Short abstract
Two genome sequences of diatoms, marine single-celled photosynthetic eukaryotes that often have ornate cell walls, give insights into their metabolic and signaling systems.
Abstract
The results of two published genome sequences from marine diatoms provide basic insights into how these remarkable organisms evolved to become one of the most successful groups of eukaryotic algae in the contemporary ocean.
Diatoms are one of the most successful clades of eukaryotic, single-celled photosynthetic organisms in the contemporary ocean [1]. Their hallmark feature is an ornate, siliceous cell wall (Figure 1). Diatoms often form extensive blooms in temperate and boreal seas. Their productivity supports most of the world's fisheries and their fossilized remains are the major source of petroleum.
Figure 1.
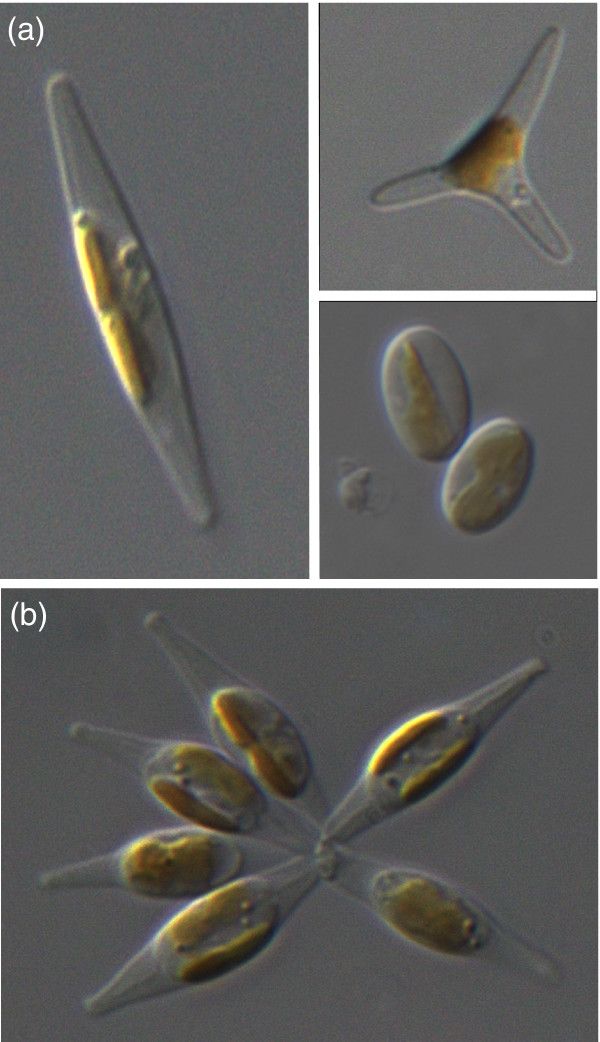
The pennate diatom Phaeodactylum tricornutum. (a) Light micrographs showing the three morphotypes of P. tricornutum: left, fusiform; top right, triradiate; bottom right, oval. (b) Light micrographs of a small cluster of cells of P. tricornutum. Each cell is approximately 15 μm in length. Images courtesy of Alessandra De Martino.
Diatoms are secondary symbionts, derived from the engulfment by a heterotrophic eukaryote host cell of a red alga, which then became integrated as a plastid [2]. Although their chromalveolate ancestor probably arose over a billion years ago [3], long before evidence of animal life, the first diatoms do not appear in the fossil record until about 146 million years ago and rose to ecological prominence only about 35 million years ago. Two major clades of diatoms are distinguished by 'body' plans: a radially symmetrical 'centric' form (Figure 2), which is ancestral to a bilaterally symmetrical 'pennate' form (Figure 1). Together, these two groups comprise about 20,000 morphological species [4], although it is believed, on the basis of molecular genetic analyses, that there are over 100,000 cryptic species [5]. In an effort to elucidate how diatoms evolved and rose to ecological prominence, the genomes of two species have been completely sequenced at the Joint Genome Institute: Thalassiosira pseudonana (Figure 2), a centric species [6], and Phaeodactylum tricornutum (Figure 1), a distantly related, recently evolved pennate species [7]. Although these two species diverged over 90 million years ago, about 60% of their genome is shared. Here we briefly review what the genomic analyses have revealed so far. Several other diatom genome sequences are in the pipeline; these include the psychrophilic diatom Fragilariopsis cylindrus, which is common in polar seas and sea ice, and Pseudonitzschia multiseries, which produces the neurotoxin domoic acid.
Figure 2.
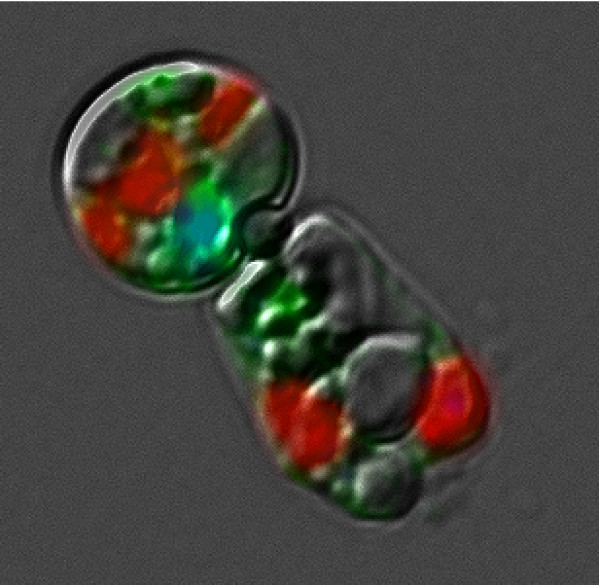
Merged differential interference contrast and epifluorescence microscope image of two cells of the centric diatom Thalassiosira pseudonana. Red, chlorophyll autofluorescence; blue, DAPI staining showing the nucleus; green, overexpressed green fluorescent protein (GFP) derived from transforming the cell with a GFP gene. The cell is shaped like a long can. The circular cell is a valve (end-on) view; the diameter is about 5 μm. The adjacent cell is lying on its side.
Basic genome structure and modes of evolution
The vegetative cells of diatoms are diploid, and the genomes are relatively large, containing approximately 30 megabases with 10,000-12,000 predicted genes. Approximately 95% of the DNA is non-coding (Table 1). Diatoms are one of the most rapidly evolving eukaryotic taxa on Earth [8]. The rapid tempo of evolution is suggested to be due to a high proportion of long terminal repeat (LTR) retrotransposons and other transposable elements as well as insertion/deletion mutation (indels). The prevalence of transcripts from LTR retrotransposons in several diatom expressed sequence tag (EST) libraries [9] is hypothesized to be related to their possible role in adaptation to stress conditions, especially nutrient limitation (Maumus F, Allen AE, Jabbari K, Vardi A, Bowler C, unpublished observations). The P. tricornutum genome contains over 50% of the introns found in T. pseudonana, whereas the latter shares less than 10% of conserved intron positions present in the chromalveolate ancestor. Moreover, the evolution of indels in T. pseudonana appears to be extremely rapid and follows a logistic rate that is proportional to genome size [8,10]. Unlike in multicellular plants, however [11], large scale duplication events do not seem to have a pivotal role in the evolution of diatom genomes, as shown by the similar numbers of genes in the two species (Table 1).
Table 1.
Comparison of the genome properties of Thalassiosira pseudonana and Phaeodactylum tricornutum genomes*
| Thalassiosira pseudonana | Phaeodactylum tricornutum | |
| Genome size (Mb) | 32.4 | 27.4 |
| Predicted genes | 11,776 | 10,402 |
| Introns | 17,880 | 8,169 |
| Number of chromosomes | 24 | 33 |
| G+C content | About 48% | About 47% |
| Percentage of genome that is non-coding | About 97% | About 94% |
| ESTs in GenBank | 61,913 | 133,871 |
A second, more surprising source of genetic variability is horizontal gene transfer (HGT). Phylogenetic analysis of P. tricornutum suggests that about 5% of the genome (587 genes) is derived from bacterial orthologs; more than half of these are shared with T. pseudonana, implying that they were acquired by diatoms early in their evolutionary history and perform essential functions [6,7]. In particular, several genes of prokaryotic origin seem to have been recruited for metabolism of organic carbon and nitrogen, including genes involved in a urea cycle that probably evolved in the primordial heterotrophic host cell before acquisition of the secondary symbiont. The mechanism of HGT in diatoms is not understood. Viral infection is one obvious pathway; indeed, several viruses, including single-stranded RNA and single- and double-stranded DNA types, have been isolated that target specific diatoms [12]. Virally mediated HGT can be inferred from the gene encoding a putative photo-receptor, phytochrome, that is clustered in the P. tricornutum genome with two viruses that infect brown algae [13]. Other mechanisms proposed to facilitate acquisition of bacterial genes by HGT include phagotrophy and association with organelles or with intracellular endosymbionts or parasites. Furthermore, 22 genes in the diatoms are of chlamydial origin [14]; these genes were hypothesized to be derived from an ancient endosymbiosis event between chlamydiae and the ancestor of primary photosynthetic eukaryotes [15].
Core metabolic pathways
In the ocean, essential nutrients such as nitrate, phosphate and silicate are brought up to the surface from the interior by wind-driven mixing (for example, storms) or deep convection. Diatoms assimilate these nutrients very rapidly in excess of their immediate growth demands, storing the nutrients in a special compartment (a vacuole) and then using them for macromolecular biosynthesis [16]. The genome sequences [6,7] have revealed the unique nature of nitrogen cycling in diatoms: a catabolic urea cycle has been identified involving ornithine and citruline and potentially yielding urea and subsequently ammonia from hydrolysis of the substrate by urease. However, diatoms do not excrete inorganic nitrogen; rather the catabolic end-products of the urea cycle are themselves returned back to anabolic pathways that initially yield glutamine and glutamate (via the glutamine synthetase/glutamate synthase (GS/GOGAT) pathway) [17]. Indeed, this efficient recycling of nutrients in diatoms was probably a major selective force for the evolution of the secondary symbiont; it prevented the original heterotrophic host cell from losing a valuable nutrient, while simultaneously photosynthesis in the newly acquired protoplast provided a steady supply of organic carbon skeletons essential for growth [4].
The primary mode of nutrition in diatoms is oxygenic photosynthesis. Although the core machinery for this process is highly conserved, it has been known since the mid-1970s that the affinity of diatoms for inorganic carbon is considerably higher than that of their primary carbon-fixing enzyme, Rubisco, for CO2, suggesting that diatoms must concentrate inorganic carbon in their cells [18]. Metabolic studies on the biochemistry of photosynthesis in the related diatom Thalassiosira weissflogii suggest that a C4-like photosynthetic pathway, in which the initial product of carbon fixation is a four-carbon molecule such as malate or oxaloacetate, indeed operates in diatoms. In this model, these molecules would subsequently be translocated to the plastid and be decarboxylated, thereby increasing the local concentration of CO2 for Rubisco [19,20]. In silico analysis of the diatom genome has revealed a complete suite of genes required for C4 metabolism [6,21], but how the system actually operates remains unclear. Sequences of the two enzymes responsible for decarboxylation of oxaloacetate and malate suggest that the proteins are targeted to the mitochondria. If so, this would require CO2 to cross six intra-cellular membranes, from its source (the mitochondria, two membranes) to its sink (the plastid, a further four membranes); a seemingly inefficient system, as the mitochondria is clearly a major intracellular source of CO2 simply as a result of respiration. The localization of the first carboxylation step in the C4 pathway is also still unclear. Determination of the cellular localization of key enzymes and of the expression of C4-related genes in cells exposed to low levels of CO2 could resolve these issues.
The formation of the silicate-based cell wall in diatoms is one of the most interesting areas of research. Silicic acid is translocated across the plasma membrane via specific transporters and is subsequently conveyed to a silica deposition vesicle, a slightly acidic environment in which the new cell wall is completely formed before it is exported by exocytosis. Silaffins and long-chain polyamines have a role in the polymerization of silica, but the mechanism of pattern formation remains unknown. Genome analysis reveals that T. pseudonana contains three silicon transporters [22] and three silaffin genes [23]. P. tricornutum, however, is an atypical diatom in that it does not have an obligate requirement of silicon for growth and exists as three distinct morphotypes: oval, triradiate and fusiform (Figure 1a). Only the oval morphotype contains a lightly silicified valve [24] and is the only diatom reported to take up the anionic form of silicon (silicate, or SiO(OH-)3), rather than the more commonly transported form, orthosilicic acid (Si(OH)4) [25]. Genome sequencing [7] revealed genes for four silicon transporters in P. tricornutum, all with strong support from ESTs, but only one silaffin-like protein.
Iron limits primary production in three major areas of the ocean: the eastern equatorial Pacific, the subarctic Pacific and the Southern Ocean. So far, 11 iron enrichment experiments have been conducted in the open ocean, covering all three environments; these involve adding iron to the sea in order to stimulate growth of phytoplankton. In all experiments, the first major group of organisms to grow following fertilization was pennate diatoms. One major factor in the ability of diatoms to take advantage of the nutrient enrichment is the vacuole, a sort of 'food pantry', which does not, as yet, have a clear genetic marker. However, analysis of the T. pseudonana and P. tricornutum genomes has revealed the presence of several Fe acquisition and storage genes in P. tricornutum that are absent from T. pseudonana. Iron acquisition in T. pseudonana seems to work through a ferroxidase/permease pathway for Fe(II) uptake. In contrast, P. tricornutum may acquire iron through a cell-surface reductase. A recent discovery of iron storage ferritin in bloom-forming pennate diatoms contributes to their success in chronically low-iron oceanic regions [26]. More data are required before we can be sure that this strategy can explain the success of pennate diatoms specifically in low Fe environments.
Signaling and regulation
Diatoms use sophisticated mechanisms to monitor and adapt appropriately to changes in environmental stress conditions [27,28]. The mosaic multi-lineage nature of the diatom genomes predicts interesting signaling pathways that are similar to features not only of plants and animals but also of prokaryotes. Both diatom genomes contain a bacterially derived two-component system composed of a novel domain organization of histidine kinase (sensor) and response regulator (transcriptional activators) [6,7]. Calcium and nitric oxide were recently shown to act as important second messengers in diatom perception and transduction of stress conditions. A novel calcium-regulated protein, induced by nitric oxide (NO) and regulating cell death, has also been identified [29]. Furthermore, a diatom alternative oxidase contains a calcium-binding EF-hand domain that is induced under iron starvation [30]. Genetic manipulation of a chloroplast-localized protein PtNOA in diatoms has revealed the interplay between sensing chemicals cues (info-chemicals), oxidative stress and cell death through NO-based signaling [31].
One of the more enigmatic aspects of evolution of protists is the emergence of programmed cell death pathways. T. pseudonana has homologs to key components of programmed cell death biochemical machinery, including metacaspases, HtrA-family proteases, apoptosis-associated nuclear factors of the E2F and DP1 families, cell death suppressor proteins and a cellular apoptosis susceptibility protein [13]. Diatom genomes contain five to six metacaspases, some of which are constitutively expressed whereas others are induced by nutrient deprivation [6,7,32]. However, T. pseudonana lacks homologs of important elements of metazoan apoptotic pathways, such as p53 and the Bcl-2 family of apoptosis regulators, as well as TIR adaptor proteins and AP-ATPases, both of which are abundant in Arabidopsis thaliana. These findings raise fundamental questions about whether T. pseudonana has a functional programmed cell death pathway in response to iron starvation [32], raising fundamental questions about how it is regulated.
Functional genomics and biotechnological applications
Genetic transformation methods have been established for both T. pseudonana and P. tricornutum [33,34]. Expression vectors for T. pseudonana have been developed that allow constitutive and inducible protein expression [34]. Also recently developed was a method for growing cultures of T. pseudonana synchronously [35], making it possible to gain insights into cell division and other metabolic processes tightly coupled to the cell cycle, such as silification. A useful tool for reverse functional genomics has also been developed in P. tricornutum that allows high-throughput cloning and expression of a target gene [36]. These tools are important advances that will enable insights into the molecular mechanisms of diatom biology that were not possible even 5 years ago. However, the field still lacks classical genetic techniques, such as a method for gene knockout. Perhaps with the newly available genome sequence and the growing interest in diatom genetics, these tools will soon become available.
Diatoms have inspired many biologists and engineers. Silicon-based nanotechnology is a multi-billion-dollar industry, but there is an increasing need for the efficient and cost-effective production of such devices, for example, in solar energy capture, charge separation in battery technologies, or even in separation technologies involving purification of gases or solutes in fluids. Diatoms provide an unparalleled system for studying the basic mechanism of silica nanofabrication because they can make complex, reproducible three-dimensional structures under ambient conditions. In addition, because diatoms have been such an important component of petroleum, potential genetic manipulation may lead to more efficient use of these organisms as biofuel feedstock. Indeed, the development of a model organism such as P. tricornutum, combined with system-level approaches for better understanding of how carbon is allocated to specific sinks, may ultimately provide a source of advanced, sustainable biofuels that does not compete with food production.
Diatom genomes are coming of age. These protists, long studied by marine biologists for their complexity and ecological success, are now becoming a source of information not only about the evolutionary history of eukaryotes, but as a potential source of nanodevices and energy for our future. These genome sequences [6,7] are the beginning of a long learning process that will potentially teach us how the complex web of metabolic processes was selected by specific clades and how we can use that information to develop a sustainable world in the coming centuries.
References
- Smetacek V. Diatoms and the ocean carbon cycle. Protist. 1999;150:25–32. doi: 10.1016/S1434-4610(99)70006-4. [DOI] [PubMed] [Google Scholar]
- Delwiche C. Tracing the thread of plastid diversity through the tapestry of life. Am Nat. 2000;154:S164–S177. doi: 10.1086/303291. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Chloroplast evolution: secondary symbiogenesis and multiple losses. Curr Biol. 2002;12:R62–R64. doi: 10.1016/S0960-9822(01)00675-3. [DOI] [PubMed] [Google Scholar]
- Falkowski PG, Katz ME, Knoll AH, Quigg A, Raven JA, Schofield O, Taylor FJ. The evolution of modern eukaryotic phytoplankton. Science. 2004;305:354–360. doi: 10.1126/science.1095964. [DOI] [PubMed] [Google Scholar]
- Kooistra WHCF, Gersonde R, Medlin LK, Mann DG. The origin and evolution of the diatoms: their adaptation to a planktonic existence. In: Falkowski PG, Knoll AH, editor. Evolution of Primary Producers in the Sea. New York: Academic Press; 2007. pp. 207–249. [Google Scholar]
- Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou S, Allen AE, Apt KE, Bechner M, Brzezinski MA, Chaal BK, Chiovitti A, Davis AK, Demarest MS, Detter JC, Glavina T, Goodstein D, Hadi MZ, Hellsten U, Hildebrand M, Jenkins BD, Jurka J, Kapitonov VV, Kröger N, Lau WW, Lane TW, Larimer FW, Lippmeier JC, Lucas S. The genome diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science. 2004;306:79–86. doi: 10.1126/science.1101156. [DOI] [PubMed] [Google Scholar]
- Bowler C, Allen AE, Badger JH, Grimwood J, Jabbari K, Kuo A, Maheswari U, Martens C, Maumus F, Otillar RP, Rayko E, Salamov A, Vandepoele K, Beszteri B, Gruber A, Heijde M, Katinka M, Mock T, Valentin K, Verret F, Berges JA, Brownlee C, Cadoret JP, Chiovitti A, Choi CJ, Coesel S, De Martino A, Detter JC, Durkin C, Falciatore A. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature. 2008. doi:10.1038/nature07410. [DOI] [PubMed]
- Oliver MJ, Petrov D, Ackerly D, Falkowski P, Schofield OM. The mode and tempo of genome size evolution in eukaryotes. Genome Res. 2007;17:594–601. doi: 10.1101/gr.6096207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diatom EST Database http://www.biologie.ens.fr/diatomics/EST3/
- Roy SW, Penny D. A very high fraction of unique intron positions in the intron-rich diatom Thalassiosira pseudonana indicates widespread intron gain. Mol Biol Evol. 2007;24:1447–1457. doi: 10.1093/molbev/msm048. [DOI] [PubMed] [Google Scholar]
- De Bodt S, Maere S, Peer Y Van de. Genome duplication and the origin of angiosperms. Trends Ecol Evol. 2005;20:591–597. doi: 10.1016/j.tree.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Nagasaki K. Dinoflagellates, diatoms and their viruses. J Microbiol. 2008;46:235–243. doi: 10.1007/s12275-008-0098-y. [DOI] [PubMed] [Google Scholar]
- Montsant A, Allen AE, Coesel S, De Martino A, Falciatore A, Mangogna M, Siaut M, Heijde M, Jabbari K, Maheswari U, Rayko E, Vardi A, Apt KE, Berges JA, Chiovitti A, Davis AK, Thamatrakoln K, Hadi MZ, Lane TW, Lippmeier JC, Martinez D, Parker MS, Pazour GJ, Saito MA, Rokhsar DS, Armbrust EV, Bowler C. Identification and comparative genomic analysis of signaling and regulatory components in the diatom Thalassiosira pseudonana. J Phycol. 2007;43:585–604. doi: 10.1111/j.1529-8817.2007.00342.x. [DOI] [Google Scholar]
- Becker B, Hoef-Emden K, Melkonian M. Chlamydial genes shed light on the evolution of photoautotrophic eukaryotes. BMC Evol Biol. 2008;8:203. doi: 10.1186/1471-2148-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JL, Gogarten JP. Did an ancient chlamydial endosymbiosis facilitate the establishment of primary plastids? Genome Biol. 2007;8:13. doi: 10.1186/gb-2007-8-6-r99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AE, Vardi A, Bowler C. An ecological and evolutionary context for integrated nitrogen metabolism and related signaling pathways in marine diatoms. Curr Opin Plant Biol. 2006;9:264–273. doi: 10.1016/j.pbi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Zehr JP, Falkowski PG. Pathway of ammonium in a marine diatom determined with the radiotracer 13N. J Phycol. 1988;24:588–591. [Google Scholar]
- Falkowski PG, Raven JA. Aquatic Photosynthesis. 2. Princeton: Princeton University Press; 2007. p. 484. [Google Scholar]
- Morris I, Mukerji D. Photosynthetic carboxylating enzymes on Phaeodactylum tricornutum : assay methods and properties. Mar Biol. 1976;36:199–206. doi: 10.1007/BF00389280. [DOI] [Google Scholar]
- Reinfelder JR, Kraepiel AML, Morel FMM. Unicellular C4 photosynthesis in a marine diatom. Nature. 2000;407:996–999. doi: 10.1038/35039612. [DOI] [PubMed] [Google Scholar]
- Kroth PG, Chiovitti A, Gruber A, Martin-Jezequel V, Mock T, Parker MS, Stanley MS, Kaplan A, Caron L, Weber T, Maheswari U, Armbrust EV, Bowler C. A model for carbohydrate metabolism in the diatom Phaeodactylum tricornutum deduced from comparative whole genome analysis. PLoS ONE. 2008;3:e1426. doi: 10.1371/journal.pone.0001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamatrakoln K, Alverson AJ, Hildebrand M. Comparative sequence analysis of diatom silicon transporters: towards a mechanistic model of silicon transport. J Phycol. 2006;42:822–834. doi: 10.1111/j.1529-8817.2006.00233.x. [DOI] [Google Scholar]
- Kröger N. Prescribing diatom morphology: toward genetic engineering of biological nanomaterials. Curr Opin Chem Biol. 2007;11:662–669. doi: 10.1016/j.cbpa.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Borowitzka MA, Volcani BE. The polymorphic diatom Phaeodactylum tricornutum : ultrastructure of its morphotypes. J Phycol. 1978;14:10–21. doi: 10.1111/j.1529-8817.1978.tb00625.x. [DOI] [Google Scholar]
- Del Amo Y, Brzezinski MA. The chemical form of dissolved Si taken up by marine diatoms. J Phycol. 1999;35:1162–1170. doi: 10.1046/j.1529-8817.1999.3561162.x. [DOI] [Google Scholar]
- Marchetti A, Parker MS, Moccia LP, Ostlund EL, Arrieta A, Ribalet F, Murphy MEP, Maldonado MT, Armbrust EV. Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature. 2008 doi: 10.1038/nature07539. doi:101038/nature07539. [DOI] [PubMed] [Google Scholar]
- Falciatore A, d'Alcalà MR, Croot P, Bowler C. Perception of environmental signals by a marine diatom. Science. 2000;288:2363–2366. doi: 10.1126/science.288.5475.2363. [DOI] [PubMed] [Google Scholar]
- Vardi A, Formiggini F, Casotti R, De Martino A, Ribalet F, Miralto A, Bowler C. A stress surveillance system based on calcium and nitric oxide in marine diatoms. PLoS Biol. 2006;4:e60. doi: 10.1371/journal.pbio.0040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CC, Hwang S-PL, Chang J. Nitric oxide as a signaling factor to upregulate the death-specific protein in a marine diatom, Skeletonema costatum, during blockage of electron flow in photosynthesis. Appl Environ Microbiol. 2008;74:6521–6527. doi: 10.1128/AEM.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AE, LaRoche J, Maheswari U, Lommer M, Schauer N. Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. Proc Natl Acad Sci USA. 2008;105:10438–10443. doi: 10.1073/pnas.0711370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi A, Bidle KD, Kwityn C, Hirsh DJ, Thompson SM, Callow JA, Falkowski P, Bowler C. A diatom gene regulating nitric oxide signaling and susceptibility to diatom-derived aldehydes. Curr Biol. 2008;18:1–5. doi: 10.1016/j.cub.2008.05.037. [DOI] [PubMed] [Google Scholar]
- Bidle KD, Bender SJ. Iron starvation and culture age activate meta-caspases and programmed cell death in the marine diatom, Thalassiosira pseudonana. Eukaryotic Cell. 2008;7:223–236. doi: 10.1128/EC.00296-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apt KE, Kroth-Pancic PG, Grossman AR. Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol Gen Genet. 1996;252:572–579. doi: 10.1007/BF02172403. [DOI] [PubMed] [Google Scholar]
- Poulsen N, Chesley PM, Kröger N. Molecular genetic manipulation of the diatom Thalassiosira pseudonana (Bacillariophyceae). J Phycol. 2006;42:1059–1065. doi: 10.1111/j.1529-8817.2006.00269.x. [DOI] [Google Scholar]
- Hildebrand M, Frigeri LG, Davis AK. Synchronized growth of Thalassiosira pseudonana (Bacillariophyceae) provides novel insights into cell wall synthesis processes in relation to the cell cycle. J Phycol. 2007;43:730–740. doi: 10.1111/j.1529-8817.2007.00361.x. [DOI] [Google Scholar]
- Siaut M, Heijde M, Mangogna M, Montsant A, Coesel S, Allen A, Manfredonia A, Falciatore A, Bowler C. Molecular toolbox for studying diatom biology in Phaeodactylum tricornutum. Gene. 2007;406:23–25. doi: 10.1016/j.gene.2007.05.022. [DOI] [PubMed] [Google Scholar]