Abstract
Sphingosine 1-phosphate (S1P), a lysophospholipid mediator that signals through G protein-coupled receptors, regulates a wide plethora of biological responses such as angiogenesis and immune cell trafficking. Detection and quantification of S1P in biological samples is challenging due to its unique physicochemical nature and occurrence in trace quantities. In this report, we describe a new method to selectively enrich S1P and dihydro-S1P from biological samples by the Fe3+ gel immobilized metal affinity chromatography (IMAC). The eluted S1P from IMAC was dephosphorylated, derivatized with o-phthalaldehyde (OPA), and detected by high-performance liquid chromatography (HPLC) coupled to a fluorescence detector. IMAC purification of S1P was linear for a wide range of S1P concentration. Using this assay, secretion of endogenous S1P from endothelial cells, fibroblasts and colon cancer cells was demonstrated. We also show that dihydro-S1P was the major sphingoid base phosphate secreted from HUVEC over expressed with Sphk1 cDNA. Pharmcological antagonists of ABC transporters, glyburide and MK-571 attenuated endogenous S1P release. This assay was also used to demonstrate that plasma S1P levels were not altered in mice deficient for ABC transporters, Abca1, Abca7 and Abcc1/Mrp1. IMAC-based affinity-enrichment coupled with a HPLC-based separation and detection system is a rapid and sensitive method to accurately quantify S1P.
Keywords: Sphingosine 1-phosphate (S1P), Immobilized metal affinity chromatography (IMAC), Endothelial cells, Plasma S1P, Sphingosine kinase (Sphk), ABC transporters, High-performance liquid chromatography (HPLC)
1. Introduction
Sphingosine 1-phosphate (S1P) is a bioactive lysophospholipid that regulates multiple biological processes. For example, S1P regulates angiogenesis [1,2], vascular permeability [3,4] vascular tone [5], heart rate [6], immune cell trafficking [7,8], inner ear development [9–11], mast cell function [12,13] and tumor growth [14,15]. Its actions are brought about by signaling through a family of G protein-coupled receptors termed as S1P1–S1P5 [16]. Despite the fact that S1P is a multifunctional mediator, the regulation of S1P synthesis and export from cells is poorly understood.
Mammalian blood contains high levels (~1 μM) of S1P, which is bound to its chaperones—high-density lipoprotein (HDL) and albumin [17]. In contrast, tissue levels of S1P are low and therefore a concentration gradient of S1P exists between blood and interstitial fluids. However, mechanisms involved in the establishment and maintenance of plasma S1P gradient is not clearly understood. Recently, several groups suggested that erythrocytes might contribute significant amounts of S1P into blood plasma [18–20].
To determine the cellular sources of S1P and the mechanisms involved in the secretion of S1P from cultured cells, current methods utilize either [3H]-sphingosine or inorganic [32P]-phosphate labeling of cells followed by analysis of released S1P by TLC [21–23]. A potential weakness of these methodologies is that they do not directly measure endogenous S1P. In addition, classical methods require extraction of conditioned media with large volumes of solvents, and the presence of salt in the sample needs extensive washing steps. Although mass spectrometry-based methods are highly sensitive and quantitative, this methodology is not widely available due to the expense associated with instrumentation set-up, operational costs and the expertise required.
Here we describe a simple and rapid technique to enrich S1P from biological samples. Immobilized Fe3+ is known to bind tightly to phosphorylated amino acids and phosphopeptides [24]. This so-called immobilized metal affinity chromatography (IMAC) was adapted to selectively enrich S1P from other non-phospholipids. We show that IMAC affinity chromatography coupled with HPLC and fluorescence detection is capable of efficiently detecting small quantities of S1P from biological fluids and from conditioned media.
2. Materials and methods
2.1. Reagents and materials
S1P and C17-S1P were purchased from Avanti Polar Lipids (Alabaster, AL). Lipid standards were dissolved in methanol and were stored at −20 °C. Glyburide, PHOS-Select™ iron affinity gel was purchased from Sigma (St. Louis, MO). MK-571 and Verapamil were purchased from Biomol (Plymouth, PA). [32P]-ATP (specific activity 6000 Ci/mmol) was purchased from Amersham Life Sciences Products Inc. HPLC grade solvents; methanol, acetonitrile and chloroform were purchased from Fisher Scientific (Fair Lawn, NJ). Abca1+/− breeding pair was purchased from Jackson Laboratory and bred in animal facility to derive Abca1+/+, Abca1+/− and Abca1−/− animals. Plasma samples from Abca7+/+ and Abca7−/− mice were provided by Dr. Michael L. Fitzgerald (Massachusetts General Hospital, Harvard Medical School, Boston, MA); while plasma and lymph from Abcc1+/+ (also known as Mrp1) and Abcc1−/− mouse were provided by Dr. Gwendalyn J. Randolph (Mt. Sinai School of Medicine, New York, NY). Platelet poor plasma was isolated as described earlier [25].
2.2. Cell culture
Human umbilical vein vascular endothelial cells (HUVEC) were cultured as described [26]. Cell culture medium contained 10% fetal bovine serum (FBS), M-199, antibiotics and antimycotics, 5 units/ml of heparin and 150 μg/ml of endothelial cell growth supplement. While, mouse embryonic endothelial cells (MEEC), NIH3T3, mouse embryonic fibroblast (MEF); HCT-116, HT-29 cells were cultured in DMEM with 10% fetal bovine serum (FBS) having antibiotics and antimycotics in humidified CO2 incubator.
2.3. S1P enrichment by IMAC
PHOS-Select™ iron affinity gel (40 μl of 50% beads slurry) was incubated with 2–5 ml of cell culture media, containing 5–10 pmol of internal standard C17-S1P. The binding of S1P with the IMAC resin was conducted in 250 mM acetic acid (pH 2.5–3.0) in 30% acetonitrile for 60 min at 4 °C. After washing the resin with 0.5 ml of 250 mM acetic acid (pH 2.5–3.0) in 30% acetonitrile and subsequently with 0.5 ml of deionized water, bound S1P was eluted in 0.5 ml of 150 or 400 mM ammonium hydroxide in 25% acetonitrile.
2.4. Lipid extraction and S1P analysis by HPLC
Lipid extraction and S1P analysis were carried out with minor modification of previously described procedures [27]. In brief, after removing the residual acetonitrile in a vacuum rotary evaporator, concentrated aqueous phase (~350 μl) was partitioned with 300 μl of chloroform to remove hydrophobic lipids. S1P in alkaline aqueous phase was dephosphorylated and derivatized by o-phthalaldehyde (OPA). Injection volume was 50–100 μl from 270 μl of total volume diluted with 90% acetonitrile. Solutions of 70% acetonitrile and 90% acetonitrile were used as eluent A and B, respectively. The elution protocol was composed of an initial 5 min with 50% eluent B, followed by linear gradient from 5 min (50% eluent B) to 30 min (95% eluent B), and returned to initial condition (50% eluent B) at 31 min for column equilibration and next sample injection.
2.5. Preparation of [32P]-S1P
[32P]-S1P was either prepared by incubating 25 μl blood with 10 μM sphingosine and 20 μCi [32P]-ATP in a final volume of 0.4 ml or 25 μl HEK-293 cell homogenates expressing Adeno-Sphk1 in Sphk assay buffer, and [32P]-S1P was separated by TLC [25]. Subsequently, [32P]-S1P band was scraped and extracted from silica with 2 ml of chloroform–methanol–HCl (100:200:1, v/v), washed twice in 1 ml of methanol and 10 mM HCl and the organic phase containing [32P]-S1P was dried. Finally [32P]-S1P was reconstituted in PBS containing 0.4% BSA and stored at −20 °C in aliquots until it was used.
2.6. Total recovery of S1P
PHOS-Select™ iron affinity gel (40 μl of 50% beads slurry) was incubated with [32P]-S1P (1.33 × 105 cpm; 60 nCi) diluted in S1P at 0.001, 0.020, 0.050, 0.2, 0.5, 2, 5 and 10.0 μM concentration in final volume 1.5 ml in 0.4% BSA under acidic condition containing 30% acetonitrile (v/v). Elution of bound [32P]-S1P was carried out in 400 mM ammonia containing 25% acetonitrile after washing the beads twice with binding solvent (250 mM acetic acid having 30% acetonitrile). Recovery of [32P]-S1P found in the eluent was determined by liquid scintillation counting.
2.7. Measurement of S1P in cultured cells and in conditioned media
HUVEC, MEEC (murine embryonic endothelial cells), NIH3T3, MEF (murine embryonic fibroblasts), HCT-116, HT-29 colon cancer cell lines were incubated with 2 or 4 ml of DMEM containing 20 mM HEPES-KOH pH 7.4, 10 mM sodium glycerophosphate, 5 mM sodium fluoride and 1 mM semicarbazide and 0.5% fatty acid free BSA for trapping S1P released from the cells. Cells were incubated in the trapping medium for 2 h. Accumulation of extracellular S1P was determined by spiking 5 or 10 pmol of C17-S1P to conditioned media, affinity isolation with the IMAC beads followed by the HPLC analysis of S1P was carried out as described previously [27]. While the cells were washed twice with ice cold PBS and scraped in 250 μl of PBS and S1P was isolated in presence of 5 or 10 pmol of C17-S1P and the intracellular S1P amounts were determined by HPLC protocol as described previously [27].
2.8. Adenoviral transduction
HUVEC were grown to 80% confluency and transduced with Adeno-Sphk1 at 50 plaque forming unit (PFU) per cell [28,29]. After 24 h cells were either treated with 10 μg/ml Brefeldin-A or with 500 nM PMA for 2 h and the formation and release of S1P and dihydro-S1P in the intracellular and extracellular compartments were analyzed as described above.
2.9. Inhibition of ABC transporters
HUVEC were grown in 6 cm dish and incubated with 500 μM glyburide, 20 μM Verapamil or 25 μM MK-571 to inhibit ABC-A (A1 and A7), ABC-B1 (MDR1) or ABC-C1 (MRP1), respectively. S1P released to the conditioned media was analyzed as described above.
3. Results
3.1. Binding and elution of [32P]-S1P from IMAC beads
We first optimized binding and elution of S1P by spiking [32P]-S1P to media containing 0.5% BSA and various concentrations of acetonitrile (10–90%). The binding of [32P]-S1P to IMAC was more efficient at high concentrations of acetonitrile. In contrast, [32P]-S1P from IMAC beads was eluted efficiently eluted with lower concentrations of acetonitrile. Due to volumetric considerations in handling conditioned media, we used 30 and 25% acetonitrile in binding and elution solutions, respectively. Various concentrations of [32P]-S1P (0–10 μM) in 1.5 ml of binding solution (250 mM acetic acid, 30% acetonitrile) were used to bind to the IMAC beads. Subsequently S1P was eluted in 1.0 ml of 400 mM ammonium hydroxide containing 25% acetonitrile. Elution of [32P]-S1P was linear for at least 2 log concentrations of S1P (0.1–10 μM S1P). Overall recovery of [32P]-S1P from IMAC enrichment was ~16–20% (Fig. 1).
Fig. 1.
Recovery of [32P]-S1P from IMAC beads. 60 nCi of [32P]-S1P was diluted with 0.001, 0.020, 0.050, 0.2, 0.5, 2, 5 and 10.0 μM cold S1P in 1.5 ml binding solution (250 mM acetic acid/30% acetonitrile) with 40 μl of IMAC beads. Bound [32P]-S1P was eluted in 1 ml elution solution containing 400 mM ammonia/25% acetonitrile. Recovery of [32P]-S1P (nmol) in eluent was quantified by liquid scintillation counting (n = 3).
3.2. Analysis of C17-S1P eluted from IMAC beads by HPLC
Next we quantified S1P eluted from IMAC beads with the HPLC methodology [24]. HPLC analysis of OPA derivatized C17-S1P eluted from IMAC beads showed that the signal is linear from 10 to 100 pmol (Fig. 2). As little as 5 pmol of C17-S1P diluted in 5 ml of S1P trapping medium was easily detected and quantified. These data suggested that S1P found in trace quantities could be efficiently quantified by this method.
Fig. 2.
HPLC analysis of C17-S1P eluted from IMAC beads. Increasing amount of C17-S1P (0, 5, 50 and 100 pmol) was diluted in 5 ml of S1P trapping medium (DMEM supplemented with 25 mM HEPES pH 7.4, 5 mM sodium flouride, 10 mM glycerophosphate, 1 mM semicarbazide and 0.5% fatty acid free BSA). C17-S1P was bound and eluted from IMAC beads and quantified by HPLC as described in Section 2. Note: C17-S1P peak area was linear to concentration of C17-S1P used in binding to IMAC (Y = 252.92X– 132.38, r2 = 0.999).
3.3. Synthesis and secretion of S1P from cultured cells in vitro
Next we used the IMAC method to quantify S1P from various cultured cells. We compared the ability of endothelial cells, fibroblasts and colon cancer cells to synthesize and release S1P. S1P was found in both conditioned medium and cell extracts from HUVEC and MEEC, suggesting that endothelial cells secrete S1P avidly (Fig. 3). In sharp contrast, fibroblasts and colon cancer contain lower S1P in the cell extracts and extremely low amounts in the conditioned medium (Fig. 3). In most cells endogenous dihydro-S1P could not be detected.
Fig. 3.
S1P synthesis and release from various cells in vitro. Detection of S1P in cell extracts (black filled bars) and 2 h conditioned medium (gray bars). Endothelial cells, fibroblasts, colon cancer cells were grown to confluency and S1P was quantified as described in Section 2 (n = 2–4). Note that only endothelial cells secreted S1P avidly compared to other cells.
3.4. Sphingoid base phosphate synthesis and secretion upon over expression of Sphk1 in HUVEC
Next we analyzed the sphingoid base phosphates in the cell extracts and in the conditioned medium of HUVEC transduced with Adeno-Sphk1. S1P levels in intracellular and extracellular compartments did not show significant changes (Figs. 3 and 4A). Interestingly, significant increases in dihydro-S1P levels were detected (Fig. 4B). We also tested whether the release of S1P and dihydro-S1P was vesicle mediated, by incubating HUVEC with Brefeldin-A which inhibits ER-Golgi vesicular traffic. Brefeldin-A did not inhibit the release of S1P and dihydro-S1P (Fig. 4). In addition, PMA which is known to stimulate synthesis and release of S1P [23,30], did not affect the synthesis and release of S1P and dihydroS1P in HUVEC ectopically expressing Adeno-Sphk1 (Fig. 4A and B).
Fig. 4.
Adeno-Sphk1 transduction of HUVEC results in dihydro-S1P release. Adeno-Sphk1 (50 PFU/cell) was infected to HUVEC upon reaching 80% confluency. After 24 h cells and 2 h conditioned medium were analyzed for S1P (A) and dihydro-S1P (B). Brefeldin A (10 μg/ml) and PMA (500 nM) were incubated to cells during the period of collection of secreted S1P (2 h). Black: cell extracts; gray: conditioned medium (n = 2).
3.5. Role of ABC transporters in the release of S1P from HUVEC
Recently a role for ABC-C1 (MRP1) in mast cells [31] and ABC-A1 or ABC-A7 in platelets [32] in the secretion of S1P was reported. Incubation of glyburide (inhibitor of ABC-A1 transporter) and MK-571 (inhibitor of ABC-C1/MRP1) in HUVEC blocked S1P release into conditioned media by 46.2 and 39.8%, respectively (Fig. 5). However, MDR1 (ABC-B1) inhibitor Verapamil did not affect endogenous S1P release into conditioned media (Fig. 5). These data suggest that both ABC-A1 and ABC-C1 might play a role in the release of S1P from HUVEC in vitro.
Fig. 5.
Effect of ABC transporter inhibitors on S1P release from HUVEC. Confluent HUVEC were incubated for 2 h in absence and presence of 25 μM MK-571 (ABC-C1/MRP1 inhibitor), 500 μM glyburide (ABC-A1) or 20 μM Verapamil (ABC-B1/MDR1) to inhibit various ABC transporters and the amount of S1P released into the conditioned media was measured as described in Section 2 (n = 3).
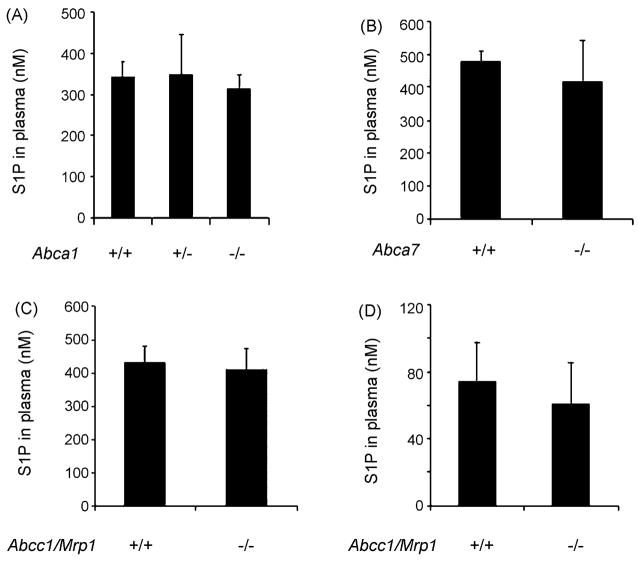
3.6. Analysis of plasma S1P levels in various ABC transporter null mice
Next we analyzed the plasma S1P levels from Abca1, Abca7 and Abcc1/Mrp1 null mice. Plasma S1P levels from Abca1, Abca7 and Abcc1/Mrp1 homozygous null mice did not show significant changes when compared to the wild type littermate controls (Fig. 6). S1P content in lymph of wild type C57BL/6 mice was 77.2 ± 36.7 pmol/100 mg wet weight and which corresponded to 18% of plasma S1P level (Fig. 6D). However there were no significant differences in lymph S1P levels between wild type and Abcc1/Mrp1 null mice (Fig. 6D). These data suggested that none of the ABC transporters analyzed was rate limiting for the maintenance of plasma S1P in vivo.
Fig. 6.
Plasma S1P levels in mutant strains of ABC transporters and S1P level in lymph of ABC-C1 mutant. S1P for plasma and lymph of indicated mice were quantified as described in the Section 2 (n = 3–4).
4. Discussion
In this report, we demonstrate the utility of IMAC beads in the enrichment of S1P released from cultured cells and from biological fluids such as plasma and lymph. In particular, Fe3+ gel IMAC has high affinity of the phosphate moiety and has been used to purify phosphopeptides in various studies focused on the analysis of cellular proteomes [24]. Our method for binding and elution of S1P is simple, quantitative and linear for at least up to 2 logs of S1P concentration (0–10 μM; Fig. 1). The advantage of this method is that trace amounts of S1P from large sample volume could be enriched efficiently. The capacity of binding of IMAC beads is ~160 nmol of S1P per 40 μl of beads, which is well above the physiological quantities of S1P found in biological systems.
By using the IMAC technique coupled with quantitative HPLC analysis, we have determined that S1P is released from variety of cultured cells. Further we have shown that endothelial cells synthesize and secrete endogenous S1P more efficiently compared to fibroblasts and colon cancer cell lines suggesting cellular specificity for S1P synthesis and secretion (Fig. 3). In addition, IMAC is also useful in quantifying both S1P and dihydro-S1P as over expression of Adeno-Sphk1 in HUVEC resulted in the significant stimulation of synthesis and secretion of only dihydro-S1P but not S1P into conditioned media (Figs. 3 and 4). Similar observations were reported in earlier studies where in the transduction of Adeno-Sphk1 in various mammalian cells [33] or over expression of Sphk1 in F9 cells [34], increased the formation of dihydro-S1P in the intracellular compartment [33,34]. However, these authors did not observe the release of (dihydro)S1P to the conditioned media. The apparent difference from our study could be attributed to differences in assay procedures to trap S1P released from cells.
The release of S1P and dihydro-S1P does not appear to require ER-Golgi vesicular traffic, as treatment of HUVEC with Brefeldin-A did not attenuate the release of both S1P and dihydro-S1P (Fig. 4). In addition treatment of PMA which is shown to stimulate S1P synthesis and its release in HEK293 cells expressing Sphk1 cDNA [23,30], did not stimulate synthesis and release of S1P and (dihydro)S1P significantly in HUVEC transformed with Adeno-Sphk1 (Fig. 4).
Recently a role for ABC-C1 (MRP1) in mast cells [31] and ABC-A1 or ABC-A7 in platelets [32] for S1P secretion has been proposed. Analysis of S1P from the conditioned media of HUVEC treated with various inhibitors of ABC transporters suggested a role for ABC-A1 and ABC-C1 in the secretion of S1P from HUVEC (Fig. 5). Since these pharmacological inhibitors lack specificity, we also analyzed the plasma S1P levels from Abca1, Abca7 and Abcc1/Mrp1 null mice and found no significant changes in S1P levels as compared to its wild type counter parts (Fig. 6). These data suggested that either these transporters are not required in the maintenance of plasma S1P. Alternatively, compensation by other transporters should also be considered.
In summary, by using IMAC we have shown that S1P can be enriched, eluted and quantified at wide range of S1P concentrations. We have also successfully demonstrated the utility of this technique to analyze sphingoid base phosphates released to conditioned media from a variety of cultured cells, and in situations such as over expression of Sphk1 and upon treatment of cells with various pharmacological reagents. We further suggest that this method could be successfully employed for the analysis of other phosphorylated lipids (for example, lysophosphatidic acid, phosphatidic acid, ceramide phosphate) in addition to S1P and dihydro-S1P.
Acknowledgments
This work was supported by NIH grants HL67330 and HL70694 to TH, and Korea Research Foundation grant (E00212) and the Korean Government (MOEHRD)(The Regional Research Universities Program/Chungbuk BIT Research-Oriented University Consortium) for YML. We express our sincere appreciation to Drs. Michael L. Fitzgerald (MGH, Boston, MA) and Gwendalyn Randolph (Mt. Sinai School of Medicine, New York) for the gift of plasma samples from mutant mice.
Abbreviations
- HPLC
high-performance liquid chromatography
- HUVEC
human umbilical vein endothelial cells
- IMAC
immobilized metal affinity chromatography
- MEEC
mouse embryonic endothelial cells
- MEF
mouse embryonic fibroblast
- S1P
sphingosine 1-phosphate
- S1Px
sphingosine 1-phosphate receptor X
References
- 1.Lee MJ, Thangada S, Claffey KP, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99(3):301–12. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 2.Lee MJ, Thangada S, Paik JH, et al. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol cell. 2001;8(3):693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- 3.English D, Kovala AT, Welch Z, et al. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothe-lial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J Hematother Stem Cell Res. 1999;8(6):627–34. doi: 10.1089/152581699319795. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez T, Estrada-Hernandez T, Paik JH, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278(47):47281–90. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 5.Dantas AP, Igarashi J, Michel T. Sphingosine 1-phosphate and control of vascular tone. Am J Physiol. 2003;284(6):H2045–52. doi: 10.1152/ajpheart.01089.2002. [DOI] [PubMed] [Google Scholar]
- 6.Sanna MG, Liao J, Jo E, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279(14):13839–48. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 7.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science (New York, NY) 2002;296(5566):346–9. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 8.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science (New York, NY) 2005;309(5741):1735–9. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 9.Kono M, Belyantseva IA, Skoura A, et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. J Biol Chem. 2007;282(14):10690–6. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- 10.Herr DR, Grillet N, Schwander M, Rivera R, Muller U, Chun J. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J Neurosci. 2007;27(6):1474–8. doi: 10.1523/JNEUROSCI.4245-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLennan AJ, Benner SJ, Andringa A, et al. The S1P2 sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hear Res. 2006;220(1–2):38–48. doi: 10.1016/j.heares.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Jolly PS, Bektas M, Olivera A, et al. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199(7):959–70. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivera A, Mizugishi K, Tikhonova A, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26(3):287–97. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Chae SS, Paik JH, Furneaux H, Hla T. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest. 2004;114(8):1082–9. doi: 10.1172/JCI22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visentin B, Vekich JA, Sibbald BJ, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9(3):225–38. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92(5):913–22. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 17.Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta. 2002;1582(1–3):132–7. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- 18.Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science (New York, NY) 2007;316(5822):295–8. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 19.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21(4):1202–9. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 20.Ito K, Anada Y, Tani M, et al. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem Biophys Res Commun. 2007;357(1):212–7. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 21.Olivera A, Kohama T, Edsall L, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147(3):545–58. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson KR, Johnson KY, Becker KP, Bielawski J, Mao C, Obeid LM. Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. J Biol Chem. 2003;278(36):34541–7. doi: 10.1074/jbc.M301741200. [DOI] [PubMed] [Google Scholar]
- 23.Pitson SM, Moretti PA, Zebol JR, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22(20):5491–500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson L, Porath J. Isolation of phosphoproteins by immobilized metal (Fe3+) affinity chromatography. Anal Biochem. 1986;154(1):250–4. doi: 10.1016/0003-2697(86)90523-3. [DOI] [PubMed] [Google Scholar]
- 25.Venkataraman K, Thangada S, Michaud J, et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397(3):461–71. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hla T, Maciag T. Cyclooxygenase gene expression is down-regulated by heparin-binding (acidic fibroblast) growth factor-1 in human endothelial cells. J Biol Chem. 1991;266(35):24059–63. [PubMed] [Google Scholar]
- 27.Min JK, Yoo HS, Lee EY, Lee WJ, Lee YM. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem. 2002;303(2):167–75. doi: 10.1006/abio.2002.5579. [DOI] [PubMed] [Google Scholar]
- 28.Ancellin N, Colmont C, Su J, et al. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277(8):6667–75. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- 29.Limaye V, Li X, Hahn C, et al. Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood. 2005;105(8):3169–77. doi: 10.1182/blood-2004-02-0452. [DOI] [PubMed] [Google Scholar]
- 30.Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA) J Biol Chem. 2002;277(38):35257–62. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- 31.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103(44):16394–9. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi N, Nishi T, Hirata T, et al. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47(3):614–21. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Berdyshev EV, Gorshkova IA, Usatyuk P, et al. De novo biosynthesis of dihydrosphingosine-1-phosphate by sphingosine kinase 1 in mammalian cells. Cell Signal. 2006;18(10):1779–92. doi: 10.1016/j.cellsig.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Kariya Y, Kihara A, Ikeda M, et al. Products by the sphingosine kinase/sphingosine 1-phosphate (S1P) lyase pathway but not S1P stimulate mitogenesis. Genes Cells. 2005;10(6):605–15. doi: 10.1111/j.1365-2443.2005.00862.x. [DOI] [PubMed] [Google Scholar]