Abstract
BALB-neuT mice expressing an activated rat c-erbB-2/neu transgene under the mouse mammary tumor virus long terminal repeat show enhanced hematopoiesis with hyperproduction of myeloid-derived suppressor cells (MDSC) because of vascular endothelial growth factor (VEGF) secreted by the tumor. Here, we show that both tumor and stromal cells express matrix metalloproteinase-9 (MMP-9), thereby increasing the levels of pro–MMP-9 in the sera of tumor-bearing mice. Treatment with amino-biphosphonates impaired tumor growth, significantly decreased MMP-9 expression and the number of macrophages in tumor stroma, and reduced MDSC expansion both in bone marrow and peripheral blood by dropping serum pro–MMP-9 and VEGF. We dissected the role of tumor-derived MMP-9 from that secreted by stromal leukocytes by transplanting bone marrow from MMP-9 knockout mice into BALB-neuT mice. Although bone marrow progenitor–derived MMP-9 had a major role in driving MDSC expansion, amino-biphosphonate treatment of bone marrow chimeras further reduced both myelopoiesis and the supportive tumor stroma, thus enhancing tumor necrosis. Moreover, by reducing MDSC, amino-biphosphonates overcome the tumor-induced immune suppression and improved the generation and maintenance of antitumor immune response induced by immunization against the p185/HER-2. Our data reveal that suppression of MMP-9 activity breaks the vicious loop linking tumor growth and myeloid cell expansion, thus reducing immunosuppression. Amino-biphosphonates disclose a specific MMP-9 inhibitory activity that may broaden their application above their current usage.
Introduction
Alteration in bone marrow hematopoiesis and immune response almost invariably accompanies tumor growth. Myeloid cell expansion often occurs in response to tumor-secreted cytokines and growth factors that foster bone marrow activity (1, 2). Such expanded myeloid cells contribute to tumor progression, providing both supporting stroma and immune evasion (3-8). In the tumor stroma, they provide growth factors, extracellular matrix proteins, and matrix-degrading enzymes that create new space for growing tumors by remodeling nearby tissue and by contributing to metastatic processes and angiogenesis. Moreover, they promote immune escape by inhibiting lymphocyte activation (7, 9-11) and antigen recognition (12).
Transgenic female mice expressing the transforming rat oncogene c-erbB-2 (HER-2/neu) under the mouse mammary tumor virus promoter (BALB-neuT) spontaneously develop mammary carcinomas with a progression resembling that of human breast cancer. In BALB-neuT mice, vascular endothelial growth factor (VEGF) released by the tumor is the main factor that conditions hematopoiesis toward the massive expansion of a Gr1/CD11b double-positive, immature myeloid cell population (2). These cells inhibit T-lymphocyte proliferation in response to allogeneic and T-cell antigen receptor–mediated stimulation and, because of their immunosuppressive activity, they have been named myeloid-derived suppressor cells (MDSC; ref. 13). In tumor-bearing animals, MDSC expansion is a reversible phenomenon, as tumor removal or transplantation of hyperplastic bone marrow into tumor-free mice results in normal hematopoiesis and rescue of the immune response (2).
MDSCs require matrix metalloproteinase-9 (MMP-9) for their function (5). Moreover, MMP-9 is a stromal factor that regulates the mobilization of hemopoietic stem cells from the bone marrow niche by solubilizing the membrane-bound form of c-KitL (14, 15). Because it remodels the extracellular matrix and promotes sprouting and growth of new blood vessels by making VEGF available to the VEGFR-2/flk receptor on endothelial cells, MMP-9 is also a linchpin in tumor progression (15-18).
A logical therapeutic approach to cancer would therefore include inhibiting MMP-9. However, MMP inhibitors have failed or shown toxicity in several clinical trials (19, 20). Amino-biphosphonates offers an interesting alternative. Their current application in cancer therapy for treatment of bone metastases and associated pain owes to their well-known toxic effect on osteoclasts and inhibition of tumor adhesion to mineralized matrices (21, 22). In addition to these activities, amino-biphosphonates inhibit MMP-9 expressed by the cells in the tumor stroma and thereby, impair VEGF availability, thus reducing tumor angiogenesis (23). These observations suggest that amino-biphosphonates potentially could be used to inhibit some MMP-9 effects perhaps without the toxicity of less selective inhibitors. In this study, we investigated whether amino-biphosphonates can antagonize tumor-induced MDSC expansion by inhibiting the MMP-9–VEGF loop that forces hyperactive hematopoiesis. To determine the relative roles of tumor and inflammatory cell–produced MMP-9 and the relative effect of amino-biphosphonate treatment, we used bone marrow chimeras to knock down MMP-9 gene expression in cells of hemopoietic origin that become part of tumor stromal microenvironment. We tested the effects on MDSC immunosuppression by combining amino-biphosphonates with vaccination in function of tumor onset and growth, as well as induction and maintenance of anti–r-p185/ HER-2 antibody response.
Materials and Methods
Mice and treatments
BALB-neuT mice were bred and maintained in the animal facility at the Istituto Nazionale Tumori, according to the national and institutional guidelines. Normal 8 to 10-week-old BALB/c, C57BL/6, and FVB mice were purchased from Charles River. F1 hybrids were obtained by mating BALB-neuT males with C57BL/6 or FVB females. The hemizygous transgenic females were identified by PCR performed at age 3 weeks (24).
MMP-9+/− mice on C57BL/6 background were kindly provided by Dr. Leif Lund (Panum Institute, Department of Experimental Medicine, University of Copenhagen, Copenhagen, Denmark) as N17 generation. They were backcrossed to BALB/c and the N6 generation, intercrossed to obtain either homozygous MMP-9−/− and heterozygous MMP-9+/− offspring to be used as bone marrow donors.
Zoledronate (Zometa; Novartis Europharm, Ltd.) at a dose of 0.1 mg/kg or pamidronate (Faulding Pharmaceuticals) at a dose of 2 mg/kg were diluted in saline and administered daily s.c. 5 days a week. Mice were weighed weekly, and the drug concentration was adjusted to their effective weight. Control mice received 0.2 mL of saline daily s.c. To control treatment nephrotoxicity, mice creatinine serum level was monitored after 12, 17, and 23 weeks of treatment by the colorimetric Jaffè method (25).
Immunotherapy was administered to (FVB×BALB–neuT)F1 females starting at 7 weeks and repeated at ages 9, 11, and 13 weeks. Plasmid DNA encoding the extracellular and transmembrane domain (ECD-TM) of the rat p185-HER-2 (26) was prepared using the EndoFree Plasmid Giga kit (Qiagen SpA) according to the manufacturer’s instruction. Mice under ketamine/ xylazine anesthesia were injected into the dorsal side of both ears with 0.05 mg of DNA in 0.05 mL of saline. A group of mice that received DNA vaccination were already treated with Zoledronate 0.1 mg/d s.c. starting at age 4 weeks.
All treated and control transgenic females were monitored weekly for mammary tumor development and progression and for myeloid cell expansion in the peripheral blood (PB). Mammary tumors were measured by calipers and tumor volume expressed as (l2 × L), where l represents the shorter diameter and L the longer one. The sum of the single tumor volumes represented the overall tumor volume, whereas tumor multiplicity was the number of mammary glands with a measurable tumor.
Immunohistochemistry and cytofluorimetric analysis
For immunohistochemistry, mammary tumors were included in optimum cutting temperature compound and snap frozen in liquid nitrogen. Acetone-fixed 5-μm cryostat sections were placed in 3% H2O2/methanol solution for 5 min to block endogenous peroxidase activity, rinsed in PBS, blocked in 5% FCS for 20 min, and immunostained with Abs anti-CD11b/CD18 (clone M1/70.5; BD Bioscience), anti–Gr-1 (clone RB6-8C5; BD Bioscience), anti-F4/80 (clone CI, A3-1; Caltag/Invitrogen), anti–MMP-9 (MAB 13415; Chemicon International), anti-CD31 (platelet/endothelial cell adhesion molecule 1; BD Bioscience).
After washing, sections were overlaid with biotinylated antimouse or antirat Ab (Vector Laboratories) for 30 min. Unbound immunoglobulin was removed by washing, and slides were incubated with avidin-peroxidase complex (DAKO) and revealed with 3,3′-diaminobenzidine (Sigma Aldrich). Sections were counterstained with Mayer’s hematoxylin, dehydrated in graded alcohol (70%, 95%, and 100% ethanol), and mounted in BDH mounting medium (Merck). All immunostainings were repeated five times with multiple sections, including negative controls for determination of background staining. All images were digitally captured on a microscope (Eclipse E-1000; Nikon) equipped with a digital camera (DXM1200; Nikon) and analyzed using ACT-1 software.
Analysis of circulating MDSC was done as described (2): 0.2 mL of PB was collected from retroorbital sinus and mixed with equal volume of 5 mmol/L EDTA, erythrocytes were lysed by hypotonic shock in NH4Cl lysis buffer, and leukocytes were counted. Cells were blocked with 10 μg/mL of rat antimouse CD16/CD32 Ab (clone 2.4G2; BD PharMingen) before staining with the combination of rat antimouse FITC-Gr-1 and PE-CD11b Abs (Caltag/Invitrogen) at 5 μg/mL (5 μg/106 cells) in 2% bovine serum albumin (BSA), 0.05% azide in PBS for 40 min in ice. Cells were washed thrice in PBS-2% BSA and analyzed by FACScalibur (Becton Dickinson) for double-positive cells.
To analyze the tumor stroma, spontaneous mammary tumors were removed, minced in 2 mL Hank’s Balanced Salt Solution (HBSS) buffer containing collagenase D (1 mg/mL), and incubated 40 min at 37°C. The enzymatic digestion was stopped with 2 mL of HBBS/5 mol/L EDTA, and the tumor was reduced to a single-cell suspension by gently pipetting with syringe and then filtering through a 70-μm cell strainer (Falcon; Becton Dickinson Labware Europe). Cells were processed and immunostained as described above with combinations of PE-CD11b with FITC-Gr-1, FITC-F4/80 (all from Caltag) or FITC-CD11c (clone HL-3; BD PharMingen), or combination of FITC- and PE-conjugated isotypic controls (BD PharMingen). Analysis was performed on a FACSCalibur E2551 cytometer (Becton Dickinson). Data were collected on 104 viable cells and analyzed using CellQuest software.
VEGF and pro–MMP-9 detection
Blood from treated and untreated mice was collected from the retroorbital sinus and allowed to clot at room temperature for 2 h. After centrifugation at 2,000 rpm for 10 min, serum was transferred, aliquoted, and stored frozen until assayed. Serum VEGF and pro–MMP-9 were measured by sandwich ELISA using the Murine VEGF Development kit (Peprotech, Inc.) and the Quantikine pro–MMP-9 ELISA kit (R&D System), respectively, according to manufacturer’s instruction.
Bone marrow colony forming units assay
Bone marrow cells were obtained by flushing femurs and tibiae with 1 mL of Iscove’s modified Dulbecco’s medium (IMDM; Life Technologies Invitrogen Corp.) supplemented with 2% heat-inactivated FCS (Bio Whittaker Europe), using a 22-g needle attached to a 1-mL syringe. Bone marrow cells were gently aspirated several times using the same syringe to obtain a single-cell suspension, and erythrocytes were removed by hypotonic lysis with NH4Cl buffer.
Bone marrow cell suspensions were diluted to 2 × 105 cells/mL in IMDM 2% FCS medium, and 0.3 mL of cells were mixed with 3 mL of MethoCult GF M3434 complete methylcellulose medium (StemCell Technologies, Inc.). Cells were plated in duplicate in 35-mm dishes, at 2 to 2.5 × 104 cells/dish, and incubated for 13 days at 37°C and a 5% CO2-humidified incubator. Colonies were scored for granulocyte-macrophage colony-forming unit (CFU-GM), colony-forming unit-granulocyte, erythrocyte, monocyte, megakaryocyte (CFU-GEMM), and burst-forming unit-erythroid (BFU-E) morphology at days 7, 10, and 13, and the sum of all three types was recorded as bone marrow–colony-forming unit (CFU).
Bone marrow transplantation
Groups of 7- to 8-week-old (C57Bl6xBALB-neuT)F1 females were lethally irradiated with a total dose of 950 cGy given in two doses with a 3-h interval. Mice were injected i.v. with bone marrow cells (107 cells/mouse) obtained from MMP-9−/− or MMP-9+/− mice.
Bone marrow engraftment was monitored by immunofluorescence for disappearance of MHC H-2Kb and expression of H-2Kd on PB lymphocytes. Nontransplanted irradiated control mice died within 2 weeks.
Half of the transplanted mice were treated daily with Zoledronate starting at age 7 weeks, whereas the others were injected daily with saline.
Cytofluorimetric evaluation of anti–r-p185/HER-2 response
Sera were collected from treated and control (FVB×BALB-neuT)F1 mice beginning 4 weeks after the last DNA vaccination and every 15 days thereafter. The presence of anti–r-p185/HER-2 antibodies was analyzed by flow cytometry, using the N202-1A and N202-1E cell lines, respectively, positive and negative for r-p185/HER-2 (27). Cells (5 × 105) were incubated for 30 min in ice with 0.1 mL of test sera or control normal serum diluted in PBS-2% BSA-0.05% azide. After washing in the same buffer, cells were incubated for 30 min with 1:60 dilution of biotinylated goat antimouse IgG (Amersham) followed by 1:150 dilution of streptavidin-PE (BD PharMingen). Cells were washed, resuspended in PBS-2% BSA, and analyzed by a FACSCalibur E2551 cytometer (Becton Dickinson). Data were collected on 104 viable cells and analyzed using CellQuest software. Sera recognizing only N202-1A and not N202-1E were considered positive for anti–r-p185/HER-2 antibody response.
The specific serum-binding potential, calculated according to Quaglino et al. (28), is as follows: (% of positive cells with test serum × fluorescence mean)−(% of positive cells with control serum × fluorescence mean) × serum dilution.
Statistical analysis
Statistical analysis was performed with GraphPad Prism software (GraphPad Software, Inc.). Data comparing differences between two groups were assessed using unpaired two-tailed Student’s t test with 95% confidence interval and the nonparametric Mann-Whitney test. Differences were considered significant when the P value is <0.05.
Results
MMP-9 expression in BALB-neuT mice and its down-modulation by amino-biphosphonates in mammary tumors
BALB-neuT mice develop multiple, multifocal mammary carcinomas whose progression is associated with expansion of MDSC and increased serum level of VEGF (2). Because VEGF availability to its receptor VEGFR-2/Flk is regulated by MMP-9 (17), we investigated MMP-9 expression during mammary tumor progression. We detected increased levels of pro–MMP-9 in sera of tumor-bearing, but not tumor-free, BALB-neuT females by ELISA (Fig. 1A), and immunohistochemistry revealed high levels of MMP-9 expression in leukocytes scattered within the stroma, as well as a lower expression in tumor cells of untreated mice (Fig. 1B). MMP-9 expression by mammary tumor cells was confirmed by Western blot analysis of tumor cell lines derived from primary tumors (Supplementary Fig. S1). To test whether MMP-9 expression, VEGF secretion, and MDSC expansion are related, we treated BALB-neuT mice with amino-biphosphonates that have been reported to reduce MMP-9 production by inhibiting the mevalonate pathway in normal- and tumor-infiltrating macrophages (23, 29). According to their relative efficacy (30), pamidronate and zole-dronate were given at 2 and 0.1 mg/kg, respectively, starting at ages 4, 7, or 12 weeks and continued daily to the age of 28 weeks. Although prolonged amino-biphosphonate administration can induce nephrotoxicity, creatinine serum levels monitored after 12, 17, and 23 weeks of treatment and weekly body weight did not show difference between treated and control mice (data not shown), indicating that repeated administration of zoledronate or pamidronate at the indicated doses has apparently no toxicity in mice.
Figure 1.
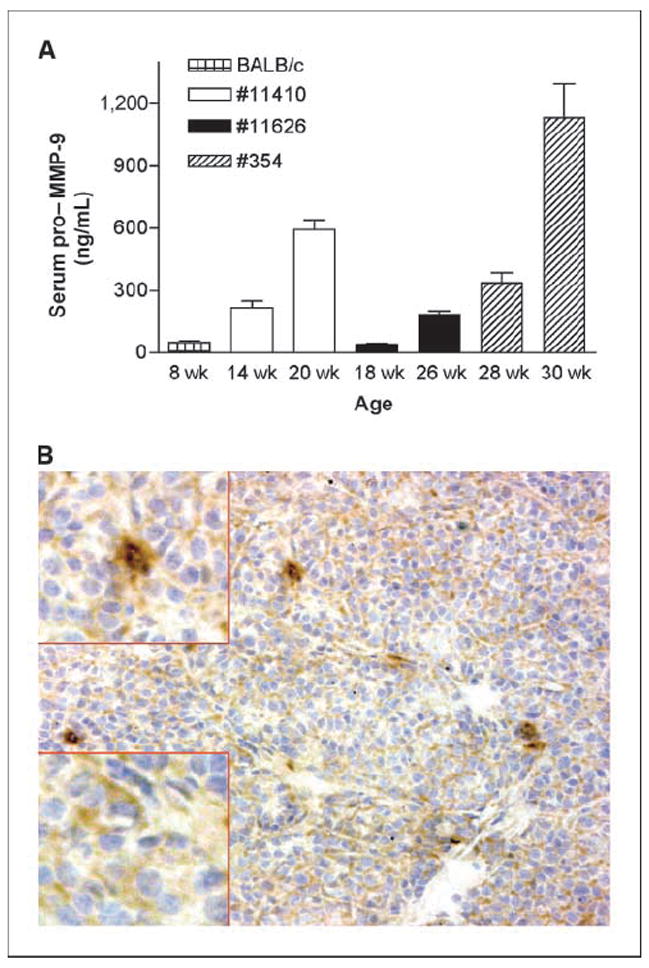
MMP-9 expression in BALB-neuT mice. A, pro–MMP-9 serum levels detected in single BALB-neuT by ELISA. Serum pro–MMP-9 increased along with age and tumor development in three representative females. Bars, SD. B, MMP-9 in a mammary tumor analyzed by immunohistochemistry. Both tumor cells and stromal macrophages produce MMP-9, although at different levels: tumor cells show a diffuse cytoplasmic positivity (bottom left, inset), whereas macrophages have a stronger and granular positivity (top left, inset; magnification, ×20; inset magnification, ×40).
Mice treated before (4 wk) or at the time of hyperplasia (7 wk) with either pamidronate or zoledronate showed delayed tumor onset, reduced number of transformed mammary glands, as well as decreased volume of outgrowing tumors. Also at 12 weeks, when mice have already numerous in situ carcinomas detectable by whole-mount analysis, the treatment was still effective in reducing the overall tumor volume (Fig. 2A). The group of mice treated from the age of 4 weeks showed a statistically significant reduction in the overall tumor volume at any time point compared with the other groups of treatment, suggesting that amino-biphosphonates act early during mammary cells transformation or that the more prolonged is the treatment, the stronger is the tumor growth inhibition (Fig. 2B). In absence of any significant differences between pamidronate and zoledronate, we continued our study with zoledronate and tested its effect on MMP-9, trying to distinguish tumor- and stroma-derived MMP-9.
Figure 2.
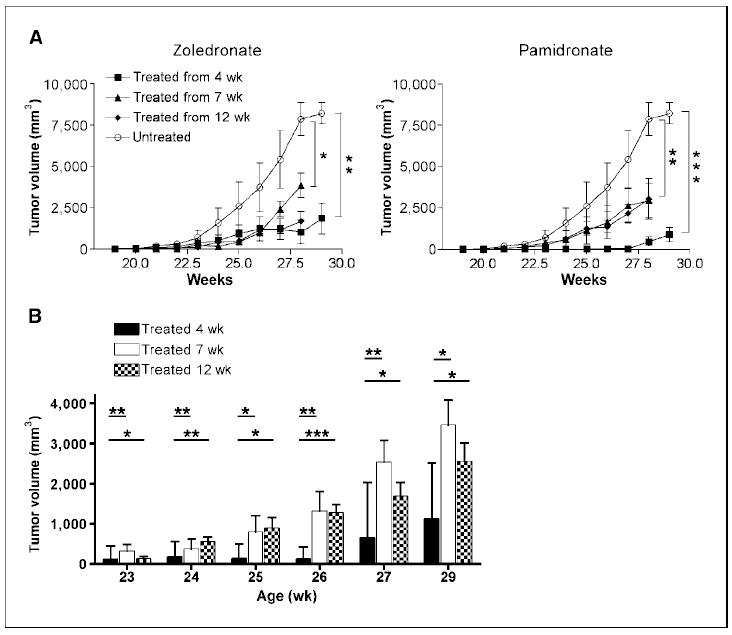
Effect of amino-biphosphonate treatment in BALB-neuT mice. Groups of BALB-neuT mice were treated five times per week with zoledronate or pamidronate, or with saline (untreated) starting at ages 4, 7, or 12 wk. The experiment was repeated twice to thrice with five to six animals per group of treatment, and the results were combined. A, tumor growth, expressed as the sum of the single tumor volumes, was significantly inhibited in all treatment groups compared with untreated controls. *, P = 0.01; **, P = 0.005 at 28 wk; **, P = 0.003; ***, P = 0.0001 at 29 wk. Bars, SE. B, weekly comparison of tumor volumes among different treatment schedules. Treatment started at age 4 wk significantly impairs tumor growth compared with both treatments started later; *, P = 0.01; **, P < 0.007; ***, P = 0.0006. Data from mice treated with zoledronate and pamidronate are combined. Bars, SE.
To test whether the expression of MMP-9 in tumors from BALB-neuT mice was responsible for the increased VEGF detectable in serum and for myeloid cell expansion during tumor progression, we examined the amino-biphosphonate–treated mice in greater detail. Regardless of the starting age, zoledronate treatment significantly decreased both pro–MMP-9 (P = 0.0009) and VEGF (P = 0.001) in the serum of tumor-bearing BALB-neuT mice (Fig. 3A) along with decreased tumor size, suggesting that impaired MMP-9 production within the tumor environment results in a reduction of VEGF detectable systemically. This confirms the key role of MMP-9 in the regulation of VEGF availability for the cognate receptors (17) and of VEGF-mediated recruitment of myeloid cells to sites of inflammation (31, 32).
Figure 3.
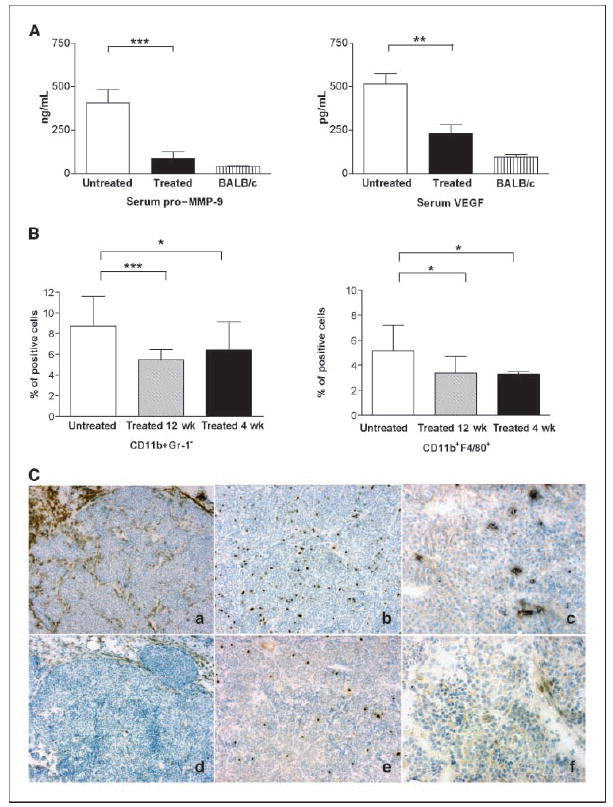
Zoledronate treatment reduces tumor-secreted factors and alters cell composition and function of tumor stroma. A, pro–MMP-9 and VEGF evaluation in tumor-bearing BALB-neuT. Sera from treated (n = 13) and untreated (n = 12) BALB-neuT mice were analyzed for pro–MMP-9 and VEGF content by ELISA and compared with normal BALB/c serum levels. ***, P = 0.0009; **, P = 0.001. Bars, SD. B, cytofluorimetric analysis of tumor stroma. Single-cell suspensions from primary tumors (n = 16–19) were labeled with CD11b-PE and Gr-1-FITC or F4/80-FITC antibodies. The mean percent of double-positive cells showed a significant reduction of CD11b/Gr-1 (***, P = 0.0003; *, P = 0.033) and CD11b/F4/80 (*, P = 0.04) expressing macrophages in the stroma of treated versus control mice. Bars, SD. C, immunohistochemistry analysis of untreated (top) and treated (bottom) mammary tumors stained with F4/80-(a–d), Gr-1– (b–e), and MMP-9–specific (c–f) antibodies. Zoledronate decreased the number of F4/80-positive macrophages (d versus a) and Gr-1–positive cells (e versus b) in the tumor stroma and dramatically reduced the MMP-9 expression in tumor and stroma cells (f versus c). Magnification, ×10.
In fact, amino-biphosphonate treatment also significantly reduced the infiltration of inflammatory cells that stained positively for CD11b, Gr-1, and F4/80 within the tumor stroma, as shown by fluorescence-activated cell sorting analysis (Fig. 3B). Immunostaining confirmed that amino-biphosphonates reduced tumor infiltrating and stromal leukocytes and inhibited MMP-9 expression in both stroma and tumor cells (Fig. 3C).
However, despite VEGF reduction, CD31 immunostaining of mammary tumors of equal size from either treated or untreated mice did not show difference in blood vessel number, distribution, and organization (data not shown). This suggests that decreased levels of VEGF, per se, did not impair tumor angiogenesis, likely because of redundant proangiogenic circuits existing in tumors (33).
Effects of zoledronate on hematopoiesis driven by tumor-secreted VEGF
Based on the above results, we next investigated whether amino-biphosphonates have any activity on tumor-induced hyperactive bone marrow hematopoiesis. Zoledronate treatment started at age 4 weeks significantly reduced myeloid cell expansion in the peripheral blood (Fig. 4A). However, Gr-1/CD11b–positive cells remaining in treated mice were as immunosuppressive as those from control mice, when added to CD3-stimulated BALB/c splenocytes at the same ratio, confirming their MDSC nature (data not shown).
Figure 4.
Zoledronate inhibits tumor-enhanced bone marrow (BM) hematopoiesis. A, the expansion of Gr-1/CD11b double-positive cells during tumor progression was significantly reduced in the PB of treated versus control BALB-neuT. *, P = 0.01; **, P = 0.003; bars, SE. B, the treatment modified the ratio between the number of circulating Gr-1/CD11b–positive cells and the overall tumor volume. The tumor mass required to induce similar number of circulating MDSC is larger in treated than in untreated mice. Bars, SD. C, the total number of bone marrow colonies obtained from BALB-neuT in methylcellulose (CFU-GEMM, CFU-GM, and BFU-E) was significantly reduced by zoledronate and similar to that of tumor-free BALB/c. **, P = 0.0019; ***, P = 0.0005. Bars, mean. D, chronic zoledronate treatment showed no toxic effect on normal bone marrow hematopoiesis of BALB/c mice, as measured by the sum of bone marrow colonies obtained in methylcellulose. Bars, mean.
Although smaller tumors should release lower amount of factors conditioning the bone marrow, the observed decrease was even higher than that expected from the decreased tumor size; it is of note that to obtain a similar number of circulating Gr-1/CD11b–positive cells, zoledronate-treated tumors would have to be six to eight times larger than untreated tumors (Fig. 4B). These data indicate that zoledronate impairs the bone marrow conditioning in addition to its effects on tumor size.
To determine the nature of this inhibitory effect, we asked whether amino-bisphosphonate affects the number of clonogenic colony-forming progenitor cells present in bone marrow. The clonogenic assay in methylcellulose medium showed a significant reduction (P = 0.0005) in the total number of colonies from bone marrow of treated than untreated mice (Fig. 4C). Particularly the number of CFU-GM and CFU-GEMM was significantly decreased in bone marrow from treated mice (P = 0.0056 and P < 0.0001, respectively; data not shown). These results confirm that amino-biphosphonates impair both expansion of bone marrow progenitor cells and tumor growth. To rule out any toxic effect of amino-biphosphonates on normal hemopoietic precursors, we treated BALB/c mice with zoledronate for 16 weeks before testing their bone marrow cells for colony formation. Virtually identical number of colonies were obtained from bone marrow of treated and untreated mice, indicating that the action of zoledronate is confined to tumor-enhanced but not normal hematopoiesis (Fig. 4D).
Genetic ablation of MMP-9 in bone marrow–derived stromal cells dissects the effects of zoledronate on tumor growth and bone marrow hematopoiesis
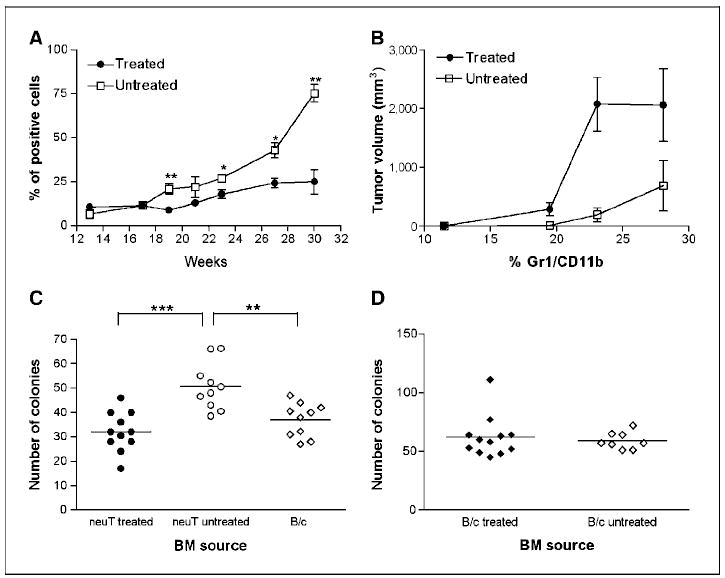
To investigate the distinct roles of MMP-9 produced by the tumor cells (low level expressed by almost every cell) and by the stromal leukocytes (high level in just a few cells scattered throughout the tumor; Fig. 1B), during tumor development and hemopoietic conditioning, we produced bone marrow chimeras. Bone marrow cells from MMP-9 knockout (MMP-9−/−) or competent (MMP-9+/−) mice were transplanted into transgenic mice, and tumor onset and progression as well as bone marrow conditioning were monitored. In both types of chimera, the tumor-derived MMP-9 would be present.
Spontaneous mammary tumors developed in bone marrow chimeras transplanted with either MMP-9−/− or MMP-9+/− bone marrow, with similar kinetics and progression (Fig. 5A; open symbols). Immunohistochemistry revealed a comparable number and distribution of CD11b and F4/80 positive cells in tumors from both types of chimeras (Supplementary Fig. S2A–B). In parallel with tumor development, MMP-9+/−>neuT chimeric mice underwent hematopoietic expansion showing increased number of bone marrow colonies, whereas in the MMP-9−/−>neuT chimeras, the colony number was not significantly different from bone marrow of normal BALB/c mice (Fig. 5B; open symbols). These data suggest that leukocyte-produced MMP-9 is dispensable for infiltration, stroma organization, and outgrowth (Fig. 5A) but is necessary to trigger bone marrow hyperplastic hematopoiesis through VEGF availability (Fig. 5B).
Figure 5.
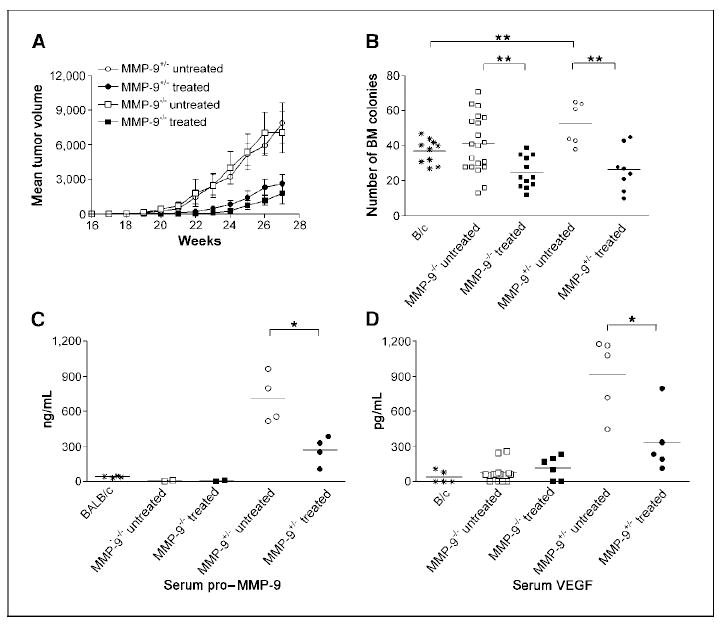
Dissection of tumor and stroma MMP-9 functions and response to zoledronate in bone marrow chimeras. A, lethally irradiated transgenic mice transplanted with bone marrow from MMP-9−/− or MMP-9+/− donors were treated with zoledronate or saline (untreated) and monitored for tumor growth. The experiment was repeated twice with six mice per group, and results were combined. MMP-9 expression by stroma cells of bone marrow origin was irrelevant for tumor onset and growth (open symbols), and zoledronate inhibited tumor growth in both bone marrow chimeras (filled symbols). Bars, SE. B, tumor outgrowth was associated with increased hematopoiesis in mice transplanted with MMP-9+/− (P = 0.005) but not with MMP-9−/− bone marrow, as shown by the total number of bone marrow colonies in methylcellulose. Zoledronate treatment significantly reduced the number of bone marrow colonies in both chimeras, normalizing hematopoiesis in tumor bearers. **, P = 0.002 to 0.003. Bars, mean. C and D, levels of pro–MMP-9 and VEGF detected by ELISA in sera of tumor-bearing bone marrow chimeras treated with zoledronate or saline (untreated). Zoledronate reduced the level of pro–MMP-9 and VEGF in mice chimeras receiving MMP-9+/− but not MMP-9−/− donor bone marrow. *, P = 0.01. Bars, mean.
Indeed, pro–MMP-9 and VEGF serum levels were increased in tumor-bearing MMP-9+/−>neuT chimeras but not in MMP-9−/−>neuT chimeras (Fig. 5C–D; open symbols). In the MMP-9−/−>neuT chimeras, the low amount of MMP-9 produced by tumor cells is insufficient to increase VEGF availability to the threshold necessary to activate the tumor-enhanced bone marrow hematopoiesis. In these mice, the radio-resistant stroma within bone marrow produces enough MMP-9 to support normal proliferation and differentiation of hematopoietic stem cells (Fig. 5B; refs. 15, 34).
Treatment with zoledronate inhibited tumor growth in both MMP-9+/−>neuT and MMP-9−/−>neuT bone marrow chimeras (Fig. 5A; filled symbols), and dramatically decreased the number of CD11b- and F4/80-positive cells within tumor stroma (Supplementary Fig. S2C–D). Treated tumors were necrotic regardless of the MMP-9 genotype of stroma cell. In MMP-9+/−>neuT mice, zoledronate significantly decreased serum pro–MMP-9 (P = 0.011) and VEGF (P = 0.018; Fig. 5C–D, respectively) and reduced the number of bone marrow methylcellulose colonies (P = 0.002; Fig. 5B).
In the MMP-9−/−>neuT chimeras treated with zoledronate, the normal levels of pro–MMP-9 and VEGF did not decrease (Fig. 5C–D) but the number of bone marrow CFU were significantly decreased (P = 0.003; Fig. 5B), suggesting that zoledronate have some effects on the MMP-9 produced by the radio-resistant bone marrow stroma (34).
Because MMP-9 is required for bone marrow recovery after bone marrow ablation, we tested whether amino-biphosphonates have any suppressive effects on normal hematopoiesis. We treated BALB-neuT and BALB/c mice with zoledronate and then ablated the bone marrow using 5-fluorouracil (5FU). 5FU treatment severely decreased cell number in peripheral blood, spleen, and bone marrow within 6 days, as well as bone marrow and spleen colonies in methylcellulose. Fourteen days later, the recovery of bone marrow colonies (CFU-GM, CFU-GEMM, and BFU-E) was normal in BALB/c mice regardless zoledronate treatment, whereas such recovery was impaired in treated BALB-neuT mice (Supplementary Fig. S3). These data confirm that zoledronate has no suppressive effect on normal hematopoiesis, although it reduces the effect of excessive MMP-9 produced at tumor site.
Zoledronate treatment improves immunotherapy outcome by reducing MDSC
The MMP-9/VEGF loop induces MDSC, which are reduced by zoledronate treatment. We therefore asked whether such MDSC reduction might improve the response to immunotherapy. To assess whether there was synergy between zoledronate and immunotherapy, we used conditions in which immunotherapy alone is insufficient to prevent tumor onset. BALB-neuT mice were crossed to FVB because this F1 hybrid develops accelerated tumorigenesis. Groups of (FVB×BALB-neuT)F1 females were either treated or not with zoledronate starting at age 4 weeks and vaccinated with a plasmid DNA encoding the rat p185/Her-2 ECD-TM four times at ages 7, 9, 11, and 13 weeks.
Zoledronate alone reduced tumor outgrowth, confirming the results of Fig. 2 in a different genetic background. Also, immunotherapy as single treatment delayed tumor onset, although it was unable to protect mice from tumor outgrowth (data not shown).
Combining zoledronate with immunotherapy further delayed tumor onset and significantly inhibited tumor growth at any time point, compared with untreated controls (P = 0.002–0.004; data not shown). Although zoledronate did not improve the outcome of vaccination using this schedule, a higher number of mice receiving the combination therapy had smaller tumor mass along the observation time (Fig. 6A). Evaluation of groups of mice bearing the same number of transformed mammary glands, regardless of mouse age, showed a cumulative tumor volume significantly smaller in mice receiving zoledronate in addition to DNA vaccination than in mice receiving DNA vaccination alone (Fig. 6B).
Figure 6.
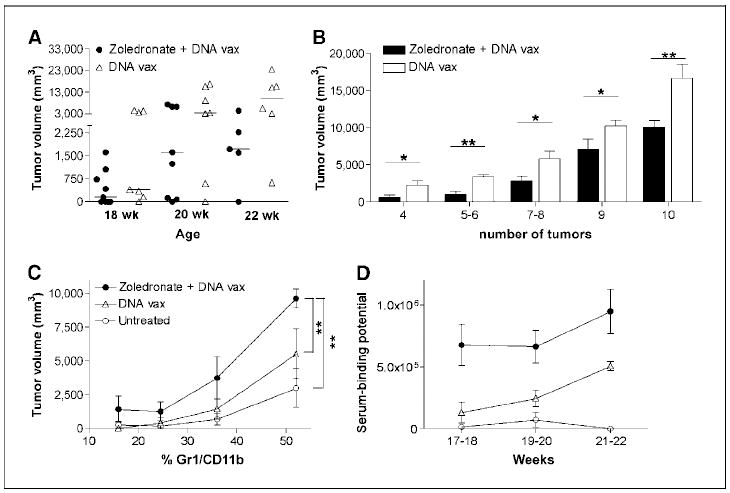
Zoledronate adjuvant effects to antitumor immunotherapy. (FVB×BALB-neuT)F1females underwent four cycles of DNA vaccination (DNA vax) with or without zoledronate treatment. Tumor growth, MDSC expansion, and anti–r-HER-2 antibody response was monitored and compared with untreated controls. A, at advanced tumor stages, between ages 18 and 22 wk, mice vaccinated and treated with zoledronate had a smaller tumor mass than mice treated with vaccination alone. Bars, median. B, cumulative tumor volume analyzed in treated mice bearing the same number of transformed mammary glands. The addition of zoledronate to DNA vaccination significantly reduced the overall tumor size. *, P = 0.01 to 0.04; **, P = 0.003 to 0.005; bars, SE. C, the correlation between tumor volume and percentage of Gr-1/CD11b–positive cells in the PB highlighted the significant reduction of MDSC in mice treated with zoledronate and vaccination compared with mice vaccinated and untreated controls. **, P = 0.006 to 0.008. Bars, SD. D, antibody-mediated immune response to r-HER-2, measured as serum-binding potential to r-p185/HER-2–positive tumor cell line. Zoledronate-treated and vaccinated mice had significantly higher titer of anti–r-HER-2 antibody than mice treated with vaccination alone or untreated. Bars, SD.
The number of MDSC in the peripheral blood was significantly reduced in all treated mice compared with untreated controls (P = 0.01–0.0004). When the percentage of circulating MDSC is correlated with the tumor volume, mice vaccinated and treated with zoledronate had a disproportionately decreased level of MDSC and, thus, needed a significant larger tumor size to induce the same percentage of circulating MDSC than untreated (P = 0.008) or vaccinated mice (P = 0.006; Fig. 6C).
Because in this immunotherapy model, DNA vaccination induces an antibody response against the rat p185/HER-2, mice were tested for the presence of antibody recognizing the HER-2 antigen. As expected, immunotherapy alone induced a humoral anti–rat p185/ HER-2 response within 3 weeks after the last immunization. Addition of zoledronate to DNA vaccination significantly increased the titer of anti–r-p185/HER-2 Ab titer (Fig. 6D). This result suggests that zoledronate-induced MMP-9 down-regulation inhibits MDSC generation, thus improving the antitumor immune response triggered by DNA vaccination.
Discussion
In this study, we provide evidence for a vicious cycle that connects tumor and bone marrow through MMP-9, produced in the tumor microenvironment mainly by stromal cells of bone marrow origin. The MMP-9 makes VEGF available to bone marrow for mobilizing hemopoietic stem cells toward enhanced myelopoiesis. Our data show that MMP-9 increases with tumor progression, and in parallel, the level of VEGF in serum increases. Inhibiting MMP-9 expression at tumor site breaks this loop and normalizes hematopoiesis, thus reducing myeloid support to tumor stroma and MDSC generation. As MMP-9 inhibitor, we used amino-biphosphonates, namely pamidronate and zoledronate. This class of biphosphonates interferes with the proteins prenylation and with the conversion of mevalonate to geranylgeranylpyrophosphate necessary for cholesterol synthesis (29). Therefore, they act on multiple metabolic pathways and, depending on cell type, have different effects: induce apoptosis and impair cell migration and adhesion to mineralized supports in vitro (22, 35), inhibit metastasis spread especially to bone (36, 37), and inhibit tumor neoangiogenesis by impairing VEGF signaling and MMP-9 expression by stromal macrophages (23) in vivo.
Treatment with amino-biphosphonates induced growth inhibition in BALB-neuT mice model of aggressive autologous tumorigenesis. Besides inhibiting MMP-9 expression, amino-biphosphonates reduced the number of CD11b- and F4/80-positive leukocytes in tumor stroma, thus depriving the tumor from the trophic effect provided by macrophages. The invasion of premalignant mammary lesions by macrophages precedes the angiogenic switch and is necessary for the progression to carcinoma in the PyMT model (38). This fits with the idea that tumor-educated macrophages promote tumor progression by secreting growth factors, cytokines, MMPs, and nitric oxide for proliferation and angiogenesis (39-41). Even macrophages recruited within tumors by adenovirus-mediated chemokine gene expression acquire the same M2 phenotype of preexisting macrophages, and tumor-associated macrophages are viewed as target for cancer therapy (42).
Our study shows that decreasing pro–MMP-9 serum levels reduces VEGF availability for angiogenesis and myelopoiesis. Of interest, the effect on bone marrow hematopoiesis seems to precede any effect on tumor growth. Indeed, to obtain a similar number of circulating MDSC, mice treated with zoledronate should have a tumor mass six to eight times larger than that of untreated mice. The decrease of MDSC was due to the normalization of hematopoiesis, as shown by counting methylcellulose colonies from bone marrow of treated tumor-bearing mice: particularly CFU-GM and CFU-GEMM precursors of circulating MDSC were significantly decreased (P = 0.005 and P < 0.0001, respectively).
Interestingly, zoledronate had no effect on normal hematopoiesis because chronically treated BALB/c mice had normal bone marrow CFU number and type, and displayed a normal recovery from temporary aplasia induced by the cytotoxic drug 5-FU. Unexpectedly and in contrast to the results in a cervical carcinoma model (23), the decreased MMP-9 and VEGF at tumor site did not impair angiogenesis. It is possible that the redundancy of proangiogenic circuits can bypass the block of VEGF perhaps through up-regulation of fibroblast growth factor, ephrin, and angiopoietin-1 produced by tumor and endothelial cells (33).
To dissect the role of MMP-9 produced by either tumor or stroma cells of hemopoietic origin and their relative sensitivity to zoledronate, we used bone marrow chimeras to knock down MMP-9 in bone marrow–derived cells. Regardless of the donor genotype, BALB-neuT chimeras developed tumors with similar onset and progression. Tumors also showed similar stroma composition in terms of infiltrating F4/80-CD11b cells. Thus, it might be possible that the MMP-9 produced by tumor cells is sufficient for the local tissue remodeling and angiogenesis. In contrast, the higher amount of MMP-9 produced by the cells of myeloid origin that infiltrate the tumor had distant effects on bone marrow; its knock down in MMP-9−/−>neuT chimeras reduced VEGF serum level and normalized hematopoiesis.
Zoledronate inhibited tumor growth in both MMP-9−/− and MMP-9+/−>BALB-neuT chimeras, decreasing the number of macrophages within the tumor and breaking the vicious circle between tumor stroma and bone marrow. In MMP-9−/− chimeras, zoledronate further decreased the number of CFU obtained from bone marrow without being toxic on normal hematopoiesis, neither was zoledronate toxic on hematopoiesis that rescued temporary aplasia induced by 5FU. Because tumors are responsible for enhanced hematopoiesis in tumor-bearing mice, normalization of bone marrow CFU suggests that zoledronate acts preferentially on tumor, while sparing bone marrow hemopoietic stem cells.
Finally, MDSC reduction in peripheral blood and secondary lymphoid organs contributes to the induction of immune response after vaccination immunotherapy.
Clinical trials with various MMP inhibitors (MMPI) were mostly unsuccessful (16, 19). Because several MMPs contribute differently to the various stages of tumor progression, the effect of MMPI may be quite different in early and late tumor stages. For this particular reason, MMP-9 has been reconsidered as a more specific target for pharmacological inhibitors (16, 20). Amino-biphosphonates represent a class of MMPI with excellent safety and tolerance (43), and display therapeutic effects beyond MMP-9 inhibition. In cancer patients, a single dose of pamidronate and zoledronate decreases serum levels of VEGF, platelet-derived growth factor, transforming growth factor β, and MMP-2 (44-46); thus supporting the idea of their multifactorial effects from blocking osteoclast bone resorption and CXCR-4–guided metastases to inhibition of MMP-9 and activation of γ-δ T lymphocytes (47-49). The tumor growth inhibition described here may have contributions from several of these mechanisms of action of amino-biphosphonates. Our study was focused on the effect of amino-biphosphonates on MMP-9 responsible for the tumor–bone marrow loop in BALB-neuT model. Mice have shown no toxicity to prolonged treatment and to a cumulative dose that might be toxic in humans (50). Future experiments will dose zoledronate to clinical-safe regimen to test the effective preclinical value of our findings. Nevertheless, in the BALB-neuT model of spontaneous mammary carcinoma, we can conclude that MMP-9 inhibition breaks the vicious loop linking tumor and bone marrow in a reciprocal provision of growth factor (VEGF) and cells fostering tumor growth (macrophage) and immune suppression (MDSC), and that amino-biphosphonates can be used as MMPI. Our results suggest the possibility to use the amino-biphosphonates MMPI activity as adjuvant to contrast tumor-induced hematopoietic and immunological disorders.
Supplementary Material
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Acknowledgments
Grant support: Associazione Italiana per la Ricerca sul Cancro and Italian Ministry of Health, and National Cancer Institute grants P01 CA072006 and U01 CA105379 (Z. Werb).
References
- 1.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 2.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/ c mice suppresses immune reactivity. Blood. 2003;102:2138–45. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 3.Bronte V, Apolloni E, Cabrelle A, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–46. [PMC free article] [PubMed] [Google Scholar]
- 4.Man AK, Young LJ, Tynan JA, et al. Ets2-dependent stromal regulation of mouse mammary tumors. Mol Cell Biol. 2003;23:8614–25. doi: 10.1128/MCB.23.23.8614-8625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–21. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 6.Jodele S, Chantrain CF, Blavier L, et al. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005;65:3200–8. doi: 10.1158/0008-5472.CAN-04-3770. [DOI] [PubMed] [Google Scholar]
- 7.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398–406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 8.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–95. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 10.Terabe M, Matsui S, Park JM, et al. Transforming growth factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–99. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 12.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–35. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Bronte V, Chen SH, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:S37–43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 17.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foda HD, Zucker S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov Today. 2001;6:478–82. doi: 10.1016/s1359-6446(01)01752-4. [DOI] [PubMed] [Google Scholar]
- 19.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 20.Overall CM, Kleifeld O. Tumour microenvironment -opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–39. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 21.Clezardin P, Ebetino FH, Fournier PG. Bisphosphonates and cancer-induced bone disease: beyond their antiresorptive activity. Cancer Res. 2005;65:4971–4. doi: 10.1158/0008-5472.CAN-05-0264. [DOI] [PubMed] [Google Scholar]
- 22.Coxon JP, Oades GM, Kirby RS, Colston KW. Zoledronic acid induces apoptosis and inhibits adhesion to mineralized matrix in prostate cancer cells via inhibition of protein prenylation. BJU Int. 2004;94:164–70. doi: 10.1111/j.1464-4096.2004.04831.x. [DOI] [PubMed] [Google Scholar]
- 23.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–33. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boggio K, Nicoletti G, Di Carlo E, et al. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med. 1998;188:589–96. doi: 10.1084/jem.188.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffè M. Uber den Niederschag, welchen Pikrinsaure in normalemHarn erzeugt und uber eine neue Reaktion des Kreatinins. Z Physiol Chem. 1886;10:391–400. [Google Scholar]
- 26.Rovero S, Amici A, Carlo ED, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–42. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 27.Nanni P, Pupa SM, Nicoletti G, et al. p185(neu) protein is required for tumor and anchorage-independent growth, not for cell proliferation of transgenic mammary carcinoma. Int J Cancer. 2000;87:186–94. [PubMed] [Google Scholar]
- 28.Quaglino E, Rolla S, Iezzi M, et al. Concordant morphologic and gene expression data show that a vaccine halts HER-2/neu preneoplastic lesions. J Clin Invest. 2004;113:709–17. doi: 10.1172/JCI19850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luckman SP, Coxon FP, Ebetino FH, Russell RG, Rogers MJ. Heterocycle-containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: evidence from structure-activity relationships in J774 macrophages. J Bone Miner Res. 1998;13:1668–78. doi: 10.1359/jbmr.1998.13.11.1668. [DOI] [PubMed] [Google Scholar]
- 30.Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19:558–67. doi: 10.1200/JCO.2001.19.2.558. [DOI] [PubMed] [Google Scholar]
- 31.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–43. [PubMed] [Google Scholar]
- 32.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Bianco P, Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–8. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oades GM, Senaratne SG, Clarke IA, Kirby RS, Colston KW. Nitrogen containing bisphosphonates induce apoptosis and inhibit the mevalonate pathway, impairing Ras membrane localization in prostate cancer cells. J Urol. 2003;170:246–52. doi: 10.1097/01.ju.0000070685.34760.5f. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki A, Boyce BF, Story B, et al. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res. 1995;55:3551–7. [PubMed] [Google Scholar]
- 37.Hashimoto K, Morishige K, Sawada K, et al. Alendronate inhibits intraperitoneal dissemination in in vivo ovarian cancer model. Cancer Res. 2005;65:540–5. [PubMed] [Google Scholar]
- 38.Lin EY, Li JF, Gnatovskiy L, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–46. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 39.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 40.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 41.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 42.Colombo MP, Mantovani A. Targeting myelomonocytic cells to revert inflammation-dependent cancer promotion. Cancer Res. 2005;65:9113–6. doi: 10.1158/0008-5472.CAN-05-2714. [DOI] [PubMed] [Google Scholar]
- 43.Fisher JF, Mobashery S. Recent advances in MMP inhibitor design. Cancer Metastasis Rev. 2006;25:115–36. doi: 10.1007/s10555-006-7894-9. [DOI] [PubMed] [Google Scholar]
- 44.Santini D, Vincenzi B, Avvisati G, et al. Pamidronate induces modifications of circulating angiogenetic factors in cancer patients. Clin Cancer Res. 2002;8:1080–4. [PubMed] [Google Scholar]
- 45.Santini D, Vincenzi B, Dicuonzo G, et al. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin Cancer Res. 2003;9:2893–7. [PubMed] [Google Scholar]
- 46.Ferretti G, Fabi A, Carlini P, et al. Zoledronic-acid-induced circulating level modifications of angiogenic factors, metalloproteinases and proinflammatory cytokines in metastatic breast cancer patients. Oncology. 2005;69:35–43. doi: 10.1159/000087286. [DOI] [PubMed] [Google Scholar]
- 47.Das H, Wang L, Kamath A, Bukowski JF. Vγ2Vδ2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood. 2001;98:1616–8. doi: 10.1182/blood.v98.5.1616. [DOI] [PubMed] [Google Scholar]
- 48.Conti L, Casetti R, Cardone M, et al. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated γδ T cells: role of CD86 and inflammatory cytokines. J Immunol. 2005;174:252–60. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- 49.Miyagawa F, Tanaka Y, Yamashita S, Minato N. Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human γ δ T cells by aminobisphosphonate antigen. J Immunol. 2001;166:5508–14. doi: 10.4049/jimmunol.166.9.5508. [DOI] [PubMed] [Google Scholar]
- 50.Daubine F, Le Gall C, Gasser J, Green J, Clezardin P. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J Natl Cancer Inst. 2007;99:322–30. doi: 10.1093/jnci/djk054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).


