Abstract
Host defenses, while effecting viral clearance, contribute substantially to inflammation and disease. This double action is a substantial obstacle to the development of safe and effective vaccines against many agents, particularly respiratory syncytial virus (RSV). RSV is a common cold virus and the major cause of infantile bronchiolitis worldwide. The role of αβ T cells in RSV-driven immunopathology is well studied, but little is known about the role of “unconventional” T cells. During primary RSV challenge of BALB/c mice, some Vγ7+ γδ T cells were present; however, immunization with a live vaccinia vector expressing RSV F protein substantially enhanced Vγ4+ γδ T cell influx after RSV infection. Harvested early, these cells produced IFN-γ, TNF, and RANTES after ex vivo stimulation. By contrast, those recruited 5 days after challenge made IL-4, IL-5, and IL-10. Depletion of γδ T cells in vivo reduced lung inflammation and disease severity and slightly increased peak viral replication but did not prevent viral clearance. These studies demonstrate a novel role for γδ T cells in the development of immunopathology and cellular influx into the lungs after immunization and RSV challenge. Though a minor population, γδ T cells have a critical influence on disease and are an attractive interventional target in the alleviation of viral lung disease.
Respiratory syncytial virus (RSV)3 is responsible for most cases of infantile bronchiolitis, making it the most common cause of infant hospitalization in the developed world (1). Re-infection with RSV occurs throughout life, and is a major cause of morbidity and mortality in immunocompromised and elderly persons (2). There is growing evidence that RSV disease during infancy increases the risk of recurrent wheeze and asthma in later life (3). Despite the substantial health and economic burden created by RSV illness there is currently no safe and effective vaccine (4).
In the 1960s, the administration of formalin-inactivated RSV resulted in exacerbated disease and two deaths upon natural RSV infection in later childhood. Analysis of the post mortem lung tissue demonstrated extensive pulmonary infiltration with mononuclear cells, neutrophils and eosinophils. In mice, immune augmentation is seen after RSV challenge in animals that have been vaccinated with formalin-inactivated RSV or with recombinant vaccinia virus vectors expressing individual RSV Ags. This immune exacerbation is thought to be caused by primarily RSV-specific αβTCR+ memory T cells, although there may also be a role for disease-enhancing Ab (5-9).
The development of safe and effective vaccines for RSV would be accelerated by a more complete understanding of the immunological mechanisms of disease enhancement. The fusion (F) protein of RSV is known to elicit strong CD4 and CD8 T cell responses as well as potentially protective neutralizing B cell responses (8, 9). Most experimental RSV vaccines use the RSV F protein (10-14), but none has lead to a successful vaccine for clinical use. Although the induction of Ab and conventional MHC-restricted peptide-specific αβ T cells by F is well understood, far less is known about the role of unconventional cells, such as γδ T cells.
However, there are intriguing indications that such cells may play an important part in regulating the response to viral infections of the lung. γδ T cells are disproportionately associated with internal and external body surfaces, frequently expressing particular Vγ-Vδ pairings: the human gut is rich in Vγ1Vδ1+ cells while the peripheral blood is contains Vγ9Vδ2+ cells; the murine skin is enriched in Vγ5Vδ1+ cells, while most of those in the circulation express either Vγ1 or Vγ4 (15-17). Although mice depleted of γδ T cells remain resistant to most microbial challenges, they show substantial alterations in patterns of immunopathology (18-20). γδ T cells from the peripheral blood of RSV-infected infants produce less IFN-γ and more IL-4 after stimulation than do equivalent cells from reovirus-infected infants. Although the percentage of γδ T cells producing IFN-γ increases during convalescence in children who recover fully, this is not seen in children who develop postbronchiolitic wheeze (21) indicating that γδ T cells may influence the inflammation that accompanies challenge and the development of long-term effects. Although numerically scarce by comparison to conventional T cells, γδ cells can contain very high levels of potent effector and regulatory mediators that may be released rapidly upon stimulation (22-24), thereby influence the development of other immune responses.
To test the contribution of γδ T cells to RSV disease, mice were infected with RSV with or without immunization with recombinant vaccinia virus expressing RSV F protein. After challenge of sensitized mice, Vγ4+ γδ T cells were recruited to the lungs; these produced IFN-γ, RANTES, IL-10, IL-4, and IL-5 in a time-dependent manner. In vivo depletion of γδ T cells slightly (but significantly) increased peak viral replication during secondary challenge of vaccinated mice without compromising viral clearance, yet greatly reduced the severity of vaccine-enhanced disease. Thus, although γδ T cells are a numerically small population of cells, they have a major role in driving inflammation and in directing the magnitude and severity of disease upon RSV challenge. Therefore, inhibition of γδ T cell activation is a promising target for alleviating vaccine-augmented RSV disease.
Materials and Methods
Mice and virus stocks
Eight- to ten-week-old female BALB/c mice were purchased from Harlan Olac and kept in pathogen-free conditions. RSV (A2 strain) and recombinant vaccinia virus (rVV) expressing the fusion protein (-F) of RSV or control β-galactosidase (rVV-β-gal) were grown as previously described (8). All work was ethically approved and licensed appropriately.
Mouse challenge and treatment
Anesthetized mice were scarified on the rump with 3 × 106 PFU of recombinant vaccinia virus in 10 μl. Two to three weeks later, mice were challenged intranasally with 2 × 106 PFU RSV in a final volume of 50 μl. For depletion experiments, mice were injected i.p. with 0.5 mg anti-Cγ mAb (UC7–13D5, hamster IgG) or control (Purified hamster IgG, Jackson ImmunoResearch Laboratories,) starting 1 day before and repeated 2 days after RSV challenge. They were weighed and monitored daily. At various time points groups of mice were killed by injection of 3 mg pentobarbitone (i.p.) and exsanguinated via the femoral vessels before dissection.
Cell and tissue recovery
Bronchoalveolar lavage (BAL) and lung cells and BAL fluid were obtained as described (8). In brief, 100 μl from each BAL fluid was cytocentrifuged onto glass slides for H&E staining. Cells were resuspended at 106 cells/ml in RPMI 1640 containing 10% FCS, 2 mM/ml l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin (R10F). In some experiments lung tissue was snap-frozen in liquid nitrogen for later RNA extraction.
Flow cytometric analysis of cell surface and intracellular Ags
After blocking cells with anti-CD16/32 Abs (Fc block, BD Biosciences), surface stains were performed with anti-TCR δ-chain-PE (GL3, BD Biosciences) and anti-CD3ε-FITC (Caltag), or anti-Vγ4, 5, or 7 mAb (courtesy of JA Bluestone) for 30 min at 4°C, followed by anti-hamster IgG-FITC (BD Biosciences) and fixed with 2% formaldehyde. For intracellular cytokine stains, 106 live cells/ml were incubated with plate-bound anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) for 6 h at 37°C in the presence of Brefeldin A (10 μg/ml), and stained for surface Ags and fixed as above. Cells were then permeabilized with 0.5% saponin in PBS containing 1% BSA and 0.1% azide for 10 min and then stained with allophycocyaninconjugated anti-IFN-γ (XMG1.2, BD Biosciences), PE-conjugated anti-IL-4 (11B11, BD Biosciences), PE-conjugated anti-IL-5 (TRFK5, BD Biosciences), PE-conjugated anti-IL-10 (JES5–16E3, BD Biosciences), QR-conjugated anti-RANTES (RandD Systems) or FITC-conjugated anti-TNF (MP6-XT22, BD Biosciences) in PBS with BSA and azide. Samples were analyzed on a Coulter EPICS Elite or FACS LSR flow cytometer (BD Biosiences), collecting data on at least 40,000 gated cells.
In vitro cytokine production from lung cells
Cells (4 × 105 per well) were cultured for 72 h in the presence of medium alone, RSV (MOI 2.0), or plate-bound αCD3/TCRβ (5 μg/ml) Ab in 0.2 ml R10F medium in 96-well plates. After 72 h, the supernatants were harvested and stored at −80°C for later cytokine analysis. IFN-γ, IL-4, and RANTES, were quantified using paired Abs from BD Pharmingen. In brief, microtiter plates were coated with 100 μl of capture Ab overnight at 4°C. After three washes with PBS containing 0.5% Tween 20, plates were blocked with 200 μl of PBS-1% BSA and left for 2 h at room temperature. Samples and standards (diluted in PBS with 1% BSA and 0.05% Tween 20) were incubated overnight at 4°C. After four washes, bound cytokine was detected using biotinylated Abs, then avidin-HRP, followed by O-phenylenediamine dihydrochloride, read at 490 nm. Standard curves were used to calculate concentrations. The assay for IL-4 covered the range 7.8 to 2000 pg/ml; for RANTES, 10 to 2500 pg/ml, and for IFN-γ, 31.3 to 10000 pg/ml.
Antibody ELISA
Total IgA and IgE in BAL were quantified using sandwich ELISA (BD Biosciences).
Nested PCR analysis of lung V gene segment usage of γδ T cells
Total RNA was extracted from snap-frozen whole lung as previously described (17) before ethanol precipitation. Six micrograms of total RNA was used to synthesize cDNA from random hexonucleotides. The integrity of cDNA was initially tested using the house keeping gene β-actin. PCR cycles were performed in a tetrad DNA thermal cycler. One microliter cDNA, 2.5 μl forward primer (2.5 μM), 2.5 μl reverse primer (2.5 μM), 2.5 μl dNTPs (2.5 μM), 0.5–2 μl MgCl2 (25 mM) (Promega), 2.5 μl 10×PCR buffer (Promega), and 0.15 μl TaqDNA Polymerase (5u/μl) (Promega) were added in a total reaction volume of 25 μl and made up with dH2O. After the initial denaturation at 94°C for 3 min, cycles comprised denaturation at 94°C for 30 s, primer annealing between 58 and 60°C for 45 s, and primer extension at 72°C for 1 min, repeated 39 times for Vγ and Vδ PCRs and 26 times for control β-actin. PCR products were then extended at 72°C for 10 min. For each nested PCR primer set, the positive control cDNAs from thymus, skin, and gut were also included, plus a negative control.
Quantitative analysis of viral load
Total RNA was extracted from lungs stored in TRIzol. One microgram total RNA was used to synthesize cDNA using random hexanucleotides in 20 μl total volume. PCR was used to detect the L gene as previously described (25), using standard L plasmid (101 to 107 copies) and a non-template controls, with 2 μg cDNA was per reaction in 25 μl total volume. Concentrations were: forward primer: 900 nM); reverse primer: 300 nM; and probe: 100 nM. Cycles were 1 cycle of 50°C for 2 min, 1 cycle of 95°C for 10 min, and 40 cycles of 95°C for 15 s.
Statistical analysis
A Student t test was applied using GraphPad Prism v4.00 and statistical significance assumed at p < 0.05.
Results
Enhanced influx of lung γδ T cells in vaccine-augmented RSV disease
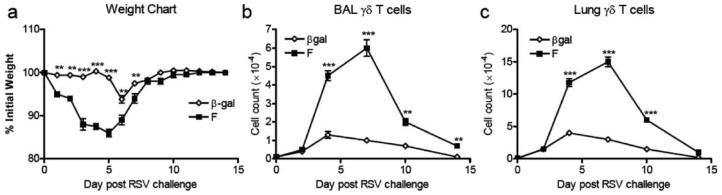
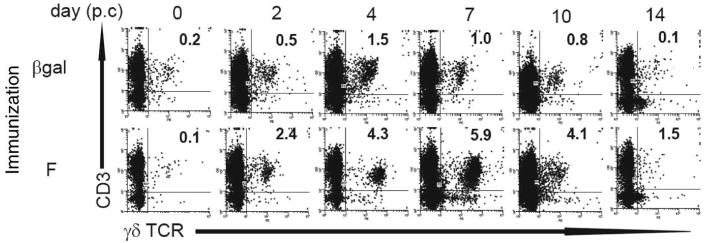
Immunization of mice with a vaccinia vector expressing RSV F protein (rVV-F) induces CD4, CD8, and B cell memory that increase illness (as reflected by weight loss) after RSV challenge, compared with vaccinia vector encoding control Ag βgal (Fig. 1a). Before RSV challenge, we estimate that on average there were only about 100 CD3+ γδ TCR+ T cells in BAL and ~1000 in the whole lung. They increased transiently in β-gal-immunized mice after RSV challenge, peaking at ~1.2 × 104 cells in BAL (Fig. 1b) and ~4.22 × 104 cells in lung (Fig. 1c) on day 4. In contrast, mice immunized against RSV F protein showed substantially enhanced γδ T cell influx to the BAL (Fig. 1b) and lungs (Fig. 1c) up to day 10 post RSV challenge. FACS analysis showed an influx of CD3+ γδTCR+ cells to the BAL (Fig. 2); CD3+ γδTCR+ cells were almost exclusively CD4−, CD8−, CD45RBlow, and CD44high in both BAL and lungs (data not shown).
FIGURE 1.
γδ T cell response to RSV challenge is enhanced by prior immunization. Mice were scarified with rVV-β-gal, or –F on day 0 and challenged intranasally with RSV on day 14. Weights of β-gal (control) immunized ( ) and F-primed (■) mice were monitored daily and the percent original body weight calculated (a). The number of TCRδ+CD3+ cells in the lymphoid gate from the BAL (b) or lung (c) are shown. Experiments were performed at least three times containing five mice per group.
) and F-primed (■) mice were monitored daily and the percent original body weight calculated (a). The number of TCRδ+CD3+ cells in the lymphoid gate from the BAL (b) or lung (c) are shown. Experiments were performed at least three times containing five mice per group.
FIGURE 2.
Representative FACS analysis of TCRδ+CD3+ cells from the BAL of mice. Mice were scarified with rVV-β-gal (control), or rVV-F on day 0 and challenged intranasally with RSV on day 14. TCRδ+CD3+ cells in the BAL were identified by flow cytometry. Representative dot plots are shown for mice immunized with rVV-βgal or rVV-F over the time course of RSV infection. The percentage of TCRδ+CD3+ cells in the lymphoid gate (identified by size and granularity) is shown for each plot. Experiments were performed at least three times containing five mice per group.
Vγ expression by γδ T cells
To examine Vγ and Vδ expression, nested PCR was performed on cDNA from whole lung tissue taken at days 4 and 7 post challenge (p.c.). In βgal-immunized mice, no signal was detected for Vγ5-Jγ1 or Vγ6-Cγ1, but Vγ4-Jγ1 gave weak signals 4 and 7 days after RSV challenge. A stronger signal was detected for Vγ7-Jγ1 (data not shown). In rVV-F-immunized mice on day 4 of challenge, a weak signal was detected for Vγ1-Jγ4, but Vγ4-Jγ1 signals were substantially enhanced. Both clonotypes are associated with infiltrating cells (26) and the signals were maintained at day 7; no signal was detected for Vγ7-Jγ1 at any time point. This indicated that immunization with RSV F protein induced influx of different γδ T cell clonotypes, compared with control mice with more rapid kinetics.
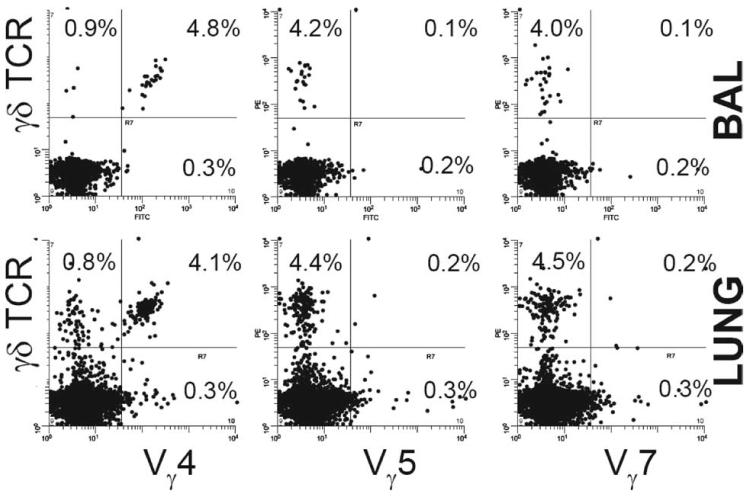
To confirm expression of these clonotype TCR at the cell surface, Abs against Vγ4 (identifying cells normally associated with the systemic compartment), Vγ5 (associated with skin), and Vγ7 (associated with the gut) (27) were used to clonotype BAL γδ cells of mice immunized with RSV F protein, 4 days post RSV challenge. Very few Vγ5+ or Vγ7+ cells were found, but >80% expressed Vγ4, consistent in an infiltration of cells from the circulation (Fig. 3).
FIGURE 3.
Clonotypic analysis of γ-chain expression by γδ T cells. Mice were primed with rVV-F and challenged with RSV on day 14. BAL and lung samples were harvested on day 4 after challenge. BAL and lung cells from infected mice were counted and stained with PE-conjugated anti-γδ TCR and either purified anti-Vγ4, -Vγ5, or -Vγ7 hamster mAbs. Cells were then stained with FITC-conjugated anti-hamster Ab and analyzed. Representative dot plots are shown. Percentage BAL cells that expressed the detected γ-chain are shown. At least 20,000 cells were collected from each sample. Experiments were performed at least three times containing five mice per group.
Cytokine profile of lung γδ T cells during challenge
FACS analysis of intracellular cytokines showed that before RSV challenge, the rare γδ cells in the lungs of rVV-F-immunized mice exhibited a ‘Th1’ cytokine profile with ~30% producing IFN-γ or TNF and 65% expressing RANTES after αCD3/αCD28 restimulation (Fig. 4a). Almost all γδ T cells produced IFN-γ and TNF simultaneously. At this time, no γδ T cells contained detectable IL-4, IL-5, or IL-10 (Fig. 4b). However, after RSV challenge of rVV-F-immunized mice lung γδ cells showed a decline in Th1 cytokine production and a rapid increase in Th2 cytokines. By day 5, <10% of γδ T cells produced IFN-γ or TNF-α while ~15% produced IL-10, ~10% produced IL-4, and IL-5 also increased. These increases were sustained for ~7 days. RANTES production by γδ cells also fell initially but had largely recovered within 7 days; however, Th1 cytokine production was still depressed 14 days after challenge. Thus, lung γδ T cells from F-primed mice produce Th1 cytokines and proinflammatory chemokines early after RSV challenge, and then switch to Th2 and suppressive cytokines at the peak of disease severity.
FIGURE 4.
Cytokine content of lung γδ T cells. Mice were primed with rVV-F and challenged with RSV on day 14. Lung cells were harvested at various time points, restimulated as described in Materials & Methods, stained with anti-C-γδ-PE, permeabilized, and stained for IFN-γ, RANTES, TNF (a) and IL-4, IL-5, or IL-10 (b). Isotype controls for each Ab were used to determine specific staining. The percentage of lung γδ T cells producing each cytokine is shown. Five mice were analyzed at each time point. C, Representative dot plots (right) and their respective isotype controls (left) from individual mice are shown (day 0 for IFN-γ, day 7 for other cytokines). Experiments were performed at least three times containing five mice per group.
Depletion of γδ T cells in vivo reduces disease severity after RSV challenge
To determine whether γδ T cells alone regulated disease severity, mice were depleted of γδ T cells by treatment with a mAb specific for Cγ (28) 13 days after rVV-F immunization. Flow cytometry confirmed that γδ T cells were efficiently depleted in the lung and the BAL (Fig. 5, a and b), and that two Ab doses were sufficient to deplete γδ T cells for more than 14 days.
FIGURE 5.
Effect of γδ T cell depletion on weight loss in RSV-challenged, immunized mice. Mice primed with rVV-F, were after 14 days treated with isotype control (open symbols) or αCγ mAb UC7–13D5 (■) −1 and +2 day relative to RSV challenge. On day 4 after RSV challenge BAL cells were stained for γδ T cells as described in Fig. 1. γδ T cells were present in mice treated with control Ab (a) but not detected in those treated with αCγ mAb (b). Mouse weights are shown as mean ± SEM of starting weights (c). Total RNA was extracted from lungs of immunized, challenged mice at various time points and treated as described in Materials and Methods. The number of viral genome copies was based on detection of the L gene DNA sequence; plasmids (107 to 101 copies) containing the L gene were used as standards and a nontemplate control as the negative (d). Results are shown as mean ± SEM of five mice per group of TCRγδ depleted mice (■) or controls (open symbols). *, p < 0.05; **, p < 0.02; ***, p < 0.001.
Weight loss was greatly inhibited in anti-Cγ treated mice (Fig. 5c), resembling that seen after RSV challenge of nonimmunized mice (β-gal in Fig. 1a). Viral replication was detectable despite immunization, but peak viral load was reduced from ~106 copies/mouse in primary infection to ~1.3 × 104 copies/mouse. Immunized, anti-Cγ treated mice showed a small but significant increase in viral L gene copy number (to 2.6 × 104; p < 0.05) at day 4, which fell to baseline by day 7 (Fig. 5d). Therefore, γδ T cells are critical to the development of enhanced disease observed after immunization with RSV F protein and contribute to controlling peak viral load, they do not affect eventual viral clearance.
Depletion of γδ T cells in vivo reduces IFN-γ, IL-4, and RANTES production in the lung
To show whether γδ T cell depletion reduced disease severity because of mediator release, we examined the effects of depletion on production of IFN-γ and IL-4 and RANTES. Anti-Cγ treatment had no effect on BAL IFN-γ (Fig. 6a) or IL-4 (Fig. 6b), but did significantly reduce RANTES levels at day 2 (Fig. 6c). There was no significant effect on BAL mediators on days 7, 10, or 14. Measuring RSV-induced release of mediators from isolated lung cells, in vivo depletion reduced the production of IFN-γ (Fig. 6d), IL-4 (Fig. 6e), and RANTES (Fig. 6f) by cells from these same mice on day 4 (the time at which the greatest difference in weight loss was seen). By day 10, levels of release were lower and only IL-4 release showed a significant effect of prior in vivo depletion (Fig. 6e). Thus, γδ T cells are important in early airway RANTES release, and in later viral Ag-specific release of multiple mediators from interstitial lung cells.
FIGURE 6.
Cytokine levels in bronchial lavage fluid and supernatant of cultured lung cells. Mice were immunized with rVV-F and challenged with RSV with and without in vivo γδ T cell depletion, as described in the legend to Fig. 5. Sequential BAL samples were taken after RSV challenge and tested for IFN-γ (a), IL-4 (b), and RANTES (c) by sandwich ELISA. On day 4 and 10, cells were extracted from whole lungs and stimulated with RSV Ag. IFN-γ (d), IL-4 (e), and RANTES (f) release into supernatants was detected by sandwich ELISA.
Depletion of γδ T in vivo reduces BAL and lung cell influx after RSV challenge
To show whether γδ T cell depletion reduced disease severity because of reduced cell influx to the lung compartment, we examined the effects of depletion on other immune responses. Anti-Cγ treated mice had significantly reduced cell counts in both the BAL (Fig. 7a) and lungs (Fig. 7d). This decrease was associated with a strong inhibition in CD8 T cell influx on days 10 and 16 (Fig. 7b). There were striking decreases in BAL B cell numbers on days 4 through 16 (Fig. 7c) and IgE levels in the BAL fluid showed a significant reduction on day 10 compared with controls (Fig. 7g). IgA levels were unaffected by depletion (Fig. 7h). These findings are in accord with evidence that γδ cells promote B cell help and germinal center formation (29, 30). There were significant but transient decreases in CD4 T cells (Fig. 7e) and NK cell influx was also reduced (Fig. 7f). We observed no change in the recruitment of granulocytes (data not shown). Thus, γδ T cells promote influx of both innate and adaptive immune cells, possibly in a RANTES-dependent manner.
FIGURE 7.
Cell phenotypes and Ab production in the BAL and lungs of immunized, RSV-challenged mice with and without depletion. Total BAL (a) and lung (d) cell recoveries were counted by trypan blue exclusion. BAL cells were stained for CD8 (b), CD4 (e), B220 (c), or DX5 (f) surface markers. BAL supernatants were stored at −70°C before total IgE (g) and IgA (h) detection using biotinylated Abs according to the manufacturer's instructions (as described in Materials and Methods). Results are mean ± SEM of five mice per group of TCRγδ-depleted mice (■) or controls (open symbols). *, p < 0.05; **, p < 0.02.
Discussion
Our results show that γδ T cells have remarkable effects on the scale and nature of the inflammatory response to RSV challenge. Although primary RSV infection caused a small and transient rise in the number of γδ T cells, infection after immunization with RSV F protein lead to a sustained accumulation of γδ T cells expressing a spectrum of Th1 and Th2 cytokines and proinflammatory chemokines. Crucially, depletion of γδ T cells in such sensitized mice greatly reduced disease severity, lung cytokine production, and cell infiltration. Therefore, although γδ T cells are numerically rare they contribute substantially to immune-augmented RSV disease.
Although we found that γδ T cells were much more numerous in mice with disease enhanced by prior vaccination with vaccinia expressed F, this was not just a feature of immune enhanced disease. In mice immunized with vaccinia expressing the RSV M2 protein there is sustained, substantial enhancement of disease (31) but a notable lack of γδ T cells (data not shown). It is possible that γδ T cell activation is critically dependent on memory CD4 T cells, since F (but not M2) induces a strong CD4 T cell response, much greater than that seen in primary infection. In chronic malaria, depletion of CD4 T cells results in a marked decrease of splenic γδ T cell numbers and exacerbation of parasitemia (32). Therefore, γδ T cells and CD4 T cells may act together to enhance RANTES production, cell influx, and disease.
It also seems that γδ T cells may act in concert with NK cells in the control of viral load. Depletion with anti-Cγ Ab resulted in an increase in peak viral RNA load, accompanied by a reduced NK cell influx. It is known that NK cells are important for viral clearance in this model (33), so it is possible that the antiviral action of γδ T cells is caused by effects on NK cells. Alternatively, it is possible that γδ T cells control viral replication via direct cytotoxic effector mechanisms. However, viral load is always very low in secondary infection of vaccinated mice (compared with primary infection of naive mice) and clearance was complete at day 7 in all groups regardless of depletion, probably due to the antiviral effects of Ab, CD8 T cells, and NK cells (5, 33).
We found that ~80% of lung γδ T cells expressed Vγ4, suggesting that they are recruited from the circulatory pool. This has been observed in other models (34). For example, Lahn et al. found that a small population (1–2 × 104 cells) of Vγ4+ CD8α+β+ γδ T cells inhibit airway hyperresponsiveness after OVA sensitization and challenge in mice, even in the absence of αβ T cells. Upon stimulation, these cells secrete IFN-γ and their depletion exacerbates airway hyperresponsiveness upon subsequent OVA challenge (35). In murine Coxsackie virus B3-induced myocarditis, Huber et al showed opposing roles for two different clonotypes in that Vγ4+ γδ T cells were associated with disease susceptibility, whereas Vγ1+ γδ T cells were associated with disease resistance (36). Depletion of Vγ4+ cells, CD4 T cells, or CD8 T cells abolished myocarditis. In our studies, Vγ1+ γδ T cells were very scarce. The association of Vγ4+ cells with enhanced lung disease has not previously been described.
Previous studies have demonstrated that γδ T cells produce Th1 or Th2 cytokines, depending on the environment in which the develop (29). For example, Vγ9/Vδ2 T cells stimulated with the low m.w. Ag HMB-PP produce RANTES in the presence of either IL-15 or IL-21, but also produce TNF, IFN-γ, or IL-4 in the presence of IL-15 (37). Our studies show that lung γδ T cells from RSV-F-immunized, RSV-challenged mice produce IFN-γ, TNF, and RANTES early after RSV challenge and IL-4, –5, and –10 after day 5. These data suggest that γδ T cells initially promote the Th1 response observed in F-immunized mice, but later change to later produce regulatory/Th2 cytokines. This change is highlighted in the lung, where both IFN-γ and IL-4 production were reduced at day 4 (p.c.), however only IL-4 was still reduced by day 10 (p.c.). These changes in the lung may reflect the changing profile of the Vγ4+ γδ T cells over time. To our knowledge this is the first time that a regulatory role for γδ T cells has been described and highlights a potentially crucial role for γδ T cells in controlling immunopathology.
There is growing clinical evidence that γδ T cells have an equally important role in human airway disease as γδ T cells are present and active in the mucosa of patients with allergic rhinitis (38), in the BAL of patients with allergic asthma (39) and in the peripheral blood of RSV-infected infants (21). Our results clearly indicate that γδ cells have modest antiviral effects but strongly enhance the severity of viral lung disease, and reveal a hitherto unappreciated role for γδ T cells in the development of viral immunopathology. Although greatly outnumbered by other cells, they play a surprisingly pivotal role in the control of disease. Therefore, we believe that γδ T cells could be an important target for therapeutic intervention in RSV disease or infants, and that strategies that inhibit their actions would produce clinical benefits without significantly compromising viral clearance.
Acknowledgments
We thank S. Wythe and Professor B. Askonas for guidance, and E. Theodoridis for assistance with some of the experiments and critical reading of this manuscript.
Footnotes
Publisher's Disclaimer: The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This work was funded by Wellcome Trust (U.K.) Program Grants (to P.O. and AH.).
Abbreviations used in this paper: RSV, respiratory syncytial virus; F, fusion; rVV, recombinant vaccinia virus; rVV-F, recombinant vaccinia virus expressing RSV F protein; BAL, bronchoalveolar lavage; p.c., post challenge.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Hall CB. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 2.Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 2000;13:371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigurs N. Epidemiologic and clinical evidence of a respiratory syncytial virus-reactive airway disease link. Am. J. Respir. Crit. Care Med. 2001;163:S2–S6. doi: 10.1164/ajrccm.163.supplement_1.2011109. [DOI] [PubMed] [Google Scholar]
- 4.Crowe JE. Respiratory syncytial virus vaccine development. Vaccine. 2001;20(Suppl. 1):S32–S37. doi: 10.1016/s0264-410x(01)00287-0. [DOI] [PubMed] [Google Scholar]
- 5.Cannon MJ, Openshaw PJM, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connors M, Giese NA, Kulkarni AB, Firestone C-Y, Morse HC, III, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin- 4 (IL-4) and IL-10. J. Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Openshaw PJM, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int. Immunol. 1992;4:493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 9.Alwan WH, Kozlowska WJ, Openshaw PJM. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan X, Hurwitz JL, Krishnamurthy S, Takimoto T, Boyd K, Scroggs RA, Surman S, Portner A, Slobod KS. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine. 2007;25:8782–8793. doi: 10.1016/j.vaccine.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mok H, Lee S, Utley TJ, Shepherd BE, Polosukhin VV, Collier ML, Davis NL, Johnston RE, Crowe JE., Jr. Venezuelan equine encephalitis virus replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. J. Virol. 2007;81:13710–13722. doi: 10.1128/JVI.01351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott MB, Chen T, Terio NB, Chong SY, Abdullah R, Luckay A, Egan MA, Boutilier LA, Melville K, Lerch RA, et al. Alphavirus replicon particles encoding the fusion or attachment glycoproteins of respiratory syncytial virus elicit protective immune responses in BALB/c mice and functional serum antibodies in rhesus macaques. Vaccine. 2007;25:7132–7144. doi: 10.1016/j.vaccine.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 13.Ternette N, Tippler B, Uberla K, Grunwald T. Immunogenicity and efficacy of codon optimized DNA vaccines encoding the F-protein of respiratory syncytial virus. Vaccine. 2007;25:7271–7279. doi: 10.1016/j.vaccine.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Cyr SL, Jones T, Stoica-Popescu I, Brewer A, Chabot S, Lussier M, Burt D, Ward BJ. Intranasal proteosome-based respiratory syncytial virus (RSV) vaccines protect BALB/c mice against challenge without eosinophilia or enhanced pathology. Vaccine. 2007;25:5378–5389. doi: 10.1016/j.vaccine.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Havran WL, Chien Y-H, Allison JP. Recognition of self antigens by skin-derived T cells with invariant γδ antigen receptors. Science. 1991;252:1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 16.Parker CM, Groh V, Band H, Porcelli SA, Morita C, Fabbi M, Glass D, Strominger JL, Brenner MB. Evidence for extrathymic changes in the T cell receptor γ/δ repertoire. J. Exp. Med. 1990;171:1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McVay LD, Carding SR, Bottomly K, Hayday AC. Regulated expression and structure of T cell receptor γ/δ transcripts in human thymic ontogeny. EMBO J. 1991;10:83–91. doi: 10.1002/j.1460-2075.1991.tb07923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu YX, Roark CE, Kelly K, Drevets D, Campbell P, O'Brien R, Born W. Immune protection and control of inflammatory tissue necrosis by γδ T cells. J. Immunol. 1994;153:3101–3115. [PubMed] [Google Scholar]
- 19.Mukasa A, Hiromatsu K, Matsuzaki G, O'Brien R, Born W, Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of αβ and γδ T cells. J. Immunol. 1995;155:2047–2056. [PubMed] [Google Scholar]
- 20.Mukasa A, Lahn M, Pflum EK, Born W, O'Brien RL. Evidence that the same γδ T cells respond during infection-induced and autoimmune inflammation. J. Immunol. 1997;159:5787–5794. [PubMed] [Google Scholar]
- 21.Aoyagi M, Shimojo N, Sekine K, Nishimuta T, Kohno Y. Respiratory syncytial virus infection suppresses IFN-γ production of γδ T cells. Clin. Exp. Immunol. 2003;131:312–317. doi: 10.1046/j.1365-2249.2003.02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 23.Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O'Brien R. Immunoregulatory functions of γδ T cells. Adv. Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- 24.Fahrer AM, Konigshofer Y, Kerr EM, Ghandour G, Mack DH, Davis MM, Chien YH. Attributes of γδ intraepithelial lymphocytes as suggested by their transcriptional profile. Proc. Natl. Acad. Sci. USA. 2001;98:10261–10266. doi: 10.1073/pnas.171320798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J. Exp. Med. 2002;196:1381–1386. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyes S, Hayday A. Disparate types of γδ T cell. Res. Immunol. 1990;141:582–587. doi: 10.1016/0923-2494(90)90060-c. [DOI] [PubMed] [Google Scholar]
- 27.Lahn M. The role of γδ T cells in the airways. J. Mol. Med. 2000;78:409–425. doi: 10.1007/s001090000123. [DOI] [PubMed] [Google Scholar]
- 28.Peterman GM, Spencer C, Sperling AI, Bluestone JA. Role of γδ T cells in murine collagen-induced arthritis. J. Immunol. 1993;151:6546–6558. [PubMed] [Google Scholar]
- 29.Wen L, Barber DF, Pao W, Wong FS, Owen MJ, Hayday A. Primary γδ cell clones can be defined phenotypically and functionally as Th1/Th2 cells and illustrate the association of CD4 with Th2 differentiation. J. Immunol. 1998;160:1965–1974. [PubMed] [Google Scholar]
- 30.Horner AA, Jabara H, Ramesh N, Geha RS. γ/δ T lymphocytes express CD40 ligand and induce isotype switching in B lymphocytes. J. Exp. Med. 1995;181:1239–1244. doi: 10.1084/jem.181.3.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartholdy C, Olszewska W, Stryhn A, Thomsen AR, Openshaw PJ. Gene-gun DNA vaccination aggravates respiratory syncytial virus-induced pneumonitis. J. Gen. Virol. 2004;85:3017–3026. doi: 10.1099/vir.0.80098-0. [DOI] [PubMed] [Google Scholar]
- 32.van der Heyde HC, Batchelder JM, Sandor M, Weidanz WP. Splenic γδ T cells regulated by CD4+ T cells are required to control chronic Plasmodium chabaudi malaria in the B-cell-deficient mouse. Infect. Immun. 2006;74:2717–2725. doi: 10.1128/IAI.74.5.2717-2725.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hussell T, Openshaw PJM. IL-12 activated NK cells reduce lung eosinophilia to the attachment protein of respiratory syncytial virus but do not enhance the severity of illness after lung challenge in CD8 cell-immunodeficient conditions. J. Immunol. 2000;165:7109–7115. doi: 10.4049/jimmunol.165.12.7109. [DOI] [PubMed] [Google Scholar]
- 34.Findly RC, Roberts SJ, Hayday AC. Dynamic response of murine gut intraepithelial T cells after infection by the coccidian parasite Eimeria. Eur. J. Immunol. 1993;23:2557–2564. doi: 10.1002/eji.1830231027. [DOI] [PubMed] [Google Scholar]
- 35.Lahn M, Kanehiro A, Takeda K, Terry J, Hahn YS, Aydintug MK, Konowal A, Ikuta K, O'Brien RL, Gelfand EW, Born WK. MHC class I-dependent Vγ4+ pulmonary T cells regulate αβ T cell-independent airway responsiveness. Proc. Natl. Acad. Sci. USA. 2002;99:8850–8855. doi: 10.1073/pnas.132519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huber SA, Graveline D, Newell MK, Born WK, O'Brien RL. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J. Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 37.Eberl M, Engel R, Beck E, Jomaa H. Differentiation of human γ-δ T cells towards distinct memory phenotypes. Cell. Immunol. 2002;218:1–6. doi: 10.1016/s0008-8749(02)00519-1. [DOI] [PubMed] [Google Scholar]
- 38.Pawankar RU, Okuda M, Suzuki K, Okumura K, Ra C. Phenotypic and molecular characteristics of nasal mucosal γδ T cells in allergic and infectious rhinitis. Am. J. Respir. Crit. Care Med. 1996;153:1655–1665. doi: 10.1164/ajrccm.153.5.8630617. [DOI] [PubMed] [Google Scholar]
- 39.Spinozzi F, Agea E, Bistoni O, Forenza N, Monaco A, Falini B, Bassotti G, De Benedictis F, Grignani F, Bertotto A. Local expansion of allergen-specific CD30+Th2-type γδ T cells in bronchial asthma. Mol. Med. 1995;1:821–826. [PMC free article] [PubMed] [Google Scholar]