Abstract
Hypoxia-inducible factor-1 (HIF-1) has been suggested to play a major role in tumor radioresistance. However, the mechanisms through which irradiation regulates HIF-1α expression remain unclear. The purpose of this study was to investigate the mechanisms that mediate HIF-1 activation and thus radioresistance. Here we show that irradiation induces survival and angiogenic activity in a subset of radioresistant lung cancer cell lines by elevating HIF-1α protein expression. Radiation induced HIF-1α protein expression mainly through two distinct pathways, including an increase in de novo protein synthesis via activation of PI3K/Akt/mTOR and stabilization of HIF-1α protein via augmenting the interaction between heat shock protein 90 (Hsp90) and HIF-1α protein. While the PI3K/Akt/mTOR pathway was activated by irradiation in all the lung cancer cells examined, the HSP90-HIF-1α interaction was enhanced in the resistant cells only. Inhibition of Hsp90 function by 17-AAG or deguelin, a novel natural inhibitor of HSP90, suppressed increases in HIF-1α/Hsp90 interaction and HIF-1α expression in radioresistant cells. Furthermore, combined treatment of radiation with deguelin significantly decreased the survival and angiogenic potential of radioresistant lung cancer cells in vitro. We finally determined in vivo that systemic administration of deguelin resulted in profound inhibition of tumor growth and angiogenesis when combined with radiation. These results provide a strong rationale to target Hsp90 as a means to block radiation-induced HIF-1α and thus to circumvent radioresistance in lung cancer cells.
Keywords: HIF-1α, Hsp90, Radiation, resistance, deguelin
Introduction
Researchers have made extensive efforts to understand the mechanisms underlying radio-resistance and to discover novel molecular targets whose modulation could enhance radio-therapeutic response (1). Radiation increases hypoxia-inducible factor (HIF)-1 activity, which has been suggested to be dominant governor of tumor response to irradiation through multiple mechanisms (2), causing radioprotection of the tumor vasculature (3–5). Blockade of HIF-1 significantly increased tumor radiosensitivity by enhancing vascular destruction (2). Overexpression of HIF-1α in tumor biopsy samples has been associated with a poor response to radiotherapy in multiple cancer types (6). Moreover, cancer cells without functional HIF-1α (7, 8) and tumor xenografts of HIF-1α–null cells are more radiosensitive than their HIF-1α expressing counterparts (7).
HIF-1 is a heterodimer composed of the HIF-1α and HIF-1β subunits (9). While HIF-1β is constitutively expressed, HIF-1α expression is induced by hypoxia, oxidative stress, and oncogenes via multiple oxygen-dependent and -independent mechanisms (10–12). The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is involved in HIF-1α protein expression (12–15). HIF-1α protein stability is affected by its interaction with heat shock protein 90 (Hsp90), an ATPase-directed molecular chaperone that controls folding and stabilization of several client proteins (16–18). HIF-1 binds to hypoxia response elements in the nucleus, stimulating the expression of genes involved in tumor metabolism, growth, and angiogenesis, including vascular endothelial growth factor (VEGF), most glycolytic enzymes, and insulin-like growth factor 2 (9, 12, 19). VEGF further induces cells to release bioactive basic fibroblast growth factor (20), which is also pro-angiogenic. A recent study elegantly demonstrated that radiation leads to HIF-1 activation and upregulating proangiogenic factor VEGF by modulating reactive oxygen species (ROS) (2). HIF-1 impacts radioresistance by modulating the survival and angiogenic activities of lung cancer cells through the activation of anaerobic metabolism (21). These findings suggest that HIF-1 protects tumors from radiation damage directly and indirectly, providing a strong rationale to target HIF-1 to improve tumor response to radiotherapy. However, other findings—for example, HIF-1 radiosensitizes tumors by promoting ATP metabolism, cell proliferation, and p53 activation (22)—indicate that its role in tumor radiosensitivity is complex. Further, very little is known about how radiation induces HIF-1 activation.
In the studies described within, we sought to determine whether HIF-1α contributes to radioresistance in lung cancer cells, to investigate whether strategies for blocking HIF-1α expression have an overall positive or negative impact on radiotherapy in a preclinical model of lung cancer, and to identify the mechanisms involved in radiation-induced HIF-1 activation in lung cancer cells.
Materials and Methods
Cells, Animals, and Materials
Human non–small-cell lung cancer cell (NSCLC) lines H1299, H226B, H226Br, and H460, human small-cell lung cancer cell (SCLC) line H182 (American Type Culture Collection, Manassas, VA), and human umbilical vein endothelial cells (HUVECs; Cambrex BioScience, Walkersville, MD) were maintained as described elsewhere (16, 23). Six-week-old female athymic nude mice were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Deguelin (Gaia Chemical Corporation, Gaylordsville, CT) was prepared as a 20-mM stock solution in dimethyl sulfoxide and stored at −20°C until it was needed. Bevacizumab (Avastin), a monoclonal anti-VEGF antibody, was purchased from Genentech (South San Francisco, CA).
Irradiation and Clonogenic Assay
For the clonogenic assay, the cells were counted and known numbers were reseeded in three wells of six-well culture dishes for each dose of radiation or described condition after irradiation under a Cs137 gammạ radiation source. After being incubated for 7 to 10 days, colonies were fixed with cold methanol, stained with hematoxylin, and counted. The percentage of colonies for each radiation dose test condition was calculated by dividing the number of cultured test colonies by the number of the appropriate non-irradiated control colonies. Five hours after the indicated dose of radiation, the cells were treated with LY294002 (30 µM), cycloheximide (0.4 nM), PD98059 (1µM), N-acetyl-cysteine (NAC) (10 mM), rapamycin (5 nM), or 17AAG (1, 2.5, or 5 µM) for 2 hours. The lung cancer cell lines growing in log phase were transfected with control-scrambled (Si-Scr, Dharmacon, CO) or HIF-1α small interfering RNA (Si-Hif-1) (GGGUAAAGAACAAAACACA-dTdT, Dharmacon, CO) and irradiated after 36 hours of the transfection and then subjected to the clonogenic assay or immunoblotting. The results were normalized to the scrambled RNA transfected control.
The surviving fraction was calculated as the ratio of the plating efficiency of the treated cells to that of control cells. The surviving fraction for combined treatment was normalized by that for HIF-siRNA or deguelin treatment alone. The dose enhancement factor (DEF) was calculated as the dose (Gy) of radiation that yielded a surviving fraction of 0.5 for control (Si-Scr treated or vehicle-treated) divided by that for the treated group (Si-HIF or deguelin). We interpreted DEF 1 as an additive and more than 1 as supra-additive effect.
Conditioned Medium (CM) and HUVEC Proliferation Assays
The cancer cells were cultured in growth medium with or without 100 nM deguelin for 12 hours and then irradiated or left un-irradiated. The cells were washed twice and then incubated with serum-free medium (4 ml) for another 24 hours. The resulting CM was collected and concentrated as described elsewhere (16). HUVECs were added with the CM. When indicated, HUVECs were treated with CM in the presence of bevacizumab (30 ug/ml) or irradiated at 6 Gy before being cultured in CM.
Reverse Transcriptase-Polymerase Chain Reaction Assay
Total RNA was prepared from whole-cell lysates, and reverse transcriptase-polymerase chain reactions were performed as described elsewhere (16). The primer sequences were: 5′-CCATGAACTTTCTGCTGTCTT-3′ (sense) and 5′-ATCGCATCAGGGCACACAG-3′ (antisense) for VEGF and 5′-GACTGGCAGGGGGAGAAACAA-3′ (sense) and 5′-ATGGCACAGTGGATGGGACAA-3′ (antisense) for aFGF and 5′-GGTGAAGGTCGGTGTGAACGGATTT-3′ (sense) and 5′-AATGCCAAAGTTGTCATGGATGACC-3′ (antisense) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Immunoprecipitation and Coimmunoprecipitation Assays
Whole-cell lysates were prepared as previously described (18). After clearing, the supernatants were transferred to fresh tubes. Total protein was incubated with 1 µg of anti-HIF-1α antibody (Santa Cruz Biotechnology, CA) or anti-Hsp90α antibody (Stressgene, Canada) at 4°C for overnight and then added to 50% protein G-Sepharose beads (Amersham Pharmacia Biotech AB, Uppsala, Sweden). The mixture was incubated at 4°C for 1 hour, washed three times with the lysis buffer and twice with phosphate-buffered saline, boiled in SDS reducing gel loading buffer, and separated using SDS-PAGE. HIF-1α and Hsp90α levels were assessed using immunoblot analysis with the corresponding antibodies.
In Vivo Tumor Growth Delay Assay, Immunohistochemical analysis, Immunoblot Assays
Described in supplementary data.
Statistical Analysis
Means ± standard error of the mean (SEM) from at least triplicate assays were calculated using Microsoft Excel 2003 (Microsoft Corporation, Redmond, WA). The two-tailed Student’s t-test was used to calculate P values. P values of < 0.05 were considered statistically significant.
Results
Differential Survival and Angiogenic Responses of Lung Cancer Cells after Irradiation
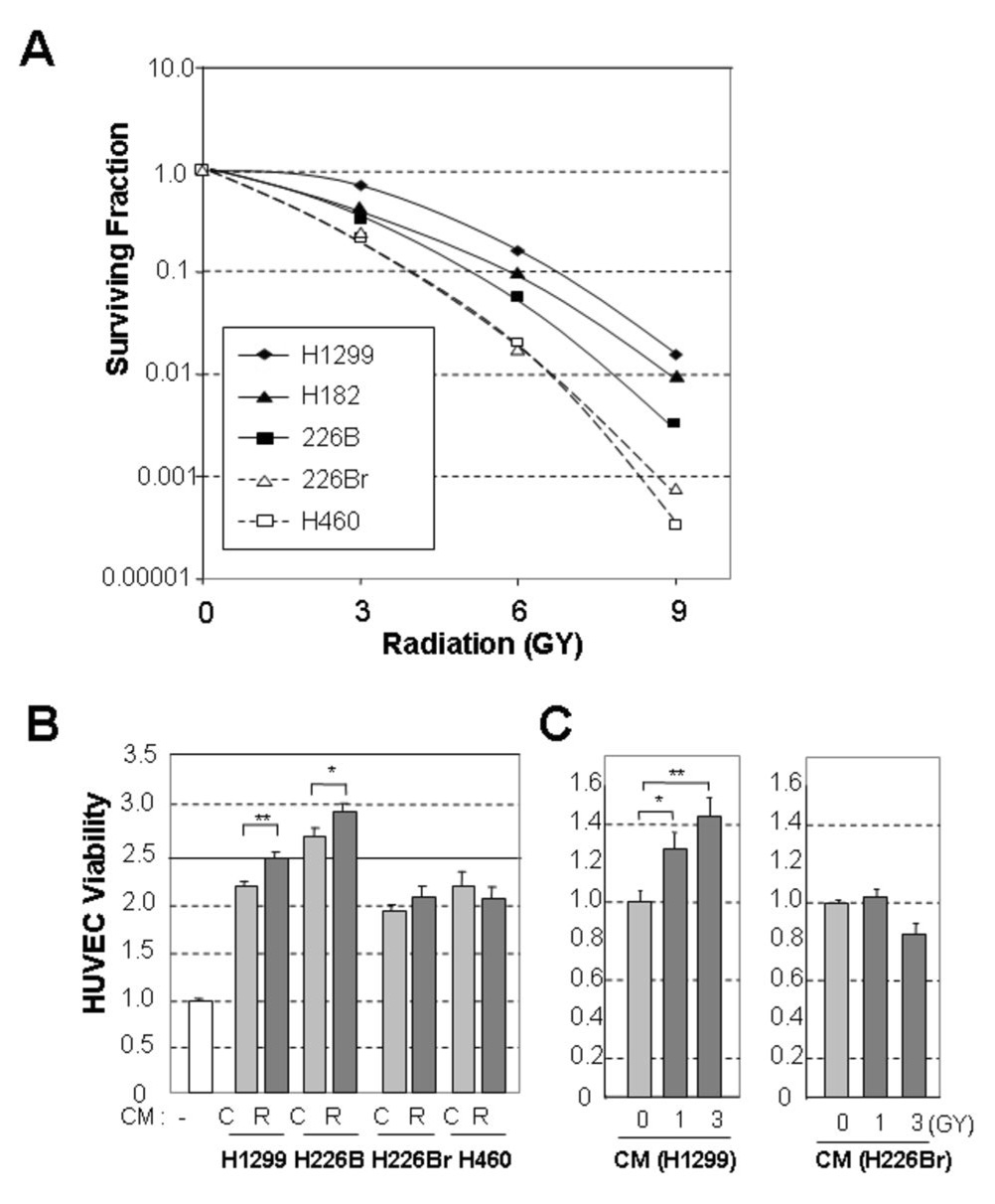
We assessed radiosensitivity of a subset of NSCLC cell lines (H1299, H460, H226B, and H226Br) and a SCLC cell line (H182) after treating them with various doses of radiation. The clonogenic cell-survival assay revealed that H1299 cells were the most radioresistant and that H226Br and H460 cells were more radiosensitive than the other cell lines (Fig. 1, A). A previous study claimed a critical role of p53 in radiosensitivity (24). Among the cells we examined, however, H1299, H226B andH226Br have mutant p53, while H460 cells have wild-type p53 (25), suggesting that other factors in addition to p53 governed the radiosensitivity of these cells.
Fig. 1. Lung cancer cells have different degrees of radiosensitivity.
A, Clonogenic survival curve demonstrating the relative radioresistance of five lung cancer cell lines (H1299, H182, H226B, H460, and H226Br). H1299 cells were most radioresistant, H182 and H226B cells were moderately radioresistant, and H460 and H226Br cells were relatively radiosensitive. B, Radiation-induced angiogenic activity of radioresistant and radiosensitive lung cancer cells. Significantly increased human umbilical vein endothelial cell (HUVEC) viability by the conditioned medium (CM) from radioresistant cancer cells. C, Irradiated HUVECs which mimics tumor environment were maximally viable when cultured in the radiated, H1299-derived CM but not in H226Br derived CM. The results are expressed as the mean ± standard error of the mean (SEM) of four replicate experiments. (*, P<0.05; **, P<0.01). Each control group (no treatment of CM, basal medium, and RPMI only) was set to 1.0.
We next investigated whether the lung cancer cells’ response to radiation was associated with their angiogenic activity by testing the effects of the CM from the irradiated NSCLC cells on HUVEC viability. Compared to the endothelial basal medium, the CM from non-irradiated H1299, H226B, H226Br, and H460 cells increased the viability of HUVECs, indicating the presence of cancer cell-derived growth factors for HUVECs (Fig. 1, B). The CM from irradiated H1299 and H226B cells further significantly enhanced viability of HUVECs, while the CM from irradiated H226Br and H460 cells provided no significant additional benefit. The CM from irradiated H1299 cells increased viability of HUVECs even more when the HUVECs had been pre-irradiated (Fig. 1, C), while the CM from H226B did not. In subsequent experiments, therefore, we divided the cancer cells into two groups: resistant (H1299 and H226B, and H182) and sensitive (H226Br and H460) cells.
Radiation-Induced Expression of HIF-1α Radioresistance in Lung Cancer Cells
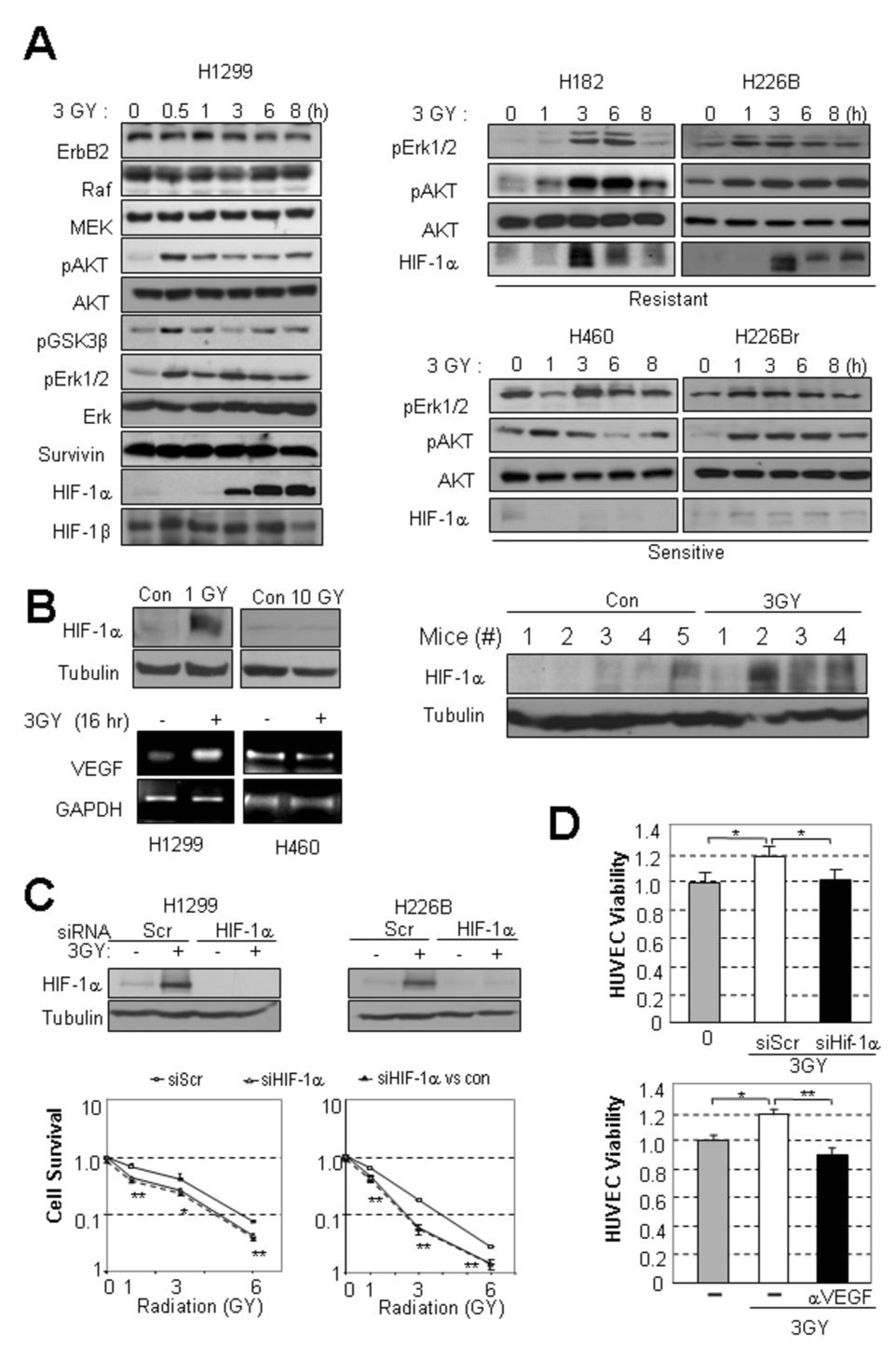
To investigate the mechanisms responsible for radiosensitizing lung cancer cells, we tested the effects of radiation on the expression of ErbB2, Raf, MEK, phosphorylated Akt (pAkt), phosphorylated glycogen synthase kinase (GSK)-3β, phosphorylated Erk1/2 (pErk1/2), survivin, and HIF-1α, all of which are involved in cell proliferation and survival against diverse stimuli. The expression of pAkt, pGSK-3β, pErk1/2 and HIF-1α was induced in H1299 cells 30 minutes to 3 hours after administering a single 3-Gy dose of radiation (Fig. 2, A left). Expression of ErbB2, Raf, MEK, Akt, Erk, Surviviṇ, and HIF-1β remained unchanged. The other radioresistant H182 and H226B cells also showed increased expression of HIF-1α, pAkt, and pErk1/2 following irradiation (Fig. 2, A, right upper). Irradiation induced increases in pAkt and pErk1/2 expression with no detectable change in HIF-1α expression in radiosensitive H460 and H226Br cells (Fig. 2, A, right lower). HIF-1α expression was induced by radiation as low as 1 Gy dose. However, 10 Gy, a dose that induced more than 90% of decrease in surviving fraction in the clonogenic assay, did not affect HIF-1α expression (Fig. 2, B, left upper). RT-PCR analysis revealed that the radiation-induced HIF-1α expression was well correlated with increase in VEGF level (Fig. 2, B, left lower). To test whether radiation increases HIF-1α expression in vivo, we analyzed irradiated H1299 xenograft tumors established in nude mice and found that HIF-1α expression markedly increased in the tumor tissues (Fig. 2, B, right).
Fig. 2. HIF-1 was activated and required for radioresistance in radioresistant cell lines.
A, Correlation between the radioresistance and induction of HIF-1α. HIF-1α expression increased in response to irradiation in H1299 cells, the most radioresistant cell line examined (left). ErbB2, Raf, MEK, HIF-1β, pAkt, Akt, pGSK3β, pErk1/2, Erk, and survivin expression are also shown. While HIF-1α expression increased in radioresistant cell lines H182 and H226B, it did not increase in radiosensitive cell lines H460 and H226Br ; pAkt and pErk1/2 were activated regardless of radioresistance (right). B, HIF-1α expression in H1299 cells after irradiation at 1 Gy and 10 Gy. VEGF mRNA expression in irradiated H1299 and H460 cells. In vivo induction of HIF-1α by irradiation from H1299 xenograft tumors after irradiation (n=4, 3 GY) or no treatment (n=5). C, HIF-1α expression was required for the survival of radioresistant lung cancer cells after irradiation. Compared with transfection of scrambled siRNA (siScr), transfection of HIF-1α siRNA (siHIF-1α) significantly sensitized H1299 and H226B cells to radiation. The dotted line shows HIF-1α expression before correction relative to the effect of siHIF-1α alone, and the line with open squares shows the normalized values. The dose enhancement factors (DEF) were 2.55 and 1.57 in the H1299 and H226B cells, respectively. D, HIF-1α expression was required for HUVEC survival. siHIF-1α transfection disrupted the radiation-enhanced viability of HUVECs cultured in H1299 CM . The radiated CM enhanced HUVEC viability was inhibited by anti-VEGF blocking antibody. (*, P<0.05; **, P<0.01).
To determine whether radiation-induced HIF-1α expression caused radioresistance in lung cancer cell lines, we assessed the viability and angiogenic activities of the radioresistant H1299 and H226B cell lines, in which HIF-1α expression had been silenced by transfection of HIF-1α siRNA (Fig. 2, C, left). Both H1299 and H226B cells transfected with HIF-1α siRNA showed significantly decreased survival after irradiation compared to cells transfected with control scrambled siRNA (siScr) (Fig. 2, C) ; the DEFs at the survival rate 0.5 for the H1299 and H226B cells transfected by the HIF-1α siRNA were 2.55 and 1.57, respectively, suggesting a supra-additive effect of the combination. In contrast, HIF-1α siRNA transfection did not affect the radiation-induced effects on H460 cells (DEF, 1.0, Supplemental Figure 1, A). Mirroring this result, HUVECs incubated with the CM from H1299 cells that had been transfected with HIF-1α siRNA and then irradiated also showed significantly decreased viability compared to those incubated with the CM from the siSCR-transfected and irradiated H1299 cells (Fig. 2, D). Addition of anti-VEGF monoclonal antibody, bevacizumab, completely abolished the CM’s enhancement of HUVEC viability (Fig. 2, D,), indicating that the radiation-induced HIF-1α increased VEGF production and thereby conferred angiogenesis-stimulating activity to the lung cancer cells. These results suggest that the radiosensitivity of lung cancer cells varies, at least in part as a function of post–radiotherapy-induced HIF-1α expression, which increases the survival and angiogenic potential of lung cancer cells.
De Novo Protein Synthesis through the PI3K/Akt/mTOR Pathway and Increased Stabilization by Hsp90 Chaperone Function Required for Radiation-induced HIF-1α Expression
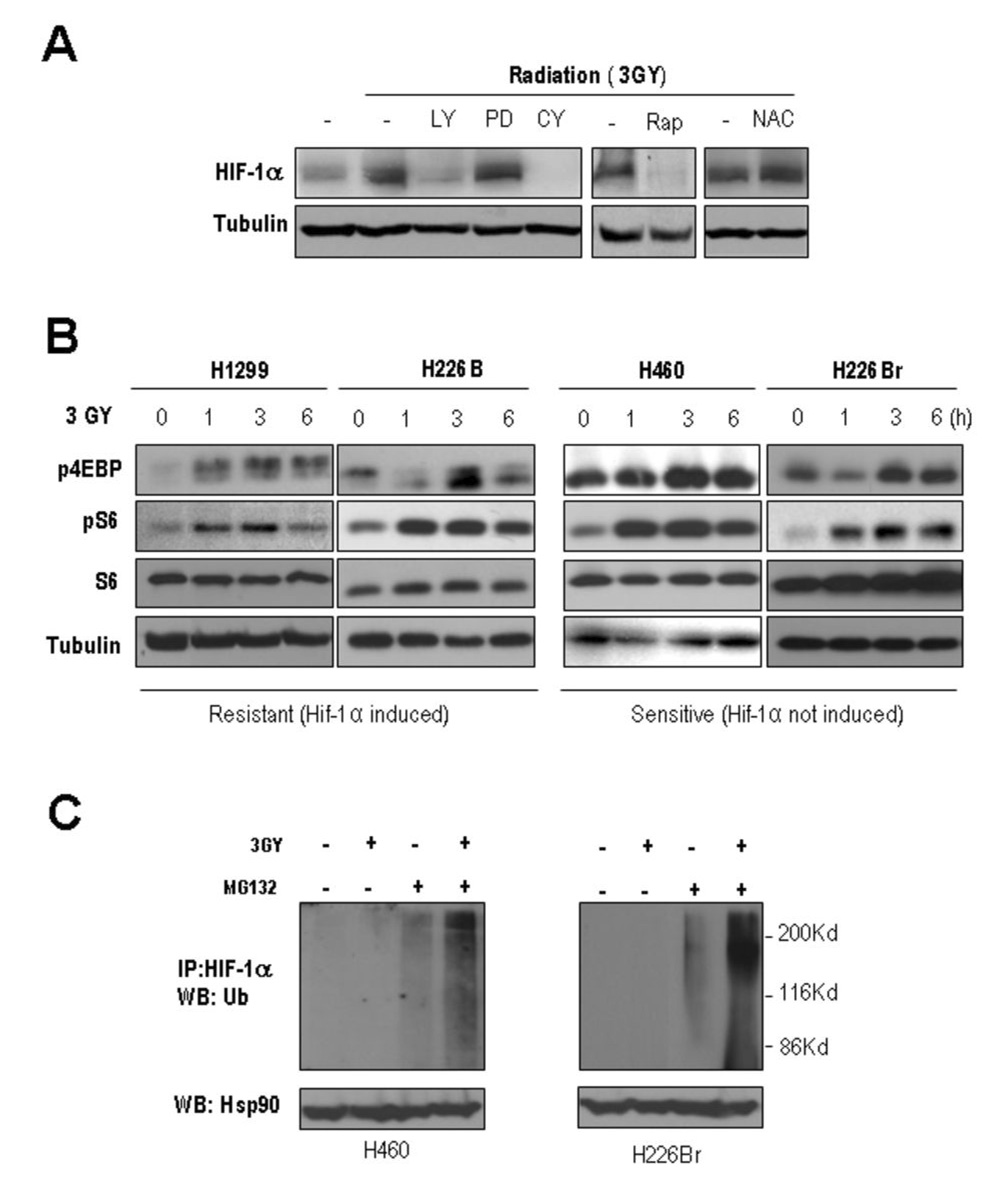
We investigated the mechanism that mediated the radiation-induced increase in HIF-1α expression in lung cancer cells. PI3K and Erk1/2 have been shown to activate mTOR, which does a key role in protein synthesis (26). Having linked radiation-induced PI3K/Akt and Erk1/2 activation (Fig. 2, A) with an increase in HIF-1α expression, we determined the effects of the pharmacologic inhibitors of PI3K (LY294002), mitogen-activated protein kinase (PD098059), peptidyl transferase of 60S ribosome (cycloheximide), and mTOR (rapamycin) on HIF-1α expression in irradiated H1299 cells. Treating irradiated H1299 cells with LY294002, cycloheximide, and rapamycin effectively inhibited the radiation-induced HIF-1α expression, while the HIF-1α protein level remained elevated after the PD98059 treatment (Fig. 3, A), suggesting that radiation induced HIF-1α expression is, at least in part, through PI3K/Akt/mTOR-mediated de novo protein synthesis. We then correlated the radiation-induced mTOR activity with the radiosensitivity of lung cancer cells by assessing radiation-mediated changes in the levels of pS6 and p4EBP, surrogate indicators of mTOR activity, in the radioresistant and radiosensitive cell lines (Fig. 3, B). All the cells showed clear increases in pS6 and p4EBP following irradiation, with no detectable changes in unphosphorylated S6, indicating the presence of mechanisms other than the translational regulation for mediating the increased level of HIF-1α protein in the radioresistant lung cancer cells.
Fig. 3. Induction of HIF-1α expression required protein translation machinery and modulation of the protein stability.
A, Pharmacological inhibition of signaling for HIF-1α synthesis. Five hours after their irradiation , H1299 cells were treated with the inhibitors LY294002 (LY), PD98059 (PD), cyclohexamide (CY), N-acetyl cysteine (NAC), and rapamycin (Rap). After 2 hours, HIF-1α expression was examined. B, Activation of protein synthesis signaling by irradiation. pS6 and p4EBP showed radiation-induced increases in all the cell lines examined. C, Proteosomal degradation suppressed the induction of HIF-1α in the sensitive cell line. H460 and H226Br cells were treated with a proteasome inhibitor MG132 right after irradiation. The shifted bands, thus ubiquitinated, had increased intensity in the irradiated cells.
Since HIF-1α protein is degraded mainly through the ubiquitin-proteasome pathway, we assessed whether a proteasome inhibitor, MG132, affects the HIF-1α protein level in radiosensitive H460 and H226Br cells. Treatment with MG132 (10 µM) resulted in the formation of polyubiquitinated, higher-molecular-weight forms of HIF-1α that were further increased by irradiation in normoxic H460 and H226Br cells (Fig. 3, C). We reasoned that the mechanisms involved in proteasome-mediated degradation of HIF-1α protein might prevent HIF-1α protein levels from increasing in radiosensitive cells. Radiation has been known to modulate ROS (2), which is implicated in HIF-1α stabilization (27). However, treatment with the ROS inhibitor NAC did not affect the HIF-1α protein level in the irradiated H1299 cells (Fig. 3, A), indicating that radiation induces HIF-1α protein stabilization through the ROS-independent mechanisms.
Differential Modulation of HSP90-HIF-α Interaction Regulates the Activation of HIF-1α Expression by Radiation
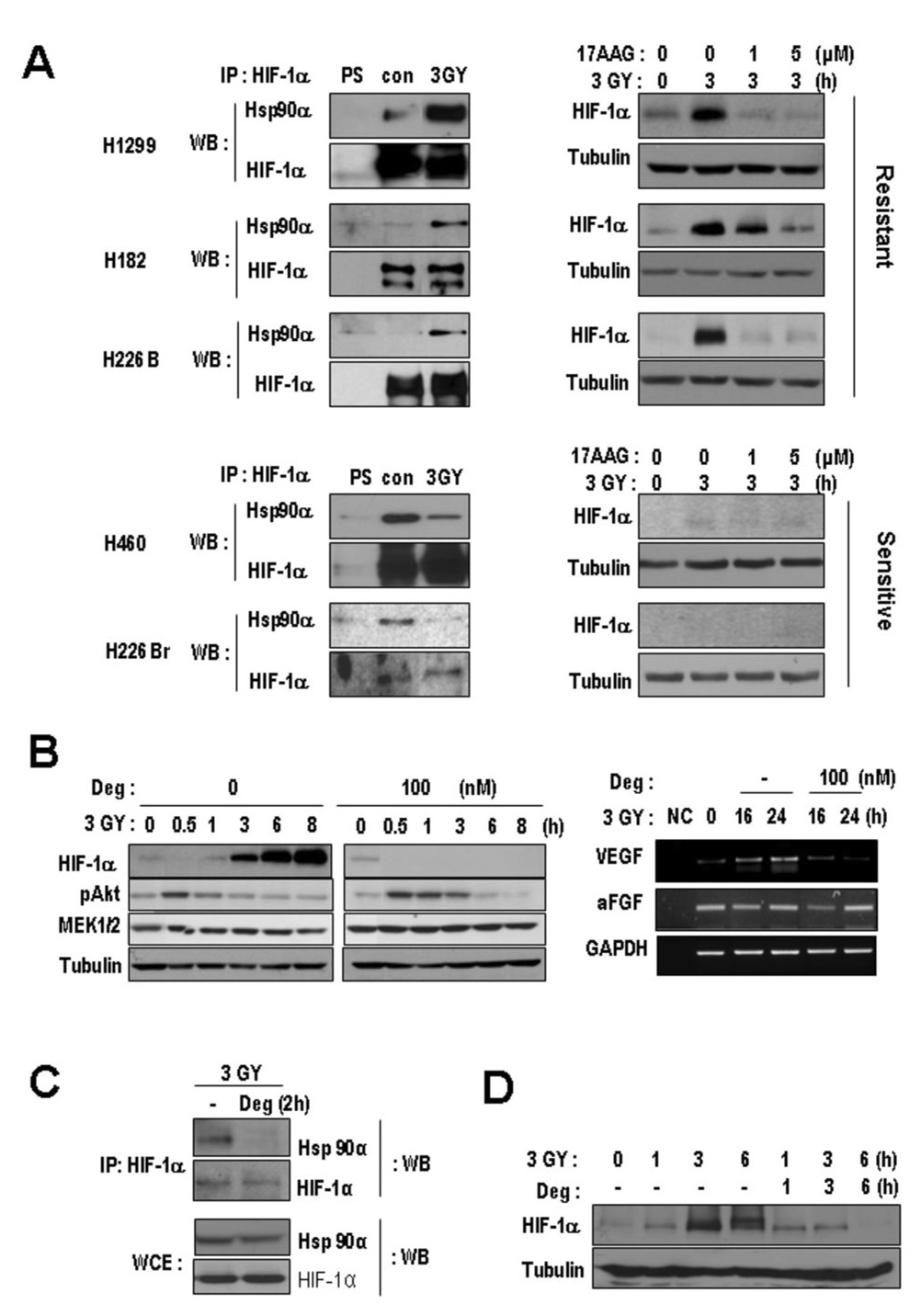
We assessed whether Hsp90 regulates HIF-1α expression after irradiation. Because irradiation did not change the total level of Hsp90 protein in H1299 cells (data not shown), we examined the effects of irradiation on the interaction between HIF-1α and Hsp90 by coimmunoprecipitation assays. Surprisingly, the all three radioresistant lines showed an increased interaction between these two proteins after irradiation, whereas the radiosensitive lines showed a markedly decreased interaction (Fig. 4, A, left). Furthermore, when Hsp90 function was blocked with 17-AAG (28), HIF-1α protein expression was significantly decreased in irradiated radioresistant cells (Fig. 4, A, right upper). Neither radiation nor 17-AAG affected HIF-1α protein expression significantly in the radiosensitive cells (Fig. 4, A, right lower). We further confirmed that deguelin, a natural Hsp90 inhibitor (18), suppressed radiation-induced HIF-1α expression. In our previous report, we found that deguelin may be more potent than 17-AAG (18), at least in vitro. When irradiated H1299 cells were pre-treated with deguelin for 12 hours, induction of HIF-1α protein (Fig. 4, B, left) and VEGF mRNA (Fig. 4, B, right) was suppressed, while aFGF and GAPDH mRNA remained unchanged.
Fig. 4. The modulation of interaction between HIF-1α and Hsp90α after irradiation associated with radioresistance and HIF-1induction.
A, The interaction between HIF-1α and Hsp90α was examined using coimmunoprecipitation (Co-IP) (left); cell lysates from the indicated cells irradiated at 3 Gy and cell lysates from non-irradiated cells (con) were harvested at 3 hours after irradiation. The anti-HIF-1α antibody immunoprecipitates were subjected to Western blotting (WB) with anti-Hsp90. Hsp90/HIF-1α interaction increased after irradiation in all radioresistant cell lines, while it decreased in all radiosensitive cell lines. PS, preimmune serum. Hsp90 function is essential for radiation-induced HIF-1α induction (right). Cells were treated with 17- AAG with 3 Gy radiation or non-irradiated (con), and the protein level of HIF-1α was examined. 17-AAG downregulated HIF-1α expression after radiation in the radioresistant cells, while it didn’t in the sensitive cells. B, Pretreatment of Deguelin (Deg) suppressed the expression of HIF-1α but not the activated pAKT and MEK1/2 (left). Deguelin suppressed radiation-induced expression of VEGF (right). Reverse transcriptase-polymerase chain reaction analysis of VEGF mRNA was performed using total RNA prepared from irradiated H1299 cells treated with or without deguelin; VEGF mRNA was decreased, while aFGF and GAPDH mRNA did not vary. C, The Hsp90α/HIF-1α interaction was abolished by deguelin treatment (100 nM) in irradiated H1299 cells within 2 hours. D, Three- and six-hour treatments with deguelin blocked HIF-1α expression.
We then tested whether deguelin could abolish radiation-induced interaction between HIF-1α and Hsp90. Indeed, deguelin effectively disrupted the radiation-induced interaction between Hsp90α and HIF-1α (Fig. 4, C) and suppressed HIF-1α protein expression (Fig. 4, D) in H1299 cells. Together, these data suggest that Hsp90 function was essential to the radiation-mediated increase in HIF-1 HIF-1α protein levels in radioresistant cells.
In Vitro and In Vivo Suppression of Radioresistance of Lung Cancer Cells by Blockade of Hsp90 Function with Deguelin
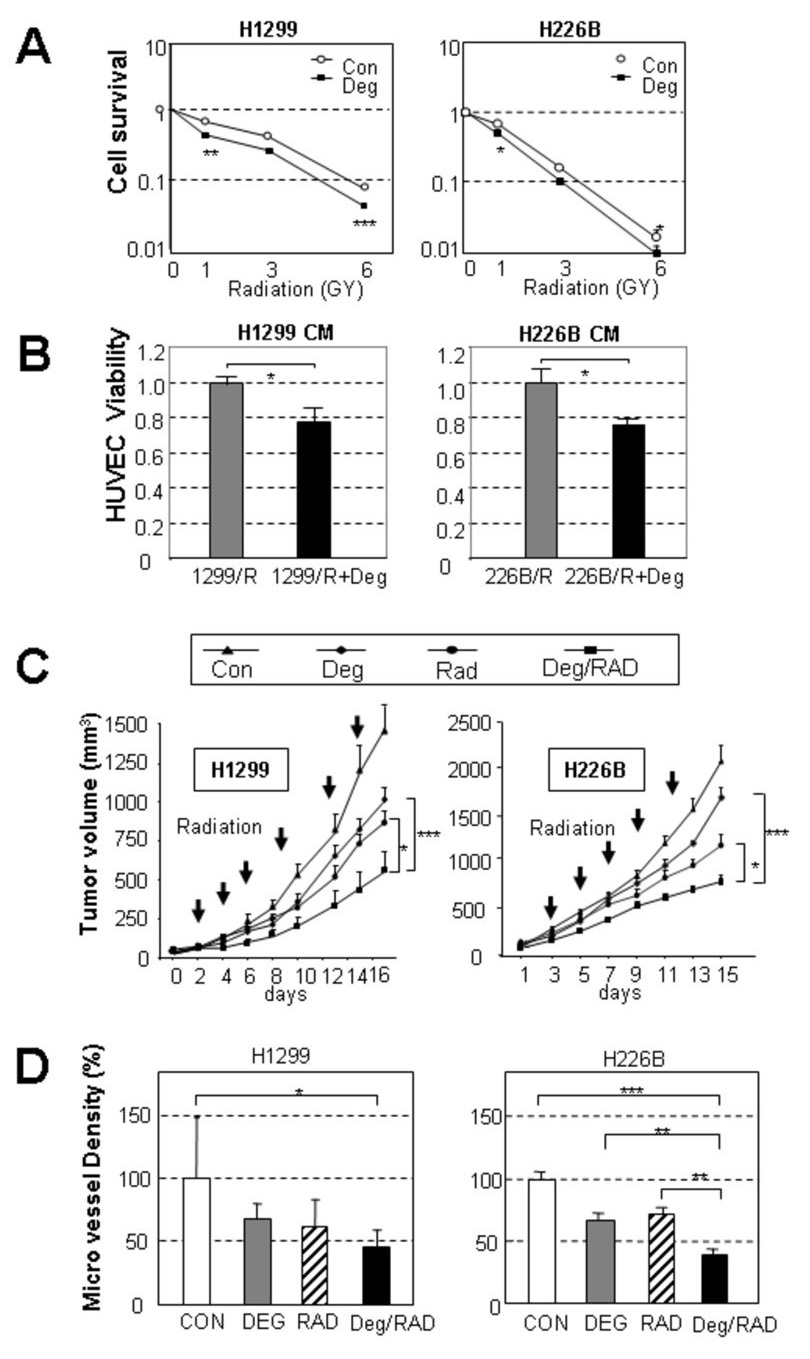
We next sought to determine whether a decrease in HIF-1α expression via suppression of Hsp90 function reduces the survival and angiogenic potential of the radioresistant cells. The clonogenic assay revealed that pretreatment with deguelin (100 nM) rendered H1299 and H226B cells significantly more sensitive to radiation than untreated cells (Fig. 5, A). DEFs for H1299 and H226B were 2.17 and 1.48, respectively, demonstrating the supra-additive effect of deguelin and radiation treatments. Moreover, HUVECs showed significantly lower viability when incubated with the CM from deguelin-treated H1299 or H226B cells compared to those incubated with the CM from untreated cells (Fig. 5, B). When we performed the same assays with a sensitive cell line, H226Br, deguelin treatment showed no enhanced effects (Supplemental Fig. 1, B and C). Cells from another radioresistant irradiated line (H182) showed that deguelin had a supra-additive effect (DEF 1.82) (Supplementary Fig. 1, D).
Fig. 5. Deguelin sensitized radioresistant cancer cells and suppressed their angiogenic potential in vitro and in vivo.
A, Clonogenic cell survival assay of H1299 and H226B cells after deguelin pre-treatment and irradiation. Because deguelin itself changed the plating efficiency, the survival fraction is shown after normalization to the plating efficiency with deguelin treatment alone. Deguelin significantly sensitized H1299 (DEF, 2.27) and H226B (DEF, 1.48) against irradiation. Results are expressed as the mean ± SEM of three independent experiments. B, Angiogenic activity of the CM from H1299 and H226B cells, which had been left untreated or treated with deguelin. The CM from the cells pretreated with deguelin significantly decreased angiogenic activity. The results are expressed as the mean ± SEM of six replicate experiments. C, H1299 and H226B (5 × 106) xenograft tumor cells were sensitized by combined deguelin and radiation treatment. Tumors in the combined treatment group were significantly smaller than those in deguelin or radiation (Rad) alone group. D, Microvessel density in the xenograft tumors that were harvested from the mice in C. The CD31-positive cells in a microscopic field (2500 µm2) were counted, and their density relative to the control is shown. Only the combined treatment showed a significant decrease from the control in H1299 xenograft while single treatment mildly decreased (left). Single treatments showed a decrease compared to control in H226B xenograft tumors. The combined treatment significantly decreased the values compared to each single treatment (right). (*, P<0.05; **, P<0.01; ***, P< 0.001).
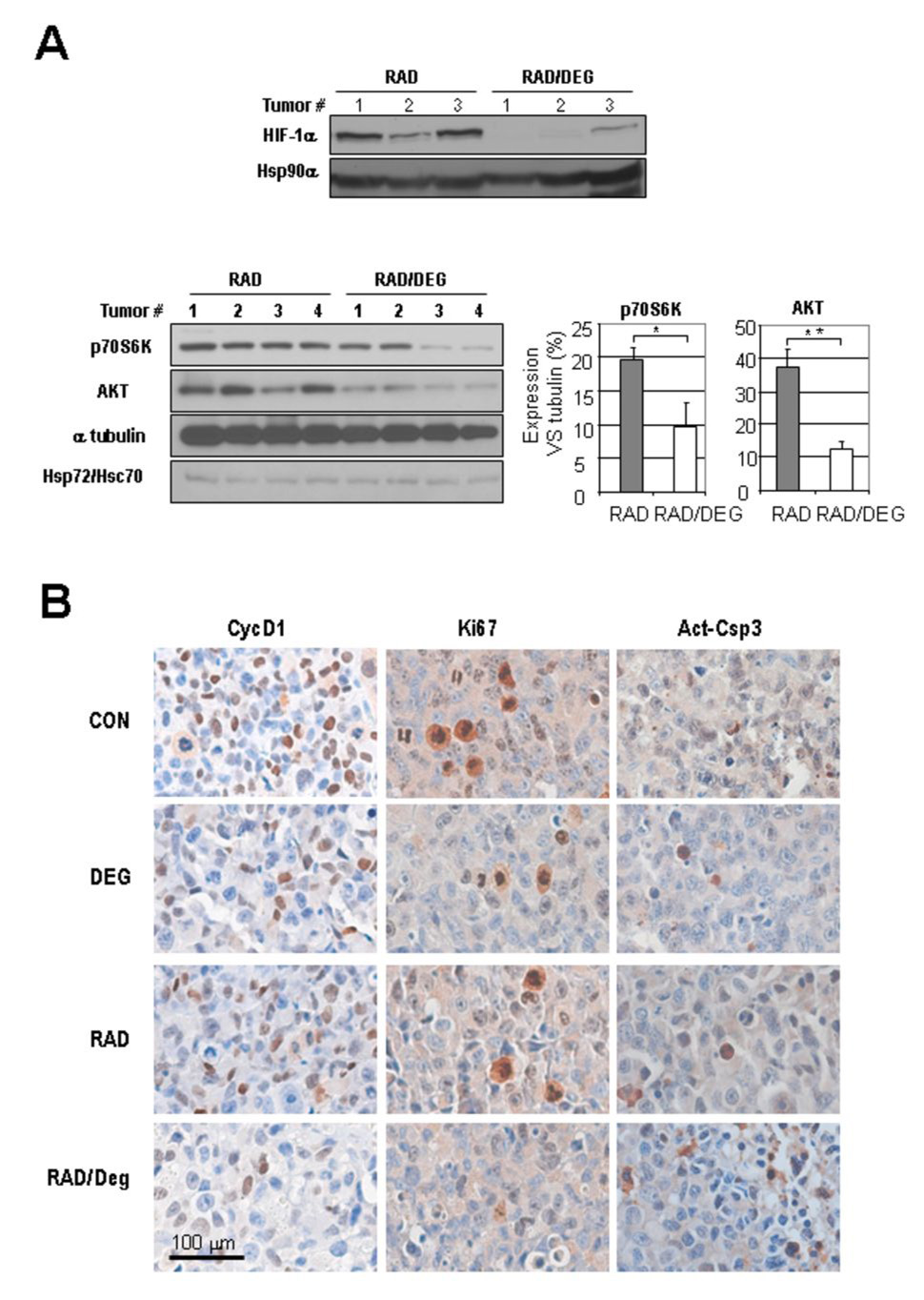
To determine whether inhibition of Hsp90 function effectively induce radiosensitivity in NSCLC in vivo, we treated athymic nude mice bearing H1299 or H226B xenografts with radiation, deguelin, or both. The treatments of deguelin or radiation alone mildly decreased the tumor growth. The combined treatment with radiation and deguelin suppressed the tumor growth significantly greater than that conferred by the single treatments (Fig. 5, C). Furthermore, compared to untreated control and/or single agent treatments, the combination modality showed significantly decreased microvessel density in the tumor, as detected by the CD31 staining in the H1299 xenograft tumors (Fig. 5, D). We examined the effect of deguelin on radiation-induced HIF-1α and Akt in tumor environment. We found that 3 day treatment with deguelin and radiation (1.25 Gy, twice) leads to the decreases in the expressions of HIF-1α, p70S6K, and AKT (Fig. 6 A) with no noticeable change in Hsp72/Hsc70, which was reported to be increased by blocking of HSP90 function.(29), as well as HSP90α. We also performed IHC assay to examine the effects of the combined treatment on cell cycle and apoptosis (Fig. 6B). Cyclin D1 and Ki-67, two cell cycle markers decreased by single treatment of deguelin or radiation, showed a further decrease by the combined treatments. In addition, apoptotic cells were more frequently observed after the combined treatment compared to single agent treatments, as determined by the IHC analysis on active caspase 3 (Act-Csp3). Quantification of the staining suggested an additive rather than synergistic effect of the combined treatment compared to the single treatments (Supplemental Fig. 2). Overall, these findings indicate that deguelin treatment improves tumor response to radiation by regulating molecules involved in cell cycle and apoptosis.
Fig. 6. In vivo effect of deguelin in radiated H1299 Xenograft tumors.
A, Expression of HIF-1α expression (upper panel), known HIF-1 target genes, Hsp72/Hsc70 (lower panel) in radiated Xenograft tumors by deguelin. The average of relative density against tubulin is shown in graph with standard error of means. (*, P<0.05 ; **, P<0.01). B, Changes in cyclin D1 and Ki-67 and the apoptosis marker active caspase-3. Consistently less staining of cell cycle markers and more apoptosis were detected in cells receiving the combined treatment than in those receiving the single treatments.
Discussion
The studies reported herein demonstrate, for the first time, that radiation increases HIF-1α protein levels in a subset of radioresistant lung cancer cells through collaborative actions of two mechanisms: activation of PI3K/Akt/mTOR, which leads to stimulation of de novo synthesis of HIF-1α, and stimulation of Hsp90 function, which leads to stabilization of HIF-1α protein. Therefore, agents that block PI3K/Akt/mTOR and Hsp90 function are likely candidates to decrease radioresistance by suppressing HIF-1α and VEGF expression and thus inhibiting survival and angiogenic potential of lung cancer cells.
Extensive efforts have been made to understand how radiation induces radioresistance. Previous work has suggested protection of tumor vasculature is a critical determinant of overall response to radiotherapy (2–5). Therefore, targeting the signaling pathways that are involved in the secretion of radioprotective factors such as VEGF may prove a promising strategy to induce tumor radiosensitization. Alternative strategies may include targeting the molecules, such as HIF-1, which initiate the radioprotective response (2). Previous studies have shown that HIF-1 render pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation (21). Conversely, HIF-1 has shown to exert apoptotic activities by up-regulating the expression of a proapaptotic protein (30), and by stabilizing p53 (31). Moeller et al., also demonstrated that HIF-1 may have a radiosensitizing effect on tumors by promoting p53 activation (22), suggesting that targeting HIF-1 could be beneficial for the survival of certain cancer cells after irradiation (22) and that the overall impact of p53 in cancer cell response to radiation seems to be dependent on the cellular context.
Focusing on the mechanisms for adioresistance in lung cancer cells, we observed that treatment with clinically relevant doses of radiation (1 or 3 Gy) induced markedly HIF-1α expression in a subset of normoxic lung cancer cell lines in vitro, which increased the cells’ viability and angiogenic potential. We further demonstrated significantly improved suppression of the viability and angiogenic activities of lung cancer cells in which HIF-1α expression had been silenced by siRNA transfection. A single high dose of radiation (10 Gy) did not induce HIF-1α expression but rather provoked extensive cancer cell death; however, concomitant lethal damage in normal bronchial epithelial cells would argue against the use of high-dose radiation for lung cancer therapy. Therefore, it is likely that radiation-induced increase in HIF-1α expression could contribute to induce radioresistence
We then assessed the mechanisms through which radiation induced HIF-1α expression in radioresistant lung cancer cells. It has been known that HIF-1α protein is regulated in both an oxygen-dependent and -independent manner (32). A previous study demonstrated that irradiation induced HIF-1 expression in response to ROS in hypoxia-reoxygeneation strategy, in vitro and in vivo, but it did not activate HIF-1 in a normoxic environment (2). Surprisingly, our results showed high levels of HIF-1α induction in several irradiated lung cancer cells in normoxic environments. Moreover, radiation-induced increases in HIF-1α and VEGF expression in those cells were not affected by the treatment with NAC, a ROS scavenger. These findings led us to investigate the mechanisms involved in radiation-induced HIF-1α expression in normoxic lung cancer cells. Given the role of PI3K/Akt/mTOR in HIF-1α protein expression, we first assumed that the enhanced translation of its preexisting transcripts caused radiation-induced increases in HIF-1α protein expression(2). However, we observed PI3K/Akt/mTOR activation in all irradiated lung cancer cell lines used in our study. The lack of correlation between pathway activation and the cell lines’ radiosensitivity indicated that additional mechanisms are involved in the radiation-induced increase of HIF-1α protein expression.
Our ongoing efforts to determine the additional mechanisms have provided evidences that Hsp90 plays a major role in radiation-induced HIF-1α expression and radioresistance in lung cancer cells. We observed that 1) irradiation increased the HSP90α and HIF-1α interaction in radioresistant H1299, H226B and H182 cells but decreased the interaction in radiosensitive H460 and H226Br cells; 2) treatment with Hsp90 inhibitors 17-AAG and deguelin, which block Hsp90 by competing with ATP for the ATP-binding pocket on Hsp90α (18), suppressed radiation-mediated increases in HIF-1α and VEGF expression; 3) deguelin treatment prevented induction of HIF-1α expression in H1299 and H226B xenograft tumors after a prolonged series of repeated irradiation, leading to effectively reduced tumor angiogenesis and growth. Therefore, it appears that radiation induces HIF-1α expression in normoxic lung cancer cells through dual mechanisms ; 1) stimulation of de novo protein synthesis of HIF-1α via PI3K/Akt/mTOR signaling pathways, 2) increases in HIF-1α stabilization via induction of HIF-1α and Hsp90 interaction. These findings provide a significant step toward understanding the mechanism that mediates radiation-induced HIF-1 activation.
In conclusion, we provide evidence here for the first time that radiation-enhanced Hsp90 chaperone function has a major role in increases in HIF-1α and VEGF protein levels in lung cancer cells, providing them with survival and angiogenic potential. Our results: 1) radiation modifies the interaction between HIF-1α and Hsp90 ; 2) among several HSP90 clients, only HIF-1α and pAkt were induced by radiation ; 3) despite of radiation-mediated increase in HIF-1α and pAkt, there was no correlation between increase in Akt activation and radiosensitivity of the NSCLC cells used in our study (Fig. 2A) strongly suggest the role of Hsp90 function in induction of HIF-1 activity and radio-resistance in NSCLC cells. Our findings provide a strong rationale for establishing therapeutic strategies for the use of inhibitors of Hsp90 (1, 33–35) in combination with radiation therapy.
We have previously demonstrated that the effects of deguelin alone on numerous HSP90 client portions in a variety of human cancer cell types (18). HSP90 client proteins could have different function depending on cellular context. Considering the role of HSP90 client proteins in the radio-responses, the concomitant decrease in the multiple HSP90 client proteins that were exempted in our current study could have contributed to the deregulin/17AAG-mediated radio-sensitization (33, 34, 36). The mild improvement by the combined treatment than the single treatments on the tumor xenograft assay (Fig. 5 and 6) may represent the contribution of broad downregulation of HSP90 client proteins by deguelin.
Further investigations on the mechanism through which radiation stimulates Hsp90 chaperone function and HIF-1 activity in radioresistant lung cancer cells are warranted.
Acknowledgments
Grant support: NIH/ R01 CA100816-01 and R01 CA109520-01 (to H. Y. Lee). American Cancer Society/ RSG-04-082-01-TBE 01 (to H. Y. Lee)
References
- 1.Camphausen K, Tofilon PJ. Combining radiation and molecular targeting in cancer therapy. Cancer Biol Ther. 2004;3:247–250. [PubMed] [Google Scholar]
- 2.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 3.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer research. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 4.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 5.Camphausen K, Moses MA, Beecken WD, Khan MK, Folkman J, O'Reilly MS. Radiation therapy to a primary tumor accelerates metastatic growth in mice. Cancer research. 2001;61:2207–2211. [PubMed] [Google Scholar]
- 6.Aebersold DM, Burri P, Beer KT, et al. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer research. 2001;61:2911–2916. [PubMed] [Google Scholar]
- 7.Unruh A, Ressel A, Mohamed HG, et al. The hypoxia-inducible factor-1 alpha is a negative factor for tumor therapy. Oncogene. 2003;22:3213–3220. doi: 10.1038/sj.onc.1206385. [DOI] [PubMed] [Google Scholar]
- 8.Williams KJ, Telfer BA, Xenaki D, et al. Enhanced response to radiotherapy in tumours deficient in the function of hypoxia-inducible factor-1. Radiother Oncol. 2005;75:89–98. doi: 10.1016/j.radonc.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends in Molecular Medicine. 2002;8:S62–S67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 10.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 11.Raval RR, Lau KW, Tran MG, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. The Journal of biological chemistry. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 14.Skinner HD, Zheng JZ, Fang J, Agani F, Jiang B-H. Vascular Endothelial Growth Factor Transcriptional Activation Is Mediated by Hypoxia-inducible Factor 1{alpha}, HDM2, and p70S6K1 in Response to Phosphatidylinositol 3-Kinase/AKT Signaling. J Biol Chem. 2004;279:45643–45651. doi: 10.1074/jbc.M404097200. [DOI] [PubMed] [Google Scholar]
- 15.Sandau KB, Faus HG, Brune B. Induction of Hypoxia-Inducible-Factor 1 by Nitric Oxide Is Mediated via the PI 3K Pathway. Biochemical and biophysical research communications. 2000;278:263–267. doi: 10.1006/bbrc.2000.3789. [DOI] [PubMed] [Google Scholar]
- 16.Han JY, Oh SH, Morgillo F, et al. Hypoxia-inducible factor 1alpha and antiangiogenic activity of farnesyltransferase inhibitor SCH66336 in human aerodigestive tract cancer. J Natl Cancer Inst. 2005;97:1272–1286. doi: 10.1093/jnci/dji251. [DOI] [PubMed] [Google Scholar]
- 17.Katschinski DM, Le L, Schindler SG, Thomas T, Voss AK, Wenger RH. Interaction of the PAS B domain with HSP90 accelerates hypoxia-inducible factor-1alpha stabilization. Cell Physiol Biochem. 2004;14:351–360. doi: 10.1159/000080345. [DOI] [PubMed] [Google Scholar]
- 18.Oh SH, Woo JK, Yazici YD, et al. Structural basis for depletion of heat shock protein 90 client proteins by deguelin. J Natl Cancer Inst. 2007;99:949–961. doi: 10.1093/jnci/djm007. [DOI] [PubMed] [Google Scholar]
- 19.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 20.Jonca F, Ortega N, Gleizes PE, Bertrand N, Plouet J. Cell release of bioactive fibroblast growth factor 2 by exon 6-encoded sequence of vascular endothelial growth factor. The Journal of biological chemistry. 1997;272:24203–24209. doi: 10.1074/jbc.272.39.24203. [DOI] [PubMed] [Google Scholar]
- 21.Akakura N, Kobayashi M, Horiuchi I, et al. Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer research. 2001;61:6548–6554. [PubMed] [Google Scholar]
- 22.Moeller BJ, Dreher MR, Rabbani ZN, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Oh SH, Woo JK, Jin Q, et al. Identification of novel antiangiogenic anticancer activities of deguelin targeting hypoxia-inducible factor-1 alpha. Int J Cancer. 2007 doi: 10.1002/ijc.23075. [DOI] [PubMed] [Google Scholar]
- 24.Lee JM, Bernstein A. p53 mutations increase resistance to ionizing radiation. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5742–5746. doi: 10.1073/pnas.90.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsudomi T, Steinberg SM, Nau MM, et al. p53 gene mutations in non-small-cell lung cancer cell lines and their correlation with the presence of ras mutations and clinical features. Oncogene. 1992;7:171–180. [PubMed] [Google Scholar]
- 26.Sunavala-Dossabhoy G, Fowler M, De Benedetti A. Translation of the radioresistance kinase TLK1B is induced by gamma-irradiation through activation of mTOR and phosphorylation of 4E-BP1. BMC Mol Biol. 2004;5:1. doi: 10.1186/1471-2199-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandel NS, McClintock DS, Feliciano CE, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 28.Schulte TW, Neckers LM. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer chemotherapy and pharmacology. 1998;42:273–279. doi: 10.1007/s002800050817. [DOI] [PubMed] [Google Scholar]
- 29.Shen HY, He JC, Wang Y, Huang QY, Chen JF. Geldanamycin induces heat shock protein 70 and protects against MPTP-induced dopaminergic neurotoxicity in mice. The Journal of biological chemistry. 2005;280:39962–39969. doi: 10.1074/jbc.M505524200. [DOI] [PubMed] [Google Scholar]
- 30.Guo K, Searfoss G, Krolikowski D, et al. Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell Death Differ. 2001;8:367–376. doi: 10.1038/sj.cdd.4400810. [DOI] [PubMed] [Google Scholar]
- 31.An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998;392:405–408. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 32.Liu YV, Semenza GL. RACK1 vs. HSP90: competition for HIF-1 alpha degradation vs. stabilization. Cell Cycle. 2007;6:656–659. doi: 10.4161/cc.6.6.3981. [DOI] [PubMed] [Google Scholar]
- 33.Bisht KS, Bradbury CM, Mattson D, et al. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer research. 2003;63:8984–8995. [PubMed] [Google Scholar]
- 34.Russell JS, Burgan W, Oswald KA, Camphausen K, Tofilon PJ. Enhanced cell killing induced by the combination of radiation and the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin: a multitarget approach to radiosensitization. Clin Cancer Res. 2003;9:3749–3755. [PubMed] [Google Scholar]
- 35.Shintani S, Zhang T, Aslam A, Sebastian K, Yoshimura T, Hamakawa H. P53-dependent radiosensitizing effects of Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin on human oral squamous cell carcinoma cell lines. Int J Oncol. 2006;29:1111–1117. doi: 10.3892/ijo.29.5.1111. [DOI] [PubMed] [Google Scholar]
- 36.Enmon R, Yang WH, Ballangrud AM, et al. Combination treatment with 17-N-allylamino-17-demethoxy geldanamycin and acute irradiation produces supra-additive growth suppression in human prostate carcinoma spheroids. Cancer research. 2003;63:8393–8399. [PubMed] [Google Scholar]