Abstract
Supplemental oxygen, used to treat pulmonary insufficiency in newborns, contributes to the development of bronchopulmonary dysplasia (BPD). Cytochrome P4501A enzymes are induced by hyperoxia in animal models, but their role in human systems is unknown. Here we investigated the molecular mechanisms of induction of CYP1A1 by hyperoxia in human lung cell lines. Three human lung cell lines were exposed to hyperoxia (95% O2) for 0-72 hours, and CYP1A1 activities, apoprotein contents, and mRNA levels were determined. Hyperoxia significantly induced CYP1A1 activity and protein contents (2-4 fold), and mRNA levels (30-40 fold) over control in each cell line. Transfection of a CYP1A1 promoter/luciferase reporter construct, followed by hyperoxia (4-72 h), showed marked (2-6 fold) induction of luciferase expression. EMSA and siRNA experiments strongly suggest that the Ah receptor (AHR) is involved in the hyperoxic induction of CYP1A1. MTT reduction assays showed attenuation of cell injury with the CYP1A1 inducer beta-naphthoflavone (BNF). Our results strongly suggest that hyperoxia transcriptionally activates CYP1A1 expression in human lung cell lines by AHR-dependent mechanisms, and that CYP1A1 induction is associated with decreased toxicity. This novel finding of induction of CYP1A1 in the absence of exogenous AHR ligands could lead to novel interventions in the treatment of BPD.
Keywords: Hyperoxia, CYP1A1, Oxidant injury, Human cell lines, Bronchopulmonary dysplasia
INTRODUCTION
Supplemental oxygen is used extensively in the treatment of pulmonary insufficiency in pre-term and term neonates (Northway and Rosan, 1968; Fisher, 1980). Considerable evidence links oxygen exposure to the development of neonatal diseases such as bronchopulmonary dysplasia (BPD), a major cause of morbidity and mortality in premature infants (Northway and Rosan, 1968; Bland and Coalson, 2000). It is known that hyperoxia causes lung injury in animal models (Sindhu et al., 2000), and similar lung injury may occur in neonates undergoing supplemental oxygen therapy. The molecular mechanisms responsible for oxygen toxicity are not completely understood, but reactive oxygen species (ROS) such as superoxide anion, hydroxyl radical, and hydrogen peroxide are known to play a role (Moorthy et al., 1997; Morel and Barouki, 1998; Yang et al., 1999; Moorthy et al., 2000). The proposed mechanism of injury involves the oxidation/peroxidation of lung lipids, proteins, or other molecules (Freeman and Crapo, 1981). Scavenging of the ROS by cytochrome P450 enzymes may modulate hyperoxic lung injury (Moorthy et al., 1997; Moorthy et al., 2000).
The cytochrome P450 (CYP) system of enzymes is a superfamily of heme-containing proteins that is involved in the metabolism of a number of exogenous and endogenous compounds (Guengerich, 2004). The CYP1A1 isoform is classically induced by planar aromatic hydrocarbons, such as benzo[α]pyrene found in cigarette smoke, or 3-methylcholanthrene (3MC) (Whitlock, 1999). Previous work in rodent models has shown that hyperoxia also induces CYP1A1 (Okamoto et al., 1993; Sindhu et al., 2000), but the mechanism involved is not clearly understood. In the classic model of CYP1A1 induction, the ligand binds to the aryl-hydrocarbon receptor (AHR), a cytosolic protein, and causes translocation of the AHR-ligand complex into the nucleus. There, it forms a heterodimer with the aryl-hydrocarbon nuclear translocator (ARNT), and this heterodimer activates a battery of genes that are under the control of the Ah gene locus (Nebert et al., 2000). Included in this gene battery are CYP1A1, CYP1A2, glutathione S-transferase-α, NAD(P)H-quinone reductase-1, UDP-glucuronosyl transferase, and aldehyde dehydrogenase. CYP1A1 induction involves the interaction of the AHR-ligand-ARNT complex with multiple Ah-responsive elements (AHREs) within the CYP1A1 gene promoter region to activate transcription (Whitlock et al., 1996; Whitlock, 1999). The mechanisms involved in the hyperoxic induction of CYP1A1 are not fully understood, but an AHR-dependent mechanism has been proposed (Okamoto et al., 1993; Couroucli et al., 2002; Jiang et al., 2004).
Whereas induction of CYP has been implicated in the potentiation of hyperoxic lung injury (Hazinski et al., 1989; Hazinski et al., 1995), several groups have shown that CYP1A1 may play a protective role. Pretreatment of rats (Mansour, 1988a) or mice (Mansour, 1988b) with inducers of CYP1A enzymes attenuates hyperoxic lung injury. In lamb studies, pretreatment with cimetidine, a noncompetitive inhibitor of CYP activity, attenuates hyperoxic lung injury (Hazinski et al., 1989). It has been shown, however, that cimetidine inhibits CYP2A6 and CYP2C11, but not CYP1A1 (Levine et al., 1998). Additionally, work from our laboratory showed that pretreating rats with the inhibitor of CYP1A, aminobenzotriazole (ABT), followed by subsequent exposure to 95% O2 severely potentiates hyperoxic lung injury (Moorthy et al., 2000). We also reported that in adult rats, the CYP1A inducer β-naphthoflavone (BNF) attenuates hyperoxic lung injury (Sinha et al., 2005). Further, we also showed that there exists an inverse correlation between pulmonary and hepatic CYP1A expression and the extent of lung injury (Sinha et al., 2005). These observations support the theory that CYP1A1 and CYP1A2 may play protective roles against oxygen-mediated lung injury.
It is known that animals exposed to hyperoxia are able to upregulate the pulmonary and hepatic CYP1A1 and 1A2 expression for a period of up to 48 hours (Moorthy et al., 1997; Moorthy et al., 2000; Sindhu et al., 2000). By 60 hours, the animals develop severe respiratory distress (Moorthy et al., 2000; Couroucli et al., 2002). When AHR (-/-) mice are exposed to hyperoxia, they do not display an increase in endogenous CYP1A1 expression as compared to wild-type mice, and are more susceptible to lung injury and inflammation than similarly exposed wild-type mice (Jiang et al., 2004). Whether CYP1A1 is similarly regulated, and what role it may play in modulating hyperoxic injury in human tissues and cells is not known. Therefore, in this investigation we tested the hypotheses that (i) hyperoxia induces CYP1A1 expression in human pulmonary cell lines by AHR-dependent mechanisms, and (ii) CYP1A1 modulates hyperoxic cell toxicity in these cells.
METHODS
Cell Lines
The following cell lines were purchased from the American Type Culture Collection™ (Manassas, VA): A549, BEAS-2B, and H441. The A549 line was maintained in DMEM-F12 medium (Invitrogen™, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Gemini Bio-Products BenchMark™, West Sacramento, CA). The BEAS-2B and H441 lines were maintained in RPMI-1640 medium (Invitrogen™, Carlsbad, CA) supplemented with 10% FBS. Penicillin-Steptomycin (Invitrogen™, Carlsbad, CA) was added to the media for each cell line. All cell lines were maintained in a Forma Scientific™ water-jacketed incubator at 37°C, in room air with 5% carbon dioxide. Hyperoxia experiments were conducted in a plexiglass, sealed chamber into which a mixture of 95% oxygen and 5% carbon dioxide was circulated. The chamber was placed in a Forma Scientific™ water-jacketed incubator at 37°C.
Experimental Design
Hyperoxia experiments
For each cell line, 103 cells were plated into 96-well tissue culture plates (BD BioSciences™, San Jose, CA). Some plates were placed in room air, and some plates were placed in the hyperoxia chamber described above. At 24, 48, and 72 hours, one plate from each condition was removed, and after decanting the growth medium, the cells were washed twice with Dulbecco's Phosphate Buffered Saline (PBS) (Invitrogen™, Carlsbad, CA) without calcium chloride and without magnesium chloride (250 μL per well). There were at least 3 independent experiments for each time point.
EROD Assays
The method for performing ethoxyresorufin-O-deethylase (EROD) assays (CYP1A1 activity) and obtaining protein concentration simultaneously was adapted from Kennedy et al. (Kennedy et al., 1995). The cells on the plates exposed to normoxia or hyperoxia were assayed according to the following procedure. Sodium phosphate buffer (62.5 μL; 50 mM, pH 8.0) was added to each of the wells. Some wells were used as blanks, and received 75 μL of sodium phosphate buffer. Ethoxyresorufin (25 μL of a methanol solution that was diluted 30-fold in sodium phosphate buffer immediately before addition to the wells, final concentration of 5 μM, Sigma™, St. Louis, MO) was added to each of the wells. The plates were placed in a 37°C incubator for a 15 minute pre-incubation period, during which BSA (Sigma™, St. Louis, MO) and resorufin (Sigma™, St. Louis, MO) standards were prepared in a separate 96-well plate. Resorufin standards were prepared by diluting a methanol solution of resorufin approximately 20-fold in sodium phosphate buffer immediately prior to addition to the wells. The BSA stock solution was prepared in sodium phosphate buffer as well, and subsequent dilutions were also made with sodium phosphate buffer.
To start the EROD reactions, NADPH (12.5 μL of a 13.4 mM solution in sodium phosphate buffer, final concentration 1 mM, Sigma™, St. Louis, MO) was added to all reaction wells. Plates were incubated for 10 minutes at 37°C, and reactions were stopped with acetonitrile (75 μL, Fisher Scientific, Sigma, St. Louis, MO) that contained fluorescamine (Fluka™, Sigma, St. Louis, MO) at a concentration of 300 μg/mL. The 96-well plates were read in a SpectraMax GeminiXS™ fluorescence plate reader. Excitation and emission wavelengths of 530 nm and 590 nm, respectively, were used to determine resorufin concentration in each test well, and excitation and emission wavelengths of 400 nm and 460 nm, respectively, were used to determine total protein content in each well.
Luciferase Assays
A 1.6kb fragment of the wild-type human CYP1A1 gene promoter was cloned into a pGL3 plasmid (Promega®, Madison, WI), upstream of the firefly luciferase reporter gene. This construct was co-transfected with the Renilla luciferase (pRL, used as an internal control) into each of the cell lines (103 cells plated into each well of a 96-well tissue culture plate) for 24 h, and the transfected cells were exposed to hyperoxia or maintained in room air (transfected with Lipfectamine 2000 Transfection Reagent, Invitrogen™, Carlsbad, CA). After 4, 8, 12, 24, 48, and 72 hours of hyperoxia or normoxia, cells were processed and firefly and Renilla luciferase activities were measured as per the protocol outlined in the Dual-Luciferase Assay Kit (Promega®, Madison, WI). Firefly luciferase activity was normalized to Renilla luciferase activity.
Cell Count and Viability Assays
For each cell line, 103 cells were plated into each well of a 96-well tissue culture plate (BD Biosciences™, San Jose, CA). Three plates were prepared for each time point (24, 48, and 72 hours) for each cell line. Each row of cells was treated with one of the following regimens: 1) Dimethylsulfoxide (DMSO) vehicle (Sigma™, St. Louis, MO); 2) 10 μM 3', 4'-dimethoxyflavone (DMF, an AHR antagonist (Alfa Aesar®, Ward Hill, MA); 3) 10 μM α-naphthoflavone (ANF, selective CYP1A1 inhibitor (Sigma™, St. Louis, MO); 4) 10 μM BNF, an AHR agonist (Sigma™, St. Louis, MO); 5) Co-treatment with ANF and BNF, each at 10 μM concentration. At each time point, MTT reduction assays were performed to assess cell count and viability, as outlined in the MTT Reduction Assay protocol (American Type Culture Collection™, Manassas, VA). Standard curves of cell count vs. OD570nm were prepared for each cell line.
Western Blot Analysis
The whole-cell suspension (100 μg protein) was subjected to SDS-polyacrylamide gel electrophoresis in 7.5% polyacrylamide gels. The separated proteins were transferred to polyvinylidene difluoride membranes, followed by Western blotting (all materials acquired from Bio-Rad™, Hercules, CA). For Western blot analysis, a monoclonal mouse anti-human IgG antibody to CYP1A1 (gift from P. E. Thomas) and goat anti-mouse IgG conjugated with horseradish peroxidase (Bio-Rad™, Hercules, CA), were used as primary and secondary antibodies respectively, as previousely described (Moorthy et al., 2000; Couroucli et al., 2002; Jiang et al., 2004). Western Blot ECL Kit (Amersham Biosciences, Buckinghamshire, UK) was used as outlined in the protocol, and the membranes were exposed to Hyperfilm™ ECL (Amersham Biosciences, Buckinghamshire, UK), and developed.
Real time RT-PCR Assays
RNA was isolated using a modification of the procedure from Chomczynski et al. (Chomczynski and Sacchi, 1987). The RNA pellet was resuspended in 100 μl of diethyl pyrocarbonate (DEPC, Sigma™, St. Louis, MO) treated water. Total RNA (50 ng), isolated from cells that were room air-maintained or exposed to hyperoxia, was subjected to one step real time quantitative TaqMan® RT-PCR. ABI PRISM 7700 Sequence Detection System was used for the RT-PCR reactions. Gene-specific primers in the presence of TaqMan® reverse transcription reagents and RT reaction mix (Applied Biosystems, Foster City, CA) were used to reverse transcribe RNA, and TaqMan® Universal PCR Master Mix (Applied Biosystems, Foster City, CA) and Assays-On-Demand Gene Expression probes were used for PCR amplification. Following an RT hold for 30 minutes at 48°C, the samples were denatured at 95°C for 10 minutes. The thermal cyclin g step was for 40 cycles at 95°C for 15 s, and 40 cycles at 60°C for 1 minute. As an int ernal control, 18S RNA was used (Applied Biosystems, Foster City, CA). Quantitative values were obtained from the threshold PCR cycle number (Ct) at which the increase in signal was associated with an exponential growth of PCR product after it starts to be detected. The relative mRNA levels for CYP1A1 were normalized to their 18S content. The relative expression levels of the target gene were calculated according to the equation 2-ΔcT, where ΔcT = Ct (target gene) - Ct (18S gene) (Jiang et al., 2004).
Electrophoretic Mobility Shift Assays
Nuclear protein extracts from room air-maintained and hyperoxia exposed cells were prepared using the NE-PER® Nuclear and Cytoplasmic Extraction Reagents kit (Pierce Biotechnology, Rockford, IL). The nuclear protein extracts were stored at -80°C until use . EMSAs were performed using the LightShift® Chemiluminescent EMSA Kit (Pierce Biotechnology, Rockford, IL). Total 15 μg of nuclear extract was incubated at room temperature for 20 minutes with 3'-biotin end-labeled double-stranded oligonucleotide probe containing the consensus sequence for binding of the AHR/ARNT heterodimer. The DNA fragments that were used to make the double-stranded oligonucleotide probe were 5'-GATCCGGCTCTTGTCACGCAACTCCGAGCTCA-3' and 5'-GATCTGAGCTCGGAGTTGCGTGAGAAGAGCCG-3', and were 3'-biotin end-labeled using the Biotin 3' End DNA Labeling Kit (Pierce Biotechnology, Rockford, IL). The double-stranded probe was made by annealing the two single-stranded 3'-biotin end-labeled fragments. For competition experiments, nuclear extracts were incubated with 25-fold excess of unlabeled probe before addition of the labeled probe. The samples were separated by non-denaturing polyacrylamide gel electrophoresis (6% gel) at 150V for 2 hours. The separated protein/DNA complexes were transferred to nylon membrane (Roche, Switzerland) by electrophoresis at 400 mA for 60 minutes, followed by cross-linking of the DNA to the membrane using the UV-light cross-linker (Stragene UV Crosslinker 1800, La Jolla, CA) at 120 mJ/cm2 for 60 seconds. The biotin-labeled DNA was detected by chemiluminscence using the Chemiluminescent Nucleic Acid Detection Module (Pierce Biotechnology, Rockford, IL), followed by exposure to HyperECL Film.
siRNA Knockdown of the Ah Receptor
siRNA knockdown of the Ah receptor mRNA was adopted from the protocol used by Bonzo et al. (Bonzo et al., 2007). A SMART-pool siRNA directed against the human Ah receptor (si-AhR, Cat # M-004990) was purchased from Dharmacon RNA technologies (Boulder, CO). To study the effect of knockdown of Ah receptor expression on CYP1A1 induction by hyperoxia, A549 cells were plated in 6-well plates and co-transfected for 6 hours with the CYP1A1 promoter-luciferase construct as well as pRL-TK, and either 50 nM si-AhR or 50 nM negative control siRNA (Dharmacon, Cat # D-001210-01), following the lipofectamine protocol. Cells were subsequently exposed to hyperoxia (95% O2 and 5% CO2) for 24, 48 and 72 hours. Firefly luciferase values were normalized to Renilla luciferase and reported as fold increase over control treatment. All experiments were conducted in triplicate. The knockdown of AHR in the Si-RNA transfected cells was validated by Western blotting.
Statistical analysis
Two-way analysis of variance (ANOVA), followed by modified t-testing, was used to determine if there was a significant interaction of condition (room air vs. hyperoxia) and time (24, 48, and 72 hours) on CYP1A1 activity (as measured by the EROD assay). Three-way ANOVA, followed by modified t-testing, was used to determine if there was a significant interaction of condition (room air vs. hyperoxia), plasmid (pGL3 vs. pGL3-1A1), and time (24, 48, and 72 hours) on luciferase activity. For cell viability assays, a three-way ANOVA, followed by modified t-testing, was used to determine if there was significant interaction of condition (room air vs. hyperoxia), treatment (vehicle vs. ANF, BNF, DMF, or combination ANF and BNF) and time (24, 48, and 72 hours). A p value of <0.05 was considered significant. All data were analyzed using the SPSS 12.0 software (Chicago, IL).
RESULTS
Effect of Hyperoxia on CYP1A1 Enzyme Activities and Apoprotein Levels
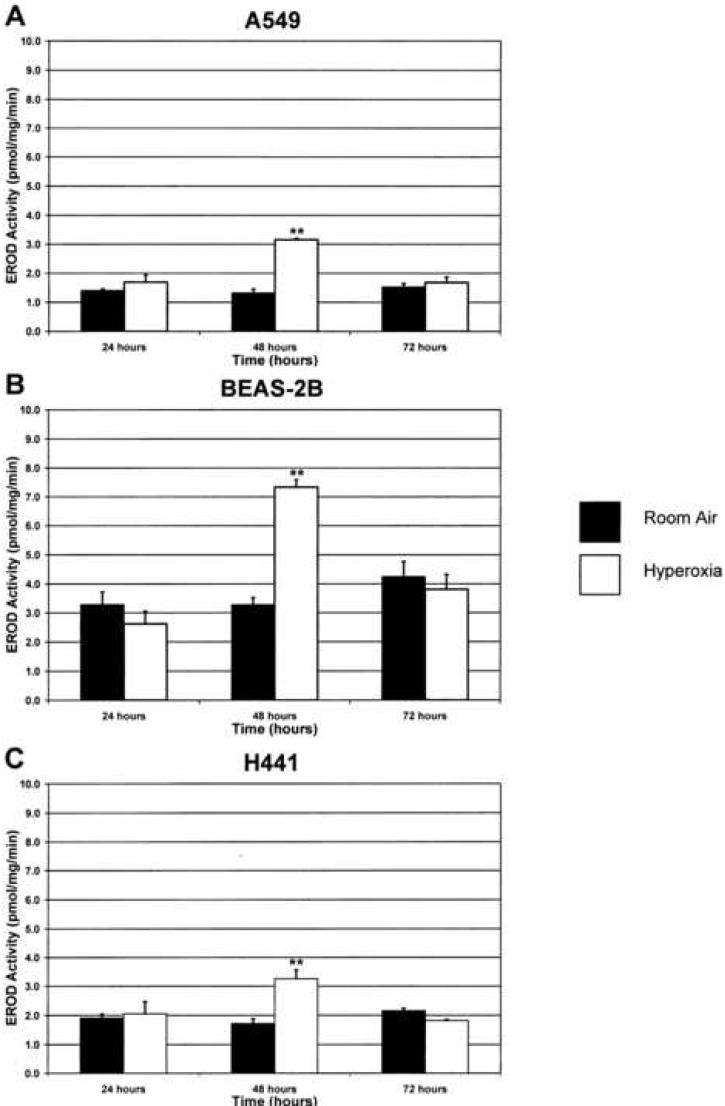
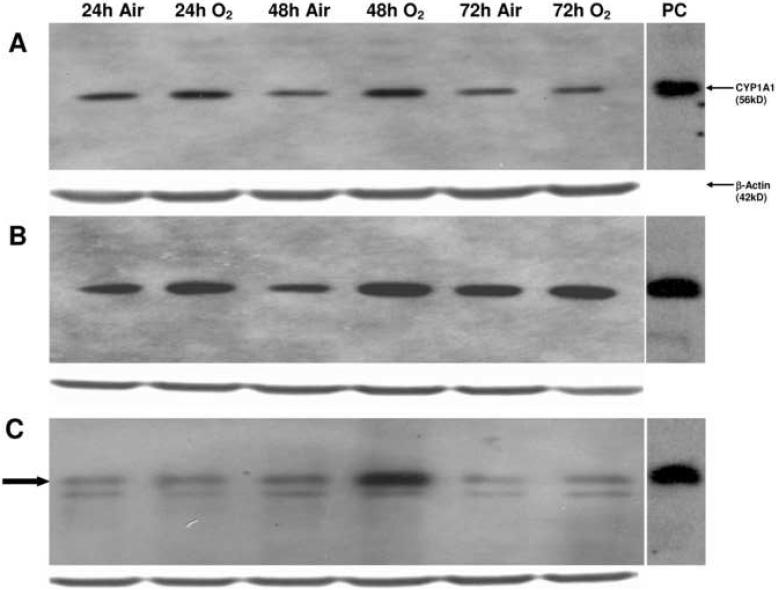
Exposure of each cell line to hyperoxia for 24 h did not alter EROD (CYP1A1) activities, compared with room air controls (Figure 1). However, in the samples exposed to 48 h of hyperoxia, the EROD activities were 2-5 times higher than room air controls (Figure 1). This induction of CYP1A1 activity was followed by a return to baseline activity by 72 h of exposure to hyperoxia. Hyperoxia-induced augmentation of CYP1A1 enzyme activity was paralleled by enhanced levels of CYP1A1 apoprotein, as analyzed by Western blotting (Figure 2). In the H441 cell line, there are two bands visible, the upper of which corresponds to the CYP1A1 positive control. As with the enzyme activities, the apoprotein levels declined to near baseline after 72 h of exposure to hyperoxia.
Figure 1. Hyperoxia induces CYP1A1 activity.
Each cell line was maintained in room air or exposed to hyperoxia as outlined in “Materials and Methods.” CYP1A1 activity was then measured using the ethoxyresorufin-O-deethylase (EROD) assay. **p<0.01 compared to room air at 48 hours or hyperoxia at 24 hours. Values represent means ± SD (n=16).
Figure 2. Hyperoxia increases apoprotein expression.
The whole cell suspensions (100 μg protein) were subjected to Western blotting using monoclonal antibodies raised against CYP1A1, as described in “Materials and Methods.” Panel A represents samples from the A549 cell line, panel B represents samples from the BEAS-2B cell line, and panel C represents samples from the H441 cell line. In the H441 cell line, the CYP1A1 protein is highlighted by the closed arrow. Under each sample lane is the corresponding β-actin blot to assess for protein loading. The positive control (labeled “PC”) was 0.5 μg of liver microsomes from mice treated with 3-methylcholanthrene.
Effect of Hyperoxia on CYP1A1 mRNA Levels
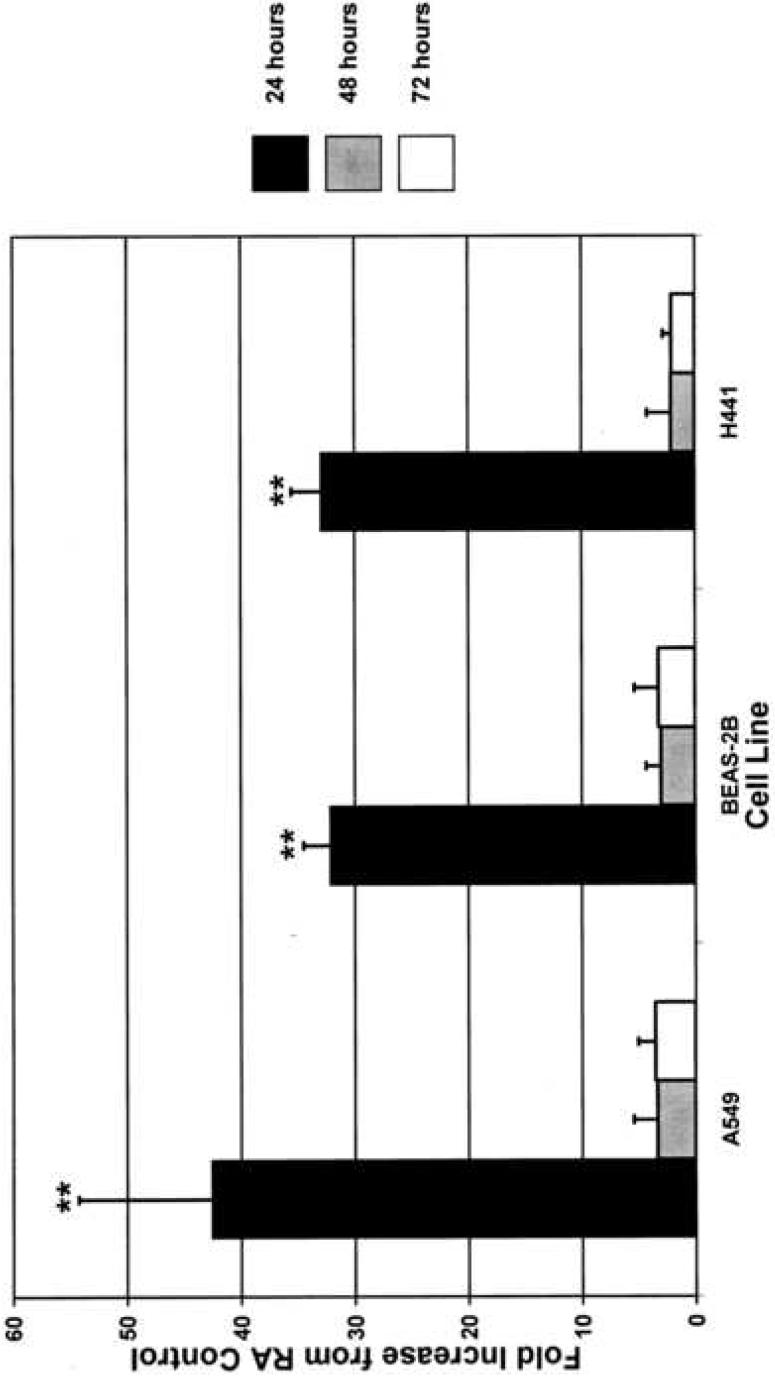
In order to determine if the enhancement of CYP1A1 activities and protein contents was preceded by an increase in its mRNA, we performed real time RT-PCR analysis from total RNA isolated from room air-maintained cells and hyperoxia exposed cells at 24, 48, and 72 hours. Quantitation of mRNA levels demonstrated that hyperoxia induced CYP1A1 mRNAs in the cell lines by 30 to 40-fold in each of the cell lines at the 24 hour time point, as compared to room air control (Figure 3). The levels of mRNAs returned to those seen in room air controls by 48 and 72 hours in each cell line. By comparison, 3-MC induced CYP1A1 mRNAs in each cell line by >900-fold, an effect that was sustained at each time point (data not shown).
Figure 3. Real time RT-PCR analysis of CYP1A1 mRNA shows significant increase in expression at 24 hours.
Each cell line was exposed to hyperoxia or maintained in room air as described in “Materials and Methods,” and CYP1A1 mRNA expression was analyzed by RT-PCR. 18S primers were used as an internal control. **p<0.01 compared to hyperoxia at 48 and 72 hours and room air at 24 hours. Values represent means ± SD (n=8).
Effect of Hyperoxia on CYP1A1 Gene Promoter-Driven Luciferase Expression
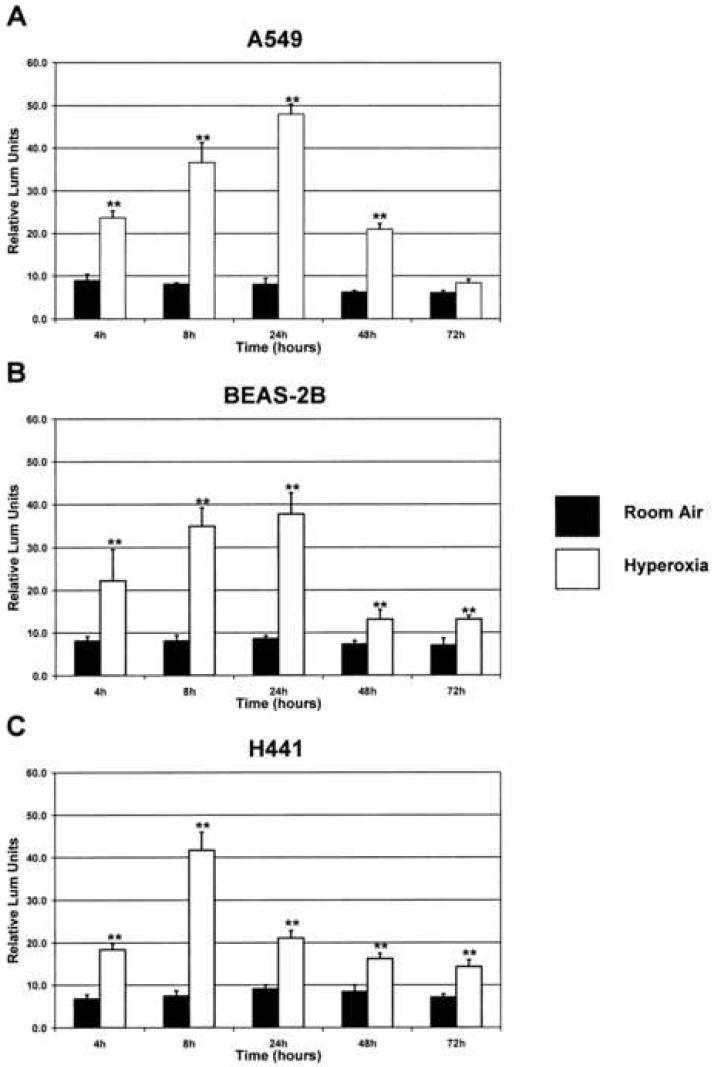
Exposure of the A549 and BEAS-2B cell lines to hyperoxia showed a significant induction of firefly luciferase activity by 4 h, under the control of the CYP1A1 gene promoter, as compared to room air controls (Figure 4A, 4B). The increased luciferase activity reached maximal levels by 24 h of exposure to hyperoxia, declining by 48 h, then subsequently returned to baseline by 72 h. Exposure of the H441 cell line to hyperoxia similarly showed a significant induction of firefly luciferase activity by 4 h, but reached maximal levels by 8 h (Figure 4C). Firefly luciferase activity began to decline by 24 h of exposure to hyperoxia, returning to baseline by 72 h.
Figure 4. Hyperoxia increases expression of the luciferase reporter gene driven by the CYP1A1 gene promoter.
A 1.6kb fragment of the human CYP1A1 gene promoter was cloned upstream of the luciferase reporter gene, and this construct was then transfected into each cell line. A plasmid containing the Renilla luciferase gene was co-transfected as an internal control. The cells were then exposed to hyperoxia or maintained in room air, and luciferase activity was measured as described in “Materials and Methods.” Results were normalized to Renilla luciferase activity. **p<0.01 as compared to respective room air controls (pGL3-1A1-RA). Values represent means ± SD (n=18).
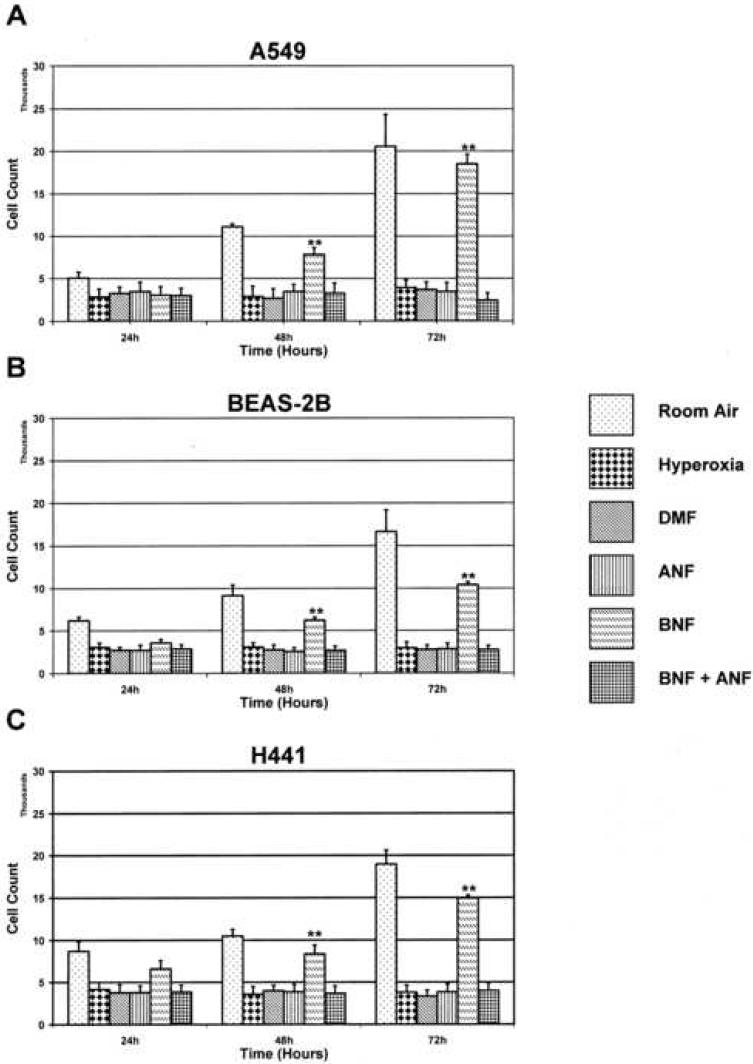
Effect of chemical-induced modulation of CYP1A1 on hyperoxic cell toxicities
Each cell line, when maintained in room air, showed an approximate doubling of cell count every 24 hours, as determined by MTT reduction assays. For each cell line, when treated with 10 μM DMF, 10 μM ANF, or co-treated with 10 μM ANF and 10 μM BNF, then subsequently exposed to hyperoxia, there was no significant change in viable cell numbers over 72 h (Figure 5). Treatment with 10 μM BNF alone, and subsequent exposure to hyperoxia, attenuated this effect, and allowed for continued cell growth (Figure 5).
Figure 5. CYP-1A1 expression modulates cell toxicity during hyperoxia exposure.
In order to determine the relative role of CYP-1A1 in modulating cell injury, each cell line was treated with either of the chemical regimens shown. Following chemical treatment, each line was exposed to hyperoxia or maintained in room air as described in “Materials and Methods.” MTT reduction assays were performed to assess cell count and viability at each time point. **p<0.01 compared to alternative treatments. Values represent means ± SD (n=16).
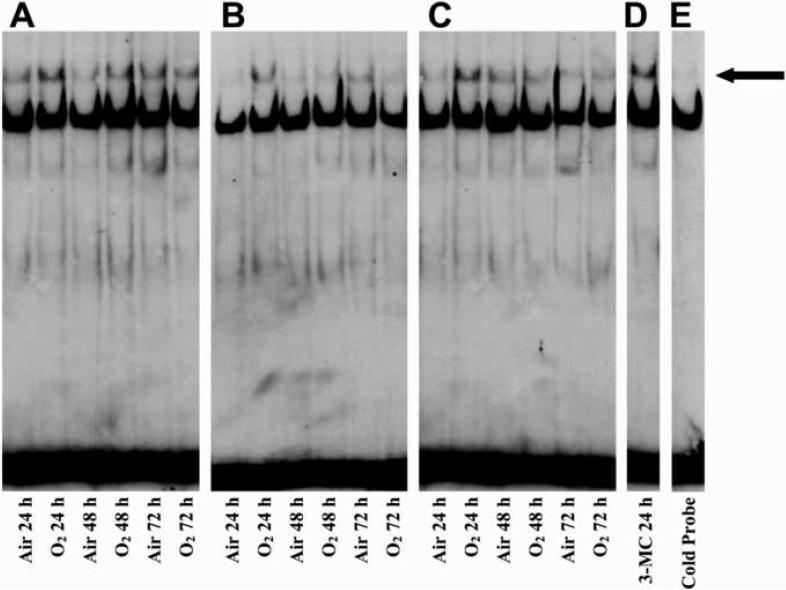
Interaction of Nuclear Proteins with Aryl-Hydrocarbon Responsive Elements (AHREs) in Hyperoxia Exposed Cell Lines
To determine whether exposure to hyperoxia induces CYP1A1 by AHR-dependent mechanisms, nuclear extracts prepared from each cell line, maintained in room air or exposed to hyperoxia, were incubated with 3' biotin end-labeled oligonucleotides that contained the AHRE consensus sequences, which are present in multiple copies within the CYP1A1 gene promoter (Okey et al., 1994). As shown in Figure 6 (Panels A, B, & C), a faint band shift was observed in room air maintained cell lines. However, exposure of each cell line to hyperoxia for 24h showed a more robust, specific band shift, which was competed off in the presence of a 25-fold excess of cold probe (Figure 6E). Comparison of EMSA of nuclear extracts prepared from hyperoxia exposed cells with that from 3-methylcholanthrene (MC) treated cells showed that the protein-DNA complex in hyperoxia exposed cells had a similar electrophoretic mobility as that observed in MC-treated cells.
Figure 6. Interaction of nuclear proteins with AhREs in hyperoxia exposed cells.
Nuclear extracts from cells maintained in room air or exposed to hyperoxia for 24, 48, or 72 hours were subjected to EMSA, as described in “Materials and Methods.” Panel A represents samples from the A549 cell line, Panel B represents samples from the BEAS-2B cell line, Panel C represents samples from the H441 cell line, Panel D represents nuclear extract from treatment of the A549 cell line with the prototypical CYP1A1 inducer 3-methylcholanthrene (3-MC) for 24 hours, and Panel E represents the nuclear extract from the BEAS-2B cell line exposed to hyperoxia for 24 hours incubated with a 25-fold excess of cold probe before being subjected to EMSA. The closed arrow indicates the shifted band.
siRNA Knowdown of the AHR Receptor
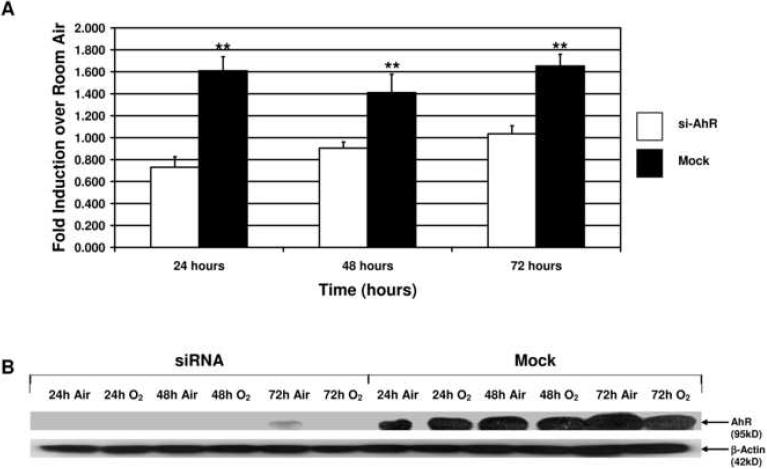
In cells that were co-transfected with siAhR, hyperoxia did not induce luciferase activities, suggesting that the CYP1A1 gene induction by hyperoxia was mediated by the AHR (Figure 7A). Western blotting experiments (Figure 7B) indicated that cells co-transfected with siAhR, but not control siRNA, blocked AHR protein expression, thereby providing evidence that the siAhR did, in fact, abrogate AHR mRNA expression, thereby reducing the expression of CYP1A1.
Figure 7. siRNA directed against AHR mRNA abolishes expression of the luciferase reporter gene driven by the CYP1A1 gene promoter.
siAhR or control siRNA was co-transfected the CYP1A1 promoter-luciferase construct as described in “Materials and Methods.” Panel A depicts fold induction over room air control of the luciferase reporter in the control siRNA (Mock) transfected cells and in the siAhR transfected cells. Results were normalized to Renilla luciferase activity. **p<0.03 as compared to siAhR. Values represent means ± SD. Panel B depicts the representative Western blot for the AhR protein in the Mock and siAhR samples. There is significant decrease in the intensity of the AhR band in the siAhR transfected cells as compared to the Mock transfections.
DISCUSSION
Pulmonary insufficiency, as encountered in preterm and term infants with various pulmonary disorders, is treated often with supplemental oxygen. It is known that hyperoxia is a risk factor in the development of BPD, a major cause of morbidity and mortality in chronically ventilated infants. Earlier work implicated CYP enzymes in the potentiation of hyperoxic lung injury (Hazinski et al., 1989). In this study, the administration of cimetidine, a known inhibitor of the CYP system, to full-term, newborn lambs attenuated hyperoxic lung injury. Subsequent work from our laboratory has shown that induction of hepatic and pulmonary CYP1A1 and CYP1A2 attenuates hyperoxic lung injury (Moorthy et al., 1997; Moorthy et al., 2000; Jiang et al., 2004; Sinha et al., 2005). In a human clinical trial with cimetidine, it has been demonstrated that, in critically ill, ventilator-dependent prematures at risk for developing BPD, this drug did not reduce early lung injury (Cotton et al., 2006). Interestingly, this group also found that the tracheal aspirate levels of F2-isoprostanes, markers of lipid peroxidation from free radical oxidant generation, were increased in the cimetidine group at 4 and 10 days after cimetidine treatment (Cotton et al., 2006), supporting the hypothesis that CYP enzymes may play a role in detoxification of F2-isoprostanes (Cotton et al., 2006). The major objective of this investigation was to establish a human cell line model for determining the mechanisms of induction of CYP1A1 by hyperoxia, in relation to oxygen toxicities in these cells. The significant increases in EROD activities (Figure 1) and CYP1A1 apoprotein levels (Figure 2) caused by exposure to hyperoxia for 48 h indicate induction of CYP1A1 expression. It should be noted that the molecular weights of human CYP1A1 and CYP1A2 are very similar (56 kD and 56.7 kD, respectively), as are the molecular weights of the mouse CYP1A1 and CYP1A2 (57.6 kD and 56.5 kD, respectively) used as our positive contols, and the conditions used in our Western blotting could not allow for separation of these proteins. We can confidently say that the band seen on Western blotting for A549 are truly CYP1A1, as it as been reported that CYP1A2 is not expressed in the A549 cell line (Shervington et al., 2007). The possibility that the bands seen on Western blot for the BEAS-2B and H441 cell lines may represent CYP1A2 is not excluded. The results of our EROD assays, which are specific for CYP1A1 activity, as well as our RT-PCR data, and finally our CYP1A1 promoter-luciferase construct transfections very strongly suggest that the bands represent CYP1A1 for each of these cell lines. Further, the lower band seen in the H441 Western blot represents non-specific binding, as the molecular weight of this band does not correlate with either CYP1A1 or CYP1A2. Although the extent of induction of CYP1A1 by hyperoxia was less than that mediated by treatment of cells with the prototype CYP1A1 inducer 3-MC (1 μM final concentration in cell culture media) in the A549 cell line (data not shown), it was still statistically significant, and may have important toxicological implications. EROD activities are known to primarily reflect catalytic activities of CYP1A1 (Moorthy et al., 1997; Moorthy et al., 2000). The observation that CYP1A1 apoprotein levels were enhanced in hyperoxia exposed cells supports the idea that the augmented EROD activities by hyperoxia reflected CYP1A1 induction.
More recent work in our laboratory has shown that oxygen-induced abnormal lung maturation, as reflected mainly by an arrest of alveolarization, is attenuated in neonatal rats by retinoic acid (Couroucli et al., 2006). We also reported sustained induction of CYP1A1 activity in the hyperoxia exposed animals at PND 8, 15, and 22, a finding that is in contradiction to earlier findings of CYP1A1 induction by hyperoxia by 48 h of exposure, followed by a decline in activity by 60 h (Okamoto et al., 1993; Moorthy et al., 1997; Couroucli et al., 2002; Couroucli et al., 2006). These seemingly contradictory findings can be explained by the fact that all of the previous studies have been conducted in adult animals, whereas Courcoucli et al (2006) looked at the effects of retinoic acid and hyperoxia on neonatal rats. Additionally, all of the human cell lines used in our work are derived from adult tissues.
The increase in CYP1A1 mRNA expression at 24 h of exposure to hyperoxia (Figure 3) was consistent with the hypothesis that CYP1A1 activities and apoprotein contents were caused, in part, by activation of CYP1A1 gene expression. Hazinski et al. (1995) showed induction of CYP1A1 mRNA by hyperoxia in cultured endothelial cells from lambs to be mediated by transcriptional mechanisms. The fact that the expression of CYP1A1 mRNA was markedly decreased between 24 and 48 h of exposure to hyperoxia (Figure 3) strongly suggests that hyperoxia-induced decline of EROD activities and apoprotein levels was caused by down-regulation of CYP1A1 expression at the pre-translational level. These findings are consistent with our findings of activation of the CYP1A1 gene promoter by hyperoxia, as determined by a firefly luciferase reporter system. It has been shown that H2O2 increases in a CYP1A1-dependent fashion in human hepatoma cells treated with benzo[α]pyrene (Morel et al., 1999). It has also been shown that exposure to hyperoxia causes the formation of H2O2 (Freeman and Crapo, 1981). It is possible, then, that CYP1A1-mediated increases in H2O2 production may have attenuated CYP1A1 gene expression by an autoregulatory loop mechanism involving down-regulation of NF-1, a protein whose binding to the NF-1 site on the basal transcription element of the CYP1A1 gene promoter is critical to the expression of the gene (Morel and Barouki, 1998; Morel et al., 1999). The possibility remains that increases in H2O2 cause oxidative degradation of the CYP1A1 enzyme, leading to a decline in EROD activity and apoprotein level.
The decrease in the expression of CYP1A1 apoprotein between 48 h and 72 h of hyperoxia exposure may also have involved other mechanisms. The half-life of the CYP1A1 protein is reported be 16 h (Shiraki and Guengerich, 1984). Therefore, the decline of apoprotein content and activities between 48 h and 72 h may have been caused by loss of immunoreactivity or function via oxidative degradation of CYP1A1 by hyperoxia. Although cellular toxicity after prolonged hyperoxic exposure may explain in part the decrease in CYP1A1 expression at 72 h, the fact that the protein expression of other enzymes, such as glutathione S-transferase-α (Moorthy et al., 1997), are not attenuated by exposure to hyperoxia suggests that the decline of induction by hyperoxia was relatively specific for CYP1A1, which may be of mechanistic relevance to hyperoxic injury. Several authors have shown that the P450 enzymes, upon degradation, are intracellular sources for redox-active iron, which might induce lung injury through increased formation of Fenton-like reactions or by propagating oxidative stress and lipid peroxidation (Kehrer and Smith, 1994; Paller and Jacob, 1994; Yang et al., 1999; Moorthy et al., 2000).
The mechanisms of induction of CYP1A1 by hyperoxia are not clearly understood. Previously, evidence has been presented that strongly suggest the presence of an endogenous ligand, based on supershifting observed on EMSA experiments (Couroucli et al., 2002). In our experiments, the electrophoretic mobility of the shifted band was similar to that of cells treated with 3-MC, suggesting that AHR-dependent mechanisms may be involved. In fact, our experiments strongly suggest involvement of the AHR, as evidenced by the abolition of luciferase activity in cells transfected with an siRNA construct directed against the AHR mRNA (Figure 7). The nature of the endogenous ligand that is formed in hyperoxic conditions is yet to be characterized.
We performed experiments involving blocking or inducing various steps in the classical pathway of CYP1A1 gene induction in an attempt to help delineate the role of this pathway in hyperoxic induction, and specifically the functional role of the CYP1A1 enzyme. In room air maintained cells, there is roughly a doubling of cell count every 24 h through 72 h. When cells were pretreated with 3', 4'-DMF, at 10 μM, an antagonist to the AHR, then subsequently exposed to hyperoxia, there was a decrease in the number of viable cells at each of the time points, suggesting an arrest of cell proliferation. Similar results were seen when cells were pretreated with ANF, at 10 μM, a specific inhibitor of the CYP1A1 enzyme. However, when the cells are pretreated with BNF, at 10 μM, a known agonist at the AHR, this effect on growth inhibition was attenuated, and there was again an approximate doubling of cell count at each time point. Since BNF is an inducer of CYP1A1 in lung cells, it is possible that this enzyme conferred protection against hyperoxic cell toxicity. Since other enzymes of the Ah gene battery such as NAD(P)H quinone reductase, glutathione S-transferase-α, and aldehyde dehydrogenase are also induced by BNF, the role of these enzymes against hyperoxic cell injury are not excluded (Moorthy et al., 1997; Das et al., 2006). Furthermore, it is not clear as to why ANF and DMF, which are mixed agonist and antagonists of the AHR, respectively or the combined treatment of ANF and BNF did not modulate arrest of cell proliferation by hyperoxia. Regardless of the mechanisms, our observed benefical effects of BNF, a flavinoid, against cellular oxygen toxicity, is intriguing and warrants further in-depth studies.
The flavinoids comprise a diverse family of chemicals that are commonly found in fruits and vegetables. A large number of these compounds, in addition to being potent modulators of expression and activity of various CYP enzymes as above, exert various effects on cell cycle regulation (Reiners et al., 1999). This regulation of cell cycle activity, such as cell cycle arrest or apoptosis, is dependent upon the concentration of the various flavinoids (Reiners et al., 1999). Several groups have looked at the cytostatic properties of various flavinoids, and have found that compounds such as ANF can cause arrest in the G1 phase of the cycle. The concentrations that are required to achieve this arrest are found to be ≥40 μM, and have no discernible effect on cell cycle regulation below 20 μM (Reiners et al., 1999). Therefore, with the concentrations of these flavinoids used in our experiments, it is unlikely that the arrest in cell proliferation seen with treatment with the different flavinoids presented here would be caused by the treatment itself, and suggests that other mechanisms are likely at play.
In conclusion, in this paper we report a novel finding, as we have demonstrated significant induction of CYP1A1 by oxygen in human pulmonary cell line models in the absence of exogenous ligands for the AHR. These studies have important implications for hyperoxic lung injury in humans. As CYP1A1 appears to attenuate hyperoxic cell injury in our pulmonary epithelial cell culture model, it is our hope that further investigation into the role of CYP1A1 in modulating hyperoxic injury in the whole animal model may lead to the development of rational strategies, involving genetic or dietary interventions, which could in turn lead to the prevention and/or treatment of BPD (chronic lung disease) in preterm and term infants undergoing supplemental oxygen therapy.
ACKNOWLEDGEMENTS
This work was in part supported by a grant from iNO Therapeutics, Inc. to KYB, grant F32 HL082431 to WJ, and RO1 grants ES 009132, HL 070921, and HL 087174, and AHA grant-in-aid (Texas Affiliate) 0655122Y to BM. The study sponsors had no involvement in study design, data collection, analysis and interpretation, writing of the report or decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bland RD, Coalson JJ. Chronic lung disease in early infancy. M. Dekker; New York: 2000. [Google Scholar]
- Bonzo JA, Belanger A, Tukey RH. The role of chrysin and the ah receptor in induction of the human UGT1A1 gene in vitro and in transgenic UGT1 mice. Hepatology. 2007;45:349–360. doi: 10.1002/hep.21481. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cotton RB, Hazinski TA, Morrow JD, Roberts LJ, Zeldin DC, Lindstrom DP, Lappalainen U, Law AB, Steele S. Cimetidine does not prevent lung injury in newborn premature infants. Pediatr Res. 2006;59:795–800. doi: 10.1203/01.pdr.0000219397.35473.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couroucli XI, Liang YW, Jiang W, Barrios R, Moorthy B. Attenuation of oxygen-induced abnormal lung maturation in rats by retinoic acid: possible role of cytochrome P4501A enzymes. J Pharmacol Exp Ther. 2006;317:946–954. doi: 10.1124/jpet.105.100677. [DOI] [PubMed] [Google Scholar]
- Couroucli XI, Welty SE, Geske RS, Moorthy B. Regulation of pulmonary and hepatic cytochrome P4501A expression in the rat by hyperoxia: implications for hyperoxic lung injury. Mol Pharmacol. 2002;61:507–515. doi: 10.1124/mol.61.3.507. [DOI] [PubMed] [Google Scholar]
- Das A, Kole L, Wang L, Barrios R, Moorthy B, Jaiswal AK. BALT development and augmentation of hyperoxic lung injury in mice deficient in NQO1 and NQO2. Free Radic Biol Med. 2006;40:1843–1856. doi: 10.1016/j.freeradbiomed.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Fisher AB. Oxygen therapy. Side effects and toxicity. Am Rev Respir Dis. 1980;122:61–69. doi: 10.1164/arrd.1980.122.5P2.61. [DOI] [PubMed] [Google Scholar]
- Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- Guengerich FP. Cytochrome P450: what have we learned and what are the future issues? Drug Metab Rev. 2004;36:159–197. doi: 10.1081/dmr-120033996. [DOI] [PubMed] [Google Scholar]
- Hazinski TA, France M, Kennedy KA, Hansen TN. Cimetidine reduces hyperoxic lung injury in lambs. J Appl Physiol. 1989;67:2586–2592. doi: 10.1152/jappl.1989.67.6.2586. [DOI] [PubMed] [Google Scholar]
- Hazinski TA, Noisin E, Hamon I, DeMatteo A. Sheep lung cytochrome P4501A1 (CYP1A1): cDNA cloning and transcriptional regulation by oxygen tension. J Clin Invest. 1995;96:2083–2089. doi: 10.1172/JCI118257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Welty SE, Couroucli XI, Barrios R, Kondraganti SR, Muthiah K, Yu L, Avery SE, Moorthy B. Disruption of the Ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes P4501A expression and exacerbates hyperoxic lung injury. J Pharmacol Exp Ther. 2004;310:512–519. doi: 10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- Kehrer J, Smith C. Free radicals in biology: sources, reactivity, and role in the etiology of human diseases. In: B F, editor. Natural Antioxidants in Human Health and Disease. Academic Press; New York: 1994. pp. 25–62. [Google Scholar]
- Kennedy SW, Jones SP, Bastien LJ. Efficient analysis of cytochrome P4501A catalytic activity, porphyrins, and total proteins in chicken embryo hepatocyte cultures with a fluorescence plate reader. Anal Biochem. 1995;226:362–370. doi: 10.1006/abio.1995.1237. [DOI] [PubMed] [Google Scholar]
- Levine M, Law EY, Bandiera SM, Chang TK, Bellward GD. In vivo cimetidine inhibits hepatic CYP2C6 and CYP2C11 but not CYP1A1 in adult male rats. J Pharmacol Exp Ther. 1998;284:493–499. [PubMed] [Google Scholar]
- Mansour H, Brun-Pascaud M, Marquetty C, Gougerot-Pocidalo MA, Hakim J, Pocidalo JJ. Protection of rat from oxygen toxicity by inducers of cytochrome P-450 system. Am Rev Respir Dis. 1988a;137:688–694. doi: 10.1164/ajrccm/137.3.688. [DOI] [PubMed] [Google Scholar]
- Mansour H, Levacher M, Azoulay-Dupuis E, Moreau J, Marquetty C, Gougerot-Pocidalo MA. Genetic differences in response to pulmonary cytochrome P-450 inducers and oxygen toxicity. J Appl Physiol. 1988b;64:1376–1381. doi: 10.1152/jappl.1988.64.4.1376. [DOI] [PubMed] [Google Scholar]
- Moorthy B, Nguyen UT, Gupta S, Stewart KD, Welty SE, Smith CV. Induction and decline of hepatic cytochromes P4501A1 and 1A2 in rats exposed to hyperoxia are not paralleled by changes in glutathione S-transferase-alpha. Toxicol Lett. 1997;90:67–75. doi: 10.1016/s0378-4274(96)03832-5. [DOI] [PubMed] [Google Scholar]
- Moorthy B, Parker KM, Smith CV, Bend JR, Welty SE. Potentiation of oxygen-induced lung injury in rats by the mechanism-based cytochrome P-450 inhibitor, 1-aminobenzotriazole. J Pharmacol Exp Ther. 2000;292:553–560. [PubMed] [Google Scholar]
- Morel Y, Barouki R. Down-regulation of cytochrome P450 1A1 gene promoter by oxidative stress. Critical contribution of nuclear factor 1. J Biol Chem. 1998;273:26969–26976. doi: 10.1074/jbc.273.41.26969. [DOI] [PubMed] [Google Scholar]
- Morel Y, Mermod N, Barouki R. An autoregulatory loop controlling CYP1A1 gene expression: role of H(2)O(2) and NFI. Mol Cell Biol. 1999;19:6825–6832. doi: 10.1128/mcb.19.10.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59:65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- Northway WH, Jr., Rosan RC. Radiographic features of pulmonary oxygen toxicity in the newborn: Bronchopulmonary dysplasia. Radiology. 1968;91:49–58. doi: 10.1148/91.1.49. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Mitsuhashi M, Fujita I, Sindhu RK, Kikkawa Y. Induction of cytochrome P450 1A1 and 1A2 by hyperoxia. Biochem Biophys Res Commun. 1993;197:878–885. doi: 10.1006/bbrc.1993.2561. [DOI] [PubMed] [Google Scholar]
- Okey AB, Riddick DS, Harper PA. The Ah receptor: mediator of the toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds. Toxicol Lett. 1994;70:1–22. doi: 10.1016/0378-4274(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Paller MS, Jacob HS. Cytochrome P-450 mediates tissue-damaging hydroxyl radical formation during reoxygenation of the kidney. Proc Natl Acad Sci U S A. 1994;91:7002–7006. doi: 10.1073/pnas.91.15.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiners JJ, Jr., Clift R, Mathieu P. Suppression of cell cycle progression by flavonoids: dependence on the aryl hydrocarbon receptor. Carcinogenesis. 1999;20:1561–1566. doi: 10.1093/carcin/20.8.1561. [DOI] [PubMed] [Google Scholar]
- Shervington A, Mohammed K, Patel R, Lea R. Identification of a novel co-transcription of P450/1A1 with telomerase in A549. Gene. 2007;388:110–116. doi: 10.1016/j.gene.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Shiraki H, Guengerich FP. Turnover of membrane proteins: kinetics of induction and degradation of seven forms of rat liver microsomal cytochrome P-450, NADPH-cytochrome P-450 reductase, and epoxide hydrolase. Arch Biochem Biophys. 1984;235:86–96. doi: 10.1016/0003-9861(84)90257-1. [DOI] [PubMed] [Google Scholar]
- Sindhu RK, Sakai H, Kikkawa Y. Effect of hyperoxia on rat pulmonary and hepatic cytochrome P450 monooxygenases. Arch Toxicol. 2000;73:540–546. doi: 10.1007/s002040050006. [DOI] [PubMed] [Google Scholar]
- Sinha A, Muthiah K, Jiang W, Couroucli X, Barrios R, Moorthy B. Attenuation of hyperoxic lung injury by the CYP1A inducer beta-naphthoflavone. Toxicol Sci. 2005;87:204–212. doi: 10.1093/toxsci/kfi226. [DOI] [PubMed] [Google Scholar]
- Whitlock JP., Jr. Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- Whitlock JP, Jr., Okino ST, Dong L, Ko HP, Clarke-Katzenberg R, Ma Q, Li H. Cytochromes P450 5: induction of cytochrome P4501A1: a model for analyzing mammalian gene transcription. Faseb J. 1996;10:809–818. doi: 10.1096/fasebj.10.8.8666157. [DOI] [PubMed] [Google Scholar]
- Yang F, Coalson JJ, Bobb HH, Carter JD, Banu J, Ghio AJ. Resistance of hypotransferrinemic mice to hyperoxia-induced lung injury. Am J Physiol. 1999;277:L1214–1223. doi: 10.1152/ajplung.1999.277.6.L1214. [DOI] [PubMed] [Google Scholar]