Abstract
Chemical models provide tools with which to simplify and study complicated biological systems. Forces and chemical processes that govern the structure, function, and interactions of a biomacromolecule can be explored with a simple, easy-to-study synthetic molecule. Chemical models of β-sheet structures have helped to elucidate the factors influencing protein structures and functions. Chemical models that mimic β-sheet quaternary structure and interactions are emerging as valuable tools with which to better understand and control protein recognition and protein aggregation.
Introduction
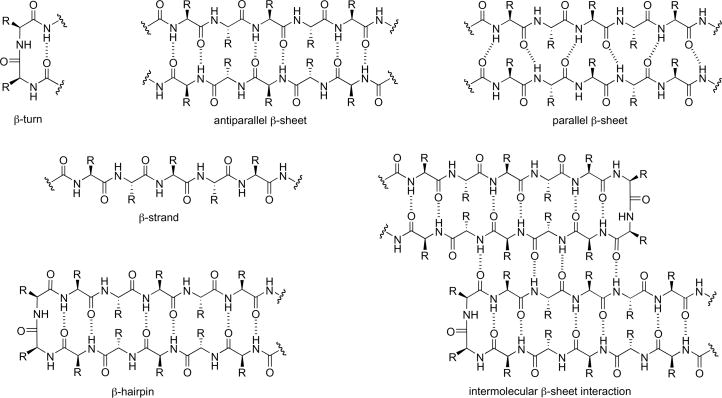
Protein β-sheet structures are formed by the lateral alignment of β-strands in parallel or antiparallel orientations and are stabilized by hydrogen bonding, hydrophobic interactions, and other forces (Figure 1). While intramolecular β-sheet interactions are associated with protein folding, intermolecular β-sheet interactions are associated with protein quaternary structure, protein-protein interactions, and peptide and protein aggregation. Not only are β-sheets important for normal biological activities, but also they are involved in many diseases including HIV, cancer, and neurodegenerative diseases. Recognizing the forces that contribute to the stability and interactions of β-sheets is essential to understanding and controling protein functions and interactions [1,2].
Figure 1.
Naturally occurring β-sheet structures and interactions.
Model systems that fold to form β-sheet-like structures or participate in intermolecular β-sheet interactions provide valuable tools to study β-sheet folding and interactions. β-Sheet folding has been studied extensively with β-hairpins and related structures [3,4,5,6,7]. Despite the importance of intermolecular β-sheet interactions, few model systems have focused on mimicking these interactions [8,9,10,11]. Artificial β-sheets are chemical models of β-sheets that contain unnatural prosthetic templates. In artificial β-sheets, β-turn mimics, β-strand mimics, and other templates help nucleate and stabilize β-sheet structures or enforce intermolecular β-sheet interactions [12,13,14,15].
This review summarizes the development of artificial β-sheets during the past two years and focuses on those that demonstrate hydrogen-bonding patterns similar to parallel or antiparallel β-sheet structures. The first two sections focus on β-sheet folding; the last two sections focus on intermolecular β-sheet interactions. This review excludes β-turn mimics and β-strand mimics that have not been incorporated into artificial β-sheets. It also excludes synthetic β-hairpins that mimic important side-chain interactions of biologically active peptides and proteins, which were recently reviewed by Robinson [16•].
β-Hairpins containing β-turn mimics
Reverse turns induce β-sheet structure in proteins by aligning protein chains. β-Turn mimics have been used extensively to nucleate β-sheet folding in β-hairpins.
Achiral α-aminoisobutyric acid (Aib) induces a β-turn conformation when combined with either a D-α-amino acid or an achiral α-amino acid. Balaram and coworkers had previously shown that Aib-DAla forms a β-turn structure in the solid state when incorporated into a peptide sequence [17]. The authors have recently shown that the conformation of peptides with a central Aib-Xaa (Xaa = DAla, DVal, DPro, or Gly) unit in solution depends on the solvent: Aib-DAla and Aib-Gly units adopt a β-turn conformation in hydrogen-bonding organic solvents and a helical conformation in organic solvents that do not participate significantly in hydrogen bonding. Aib-DPro and Aib-DVal units, on the other hand, adopt a β-turn conformation in both hydrogen-bonding and non-hydrogen-bonding organic solvents [18]. Hammer, Veglia, Barany, and coworkers have shown that both Aib-DAla and Aib-Gly adopt a β-turn conformation in water [19]. The authors have compared the conformation of one of Gellman’s peptides [20] with three different turn units: Gellman’s DPro-Gly, Balaram’s Aib-DAla, and achiral Aib-Gly (Figure 2A). These peptides all fold into superimposable β-hairpin structures in water. Collectively, these findings show that achiral turn units, like chiral turn units with appropriate chirality, can induce β-hairpin folding.
Figure 2.
β-Hairpins containing β-turn mimics. (A) β-Hairpins with Aib-based or DPro-Gly turn units. (B) β-Hairpins with extended α,β, α,γ, and α,δ turn units. (C) Light-controlled β-hairpin with azobenzene-based turn unit. (D) β-Hairpin with 1,2,3-triazole-based turn unit. (E) β-Hairpin with tetrahydroisoquinoline-based turn unit. (F) β-Hairpin with diketopiperazine-based turn unit. (G) Parallel β-sheet based on DPro-DADME diamine turn unit. (H) Parallel β-sheet based on CHDA-Gly diacid turn unit.
Balaram and coworkers have reported several expanded β-turn mimics that contain DPro in the i+1 position and a β-, γ-, or δ-amino acid in the i+2 position [21]. A peptide with a α,δ turn unit adopts a β-hairpin structure in solid state, while peptides with α,β and α,γ turn units adopt β-hairpin structures in methanol (Figure 2B).
β-Turn mimics that undergo a rapid light-triggered conformational change permit studying β-sheet folding processes. Previous studies showed that the cis isomer of an m, m′-substituted azobenzene amino acid induces a β-hairpin conformation in peptides, while the trans isomer disfavors this conformation (Figure 2D) [22,23]. The photo-induced cis-trans isomerization of the azo bond occurs efficiently in picoseconds. Moroder, Zinth, Renner, Tavan, and coworkers have monitored the photo-triggered folding-unfolding process of a peptide that contains this azobenzene turn unit by using ultrafast time-resolved mid-IR spectroscopy [24•]. Gogoll and coworkers have reported an m, m′-substituted stilbene derivative as an alternative to the photo-switchable azobenzene turn unit in linear and cyclic peptides [25]. The stilbene double bond suffers from a slow and incomplete cis-trans isomerization, but is more thermally stable than the azo bond. In linear peptides, both the cis and trans isomers of the stilbene derivative fail to induce β-hairpin structures in methanol and in DMSO. In cyclic peptides containing both the stilbene turn unit and a DPro-Gly turn unit, only the cis isomer supports β-sheet folding.
Several other research groups have reported new β-turn mimics that induce β-hairpin folding in peptides. Guan and Oh have developed a 1,4-disubstituted 1,2,3-triazole-based β-turn mimic that induces a β-hairpin-like structure for a small peptide in chloroform (Figure 2D) [26]. Silvani and coworkers have reported β-hairpin folding of small peptides containing a tetrahydroisoquinoline-based β-turn mimic in chloroform (Figure 2E) [27]. Piarulli and coworkers have developed a diketopiperazine amino acid that induces a β-hairpin conformation in peptide sequences in DMSO (Figure 2F) [28].
Chemical model systems are uniquely suited for studying parallel β-sheet folding, because small peptides composed only of α-amino acids cannot fold to form parallel β-sheets. β-Hairpin analogues containing diamine or diacid turn units provide good models with which to study parallel β-sheet folding, because they can orient peptide chains in parallel. Gellman and coworkers previously introduced the reverse-turn diamine linker DPro-DADME that aligns two C-terminally attached peptide strands in a parallel orientation in water (Figure 2G) [29]. The authors evaluated the folding thermodynamics of this model system by using a cyclic peptide as the benchmark for folding [30].
In their initial efforts to induce parallel β-sheet folding by diacid linkers, Gellman and coworkers employed succinyl-Gly as a turn unit. This diacid linker only partially further stabilizes the parallel β-sheet folding nucleated by the DPro-DADME diamine linker in the benchmark cyclic peptide described above [30]. Replacing the succinic acid with the more rigid S,R- or R,S-cis-cyclohexanedicarboxylic acid (CHDA), Gellman and coworkers have recently developed the CHDA-Gly turn unit as the first diacid linker that promotes parallel β-sheet folding in water (Figure 2H) [31•]. The CHDA-Gly diacid linker aligns two N-terminally attached peptide strands in a parallel orientation. Swapping the attached peptide strands eliminates folding by replacing favorable side-chain interactions with less favorable ones. This finding suggests that favorable side-chain interactions between parallel strands [32] are more important in folding than the reverse-turn linker or the intrinsic β-sheet propensity of the residues. The authors anticipate that combining the CHDA-Gly diacid linker and the DPro-DADME diamine linker may enable the preparation of both parallel cyclic peptides and multistranded parallel β-sheets.
Cyclic β-sheet peptides containing β-turn and β-strand mimics
Macrocyclization helps stabilize β-sheet structure in the naturally occurring peptide antibiotics gramicidin S and θ-defensin and has been used extensively to create well-folded β-sheet peptides [16•,20].
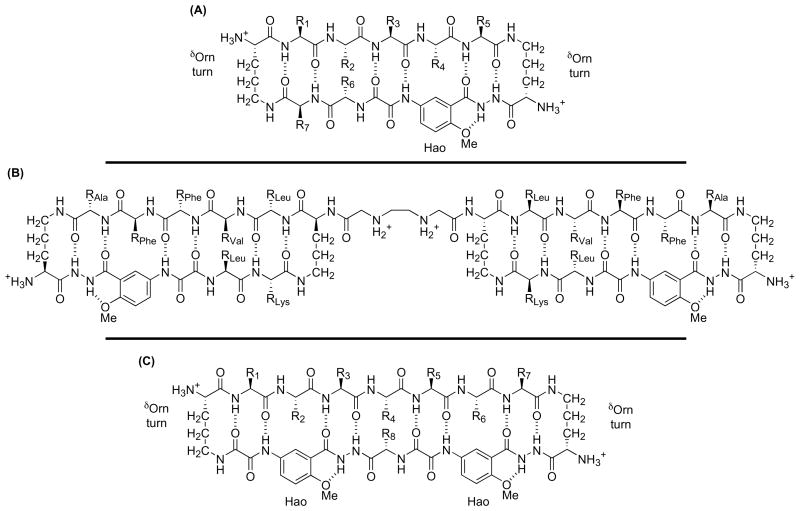
To help enforce β-sheet structure and reduce the dependence of folding on sequence, Nowick and coworkers have developed macrocyclic peptides containing β-strand and β-turn mimics [33,34,35••,36]. One class of these macrocyclic β-sheet peptides consists of a pentapeptide strand, a Hao β-strand mimic, and two δ-linked ornithine (δOrn) β-turn mimics in a 42-membered ring (Figure 3A) [33,34,36]. Many of the 42-membered-ring macrocyclic peptides adopt folded β-sheet structures in water. Nowick and coworkers are now using these peptides as ligands to bind protein β-sheets and control protein-protein interactions. In addition to imparting water solubility, the δOrn α-amino group allows preparation of bivalent structures in which a linker connects two molecules (Figure 3B). A related class of macrocyclic β-sheet peptides is described in the next section (Figure 3C)[33,35••,36].
Figure 3.
Cyclic β-sheet peptides containing β-strand and β-turn mimics. (A) 42-Membered-ring cyclic β-sheets containing Hao and δOrn units. (B) Bivalent structure based on 42-membered-ring cyclic β-sheets. (C) 54-Membered-ring cyclic β-sheets containing Hao and δOrn units.
Mimicry of β-sheet quaternary structure through non-covalent interactions
Artificial β-sheets that participate in intermolecular β-sheet interactions provide easy-to-study chemical models of the interactions that occur in complex protein assemblies and aggregates.
Nowick and coworkers previously developed chemical models that form antiparallel β-sheet dimers in non-hydrogen-bonding solvents [33,36]. Chemical models that form well-defined β-sheet dimers in water proved more challenging. Hydrogen bonding between water molecules and the amide groups of small peptides strongly competes with the hydrogen bonding associated with intermolecular β-sheet interactions. In addition, hydrophobic interactions in β-sheets can result in non-specific aggregation. Nowick and coworkers have developed 54-membered-ring cyclic peptides that mimic β-sheet quaternary structure in water through intermolecular antiparallel β-sheet interactions (Figures 3C and 4A) [33,35••,36]. These artificial β-sheets consist of a heptapeptide strand, two Hao β-strand mimics, and two δOrn turn β-mimics. Many of these peptides form a tetrameric β-sheet sandwich, which is in equilibrium with a partially folded monomer. In the tetrameric β-sheet sandwich, two edge-to-edge β-sheet dimers further dimerize through stabilizing hydrophobic interactions. Variations in the heptapeptide sequence reveal the importance of hydrophobic interactions in tetramer formation. The authors are currently exploring the interactions of this system with protein β-sheets and β-sheet aggregates.
Figure 4.
Chemical models of β-sheet quaternary structure. (A) Edge-to-edge antiparallel β-sheet dimer present in the tetrameric β-sheet sandwich of 54-membered-ring cyclic β-sheet peptides containing Hao and δOrn units. (B) Parallel β-sheet dimer containing succinic or fumaric diacid linkers and β-strand and β-turn mimics. (C) Antiparallel β-sheet-like dimer of Ach-based cyclic α,γ-peptides. (D) Antiparallel β-sheet dimer stabilized by a disulfide linkage. (E) Antiparallel β-sheet heterodimer stabilized by metal coordination.
Parallel β-sheet interactions are especially important in peptide and protein aggregation, such as β-amyloid peptide aggregation in Alzheimer’s disease. To study intermolecular parallel β-sheet interactions, Nowick and Levin have recently developed artificial β-sheets that dimerize through parallel β-sheet interactions in chloroform (Figure 4B) [36,37•]. In these β-sheets, two peptide strands are N-terminally linked with a succinic or fumaric diacid linker. An (S)-2-aminoadipic acid β-turn mimic connects the resulting linked dipeptide strand to a β-strand mimic. In the dimer, one edge of the linked dipeptide strand is blocked through intramolecular antiparallel β-sheet hydrogen bonding with the β-strand mimic, while the other edge participates in intermolecular parallel β-sheet interactions. The authors anticipate using this new model system to study side-chain interactions in parallel β-sheet systems and extending this model system to aqueous solutions.
Spontaneous stacking of certain cyclic peptides through intermolecular β-sheet-like hydrogen bonding produces nanotubes. Previously reported nanotube-forming cyclic peptides include cyclic peptides with α-amino acids of alternating chirality and cyclic β-peptides [38,39]. Selective N-methylation on one edge limits the stacking of cyclic peptides to dimer formation. Granja and coworkers have recently developed new nanotube-forming cyclic peptides composed of alternating α-amino acids and cyclic γ-amino acids with alternating chirality. Cyclic α,γ-peptides with cyclic γ-cis-amino acids, such as cis-3-aminocyclopentanecarboxylic acid (Acp) and cis-3-aminocyclohexanecarboxylic acid (Ach), form tubular structures in non-hydrogen-bonding solvents and in the solid state. N-Methylation of either the α-amino acids or cyclic γ-amino acids affords antiparallel β-sheet-like dimers (Figure 4C), with N-methylation of the cyclic γ-amino acids providing higher dimerization constants [40,41]. Heterodimerization occurs preferentially between Acp- and Ach-based cyclic peptides, and fluorescence studies of peptides bearing different chromophores allow analysis of the heterodimer-homodimer equilibrium [42,43].
Kimura and coworkers have reported parallel β-sheet stacking of cyclic β-peptides based on trans-2-aminocyclohexanecarboxylic acid and trans-pyranoid sugar amino acids in the solid state [44,45,46]; Chandrasekhar and coworkers have reported parallel β-sheet stacking of cyclic β-peptides based on cis-furanoid sugar amino acids in the solid state and in non-hydrogen-bonding solvents [47].
Many enzymes, binding proteins, and membrane pores contain β-barrel structures consisting of a tubular β-sheet comprising β-strand “staves”. In the late 1990s, Matile and coworkers introduced a class of chemical models of β-barrels comprising octaphenyl templates bearing peptide strands, which self-assemble to form tubular tetrameric structures [48]. In these structures, the octaphenyl templates act as staves and the peptide strands interdigitate to form β-sheet “hoops”. The biphenyl torsions in the octaphenyl staves create angles in the attached β-sheets and prevent aggregation, while intermolecular antiparallel β-sheet interactions stabilize the tetrameric β-barrel-like structure. Matile and coworkers have used variants of artificial β-barrels as receptors, ion channels, pores, catalysts, and sensors. The authors have recently developed a muticomponent-sensing system that combines signal-generating enzymes, signal-transducing artificial β-barrels, and signal-amplifying reactive pore-blockers [49]. This artificial tongue selectively responds to several sweet-, sour-, and umami-tasting molecules. Matile and coworkers have summarized the development and application of these artificial β-barrels in two recent reviews [50,51].
Mimicry of β-Sheet quaternary structures through covalent bonds
Many protein quaternary structures are stabilized through additional linking of protein domains by disulfide bond formation or metal coordination. Artificial β-sheets with these types of covalent or coordinative bonds provide another type of easy-to-study chemical model of the interactions that occur in complex protein assemblies and aggregates.
Disulfide bond formation among cysteine residues to form cystine occurs widely in protein quaternary structure. Linton and Cashman have demonstrated that disulfide bond formation induces β-sheet hydrogen bonding between the attached peptide strands in non-hydrogen-bonding organic solvents [52•]. A cystine dimer of a tripeptide with a central cysteine residue forms a small antiparallel β-sheet structure (Figure 4D). This result shows that a disulfide bond between a non-hydrogen-bonded cysteine pair not only acts as a linkage between β-strands, but also further stabilizes the β-sheet structure. Subsequent studies of a β-hairpin peptide model system containing cystine by Rico, Jiménez, and coworkers corroborate the stabilization of β-sheet structure by disulfide linkages between non-hydrogen-bonded cysteine pairs [53•].
Metal coordination also occurs widely in protein quaternary structure, with the protein imidazole, thiol, and carboxylate functionalities acting as metal-coordinating ligands. Breit and coworkers are creating bidentate ligands in which intermolecular hydrogen bonding brings together two monodentate ligands. The authors initially obtained homodimeric helical structures by coordinating dipeptides bearing N-terminal phosphine groups to Pt(II) or Rh(I) in chloroform [54]. The authors have subsequently obtained heterodimeric β-sheet structures by coordinating a dipeptide bearing an N-terminal phosphine group and a complementary dipeptide bearing a C-terminal phosphine group (Figure 4E) [55•]. Size complementarity of the side chains (e.g. Val and Ala), heterochirality of the peptides (e.g. LVal and DVal), and peptide length favor the heterodimeric β-sheet structures over the competing homodimeric helical structures.
Conclusion
Chemical models of β-sheet structures increase the understanding of forces involved in protein β-sheet folding and intermolecular β-sheet interactions. Unnatural templates, such as β-strand mimics, β-turn mimics, and various linkers help induce and stabilize β-sheet folding or enforce intermolecular β-sheet interactions in small artificial β-sheets. These unnatural templates furthermore help control aggregation and impart properties, such as modularity, solubility, and parallel alignment, which may be difficult to achieve with natural peptides and proteins. Within the past two decades, many chemical model systems have been developed to study β-sheet folding. A new trend focuses on chemical model systems that demonstrate intermolecular β-sheet interactions. Another trend focuses on chemical model systems that exhibit parallel β-sheet structures and interactions. The findings from these studies will help researchers better control or modify β-sheet interactions and may have profound implications for the treatment of Alzheimer’s and other neurodegenerative diseases.
Acknowledgments
The authors thank the National Institutes of Health and the National Science Foundation for grant support (GM-49076, CHE-0750523).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maitra S, Nowick JS. The amide linkage. In: Greenberg A, Breneman CM, Liebman JF, editors. Structural significance in chemistry, biochemistry, and materials science. Wiley; New York: 2000. pp. 495–518. [Google Scholar]
- 2.Remaut H, Waksman G. Protein–protein interaction through β-strand addition. Trends Biochem Sci. 2006;31:436–444. doi: 10.1016/j.tibs.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Gellman SH. Minimal model systems for β-sheet secondary structure in proteins. Curr Opin Chem Biol. 1998;2:717–725. doi: 10.1016/s1367-5931(98)80109-9. [DOI] [PubMed] [Google Scholar]
- 4.Searle MS. Peptide models of protein β-sheets: design, folding and insights into stabilising weak interactions. J Chem Soc, Perkin Trans. 2001;2:1011–1020. [Google Scholar]
- 5.Venkatraman J, Shankaramma SC, Balaram P. Design of folded peptides. Chem Rev. 2001;101:3131–3152. doi: 10.1021/cr000053z. [DOI] [PubMed] [Google Scholar]
- 6.Searle MS, Ciani B. Design of β-sheet systems for understanding the thermodynamics and kinetics of protein folding. Curr Opin Struct Biol. 2004;14:458–464. doi: 10.1016/j.sbi.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Hughes RM, Waters ML. Model systems for β-hairpins and β-sheets. Curr Opin Struct Biol. 2006;16:514–524. doi: 10.1016/j.sbi.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Quinn TP, Tweedy NB, Williams RW, Richardson JS, Richardson DC. Betadoublet: De novo design, synthesis, and characterization of a β-sandwich protein. Proc Natl Acad Sci USA. 1994;91:8747–8751. doi: 10.1073/pnas.91.19.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayo KH, Ilyina E. A folding pathway for β pep-4 peptide 33mer: From unfolded monomers and β-sheet sandwich dimers to well-structured tetramers. Protein Sci. 1998;7:358–368. doi: 10.1002/pro.5560070216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carulla N, Woodward C, Barany G. BetaCore, a designed water soluble four-stranded antiparallel β-sheet protein. Protein Sci. 2002;11:1539–1551. doi: 10.1110/ps.4440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali MH, Peisach E, Allen KN, Imperiali B. X-ray structure analysis of a designed oligomeric miniprotein reveals a discrete quaternary architecture. Proc Natl Acad Sci USA. 2004;101:12183–12188. doi: 10.1073/pnas.0401245101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nowick JS, Smith EM, Pairish M. Artificial β-sheets. Chem Soc Rev. 1996:401–415. [Google Scholar]
- 13.Nowick JS. Chemical models of protein β-sheets. Acc Chem Res. 1999;32:287–296. [Google Scholar]
- 14.Moriuchi T, Hirao T. Highly ordered structures of peptides by using molecular scaffolds. Chem Soc Rev. 2004;33:294–301. doi: 10.1039/b307632f. [DOI] [PubMed] [Google Scholar]
- 15.Loughlin WA, Tyndall JDA, Glenn MP, Fairlie DP. Beta-strand mimetics. Chem Rev. 2004;104:6085–6117. doi: 10.1021/cr040648k. [DOI] [PubMed] [Google Scholar]
- 16•.Robinson JA. β-Hairpin peptidomimetics: design, structures and biological activities. Acc Chem Res. 2008 doi: 10.1021/ar700259k. ASAP. This review describes small synthetic β-hairpins and related structures that mimic the structural and functional properties of biologically important protein and peptide epitopes. The author emphasizes the potential therapeutic use of these epitope mimics as inhibitors of protein-protein and protein-nucleic acid interactions associated with diseases like cancer and HIV. [DOI] [PubMed] [Google Scholar]
- 17.Aravinda S, Shamala N, Rajkishore R, Gopi HN, Balaram P. A crystalline β-hairpin peptide nucleated by a type I’ Aib-D-Ala β-turn: evidence for cross-strand aromatic interactions. Angew Chem Int Ed. 2002;41:3863–3865. doi: 10.1002/1521-3773(20021018)41:20<3863::AID-ANIE3863>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Rai R, Raghothama S, Sridharan R, Balaram P. Tuning the β-turn segment in designed peptide β-hairpins: Construction of a stable type I’ β-turn nucleus and hairpin–helix transition promoting segments. Biopolymers. 2007;88:350–361. doi: 10.1002/bip.20649. [DOI] [PubMed] [Google Scholar]
- 19.Masterson LR, Etienne MA, Porcelli F, Barany G, Hammer RP, Veglia G. Nonstereogenic α-aminoisobutyryl-glycyl dipeptidyl unit nucleates type I’ β-turn in linear peptides in aqueous solution. Biopolymers. 2007;88:746–753. doi: 10.1002/bip.20738. [DOI] [PubMed] [Google Scholar]
- 20.Syud FA, Espinosa JF, Gellman SH. NMR-Based quantification of β-sheet populations in aqueous solution through use of reference peptides for the folded and unfolded states. J Am Chem Soc. 1999;121:11577–11578. [Google Scholar]
- 21.Rai R, Vasudev PG, Ananda K, Raghothama S, Shamala N, Karle IL, Balaram P. Hybrid peptides: Expanding the β turn in peptide hairpins by the insertion of β-, γ-, and δ-residues. Chem — Eur J. 2007;13:5917–5926. doi: 10.1002/chem.200601562. [DOI] [PubMed] [Google Scholar]
- 22.Dong S-L, Löweneck M, Schrader TE, Schreier WJ, Zinth W, Moroder L, Renner C. A photocontrolled β-hairpin peptide. Chem — Eur J. 2006;12:1114–1120. doi: 10.1002/chem.200500986. [DOI] [PubMed] [Google Scholar]
- 23.Aemissegger A, Kräutler V, Van Gunsteren WF, Hilvert D. A photoinducible β-hairpin. J Am Chem Soc. 2005;127:2929–2936. doi: 10.1021/ja0442567. [DOI] [PubMed] [Google Scholar]
- 24•.Schrader TE, Schreier WJ, Cordes T, Koller FO, Babitzki G, Denschlag R, Renner C, Löweneck M, Dong S-L, Moroder L, Tavan P, Zinth W. Light-triggered β-hairpin folding and unfolding. Proc Natl Acad Sci USA. 2007;104:15729–15734. doi: 10.1073/pnas.0707322104. This article describes a reversible unfolding-folding process for a light-switchable β-hairpin triggered by ultrashort UV pulses and monitored by ultrafast mid-IR probing. The results confirm that transition from a collapsed hydrophobic cluster to a hydrogen-bonded β-sheet structure is the rate-determining step of the folding process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erdélyi M, Karlén A, Gogoll A. A new tool in peptide engineering: a photoswitchable stilbene-type β-hairpin mimetic. Chem — Eur J. 2006;12:403–412. doi: 10.1002/chem.200500648. [DOI] [PubMed] [Google Scholar]
- 26.Oh K, Guan Z. A convergent synthesis of new β-turn mimics by click chemistry. Chem Commun. 2006:3069–3071. doi: 10.1039/b606185k. [DOI] [PubMed] [Google Scholar]
- 27.Lesma G, Meschini E, Recca T, Sacchetti A, Silvani A. Synthesis of tetrahydroisoquinoline-based pseudopeptides and their characterization as suitable reverse turn mimetics. Tetrahedron. 2007;63:5567–5578. [Google Scholar]
- 28.Ressurreição ASM, Bordessa A, Civera M, Belvisi L, Gennari C, Piarulli U. Synthesis and conformational studies of peptidomimetics containing a new bifunctional diketopiperazine scaffold acting as a β-hairpin inducer. J Org Chem. 2008;73:652–660. doi: 10.1021/jo702072z. [DOI] [PubMed] [Google Scholar]
- 29.Fisk JD, Gellman SH. A parallel β-sheet model system that folds in water. J Am Chem Soc. 2001;123:343–344. doi: 10.1021/ja002493d. [DOI] [PubMed] [Google Scholar]
- 30.Fisk JD, Schmitt MA, Gellman SH. Thermodynamic analysis of autonomous parallel β-sheet formation in water. J Am Chem Soc. 2006;128:7148–7149. doi: 10.1021/ja060942p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Freire F, Fisk JD, Peoples AJ, Ivancic M, Guzei IA, Gellman SH. Diacid linkers that promote parallel β-sheet secondary structure in water. J Am Chem Soc. 2008;130:7839–7841. doi: 10.1021/ja802042c. This communication describes the development of a water-soluble parallel β-sheet. Using this model system, the authors confirm the amino acid pairing preferences that Hutchinson and coworkers recently obtained from statistical study of parallel β-sheets in protein crystal structures [32]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fooks HM, Martin ACR, Woolfson DN, Sessions RB, Hutchinson EG. Amino acid pairing preferences in parallel β-sheets in proteins. J Mol Biol. 2006;356:32–44. doi: 10.1016/j.jmb.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Nowick JS. What I have learned by using chemical model systems to study biomolecular structure and interactions. Org Biomol Chem. 2006;4:3869–3885. doi: 10.1039/b608953b. [DOI] [PubMed] [Google Scholar]
- 34.Woods RJ, Brower JO, Castellanos E, Hashemzadeh M, Khakshoor O, Russu WA, Nowick JS. Cyclic modular β-sheets. J Am Chem Soc. 2007;129:2548–2558. doi: 10.1021/ja0667965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Khakshoor O, Demeler B, Nowick JS. Macrocyclic β-sheet peptides that mimic protein quaternary structure through intermolecular β-sheet interactions. J Am Chem Soc. 2007;129:5558–5569. doi: 10.1021/ja068511u. This article describes a simple model system that exhibits well-defined intermolecular antiparallel β-sheet interactions in aqueous solution. Self-assembly and folding in this model system are cooperative, reminiscent of large multidomain proteins like human transthyretin. Self-assembly is also highly dependent on stabilizing hydrophobic interactions, reflecting the frailty of hydrogen bonds alone in water. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nowick JS. Exploring β-sheet structure and interactions with chemical model systems. Acc Chem Res. doi: 10.1021/ar800064f. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Levin S, Nowick JS. An artificial β-sheet that dimerizes through parallel β-sheet interactions. J Am Chem Soc. 2007;129:13043–13048. doi: 10.1021/ja073391r. This article describes the first model system that exhibits well-defined intermolecular parallel β-sheet interactions. N-Terminally linking two dipeptides with a diacid unit maximizes the number of hydrogen-bonding groups available for parallel β-sheet dimerization and minimizes the number of hydrogen-bonding groups available for antiparallel β-sheet dimerization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bong DT, Clark TD, Granja JR, Ghadiri MR. Self-assembling organic nanotubes. Angew Chem Int Ed. 2001;40:988–1011. [PubMed] [Google Scholar]
- 39.Brea RJ, Granja JR. Dekker Encyclopedia of Nanoscience and Nanotechnology. Marcel Dekker; New York: 2004. Self-assembly of cyclic peptides in hydrogen-bonded nanotubes; pp. 3439–3457. [Google Scholar]
- 40.Brea RJ, Castedo L, Granja JR. Chem Commun. 2007. Large-diameter self-assembled dimers of α,γ-cyclic peptides, with the nanotubular solid-state structure of cyclo-[(L-Leu-D-MeN-γ-Acp)4-].4CHCl2COOH; pp. 3267–3269. [DOI] [PubMed] [Google Scholar]
- 41.Amorín M, Castedo L, Granja JR. Folding control in cyclic peptides through N-Methylation pattern selection: Formation of antiparallel β-sheet dimers, double Reverse turns and supramolecular helices by 3α,γ-cyclic peptides. Chem — Eur J. 2008;14:2100–2111. doi: 10.1002/chem.200701059. [DOI] [PubMed] [Google Scholar]
- 42.Brea RJ, Amorín M, Castedo L, Granja JR. Methyl-blocked dimeric α,γ-peptide nanotube segments: formation of a peptide heterodimer through backbone-backbone interactions. Angew Chem Int Ed. 2005;44:5710–5713. doi: 10.1002/anie.200501555. [DOI] [PubMed] [Google Scholar]
- 43.Brea RJ, Vázquez ME, Mosquera M, Castedo L, Granja JR. Controlling multiple fluorescent signal output in cyclic peptide-based supramolecular systems. J Am Chem Soc. 2007;129:1653–1657. doi: 10.1021/ja066885h. [DOI] [PubMed] [Google Scholar]
- 44.Fujimura F, Fukuda M, Sugiyama J, Morita T, Kimura S. Parallel assembly of dipolar columns composed of a stacked cyclic tri-β-peptide. Org Biomol Chem. 2006;4:1896–1901. doi: 10.1039/b600407e. [DOI] [PubMed] [Google Scholar]
- 45.Fujimura F, Hirata T, Morita T, Kimura S. Columnar assembly of cyclic β-amino Acid functionalized with pyranose rings. Biomacromolecules. 2006;7:2394–2400. doi: 10.1021/bm060415y. [DOI] [PubMed] [Google Scholar]
- 46.Hirata T, Fujimura F, Horikawa Y, Sugiyama J, Morita T, Kimura S. Molecular assembly formation of cyclic hexa-β-peptide composed of acetylated glycosamino acids. Biopolymers. 2007;88:150–156. doi: 10.1002/bip.20694. [DOI] [PubMed] [Google Scholar]
- 47.Jagannadh B, Reddy MS, Rao CL, Prabhakar A, Jagadeesh B, Chandrasekhar S. Self-assembly of cyclic homo- and hetero-β-peptides with cis- furanoid sugar amino acid and β-hGly as building blocks. Chem Commun. 2006:4847–4849. doi: 10.1039/b610858j. [DOI] [PubMed] [Google Scholar]
- 48.Sakai N, Majumdar N, Matile S. Self-Assembled Rigid-Rod Ionophores. J Am Chem Soc. 1999;121:4294–4295. [Google Scholar]
- 49.Litvinchuk S, Tanaka H, Miyatake T, Pasini D, Tanaka T, Bollot G, Mareda J, Matile S. Synthetic pores with reactive signal amplifiers as artificial tongues. Nat Mater. 2007;6:576–580. doi: 10.1038/nmat1933. [DOI] [PubMed] [Google Scholar]
- 50.Sisson AL, Shah MR, Bhosale S, Matile S. Synthetic ion channels and pores (2004–2005) Chem Soc Rev. 2006;35:1269–1286. doi: 10.1039/b512423a. [DOI] [PubMed] [Google Scholar]
- 51.Sakai N, Mareda J, Matile S. Artificial β-barrels. Acc Chem Res. 2008 doi: 10.1021/ar700229r. ASAP. [DOI] [PubMed] [Google Scholar]
- 52.Cashman TJ, Linton BR. β-Sheet hydrogen bonding patterns in cystine peptides. Org Lett. 2007;9:5457–5460. doi: 10.1021/ol7023696. [DOI] [PubMed] [Google Scholar]
- 53•.Santiveri CM, León E, Rico M, Jiménez MA. Context-dependence of the contribution of disulfide bonds to β-hairpin stability. Chem — Eur J. 2008;14:488–499. doi: 10.1002/chem.200700845. This article describes the use of a β-hairpin peptide model system with a cystine in various locations to determine the effect of the disulfide bond and its location on β-sheet folding. The results from this study establish that a disulfide bond between a non-hydrogen-bonded cysteine pair stabilizes the β-sheet structure about 1 kcal mol−1. [DOI] [PubMed] [Google Scholar]
- 54.Laungani AC, Breit B. Supramolecular PhanePhos-analogous ligands through hydrogen-bonding for asymmetric hydrogenation. Chem Commun. 2008:844–846. doi: 10.1039/b716529c. [DOI] [PubMed] [Google Scholar]
- 55•.Laungani AC, Slattery JM, Krossing I, Breit B. Supramolecular bidentate ligands by metal-directed in situ formation of antiparallel β-sheet structures and application in asymmetric catalysis. Chem — Eur J. 2008;14:4488–4502. doi: 10.1002/chem.200800359. This article describes formation of antiparallel β-sheet structure through coordination of two peptide strands to a metal complex. The authors are using this β-sheet structure as a class of chiral bidentate ligands in organometallic catalysis. [DOI] [PubMed] [Google Scholar]