Abstract
Introduction
The aim of this study was to investigate whether in-hospital mortality was associated with the administered fraction of oxygen in inspired air (FiO2) and achieved arterial partial pressure of oxygen (PaO2).
Methods
This was a retrospective, observational study on data from the first 24 h after admission from 36,307 consecutive patients admitted to 50 Dutch intensive care units (ICUs) and treated with mechanical ventilation. Oxygenation data from all admission days were analysed in a subset of 3,322 patients in 5 ICUs.
Results
Mean PaO2 and FiO2 in the first 24 h after ICU admission were 13.2 kPa (standard deviation (SD) 6.5) and 50% (SD 20%) respectively. Mean PaO2 and FiO2 from all admission days were 12.4 kPa (SD 5.5) and 53% (SD 18). Focusing on oxygenation in the first 24 h of admission, in-hospital mortality was shown to be linearly related to FiO2 value and had a U-shaped relationship with PaO2 (both lower and higher PaO2 values were associated with a higher mortality), independent of each other and of Simplified Acute Physiology Score (SAPS) II, age, admission type, reduced Glasgow Coma Scale (GCS) score, and individual ICU. Focusing on the entire ICU stay, in-hospital mortality was independently associated with mean FiO2 during ICU stay and with the lower two quintiles of mean PaO2 value during ICU stay.
Conclusions
Actually achieved PaO2 values in ICU patients in The Netherlands are higher than generally recommended in the literature. High FiO2, and both low PaO2 and high PaO2 in the first 24 h after admission are independently associated with in-hospital mortality in ICU patients. Future research should study whether this association is causal or merely a reflection of differences in severity of illness insufficiently corrected for in the multivariate analysis.
Introduction
It is generally acknowledged that mechanical ventilation may cause or exacerbate lung damage in critically ill patients with acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). Many studies have examined the effects of different settings of ventilation, such as low vs high tidal volumes, prone positioning and high-frequency oscillation on outcome of intensive care unit (ICU) patients [1]. Lung-protective mechanical ventilation strategies in patients with ALI/ARDS, applying lower tidal volumes and sufficient levels of positive end expiratory pressure (PEEP) [2,3], have been shown to improve outcome.
The mode of mechanical ventilation and the oxygenation targets may influence the outcome for patients. Traditionally, arterial oxygen concentration (measured as partial oxygen pressure, PaO2) and oxygen saturation by pulse oximetry are used as targets. Common recommendations for oxygenation propose PaO2 values to be between 7.3 and 10.6 kPa [2,4]. The deleterious effects of hypoxia are well known and physicians may be mostly concerned about avoiding hypoxia and give additional oxygen 'to be on the safe side'. Hyperoxia, however, is also to be avoided as oxygen may be toxic. First, it is long known that high fraction of oxygen in inspired air (FiO2) may be toxic for the lungs. In animals, prolonged hyperoxia causes histopathological changes similar to those seen in ARDS [5]. Baboons exposed to 100% oxygen demonstrated a progressive reduction in forced vital capacity and functional residual capacity [6] and proliferative epithelial changes and interstitial fibrosis [7]. In healthy humans, exposure to 100% oxygen may lead to atelectasis, impaired mucocilliary clearance and tracheobronchitis, alveolar protein leakage and enhanced expression of leukotrienes by alveolar macrophages and increases in alveolar neutrophils [8]. Apart from its effects on the lungs, oxygen may also lead to systemic toxicity. It has been associated with an increase in vascular resistance and a decrease in cardiac output [9]. Hyperoxia may result in the generation of central nervous system, hepatic and pulmonary free radicals. Cardiopulmonary resuscitation following cardiac arrest in a canine model is associated with a worsened neurologic outcome when performed in the presence of hyperoxia vs normoxia [8,10].
The aim of the present study was to describe the present oxygenation targets applied in ICUs in The Netherlands, and to determine whether outcome of ICU patients was associated with differences in administered oxygen (FiO2) or achieved arterial PaO2.
Materials and methods
Patient data
This study is based on retrospective analysis of all consecutive patients admitted between 1 January 1999 and 30 June 2006 to the ICUs of 50 university, teaching and non-teaching hospitals in The Netherlands who were on mechanical ventilation within the first 24 h after ICU admission. Data were collected as part of the Dutch National Intensive Care Evaluation (NICE) registry. Within this registry data collection takes place in a standardised manner according to strict definitions and is subject to stringent data quality checks, which has been shown to result in a high quality of data [11]. The data have been encrypted in a way that all patient identifying information, such as name and patient identification number, has been removed. In The Netherlands, there is no need to obtain consent to make use of registries when patient identifying information is not used. According to the Dutch Medical Research Involving Human Subjects Act, there is no need for approval by ethical committees [12]. The NICE initiative is officially registered according to the Dutch Personal Data Protection Act.
The variables are used to calculate probabilities of death for each patient using the Acute Physiology and Chronic Health Evaluation (APACHE) II [13], the Simplified Acute Physiology Score (SAPS) II [14], and the Mortality Probability Models (MPM) II at admission and 24-h scoring systems [15]. In this study the SAPS II was used for case mix adjustment as previous research has shown that this scoring system fits best to the patient population of the NICE registry [16]. The database contains 108 demographic, diagnostic and physiologic variables collected within the first 24 h of ICU admission and outcome data on ICU and in-hospital mortality.
In analogy with the exclusion criteria commonly used in analyses based on the SAPS II scoring system, patients admitted after cardiac surgery, patients admitted with severe burns and patients aged under 18 were excluded from the analyses. For patients with multiple ICU admissions during a hospitalisation period only the first ICU admission was used.
In the analyses focusing on oxygenation in the first 24 h of ICU stay, information of all patients was used. For the analyses related to oxygenation during the entire ICU stay a selection of the patients was used, as only five of the ICUs participating in the NICE registry provide information to the registry database on the patient's condition on a daily basis using the Sequential Organ Failure Assessment (SOFA) score [17]. For this analysis only patients with a minimum length of ICU stay of 3 days were included into the analyses. Mean PaO2 and mean FiO2 values were calculated based on the entire ICU stay.
If more than one blood gas analysis was available for a patient during the first 24 h after ICU admission, PaO2, FiO2 and partial CO2 (PaCO2) values were from the arterial sample with the lowest PaO2/FiO2 ratio. Likewise, oxygenation data in the SOFA scores was based on samples with lowest PaO2/FiO2 ratios in the particular 24 h period.
Statistical analyses
The relation between the oxygenation parameters and in-hospital mortality was assessed using logistic regression analysis.
As PaO2 and FiO2 are both continuous variables, univariate regression analyses using polynomial functions and spline functions [18,19] were performed to investigate the relation between each of these variables and in-hospital mortality. For PaO2 a model including PaO2 as a natural spline with five degrees of freedom turned out to result in the best fit. To enhance interpretation of the results, in subsequent analyses PaO2 was categorised. As no standard cut-off points are in use for PaO2, categorisation of PaO2 values into five categories was based on the distribution of the data, using quintiles as cut-off values between categories. For FiO2 inclusion into the model in a linear fashion showed to be optimal fit.
Multivariable logistic regression analyses were performed both for the first 24 h of ICU stay and for the entire ICU stay. Potential confounders in the association of oxygenation with hospital mortality (age, SAPS II, Glasgow Coma Scale (GCS) score below 15 and admission type) were included into the models. Also a specific variable denoting the 'hospital' was included into these models to correct for potential differences in overall in-hospital mortality between the five hospitals. In the model focusing on the entire ICU stay, the PaO2/FiO2 ratio during the first 24 h of admission was added as additional confounder. In the modelling process the presence of multicolinearity between the oxygenation parameters was verified based on the standard errors of the parameters in the model.
The Standardised Mortality Ratio (SMR) was calculated as the ratio of the number of observed deaths to the number of deaths expected according to the SAPS II model [13].
In all analyses a p value of 0.05 was considered to represent a statistically significant difference. The analyses were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) and S-plus version 7.0 (Insightful Corp., Seattle, WA, USA).
Results
Analysis of data from first 24 h after admission
In total, 36,307 patients from 50 ICUs were included in the analysis. All patients were treated with mechanical ventilation within the first 24 h after ICU admission. Data on the severity of illness, reason for admission and referring specialty are given in Table 1. Mean PaO2 was 13.2 kPa (standard deviation (SD) 6.5). Mean FiO2 was 50% (SD 20). Regression analysis using PaO2 as a continuous variable showed that with increasing PaO2 the average in-hospital mortality first decreased and subsequently started to rise again (Figure 1). Figure 2 denotes the relation between PaO2 and in-hospital mortality when correcting for SAPS II (by means of the SMRs). Figures 3 and 4 show the association between SMR and FiO2 or PaO2/FiO2 ratio respectively. Multivariate regression analysis indicated that the U-shaped relation between PaO2 and mortality (modelled using a spline function) remained significant after correction for age, admission type, GCS score and severity of illness measured with the SAPS II. Multicolinearity was not found to be present for FiO2 and PaO2 values. Table 2 presents the odds ratios for FiO2 and the PaO2 quintiles in a multivariate regression model, indicating that in-hospital mortality was associated with FiO2 and PaO2 values.
Table 1.
Characteristics of patients
| Analysis of data from first 24 h after admission | Analysis of data from all admission days | |
| No of patients | 36,307 | 3,322 |
| Male (%) | 60.1 | 60.3 |
| Age in yearsa | 62.5 ± 16.1 | 62.4 ± 15.9 |
| SAPS IIa | 42.7 ± 18.4 | 47.6 ± 15.7 |
| SAPS II predicted mortalitya | 0.34 ± 0.28 | 0.42 ± 0.27 |
| GCS score below 15 (%) | 33.1 | 33.6 |
| PaO2 at admission (kPa)a | 13.2 ± 6.5b | 12.5 ± 5.5c |
| FiO2 (%)a | 50.4 ± 19.9b | 53.1 ± 18.7c |
| PaO2/FiO2 ratio (kPa)a | 29.1 ± 15.0b | 24.6 ± 12.6c |
| Admission type (%): | ||
| Medical | 48.4 | 61.0 |
| Unplanned surgery | 22.6 | 23.0 |
| Planned surgery | 28.9 | 16.0 |
| Referring specialty (%): | ||
| Internal medicine | 16.4 | 16.7 |
| Cardiology | 10.8 | 17.9 |
| Pulmonary disease | 7.4 | 10.4 |
| Neurology | 5.7 | 6.4 |
| Surgery | 33.2 | 30.6 |
| Cardiothoracic surgery | 6.4 | 1.2 |
| Neurosurgery | 6.9 | 6.1 |
| Other | 13.1 | 10.7 |
| ICU mortality | 23.0 | 21.0 |
| In-hospital mortality (%) | 31.1 | 32.7 |
aMean ± standard deviation (SD); bPaO2, FiO2 and PaO2/FiO2 ratio from sample with lowest PaO2/FiO2 ratio within 24 h after admission; cmean value of all admission days. Per day PaO2, FiO2 and PaO2/FiO2 ratio was taken from sample with lowest PaO2/FiO2 ratio.
GCS, Glasgow Coma Scale; FiO2, fraction of oxygen in inspired air; ICU, intensive care unit; PaO2, partial oxygen pressure; SAPS II, Simplified Acute Physiology Score II.
Figure 1.
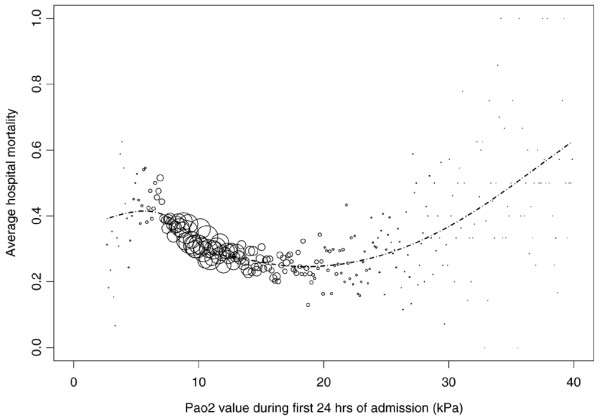
In-hospital mortality by partial oxygen pressure (PaO2) (kPa). Values were taken from blood gas analysis with lowest PaO2/fraction of oxygen in inspired air (FiO2) ratio in the first 24 h after intensive care unit (ICU) admission. The sizes of the circles represent the number of patients with the same PaO2 value. The curve represents the predicted mortality using the logistic regression equation in which the PaO2 value was incorporated using a spline function.
Figure 2.
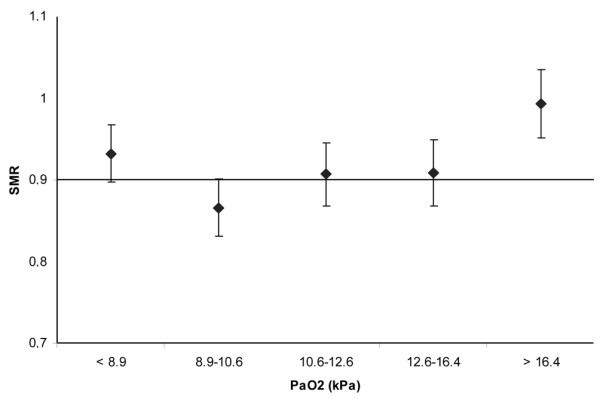
Standardised mortality ratio (SMR) by partial oxygen pressure (PaO2) (kPa). PaO2 values were taken from blood gas analysis with lowest PaO2/fraction of oxygen in inspired air (FiO2) ratio in the first 24 h after intensive care unit (ICU) admission. PaO2 values are categorised as quintiles. Error bars represent 95% confidence intervals.
Figure 3.
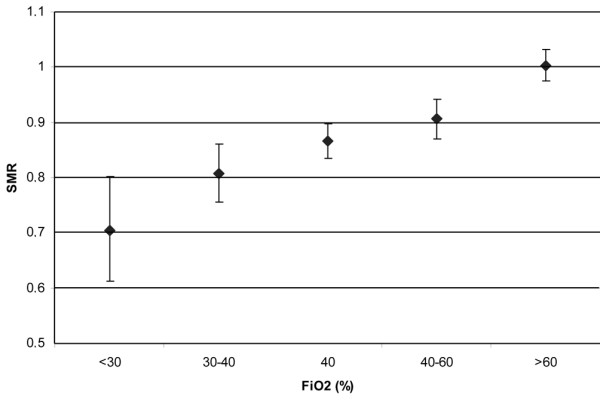
Standardised mortality ratio (SMR) by fraction of oxygen in inspired air (FiO2). FiO2 values were taken from blood gas analysis with lowest partial oxygen pressure (PaO2)/FiO2 ratio in the first 24 h after intensive care unit (ICU) admission FiO2 values are categorised as quintiles. Error bars represent 95% confidence intervals.
Figure 4.
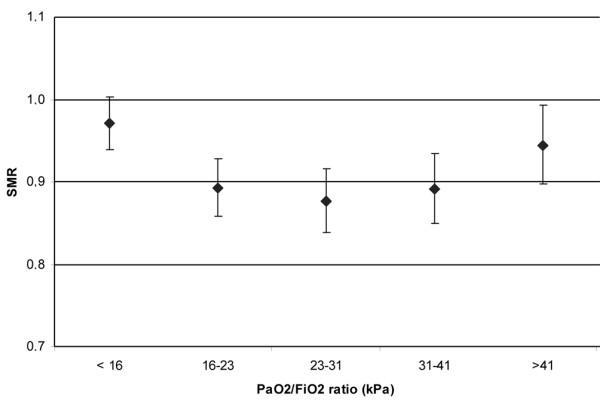
Standardised mortality ratio (SMR) by lowest partial oxygen pressure (PaO2)/fraction of oxygen in inspired air (FiO2) ratio (kPa) in the first 24 h after intensive care unit (ICU) admission. PaO2/FiO2 ratio values are categorised as quintiles. Error bars represent 95% confidence intervals.
Table 2.
Adjusted odds ratios for partial oxygen pressure (PaO2) and fraction of oxygen in inspired air (FiO2) resulting from a multivariate regression analysis on data from the first 24 h after ICU admission
| Covariate | Odds ratio | 95% Confidence interval |
| PaO2 in kPa: | ||
| < 8.9 (n = 6,937) | 1.12 | 1.03 to 1.21 |
| 8.9 to 10.6 (reference category) (n = 7,466) | 1 | |
| 10.6 to 12.6 (n = 6,430) | 1.11 | 1.02 to 1.21 |
| 12.6 to 16.4 (n = 7,278) | 1.08 | 1.00 to 1.18 |
| ≥ 16.4 (n = 8,196) | 1.23 | 1.13 to 1.34 |
| FiO2 (per 10%) | 1.12 | 1.10 to 1.13 |
Odds ratio after adjustment for the following potential confounders: age, SAPS II, GCS score below 15, admission type, individual hospital. The equation of the model is: Logit(p) = -5.419 + 0.059 × age (per 5 years) + 0.066 × SAPS II + 0.070 × I(GCS < 15) + 0.221 × I(admission type = urgent) + 0.453 × I(admission type = medical) + βhosp + 0.105 × FiO2 (per 10%) + 0.109 × I(PaO2 < 67) + 0.109 × I(80 ≤ PaO2 < 95) +0.079 × I(95 ≤ PaO2 < 123) +0.206 × I(PaO2 ≥ 123) Probability of in-hospital death = e(logit)/(1+e(logit)). Median βhos for individual hospitals was -0.12 (IQR -0.43 to 0.05).
GCS, Glasgow Coma Scale; ICU, intensive care unit; IQR, interquartile range; SAPS II, Simplified Acute Physiology Score II.
Analysis of data from all ICU admission days
For the analysis regarding entire ICU stay 3,322 patients from 5 ICUs were included. Characteristics of these patients are given in Table 1. Mean PaO2 during ICU stay was 12.4 kPa (SD 5.5). Mean FiO2 during ICU stay was 53% (SD 18). The results of the multivariate analysis are shown in Table 3. Mean FiO2 value and a mean PaO2 value lower than 10.6 kPa were associated with a higher mortality. This association was independent of the potential confounders age, SAPS II, abnormal GCS score, PaO2/FiO2 ratio at admission, admission type and hospital.
Table 3.
Adjusted odds ratios for mean partial oxygen pressure (PaO2) value and mean fraction of oxygen in inspired air (FiO2) during ICU stay resulting from a multivariate regression analysis on data from the entire ICU stay
| Covariate | Odds ratio | 95% Confidence interval |
| Mean PaO2 in kPa: | ||
| < 8.9 (n = 402) | 1.63 | 1.16 to 2.3 |
| 8.9 to 10.6 (n = 871) | 1.51 | 1.18 to 1.96 |
| 10.6 to 12.6 (n = 970) | 1.25 | 0.99 to 1.57 |
| 12.6 to 16.4 (reference category) (n = 841) | 1 | |
| > 16.4 (n = 238) | 1.04 | 0.64 to 1.68 |
| Mean FiO2 (per 10%) | 1.63 | 1.47 to 1.81 |
Odds ratio after adjustment for the following potential confounders: age, SAPS II, GCS score below 15, admission type, PaO2/FiO2 ratio at admission, and hospital. The equation of the eventual model is: Logit(p) = -7.060 + 0.090 × age (per 5 years) + 0.049 × SAPS II + 0.054 × I(GCS < 15) + 0.015 × I(admission type = urgent) + 0.161 × I(admission type = medical) + 0.004 × PaO2/FiO2 – 1.114 × I(hospital = 2) – 0.060 × I(hospital = 3) – 0.285 × I(hospital = 4) – 0.618 × I(hospital = 5) + 0.488 × mean FiO2 (per 10%) + 0.492 × I(mean PaO2 < 67) + 0.417 × I(67 ≤ PaO2 < 80) + 0.221 × I(80 ≤ PaO2 < 95) + 0.038 × I(PaO2 ≥ 123). Probability of in-hospital death = e(logit)/(1+e(logit)).
GCS, Glasgow Coma Scale; ICU, intensive care unit; SAPS II, Simplified Acute Physiology Score II.
Discussion
We found that administration of high FiO2 values in ICU patients was associated with increased in-hospital mortality. This association was found for FiO2 values in the first 24 h after admission and also for mean FiO2 during all admission days. The increased risk in patients with high FiO2 remained after correcting for SAPS II, admission type, reduced GCS score and pulmonary dysfunction measured as PaO2/FiO2 ratio. This suggests that the administration of oxygen itself could be deleterious, and that the association between high FiO2 and mortality cannot be explained by the confounding issue that highest FiO2 levels are administered in patients with severe pulmonary dysfunction.
Our observations are in accordance with prior experimental studies showing the potential toxicity of high fractions of inspired oxygen [5]. Administration of supplemental oxygen can cause lung damage. This risk is especially high in prematurely born infants, probably attributable to inadequate host defences, underdeveloped lungs and immature antioxidant systems [20]. Exposure to hyperoxia leads to diffuse pulmonary damage characterised by an extensive inflammatory response and destruction of the alveolar-capillary barrier leading to oedema, impaired gas exchange and respiratory failure [21]. Mouse lungs exposed to > 90% oxygen for 48 h were more susceptible to ventilator-induced lung injury than those exposed to room air [22]. Hyperoxia also aggravates pulmonary injury following artificial ventilation in rats using high tidal volumes [23]. Furthermore, hyperoxia impairs the innate immune response by decreased macrophage function, impaired bacterial killing and increased susceptibility to pneumonia in a Klebsiella pneumoniae model [24]. Lung injury is likely to be initiated when the rates of generation of reactive oxygen species (ROS) are increased beyond the capacities of the antioxidant defences, such as the enzymes glutathione, superoxide dismutase and catalase. Mitochondrial mediated cell injury by ROS has been identified as a critical event in both apoptotic and necrotic forms of cell death in hyperoxia [25]. Another organ that may be injured by hyperoxia is the kidney. Hyperoxic reperfusion exacerbates renal dysfunction and histopathologic injury after 30 min of complete normothermic ischaemia in rabbits. This hyperoxia associated dysfunction was prevented by the administration of the radical scavenger allopurinol [26], suggesting that oxidative injury by ROS plays a role in post-ischaemic renal failure.
Several studies focused on the role of high reperfusion oxygen tensions following cardiac arrest and resuscitation. In a canine model of 10 min of cardiac arrest, resuscitation with 21% vs 100% inspired O2 resulted in lower levels of oxidised brain lipids and improved neurological outcome [27]. In another study using the same canine model, it was shown that resuscitation with 100% O2 resulted in impaired hippocampal neuronal metabolism [28]. Proposed pathogenetic mechanisms of hyperoxia induced reperfusion injury of the brain include increased production of ROS, a high ratio of oxidised over reduced glutathione [29] and increased nitric oxide production by endothelium and neuron derived nitric oxide synthase [30].
Many studies investigated the use of 100% vs 21% oxygen for resuscitation in depressed newborn infants (that is, infants with apnoea or relative bradycardia at birth). A systematic review and meta-analysis of 10 studies reported a significant reduction in the risk of neonatal mortality and a trend towards a reduction in severe encephalopathy in newborns resuscitated with 21% O2. The reduction in mortality was also found in a subgroup analysis only including strictly randomised controlled trials and in a subgroup of studies enrolled in European countries with a lower risk of mortality than in less developed countries [31].
Human clinical studies evaluating the effects of hyperoxia in critically ill adult patients are lacking. The effects of hyperoxia in non-ICU settings are not clear. A reduction in surgical site infections by the use of hyperoxia has been reported by one study group [32], while others reported more surgical site infections in patients treated with hyperoxia [33].
An alternative explanation for the association between oxygenation and mortality in ICU patients could be that common criteria for weaning from mechanical ventilation are based on FiO2 and PEEP levels. High FiO2 and PEEP, both leading to high PaO2 values, may delay weaning from mechanical ventilation, thus negatively influencing outcome in ICU patients. Also, we cannot exclude that high PaO2 values were achieved by more invasive ventilation strategies, potentially being more injurious to the patients.
Interestingly, apart from FiO2 values, there was also a U-shaped association between achieved arterial oxygen tension (PaO2) during the first 24 h after ICU admission and mortality with higher mortality in patients with either a very low or high PaO2. That mortality is higher in patients with very low PaO2 is not unexpected and possibly related to ischaemia or to selection of the sickest patients. However, mortality was also higher in patients with highest PaO2 values, suggesting the possibility of systemic oxygen toxicity.
In our analysis of mean oxygenation during all admission days, we again found a linear association between mortality and FiO2 values. Low PaO2s were also associated with higher mortality but high PaO2s were not. The shape of the association between PaO2 and mortality was hard to assess. In our data a linear association appeared to best fit the data (data not shown). The number of patients included in this analysis was only 3,322. Only 2% of the patients had a mean PaO2 higher than 20.0 kPa. Thus, the power of our study may have been too low to detect an association between high mean PaO2 values during the ICU stay and increased mortality.
There are limitations to this study. Most importantly, it was a retrospective observational study and the association between mortality and oxygenation is not necessarily causal. Although the association appeared to be independent of a number of potential confounding covariates, we cannot exclude that, despite our efforts, there are still differences in case mix associated with oxygenation that are not taken into account in our multivariate analyses. It is possible that physicians recognised some marker of severity that was not represented in our attempts to adjust for severity, and that they purposefully gave higher concentrations of oxygen to achieve higher levels of PaO2 in these high-risk patients.
The three potential confounders that we corrected for (age, reduced GCS score, and admission type) are part of the SAPS II that was also included as covariate in the multivariate analysis. We have repeated the analyses without these three variables, adjusting for SAPS II only. This yielded similar results for the association between in-hospital mortality and PaO2 and FiO2 respectively.
We corrected for pulmonary dysfunction by including PaO2/FiO2 ratio at admission in the multivariate analysis of data from all admission days. PaO2/FiO2 ratio was not included in the analysis of data from the first 24 h after ICU admission, because including PaO2, FiO2 and PaO2/FiO2 ratio, all from the same arterial blood sample, would introduce problems by colinearity of the data. In this population, however, we performed a separate multivariate analysis substituting PaO2/FiO2 ratio for PaO2 values. Again, FiO2 appeared to be a predictor of mortality, also independent of PaO2/FiO2 ratio (OR 1.15, 95% CI 1.14 to 1.17, model not shown). PaO2/FiO2 ratio is not only influenced by pulmonary dysfunction, but also by ventilator settings, such as PEEP levels. As PEEP was not part of the NICE data collection, we could not include this possible confounder in our analysis. Prospective, controlled trials are necessary to show a causal relationship between high FiO2s and mortality.
As the association between PaO2 and mortality was U-shaped, we categorised PaO2 values for the multivariate analysis using quintiles as categories (as no standard categorisation is available). The boundaries of quintiles are chosen arbitrarily and may not be the optimal cut-off levels to discriminate between patients with low and high risk of mortality. Therefore, we repeated the same multivariate analysis (model not shown) on data from the first 24 h after ICU admission using PaO2 values categorised as deciles and found similar results.
Another finding from our study is the fact that in most patients the achieved PaO2 values are higher than the targets commonly recommended [2,4]. Although oxygen toxicity is a well known entity [34], FiO2s up to 0.5 are commonly considered 'safe' by physicians [5]. It appears that physicians are more concerned about avoiding hypoxia and ischaemia than about the risks of hyperoxia. In The Netherlands, no formal guidelines for oxygenation targets are available. This may be related to the fact that the influence of oxygenation targets has never been studied making it impossible to provide evidence-based recommendations. Based on other observational studies, it may well be that also in other countries actual PaO2s in ICU patients are higher than recommended [35,36]
Conclusion
High fractions of oxygen in the inspired air and high PaO2 values are associated with increased mortality in ICU patients. Actually achieved PaO2 values in Dutch ICU patients are higher than the PaO2 targets in some recent international recommendations. Prospective interventional studies are necessary to find out whether the association between outcome and oxygenation is causal and to provide evidence-based guidelines on oxygenation targets.
Key messages
• The weaning rate of catecholamines is usually chosen empirically by intensivists.
• Actually achieved PaO2 values in Dutch ICU patients are higher than the PaO2 targets given in recent international recommendations.
• High fractions of oxygen in the inspired air are associated with increased mortality in ICU patients on mechanical ventilation.
• Both low and high PaO2 values in the first 24 hours after ICU admission were associated with increased mortality.
• Future interventional studies are required to find out whether these associations between oxygenation and outcome are causal or due to other confounding issues.
Abbreviations
APACHE II: Acute Physiology and Chronic Health Evaluation II; FiO2: fraction of oxygen in the inspired air; MPM II: Mortality Prediction Model II; NICE: National Intensive Care Evaluation; PaO2: partial pressure of oxygen; SAPS II: Simplified Acute Physiology Score II; SOFA: Sequential Organ Failure Assessment.
Competing interests
During the period from 2002 to 2004 LP received an unrestricted educational grant from Eli Lilly Netherlands B.V. The study described in this manuscript was not conducted under the grant, and Eli Lilly Netherlands B.V. has not been involved in any part of the present study. All other authors declare that they have no competing interests.
Authors' contributions
EdJ designed the study and drafted the manuscript. LP and NdK were involved in the set-up of the study, performed the statistical analyses and helped in interpreting the results and writing the manuscript. PK was involved in the set-up of the study, interpreting the results and writing the manuscript. JJ, DdL, PvdV, RB, RdW and RW were involved in interpreting the results and writing the manuscript. All authors read and approved the final manuscript.
Contributor Information
Evert de Jonge, Email: e.dejonge@amc.uva.nl.
Linda Peelen, Email: L.M.Peelen@umcutrecht.nl.
Peter J Keijzers, Email: peterkeijzers@kpnplanet.nl.
Hans Joore, Email: J.C.A.Joore@UmcUtrecht.nl.
Dylan de Lange, Email: d.w.delange@umcutrecht.nl.
Peter HJ van der Voort, Email: p.voort3@chello.nl.
Robert J Bosman, Email: R.J.Bosman@olvg.nl.
Ruud AL de Waal, Email: ruud.de.waal@planet.nl.
Ronald Wesselink, Email: rmj.wesselink@Antonius.net.
Nicolette F de Keizer, Email: n.f.keizer@amc.uva.nl.
References
- Fan E, Needham DM, Stewart TE. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA. 2005;294:2889–2896. doi: 10.1001/jama.294.22.2889. [DOI] [PubMed] [Google Scholar]
- The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- Altemeier WA, Sinclair SE. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care. 2007;13:73–78. doi: 10.1097/MCC.0b013e32801162cb. [DOI] [PubMed] [Google Scholar]
- Fracica PJ, Knapp MJ, Piantadosi CA, Takeda K, Fulkerson WJ, Coleman RE, Wolfe WG, Crapo JD. Responses of baboons to prolonged hyperoxia: physiology and qualitative pathology. J Appl Physiol. 1991;71:2352–2362. doi: 10.1152/jappl.1991.71.6.2352. [DOI] [PubMed] [Google Scholar]
- Crapo JD, Hayatdavoudi G, Knapp MJ, Fracica PJ, Wolfe WG, Piantadosi CA. Progressive alveolar septal injury in primates exposed to 60% oxygen for 14 days. Am J Physiol. 1994;267:L797–L806. doi: 10.1152/ajplung.1994.267.6.L797. [DOI] [PubMed] [Google Scholar]
- Kavanagh BP. Goals and concerns for oxygenation in acute respiratory distress syndrome. Curr Opin Crit Care. 1998;4:16–20. doi: 10.1097/00075198-199802000-00003. [DOI] [Google Scholar]
- Lodato RF. Decreased O2 consumption and cardiac output during normobaric hyperoxia in conscious dogs. J Appl Physiol. 1989;67:1551–1559. doi: 10.1152/jappl.1989.67.4.1551. [DOI] [PubMed] [Google Scholar]
- Zwemer CF, Whitesall SE, D'Alecy LG. Hypoxic cardiopulmonary-cerebral resuscitation fails to improve neurological outcome following cardiac arrest in dogs. Resuscitation. 1995;29:225–236. doi: 10.1016/0300-9572(94)00848-A. [DOI] [PubMed] [Google Scholar]
- Arts D, de Keizer NF, Scheffer GJ, de Jonge E. Quality of data collected for severity of illness scores in the Dutch National Intensive Care Evaluation (NICE) registry. Intensive Care Med. 2002;28:656–659. doi: 10.1007/s00134-002-1272-z. [DOI] [PubMed] [Google Scholar]
- The Central Committee on Research Involving Human Subjects (CCMO) http://www.ccmo-online.nl
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- Lemeshow S, Teres D, Klar J, Avrunin JS, Gehlbach SH, Rapoport J. Mortality Probability Models (MPM II) based on an international cohort of intensive care unit patients. JAMA. 1993;270:2478–2486. doi: 10.1001/jama.270.20.2478. [DOI] [PubMed] [Google Scholar]
- Peek N, Arts DG, Bosman RJ, Voort P van der, de Keizer NF. External validation of prognostic models for critically ill patients required substantial sample sizes. J Clin Epidemiol. 2007;60:491–501. doi: 10.1016/j.jclinepi.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Vincent JL, de MA, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- Smith PL. Splines as a useful and convenient statistical tool. American Statistician. 1979;33:57–62. doi: 10.2307/2683222. [DOI] [Google Scholar]
- Harrell FE. Regression Modeling Strategies. New York, NY: Springer; 2001. [Google Scholar]
- O'Donovan DJ, Fernandes CJ. Mitochondrial glutathione and oxidative stress: implications for pulmonary oxygen toxicity in premature infants. Mol Genet Metab. 2000;71:352–358. doi: 10.1006/mgme.2000.3063. [DOI] [PubMed] [Google Scholar]
- Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980;122:123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Bailey TC, Martin EL, Zhao L, Veldhuizen RA. High oxygen concentrations predispose mouse lungs to the deleterious effects of high stretch ventilation. J Appl Physiol. 2003;94:975–982. doi: 10.1152/japplphysiol.00619.2002. [DOI] [PubMed] [Google Scholar]
- Quinn DA, Moufarrej RK, Volokhov A, Hales CA. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J Appl Physiol. 2002;93:517–525. doi: 10.1152/japplphysiol.00570.2001. [DOI] [PubMed] [Google Scholar]
- Baleeiro CE, Wilcoxen SE, Morris SB, Standiford TJ, Paine R., III Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol. 2003;171:955–963. doi: 10.4049/jimmunol.171.2.955. [DOI] [PubMed] [Google Scholar]
- Wallace KB, Eells JT, Madeira VM, Cortopassi G, Jones DP. Mitochondria-mediated cell injury. Symposium overview. Fundam Appl Toxicol. 1997;38:23–37. doi: 10.1006/faat.1997.2320. [DOI] [PubMed] [Google Scholar]
- Zwemer CF, Shoemaker JL, Jr, Hazard SW, III, Davis RE, Bartoletti AG, Phillips CL. Hyperoxic reperfusion exacerbates postischemic renal dysfunction. Surgery. 2000;128:815–821. doi: 10.1067/msy.2000.109117. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rosenthal RE, Haywood Y, Miljkovic-Lolic M, Vanderhoek JY, Fiskum G. Normoxic ventilation after cardiac arrest reduces oxidation of brain lipids and improves neurological outcome. Stroke. 1998;29:1679–1686. doi: 10.1161/01.str.29.8.1679. [DOI] [PubMed] [Google Scholar]
- Richards EM, Fiskum G, Rosenthal RE, Hopkins I, McKenna MC. Hyperoxic reperfusion after global ischemia decreases hippocampal energy metabolism. Stroke. 2007;38:1578–1584. doi: 10.1161/STROKEAHA.106.473967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand SL, Vento M, Sastre J, Lust WD, Smith MA, Perry G, Walsh M, Martin R. A new model of oxidative stress in rat pups. Neonatology. 2008;94:293–299. doi: 10.1159/000151649. [DOI] [PubMed] [Google Scholar]
- Allen BW, Demchenko IT, Piantadosi CA. Two faces of nitric oxide: implications for cellular mechanisms of oxygen toxicity. J Appl Physiol. 2008. [DOI] [PubMed]
- Saugstad OD, Ramji S, Soll RF, Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology. 2008;94:176–182. doi: 10.1159/000143397. [DOI] [PubMed] [Google Scholar]
- Greif R, Akca O, Horn EP, Kurz A, Sessler DI. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. Outcomes Research Group. N Engl J Med. 2000;342:161–167. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- Pryor KO, Fahey TJ, III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgical population: a randomized controlled trial. JAMA. 2004;291:79–87. doi: 10.1001/jama.291.1.79. [DOI] [PubMed] [Google Scholar]
- Mao C, Wong DT, Slutsky AS, Kavanagh BP. A quantitative assessment of how Canadian intensivists believe they utilize oxygen in the intensive care unit. Crit Care Med. 1999;27:2806–2811. doi: 10.1097/00003246-199912000-00033. [DOI] [PubMed] [Google Scholar]
- Young MP, Manning HL, Wilson DL, Mette SA, Riker RR, Leiter JC, Liu SK, Bates JT, Parsons PE. Ventilation of patients with acute lung injury and acute respiratory distress syndrome: has new evidence changed clinical practice? Crit Care Med. 2004;32:1260–1265. doi: 10.1097/01.CCM.0000127784.54727.56. [DOI] [PubMed] [Google Scholar]
- Kalhan R, Mikkelsen M, Dedhiya P, Christie J, Gaughan C, Lanken PN, Finkel B, Gallop R, Fuchs BD. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med. 2006;34:300–306. doi: 10.1097/01.CCM.0000198328.83571.4A. [DOI] [PubMed] [Google Scholar]


