Abstract
The focus of this study was to evaluate the therapeutic benefit of combined gastrin releasing peptide (GRP) receptor targeted-radiotherapy (TRT) with chemotherapy using the PC-3 xenograft SCID mouse model. 177Lu-DOTA-8-AOC-BBN(7-14)NH2 is a radiotherapeutic peptide which specifically targets the gastrin releasing peptide receptor over-expressed on primary and metastatic prostate cancer. The chemotherapeutic agents docetaxel and estramustine, were administered as single agents or in combination with the receptor targeted radiotherapeutic agent. Combination receptor TRT/chemotherapy studies were begun 21 days post xenografting and were conducted as multiple dose trials. The GRP receptor TRT agent was administered every 14 days and single and combination chemotherapy dose regimens were given weekly. Tumor size, body weight, and body condition score were evaluated twice weekly and a hematology profile once weekly. Therapy study tumor volumes were evaluated by way of a repeated measures analysis of variance. Tumor volume measurements at 12 days post dose administration demonstrated a statistically significant (two-tailed p value < 0.05) tumor growth suppression in all experimental groups receiving GRP receptor targeted radiotherapy, when compared to the control group. The two combined GRP receptor TRT/chemotherapy treatment groups demonstrated the greatest tumor growth suppression of all treatment groups. In comparing the two combined GRP receptor TRT/chemotherapy groups to the GRP receptor TRT alone group, a statistically significant difference was demonstrated for the combined groups by day 30, post dose administration. These data demonstrate that GRP receptor targeted radiation therapy using 177Lu-DOTA-8-AOC-BBN(7-14)NH2, employed either alone, or in combination with conventional chemotherapy can suppress the growth of androgen independent prostate cancer.
Keywords: prostate, gastrin releasing peptide, docetaxel, lutetium-177, scintigraphic imaging
INTRODUCTION
Prostate cancer is the second leading cause of cancer death in the United States.1 The American Cancer Society estimates that in 2006 alone, 234,460 men will be diagnosed with prostate cancer and an estimated 27,350 will die.1 Treatment options have varied, dependent upon stage and patient history. Early stage disease treatment options include surgery and radiation, while treatment of metastatic disease includes hormonal therapy, chemotherapy, radiation, or combinations of the above.2
The focus of our study was to investigate the utility of a combination therapy regimen for treatment of androgen-independent (hormone refractory) metastatic prostate cancer using a novel receptor targeted radiolabeled peptide, 177Lu-DOTA-8-AOC-BBN(7-14)NH2, combined with micro-tubule inhibiting chemotherapeutic agents. Androgen independent prostate cancer (AIPC) is a progressive, advanced form of prostate cancer with few limited treatment options and a poor overall prognosis. AIPC presents when prostate tumors acquire the ability to progress and grow in the absence of androgens. Therapy for AIPC includes further attempts at hormonal manipulations, often futile, chemotherapy, and radiotherapy.2,3 General survival estimates for men who have progressed to AIPC range from 9–12 months.4 Until recently, no phase III clinical trials had demonstrated significant increases in survival once a patient was diagnosed with AIPC. However, two recent phase III clinical trials in men have shown an increased survival with use of Taxotere (docetaxel)-based regimens.5,6
The utility of employing peptide based receptor targeted radiotherapeutic (TRT) agents has been elegantly demonstrated within the last decade by the results obtained in targeting somatostatin receptor positive neoplasms with radiolabeled peptides.7,8 These studies have paved the way for researchers now exploring the use of radiolabeled analogs of bombesin (BBN), gastrin releasing peptide (GRP), alpha melanocyte stimulating hormone (α-MSH), neurotensin, vasoactive intestinal peptide (VIP), gastrin/cholecystokinin (CCK), and substance P.9–11
Our laboratory is currently focused on developing new diagnostic and therapeutic radiopharmaceuticals which target the GRP receptor.10,12–15 The GRP, or BB2 receptor, is one of the four receptor subtypes belonging to the bombesin receptor family.10 The GRP receptor has been shown to be expressed in abundance on several tumors of neuroendocrine origin including prostate, breast, small cell lung and pancreatic.12–15 With respect to prostate cancer, the GRP receptor may be an ideal candidate for receptor targeted drug development based on a recent report demonstrating the GRP receptor to be overexpressed on human neoplastic prostate tissue, but absent in human prostate hyperplasia16. This data, and the availability of the PC-3 cell line, an androgen independent prostatic human tumor line that overexpresses the GRP receptor, make this an extremely interesting and applicable system to explore.17
We have previously demonstrated in mouse xenograft studies that truncated radiolabeled analogues of the peptide bombesin (BBN), successfully bind to GRP receptors expressed on PC-3 prostate tumors.10,12–15 Upon binding the GRP receptor on the cellular membrane, these compounds undergo rapid receptor mediated endocytosis and remain trapped within the tumor cell for extended periods of time.13–15 The cellular retention of these compounds is required in order to deliver an effective radiation dose to the target cell as well as to immediately adjacent cells. Using pharmacokinetic screening, we have selected the peptide DOTA-8-AOC-BBN(7-14)NH2, as possessing optimum pharmacokinetic and receptor binding properties of the GRP receptor targeted compounds currently under development in our laboratory.12,15
177Lu is an attractive isotope as the radiotherapeutic component of a receptor targeted molecule based on its ready availability and physical properties, which include the emission of a medium energy β− (0.497 MeV), a half-life of 6.71 days, and a maximum range in soft tissue of approximately 2mm.15 Preliminary studies from our laboratory have evaluated the utility of targeted GRP receptor radiotherapy in PC-3 xenograft SCID mice using 177Lu-DOTA-8-AOC-BBN(7-14)NH2, administered as a single agent.18 These studies demonstrated that a single dose of 177Lu-DOTA-8-AOC-BBN(7-14)NH2 was effective in inhibiting tumor growth and prolonging survival, with therapeutic responses being observed at dosing levels as low as 740 MBq/kg.18
In the current study, we assessed the therapeutic effects of a combination of standard chemotherapeutic regimens for treatment of AIPC with 177Lu-DOTA-8-AOC-BBN(7-14)NH2, a GRP-receptor TRT compound, using the PC-3 flank xenograft SCID mouse model. In these trials, we evaluated GRP receptor TRT in combination with the cytotoxic chemotherapeutics, docetaxel and estramustine. Docetaxel based regimens, with or without the addition of estramustine, are currently being evaluated in clinical trials to treat AIPC.19–24 Docetaxel and estramustine, when used in combination, function synergistically as microtubule inhibitors to offer an increased cytotoxic effect.23,25–27 Docetaxel is a member of the Taxane family and its major mode of action on cancer cells is due to a high affinity for tubulin, and subsequent inhibition of microtubule depolymerization.28,29 The chemotherapeutic estramustine belongs to the family of plant drugs, vinca alkaloids. Estramustine is a stable conjugate of nornitrogen mustard and estradiol, with its main therapeutic mechanism of action on tumor cells due to a weak inhibition of microtubule polymerization.19,28,30–33 The microtubule stabilization brought about by these chemotherapeutic agents leads to cellular arrest in the G2/M phase of the cell cycle, which provides these agents with both cytotoxic and radiosensitization properties which have the potential to enhance the therapeutic effects of a GRP receptor TRT compound.
MATERIALS AND METHODS
Mice and Husbandry
Four to five week old female ICR SCID (severe combined immunodeficiency) mice (Taconic, Germantown, NY) were used for the therapy study. The mice were housed five per cage in autoclaved microisolator caging, on paper-chip laboratory animal bedding (RPCO, St. Louis, MO). An exception to routine housing occurred during the initial high radiotherapy dose phase, as the mice were housed individually for three days post radiotherapy administration to reduce likelihood for cross exposure to excreted radiation in urine and feces.
The mice were housed in a temperature and humidity controlled room with a 12 hour light and 12 hour dark cycle. Mice were ad libitum fed Lab Diet 5053 irradiated rodent diet (PMI Nutrition International LLC, Brentwood, MO). Husbandry and care guidelines were adhered to as directed in the Guide for the Care and Use of Laboratory Animals. Animal studies were approved and conducted according to animal research policies at the Harry S Truman Memorial Veterans Hospital, Columbia, Missouri and in accordance with NIH PHS guidelines.
Cell line and Xenografting Procedure
The prostate carcinoma, PC-3 cell line (American Type Culture Collection, Manassas, VA) originates from a human bone metastasis.34,35 The tumor cell lines were maintained by the University of Missouri Cell and Immunobiology Core Facility. PC-3 cells were grown in base media F-12K nutrient mixture, Kaighn’s modification (Invitrogen Corporation, Carlsbad, CA). In preparation for xenografting, the PC-3 cell/media was first centrifuged to separate cells from the media. Next, the PC-3 cell pellet was combined with Dulbecco’s phosphate-buffered saline (Invitrogen Corporation, Carlsbad, CA) to reach a final concentration of 5 million cells/100μL. The SCID mice were bilaterally flank inoculated with 5 million PC-3 tumor cells per flank. The mice were xenografted while under gas anesthesia. Anesthesia was administered with a non-rebreathing apparatus (Summit Medical Equipment Company, Bend, OR) at a vaporizer setting of ~3.5% isoflurane (Baxter Healthcare Corp., Deerfield, IL) with 0.5 L/min oxygen. Mice were allowed to grow flank tumors for three weeks prior to study therapy initiation.
Chemotherapy
Docetaxel (Taxotere®, Aventis Pharmaceuticals Products Inc., Bridgewater, NJ) and Estramustine phosphate sodium (Emcyt®, Pharmacia Corporation, Kalamazoo, MI) were obtained from commercial sources. The chemotherapeutic agents were aseptically prepared for administration within a biosafety cabinet. Docetaxel was prepared as a stock solution of 10 mg/ml, per package instructions. The docetaxel stock solution (2.0 ml) was diluted with 8 ml of sterile saline to reach a final parenteral solution of 2.0 mg/ml. A parenteral estramustine solution was prepared by dissolving the contents of an estramustine capsule (140 mg/capsule) with sterile saline to reach a final concentration of 2.0 mg/ml. Docetaxel and estramustine were both administered intraperitoneally (ip), at a dosage level of 15 mg/kg. Chemotherapeutic dose levels were extrapolated from a conversion formula used to convert human dosage (mg/m2) to an approximate mouse dosage (mg/kg) and these dose levels are similar to those previously published.27,36,37 The docetaxel and docetaxel + estramustine regimens were administered once weekly (days 0, 7, 14, 21, and 28) for five weeks. The mouse dosage schedule was developed to closely resemble one human dosing cycle. In the human dose regimen, docetaxel, with or without addition of estramustine, is often administered weekly for six weeks. There was a resting period of approximately 2 weeks introduced before the cycle was resumed.38,39
Radiochemistry/Targeted Radiotherapy
177Lu was purchased from the Missouri University Research Reactor (MURR). The DOTA-BBN compound, DOTA-8-AOC-BBN(7-14)NH2 (Figure 1A) was synthesized and radiolabeled using methods previously described.12,15 Briefly, the GRP receptor binding peptide, DOTA-8-AOC-BBN(7-14)NH2, was synthesized using solid phase peptide synthesis and purified using reversed-phase HPLC. The purified peptide was radiolabeled by the addition of 50 μg of peptide dissolved in 50 μl of 0.2 M ammonium acetate brought to a final volume of 200 μl by the addition of 150 μl of 0.4 M ammonium acetate. To this solution, 50 μL of 177LuCl3 was added. The final solution was incubated at 80°C for 1 hour. 177Lu-DOTA-8-AOC-BBN(7-14)NH2 was subsequently purified and quality control performed using reversed-phase HPLC. The final product was mixed with isotonic saline to obtain the desired MBq/ml administration concentration for therapeutic evaluation and scintigraphic imaging studies.
Figure 1.
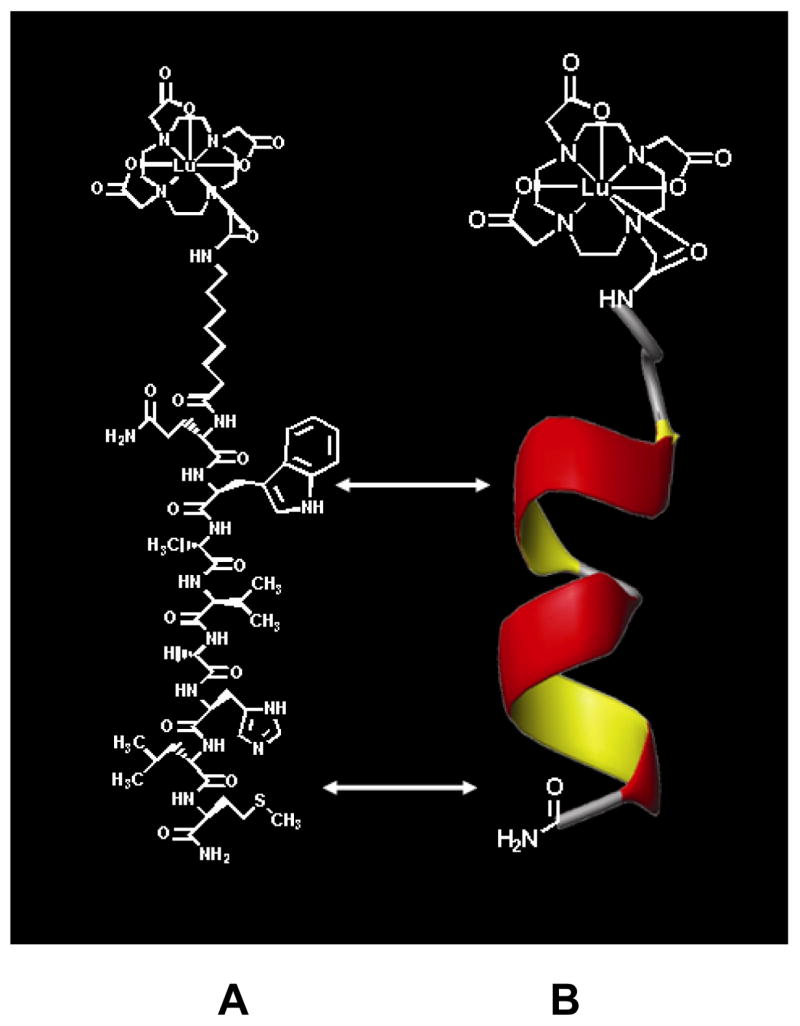
(A) Structure of 177Lu-DOTA-8AOC-BBN(7-14)NH2 shown on left; (B) Two dimensional NMR solution structure (shown at right) of the BBN(7-14)NH2 GRP receptor binding region demonstrating helical structure of the amino acids involved in binding to the GRP receptor.
The three-dimensional structure of DOTA-8-AOC-BBN(7-14)NH2, was determined using two-dimensional nuclear magnetic resonance (NMR) spectroscopy. The sample contained a 5 mM peptide dissolved in 0.6 ml of 2, 2, 2-trifluroethanol (TFE)/H2O (40%:60%, v/v) solution at a pH = 2.6. The 2D 1H NOESY, ROESY, TOCSY and 1H-13C HSQC NMR experiments were recorded at 298° K on a Varian 600 MHz spectrometer (Varian Inc., Palo Alto, CA). After completing the 1H and 13C chemical shift assignments, the 1H-1H distances were obtained from the intensity of the NOESY cross peaks. Structure calculations were carried out using a simulated annealing protocol within X-PLOR (Version 3.1, Axel T. Brunger, Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT).40 The peptide sequence, BBN(7-14)NH2, forms a helical structure in a membrane mimetic environment such as the TFE/H2O solution used in this experiment. Figure 1B was generated using MOLMOL software (ETH, Zurich, Switzerland) and demonstrates the helical structure of the BBN(7-14)NH2 peptide region appended to the 177Lu-DOTA-8-AOC metal complex and linking group.41
For receptor targeted radiotherapy studies, 177Lu-DOTA-8-AOC-BBN(7-14)NH2 was administered at 1850 MBq/kg (iv) in a volume of 0.2 mL or less. The dose chosen was based on previous work in our laboratory which demonstrated an effective therapeutic working range of 177Lu-DOTA-8-AOC-BBN(7-14)NH2 when used as a single agent radiotherapeutic drug in PC-3 xenografts.18 Dose administration of 177Lu -DOTA-8-AOC-BBN(7-14)NH2 occurred on days 0, 14, and 21.
Scintigraphic Imaging
Scintigraphic images demonstrating the selective prostate tumor targeting were obtained 48 hours post intravenous administration of approximately 2 mCi of 177Lu-DOTA-8-AOC-BBN(7-14)NH2. The planar scintigraphic images were obtained using a Siemens LEM+ gamma camera (Siemens Medical Solutions, Malvern, PA) equipped with a medium energy collimator. The images were acquired in a 512 × 512 × 8 bit format using an ADAC Micro 3300 (ADAC Laboratories, Milpitas, CA) image processing workstation. The images are displayed using a color intensity palette which represents accumulated radioactivity scaled from highest to lowest with white = very high, red = high, yellow = medium, green = low, and blue = very low. An example of the scintigraphic images thus obtained is shown in Figure 2.
Figure 2.
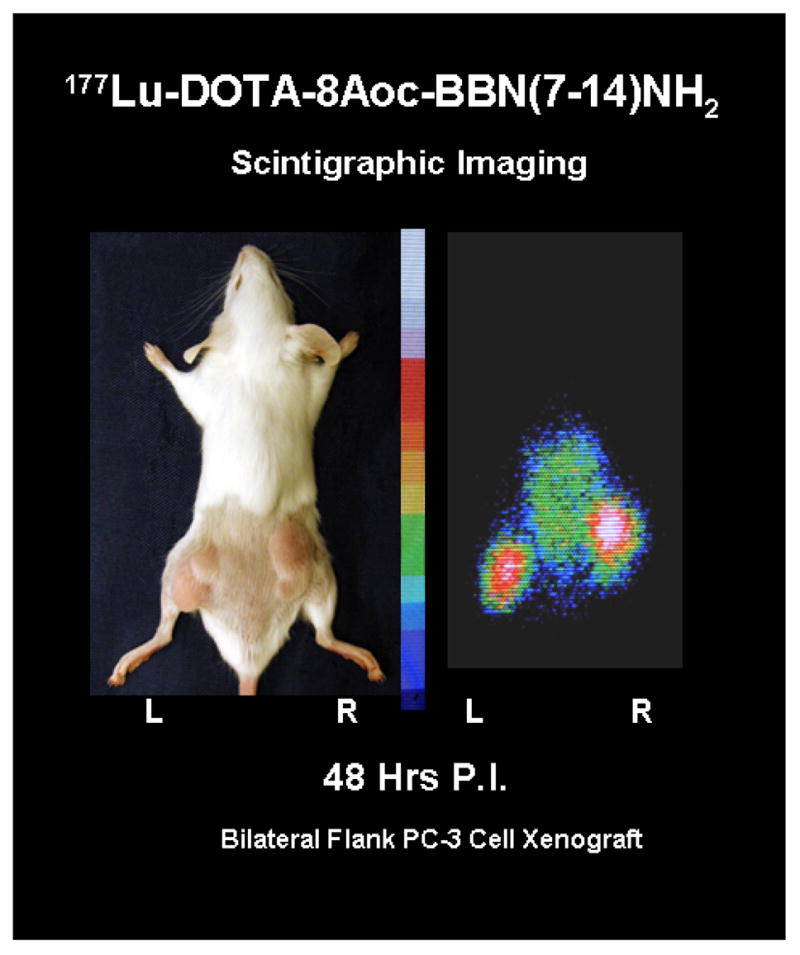
Planar nuclear scintigraph of the distribution of 177Lu-DOTA-8AOC-BBN(7-14)NH2 obtained 48 hours post radiopharmaceutical administration in a PC-3 human prostate tumor xenografted SCID mouse. The areas of highest focal intensity of 177Lu-DOTA-8AOC-BBN(7-14)NH2 correspond to PC-3 xenograft location.
Therapy Protocol
Xenografted SCID mice were randomized by weight and tumor size into 6 groups of 15 mice each. The six study groups were as follows: (1) control, (2) docetaxel, (3) docetaxel + estramustine, (4) TRT + docetaxel, (5) TRT + docetaxel + estramustine, and (6) TRT alone. Data was collected according to the following protocol: tumor volume measurements, body weights, and body condition scores (BCS) were collected one week prior to drug administration and then twice weekly until the completion of the study. A hematology profile analysis was performed one week prior to the initiation of the study and then weekly thereafter for a period of 7 weeks. Tumor measurements were collected using a Fowler-Ultra-Cal IV digital caliper (Fred V. Fowler Co. Inc, Newton, MA). Individual right and left tumor volumes were calculated using a hemiellipsoid formula, (Length × Width × Depth)/2. At three weeks post PC-3 cell inoculation, two days prior to administration of the therapeutic protocol, the flank tumors had an average volume measurement of 5–10 mm3.
Chemotherapy and radiotherapy dosing began on day 0, three weeks post xenograft inoculation. Chemotherapy was administered once weekly (days 0, 7, 14, 21, and 28) and the targeted radiotherapeutic dose was delivered on days 0, 14, and 28. Study animals scheduled to be treated with both chemotherapy and receptor TRT were given chemotherapy approximately 2 hours prior to administration of receptor TRT.
End Point Criteria
Mice were promptly removed from the study upon reaching predetermined endpoint criteria and recorded as a study death. The endpoint criteria were as follows: (1) Single (right or left) flank tumor volume ≥ 1.5cm3; (2) Body condition score (BCS) of -2 or less (Note: The mouse BCS is categorized on a scale of 1–5. A score of one is applied to a mouse that is emaciated with prominent, palpable vertebrae and a five is an obese mouse with lack of palpable vertebrae, due to subcutaneous fat. A +/− attached to the number depicts a high or low variation within the scale),42 (3) Weight loss of ≥ 20 % from high body weight; (4) Active ulceration of tumor; (5) Lastly, illness and/or observed depression.
Hematology
Blood samples were analyzed with the use of a Hemavet 850® (Drew Scientific, Inc., Oxford, CT). All blood samples were collected from the lateral saphenous vein of non-anesthetized mice. The area over the vein was shaved and the vessel was then lightly punctured using a 26 gauge needle. The blood was collected into EDTA coated 20 μL micro-capillary tubes (Drew Scientific, Inc., Oxford, CT.). Individual mouse blood samples were analyzed for platelet count (K/μL), RBC count (M/μL) and WBC count (K/μL). Normal ranges reported by the Hemavet® for laboratory mice were as follows: WBC (1.8–10.7 K/μL), RBC (6.36–9.42 M/μL), and platelet count (592–2972 K/μL). The normal ranges given by the manufacturer were used as guidelines, while the control group of 15 mice was used as the primary reference range.
Statistical Analysis
Statistical analysis of the sample group tumor volumes were calculated by way of a repeated measures analysis of variance (ANOVA). The SAS system (SAS Institute Inc., Cary, NC) was used for tumor volume statistical analysis incorporating the PROC MIXED procedure. Statistical significance was reached with a two-tailed p value of <0.05. Statistics were produced in a fashion to allow for mean tumor volume comparisons between groups at specific time points throughout the study. Statistical interpretation of tumor volumes was evaluated for days 0 to 44 of the study. Survival was evaluated by way of a Kaplan Meier analysis, using the SPSS (SPSS Inc., Chicago, IL) statistical program. Study group mean survival days and 95% confidence intervals were calculated with the Kaplan Meier survival analysis. Survival group means were compared by use of a Student t-test, and statistical significance was reached when p<0.05.
RESULTS
Tumor Suppression
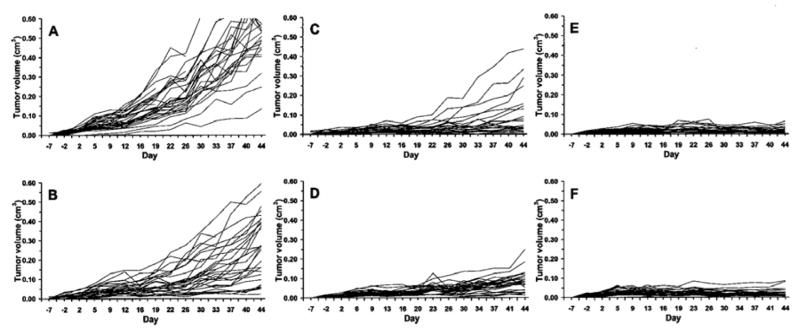
Tumor measurements were recorded twice weekly until the completion of the study. Figure 3 depicts the mean tumor volumes per group of mice, starting one week prior to dose initiation and ending at day 44 of the study. Figure 3 also provides a summary of the therapeutic dosing schedule. Tumor suppression is evident in Figure 3 amongst all treatment groups. In comparison to the control group, the combined GRP receptor TRT/chemotherapy groups showed the most dramatic regression of tumor volumes. Figure 4 demonstrates individual tumor growth trends among the study groups, labeled A–F. Each line on the graph in Figure 4 represents one flank tumor, beginning at one week prior to dose administration and ending at day 44 of the study.
Figure 3.
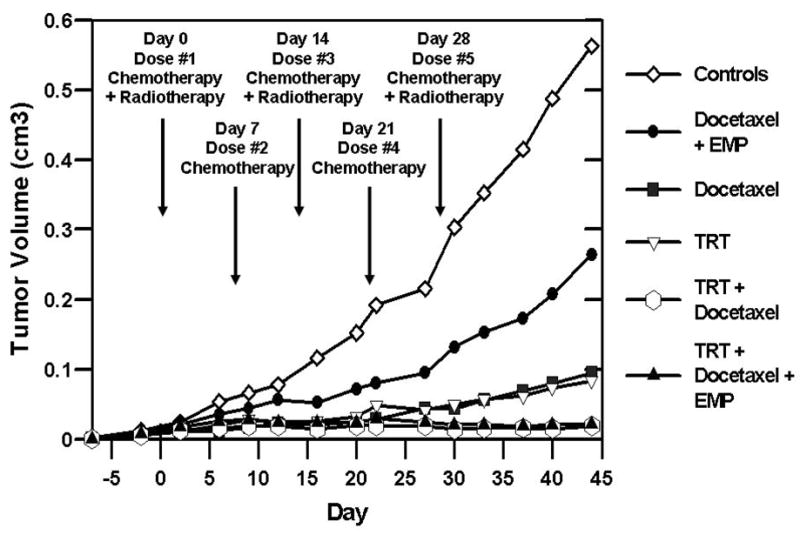
Single flank tumor mean volume (cm3) starting one week before first dose administration through day 44 of study. Dose administration occurred on days 0, 7, 14, 21, and 28. Study group tumor mean (cm3) +/- standard deviation on days 33 and 44 as follows: 0.353 ± 0.14 and 0.563 ± 0.11 for Control; 0.153 ± 0.10 and 0.264 ± 0.17 for docetaxel + estramustine (EMP); 0.056 ± 0.07 and 0.095 ± 0.11 for docetaxel; 0.057 ± 0.03 and 0.083 ± 0.05 for targeted radiotherapy (TRT); 0.015 ± 0.01 and 0.019 ± 0.02 for TRT + docetaxel; and 0.020 ± 0.01 and 0.022 ± 0.02 for TRT + docetaxel + EMP.
Figure 4.
Individual flank tumor volume (cm3) measurements. Each line depicts growth trend of one flank tumor from one week before dose administration through day 44 of study. Graphs are all scaled 0 – 0.6 (cm3). Tumor growth suppression, in comparison to control (A) group is most evident on group C–F graphs, with greatest suppression on graphs E and F. (A = Control; B = docetaxel + estramustine (EMP); C = docetaxel; D = for targeted radiotherapy (TRT); E = TRT + docetaxel; and F = TRT + docetaxel + EMP).
At day 12 post-dose initiation, each group had received one round of the therapeutic regimen. The total tumor volume (cm3) mean estimates ± standard deviation on day 12 were as follows: 0.155 ± 0.06 for the control group, 0.042 ± 0.03 for the docetaxel group, 0.114 ± 0.05 for the docetaxel + estramustine group, 0.035 ± 0.02 for the docetaxel + TRT group, 0.048 ± 0.02 for the docetaxel + estramustine + TRT group, and 0.046 ± 0.02 for the TRT group. A statistically significant (two-tailed p value <0.05) tumor growth suppression was evident on day 12, and continued through day 44 in all groups receiving TRT and the docetaxel alone group, when compared to the control group. At day 33, the various treatment groups were compared to the TRT group to evaluate for significant differences in total tumor volume (cm3) mean estimates. The mean estimates (cm3) were as follows: 0.114 ± 0.05 for TRT, 0.100 ± 0.12 for docetaxel, 0.029 ± 0.02 for docetaxel + TRT, and 0.040 ± 0.02 for docetaxel + estramustine + TRT. Total tumor volume (cm3) mean estimates at day 33 reached statistical significance (two-tailed p value <0.05) in tumor growth suppression, for the combination chemotherapy/TRT groups in comparison to the TRT and docetaxel groups. No statistical difference in the total tumor volume mean estimate was noted between the TRT group and docetaxel group on day 33. That data previously described (days 12 and 33) is demonstrated in Table 1. The information shown in Table 1 was calculated and evaluated using the SAS system, PROC MIXED procedures.
Table 1. Statistical analysis of total tumor volume means using the SAS system, PROC MIXED procedures.
| Column I
|
Column II
|
|||||
|---|---|---|---|---|---|---|
| Group* | Day** | Mean Tumor Volume (cm3)*** | Group* | Day** | Mean Tumor Volume (cm3)*** | Group Comparisons:Column I to Column II p-value (Pr > [t]) |
| A | 12 | 0.155 ± 0.06 | B | 12 | 0.114 ± 0.05 | 0.1899 |
| A | 12 | 0.155 ± 0.06 | C | 12 | 0.042 ± 0.03 | <0.0001 |
| A | 12 | 0.155 ± 0.06 | D | 12 | 0.046 ± 0.02 | <0.0001 |
| A | 12 | 0.155 ± 0.06 | E | 12 | 0.035 ± 0.02 | <0.0001 |
| A | 12 | 0.155 ± 0.06 | F | 12 | 0.048 ± 0.02 | 0.0001 |
|
| ||||||
| C | 33 | 0.100 ± 0.12 | D | 33 | 0.114 ± 0.05 | 0.6316 |
| C | 33 | 0.100 ± 0.12 | E | 33 | 0.029 ± 0.02 | 0.0116 |
| C | 33 | 0.100 ± 0.12 | F | 33 | 0.040 ± 0.02 | 0.0345 |
| D | 33 | 0.114 ± 0.05 | E | 33 | 0.029 ± 0.02 | 0.0023 |
| D | 33 | 0.114 ± 0.05 | F | 33 | 0.040 ± 0.02 | 0.0084 |
Comparison (Differences of Least Square Means) of study group from Column I to study group in Column II on a select day of the study. Comparison of study groups evaluates for statistically significant difference in total tumor volumes (p-value < 0.05, two tailed).
Groups as follows: A = Control; B = Docetaxel + Estramustine; C = Docetaxel; D = Targeted-Radiotherapy (TRT); E = TRT+ Docetaxel; F = TRT + Docetaxel + Estramustine.
Day refers to number of days post dose initiation (day 0).
Mean estimate ± SD (total tumor volume in cm3 per mouse). Standard error of statistical comparison of Column I and II is 0.020.
Percent tumor growth suppression, defined as the total tumor volume mean of treated groups/control group, was calculated at day 44. The results were as follows: In comparing treatment groups to control groups, docetaxel + TRT had 97 ± 2.4 percent tumor suppression, docetaxel + estramustine + TRT had 96 ± 2.6 percent tumor suppression, TRT had 85 ± 8.2 percent tumor suppression, docetaxel alone had 83 ± 14.4 percent tumor suppression, and docetaxel + estramustine had 53 ± 26.7 percent tumor suppression.
Kaplan Meier (Survival Analysis)
Mice were removed from the therapy study upon reaching predetermined endpoints. The mean survival day and survival 95% confidence intervals (CI) were calculated from the Kaplan Meier for each therapy study group. Figure 5A demonstrates the Kaplan Meier survival analysis for this study. Figure 5B summarizes the survival data and shows the mean survival days and 95% confidence interval for the means in each group. The mean survival day and confidence intervals for the study groups were as follows: Control mean of 50 days with CI of 40–59 days; Docetaxel + estramustine mean of 62 days with CI of 54–71 days; Docetaxel alone mean of 74 days with a CI of 65–84 days. TRT alone mean of 83 days with CI of 75–92 days; Combined TRT + docetaxel mean of 107 with CI of 98–115 days; Combined TRT + docetaxel + estramustine mean of 109 with CI of 96–123 days. A Student t-test (p<0.05) was used to compare survival means among the study groups. The TRT/chemotherapy groups and TRT group demonstrated a statistically significant survival advantage when compared to the control animals. Furthermore, the combination of TRT and chemotherapy demonstrated a statistically significant survival advantage compared to the TRT administered alone. No statistically significant survival difference was observed between the two combination TRT/chemotherapy groups.
Figure 5.
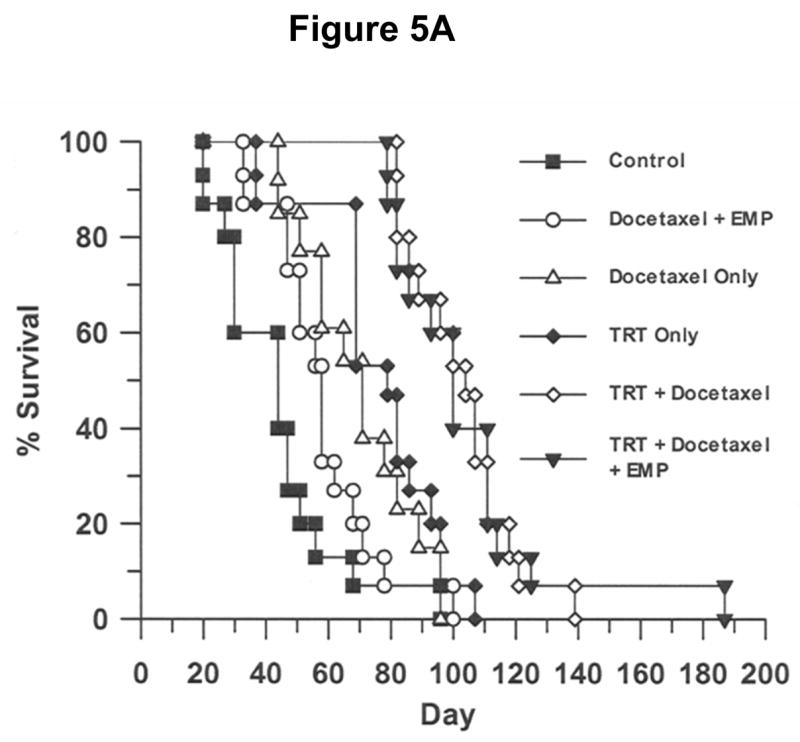
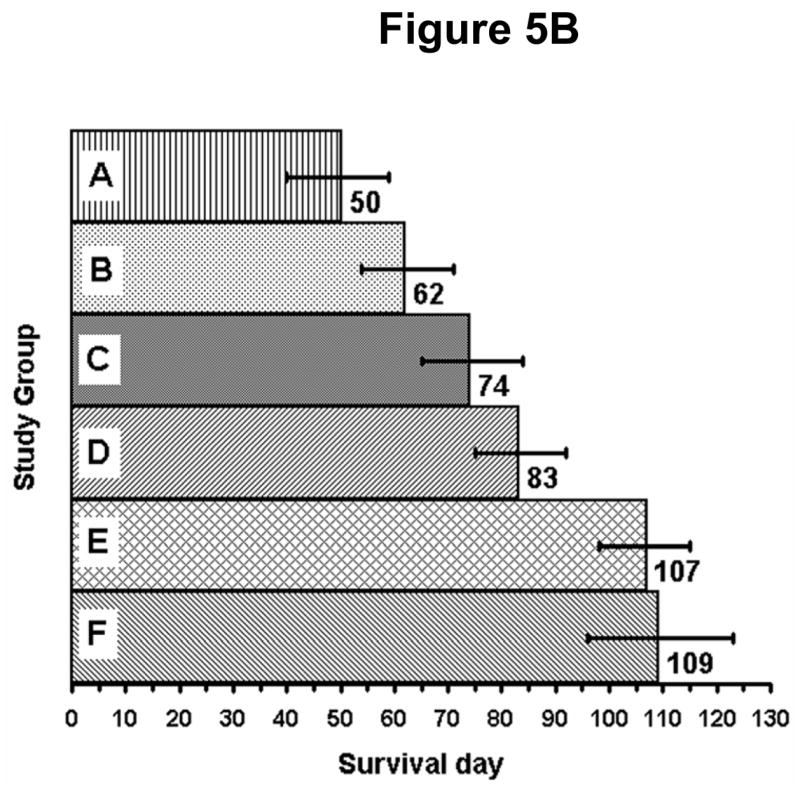
(A) Kaplan Meier survival analysis demonstrating the percent survival versus time (day). The TRT + docetaxel, TRT + docetaxel and estramustine, and TRT groups demonstrated a statistically significant survival advantage, when compared to the control animals. Statistical comparison of group survival evaluated with a Student t-Test (p<0.05). (B) Mean survival day and 95% confidence intervals for each study group. The combination of TRT/chemotherapy demonstrated a statistically significant survival advantage when compared to all other study groups. No statistically significant survival difference was observed between the two combination TRT/chemotherapy groups. Statistical comparison of group mean survival evaluated with a Student t-Test (p<0.05). (A = Control; B = docetaxel + estramustine (EMP); C = docetaxel; D = for targeted radiotherapy (TRT); E = TRT + docetaxel; and F = TRT + docetaxel + EMP).
Blood Analysis
Blood was collected on a weekly basis, beginning one week prior to dose initiation and ending two weeks after final dose administration. This analysis was performed in order to evaluate for marked myelosuppression in response to combined chemotherapy and TRT.29,43 A prior therapy study conducted in our laboratory, utilizing PC-3 implanted SCID mice demonstrated no significant myelosuppression with 177Lu-DOTA-8-AOC-BBN(7-14)NH2 when used as a single agent when administered at a dose of up to 2960 MBq/kg.18 Red blood cell (RBC) counts, white blood cell (WBC) counts, and platelet counts were evaluated in a majority of mice from each group. Average blood values for each group were calculated and comparisons made between the controls and treatment groups. No significant trends were noted with WBC counts, RBC counts, and platelet counts from the period prior to drug administration to day 34 of the study, which was 6 days after all dosing had ceased.
DISCUSSION
This study investigated the therapeutic potential of employing a GRP receptor TRT regimen using 177Lu-DOTA-8-AOC-BBN(7-14)NH2 either alone, or in combination with conventional chemotherapeutic agents in an AIPC in vivo model. Our laboratory has previously demonstrated that 177Lu-DOTA-8-AOC-BBN(7-14)NH2 can successfully target the BB2 bombesin receptor subtype, commonly referred to as the gastrin releasing peptide (GRP) receptor expressed on human prostate tumors including xenografts of PC-3 cell line origin.15,18 The scintigraphic imaging data presented in Figure 2 visually confirms the selective GRP receptor tumor targeting properties of 177Lu-DOTA-8-AOC-BBN(7-14)NH2. The scintigraphic images in Figure 2 complement the previously published pharmacokinetic data using this compound which demonstrated selective in vivo GRP receptor targeting with rapid clearance of the non-receptor targeted radiolabeled material from non-target tissues and prolonged retention of the radiotherapeutic agent in receptor expressing tumor tissue.15 The receptor specific tumor targeting and subsequent prolonged retention at the PC-3 xenograft tumor site were criteria utilized during the initial selection of 177Lu-DOTA-8-AOC-BBN(7-14)NH2 for preclinical evaluation.
The particulate emission characteristics of 177Lu make it an ideal radioisotope for use in a receptor targeted radiation therapy agent, such as described in this report. 177Lu has a medium energy β− (0.497 MeV) emission with a maximum range in soft tissue of approximately 2 mm.15 The 2 mm range in soft tissue makes 177Lu an ideal radioisotope for use in small tumors, where the damaging ionizing radiation effects stay fairly localized. Finally, the short half-life of 6.71 days makes 177Lu a manageable radioisotope with respect to dose preparation and waste disposal.
The PC-3 xenograft model was employed in these studies since this cell line is classified as an androgen independent cell line, expresses the GRP receptor, and agonists of the GRP receptor undergo rapid receptor mediated internalization following GRP receptor binding.12–15,17,34,35 The receptor specific targeting and subsequent retention of the internalized radiopharmaceutical within the target cell are critical features in the delivery of a site specific targeted radiotherapeutic agent to the tumor cell. The retention of the radiotherapeutic agent at the tumor site is essential for delivery of a cytotoxic dose of radiation to the targeted tumor cell and immediately adjacent tumor cells. Additional characteristics of the PC-3 cell line which relate to AIPC progression in man include the lack of the p53 gene, a tumor suppressor gene that is one of the most frequently mutated genes in cancer, and the expression of the Bcl-2 protein, an anti-apoptotic protein.44,45 The Bcl-2 protein is commonly over-expressed in many solid tumors, especially AIPC. Lastly, the Bcl-2 protein is thought to contribute to the persistence of malignant cells and overall tumor cell survival.45
The PC-3 xenograft tumor growth suppression and survival analysis results obtained using 177Lu-DOTA-8-AOC-BBN(7-14)NH2 in combination with chemotherapy, demonstrate a significantly improved response as compared to experimental groups receiving single agent regimens. The greatest tumor growth suppression and increased survival observed for all therapies evaluated was obtained using combined 177Lu-DOTA-8-AOC-BBN(7-14)NH2 GRP receptor TRT + chemotherapy. The combined GRP receptor TRT/chemotherapy treatment regimen demonstrated statistically significant efficacy over the use of GRP receptor TRT or chemotherapy alone, resulting in at least a 30% increase in mean survival as compared to the respective single agent therapies studied. The increased therapeutic efficacy observed in combining GRP receptor targeted radiotherapy with chemotherapy appears to be the result of a direct additive effect of the combined treatment modalities. We found no statistical evidence to suggest that use of TRT with chemotherapy resulted in a synergistic enhancement of tumor control or survival even though the chemotherapeutics employed can function as radiosensitizing agents.46–48
Docetaxel and estramustine function primarily as microtubule inhibitors, and when used together, act synergistically to enhance the cytotoxic effect on cancer cells.23,25–27 Docetaxel’s major mode of action is based on the high affinity of docetaxel for tubulin and subsequent inhibition of microtubule depolymerization. An additional mode of action for docetaxel is through induction of Bcl-2 phosphorylation.47 Estramustine displays cytotoxic activity through a weak inhibition of microtubule polymerization.28,30–33 The resulting microtubule stabilization produced by docetaxel alone, or in combination with estramustine, leads to cellular arrest of the tumor cells. More importantly for the purpose of combined TRT/chemotherapy studies, the cytotoxic properties of ionizing, radiation-damage to targeted cells can be enhanced when selective chemotherapeutic drugs which arrest the cell in the G2/M phase are employed. This is based on the fact that the G2/M phase of the cell cycle is the most radiosensitive portion of the cell cycle.47
The statistical evaluation of tumor volume measurements was carried out to day 44, post- dose initiation. This time period was chosen to best approximate the benefits of a full five week cycle of weekly chemotherapy and bi-monthly targeted radiotherapy, concluding in a two week rest period. In the design of this study we attempted to mimic one round of a typical human patient dosing schedule for weekly administration of docetaxel + estramustine, given for a period of six weeks, providing a resting period of approximately 2 weeks, then repeating this cycle. This regimen is based on docetaxel administration schedules currently under evaluation for the treatment of AIPC.20,24,38,49 Chemotherapy dosage levels were extrapolated from a conversion formula to convert human dosage (mg/m2) to an approximate mouse dosage (mg/kg).36,37 The human dose of 36 mg/m2 for docetaxel and 14 mg/kg for estramustine, in a weekly administration regimen, were the starting points for determination of mouse dosages.38,50 Prior to initiating the combined TRT/chemotherapy study, we conducted a pilot chemotherapy study to assess the safety and efficacy of the selected chemotherapeutics and establish working dose ranges in PC-3 tumor-bearing SCID mice. The results of this pilot study were consistent with dosing schemes previously described using the PC-3 model by Pienta and coworkers.27,37 Upon completion of the entire treatment regimen, we observed tumor growth in all experimental groups that had initially demonstrated tumor suppression. Following cessation of therapy, the growth of the treatment group’s tumors eventually reached the size and character of that demonstrated by tumors expressed in the more rapid, early growth non-treated control group. These results clearly warrant further laboratory evaluation employing multiple cycles of combination TRT/chemotherapy to maintain the significant tumor suppression noted following the single round of combination therapy shown in this study.
A comparison of experimental groups receiving single agent therapies of either TRT alone, or docetaxel alone demonstrated no significant difference in control of tumor growth or increased survival between these two single agent treatment groups. A comparison of the experimental groups receiving only chemotherapy demonstrated that the combination of docetaxel and estramustine was much less effective in tumor growth suppression and survival advantage than that of docetaxel alone. Possible explanations for this observation could be a result of dosing frequency, drug formulation, and drug efficacy in the PC-3 xenografted tumor models. The efficacy of estramustine may have been diminished due to administration on a weekly basis rather than three consecutive days or more each week, as is common in human therapy administration.21,49
In summary, the data from this study demonstrates that GRP receptor TRT using 177Lu-DOTA-8-AOC-BBN(7-14)NH2, employed either alone, or in combination with conventional chemotherapy, can significantly suppress the growth of androgen independent prostate cancer, as well as impart a significant survival advantage in the human prostate PC-3 xenografted SCID mouse model. These data further suggest that optimal tumor growth suppression using GRP receptor TRT may require that 177Lu-DOTA-8-AOC-BBN(7-14)NH2, or similar agents, be used in conjunction with radiosensitizing chemotherapeutic agents. Evaluation of multiple treatment regimens varying both the frequency and order of agent administration may be required to optimize the benefits of combined modality therapies of this type.
Acknowledgments
We would like to thank the U.S. Department of Veterans Affairs for support of this project, the University of Missouri Research Reactor for supplying radioisotopes, and finally, the University of Missouri Social Sciences Statistics Center for assistance and guidance in statistical evaluation of this data. The NMR spectra were acquired at the University of Missouri-Columbia NMR facility supported by NSF Grant #DBI-0070359 and NIH Grant #RO1-GM57289. The University of Missouri owns U.S. Patent No. 6,200,546 describing the GRP receptor targeting technology described within this article.
GRANT SUPPORT
This work was supported in part by funding from the American Cancer Society RSG-99-331-04-CDD, National Institute of Health grants RO1-CA90246 and an NIH Training Grant T32 RR07004.
References
- 1.American Cancer Society. Cancer Facts and Figures 2006. Atlanta: American Cancer Society; 2006. [Google Scholar]
- 2.Small EJ, Reese DM, Vogelzang NJ. Hormone-refractory prostate cancer: an evolving standard of care. Semin Oncol. 1999;26:61. [PubMed] [Google Scholar]
- 3.Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP. Chemotherapy for androgen-independent prostate cancer. Semin Urol Oncol. 2002;20(Suppl 1):31. doi: 10.1053/suro.2002.35052. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberger M, DeWit R, Berry W, et al. A multicenter phase III comparison of docetaxel (D) + prednisone (P) and mitoxantrone (MTZ) + P in patients with hormone-refractory prostate cancer. [abstr. #4] J Clin Oncol. 2004;22(Suppl 14) [Google Scholar]
- 6.Petrylak D, Tangen C, Hussain M, et al. SWOG-99-16: Randomized phase III trial of docetaxel (D)/estramustine (E) versus mitoxantrone(M)/prednisone (p) in men with androgen-independent prostate cancer (AIPC). [abstr. #3] J Clin Oncol. 2004;22(Suppl 14) [Google Scholar]
- 7.Krenning EP, Kwekkeboom DJ, Valkema R, et al. Peptide receptor radionuclide therapy. Ann New York Acad Sci. 2004;1014:234. doi: 10.1196/annals.1294.026. [DOI] [PubMed] [Google Scholar]
- 8.De Jong M, Valkema R, Jamar F, et al. Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings. Semin Nucl Med. 2002;32:133. doi: 10.1053/snuc.2002.31027. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman TJ, Quinn TP, Volkert WA. Radiometallated receptor-avid peptide conjugates for specific in vivo targeting of cancer cells. Nucl Med Biol. 2001;28:527. doi: 10.1016/s0969-8051(01)00209-8. [DOI] [PubMed] [Google Scholar]
- 10.Smith CJ, Volkert WA, Hoffman TJ. Gastrin releasing peptide (GRP) receptor targeted radiopharmaceuticals: a concise update. Nucl Med Biol. 2003;30:861. doi: 10.1016/s0969-8051(03)00116-1. [DOI] [PubMed] [Google Scholar]
- 11.Heppeler A, Froidevaux S, Eberle AN, Maecke HR. Receptor targeting for tumor localisation and therapy with radiopeptides. Curr Med Chem. 2000;7:971. doi: 10.2174/0929867003374516. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman TJ, Gali H, Smith CJ, et al. Novel series of 111In-labeled bombesin analogs as potential radiopharmaceuticals for specific targeting of gastrin-releasing peptide receptors expressed on human prostate cancer cells. J Nucl Med. 2003;44:823. [PubMed] [Google Scholar]
- 13.Smith CJ, Sieckman GL, Owen NK, et al. Radiochemical investigations of gastrin-releasing peptide receptor-specific [(99m)Tc(X)(CO)3-Dpr-Ser-Ser-Ser-Gln-Trp-Ala-Val-Gly-His-Leu-Met-(NH2)] in PC-3, tumor-bearing, rodent models: syntheses, radiolabeling, and in vitro/in vivo studies where Dpr = 2,3-diaminopropionic acid and X = H2O or P(CH2OH)3. Cancer Res. 2003;63:4082. [PubMed] [Google Scholar]
- 14.Smith CJ, Sieckman GL, Owen NK, et al. Radiochemical investigations of [188Re(H2O)(CO)3-diaminopropionic acid-SSS-bombesin(7-14)NH2]: syntheses, radiolabeling and in vitro/in vivo GRP receptor targeting studies. Anticancer Res. 2003;23:63. [PubMed] [Google Scholar]
- 15.Smith CJ, Gali H, Sieckman GL, et al. Radiochemical investigations of 177Lu-DOTA-8-Aoc-BBN[7-14]NH2: an in vitro/in vivo assessment of the targeting ability of this new radiopharmaceutical for PC-3 human prostate cancer cells. Nucl Med Biol. 2003;30:101. doi: 10.1016/s0969-8051(02)00391-8. [DOI] [PubMed] [Google Scholar]
- 16.Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999;59:1152. [PubMed] [Google Scholar]
- 17.Reile H, Armatis PE, Schally AV. Characterization of high-affinity receptors for bombesin/gastrin releasing peptide on the human prostate cancer cell lines PC-3 and DU-145: internalization of receptor bound 125I-(Tyr4) bombesin by tumor cells. Prostate. 1994;25:29. doi: 10.1002/pros.2990250105. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman TJ, Smith CJ, Owen NK, et al. Lu-177 radiolabeled peptides for targeted radiotherapy of androgen independent prostate cancer. AACR Proc. 2003;44:1292. [Google Scholar]
- 19.Hudes G. Estramustine-based chemotherapy. Semin Urol Oncol. 1997;15:13. [PubMed] [Google Scholar]
- 20.Logothetis CJ. Docetaxel in the integrated management of prostate cancer. Current applications and future promise. Oncol (Huntington) 2002;16(6Suppl 6):63. [PubMed] [Google Scholar]
- 21.Petrylak DP, Macarthur R, O’Connor J, et al. Phase I/II studies of docetaxel (Taxotere) combined with estramustine in men with hormone-refractory prostate cancer. Semin Oncol. 1999;26(5 Suppl 17):28. [PubMed] [Google Scholar]
- 22.Petrylak DP. Docetaxel (Taxotere) in hormone-refractory prostate cancer. Semin Oncol. 2000;27(2Suppl 3):24. [PubMed] [Google Scholar]
- 23.Savarese DM, Halabi S, Hars V, et al. Phase II study of docetaxel, estramustine, and low-dose hydrocortisone in men with hormone-refractory prostate cancer: a final report of CALGB 9780. Cancer and Leukemia Group B. J Clin Oncol. 2001;19:2509. doi: 10.1200/JCO.2001.19.9.2509. [DOI] [PubMed] [Google Scholar]
- 24.Small EJ. Docetaxel in prostate cancer. Anti-Cancer Drugs. 2001;12(Suppl 1):S17. [PubMed] [Google Scholar]
- 25.Sinibaldi VJ, Carducci M, Laufer M, Eisenberger M. Preliminary evaluation of a short course of estramustine phosphate and docetaxel (Taxotere) in the treatment of hormone-refractory prostate cancer. Semin Oncol. 1999;26(Suppl 17):45. [PubMed] [Google Scholar]
- 26.Sinibaldi VJ, Carducci MA, Moore-Cooper S, et al. Phase II evaluation of docetaxel plus one-day oral estramustine phosphate in the treatment of patients with androgen independent prostate carcinoma. Cancer. 2002;94:1457. doi: 10.1002/cncr.10350. [DOI] [PubMed] [Google Scholar]
- 27.Williams JF, Muenchen HJ, Kamradt JM, et al. Treatment of androgen-independent prostate cancer using antimicrotubule agents docetaxel and estramustine in combination: an experimental study. Prostate. 2000;44:275. doi: 10.1002/1097-0045(20000901)44:4<275::aid-pros3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Fukuoka K, Saijo N. Antimitotic agents. Jap J Cancer & Chemother. 1997;24:1519. [PubMed] [Google Scholar]
- 29.Vaishampayan U, Parchment RE, Jasti BR, Hussain M. Taxanes: an overview of the pharmacokinetics and pharmacodynamics. Urology. 1999;54(6ASuppl):22. doi: 10.1016/s0090-4295(99)00451-3. [DOI] [PubMed] [Google Scholar]
- 30.Gunnarsson PO, Forshell GP. Clinical pharmacokinetics of estramustine phosphate. Urology. 1984;23(6Suppl):22. doi: 10.1016/s0090-4295(84)80093-x. [DOI] [PubMed] [Google Scholar]
- 31.Hoisaeter PA. Mode of action of Emcyt. Urology. 1984;23(6Suppl):46. doi: 10.1016/s0090-4295(84)80097-7. [DOI] [PubMed] [Google Scholar]
- 32.Panda D, Miller HP, Islam K, Wilson L. Stabilization of microtubule dynamics by estramustine by binding to a novel site in tubulin: a possible mechanistic basis for its antitumor action. Proc Natl Acad Sci USA. 1997;94:10560. doi: 10.1073/pnas.94.20.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tew KD, Hartley-Asp B. Cytotoxic properties of estramustine unrelated to alkylating and steroid constituents. Urology. 1984;23(6Suppl):28. doi: 10.1016/s0090-4295(84)80094-1. [DOI] [PubMed] [Google Scholar]
- 34.Kaighn ME, Narayan KS, Ohnuki Y, et al. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urology. 1979;17:16. [PubMed] [Google Scholar]
- 35.van Bokhoven A, Varella-Garcia M, Korch C, et al. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 36.Freireich EJ, Gehan EA, Rall DP, et al. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Reports - Part 1. 1966;50:219. [PubMed] [Google Scholar]
- 37.O’Donnell RT, DeNardo SJ, Miers LA, et al. Combined modality radioimmunotherapy for human prostate cancer xenografts with taxanes and 90 Yttrium-DOTA-peptide-ChL6. Prostate. 2002;50:27. doi: 10.1002/pros.10029. [DOI] [PubMed] [Google Scholar]
- 38.Berry W, Dakhil S, Gregurich MA, Asmar L. Phase II trial of single-agent weekly docetaxel in hormone-refractory, symptomatic, metastatic carcinoma of the prostate. Semin Oncol. 2001;28(4Suppl 15):8. doi: 10.1016/s0093-7754(01)90149-6. [DOI] [PubMed] [Google Scholar]
- 39.Ferrero JM, Foa C, Thezenas S, et al. A weekly schedule of docetaxel for metastatic hormone-refractory prostate cancer. Oncol. 2004;66:281. doi: 10.1159/000078328. [DOI] [PubMed] [Google Scholar]
- 40.Brunger AT. X-PLOR manual, Version 3.1. Yale University; 1993. [Google Scholar]
- 41.Koradi R, Billeter M, Wüthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graphics. 1996;14:51. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 42.Ullman-Cullere MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci. 1999;49:319. [PubMed] [Google Scholar]
- 43.Muntzing J, Gunnarsson K. Preclinical pharmacology and toxicology of estramustine phosphate. Urology. 1984;23(6Suppl):6. doi: 10.1016/s0090-4295(84)80091-6. [DOI] [PubMed] [Google Scholar]
- 44.Arah IN, Song K, Seth P, et al. Role of wild-type p53 in the enhancement of camptothecin cytotoxicity against human prostate tumor cells. Anticancer Res. 1998;18:1845. [PubMed] [Google Scholar]
- 45.Rokhlin OW, Bishop GA, Hostager BS, et al. Fas-mediated apoptosis in human prostatic carcinoma cell lines. Cancer Res. 1997;57:1758. [PubMed] [Google Scholar]
- 46.Eklov S, Westlin JE, Rikner G, Nilsson S. Estramustine potentiates the radiation effect in human prostate tumor transplant in nude mice. Prostate. 1994;24:39. doi: 10.1002/pros.2990240109. [DOI] [PubMed] [Google Scholar]
- 47.Pienta KJ. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. Semin Oncol. 2001;28(4Suppl 15):3. doi: 10.1016/s0093-7754(01)90148-4. [DOI] [PubMed] [Google Scholar]
- 48.Stahlberg K, Kairemo K, Karonen SL, Jekunen A, Taari K, Rannikko S. Radioiodinated estramustine phosphate and estramustine binding protein antibody accumulate in the prostate of a mouse. Prostate. 1997;32:1. doi: 10.1002/(sici)1097-0045(19970615)32:1<1::aid-pros1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 49.Sitka Copur M, Ledakis P, Lynch J, et al. Weekly docetaxel and estramustine in patients with hormone-refractory prostate cancer. Semin Oncol. 2001;28(4Suppl 15):16. doi: 10.1016/s0093-7754(01)90150-2. [DOI] [PubMed] [Google Scholar]
- 50.Kreis W, Budman D. Daily oral estramustine and intermittent intravenous docetaxel (Taxotere) as chemotherapeutic treatment for metastatic, hormone-refractory prostate cancer. Semin Oncol. 1999;26(5Suppl 17):34. [PubMed] [Google Scholar]