addition, Pofut1 gene and protein expression and Pofut1 activity of the EGF4 domain of agrin were modulated during neuromuscular development. These data are consistent with a role for Pofut1 in AChR aggregation during synaptogenesis via the regulation of the synaptogenic activity of muscle agrin.
Keywords: glycosylation, agrin, synapse, neuromuscular junction, fucose, Notch
An essential step in the formation of all types of synapses is the concentration of neurotransmitter receptors in the postsynaptic membrane such that they are localized in a manner that allows efficient and robust neurotransmission by the presynaptic nerve terminal. Nowhere is this more dramatically in evidence than at the mammalian neuromuscular junction, where postsynaptic concentration of the nicotinic acetylcholine receptors (AChRs) approaches a thousand-fold relative to AChR protein expression in the extrasynaptic membrane (Sanes and Lichtman, 2001). While this concentration of AChRs is brought about by multiple cellular and molecular mechanisms, the lateral migration of extrasynaptic AChRs during development to the postsynaptic membrane requires the stimulation of signal transduction pathways in skeletal myofibers by agrin, a motor nerve-derived synaptogenic factor (Kummer et al., 2006).
Agrin is a highly glycosylated extracellular matrix protein that is essential for the formation of the mammalian neuromuscular junction (Martin, 2003b; Sanes et al., 1998b). Studies using recombinant forms of agrin have shown that high affinity AChR aggregating activity is contained within the C-terminal 45kDa fragment of this 400kDa glycoprotein (Campanelli et al., 1996; Gesemann et al., 1996; Hoch et al., 1993). This region contains two of the three laminin-like G domains, G2 and G3; (Rupp et al., 1991; Timpl et al., 2000; Tsim et al., 1992); G2 is essential for binding of agrin to α-dystroglycan (Bowe et al., 1994; Campanelli et al., 1994; Gee et al., 1994; Sugiyama et al., 1994), an important membrane receptor in skeletal muscle (Durbeej et al., 1998; Ervasti and Campbell, 1991, 1993; Ibraghimov-Beskrovnaya et al., 1992), while G3 is essential for the AChR aggregating activity of agrin (Campanelli et al., 1996; Gesemann et al., 1996; Hoch et al., 1993). G2 and G3 are linked by a single EGF repeat (EGF4), the fourth of four EGF repeats, which are all present in the C-terminal half of the protein (Rupp et al., 1991; Tsim et al., 1992).
In neurons, agrin transcripts contain 1–2 additional exons, giving the G3 domain an 8, 11, or 19 amino acid insertion at the z splice site (Ferns et al., 1992; Ferns et al., 1993; Hoch et al., 1993). Insertion of any of these sequences confers AChR clustering activity upon the neuron-specific forms of agrin (Ferns et al., 1992; Ferns et al., 1993). Agrin in skeletal muscle and other tissues lacks a splice insertion in this domain (termed z0). AChR clustering activity of z0 agrin is at least 10,000-fold less than that of neuron-specific z splice forms (Ferns et al., 1992; Ferns et al., 1993; Hoch et al 1993). Thus, muscle agrin has no AChR clustering activity in the biologically relevant concentration range. Consistent with this notion, elimination of the neuron-specific splice exons from the agrin gene is equivalent to elimination of the entire agrin locus with respect to its phenotype regarding neuromuscular formation (Burgess et al., 1999; Gautam et al., 1996).
Neuron-specific agrin stimulates AChR clustering via its activation of MuSK (Glass et al., 1996). MuSK is a transmembrane tyrosine kinase that is expressed at the neuromuscular junction in skeletal muscle and is an essential signal for synapse formation. Loss of MuSK in mice leads to failure of AChR aggregation and neuromuscular junction formation (DeChiara et al., 1996). Agrin also interacts with other proteins, including integrins (Burgess et al., 2002; Martin and Sanes, 1997) and a Na+/K+ ATPase (Hilgenberg et al., 2006). Thus the mechanisms of action of agrin are complex at a molecular level (Strochlic et al., 2005), particularly as agrin does not bind MuSK directly but utilizes as yet undescribed skeletal muscle accessory factors to activate MuSK signaling (Glass et al., 1996).
During neuromuscular development, acetylcholine receptor clustering activity that is independent of neural agrin is present within skeletal muscles. Moreover, AChR clustering can be present in skeletal muscles even when those muscles are not innervated and therefore do not have access to neuron-specific forms of agrin, but these clusters still require MuSK (Lin et al., 2001; Yang et al., 2000). AChR aggregates also appear in extrasynaptic regions of myofibers even after innervation has occurred during embryonic development (Lin et al., 2001; Yang et al., 2000). Thus, skeletal muscles naturally possess an AChR clustering activity, even though they express a form of agrin apparently lacking such activity (Hoch et al., 1993). Here we show that the muscle-specific form of agrin can possess AChR clustering activity equivalent to the neural splice forms by altering its glycosylation state. In addition, we show that the enzyme that mediates this glycosylation, Pofut1, is dynamically regulated during muscle development and that its deletion alters AChR clustering in skeletal muscle cells and in skeletal myofibers.
RESULTS
O-fucosylation mediates the AChR clustering activity of recombinant C45(z0) agrin
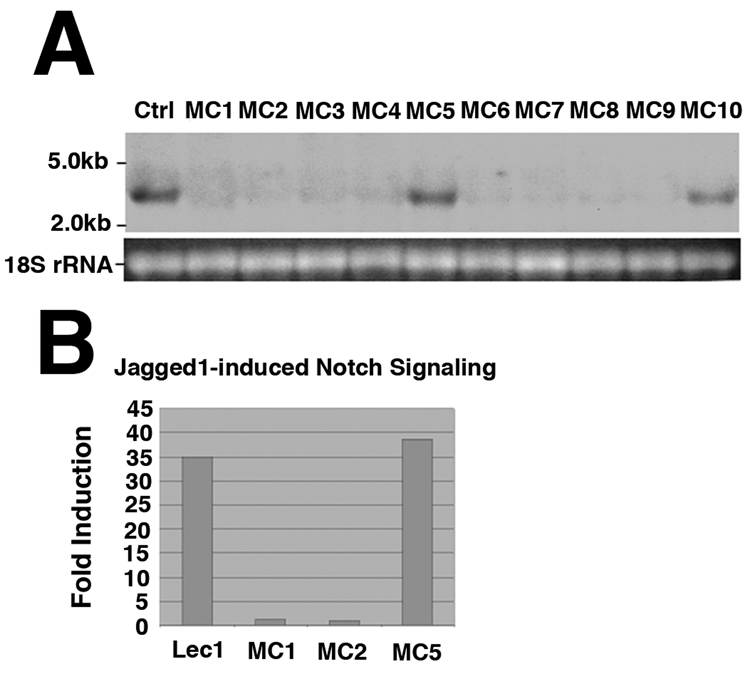
To assess roles for O-fucosylation of agrin, Lec1 CHO cells that stably expressed siRNA sequences designed to knock down Pofut1 gene expression were generated. Northern blot analysis showed no expression of mature Pofut1 mRNA in multiple Lec1 cells stably overexpressing the Pofut1 siRNA (Fig. 1A). Several of these lines (MC5 and MC10), however, had Pofut1 transcript levels equivalent to parent Lec1 cells and served as negative controls (Fig. 1A). As mammalian Notch receptors require Pofut1 for signaling activity(Shi and Stanley, 2003), we assessed the ability of the Notch ligand Jagged1 (Jag1) to induce Notch signaling in MC1, MC2 and MC5 cells (Fig. 1B). MC1 and MC2 cells, which had barely detectable Pofut1 transcripts, had a 35-fold reduction in Notch signaling, while MC5 cells, in which the Pofut1 transcript level was unchanged, were indistinguishable in Notch signaling from control Lec1 cells.
Figure 1. Lec1 cells lacking Pofut1 mRNA expression have reduced Notch signaling.
(A) Northern blot of Pofut1 transcripts in Lec1 control cells (Ctrl) or in Lec1 cells selected for stable expression of siRNA against CHO Pofut1. (B) Jagged1-induced Notch signaling in control Lec1, MC1, MC2, and MC5 cells. Loss of Pofut1 expression (A) correlated with loss of Notch signaling (B). Experiments in A and B are representative of data from 3 independent experiments with similar results.
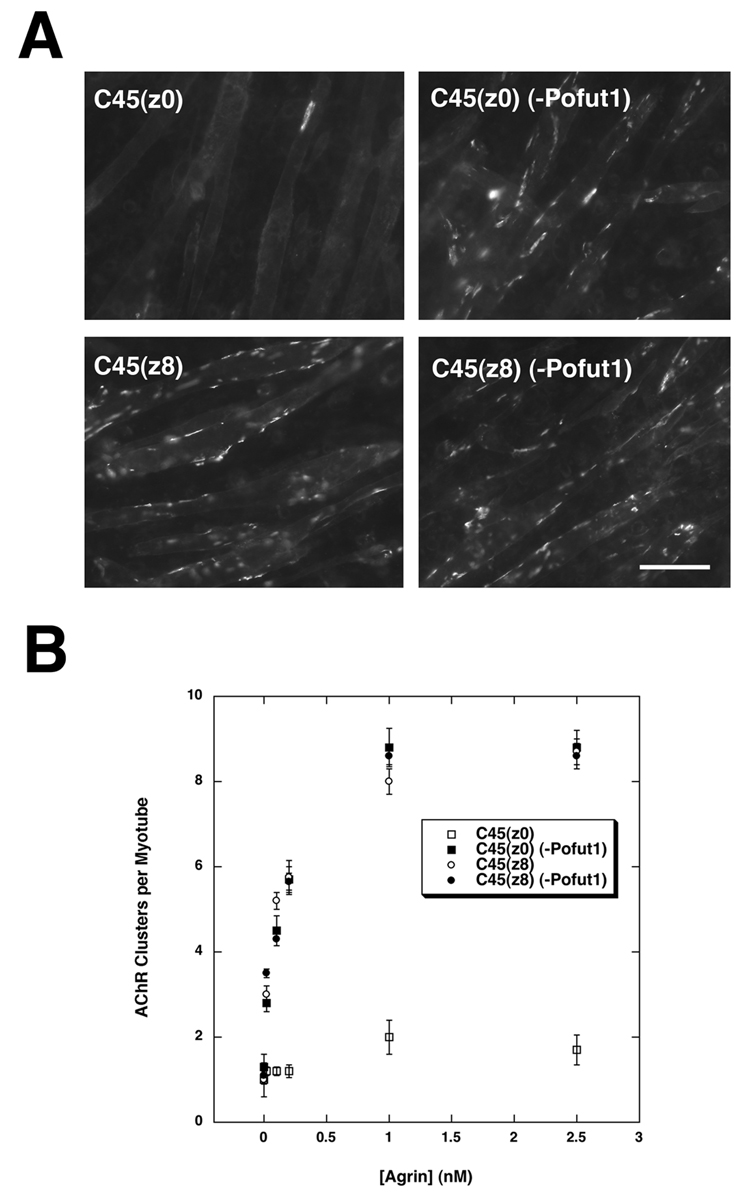
AChR clustering active (neural, z8) and inactive (muscle, z0) C45 agrin fragments possess a single consensus site for O-fucosylation by Pofut1 (C2-X4–5-(S/T)C3, where X is any amino acid, S/T is O-fucosylated, and C2 and C3 represent the second and third cysteines in the 6 cysteine EGF repeat motif (Panin et al., 2002)). To assess roles for Pofut1 in the AChR clustering activity of agrins, Lec1 cells with low to absent Pofut1 expression (MC1) or with high Pofut1 expression (MC5) were used to produce recombinant neural (C45(z8)) and muscle (C45(z0)) agrin (Figs. 2A and B). The activity of purified recombinant agrins was determined by an AChR clustering assay using the C2C12 muscle cell line, as before (Martin and Sanes, 1995). C2C12 cells are a mouse myoblast cell line derived from a satellite cell lineage that grow as myoblasts in high serum and that can be fused, when confluent, in low serum to generate myotubes that reiterate aspects of postsynaptic differentiation (Martin, 2003b). Concentration curves for C45(z8) agrin purified from MC1 (Pofut1 low) or MC5 (control) cell secretions showed no difference in AChR clustering activity, both having half-maximal activity at ca. 250pM. C45(z0) produced essentially in the absence of Pofut1 from MC1 cells displayed AChR clustering activity that was equivalent to C45(z8) agrin, again being half maximal at 250pM(Figs. 2A,B). By contrast, C45(z0) agrin purified from MC5 control cells, which possess Pofut1 activity, showed no AChR clustering activity (Figs. 2A,B). Data were quantified with regard to the number of large (>10µm) AChR clusters per myotube. No condition showed a significant change in the formation of myotubes. These data demonstrate that C45(z0) agrin activity produced in cells with low Pofut1 activity becomes equivalent to C45(z8) agrin.
Figure 2. C45(z0) agrin produced in cells lacking Pofut1 has AChR clustering activity.
(A) Rhodamine-α-bungarotoxin staining of AChR clusters in C2C12 myotube cultures incubated with 1nM neuron-specific C45(z8) or muscle-specific C45 (z0) agrin purified from MC5 cells, which express Pofut1, or MC1 cells which have no detectable Pofut1 (−Pofut1). Bar is 100µm. (B) C45(z0) (−Pofut1), C45(z8), and C45(z8) (−Pofut1) showed similar concentration curves for the induction of AChR clustering, while C45(z0) had no activity. Errors are SEM for n=6 experiments.
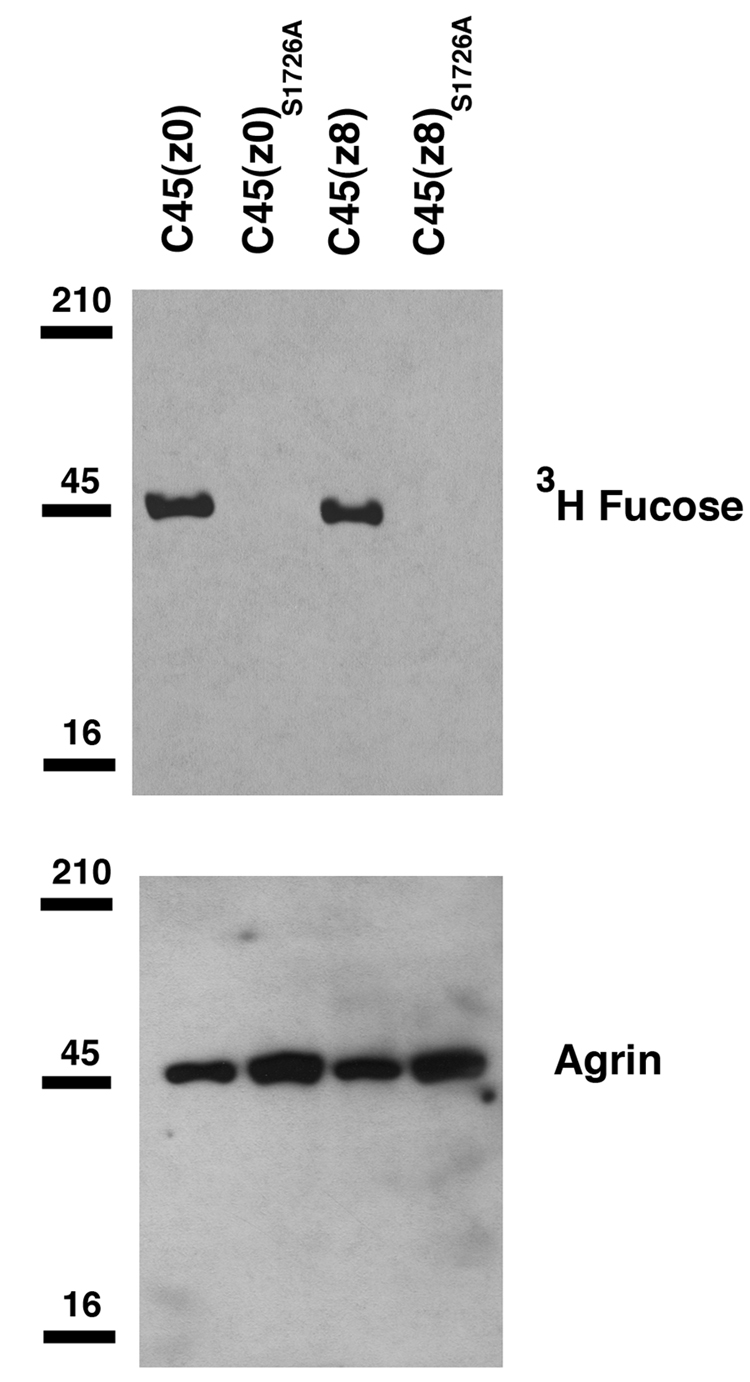
To assess protein O-fucosylation of agrin, cDNAs encoding FLAG-epitopetagged versions of C45(z0) and C45(z8) agrin were transfected into Lec1 cells labeled with 3H-fucose. Lec1 cells are a CHO cell variant containing a mutant Mgat1 gene that precludes expression of active N-acetylglucosaminyltransferase I (GlcNAcT-I; (Chen and Stanley, 2003). Mgat1 is required for the synthesis of complex and hybrid N-glycans. As a result, N-glycans from Lec1 cells remain as high mannose structures that are very poorly core fucosylated, thereby allowing O-linked fucosylation to be more specifically detected. Recombinant agrin proteins were subsequently purified from cell supernatants and the incorporation of radiolabeled fucose was determined. As expected, both C45(z0) and C45(z8) agrin were labeled with 3H-fucose (Fig. 3), though neural agrin incorporated 40±10% less than muscle agrin on a per mole basis (P<0.05, n=4). C45 agrins contain no consensus sites for N-linked glycosylation, and N-glycanase treatment of proteins did not alter their 3H-fucose incorporation (not shown). In order to verify that the gain of function for C45(z0) agrin was indeed due to a lack of fucosylation, the single consensus site for O-fucosylation in EGF4 repeat of the protein was mutated from serine to alanine (S1726A). C45(z8)S1726A or C45(z0)S1726A proteins were then produced in Lec1 cells and compared to wild type C45 proteins (Fig. 3). All 3H-fucose incorporation was eliminated from C45(z8)S1726A and C45(z0)S1726A. Thus, C45 agrin is glycosylated with fucose on serine 1726 in the EGF4 domain and this appears to be the only fucosylation site on C45 agrin.
Figure 3. 3H-fucose incorporation into C45 agrin and C45 agrinS1726A mutants in Lec1 cells.
cDNAs for FLAG-tagged C45(z0) and C45(z8) agrin were transfected into Lec1 CHO cells labeled with 3H-fucose. Secreted proteins were then purified and analyzed for 3H-fucose incorporation by autoradiography. Both C45(z0) and C45(z8) agrin incorporated 3H-fucose, though C45(z8) less so. Mutation of serine 1726 to alanine (S1726A) in either protein eliminated 3H-fucose incorporation. Data are representative of 4 experiments with similar results.
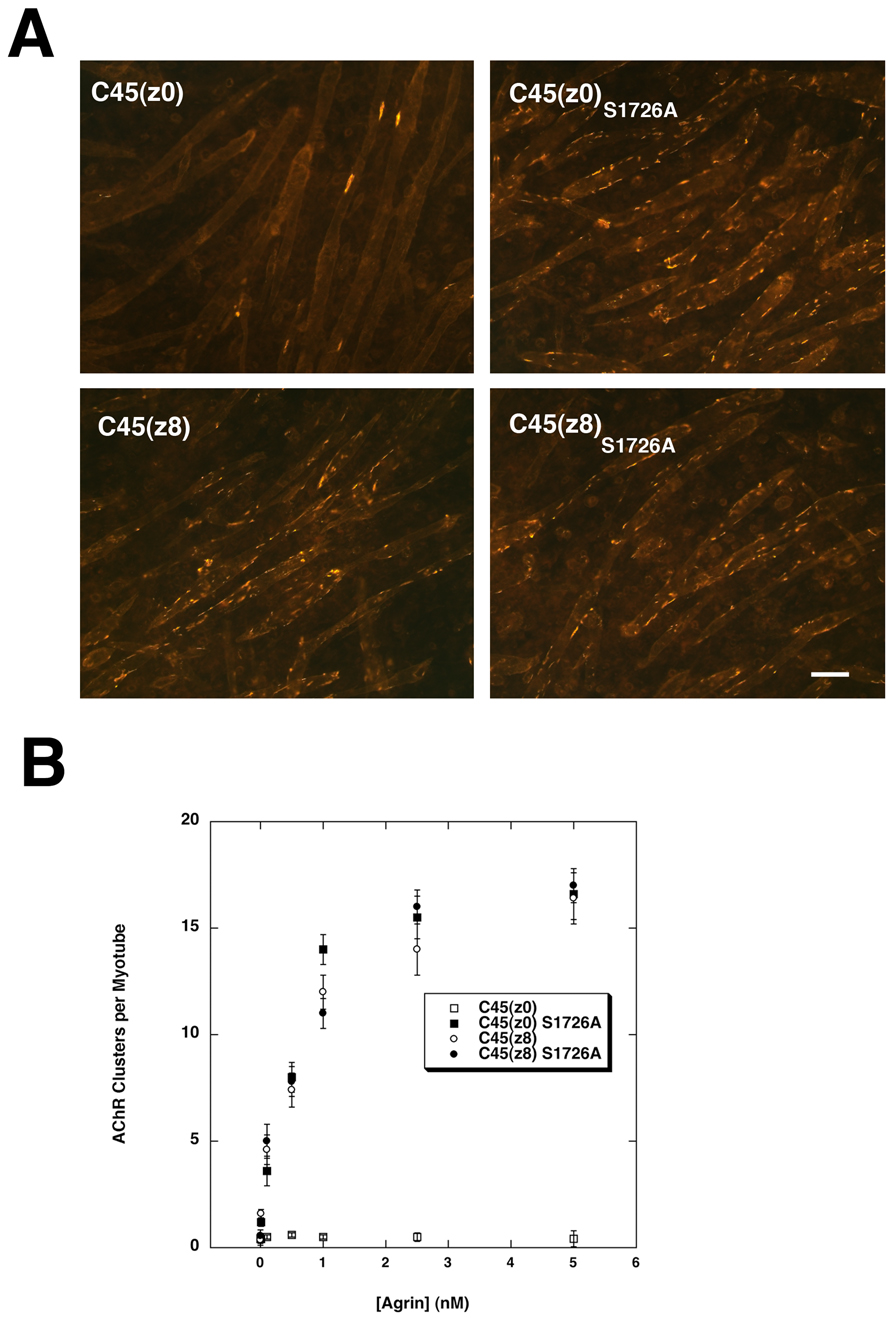
AChR clustering assays with C45(z0)S1726A gave results similar to those with C45(z0) produced in Pofut1-deficient (MC1) Lec1 cells (Figs. 4A and B). Indeed, C45(z0)S1726A induced AChR clustering that was equivalent to that induced by C45(z8) (Figs. 4A,B), with both showing half maximal activity by 500pM. Interestingly, C45(z8)S1726A was as active as C45(z8). Thus, eliminating the single consensus site for O-fucosylation on C45(z0) agrin caused it to gain AChR clustering activity to a degree normally seen with C45(z8) agrin, while C45(z8) activity was unaffected by the same mutation.
Figure 4. Mutation of the O-fucosylation site on C45 muscle agrin stimulates AChR clustering.
(A) 1nM FLAG-tagged C45(z8), C45(z8)S1726A, C45(z0), or c45(z0)S1726A were assayed for AChR clustering on C2C12 myotubes by staining with rhodamine-α-bungarotoxin. Bar is 50µm. (B) C45(z0)S1726A, C45(z8), and C45(z8)S1726A agrin showed similar concentration curves for the induction of AChR clustering, while C45(z0) agrin had no activity. Errors are SEM for n=6 experiments.
Because C45(z8) neural agrin had less fucosylation than C45(z0) muscle agrin, it was possible that some if its activity might arise from its relatively low level of fucosylation. To test this, we expressed C45(z8) agrin in the presence of excess Pofut1 by cotransfecting cDNAs with both genes into Lec1 cells. We then labeled cells with 3H-fucose, purified agrins, and measured fucose incorporation. Overexpression of Pofut1 led to a reduction in C45(z8) secretion (not shown). If scaled up to allow more protein to be produced, however, Pofut1 overexpression led to a 3.2±4-fold increase in 3H-fucose incorporation per mol C45(z8) agrin protein (P=0.01, compared to C45(z8) alone, n=3). This increase more than corrected for the 40% less 3H-fucose in C45(z8) agrin originally observed (Fig. 3). We then performed concentration curves for agrin activity using these two differentially O-fucosylated C45(z8) agrins and found no change in potency (P>0.05 for AChR clustering at 100pM, 200pM, 1nM or 2.5nM vs C45(z8) alone, n=3). Thus, the lower level of fucose on C45(z8) agrin relative to C45(z0) agrin does not correlate with C45(z8) bioactivity, but likely does reflect that C45(z8) agrin is a poorer substrate for Pofut1 than C45(z0) agrin.
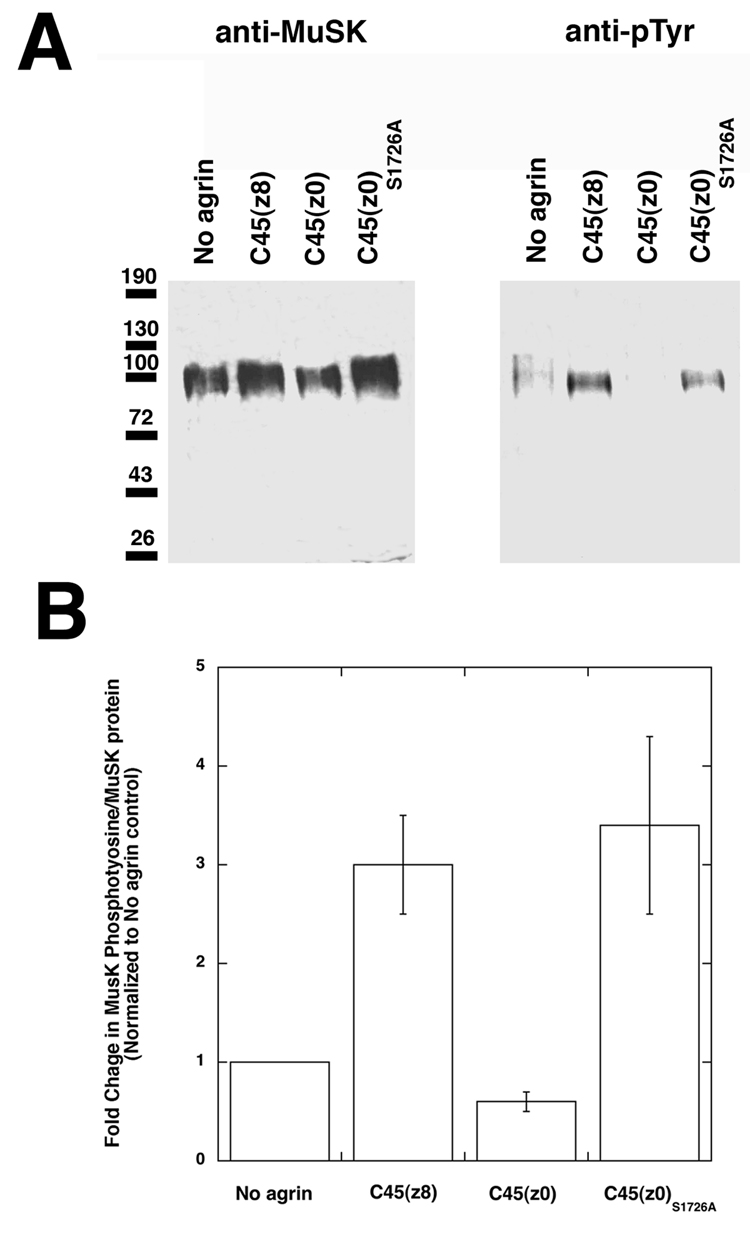
Neural agrin stimulates phosphorylation of the MuSK tyrosine kinase in skeletal muscle cells (Glass et al., 1996), and MuSK expression and phosphorylation are required for agrin activity (DeChiara et al., 1996; Herbst et al., 2002). Therefore, we next determined if the C45(z0)S1726A agrin lacking O-fucose stimulated MuSK phosphorylation (Fig. 5A and B). The time for optimal MuSK autophosphorylation in C2C12 myotubes by C45(z8) neural agrin was ~30 minutes, similar to previous studies (Herbst et al., 2002; Hopf and Hoch, 1998a, b; Watty and Burden, 2002). When recombinant C45(z8) was added to C2C12 myotube cultures, an increase in MuSK tyrosine phosphorylation was observed. As expected, C45(z0) muscle agrin containing fucose showed no activity in this assay, while C45(z0)S1726A lacking fucose stimulated MuSK phosphorylation. The degree of increased phosphotyrosine on MuSK, relative to total MuSK protein, for C45(z8) agrin was 300±52 relative to C45(z0), while it was 359±90 for C45(z0)S1726A relative to C45(z0) (n=3 for both comparisons). Thus, C45(z0)S1726A, like C45(z8), can stimulate MuSK phosphorylation, and this is consistent with a role for MuSK in C45(z0)S1726A-induced AChR clustering.
Figure 5. Activation of MuSK tyrosine phosphorylation by C45(z0)S1726A.
(A) MuSK kinase was immunoprecipitated from C2C12 myotubes after addition of 1nM FLAG-tagged C45(z8), C45(z0), or C45(z0)S1726A agrin and probed with anti-MuSK or anti-phosphotyrosine (pTyr) antibodies by Western blot. C45(z0) agrin did not increase MuSK tyrosine phosphorylation, while C45(z0)S1726A and C45(z8) agrin did. (B) The ratio of phosphotyrosine to MuSK protein is higher for C45(z8) and C45(z0)S1726A agrin relative to C45(z0) or no agrin treatment. Errors are SD for n=3.
Deletion of Pofut1 stimulates AChR clustering and expression in primary myotubes and in skeletal muscles in vivo
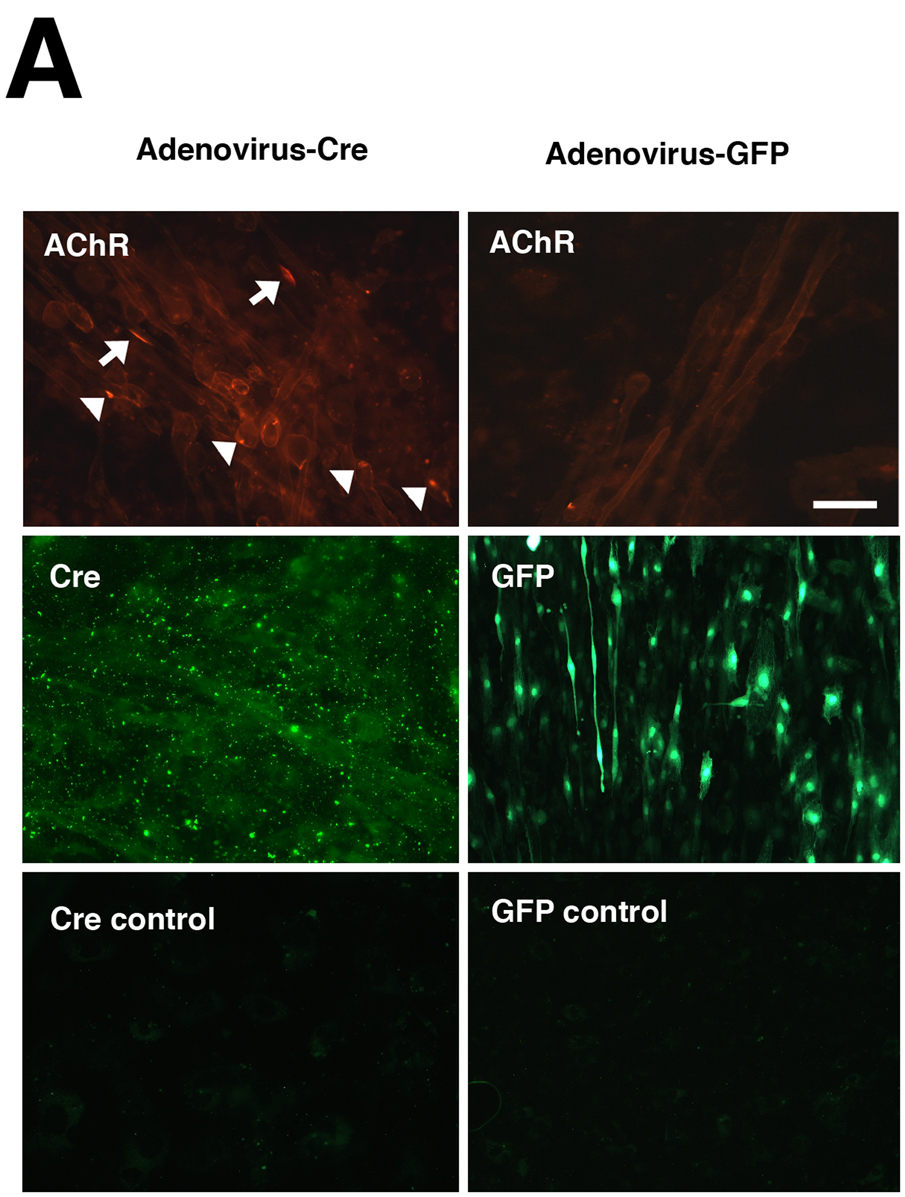
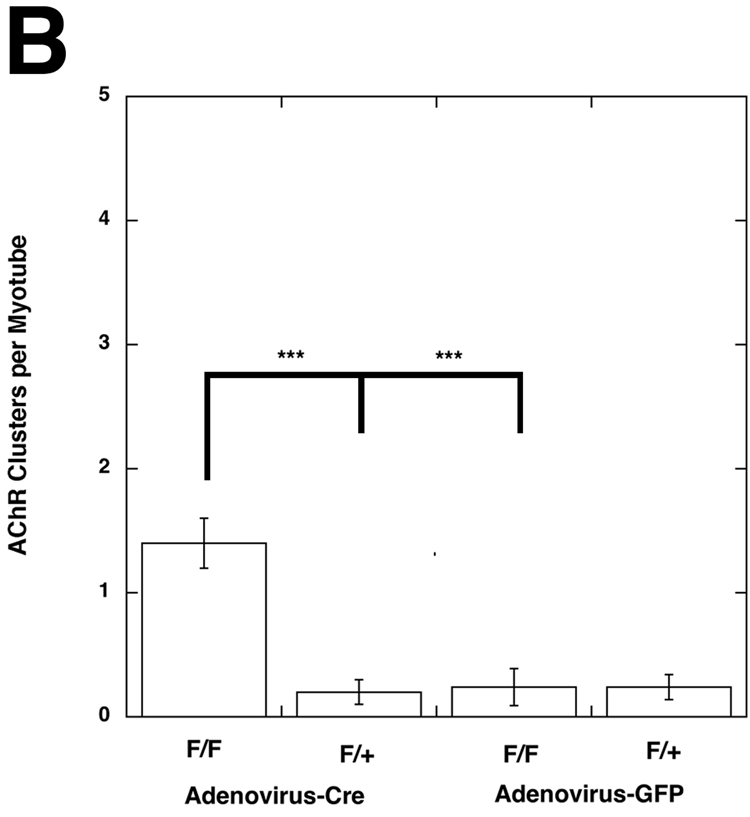
Given that loss of protein O-fucosylation caused C45(z0) agrin to gain the ability to cluster AChR in cultured myotubes, it was important to determine if ectopic AChR clustering would be stimulated in skeletal muscle following elimination of Pofut1 in vivo. We tested this in two ways. First, primary myoblast cells were isolated and grown from mice homozygous (Pofut1F/F) or hemizygous (Pofut1+/F ) for a floxed allele of Pofut1 (Shi et al., 2005). Once confluent, these cells were fused in serum-poor media to stimulate their fusion into myotubes (Fig. 6A). Myotube cultures were then infected with adenovirus bearing either Cre recombinase (to delete Pofut1) or GFP (as a control vector). Virus was incubated with myotubes at >1000 pfu/cell for 5 days. Nearly 100% infection of myotubes was obtained in all experiments. After infection, myotubes were incubated with rhodamine-α-bungarotoxin and stained AChR clusters counted (Martin and Sanes, 1995). Pofut1+/F cells expressing Cre, Pofut1+/F and Pofut1F/F cells expressing GFP showed very low numbers of AChR clusters (Fig. 6B). By contrast, Pofut1F/F muscle cells expressing Cre had a 7-fold induction in AChR clusters 4 days after infection (P<0.001 relative to similarly infected F/+ or Adenovirus-GFP infected F/F, n=4–6, Fig. 6B).
Figure 6. Induction of AChR clustering in primary myotubes after deletion of Pofut1 in vivo.
(A) Primary myotube cultures were made from mice homozyogous for a floxed allele of Pofut1 (F/F). Myotube cultures were incubated with saturating titers of Adenovirus bearing a cDNA for Cre recombinase (Adenovirus-Cre) or Adenovirus bearing a cDNA for GFP (Adenovirus-GFP). Adenovirus–Cre led to uniform, abundant, Cre expression in the majority of myotubes (middle left panel, with control (no primary antibody) lower left), and stimulated formation of both large (arrows) and small (arrowheads) acetylcholine receptor (AChR) aggregates, as evidenced by rhodamine-α-bungarotoxin staining (upper left). Adenovirus-Cre also increased overall bungarotoxin staining of myotube membranes. Adenovirus-GFP led to similar levels of infectivity (middle right), but did not increase AChR aggregates (upper right). Bar is 100µm. (B) Myotubes homozygous for floxed Pofut1 (F/F) that were infected with Adenovirus-Cre had significantly elevated levels of AChR clusters compared to similarly infected myotubes that were hemizyogous for floxed Pofut1 (F/+) or to F/F or F/+ cultures infected with Adenovirus-GFP. Errors are SEM for n=5–6 experiments.
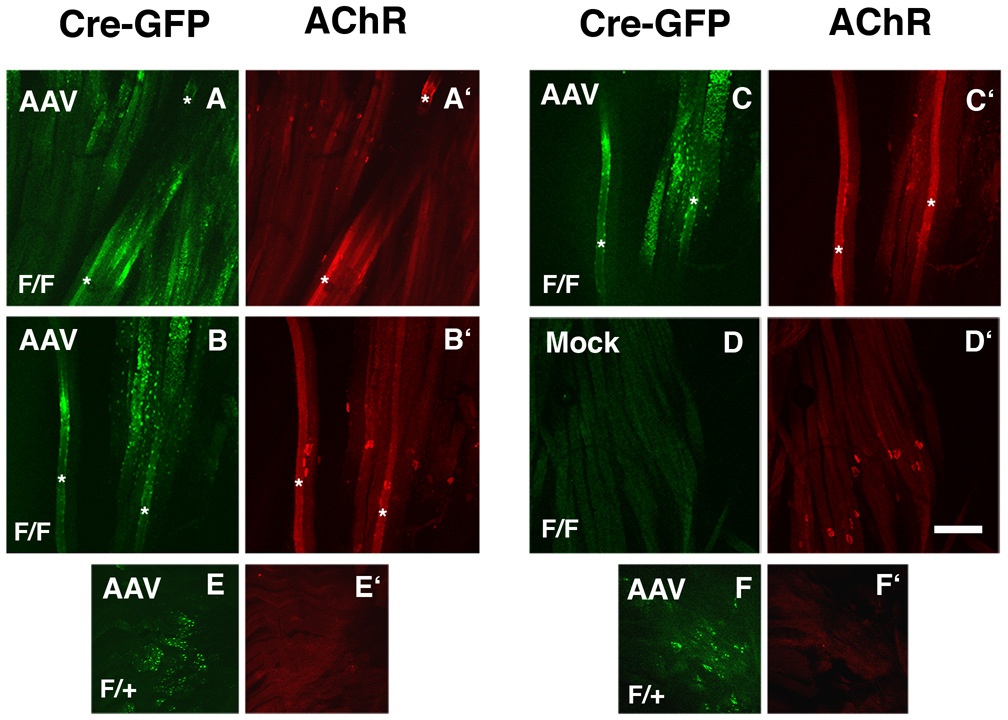
We next investigated skeletal muscles of Pofut1F/F mice (Fig. 7). Skeletal muscles of 3-month old Pofut1F/F and Pofut1+/F mice were infected with 1×1010 vector genomes (vg) of Adeno-associated virus (AAV) bearing a GFP-Cre fusion protein that allows Cre expression to be tracked by GFP fluorescence within myotubes. Eight weeks after infection, teased muscle fiber preparations were analyzed for Cre-GFP expression and for altered AChR clustering. As AAV vectors typically take a week to begin expressing a transgene following infection (Herzog et al., 1997), the experiment allowed 7 weeks for expressed Cre-GFP protein to delete Pofut1 alleles and reduce O-fucosylation. In our experience, AAV-mediated gene expression at this dose in skeletal muscle is saturated by 3–4 weeks post-infection (Xu et al., 2007b). Myofibers from Pofut1F/F mice that expressed GFP-Cre had AChR aggregates in the local vicinity of Cre expression, even when this was removed by several hundred microns from the endplate band (Fig. 7A, A’). In general myofibers with small regions of Cre-GFP expression tended to have distinct AChR aggregates, while myofibers expressing large amounts of Cre-GFP showed overexpression of AChRs along larger regions of the myofiber membrane (asterisks in Fig. 7A,A’,B,B’C,C’). In these instances, small 1–2µm AChR microaggregates were present throughout these fields of bright bungarotoxin staining. Induction of AChR clusters along such myofibers, however, was shown in serial confocal images to be generally confined to regions overexpressing Cre-GFP (Fig. 7B,B’,C,C’). This suggests that denervation was not responsible for the altered AChR clustering pattern. Expression of the same AAV vector in Pofut1F/+ myofibers gave no AChR aggregating activity and did not alter AChR expression (Fig. 7E,E’,F,F’). Mock-infected muscles in Pofut1F/F myotubes also had no Cre-GFP fluorescence in regions where neuromuscular junctions were abundant, demonstrating the specificity of the signal induced by AAV-Cre-GFP (Fig. 7D,D’). These data show that elimination of Pofut1 in adult myofibers can stimulate AChR aggregation and alter AChR density.
Figure 7. Induction of AChR clustering by deletion of Pofut1 in skeletal myofibers.
Mice homozyogous for a floxed allele of Pofut1 (F/F) or heterozygous for this allele (F/+) were infected with Adeno-associated virus (AAV) bearing a Cre-GFP fusion protein (A–E) or mock-infected control (F). Expression of Cre-GFP protein was visualized by endogenous GFP fluorescence (A–F) and AChRs were visualized by labeling with rhodamine-α-bungarotoxin (A’–F’). Expression of GFP-Cre increased AChR clustering and expression in F/F myotubes. Asterisks mark examples of myofibers with regions of high AChR expression and aggregation. B and C are different z sections taken from the same confocal stack. Bar is 200µm.
Expression of Pofut1 and agrin during neuromuscular development
To determine if Pofut1 expression might be regulated during skeletal muscle or brain development, we assessed Pofut1 and agrin mRNA and protein expression, and also Pofut1 enzyme activity on the EGF4 repeat of agrin (Figs. 8A–C). Pofut2, which O-fucosylates thrombospondin repeats, but not to EGF repeats(Luo et al., 2006), and is active in the absence of Pofut1 (Luo et al., 2006)(Shi et al., 2007), was also studied. TaqMan Real-time PCR was used to determine the expression of Pofut1, Pofut2, and agrin mRNA transcripts during embryonic (E11.5 and E15.5) and postnatal (P0, P7, P14, P21, and P42) muscle and brain development (Fig. 8A). At each developmental stage, the amount of gene expression was normalized to 18S rRNA, as before (Xu et al., 2007b). Relative changes in gene expression were then expressed compared to the signal for each individual gene at P42, the latest developmental stage analyzed (Fig. 8A). All three genes had very similar signals at P42 (not shown).
Figure 8. Relative Pofut1 and agrin mRNA and protein expression, and Pofut1 actvity, in developing skeletal muscle.
(A) mRNA was extracted from skeletal muscle or brain at embryonic (E11.5 and E15.5) or postnatal time points (P0, P7, P14, P21, and P42) and subjected to TaqMan Real-time PCR analysis of mRNA expression for Pofut1, Pofut2, or agrin vs 18S rRNA. Relative change in mRNA expression is shown normalized to expression for each gene at P42. Errors are SEM for n=6 samples with 3 measurements per sample. (B) Expression of Pofut1 and agrin protein during development were assessed by Western blot, with GAPDH as a control for protein loading and transfer. 40µg of total cell protein was separated on 4–12% SDS-PAGE gradient gels. Both Pofut1 and agrin protein expression were highest in embryonic mouse muscle, consistent with mRNA expression studies. Immunoblots using two different affinity purified Pofut1 anti-peptide antibodies (Pofut1A and C) are shown. The polyclonal antibody used to blot agrin protein recognizes the C45 domain. Arrow indicates full-length agrin protein. (C) The EGF4 peptide from agrin serves as a substrate for Pofut1 O-fucosylation by GDP-3H-Fucose. Activity was linear (upper left) and required a serine at position 1726 (upper right). Activity was higher in adult (3-month) brain or spinal cord than in skeletal muscle (lower left). In skeletal muscle, Pofut1 enzyme activity was highest during embryogenesis and declined postnatally (lower right). Errors are SEM for n=6 measurements.
In skeletal muscle, the expression of Pofut1 was 20%–30% lower than that of agrin at all stages examined, with the exception of E15.5, where it was reduced by 75% relative to agrin gene expression (P<0.001; for Pofut1/agrin at E15 relative to P0, n=6). The reduced expression of Pofut1 relative to agrin at E15.5 is consistent with the production of muscle agrin with reduced O-fucose at the precise time when AChR aggregates can form along regions of skeletal myofibers outside the neuromuscular junction (Lin et al., 2001). The change in this ratio was predominantly due to increased agrin mRNA expression at this developmental stage. By contrast, the relative expression of Pofut1 in embryonic brain was highest at E11.5 and dropped precipitously at P0, while levels of agrin and Pofut2 were highest at E15.5 and remained high until P7 (Fig. 8A). Thus, the pattern of agrin and Pofut1 expression during brain development differs from the pattern observed in skeletal muscle.
Pofut1 protein expression during skeletal muscle development mirrored changes measured in Pofut1 mRNA expression (Fig. 8B). We used two different affinity purified anti-peptide Pofut1 antibodies (Pofut1A and Pofut1C) to blot whole muscle cell protein (Fig. 8B). Both antibodies yielded similar results. Pofut1 levels were higher at E15.5 than at later developmental stages. GAPDH levels, a control for protein loading and transfer, were not changed (Fig. 8B). Expression of full-length agrin (ca. 400kDa, (Cole and Halfter, 1996)) was high from E17 to P7, but was reduced to undetectable levels by P42 (Fig. 8B). Cleavage of the C45 fragment of agrin was increased during postnatal development at the time when expression of full-length agrin was concomitantly decreased (Fig. 8B). These data are consistent with previously published immunostaining studies showing enriched expression of agrin protein in the muscle basal lamina during late embryonic and early postnatal development, with confinement of agrin to the neuromuscular synapse evident by postnatal day 14 (Hoch et al., 1993). Other studies, however, suggest that extrasynaptic agrin is present in most adult skeletal muscles, though at reduced levels relative to expression at the neuromuscular synapse (Eusebio et al., 2003). Thus, Pofut1 mRNA and protein expression were dynamically regulated during skeletal muscle development.
Last, we developed an enzyme assay to assess Pofut1 glycosylation of agrin more directly (Fig. 8C). This involved synthesizing a biotinylated peptide encoding the EGF4 repeat of agrin. Incubation of non-ionic detergent cell lysates from Pofut1-transfected HEK293T cells with the EGF4 peptide and GDP-3H-Fucose led to linear incorporation of 3H-fucose into the peptide with time. This activity required serine 1726, as a peptide with this serine changed to alanine (EGF4S1726A) showed no activity. Immunodepletion of cell lysates with a cocktail of Pofut1 antibodies also greatly diminished activity (not shown). Pofut1 had activity in adult skeletal muscle, brain, and spinal cord, with the latter two having more activity than the former. In skeletal muscle, Pofut1 activity on the EGF4 repeat was highest at E15, being 8-fold higher than at P42. Pofut1 activity declined in postnatal stages of muscle development, being lowest in adults. Thus, Pofut1 activity on EGF4 of agrin mirrored Pofut1 protein and mRNA expression during skeletal muscle development.
DISCUSSION
By examining the AChR clustering activity of C45(z0) agrin in the presence and absence of Pofut1 activity, we have identified a new role for O-fucosylation in agrin’s synaptogenic activity, and possibly also in neuromuscular development. Loss of O-fucose from recombinant C45(z0) agrin caused it to gain function with regard to AChR clustering, and this gain of function was equivalent in potency to that of the neuron-specific C45(z8) agrin protein (Fig. 2, Fig. 4), the form previously thought to exclusively possess such activity(Ferns et al., 1992; Ferns et al., 1993). This gain of function includes the ability of C45(z0) lacking fucose to stimulate MuSK phosphorylation, which suggests it may stimulate AChR clustering by a mechanism similar to that used by C45(z8) agrin (Fig. 5). Loss of O-fucosylation on C45(z8) neural agrin, however, did not alter its activity (Fig. 2–Fig. 4), nor did increasing its degree of fucosylation. Therefore, the neural splice insert conveys activity to agrin that is likely to be independent of O-fucosylation, and appears to make C45(z8) agrin a poorer substrate for Pofut1 than C45(z0) agrin.
Loss of Pofut1 in skeletal muscle increased AChR aggregation on skeletal myofibers (Fig. 7), indicating that Pofut1 is a negative regulator of AChR clustering by muscle agrin. These in vivo results are consistent with the in vitro results showing that O-fucose-deficient muscle agrin has AChR clustering activity. This suggests that muscle agrin may normally have such clustering activity, particularly during early neuromuscular development, when AChR aggregates can be formed along regions of the myofiber not opposed by a nerve terminal (Lin et al., 2001; Yang et al., 2001) and when muscle agrin is highly expressed in the extrasynaptic basal lamina (Hoch et al., 1993). As MuSK is required for muscle AChR aggregates, it is also possible, and perhaps more likely, that the activity of MuSK is what give rise to these clusters, independent of agrin. Indeed, overexpression of MuSK is sufficient, even in the absence of agrin, to induce postsynaptic membranes (Kim and Burden, 2008). Moreover, aberrant neuromuscular development in vivo appears specific to deletion of the neuron-specific splice form of agrin (Burgess et al., 1999). Thus, increased AChR clustering in Pofut1-deleted muscles may alternatively reflect an agrin-independent mechanism. Indeed, ectopic AChR clusters were found in agrin null animals, both in embryonic and neonatal periods (Gautam et al., 1996; Lin et al., 2001), suggesting that additional agrin-independent mechanisms for AChR cluster formation do exist. Other proteins recently shown to be required for neuromuscular formation, for example LRP4, may also be involved in AChR aggregation (Weatherbee et al., 2006), as could Notch receptors, which are known to be affected by Pofut1 activity (Buono et al., 2006; Okamura and Saga, 2008; Stahl et al., 2008). While unlikely, it is also possible that C45(z0)S1726A acts by forming protein oligomers with C45(z8) or related neural forms of agrin that may arise in serum or in certain cell lines.
There are several reports of the muscle-specific form of agrin possessing AChR clustering activity and these may be relevant to the mechanism of activation described here. The original studies of Scheller and colleagues using native full-length agrin demonstrated AChR aggregating activity of muscle agrin (Campanelli et al., 1991). Likewise, Godfrey and colleagues have shown that muscle agrin cDNAs overexpressed in Xenopus oocytes are equivalent to neural agrin in inducing AChR aggregation in developing frog skeletal muscles (Godfrey et al., 1999). It is intriguing to hypothesize that these experiments may have been conducted in a way that allowed for the production of muscle agrin lacking O-linked fucose, and that the lack of the suppressive activity of Pofut1 allowed such muscle-derived agrin forms to possess bioactivity. Given that recombinant agrin is very potent in AChR clustering activity, with significant activity in the 100pM range (Gesemann et al., 1996; Gesemann et al., 1995), only a relatively small amount of O-fucose-deficient muscle agrin would have to be produced to generate AChR clustering activity.
These experiments, coupled with previous findings, suggest that glycans, both on agrin and on proteins that agrin binds, may affect agrin activity (Martin, 2003b). The best example of this, perhaps, is the fact that glycans on α-dystroglycan are required for agrin binding to that protein (Michele et al., 2002). In addition, glycosylation of one of the mucin regions of agrin by the CT GalNAc transferase (Galgt2) modulates the activity of C120(y4,z8) agrin on cultured muscle cells (Xia and Martin, 2002). Such regulation is not likely to be relevant to the current study, however, as the mucin domain of C120 agrin is not present in the C45 forms used here. Burden and colleagues have shown that the N-linked glycans of MuSK also modulate, though are not required, for MuSK activity (Watty and Burden, 2002). Given that extracellular matrix proteins, including laminins and agrins, bind to muscle membrane proteins in a manner that is affected by glycosylation (Martin, 2003a) and have clear functions in neuromuscular synaptogenesis (Sanes et al., 1998a), it is likely that glycosylation will play important modulatory roles in their function. Because a particular type of glycosylation often affects many processes in a given tissue, the identification of such modulatory influences may not be readily apparent by reverse genetic approaches, where functional redundancy of glycosyltransferases, or conversely lethality, makes analysis more difficult.
EXPERIMENTAL PROCEDURES
Generation of Pofut1 knockdown Lec1 cells
The Silencer®siRNA Construction Kit (Ambion; Austin, TX) was used to prepare siRNAs to eliminate Pofut1 transcripts in Chinese hamster ovary (CHO) cells. Six different siRNAs targeting the coding region of a Chinese hamster Pofut1 cDNA were tested by transient transfection followed by northern analysis using a CHO Pofut1 coding region probe. The sequence 5’-AAGTCCTGATAAGAAGACATG-3’ gave the greatest reduction of Pofut1 transcripts, and was cloned into the vector pSilencer2.1-U6 neo following the procedures described in the pSilencer™ siRNA Expression Vectors Instruction Manual (Ambion; Austin, TX). The resulting construct, pSilencer2.1-U6 neo-Pofut1-MC, was transfected into Pro−5Lec1.3C (termed Lec1) CHO cells (Chen and Stanley, 2003) using LipofectAmine 2000 (Invitrogen; Carlsbad, CA), and selection for stable transfectants was performed using G418 (1.5 mg/ml, Germini) in alpha-MEM media containing 10% fetal calf serum (FCS; Gemini; West Sacramento, CA). Total RNA was prepared from G418-resistant isolates using Trizol (Invitrogen; Carlsbad, CA) and subjected to northern analysis using the Pofut1 cDNA probe. Clones MC1 and MC2 exhibited the least Pofut1 transcripts. Clone MC5, whose Pofut1 transcript level remained the same as parental cells, was used as a negative control.
Notch signaling assay
The co-culture Notch signaling assay was performed essentially as described (Shi et al., 2005). Duplicate cultures of Lec1, MC1, MC2, and MC5 CHO cells were plated at 2 × 105 cells per well of a 6-well plate and cultured at 37°C for 24 hr. Cells were transfected using FuGENE6 (Roche;Nutley, NJ) with 0.2 µg TP-1 Notch reporter plasmid that contains 8 copies of an RBP-J DNA binding sequence driving firefly luciferase expression, 0.05 µg of pRLtk (a plasmid with a Renilla luciferase gene driven by the thymidine kinase promoter), and 1.5 µg of empty pMIRB plasmid. After 16 hr, 2 × 106 L cells expressing rat Jagged1 (and sorted for high Jagged1 expression) or the same number of control L cells (sorted for low Jagged1 expression) were overlaid and co-cultured for ~30 hr Firefly and Renilla luciferase activities were quantitated in cell lysates using a dual luciferase assay according to the manufacturer's instructions (Promega; Madison, WI). Notch ligand-induced signaling is expressed as the ratio of Renilla-normalized firefly luciferase activity in Jagged1/L versus L cell co-cultures.
Cell culture
C2C12 cells (ATCC; Bethesda, MD) were grown in Dulbecco’s modified Eagle’s media (DMEM) with 20% FCS, and 50 µg/ml streptomycin 50 U/ml penicillin (P/S). To fuse cells into myotubes, cells were grown to confluence on 12 mm glass coverslips that had been coated with 0.2% gelatin and then placed in fusion medium (DMEM with 2% horse serum, 50 µg/ml streptomycin, and 50 U/ml penicillin) for 3–4 days. For immunoprecipitation experiments, cells were grown on 10 cm plates and fused in media containing 2% horse serum for 5–6 days. Lec1 cells were grown in DMEM containing 10% FCS and P/S. MC1, MC2, and MC5 cells were grown in alpha MEM media containing ribonucleotides and deoxyribonucleotides, 10% FCS and P/S. Primary muscle cells were cultured from homozygous (Pofut1F/F) or heterozygous (Pofut1+/F) mice (Shi et al., 2005) of 4–8 weeks. Briefly, mice were euthanized and dipped in 70% ethanol. Leg skeletal muscles were dissected and minced in dissociation buffer containing phosphate-buffered saline (PBS), 1.5 mg/ml trypsin (Irvine Scientific; Irvine, CA), 1.0 mg/ml collagenase (Sigma; St. Louis, MO), and 2 mg/ml DNAse I (Boehringer Mannheim; Indianapolis, IN) for 20 min. Tissue was titurated with a 10 ml pipette and diluted with DMEM containing 20% FCS, 4% chick embryo extract, and P/S. Cells were spun at 1000g for 3 min, retiturated in growth medium, pre-plated on tissue culture plastic for 20 min at 37°C to remove intramuscular fibroblasts, and the supernatant divided onto 12 mm glass coverlips that had been coated with 0.2% gelatin. After cells had grown to confluence, they were fused into myofibers by replacing the medium with DMEM containing 2% horse serum, and P/S.
Production and 3H-fucose-radiolabeling of recombinant agrin
FLAG-tagged or untagged cDNAs encoding C45(z8) agrin and C45(z0) agrin, C45(z0)S1726A, C45(z8)S1726A agrin were produced and purified as has been previously described (Xia and Martin, 2002). Recombinant agrin proteins were produced by transfection of the appropriate cDNA into Lec1, MC1, or MC5 cells. 10 cm plates of cells were transfected with 2 µg/ml DNA pre-complexed with Fugene 6 transfection reagent as previously described (Xia and Martin, 2002). Lack of a splice insert in the muscle agrin construct and the presence of the z8 splice insert in neural agrin was verified by PCR using primers that delineate z splice forms (Ferns et al., 1992; Ferns et al., 1993; Hoch et al., 1993). For AChR clustering assays, medium was collected 3 days after transfection and subjected to purification based on the presence of the FLAG epitope using anti-FLAG (M2) antibody-conjugated agarose, as previously described (Xia et al., 2002). Purified protein was characterized using anti-FLAG and anti-agrin immunoblotting and protein amounts were quantitated, as previously described (Xia and Martin, 2002). For 3H-fucose labeling experiments, 10 cm plates of Lec1 cells were transfected with agrin cDNAs as above. The following day, cells were labeled with 4 ml of medium containing 20 µCi/ml 3H[5,6]-fucose (Sigma; St. Louis, MO), essentially as described (Shao et al., 2003). One day later, agrin was purified from the supernatant using M2-agarose and subjected to Western blotting or autoradiography using EN3HANCE-(Perkin Elmer;Boston, MA) soaked gels to visualize labeled protein, and scintillation counting of purified pellets, as described (Shao et al., 2003).
Immunofluorescence analyses
Live C2C12 myotube cultures were labeled for 1 hr with 50 nM rhodamine-α- bungarotoxin (Molecular Probes; Eugene, OR), washed once in PBS, and fixed for 20 min in 2% paraformaldehyde. For primary muscle cultures, cells were further fixed in 2% paraformaldehyde with 0.1% Triton X-100 for 10 min. Cells were washed with PBS two times and blocked in PBS with 10% goat serum for one hr. Primary antibody (1:250 dilution of rabbit anti-Cre polyclonal antibody from Novagen; Madison, WI) or secondary antibody alone were added for one hr, washed four times for 10 min with PBS, stained with goat anti-rabbit FITC at 1:200 for one hr, washed four times for 10 min with PBS, and placed on a glass slide with paraphenylenediamene to inhibit fluorescence quenching. For Adenovirus-GFP-infected cells, GFP fluorescence was visualized using fluorescein-specific optics, while bungarotoxin was visualized using rhodamine-specific optics on a Zeiss epifluorescence microcope. For muscle cells, Adeno-associated virus (AAV)-infected or mock-infected muscles (gastrocnemius and quadriceps) were dissected, teased, washed in PBS, and fixed for 30 min in 2% paraformaldehyde. Cells were then washed in PBS, followed by PBS with 0.1 M glycine, for 4 hr, and blocked in PBS with 10% goat serum. Myofibers were labeled with 50 nM rhodamine-α-bungarotoxin for 12 hr, washed for 12 more hr in PBS, and mounted on glass slides with paraphenylenediamene to inhibit fluorescence quenching. Muscles were visualized using a Zeiss 510 Meta LSM confocal microscope, where Cre/GFP fusion protein was visualized using fluorescein-specific optics to visualize endogenous GFP fluorescence and bungarotoxin was visualized using rhodamine-specific optics.
Determination of MuSK phosphorylation
C2C12 myotube cultures were treated with purified C45(z0), C45(z0)S1726A, or C45(z8) agrin at a concentration of 1 nM for 30 min. After incubation, proteins were solubilized in lysis buffer (1% NP40, 50 mM Tris pH7.4, 150 mM NaCl, 1 mM EDTA, 1 mM NaV,10 mM NaF with protease inhibitor cocktail (Sigma; St. Louis, MO ) and lysates were immunoprecipitated with MuSK antibody (C-19, Santacruz Biotechnology;Santa Cruz, CA) overnight at 4°C. The next day, samples were incubated with protein G agarose beads (Pierce;Rockford, IL) for 2 hr, after which samples were loaded on SDS-PAGE gels and immunoblotted with anti-phosphotyrosine (4G10, Upstate Biotechnology; Lake Placid, NY). The same membrane was then stripped and immunoblotted for MuSK using a rabbit polyclonal antibody.
AChR clustering assays
C2C12 myotube cultures that had been grown on gelatin-coated glass coverslips were incubated with C45(z0), C45(z0)S1726A, C45(z8), C45(z8)S1726A agrin for 6 hr (a time period that allows maximal activation of AChR clustering)(Martin and Sanes, 1995). During the final hr, 50 nM rhodamine-α-bungarotoxin was added to label nicotinic acetylcholine receptors (AChRs). Cells were then washed in PBS, fixed in 2% paraformaldehyde, washed again in PBS, placed on glass slides, and AChR clusters analyzed as before(Martin and Sanes, 1995) using rhodamine-specfic optics.
Virus production
Adenovirus bearing Cre recombinase and Adenovirus bearing GFP were produced and purified by the Viral Vector Core at Nationwide Children’s Hospital. Adeno-associated virus (AAV) bearing a Cre recombinase-GFP fusion protein was produced and purified using triple transfection methods (Xiao et al., 1998) by the Viral Vector Core at Nationwide Children’s Hospital.
Mice
Mice carrying a floxed allele of the Pofut1 gene were previously described (Shi et al., 2005).
RNA Isolation and Real-Time PCR measurement of gene expression
Embryonic (E11.5 and E15.5) and postnatal (P0, P7, and P42) brain and skeletal muscle tissues were harvested in RNALater (Ambion; Austin, TX) and kept frozen at −80°C until RNA isolation. Total RNA was extracted using Trizol reagent (Invitrogen; Carlsbad, CA) following manufacturer’s instructions and was further purified by passing through silica-gel-based membrane (RNeasy-Mini; Qiagen, Valencia, CA). The integrity of RNA was determined by capillary electrophoresis using 6000 Nano LabChip kit on a Bioanalyzer 2100 (Agilent;Palo Alto, CA). RNA content was measured using a ND-1000 spectrophotometer (Nanodrop; Wilmington, DE). No RNA degradation was evident in any of the samples.
3 µg of total RNA was reverse transcribed using High Capacity cDNA Archive Kit (Applied Biosystems; Foster City, CA) in a 100 µL reaction following the instructions provided. Real-time PCR was performed, in triplicate, on a TaqMan ABI 7500 Sequence Detection System (Applied Biosystems; Foster City, CA). Primers and probes for 18S ribosomal RNA (Product No. 4308329), Agrin (Product No. Mm01545840_m1), Pofut1 (Product No.Mm01240154_m1), and Pofut2 (Product No. Mm00518823_m1) were purchased as pre-developed 20X TaqMan assay reagents from Applied Biosystems (Foster City, CA). Each 25 µL PCR reaction mix consisted of 1X primer-probe mix, 1X TaqMan Universal PCR master mix with AmpliTaq Gold DNA polymerase, uracil-N-glycosylase (AmpErase), dNTPs with dUTP, and a passive reference to minimize background fluorescence fluctuations. After an initial hold of 2 min at 50°C to allow activation of AmpErase and 10 minutes at 95°C to activate the AmpliTaq polymerase, the samples were cycled 40 times at 95°C for 15 s and 60°C for 1 min. Gene expression was determined as relative changes by the 2−ΔΔCt method (Livak and Schmittgen, 2001) and the data are presented as fold difference normalized to 18S ribosomal RNA. All measures were done in triplicate for each data point. Relative changes in gene expression during development were normalized to the 2−ΔΔCt value at P42 to determine the fold change.
Infection of muscle cells with Adenovirus-Cre or Adenovirus-GFP
Primary myoblasts were isolated from adult mice, aged 6–10 weeks, that were homozygous or heterozygous for the Pofut1 floxed allele (F/F or F/+), as previously described (Xia et al., 2002). Myoblasts were grown to confluence on 10 cm gelatin-coated glass coverslips and fused into myotubes for 4 days. Cells were then infected with 109pfu/well of Adenovirus-Cre or of Adenovirus-GFP in low serum media. Virus was left on the cells for 5–6 days. One hr prior to fixation, cells were incubated live with 50 nM rhodamine-α-bungarotoxin at 37°C in normal fusion media (DMEM, 2% horse serum, P/S). Cells were then washed with PBS, fixed in 2% paraformaldehyde for 20 min and in 2% paraformaldehyde with 0.1% Tween-20 for 10 min Cells infected with Adenovirus-Cre were stained with anti-Cre antibody (Novagen; Madison, WI) and appropriate secondary antibody. Cells infected with Adenovirus-GFP were fixed in 2% paraformaldehyde, washed with PBS, and analyzed.
Intramuscular injection of AAV-Cre/GFP
AAV-Cre/GFP (1×1010vg) was injected in 50 µl of sterile PBS into the left gastrocnemius muscle and into the left quadriceps muscle of 3 month-old mice homozygous or heterozygous for the Pofut1 floxed allele (F/F or F/+). Sterile PBS (50µl) was injected into the right gastrocnemius and quadriceps muscle of the same animals as mock control (n=3–4 age-matched for each condition and each muscle type). Two months after injection, mice were sacrificed and muscles dissected. Muscles were teased further and stained with rhodamine-α-bungarotoxin using whole mount methods as previously described (Jayasinha et al., 2003).
Production of Pofut1 anti-peptide polyclonal antibodies
Pofut1 antibodies were raised by immunizing rabbits with the following peptides against mouse Pofut1 sequence; Pofut1A, CQRFPAKEHPVLALPG (aa 186–199), Pofut1B, CQFPVLEEHRELQKY (203–216) and Pofut1C, CFGMDRPSQLRDEF (381–393) (Sigma antibody production, St. Louis MO). Sera from immunized rabbits were purified using SulfoLink® Immobilization Kit for Peptides (Pierce, IL) according to the manufacturer’s instructions. Each peptide immunogen was immobilized on column through thioether bond and antibody with affinity for the peptide was purified and eluted using methods previously described (Xia et al., 2002). Blockage with each peptide (as before (Xia et al., 2002)) eliminated Pofut1 bands on Western blots (not shown).
Assay of Pofut1 activity on the EGF4 repeat of agrin
To test fucosylation activity of Pofut1 in vitro, the peptide of the fourth EGF domain of agrin (EGF4) was used as an acceptor substrate with biotin conjugated at the N-terminus (Biotin-DHPCTQALGNPCLNGGSCVPREATYECLCPGGFSGLHCEKG) (AnaSpec; San Jose, CA). Cell lysates from HEK293T cells transfected with a cDNA for CHO Pofut1 were prepared with lysis buffer (50mM Tris, pH 7.4, 150mM NaCl, 1% NP-40 and protease inhibitor cocktail) and protein amount quantitated in triplicate as before(Xia et al., 2002). 80µg of total cell lysates were diluted from concentrated extracts into the following buffer for activity assays: 100mM Imidazole pH7.0, 50mM MnCl2, 0.1mM GDP-Fucose with 40,000 cpm (15–35 Ci/mol) of GDP-(2,3) 3H-Fucose (Perkin Elmer, MA) and 20µM biotinylated EGF4 peptide. Biotin-EGF4S1726A, which is mutated at serine residue 1726 to alanine, thereby eliminating the consensus Pofut1 O-fucosylation site, was used as a control and never yielded any positive signal. Reactions were incubated at 37°C for 2–4hr and then stopped by adding cold 0.25M EDTA, pH8.0. Reactions up to 4 hours remained linear with regard to Pofut1 activity. Biotinylated peptides were pulled down with Streptavidin-agarose (Pierce, IL) and radioactivity was measured with scintillation counter. HEK293T cell lysates without Pofut1 transfection and reactions without peptides were used as controls, and never exceeded 20% of signal from transfected cells (not shown). In addition, over half of the activity could be immunodepleted using anti-Pofut1 antibodies (not shown). To measure Pofut1 activity during development, E15, E17, E20, P0, P7, P14, P21 and P42 leg muscles were dissected dissolved in lysis buffer (50mM Tris, pH 7.4, 150mM NaCl, 1% NP-40 and protease inhibitor cocktail, as before (Xu et al., 2007a). 100µg of protein lysates were used for Pofut1 activity assay, as above, with or without biotin-EGF4. Similar extractions were performed on adult skeletal muscle, brain, and spinal cord preparations for those comparisons. Reactions were incubated at 37°C for 4hr and then stopped by adding cold 0.25M EDTA, pH8.0. Biotin-EGF4 peptides were pulled down with Streptavidin-agarose (Pierce, IL) and radioactivity was measured with scintillation counter.
Statistics
Significance was assessed using a paired Student’s t test, with equal weighting between samples. Such determinations are described in the text of the results section.
Acknowledgements
This work was supported by NCI grant RO1 95022 to P.S. and NIH grants RO1 AR050202 and RO1 AR047922 to P.T.M. We thank Jihua Chen (Albert Einstein College of Medicine) for the Chinese hamster Pofut1 cDNA sequence, K. Reed Clark (The Research Institute at Nationwide Children’s Hospital) for Adenovirus-GFP, Bing Xia (University of California, San Diego) for help with preliminary experiments, and Neha Singhal (The Research Institute at Nationwide Children’s Hospital) for production of Pofut1 antibodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bowe MA, Deyst KA, Leszyk JD, Fallon JR. Identification and purification of an agrin receptor from Torpedo postsynaptic membranes: a heteromeric complex related to the dystroglycans. Neuron. 1994;12:1173–1180. doi: 10.1016/0896-6273(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Buono KD, Robinson GW, Martin C, Shi S, Stanley P, Tanigaki K, Honjo T, Hennighausen L. The canonical Notch/RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev Biol. 2006;293:565–580. doi: 10.1016/j.ydbio.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Burgess RW, Dickman DK, Nunez L, Glass DJ, Sanes JR. Mapping sites responsible for interactions of agrin with neurons. J Neurochem. 2002;83:271–284. doi: 10.1046/j.1471-4159.2002.01102.x. [DOI] [PubMed] [Google Scholar]
- Burgess RW, Nguyen QT, Son YJ, Lichtman JW, Sanes JR. Alternatively spliced isoforms of nerve- and muscle-derived agrin: their roles at the neuromuscular junction. Neuron. 1999;23:33–44. doi: 10.1016/s0896-6273(00)80751-5. [DOI] [PubMed] [Google Scholar]
- Campanelli JT, Gayer GG, Scheller RH. Alternative RNA splicing that determines agrin activity regulates binding to heparin and alpha-dystroglycan. Development. 1996;122:1663–1672. doi: 10.1242/dev.122.5.1663. [DOI] [PubMed] [Google Scholar]
- Campanelli JT, Hoch W, Rupp F, Kreiner T, Scheller RH. Agrin mediates cell contact-induced acetylcholine receptor clustering. Cell. 1991;67:909–916. doi: 10.1016/0092-8674(91)90364-5. [DOI] [PubMed] [Google Scholar]
- Campanelli JT, Roberds SL, Campbell KP, Scheller RH. A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell. 1994;77:663–674. doi: 10.1016/0092-8674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Chen W, Stanley P. Five Lec1 CHO cell mutants have distinct Mgat1 gene mutations that encode truncated N-acetylglucosaminyltransferase I. Glycobiology. 2003;13:43–50. doi: 10.1093/glycob/cwg003. [DOI] [PubMed] [Google Scholar]
- Cole GJ, Halfter W. Agrin: an extracellular matrix heparan sulfate proteoglycan involved in cell interactions and synaptogenesis. Perspect Dev Neurobiol. 1996;3:359–371. [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, Smith C, DiStefano PS, Glass DJ, Burden SJ, Yancopoulos GD. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Henry MD, Campbell KP. Dystroglycan in development and disease. Curr Opin Cell Biol. 1998;10:594–601. doi: 10.1016/s0955-0674(98)80034-3. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio A, Oliveri F, Barzaghi P, Ruegg MA. Expression of mouse agrin in normal, denervated and dystrophic muscle. Neuromuscul Disord. 2003;13:408–415. doi: 10.1016/s0960-8966(03)00036-1. [DOI] [PubMed] [Google Scholar]
- Ferns M, Hoch W, Campanelli JT, Rupp F, Hall ZW, Scheller RH. RNA splicing regulates agrin-mediated acetylcholine receptor clustering activity on cultured myotubes. Neuron. 1992;8:1079–1086. doi: 10.1016/0896-6273(92)90129-2. [DOI] [PubMed] [Google Scholar]
- Ferns MJ, Campanelli JT, Hoch W, Scheller RH, Hall Z. The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron. 1993;11:491–502. doi: 10.1016/0896-6273(93)90153-i. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994;77:675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Gesemann M, Cavalli V, Denzer AJ, Brancaccio A, Schumacher B, Ruegg MA. Alternative splicing of agrin alters its binding to heparin, dystroglycan, and the putative agrin receptor. Neuron. 1996;16:755–767. doi: 10.1016/s0896-6273(00)80096-3. [DOI] [PubMed] [Google Scholar]
- Gesemann M, Denzer AJ, Ruegg MA. Acetylcholine receptor-aggregating activity of agrin isoforms and mapping of the active site. J Cell Biol. 1995;128:625–636. doi: 10.1083/jcb.128.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, DiStefano PS, Valenzuela DM, DeChiara TM, Yancopoulos GD. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- Godfrey EW, Roe J, Heathcote RD. Overexpression of agrin isoforms in Xenopus embryos alters the distribution of synaptic acetylcholine receptors during development of the neuromuscular junction. Dev Biol. 1999;205:22–32. doi: 10.1006/dbio.1998.9104. [DOI] [PubMed] [Google Scholar]
- Herbst R, Avetisova E, Burden SJ. Restoration of synapse formation in Musk mutant mice expressing a Musk/Trk chimeric receptor. Development. 2002;129:5449–5460. doi: 10.1242/dev.00112. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Hagstrom JN, Kung SH, Tai SJ, Wilson JM, Fisher KJ, High KA. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci U S A. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenberg LG, Su H, Gu H, O'Dowd DK, Smith MA. Alpha3Na+/K+-ATPase is a neuronal receptor for agrin. Cell. 2006;125:359–369. doi: 10.1016/j.cell.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Hoch W, Ferns M, Campanelli JT, Hall ZW, Scheller RH. Developmental regulation of highly active alternatively spliced forms of agrin. Neuron. 1993;11:479–490. doi: 10.1016/0896-6273(93)90152-h. [DOI] [PubMed] [Google Scholar]
- Hopf C, Hoch W. Dimerization of the muscle-specific kinase induces tyrosine phosphorylation of acetylcholine receptors and their aggregation on the surface of myotubes. J Biol Chem. 1998a;273:6467–6473. doi: 10.1074/jbc.273.11.6467. [DOI] [PubMed] [Google Scholar]
- Hopf C, Hoch W. Tyrosine phosphorylation of the muscle-specific kinase is exclusively induced by acetylcholine receptor-aggregating agrin fragments. Eur J Biochem. 1998b;253:382–389. doi: 10.1046/j.1432-1327.1998.2530382.x. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Jayasinha V, Nguyen HH, Xia B, Kammesheidt A, Hoyte K, Martin PT. Inhibition of dystroglycan cleavage causes muscular dystrophy in transgenic mice. Neuromuscul Disord. 2003;13:365–375. doi: 10.1016/s0960-8966(03)00040-3. [DOI] [PubMed] [Google Scholar]
- Kim N, Burden SJ. MuSK controls where motor axons grow and form synapses. Nat Neurosci. 2008;11:19–27. doi: 10.1038/nn2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo Y, Nita-Lazar A, Haltiwanger RS. Two distinct pathways for O-fucosylation of epidermal growth factor-like or thrombospondin type 1 repeats. J Biol Chem. 2006;281:9385–9392. doi: 10.1074/jbc.M511974200. [DOI] [PubMed] [Google Scholar]
- Martin PT. Dystroglycan glycosylation and its role in matrix binding in skeletal muscle. Glycobiology. 2003a;13:55R–66R. doi: 10.1093/glycob/cwg076. [DOI] [PubMed] [Google Scholar]
- Martin PT. Glycobiology of the neuromuscular junction. J Neurocytol. 2003b;32:915–929. doi: 10.1023/B:NEUR.0000020632.41508.83. [DOI] [PubMed] [Google Scholar]
- Martin PT, Sanes JR. Role for a synapse-specific carbohydrate in agrin-induced clustering of acetylcholine receptors. Neuron. 1995;14:743–754. doi: 10.1016/0896-6273(95)90218-x. [DOI] [PubMed] [Google Scholar]
- Martin PT, Sanes JR. Integrins mediate adhesion to agrin and modulate agrin signaling. Development. 1997;124:3909–3917. doi: 10.1242/dev.124.19.3909. [DOI] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Saga Y. Pofut1 is required for the proper localization of the Notch receptor during mouse development. Mech Dev. 2008 doi: 10.1016/j.mod.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Panin VM, Shao L, Lei L, Moloney DJ, Irvine KD, Haltiwanger RS. Notch ligands are substrates for protein O-fucosyltransferase-1 and Fringe. J Biol Chem. 2002;277:29945–29952. doi: 10.1074/jbc.M204445200. [DOI] [PubMed] [Google Scholar]
- Rupp F, Payan DG, Magill-Solc C, Cowan DM, Scheller RH. Structure and expression of a rat agrin. Neuron. 1991;6:811–823. doi: 10.1016/0896-6273(91)90177-2. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Apel ED, Burgess RW, Emerson RB, Feng G, Gautam M, Glass D, Grady RM, Krejci E, Lichtman JW, Lu JT, Massoulie J, Miner JH, Moscoso LM, Nguyen Q, Nichol M, Noakes PG, Patton BL, Son YJ, Yancopoulos GD, Zhou H. Development of the neuromuscular junction: genetic analysis in mice. J Physiol Paris. 1998a;92:167–172. doi: 10.1016/s0928-4257(98)80004-1. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Apel ED, Gautam M, Glass D, Grady RM, Martin PT, Nichol MC, Yancopoulos GD. Agrin receptors at the skeletal neuromuscular junction. Ann N Y Acad Sci. 1998b;841:1–13. doi: 10.1111/j.1749-6632.1998.tb10905.x. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Shao L, Moloney DJ, Haltiwanger R. Fringe modifies O-fucose on mouse Notch1 at epidermal growth factor-like repeats within the ligand-binding site and the Abruptex region. J Biol Chem. 2003;278:7775–7782. doi: 10.1074/jbc.M212221200. [DOI] [PubMed] [Google Scholar]
- Shi S, Ge C, Luo Y, Hou X, Haltiwanger RS, Stanley P. The threonine that carries fucose, but not fucose, is required for Cripto to facilitate Nodal signaling. J Biol Chem. 2007;282:20133–20141. doi: 10.1074/jbc.M702593200. [DOI] [PubMed] [Google Scholar]
- Shi S, Stahl M, Lu L, Stanley P. Canonical Notch signaling is dispensable for early cell fate specifications in mammals. Mol Cell Biol. 2005;25:9503–9508. doi: 10.1128/MCB.25.21.9503-9508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Stanley P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc Natl Acad Sci U S A. 2003;100:5234–5239. doi: 10.1073/pnas.0831126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M, Uemura K, Ge C, Shi S, Tashima Y, Stanley P. Roles of Pofut1 and O-fucose in mammalian Notch signaling. J Biol Chem. 2008;283:13638–13651. doi: 10.1074/jbc.M802027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strochlic L, Cartaud A, Cartaud J. The synaptic muscle-specific kinase (MuSK) complex: new partners, new functions. Bioessays. 2005;27:1129–1135. doi: 10.1002/bies.20305. [DOI] [PubMed] [Google Scholar]
- Sugiyama J, Bowen DC, Hall ZW. Dystroglycan binds nerve and muscle agrin. Neuron. 1994;13:103–115. doi: 10.1016/0896-6273(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Timpl R, Tisi D, Talts JF, Andac Z, Sasaki T, Hohenester E. Structure and function of laminin LG modules. Matrix Biol. 2000;19:309–317. doi: 10.1016/s0945-053x(00)00072-x. [DOI] [PubMed] [Google Scholar]
- Tsim KW, Ruegg MA, Escher G, Kroger S, McMahan UJ. cDNA that encodes active agrin. Neuron. 1992;8:677–689. doi: 10.1016/0896-6273(92)90089-v. [DOI] [PubMed] [Google Scholar]
- Watty A, Burden SJ. MuSK glycosylation restrains MuSK activation and acetylcholine receptor clustering. J Biol Chem. 2002;277:50457–50462. doi: 10.1074/jbc.M208664200. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006;133:4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- Xia B, Hoyte K, Kammesheidt A, Deerinck T, Ellisman M, Martin PT. Overexpression of the CT GalNAc transferase in skeletal muscle alters myofiber growth, neuromuscular structure, and laminin expression. Dev Biol. 2002;242:58–73. doi: 10.1006/dbio.2001.0530. [DOI] [PubMed] [Google Scholar]
- Xia B, Martin PT. Modulation of agrin binding and activity by the CT and related carbohydrate antigens. Mol Cell Neurosci. 2002;19:539–551. doi: 10.1006/mcne.2001.1095. [DOI] [PubMed] [Google Scholar]
- Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Camboni M, Martin PT. Postnatal overexpression of the CT GalNAc transferase inhibits muscular dystrophy in mdx mice without altering muscle growth or neuromuscular development: Evidence for a utrophin-independent mechanism. Neuromuscul Disord. 2007a doi: 10.1016/j.nmd.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Chandrasekharan K, Yoon JH, Camboni M, Martin PT. Overexpression of the cytotoxic T cell (CT) carbohydrate inhibits muscular dystrophy in the dyW mouse model of congenital muscular dystrophy 1A. Am J Pathol. 2007b;171:181–199. doi: 10.2353/ajpath.2007.060927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Arber S, William C, Li L, Tanabe Y, Jessell TM, Birchmeier C, Burden SJ. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30:399–410. doi: 10.1016/s0896-6273(01)00287-2. [DOI] [PubMed] [Google Scholar]
- Yang X, Li W, Prescott ED, Burden SJ, Wang JC. DNA topoisomerase IIbeta and neural development. Science. 2000;287:131–134. doi: 10.1126/science.287.5450.131. [DOI] [PubMed] [Google Scholar]