Abstract
Cytotoxic T cells (CTLs) and natural killer cells (NKs) both kill virus-infected cells and tumor cells by releasing the cytoxic contents of their lytic granules. We recently demonstrated a role for calcineurin in lytic granule exocytosis in TALL-104 human leukemic CTLs[1]. However, whether calcineurin plays a similar role in NK lytic granule release is not known. We tested whether calcineurin is involved in lytic granule exocytosis in human leukemic NK-92 cells using immunosuppressive drugs that block calcineurin function and by overexpressing a constitutively active calcineurin fusion protein. Our results indicate that calcineurin does play a role in lytic granule exocytosis in NK-92 cells, and suggest that, as was the case in TALL-104 cells, there are likely to be multiple calcium-dependent steps.
Keywords: Signal transduction, Calcineurin, flow cytometry, cytotoxicity, lytic granules, granule exocytosis
Introduction
Cytotoxic T cells and natural killer cells play important roles in killing virus-infected and tumor cells. NKs are members of the innate immune system that kill target cells without prior antigen sensitization, while CTLs are members of the adaptive immune system that require prior antigen sensitization for their cytolytic activity (reviewed in[2] [3]). One of the key mechanisms these cells use to kill target cells is the release of lytic granules, secretory lysosomes that harbour cytolytic agents such as perforin (a pore forming peptide) and granzymes (serine proteases that trigger target cell DNA degradation) in their central core (reviewed in [4; 5]). Despite its immunological significance, knowledge of the signalling events that control lytic granule exocytosis remains fairly rudimentary.
CTLs are activated by T cell receptor recognition of antigen bound to MHC class I, while NK cells are activated by integrating a set of activating and inhibiting signals [6]. However, phospholipase C (PLCγ) activation is a common downstream event in the signal transduction cascades in both cell types [6; 7]. PLCγ cleaves phosphotidyl inositiol bisphosphate (PIP2) to inositol triphosphate (IP3) and diacylglycerol (DAG). Drugs that trigger calcium influx (such as ionomycin or thapsigargin) in conjunction with a DAG analog, phorbol myristate acetate (PMA), have been shown to trigger lytic granule exocytosis independent of receptor activation in both CTLs and NKs [8; 9; 10; 11; 12].
We recently confirmed the idea that calcineurin plays a role in CTL lytic granule exocytosis using TALL-104 human leukemic CTLs [1]. Based on the similarities in downstream signalling, we hypothesized that granule exocytosis in NK cells is also likely to be calcineurin-dependent. We tested this using a human leukemic natural killer cell line, NK-92. We measured exocytosis in response to thapsigargin (TG) and PMA stimulation using an assay that measures via antibody binding the externalization of lysosome-associated membrane protein 1 (LAMP-1) that occurs as a consequence of granule exocytosis. Our strategy was similar to the one we used previously to assess the calcineurin-dependence of granule exocytosis in CTLs [1]. Our results suggest that calcineurin activity is required for NK-92 lytic granule exocytosis, but is only one calcium-dependent step.
Experimental Procedures
Chemicals and reagents
Fetal calf serum was from Atlas Biologicals (Ft. Collins, CO), Horse serum was from Invitrogen. Thapsigargin and PMA were from Alexis Biochemicals (San Diego, CA). PE conjugated Mouse IgG anti-CD107a (clone H4A3) was purchased from BD Biosciences (San Diego, CA).
Cells and solutions
NK-92 cells were obtained from American Type Culture Collection (Rockville, MD) and grown in Alpha minimum essential medium supplemented with 12.5% FCS 12.5% Horse serum, 100 IU IL-2 and 50 μ beta mercaptoethanol, in a humidified incubator at 37 C in 5% CO2. Experiments were conducted in complete cell culture medium. Serum contains variable amounts of calcium, making the precise determination of calcium concentration difficult. However, if we assume that the calcium level in complete medium was ~ 2mM, we expect the free extracellular calcium concentration to be ~ 500mM after the addition of 1.5mM EGTA, 250mM after the addition of 1.75mM EGTA, less than 1 μM after the addition of 2mM EGTA and less than 10 nM after the addition of 10mM EGTA (calculated using the program “EGTA calculator”; see http://brneurosci.org/egta.html).
Immunocytochemistry and flow cytometry
For immunolocalization experiments, cells were prepared following a published protocol using methanol/acetone fixation and permeabilization [13]. A Leica SP-2 confocal system was used to obtain images. For flow cytometry, cells were first washed with medium and then stimulated with 50 nM PMA and 1 μM TG either in medium or in Normal Ringers for one hour at 37 C in the presence of anti-LAMP-1 antibody. Anti-LAMP was used at a final concentration of 0.09 micrograms per test. Flow cytometry was performed on a FACSCalibur at the University of Connecticut at Storrs Flow Cytometry and Confocal Microscopy Facility. Data were analyzed using FlowJo (TreeStar, Ashland, OR). For multi-colour experiments data were procured without hardware compensation and later compensated off-line using FlowJo using data from single colour samples.
cDNA constructs and transfections
5 × 10^6 NK-92 cells were transfected with an Amaxa Nucleofector (Amaxa Biosystems, Gaithersburg, MD) using program A-24 and solution V. Experiments were performed 14–16 h post-transfection. The mutant constitutively active calcium-independent calcineurin construct has been described previously [1].
Statistics
Statistical significance was assessed using repeated measures ANOVA (Instat, Graphpad Software, San Diego, CA).
Results
LAMP-1 externalization as a marker for NK lytic granule exocytosis
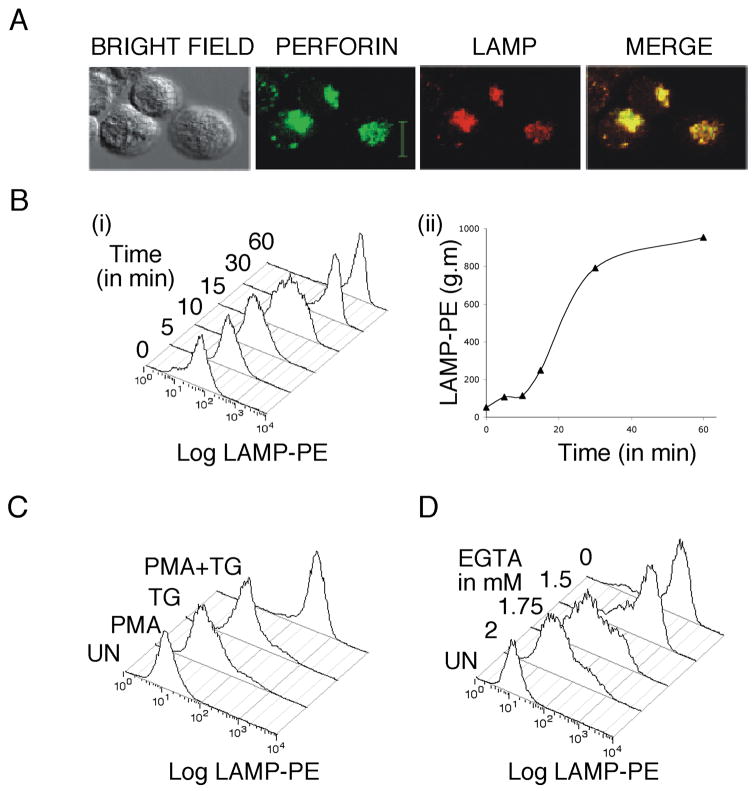
LAMP externalization has been used previously to monitor NKP44-stimulated degranulation in NK-92 cells [14]. We confirmed that LAMP externalization can be used to monitor lytic granule exocytosis in NK-92 cells. We found good co-localization between LAMP and perforin staining in unstimulated NK-92 cells (Figure 1A). When we stimulated NK-92 cells with TG and PMA and measured LAMP staining at various time points (cells were fixed at specific time points and then stained for 45 min with LAMP antibody), we found a time-dependent increase in fluorescence intensity that was maximal at 60 minutes after stimulation (Figure 1B). Treating cells with PMA or TG alone did not trigger lytic granule exocytosis (Figure 1C). When we varied extracellular calcium levels in cell culture medium by adding different amounts of EGTA, a calcium chelator, we found stimulation in reduced calcium concentrations reduced lytic granule exocytosis (Figure 1D). NK-92 cells responded better to stimulation in cell culture medium than in Normal Ringer’s solution (data not shown), but we also observed Ca2+ dependent LAMP staining when we stimulated the cells in Ringer’s solutions containing either 2mM Ca2+ or 0.5mM Ca2+. Taken together, the results of Figure 1 are consistent with the idea that LAMP staining can be used to monitor degranulation in NK-92 cells, and that degranulation can be stimulated by calcium influx and PMA.
Figure 1. LAMP externalization as a marker for NK-92 lytic granule exocytosis.
A) Unstimulated NK-92 cells were fixed and permeabilized and stained for LAMP-1 and perforin. Scale bar denotes 10 μM. B) i) NK-92 cells were stimulated with TG and PMA and LAMP externalization was measured at various time points. Histograms of anti-LAMP fluorescence are shown. ii) Geometric mean (g.m.) from the experiment in (i) plotted vs. time. C) Representative histograms of anti-LAMP staining intensity showing the effects of stimulating cells with TG, PMA or both TG+PMA on granule release. D) Representative histograms of anti-LAMP staining intensity showing the effects of varying calcium levels on lytic granule exocytosis.
Cyclosporin A and FK 506 inhibit NK granule exocytosis
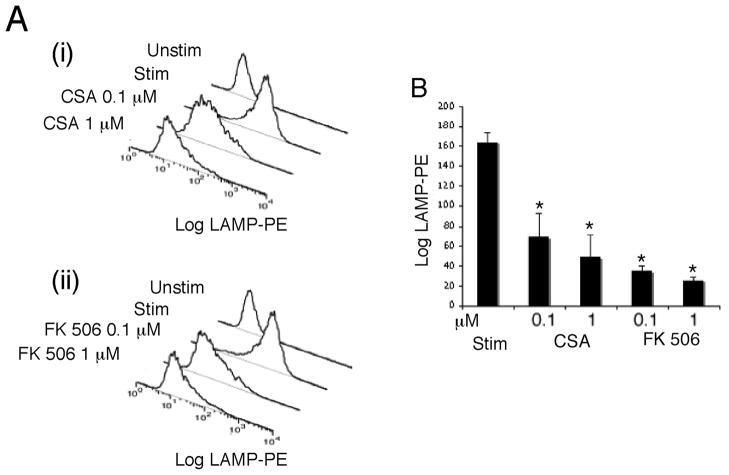
Next, we tested the effects of cyclosporin A (CsA) and FK 506 on NK lytic granule exocytosis (Figure 2). These drugs, when bound to cyclophilins and FK 506 binding proteins, respectively, act as potent inhibitors of calcineurin [15; 16]. NK-92 cells were pre-treated with varying concentrations of drugs for 30 minutes and then stimulated in their presence while monitoring granule release via the LAMP assay. Both drugs reduced NK lytic granule exocytosis, with FK 506 having a more pronounced effect in accordance with the known affinity of drug-immunophilin complexes.
Figure 2. Immunosuppressive drugs inhibit lytic granule exocytosis in NK-92 cells.
Cells were pre-treated with the indicated concentration of CsA or FK 506 for 30 min and then stimulated with TG and PMA in the presence of the drugs. LAMP externalization assays were used to monitor granule exocytosis. A) Representative histograms of LAMP fluorescence for the different conditions. B) Quantifications for three such experiments. Data values that differ from the stimulated condition at p < 0.05 is indicated by *.
Effect of expressing mutant Ca2+ independent constitutively active calcineurin A on NK-92 lytic granule exocytosis
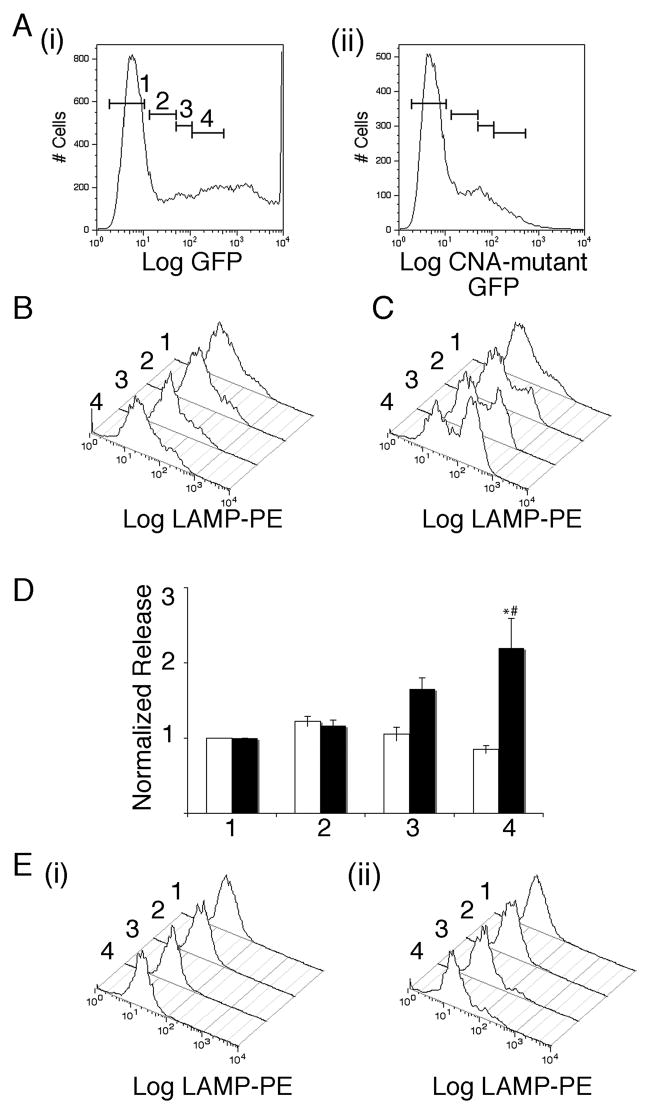
We tested the effect of overexpressing a constitutively active calcium-independent calcineurin A fused to GFP (used in our earlier work in CTLs [1]) on lytic granule exocytosis (Figure 3). We stimulated cells with TG and PMA in cell culture medium to which we added 1.75 mM EGTA to reduce extracellular calcium and aid us in detecting any enhancement of granule exocytosis. There was increased LAMP staining in cells expressing high levels of mutant calcineurin compared to untransfected cells or cells expressing comparable levels of control GFP vector (Figure 3 A–D).
Figure 3. Effects of expressing mutant constitutively active calcium-independent calcineurin A on NK-92 lytic granule exocytosis.
A i) Representative plots of GFP fluorescence for cells transfected with GFP. The bars indicate gating regions used in analyzing LAMP externalization in panels below. ii) Representative plots of mutant calcineurin construct fused to GFP. B) Representative histograms of LAMP fluorescence for cells transfected with GFP and then stimulated with TG and PMA in medium supplemented with 1.75 mM EGTA for the various gating regions shown in A. Histograms are arranged with untransfected cells at the back and moving to front corresponds to increasing levels of expression. C) As in B for cells transfected with mutant calcineurin. D) Normalized release for four such experiments for the cells in the different gating regions. White bars denote GFP transfected cells and black bars denote mutant calcineurin transfected cells. Data were normalized to the level of release observed in stimulated cells from the GFP negative population. Normalized LAMP fluorescence values of mutant-calcineurin transfected cells that differ at p<0.05 level from untransfected cells is indicated by * and those that differ from equally GFP expressing cells is indicated by #. E) (i) Representative histograms of LAMP fluorescence for cells transfected with GFP and then stimulated with TG and PMA in 10mM EGTA containing medium for the various gating regions.(ii) As in (i) but for cells transfected with mutant calcineurin.
As mutant calcineurin is calcium independent we were able to test whether activation of calcineurin is the only calcium dependent step in lytic granule exocytosis in NK-92 cells. If it is, then overexpressing this mutant should trigger exocytosis independent of calcium influx. We stimulated NK-92 cells in an essentially calcium-depleted medium containing 10 mM EGTA, and found that even in cells that expressed high levels of the mutant calcium-independent calcineurin, there was no response (Figure 3E). This indicates that additional proteins are likely involved in conferring calcium dependency to lytic granule exocytosis in NK-92 cells. Future work will be required to identify these proteins.
Discussion
We report three main conclusions in this work. First, immunosuppressant drugs inhibit lytic granule exocytosis in NK-92 cells. Second, calcineurin is involved in NK-92 lytic granule exocytosis. Third, as in TALL-104 cells, NK-92 lytic granule exocytosis likely involves multiple calcium-dependent steps, since expressing mutant calcineurin did not render exocytosis calcium-independent. Our work has only tested calcineurin dependence of exocytosis in a singe leukemic NK cell line. Additional work will be required to confirm these results in primary NK cells.
Several previous studies have examined the effects of immunosuppressants on target cell killing by NKs, with conflicting results. Two studies reported inhibition of target cell killing by CsA, but only after treating cells with drugs for 8 or more hours [17; 18], while a third study found no effects of CsA [19], and a fourth more recent one found that CsA actually increased cytotoxicity, an effect attributed to alteration in levels of expression of plasma membrane receptors [20]. One of the studies mentioned above reported that FK506 was without effect [17]. We found that both cyclosporin A and FK506 blocked NK-92 lytic granule exocytosis, and that only brief incubations were required. It may be that calcineurin-dependent granule exocytosis is a property limited to IL-2 dependent killer cells, as it is manifested in CTLs [1; 21] and NK-92 cells, which require IL-2 for growth and cytotoxicity. If this is the case, then we note that effects on lytic granule exocytosis are unlikely to contribute to the clinical efficacy of these drugs, as upstream effects on helper T cell IL-2 production will supersede them. If not the effects on NK lytic granule exocytosis may contribute to the clinical efficacy of the immunosuppressive drugs. Alternatively, it may be that receptor-stimulated responses are not calcineurin-dependent in NK cells. However, this possibility seems unlikely to us, based on the generally conserved signal transduction cascades in CTLs and NK cells. Finally, it may be that confounding effects (such as the aforementioned alteration in receptor expression) obscure effects of calcineurin inhibitors when target cell killing is used as the assay endpoint.
Their high lytic activity and the ease with which they can be grown have lead to the idea that NK-92 cells might be useful in cell-based immunotherapies (they have been considered for clinical trials [22]). These same properties have contributed to the use of NK-92 cells as a model for NK cell signal transduction in a number of studies e.g. [13; 23]. The present work suggests that NK-92 cells will likely be useful in experiments designed to investigate the molecular basis of the calcineurin-dependence of lytic granule exocytosis. NFAT is a well-characterized substrate of calcineurin that regulates gene transcription and cytokine production in helper T cells [24]. However, lytic granule exocytosis involves release of preformed granules without the need for additional protein synthesis. NFAT is therefore unlikely to be involved in the granule release step [1]. NK-92 cells might provide an advantageous system for applying biochemical approaches to identifying the currently-unknown calcineurin substrate(s) involved in lytic granule exocytosis. They also might be useful for identifying the additional, non-calcineurin Ca2+-sensitive proteins that are likely involved.
Acknowledgments
We would like to thank Dr. Carol Norris and the University of Connecticut Flow Cytometry and Confocal Microscopy Facility. We also thank Dr. Hans Klingemann (Tufts- New England Medical Center, Boston, MA) for advice on culturing NK-92 cells. This work was supported by NIH Grant R01 AI054839 to A.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grybko MJ, Bartnik JP, Wurth GA, Pores-Fernando AT, Zweifach A. Calcineurin activation is only one calcium-dependent step in cytotoxic T lymphocyte granule exocytosis. J Biol Chem. 2007;282:18009–18017. doi: 10.1074/jbc.M702222200. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 3.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 4.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 5.Peters PJ, Geuze HJ, Van der Donk HA, Slot JW, Griffith JM, Stam NJ, Clevers HC, Borst J. Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. Eur J Immunol. 1989;19:1469–1475. doi: 10.1002/eji.1830190819. [DOI] [PubMed] [Google Scholar]
- 6.Bryceson YT, March ME, Ljunggren H-G, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radoja S, Frey AB, Vukmanovic S. T-cell receptor signaling events triggering granule exocytosis. Crit Rev Immunol. 2006;26:265–290. doi: 10.1615/critrevimmunol.v26.i3.40. [DOI] [PubMed] [Google Scholar]
- 8.Alter G, Malenfant J, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Lancki DW, Weiss A, Fitch FW. Requirements for triggering of lysis by cytolytic T lymphocyte clones. J Immunol. 1987;138:3646–3653. [PubMed] [Google Scholar]
- 10.Esser MT, Haverstick DM, Fuller CL, Gullo CA, Braciale VL. Calcium signaling modulates cytolytic T lymphocyte effector functions. J Exp Med. 1998;187:1057–1067. doi: 10.1084/jem.187.7.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pores-Fernando AT, Bauer RA, Wurth GA, Zweifach A. Exocytic responses of single leukaemic human cytotoxic T lymphocytes stimulated by agents that bypass the T cell receptor. J Physiol (Lond) 2005;567:891–903. doi: 10.1113/jphysiol.2005.089565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyubchenko TA, Wurth GA, Zweifach A. The actin cytoskeleton and cytotoxic T lymphocytes: evidence for multiple roles that could affect granule exocytosis-dependent target cell killing. J Physiol (Lond) 2003;547:835–847. doi: 10.1113/jphysiol.2002.033522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang K, Zhong B, Gilvary DL, Corliss BC, Hong-Geller E, Wei S, Djeu JY. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- 14.Ho JW, Hershkovitz O, Peiris M, Zilka A, Bar-Ilan A, Nal B, Chu K, Kudelko M, Kam YW, Achdout H, Mandelboim M, Altmeyer R, Mandelboim O, Bruzzone R, Porgador A. H5-Type influenza virus hemagglutinin is functionally recognized by the natural killer-activating receptor NKp44. J Virol. 2008;82:2028–2032. doi: 10.1128/JVI.02065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao A, Luo C, Hogan P. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 16.Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cells. 2004;18:1–9. [PubMed] [Google Scholar]
- 17.Wasik M, Gorski A, Stepien-Sopniewska B, Lagodzinski Z. Effect of FK506 versus cyclosporine on human natural and antibody-dependent cytotoxicity reactions in vitro. Transplantation. 1991;51:268–270. doi: 10.1097/00007890-199101000-00045. [DOI] [PubMed] [Google Scholar]
- 18.Introna M, Allavena P, Spreafico F, Mantovani A. Inhibition of human natural killer activity by cyclosporin A. Transplantation. 1981;31:113–116. doi: 10.1097/00007890-198102000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Shao-Hsien C, Lang I, Gunn H, Lydyard P. Effect of in vitro cyclosporin. A treatment on human natural and antibody-dependent cell-mediated cytotoxicity. Transplantation. 1983;35:127–129. doi: 10.1097/00007890-198302000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Grzywacz B, Sukovich D, McCullar V, Cao Q, Lee AB, Blazar BR, Cornfield DN, Miller JS, Verneris MR. The unexpected effect of cyclosporin A on CD56+CD16− and CD56+CD16+ natural killer cell subpopulations. Blood. 2007;110:1530–1539. doi: 10.1182/blood-2006-10-048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutz JP, Fruman DA, Burakoff SJ, Bierer BE. A role for calcineurin in degranulation of murine cytotoxic T lymphocytes. J Immunol. 1993;150:2591–2598. [PubMed] [Google Scholar]
- 22.Tonn T, Becker S, Esser R, Schwabe D, Seifried E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res. 2001;10:535–544. doi: 10.1089/15258160152509145. [DOI] [PubMed] [Google Scholar]
- 23.Jiang K, Zhong B, Gilvary DL, Corliss BC, Vivier E, Hong-Geller E, Wei S, Djeu JY. Syk regulation of phosphoinositide 3-kinase-dependent NK cell function. J Immunol. 2002;168:3155–3164. doi: 10.4049/jimmunol.168.7.3155. [DOI] [PubMed] [Google Scholar]
- 24.Masuda ES, Imamura R, Amasaki Y, Arai K, Arai N. Signalling into the T-cell nucleus: NFAT regulation. Cell Signal. 1998;10:599–611. doi: 10.1016/s0898-6568(98)00019-9. [DOI] [PubMed] [Google Scholar]