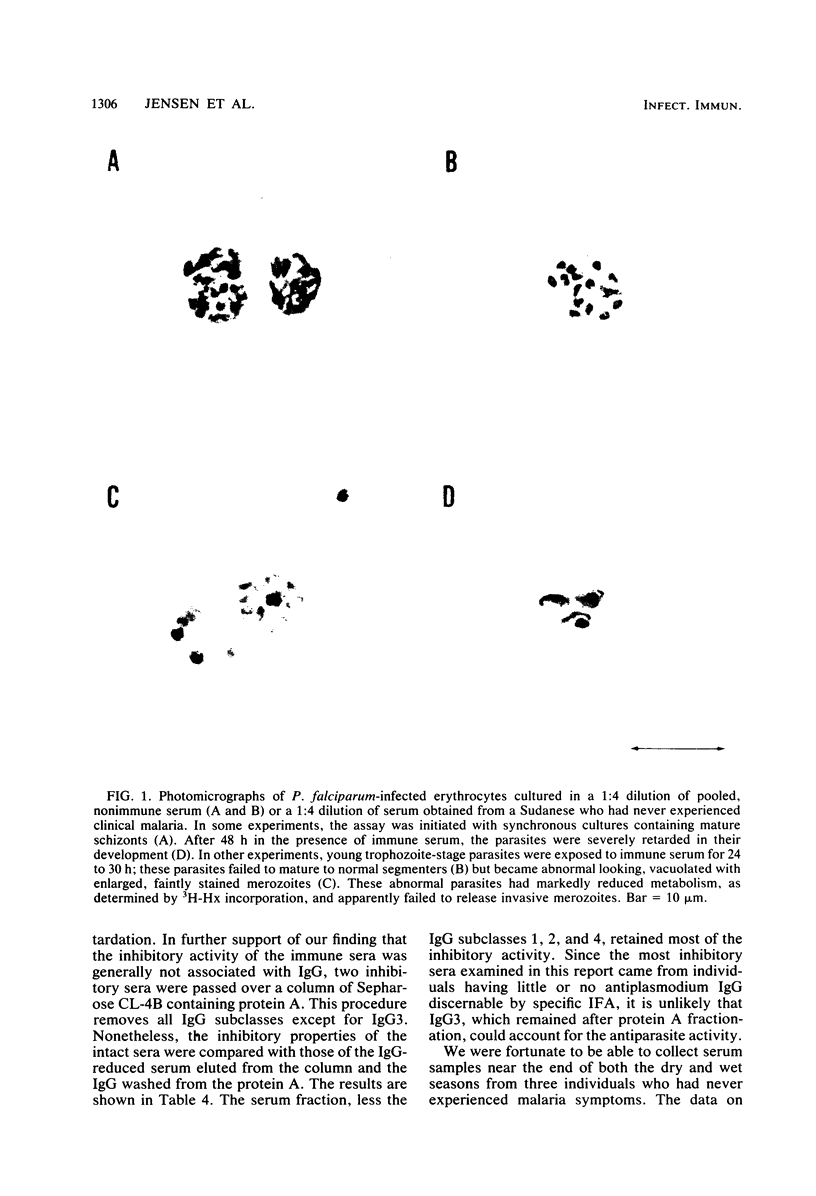
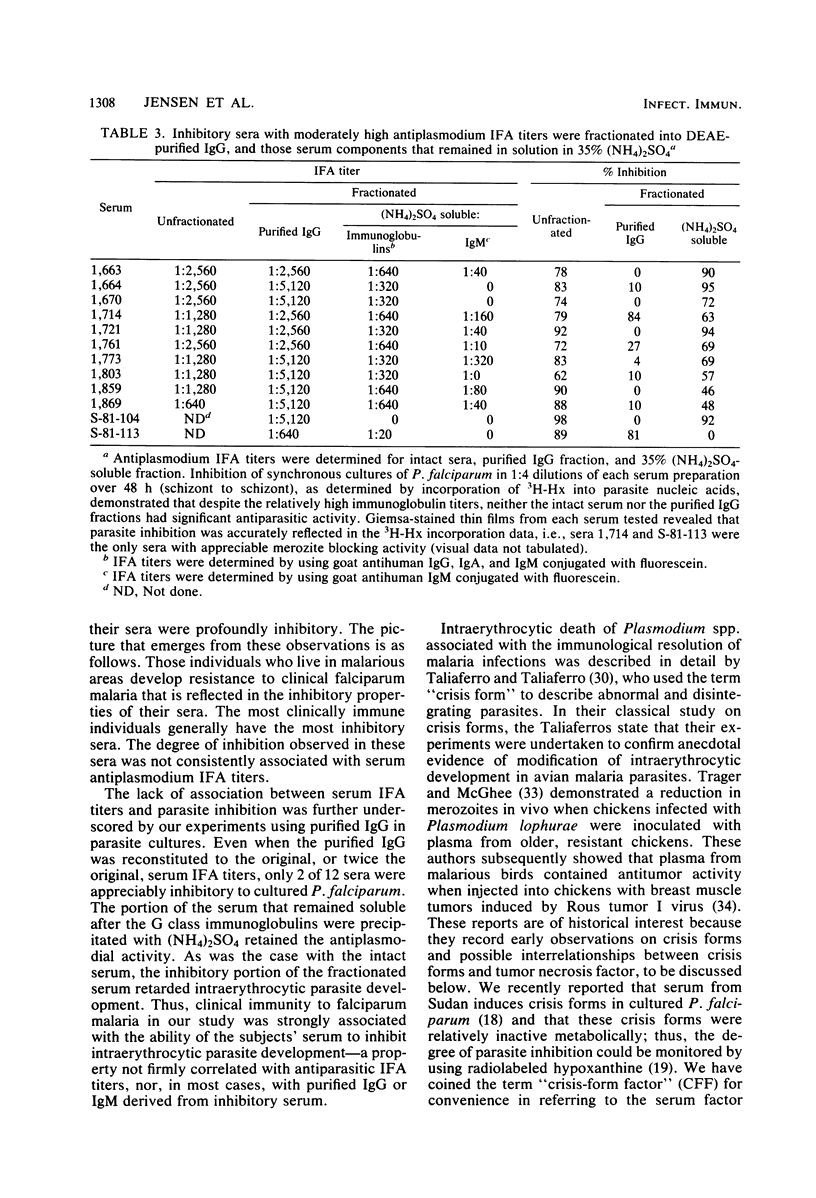
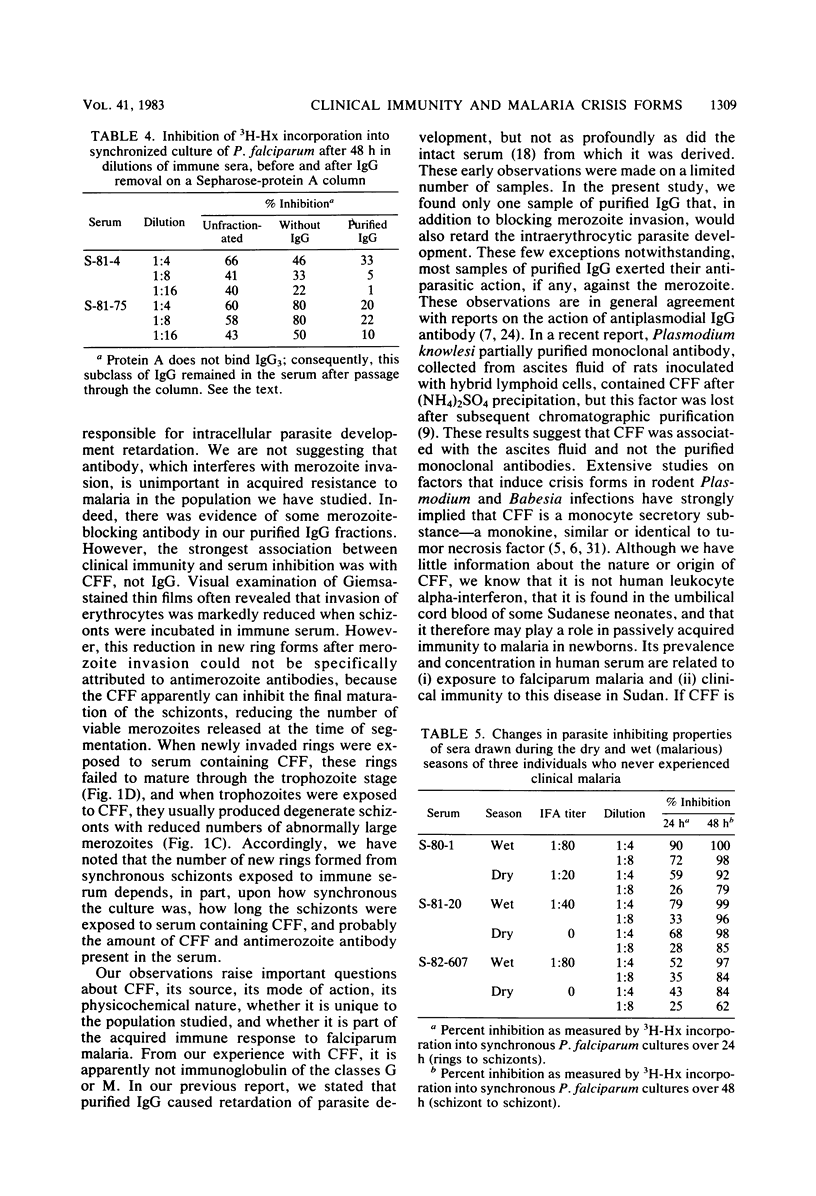
Abstract
Clinical histories with regard to falciparum malaria were collected from adults living in holo-, hyper-, and hypoendemic areas of Sudan and matched to serum samples which were assayed for antiparasitic activity in cultures of Plasmodium falciparum. The adult population of the endemic areas could be divided into three groups based on oral histories: those who never experience falciparum malaria; those with a childhood history of malaria, who experience only mild occasional malaria as adults; and those who suffer serious recurring malaria symptoms. In vitro parasite inhibition was greatest with sera from individuals with no clinical histories of malaria, and generally, more inhibition was noted in sera from holoendemic versus hyperendemic areas. Serum from hypoendemic urban Khartoum was not inhibitory. There was no relationship between serum indirect fluorescent antibody titers and parasite inhibition, but there was strong association between clinical immunity and intraerythrocytic parasite inhibition resulting in "crisis" forms. Purified immunoglobulin G was not strongly associated with crisis forms, which were consistently associated with fractions of immune serum remaining after immunoglobulin removal. Thus, it appears that clinical immunity to malaria in Sudan is based on nonantibody serum factors, possibly associated with cell-mediated immunity. Human leukocyte alpha-interferon had no inhibitory effects on cultured P. falciparum. Some umbilical cord sera were profoundly inhibitory, producing crisis forms, whereas others were not inhibitory, suggesting that factors that induce crisis forms may play a role in protecting neonates from falciparum malaria.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C. Protection afforded by sickle-cell trait against subtertian malareal infection. Br Med J. 1954 Feb 6;1(4857):290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. V., Anders R. F., Stace J. D., Alpers M. P., Mitchell G. F. Immunoprecipitation of biosynthetically-labelled proteins from different Papua New Guinea Plasmodium falciparum isolates by sera from individuals in the endemic area. Parasite Immunol. 1981 Winter;3(4):283–298. doi: 10.1111/j.1365-3024.1981.tb00407.x. [DOI] [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Campbell G. H., Mrema J. E., O'Leary T. R., Jost R. C., Rieckmann K. H. In vitro inhibition of the growth of Plasmodium falciparum by Aotus serum. Bull World Health Organ. 1979;57 (Suppl 1):219–225. [PMC free article] [PubMed] [Google Scholar]
- Chulay J. D., Haynes J. D., Diggs C. L. Inhibition of in vitro growth of Plasmodium falciparum by immune serum from monkeys. J Infect Dis. 1981 Sep;144(3):270–278. doi: 10.1093/infdis/144.3.270. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Butcher G. A. Properties of protective malarial antibody. Immunology. 1970 Aug;19(2):369–383. [PMC free article] [PubMed] [Google Scholar]
- Deans J. A., Alderson T., Thomas A. W., Mitchell G. H., Lennox E. S., Cohen S. Rat monoclonal antibodies which inhibit the in vitro multiplication of Plasmodium knowlesi. Clin Exp Immunol. 1982 Aug;49(2):297–309. [PMC free article] [PubMed] [Google Scholar]
- Freeman R. R., Trejdosiewicz A. J., Cross G. A. Protective monoclonal antibodies recognising stage-specific merozoite antigens of a rodent malaria parasite. Nature. 1980 Mar 27;284(5754):366–368. doi: 10.1038/284366a0. [DOI] [PubMed] [Google Scholar]
- Friedman M. J. Erythrocytic mechanism of sickle cell resistance to malaria. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1994–1997. doi: 10.1073/pnas.75.4.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen A. A., Jr, Jensen J. B. Mitogenic activity of extracts from continuous cultures of Plasmodium falciparum. Am J Trop Med Hyg. 1982 May;31(3 Pt 1):441–448. doi: 10.4269/ajtmh.1982.31.441. [DOI] [PubMed] [Google Scholar]
- Green T. J., Morhardt M., Brackett R. G., Jacobs R. L. Serum inhibition of merozoite dispersal from Plasmodium falciparum schizonts: indicator of immune status. Infect Immun. 1981 Mar;31(3):1203–1208. doi: 10.1128/iai.31.3.1203-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Oduloju A. J., Platts-Mills T. A. Partial characterization of a malaria mitogen. Trans R Soc Trop Med Hyg. 1979;73(2):178–182. doi: 10.1016/0035-9203(79)90204-9. [DOI] [PubMed] [Google Scholar]
- Hall C. L., Haynes J. D., Chulay J. D., Diggs C. L. Cultured Plasmodium falciparum used as antigen in a malaria indirect fluorescent antibody test. Am J Trop Med Hyg. 1978 Sep;27(5):849–852. doi: 10.4269/ajtmh.1978.27.849. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Boland M. T., Akood M. Induction of crisis forms in cultured Plasmodium falciparum with human immune serum from Sudan. Science. 1982 Jun 11;216(4551):1230–1233. doi: 10.1126/science.7043736. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Boland M. T., Hayes M., Akood M. A. Plasmodium falciparum: rapid assay for in vitro inhibition due to human serum from residents of malarious areas. Exp Parasitol. 1982 Dec;54(3):416–424. doi: 10.1016/0014-4894(82)90051-0. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Capps T. C., Carlin J. M. Clinical drug-resistant falciparum malaria acquired from cultured parasites. Am J Trop Med Hyg. 1981 May;30(3):523–525. doi: 10.4269/ajtmh.1981.30.523. [DOI] [PubMed] [Google Scholar]
- Jensen J. B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg. 1978 Nov;27(6):1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Trager W. Plasmodium falciparum in culture: use of outdated erthrocytes and description of the candle jar method. J Parasitol. 1977 Oct;63(5):883–886. [PubMed] [Google Scholar]
- Kilejian A., Abati A., Trager W. Plasmodium falciparum and Plasmodium coatneyi: immunogenicity of "knob-like protrusions" on infected erythrocyte membranes. Exp Parasitol. 1977 Jun;42(1):157–164. doi: 10.1016/0014-4894(77)90073-x. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Luzzatto L., Usanga F. A., Reddy S. Glucose-6-phosphate dehydrogenase deficient red cells: resistance to infection by malarial parasites. Science. 1969 May 16;164(3881):839–842. doi: 10.1126/science.164.3881.839. [DOI] [PubMed] [Google Scholar]
- Myler P., Saul A., Mangan T., Kidson C. An automated assay of merozoite invasion of erythrocytes using highly synchronized Plasmodium falciparum cultures. Aust J Exp Biol Med Sci. 1982 Feb;60(Pt 1):83–89. doi: 10.1038/icb.1982.6. [DOI] [PubMed] [Google Scholar]
- Nurse G. T. Iron, the thalassaemias, and malaria. Lancet. 1979 Nov 3;2(8149):938–940. [PubMed] [Google Scholar]
- Playfair J. H. Immunity to malaria. Br Med Bull. 1982 May;38(2):153–159. doi: 10.1093/oxfordjournals.bmb.a071752. [DOI] [PubMed] [Google Scholar]
- Reese R. T., Motyl M. R., Hofer-Warbinek R. Reaction of immune sera with components of the human malarial parasite, Plasmodium falciparum. Am J Trop Med Hyg. 1981 Nov;30(6):1168–1178. doi: 10.4269/ajtmh.1981.30.1168. [DOI] [PubMed] [Google Scholar]
- Reese R. T., Motyl M. R. Inhibition of the in vitro growth of Plasmodium falciparum. I. The effects of immune serum and purified immunoglobulin from owl monkeys. J Immunol. 1979 Oct;123(4):1894–1899. [PubMed] [Google Scholar]
- TRAGER W., MCGHEE R. B. Inhibition of chicken tumor I by plasma from chickens infected with an avian malaria parasite. Proc Soc Exp Biol Med. 1953 Jun;83(2):349–352. doi: 10.3181/00379727-83-20356. [DOI] [PubMed] [Google Scholar]
- Taverne J., Dockrell H. M., Playfair J. H. Endotoxin-induced serum factor kills malarial parasites in vitro. Infect Immun. 1981 Jul;33(1):83–89. doi: 10.1128/iai.33.1.83-89.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]



