SUMMARY
Hematopoietic stem cells within the bone marrow exist in a quiescent state. They can differentiate and proliferate in response to hematopoietic stress (e.g., myelosuppression), thereby ensuring a well-regulated supply of mature and immature hematopoietic cells within the circulation. However, little is known about how this stress response is coordinated. Here, we show that plasminogen (Plg), a classical fibrinolytic factor, is a key player in controlling this stress response. Deletion of Plg in mice prevented hematopoietic stem cells from entering the cell cycle and undergoing multilineage differentiation after myelosuppression, leading to the death of the mice. Activation of Plg by administration of tissue-type plasminogen activator promoted matrix metalloproteinase-mediated release of Kit ligand from stromal cells, thereby promoting hematopoietic progenitor cell proliferation and differentiation. Thus, activation of the fibrinolytic cascade is a critical step in regulating the hematopoietic stress response.
INTRODUCTION
Hematopoiesis is initiated by multipotent hematopoietic stem cells (HSCs). The blood-forming system is able to respond efficiently to hematological stresses such as bleeding, inflammation, or exposure to irradiation or cytotoxic agents by expanding the HSC population (Heissig et al., 2005b). Thus, HSC proliferation and differentiation must be highly adaptive to ensure both a durable production of progenitor populations during steady-state hematopoiesis and extensive, stress-induced proliferation without depletion of the stem cell pool. A major challenge in stem cell biology is to elucidate the factors contributing to this fine-tuned adaptation to stress or cell loss.
The bone marrow (BM) is the primary hematopoietic site in the adult. For example, the BM stromal compartment harbors extracellular matrix (ECM) and cytokines that are capable of influencing self-renewal, proliferation, and differentiation of hematopoietic stem and progenitor cells (HSPCs).
The serine protease plasmin plays an important role in fibrinolysis and can degrade ECM molecules (Herren et al., 2003). Plasminogen (Plg), its inactive proenzyme, can be activated by tissue-type plasminogen activator (tPA), urokinase-type plasminogen activator (uPA), and other serine proteases. Mice with homozygously disrupted Plg genes are predisposed to severe thrombosis and develop intra- and extravascular fibrin deposition in many tissues (Bugge et al., 1995). During fibrinolysis, plasmin can participate in the consecutive activation of matrix metalloproteinases (MMPs) (Lijnen et al., 1998b; Liu et al., 2005).
In this study, we provide mechanistic data demonstrating that Plg−/− mice exhibit defects in hematopoietic reconstitution after myelosuppression, owing to a defect in HSPC proliferation. The absence of Plg results in impaired long-term survival after BM transplantation.
RESULTS
Plasmin Generation Is Required for Hematopoietic Regeneration
We took advantage of mice with homozygously disrupted Plg genes (Bugge et al., 1995) and a model of stress hematopoiesis to investigate the role of Plg in hematopoiesis. We stained BM sections for Plg during the early recovery phase (2 or 3 days after myelosuppression) after chemotherapy and found positively stained areas along bone-lining cells (Figure 1A). We therefore hypothesized that plasmin(ogen) regulates hematopoietic reconstitution after myelosuppression.
Figure 1. Delayed Bone Marrow Cell Recovery in Plg−/− Mice after Myelosuppression.
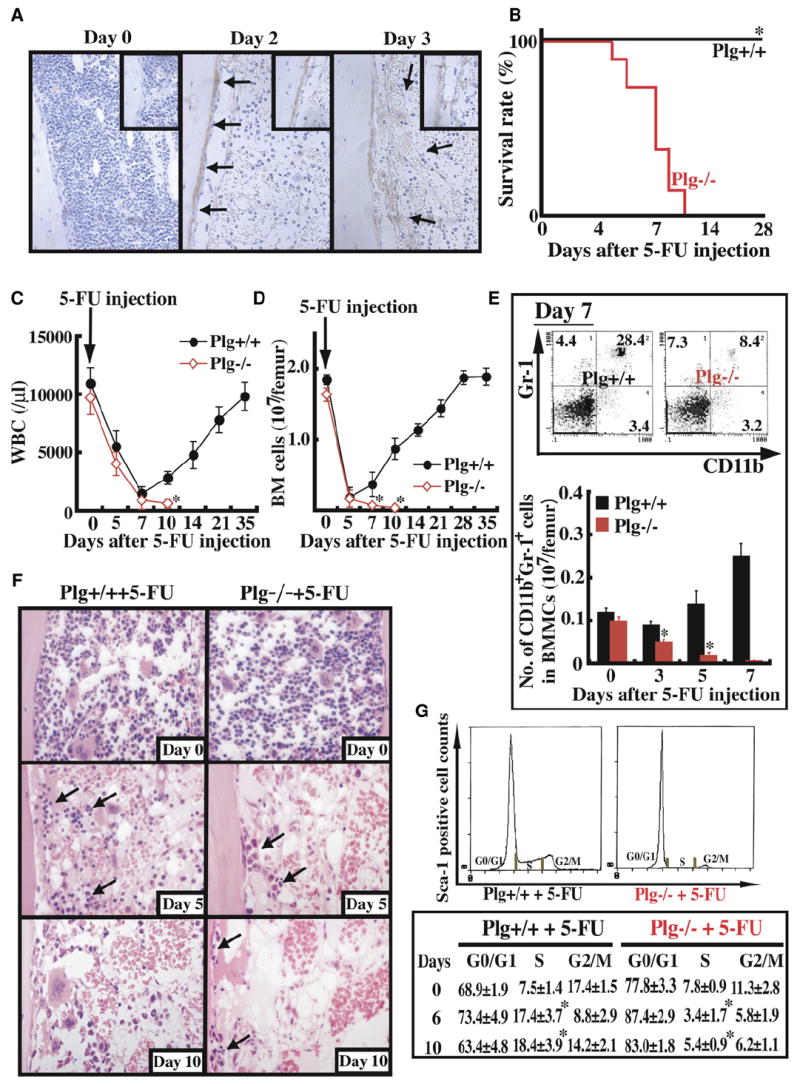
(A–G) Plg+/+ (n = 12) and Plg−/− mice (n = 12) received a single dose of the myelosuppressive agent 5-FU i.v.
(A) Immunohistochemical staining for Plg/plasmin (brown staining) in bone marrow (BM) sections from Plg+/+ and Plg−/− mice treated with 5-FU. Magnification ×100.
(B) Survival of 5-FU-treated mice was assessed daily.
(C) WBC counts were counted. Error bars represent standard error of the mean (SEM).
(D) Total number of BMMCs per femur was assessed. Error bars represent SEM.
(E) BM cells were stained for the myeloid markers CD11b-FITC and Gr-1-PE and analyzed by FACS (upper panel). Absolute numbers of CD11b+/Gr-1+ BM cells per femur were calculated (lower panel). Error bars represent SEM.
(F) H&E staining of BM sections after 5-FU treatment. Arrows depict hematopoietic cells. Magnification ×200.
(G) DNA content of Sca-1+ BM cells was determined after 5-FU treatment (n = 3 per group and time point). *p < 0.05.
Myelosuppression induced by 5-fluorouracil (5-FU) results in apoptosis of cycling HSPCs but does not affect HSCs in G0 (Hattori et al., 2002). All Plg−/− mice receiving 5-FU succumbed by day 10 after 5-FU injection, whereas Plg+/+ mice survived the 5-FU treatment (Figure 1B) and exhibited recovery of white blood cells (WBCs) (Figure 1C), platelets (see Figure S1A in the Supplemental Data available with this article online) and BM cells (Figure 1D). During hematopoietic regeneration, quiescent (G0/G1) stem cells enter the cell cycle and can differentiate. Regeneration of total CD11b+/Gr-1+ myeloid BM cells (Figure 1E) and immature megakaryocyte progenitors at the 2N and 4N stage (Figure S1B) occurred in the Plg+/+ mice, but not in Plg−/− mice. Hematopoietic recovery was delayed in Plg−/− mice as compared to Plg+/+ mice (Figure 1F). Although the percentage of Sca-1+ cells in S phase of the cell cycle was similar in Plg+/+ and Plg−/− mice under steady-state conditions (Figure 1G), fewer Sca-1+ cells shifted into S phase in Plg−/− mice as compared to wild-type (WT) cells upon myelosuppression. Thus, cell-cycle transition and the stress-induced cell regeneration did not occur in Plg-deficient mice. Treatment with 5-FU did not result in death of mice singly deficient in uPA (n = 5), tPA (n = 5), or urokinase plasminogen activator receptor (uPAR) (n = 6) (data not shown). However, one out of three uPA/tPA double-deficient mice died within the first few days after 5-FU injection (data not shown).
Plg-Mediated Activation of MMPs in the BM Promotes the Release of KitL
Plg activation can lead to activation of MMPs, especially MMP-9 (Lijnen et al., 1998b). We found higher levels of proMMP-2 and proMMP-9 but, more importantly, active MMP-9 in supernatants of equal numbers of cultured BM cells derived from Plg+/+ animals 5 and 10 days after 5-FU treatment, as compared to Plg−/− mice (Figure 2A). Levels of immunoreactive proMMP-9 were attenuated in BM (Figure 2B), and less active MMP-9 was found in the plasma of Plg−/− mice treated with 5-FU (Figure 2C).
Figure 2. MMP Activation and KitL Release Are Impaired in Plg−/− Mice after Myelosuppression, Resulting in BM Recovery Failure.
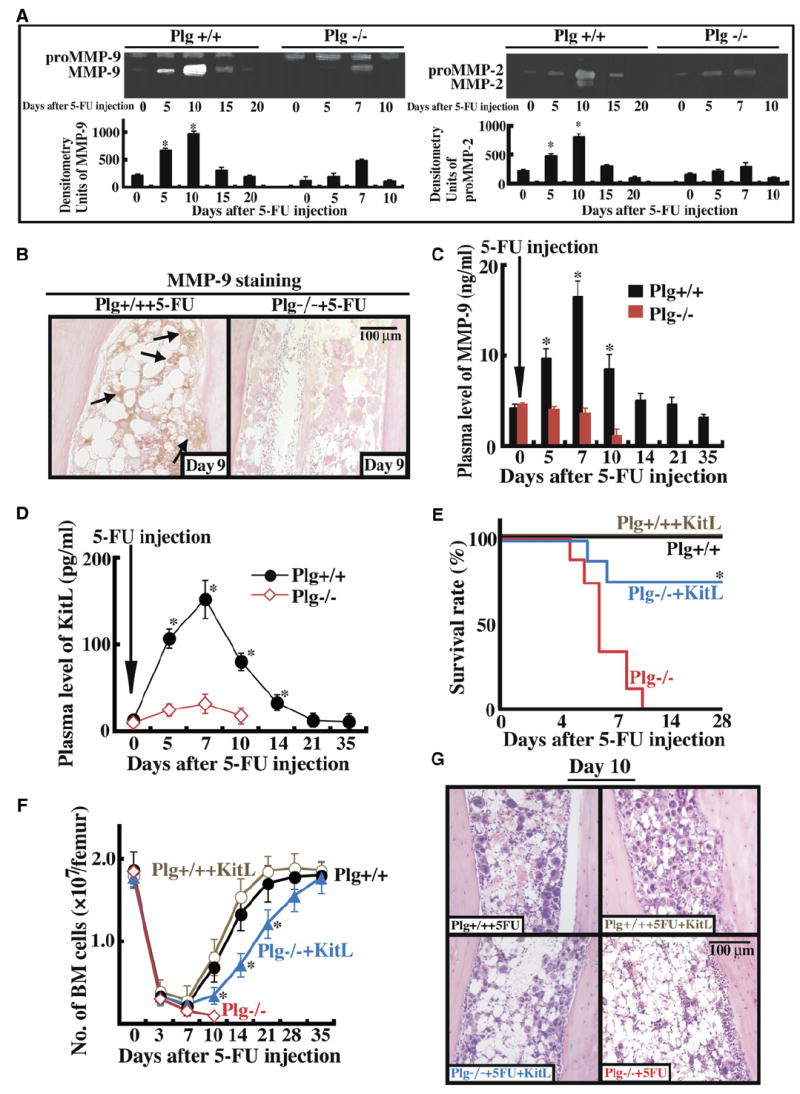
(A–D) Plg+/+ and Plg−/− mice were injected with a single dose of 5-FU i.v. (A) BM cells (three mice per time point) were cultured in serum-free medium overnight. Cell supernatants were assayed for proMMP-9 (103 kDa), active MMP-9 (86 kDa), proMMP-2 (72 kDa), and active MMP-2 (62 kDa) by gelatin zymography. Error bars represent standard deviation. (B) Immunohistochemistry of BM sections 9 days after 5-FU injection for proMMP-9 with positive staining in the BM stromal compartment of Plg+/+, but less in the BM stromal compartment of Plg−/− mice. Magnification ×200. (C and D) Plasma obtained from peripheral blood (PB) was assayed for active MMP-9 (C) or KitL (D) by ELISA (p < 0.05). Error bars represent SEM. (E–G) Plg+/+ and Plg−/− mice were left untreated (n = 8) or received a single injection of 5-FU i.v. with/without daily i.v. injections of recombinant KitL (n = 6). (E) Survival of treated animals was determined daily. (F) Total number of BM cells per femur was assessed. Error bars represent SEM. (G) H&E staining of BM sections 10 days after 5-FU treatment. Magnification ×200. *p < 0.05.
If plasmin activates MMPs, then plasma levels of KitL, a cytokine implicated in hematopoietic regeneration that can be regulated by MMP-9 (Heissig et al., 2002), should be low in Plg−/− mice after 5-FU treatment, as indeed was the case (Figure 2D). We could implicate the lack of hematopoietic recovery after myelosuppression to impaired release of KitL in Plg−/− mice because daily injections of recombinant KitL partly restored the survival of Plg−/− mice (Figure 2E) and promoted BM cell recovery (Figures 2F and 2G). Similarly, pretreatment of mice with recombinant KitL restored BM cell-cycle progression in Plg−/− cells to levels observed in Plg+/+ cells (S, G2M phase, Plg+/+ Sca-1+ cells 11.4% versus Plg−/− Sca-1+ cells 9.5%, n = 3; not significant; 5 days after the start of injection). Thus, the defective hematopoietic recovery in Plg−/− mice can be attributed at least in part to the inability to produce amounts of soluble KitL sufficient to induce hematopoietic cell-cycle progression.
Administration of Recombinant tPA Promotes Plg Accumulation and MMP Activation within the BM
Next, we investigated whether BM cells can produce Plg or Plg activators. Plg mRNA was detectable in liver cells, but not in freshly isolated BM lin− or lin+ cells or in cultured BM stroma (Figure 3A). tPA was strongly expressed in BM stromal cells of both Plg+/+ and Plg−/− mice and cultured MS-5 stromal cells and was weakly expressed in freshly isolated lin+ and lin− cells of both Plg+/+ and Plg−/− mice. Immunoreactive tPA was found throughout the BM, including stromal cells, endothelial cells, and some hematopoietic cells (Figure 3B).
Figure 3. tPA-Mediated MMP Activation Releases KitL from Stromal Cells.
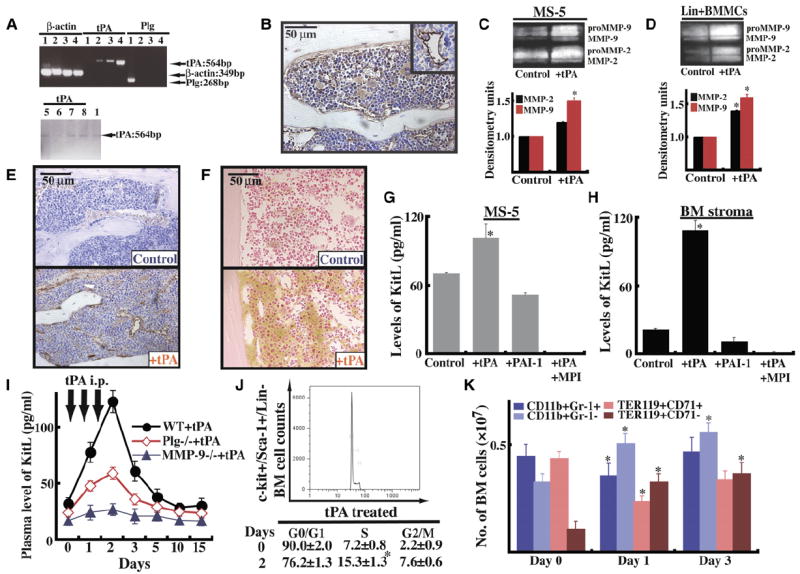
(A) RT-PCR for tPA and Plg in liver (sample 1; positive control for Plg), 4-week-old BM stroma of Plg+/+ mice (sample 2) and of Plg−/− mice (sample 3), MS-5 cells (sample 4), freshly isolated BM-derived lin− cells from Plg+/+ mice (sample 5), or Plg−/− mice (sample 6), as well as freshly isolated BM-derived lin+ cells from Plg+/+ mice (sample 7) or Plg−/− mice (sample 8). Agarose gel of one representative experiment.
(B) Immunohistochemistry for tPA in BM sections of Plg+/+ mice under steady-state conditions (magnification ×200). (Insert) Vessel stained positive for tPA.
(C–F) MS-5 cells (C) or lin+ BMMCs from Plg+/+ mice (D) were cultured overnight with/without tPA under serum-free conditions. Supernatants were analyzed by zymography for MMP-2 and MMP-9. Error bars represent standard deviation. Immunohistochemistry for Plg (E) and MMP-9 (F) in BM sections of Plg+/+ mice 3 days after starting tPA treatment (magnification ×200).
(G and H) Confluent MS-5 stromal cells (G) or Plg+/+ primary BM stroma cells (H) were cultured overnight in serum-free medium (n = 3) in the presence of recombinant tPA, recombinant PAI-1, and MPI (CGS 27023A) with or without tPA. Supernatants were collected and analyzed for KitL by ELISA (n = 3, p < 0.05). Error bars represent SEM.
(I–K) Plg−/− and MMP-9−/− mice and littermate mice were injected with a single dose of tPA or PBS daily from day 0–2 i.p. (I) Blood was drawn after tPA administration as indicated. Plasma samples were analyzed for KitL by ELISA (n = 6; ELISA was performed twice). Error bars represent SEM. (J) BM cells were collected on day 0 and day 2. Sca-1/ c-Kit+/Lin− (KSL) cells were costained with PI. Cell-cycle analysis was performed on KSL cells (n = 3). (K) BMMCs of tPA- and non-tPA-treated animals (n = 3) were incubated with anti-Ter119 and anti-CD71 antibodies. Proerythroblasts stained positive for CD71hiTer119hi, whereas erythroblasts stained positive for CD71loTer119hi. Granulocytic cells are positive for CD11b and Gr-1, whereas BM-derived monocytic cells showed positive staining for CD11b and expressed little or no Gr-1. Absolute numbers are given. Error bars represent SEM.
We reasoned that tPA, through activation of Plg, indirectly could activate MMPs in BM stromal cells. Active MMP-9 and MMP-2 were detectable by zymography in MS-5 (Figure 3C) and freshly isolated lin+ cells from Plg+/+ animals (Figure 3D) incubated with recombinant tPA. Immunoreactive Plg was detectable 1 hr after injection of recombinant tPA in close vicinity to sinusoidal BM vessels (Figure 3E), while hematopoietic and stroma cells throughout the BM of tPA-treated animals expressed immunoreactive MMP-9 (Figure 3F).
tPA Treatment Augments the Release of Soluble KitL
Since activation of MMP-9 modulates the c-Kit/KitL pathway (Heissig et al., 2002), we assessed whether tPA promotes KitL release, which could drive hematopoietic regeneration. tPA treatment augmented the release of KitL from MS-5 cells (Figure 3G) and primary BM stromal cells (Figure 3H). Of importance, the tPA-mediated release of KitL from stromal cells took place via MMP activation: addition of a MMP inhibitor inhibited KitL release from stromal cells. When MS-5 cells were treated with PAI-1, the natural inhibitor of tPA, KitL release was lower than in control supernatants.
If Plg is important for KitL release and tPA converts Plg into plasmin, increased KitL plasma levels should be found in tPA-treated animals, which indeed was the case (Figure 3I). Similarly, KitL levels rose above baseline levels after tPA treatment, even in Plg−/− mice. Nonenzymatic mutant tPA has been reported to induce MMP-9 expression, suggesting that some actions of tPA are independent of its proteolytic activity (Hu et al., 2006). When MMP-deficient mice were injected with tPA, KitL plasma levels did not rise, indicating that tPA-mediated KitL release required MMP-9 activation (Figure 3I).
KitL in combination with other cytokines can push quiescent stem cells into cell cycle. More lin−/Sca-1/c-Kit+ BM cells entered the S phase of the cell cycle in BM cells of tPA-treated mice as compared to carrier-treated animals (Figure 3J). These data indicate that administration of tPA leads to activation of MMPs, necessary for the release of KitL from BM stromal cells. KitL in turn pushes noncycling cells into cell cycle.
tPA Promotes Erythroid, Myelomonocytic, and Megakaryocytic Cell Differentiation
Committed erythroid cells undergo several steps of differentiation from erythroid progenitors, forming EPO-responsive erythroblasts, which differentiate ultimately to erythrocytes. Erythroid differentiation can be monitored by using the markers CD71 and Ter119 (Socolovsky et al., 2001). CD71 is expressed in hematopoietic cells including BFU-Es, CFU-Es, and proerythroblasts, whereas Ter119 is a late erythroid marker expressed in erythroblasts. Within the BM of nontreated mice 0.44 ± 0.01 × 107 cells were found within the Ter119hiCD71hi population (proerythroblast) and 0.11 ± 0.002 × 107 cells in the later stage Ter119hiCD71lo (erythroblast), as shown in Figure 3K. BM cells of tPA-treated animals showed enhanced erythroid differentiation, with 0.33 ± 0.007 ×107 erythroblasts and 0.24 ± 0.002 × 107 cells found within the Ter119hiCD71hi stage.
Granulocytic cells of adult mouse BM express CD11b and high amounts of Gr-1. BM monocytic cells express CD11b and little or no Gr-1 (Lagasse and Weissman, 1996). We assessed CD11b and Gr-1 expression in adult BM with flow cytometry. The absolute number of CD11b+Gr-1hi cells decreased in tPA-treated mice on day 1 and to a lesser extent on day 3, whereas CD11b+Gr-1lo cells increased as compared to untreated animals (0.36 ± 0.007 versus 0.51 ± 0.01 × 107 on day 1).
The differentiation of megakaryocytes is characterized by polyploidization and cytoplasmic maturation leading to platelet production. The percentage of polyploid megakaryocytese of 32 and 64N ploidy increased in tPA-treated mice as compared to controls (Figure S1C). These data indicate that administration of tPA promotes erythroid, myelomonocytic, and megakaryocytic cell differentiation in vivo. But further studies will be needed to understand the underlying mechanism.
tPA-Mediated HSPC Expansion Requires the Presence of KitL
To assess whether tPA can act as a hematopoietic cytokine, we evaluated HSPC expansion both in vitro and in vivo. tPA administration in vivo augmented the number of BM cells in WT controls, but this was attenuated in both Plg-deficient and MMP-9-deficient mice (Figure 4A). tPA increased the number of spleen colony-forming units day 8 (CFU-Sd8, Figure 4B), of long-term culture-initiating cells (LTC-IC, Figure 4C), and of the c-Kit+/Sca-1+/lin− (KSL) cell fraction in BM cells of treated animals (Figure 4D). Long-term injection of tPA kept BM cell numbers persistently elevated above steady-state values (Figure 4E). These data indicate that tPA, through activation of Plg, influences progenitor cell proliferation.
Figure 4. tPA Promotes HSPC Proliferation in KitL-Competent Mice.
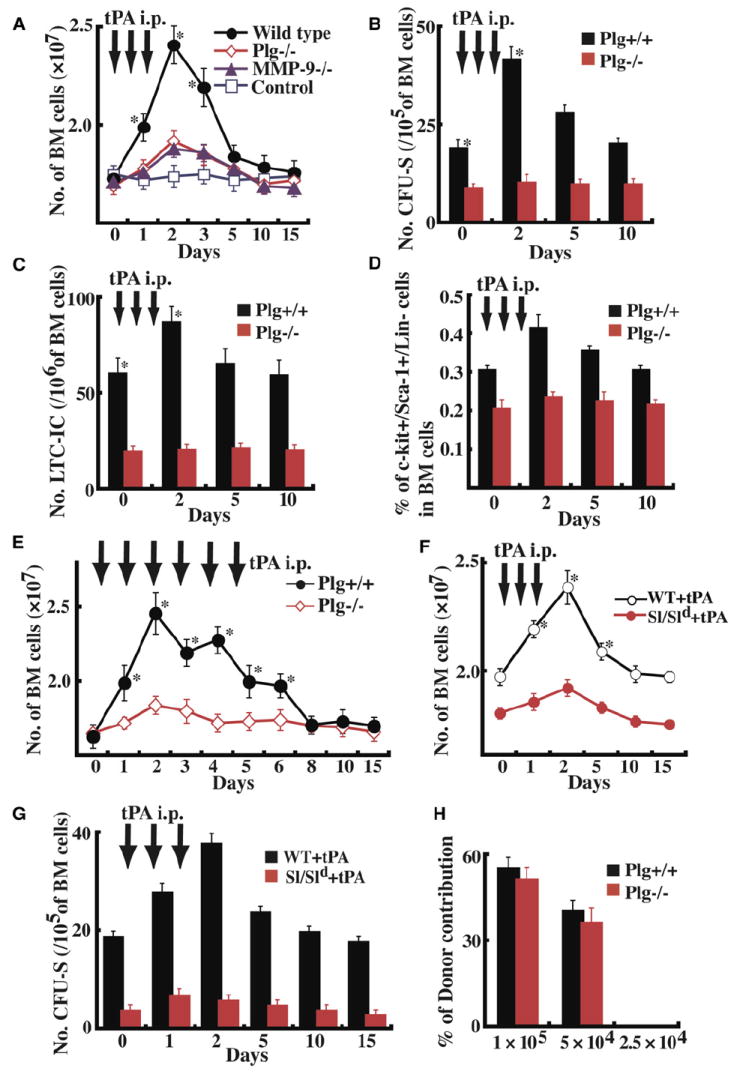
(A) Plg-deficient, MMP-9-deficient, and littermate mice were injected with recombinant tPA i.p. from day 0–2 (short-term effect), and BM cells were counted. BM cells of Plg+/+ and Plg−/− cells were analyzed in a CFU-S assay ([B], n = 9), LTC-IC assay ([C], n = 9), and by FACS for the presence of KSL cells ([D], n = 9). (E) Plg+/+ and Plg−/− mice were injected with recombinant tPA from day 0–5 (long-term effect), and BM cells were counted (n = 9). (F and G) Sl/Sld and WBB6F1 mice were treated with/without tPA (n = 5). BM cell numbers per femur (F) and the number of CFU-S were determined (G). (H) Mouse PB cell donor contribution transplantation of BM cells from Plg+/+ and Plg−/− BM CD45.2+ cells at limiting cell dilutions into CD45.1+ recipient mice (n = 9 per cell concentration); *p < 0.05. Error bars represent SEM.
KitL can expand hematopoietic CFCs of multiple types in the BM (Andrews et al., 1992) and increase marrow cellularity. To test if tPA-mediated hematopoietic cell proliferation is dependent on KitL in vivo, we treated KitL-deficient Sl/Sld mice and WBB6F1+/+ control mice with tPA. BM cellularity (Figure 4F) and the number of immature CFU-Sd8 progenitor cells (Figure 4G) increased in WBB6F1+/+ animals by day 2 after tPA injections, but not in Sl/Sld mice. These studies show that tPA-mediated hematopoietic cell proliferation requires the presence of KitL.
Plg-Deficient Mice Do Not Have an Intrinsic Hematopoietic Stem Cell Defect
We noticed that, in the steady state, Plg−/− mice showed a reduced number of immature cells including LTC-IC, CFU-Sd8, and Sca-1+/c-Kit+ cells in BM as compared to controls (Figures 4B–4D). The percentage of side population (SP) cells within the BM was lower in Plg−/− as compared to Plg+/+ mice (0.018% ± 0.001% versus 0.051% ± 0.005%; n = 9, p < 0.0001). Even though LTC-IC, CFUSd8, and SP cells show enrichment for HSCs, they still contain a significant number of progenitor cells. For example, the SP cell fraction is enriched between 1000-fold and 3000-fold for HSCs (Goodell et al., 1996).
To date, transplantation assays are still regarded as the most stringent assay for the quantification of stem cells. To ensure that the decreased number of progenitor cells was due to extrinsic factors (e.g., the lack of KitL within the hematopoietic microenvironment) rather than an intrinsic stem cell defect, we performed competitive repopulation experiments with the congenic CD45.1/CD45.2 alleles to distinguish donor and competitor cells. We transplanted various cell numbers of BM mononuclear cells (BMMCs) from Plg+/+ and Plg−/− mice into congenic CD45.1 lethally irradiated recipient mice. A minimum of 5 × 104 transplanted cells of either Plg+/+ and Plg−/− cells were necessary to detect donor cells in the PB cell fraction after 90 days. Plg−/− cells showed identical engraftment in WT CD45.1 recipients (Figure 4H). Transplantation of CD45.2+ KSL cells (10, 50, 100) from Plg+/+ and Plg−/− mice together with CD45.1 competitor cells resulted in identical three-lineage hematopoietic engraftment (Figure S1D) when analyzed 7 months after transplantation. The frequency of competitive repopulation units (CRUs) in Plg+/+ and Plg−/− KSL cells was identical (Figure S1E). These data indicate that Plg-deficient mice do not have a functional HSC but rather a progenitor cell defect.
Plg and MMP-9 Are Necessary for tPA-Mediated BM Reconstitution
To address the question of whether tPA-mediated hematopoiesis requires Plg and/or MMP-9, we treated Plg- and MMP-deficient with 5-FU on day 0 followed by daily injections with or without recombinant tPA. tPA did not improve survival (Figure 5A), recovery of WBC (Figure 5B), or the number of BM cells (Figure 5C) in Plg−/− mice. Of interest, stronger Plg/plasmin staining was detectable by immunohistochemistry after the administration of recombinant tPA into Plg+/+ mice in BM areas close to the bone (Figure 5D).
Figure 5. tPA-Mediated BM Reconstitution Requires Both Plg and MMP Activation.
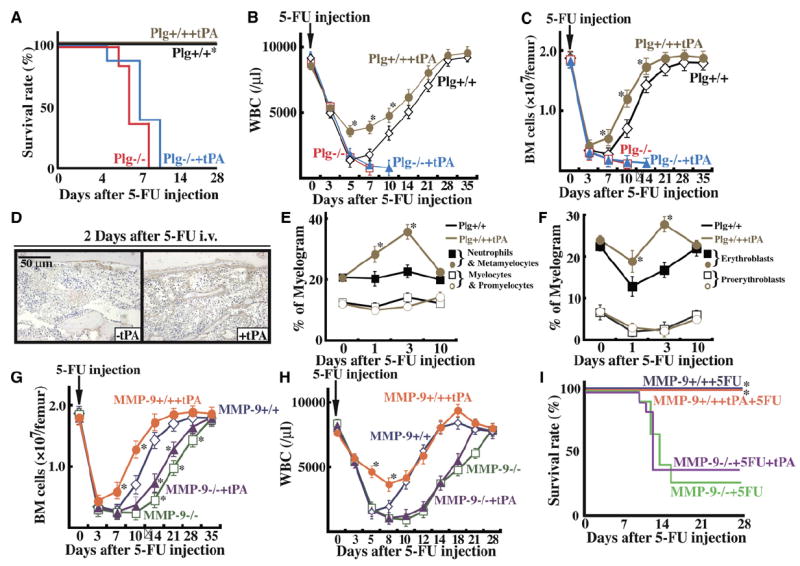
Plg+/+ and Plg−/− mice (A–F) and MMP-9+/+ and MMP-9−/− mice (G–I) were injected with a single dose of 5-FU followed by daily injections with recombinant tPA or carrier. (A) Survival was assessed daily in Plg+/+ (n = 9 per group) and Plg−/− mice (n = 8 per group). WBC counts (B) and BM cell numbers per femur (C) were determined. Error bars represent SEM. (D) BM sections of Plg+/+ mice coinjection with or without tPA were stained for Plg 2 days after 5-FU treatment (brown staining). Plg staining was found along bone-lining cells. (E and F) BM myelogram was performed. Percentage of myeloid (E) and erythroid (F) BM cells are given. (G–I) BM cell numbers per femur (G), WBC counts (H), and survival (I) were assessed in MMP-9+/+ and MMP-9−/− mice (n = 12). *p < 0.05. (E–H) Error bars represent SEM.
To elucidate why tPA-treated Plg+/+ mice showed faster BM recovery and higher leukocyte counts, we examined BM cell myelograms. In myelogram analysis, percentages of late myeloid cells, such as neutrophils and metamyelocytes, increased in tPA-treated mice as compared with nontreated littermates (Figure 5E). Similarly, the number of erythroblasts increased at the expense of the more immature proerythroblasts (Figure 5F). tPA did not promote cell differentiation in Plg−/− mice (data not shown).
Similarly, in 5-FU-treated MMP-9-deficient mice, the recovery of BM cells (Figure 5G) or WBCs (Figure 5H) was delayed, and death after 5-FU (Figure 5I) could not be rescued/prevented using daily injections of recombinant tPA. Death occurred in about 70% of MMP-9−/− mice. Thus, the effect of tPA on hematopoietic reconstitution after myelosuppression requires the presence of both Plg and MMP-9 and possibly other MMPs.
Fibrinolytic Factors Expressed within the BM Promote Hematopoietic Cell Proliferation and Differentiation
To elucidate whether the observed tPA effects on hematopoietic cells were mediated directly by tPA or indirectly, e.g., via BM stromal cells, we examined the effect of tPA on hematopoietic cell expansion in vitro. tPA improved proliferation of lin− cells in stromal-cell-based cultures (MS-5 feeder layer) (Figure 6A), but not in the absence of a feeder layer (Figures S1F and S1G). In synergy, tPA and KitL expanded the number of progenitors in stromal-cell-based cultures. Comparable numbers of progenitor colonies emerged in cultures set up with Plg+/+ or Plg−/− lin− cells (Figure 6A).
Figure 6. Plg-Deficient Mice Have a Defective BM Stromal Compartment due to Impaired Release of KitL.
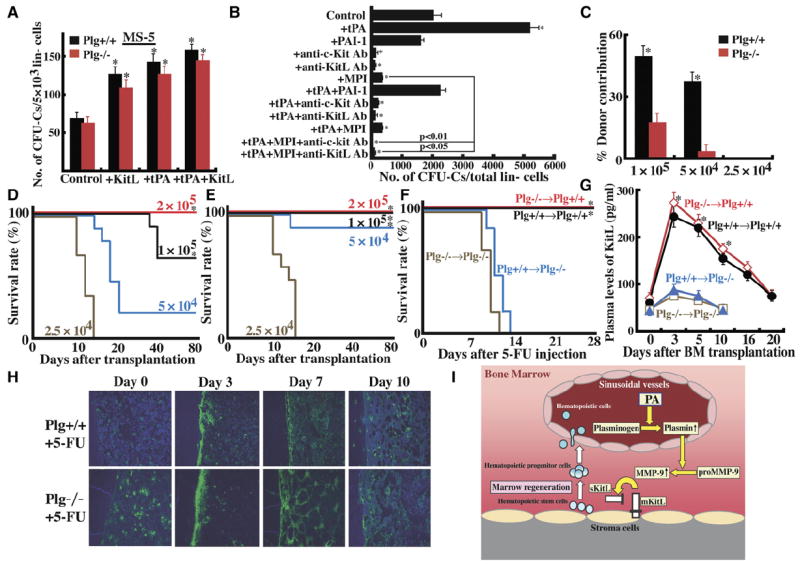
(A) Lin− cells from Plg+/+ and Plg−/− mice were cultured in the presence or absence of tPA with or without KitL on MS-5 stromal cells. After 7 days, cells were harvested and subjected into a progenitor assay (n = 6).
(B) Lin− cells from Plg+/+ mice were cultured on confluent MS-5 stromal cells in serum-free medium (n = 3). Recombinant tPA, recombinant PAI-1, antibodies against c-Kit or KitL, or a matrix metalloproteinase inhibitor (MPI; CGS 27023A) was added to the cultures for 5 days. Cells were collected and subjected to a progenitor assay.
(C–E) BM cells from CD45.1 congenic donor mice were transplanted at limiting cell dilutions into lethally irradiated Plg+/+ or Plg−/− CD45.2+ recipient mice (n = 6). (C) Mouse PB cell donor contribution (CD45.1+ cells) was assessed 80 days after transplantation of CD45.1 donor cells into Plg+/+ or Plg−/− recipient mice. (A–C) Error bars represent SEM. Survival of Plg−/− recipient mice ([D], n = 10) and Plg+/+ recipient mice ([E], n = 9) was monitored daily.
(F and G) Lethally irradiated Plg+/+ or Plg−/− mice were transplanted with BM cells from donor Plg+/+ or Plg−/− mice 16 days prior to 5-FU injection (n = 9 in each group). After cell engraftment, a single dose of 5-FU was injected on day 0. (F) Survival of transplanted mice was monitored (*p < 0.05, comparing survival of Plg+/+ recipient mice to Plg−/− recipient animals). (G) Blood was drawn after 5-FU administration. Plasma samples were analyzed for KitL by ELISA (n = 3). Error bars represent SEM.
(H) Immunohistochemical staining for fibrin (green fluorescence) in BM sections from Plg+/+ and Plg−/− mice treated with a single injection of 5-FU i.v.
(I) PA-mediated activation of Plg generates plasmin after induction of hematopoietic stress within the BM. Plasmin in turn can activate MMPs, especially MMP-9, thereby releasing chemo/cytokines, like KitL. These factors can regulate the hematopoietic stress response by promoting HSPC proliferation, differentiation, and BM reconstitution. *p < 0.05.
If c-Kit/KitL signaling following tPA treatment promotes hematopoietic cell proliferation, blocking the signaling should prevent tPA-induced progenitor proliferation. Indeed, addition of neutralizing antibodies against c-Kit and/or KitL prevented the tPA-mediated generation of CFU-Cs from lin− cells in stromal-cell-based cultures (Figure 6B). Likewise, addition of PAI-1, the natural inhibitor of tPA to these cultures, abolished tPA-mediated CFU-C formation. Confirming our in vivo data, addition of a synthetic MPI completely blocked progenitor production and prevented tPA-mediated progenitor expansion. These data imply that tPA can modulate progenitor cell expansion by promoting the release of KitL.
tPA affected hematopoietic cells only in the presence of stromal cells. So do Plg-deficient mice have a stromal cell defect causing the observed hematopoietic phenotype? When CD45.1 donor cells were transplanted into Plg+/+ or Plg−/− recipients, donor cell contribution to PB leukocytes 80 days after transplantation was lower in Plg−/− than in Plg+/+ recipient mice (Figure 6C). This was especially apparent at limiting cell dilutions and correlated with decreased mouse survival at 80 days after transplantation of CD45.1 donor cells in Plg−/− recipient mice (Figure 6D). Fewer donor cells were necessary to promote survival in Plg+/+ recipient mice (Figure 6E). These data imply that the absence of Plg in the BM is responsible for the reduced hematopoietic cell proliferation in Plg−/− mice.
To overcome the problem of reduced cell proliferation in a Plg-deficient microenvironment, we transplanted very high numbers of BM cells (2 × 107 cells/mouse) from Plg−/− or Plg+/+ mice into lethally irradiated Plg−/− or Plg+/+ mice. Under these conditions, all mice survived and showed engraftment after 4 weeks (WBC counts of WT cells transplanted into WT mice 7933 ± 945/μL blood; KO cells into WT mice 7133 ± 1815 μl blood; WT cells into KO mice 6400 ± 1697 μl blood; KO cells into KO mice 7700 ± 1556/μL blood). Then, chimeric mice were treated with the myelosuppressive agent 5-FU. Plg-deficient mice transplanted with either Plg+/+ or Plg−/− BM donor cells succumbed within the first 2 weeks after induction of myelosuppression (Figure 6F; n = 9 per group). In contrast, all Plg WT mice transplanted with either Plg+/+ or Plg−/− BM donor cells survived (n = 9 per group), emphasizing a critical role for Plg in the nonhematopoietic BM compartment. We then asked if the impaired survival and cell proliferation of HSPCs within the Plg-deficient BM stromal compartment was due to reduced production of KitL. Lower KitL levels were detected in Plg−/− recipient chimeric mice as compared to Plg+/+ recipient controls (Figure 6G). These data underscore the importance of KitL release and maybe other yet-to-be-defined factors from BM stromal cells in a Plg-competent environment for BM reconstitution after myelosuppression with 5-FU.
Binding of Plg to fibrin enhances Plg activation (Camiolo et al., 1971). Immunohistochemical staining of BM sections from WT animals after myelosuppression revealed that, during the hematopoietic regeneration phase, fibrin was found in close vicinity of bone-lining osteoblasts and later in vascular areas, which might be part of the recently proposed vascular niche (Figure 6H) (Heissig et al., 2005a). Fibrin deposition was observed in BM of Plg−/− and, to a lesser extent, Plg+/+ mice after myelosuppressive stress.
Our data indicate that activation of Plg in the regeneration phase after myelosuppression results in an orderly activation of another protease cascade, like MMPs (Figure 6I). This proteolytic mixture helps to make growth factors bioavailable, which in a fine-tuned manner can modulate hematopoietic cell proliferation and differentiation.
DISCUSSION
The cellular BM microenvironment mediates the stem cell choice of self-renewal versus differentiation. Whereas self-renewal is important to maintain the stem cell pool, rapid recruitment of HSCs into cell cycle followed by proliferation, and differentiation allows the organism to adapt to hematopoietic stress—cell loss after blood loss or myelosuppression—with the formation of new blood cells. In this study, we provide genetic, functional, and biochemical evidence that the orderly activation of two separate protease systems, the Plg/PA fibrinolytic system and the MMP system after hematopoietic stress, allows hematopoietic cell proliferation and differentiation to take place, a process in part mediated by the release of KitL (Figure 6I). Collectively, these data introduce a novel paradigm by which fibrinolytic enzymes mediate systemic and localized effects in the BM and establish a novel role for Plg activation in hematopoiesis.
Local Accumulation of Components of the Plasminogen System within the BM
We found local accumulation of Plg/plasmin within the BM stromal compartment after myelosuppression and demonstrated endogenous tPA expression within BM stromal and hematopoietic cells. The presence of fibrinolytic factors within the BM in vivo suggests that localized proteolysis occurs during BM cell reconstitution.
Plasmin Activates MMP-9 in the BM
But what is the function of proteolysis in stress hematopoiesis, and what are its mediators? Plasmin can cleave proMMPs (MMP-9, MMP-12, MMP-1) (Lijnen et al., 1998a). Here, we show that tPA either provided by BM cells or exogenously administered causes MMP activation, namely MMP-2 and MMP-9 in cultured BM stromal cells. MMPs have been identified as downstream targets for plasmin activation in other cells, e.g., at the nerve injury site (Zou et al., 2006).
The Fibrinolytic Factor tPA Modulates Hematopoiesis by Releasing KitL
KitL is a growth factor for primitive hematopoietic cells and multiple differentiating lineages, acting in synergy with other cytokines to promote cell proliferation and differentiation. KitL is synthesized as a transmembrane protein that may be processed to produce soluble KitL (Huang et al., 1990). We demonstrated that MMP-9 activation can promote the shedding of KitL (Heissig et al., 2002). Here, we show that tPA indirectly via Plg and MMP activation is able to release KitL from stromal cells.
Exogenous administration of recombinant tPA promotes myeloid, erythroid, and megakyaryocytic cell differentiation and progenitor proliferation. The importance of KitL for these tPA-mediated effects on hematopoiesis was supported by experiments using Sl/Sld mice, where tPA-mediated BM cell and progenitor proliferation did not occur. Similarly, impaired BM regeneration seen in Plg−/− mice was due to an impaired compensatory increase in KitL after myelosuppression, as administration of KitL induced BM reconstitution and rescued Plg-deficient mice.
Plg activation can occur via tPA, uPA, and plasma kallikrein (Lund et al., 2006) and has been implicated in cell differentiation, like myoblast fusion and differentiation (Suelves et al., 2002) or adipocyte differentiation (Selvarajan et al., 2001).
Rebleeding has been reported after tPA treatment in ischemic stroke patients (Cheng et al., 2006), which could stimulate HSPC proliferation (Hellman and Grate, 1967). Therefore, it is conceivable that bleeding could have affected the outcome of our study. But no bleeding was observed after tPA administration into healthy, nonischemic mice. tPA-mediated progenitor proliferation was also observed in vitro, where bleeding can be excluded.
tPA-Mediated Effects on Hematopoietic Cells Require a Functional Microenvironment
KitL expressed in the stromal compartment is a key factor to maintain hematopoiesis. We observed reduced engraftment of either Plg−/− or Plg+/+ HSPCs into Plg−/− recipient mice, which was not due to impaired HSPC adhesion (data not shown) but due to a BM microenvironment, which could not provide KitL to support long-term hematopoiesis. The effects of a defective microenvironment are best known from studies on Sl/Sld mice. When cells had been transplanted very early into a non-KitL defective environment, long-term repopulating activity was identical in Sl/Sld and WT mice (Barker, 1997). Persistence of HSCs in a defective environment resulted in progressive loss of HSC activity (Barker, 1994).
Similar to Sl/Sld mice, Plg−/− mice showed reduced numbers of hematopoietic progenitors, whereas the HSC compartment (cells with long-term repopulating capacity) was functionally normal. Our data indicate that Plg plays a role in cell differentiation and progenitor proliferation rather than HSC-associated self-renewal. Therefore, myelosuppression models can reveal the hematopoietic phenotype. The early death observed in Plg−/− mice after myelosuppression is most likely due to lack of cell differentiation and progenitor proliferation, as differentiated cells rather than HSCs ensure hematopoietic recovery and survival 10 days after injection of the myelosuppressive agent. Further studies will show if, aside from KitL, other growth factors are altered in Plg−/− mice.
This study has implications for the control of various diseases. Plg activation is associated with cancer invasion and metastasis (Andreasen et al., 2000). It is possible that plasmin generated in tumors activates MMPs and releases KitL, a process that could promote metastasis or angiogenesis (Kijima et al., 2002; Heissig et al., 2003). Further studies will be necessary to evaluate this. tPA is in clinical use in the treatment of myocardial infarction (Andersen et al., 2003; Wilcox, 1996). Its positive effect on myocardial reperfusion had been thought to be due to its fibrinolytic effects. We speculate that part of its effects after myocardial infarction is driven by KitL and its effect on hematopoietic cells. Finally, tPA can potentially be used for stem cell harvesting and might be used to expand HSPCs.
Conclusions
Activation of Plg after hematopoietic stress (Figure 6I) leads to activation of another protease cascade, namely MMPs. Activation of MMP-9 releases, e.g., KitL, which in turn controls hematopoietic cell proliferation and differentiation. This process helps to replenish hematopoietic cells within the BM. The involvement of the fibrinolytic system in the regulation of adult stress hematopoiesis represents a new paradigm with important implications for regenerative medicine.
EXPERIMENTAL PROCEDURES
In Vivo Assays
Animals
Age- (6–8 week) and sex-matched Plg+/+ and Plg−/− mice were obtained by heterozygous breeding of mice backcrossed into a C57BL/6J background (Bugge et al., 1995). MMP-9+/+ and MMP-9−/− mice were used after more than ten backcrosses into CD1 mice (Heissig et al., 2002). Animal procedures were approved by the Animal Care Committee of Juntendo University. KitL WT (WBB6F1) and KitL-deficient (Sl/Sld), CD45.1 congenic, and C57BL/6 mice were purchased from SLC (Japan).
Myelosuppression Model
Plg+/+ and Plg−/− mice, MMP-9+/+ and MMP-9−/− mice, or Plg chimeric mice were injected with the myelosuppressive agent 5-FU (250 mg/kg body weight, Kyowa Hakko Kogyo, Tokyo, Japan). Survival was monitored daily.
Chimeric BM Transplantation Model
Plg+/+ or Plg−/− animals were lethally irradiated (900 cGy) and 6 hr later underwent transplantation with 2 × 107 BMMCs from either Plg+/+ or Plg−/− animals.
Competitive Transplantation Experiments
Lethally irradiated Ly-5.1 mice were injected with 10, 50, or 100 KSL cells (ten recipient mice per cell concentration) from Plg+/+ and Plg−/− mice together with 5 × 104 CD45.1 BM competitor cells. Percentage of donor-derived three-lineage contribution in PBMCs using antibodies against CD45.2, Gr-1, B220, or CD3 was assessed.
Long-Term Engraftment
1 × 105, 4 × 104 or 2.5 × 104 BM cells from Plg+/+ or Plg−/− mice (CD45.2+; six mice each) were transplanted into lethally irradiated (900 cGy) CD45.1 syngenic mice together with a constant number of competitor cells (5 × 105). In another experiment, 1 × 105, 4 × 104, or 2.5 × 104 CD45.1+ BM cells together with 5 × 105 CD45.1 competitors were transplanted into lethally irradiated congenic Plg+/+ or Plg−/− (CD45.2+) recipient mice. The percent of donor contribution was calculated by measuring CD45.1+ and CD45.2+ on PB leukocytes in recipient mice 90 days posttransplant.
tPA Administration In Vivo
Mice were injected with human recombinant tPA (kindly provided by Eisai, Tokyo, Japan) intraperitoneally at a concentration of 1.25 × 106 IU = 1 mg/kg body weight daily or PBS for a total of 3 days. (Recommended tPA dosage in humans for clinical use is 10 mg/kg.) Blood and plasma samples were collected.
Peripheral Blood Analysis
Blood was collected from mice by retro-orbital bleeding using heparinized capillaries. WBC counts were determined. PBMCs were isolated from heparinized blood after centrifugation over a discontinuous gradient using lympholyte-M (Cedarlane, Ontario, Canada). Plasma samples were stored at −80°C until further analysis.
CFU-S Assay
Mobilized PBMCs were obtained and subjected to a CFU-S assay as previously described (Hattori et al., 2001). Mice were sacrificed on day 8. The number of visible colonies was counted.
Histological Assessment
Deparaffinized BM sections (3–5 μm) were stained for fibrin(ogen) followed by a secondary Alexa 488-labeled antibody (Molecular Probes, Inc) or with hematoxylin and eosin (H&E). BM sections were stained for MMP-9 (7-11C) as described (Heissig et al., 2002). BM sections were pretreated with hyaluronidase (for Plg) or Proteinase K (for tPA), blocked, and incubated with a rabbit anti-human Plg polyclonal Ab (H-90, Santa Cruz, dilution 1/100) or rabbit anti-human tPA IgG fraction (INR, dilution 1/100) for 1 hr. Following a catalyzed signal amplification II system (Dako), slides were developed with DAB and counterstained with H&E.
In Vitro Assays
Lin− Cell Separation
Murine BM cells were obtained after flushing their femurs and tibiae. Cells were stained using a lin− cell separation kit (Stem Cell Technologies, Vancouver). After running through two MACS columns (Miltenyi Biotec), more than 90% of the separated lin− cells were c-Kit/Sca-1 double-positive cells by FACS.
Hematopoietic Progenitor Assay
PBMCs (105 cells/plate) or lin− cells (5 ×103 cells/plate) were plated in triplicate in 1 ml of a commercially available methylcellulose-based assay (Methocult, Stem Cell Technologies, Vancouver).
Culture Supernatants
(1) BM cells (1 × 106) from 5-FU treated Plg+/+ and Plg−/− mice were cultured overnight in serum-free medium. (2) MS-5 cells were grown to confluency in 6-well plates (Falcon) and then exposed overnight in serum-free medium to tPA (5 μg/ml CleactorR). (3) BM-derived lin+ cells (1 × 105) from Plg+/+ mice were cultured in serum-free medium overnight with or without recombinant tPA (5 μg/ml, CleactorR). Culture supernatants were collected. Supernatants (1–3) were analyzed by substrate zymography or ELISA. Or confluent MS-5 cells or primary stromal cells were cultured in the presence of tPA (5 μg/ml, CleactorR) with or without PAI-1 (2 μg/ml, Calbiochem) or MMP inhibitor (CGS 27023A, Novartis, Basel, Switzerland, 1 nM daily). Supernatants were stored at −30°C and analyzed for the release of KitL by ELISA.
Stromal-Based Expansion Cultures
1 × 105 lin− cells from BM of Plg+/+ mice were cocultured with a confluent layer of stromal cells (MS-5). Recombinant tPA (5 μg/ml, daily, CleactorR) was added to cultures with or without KitL (2 μg/ml, every other day). Seven days later, adherent cells were retrieved using trypsin (Sigma) and pooled with the nonadherent cells. Hematopoietic cells were counted and subjected to a progenitor assay.
Suspension Culture
BM-derived lin− cells (1.0 × 105) from Plg+/+ mice were cultured in serum-free medium for 4 days in the presence/absence of recombinant tPA (1 or 5 μg/ml, CleactorR, Eisai Co, Japan) plus or minus recombinant KitL (2 μg/ml, PeproTech) daily added to the cultures. Cells were harvested, counted, and subjected to a progenitor cell assay.
Proliferation Blocking Experiment
Lin− cells (1.2 × 105) were isolated from the BM of Plg+/+ mice and cocultured with MS-5 stromal cells in the presence/absence of recombinant tPA (5 ug/ml, CleactorR) with or without blocking antibodies against c-Kit (clone ASK, final concentration 0.5 mg/ml), against SCF (KitL, final concentration 0.5 μg/ml, R & D Systems), with or without PAI-1 (2 μg/ml, Calbiochem), or the MMP inhibitor (1 nM, CGS 27023A, Novartis, Basel, Switzerland) daily. The adherent and nonadherent cells collected after 5 days of culture. After counting, hematopoietic cells were subjected to a hematopoietic progenitor assay.
Substrate Zymography
Supernatants were treated with gelatin-agarose beads and processed through sodium dodecyl sulfate polyacrylamide gel electrophoresis as described (Heissig et al., 2002) A pro-MMP-2 standard (Oncogene Research Products, Cambridge, MA) was used to determine the molecular weight of gelatinolytic bands.
RNA Extraction and RT-PCR Analysis
Total RNAs were extracted from 4-week-old LTC stroma and MS-5 cells using Trizol reagent. RT-PCR was performed using One Step RNA PCR Kit (AMC) (TaKaRa Shuzo Co., Japan). β-actin primers 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ (sense strand) and 5′-TAAAA CGCAGCTCAGTAACAGTCCG-3′ (antisense strand) were used to test the integrity of the total RNA extracted (349 bp product). Primer sequences for tPA were 5′-CTGAGGTCACAGTCCAAGCAATGT-3′ (sense primer) and 5′-GCTCACGAAGATGATGGTGTAAAGA-3′ (antisense), yielding a 564 bp product. Primer sequences for plasminogen were 5′-TGTGGGCTCTAAAGATGGAACTCC-3′ (sense primer) and 5′-GACAAGGGGACTCGCTGGATGGCTA-3′ (antisense primer), yielding a product of 268 bp.
FACS Analysis
BM cells of Plg+/+ and Plg−/− mice were incubated with CD11b-FITC and Gr-1-PE antibodies and analyzed by FACS. BM cell ploidy was analyzed using FACS after cell fixation with ethanol and labeling with propidium iodide (PI). For cell-cycle analysis, whole BM cells were labeled with Sca-1-FITC and stained with PI (Molecular Probes, OR) as described (Heissig et al., 2002).
BM cells of untreated and tPA-treated Plg+/+ mice were stained with CD11b-FITC and Gr-1-PE or CD71-FITC and Ter119-PE antibodies and analyzed by FACS. Next, lin− BM cells were stained with Sca-1-FITC and PI before cell-cycle analysis was performed. For SP cell analysis, BM cells were subjected to FACS (BD Biosciences) by using the MoFlo (DakoCytomation) with the following filter sets: 405/30 bandpass (BP) to detect Hoechst blue, 585/42 BP to detect Hoechst red, and a 440 nm long-pass dichromic filter to separate Hoechst red from Hoechst blue. A subset of cells treated with verapamil confirmed the identity of the SP gate.
Immunoassay for KitL, proMMP-9, and Active MMP-9
In plasma or culture supernatants, KitL (SCF) was measured using commercially available ELISA (R & D Systems). Active MMP-9 was analyzed using the MMP-9 Activity Assay Biotrak System ELISA (Amersham Biosciences, UK).
Statistical Analysis
Results are expressed as mean ± SEM (standard error of mean). Data were analyzed using the unpaired two-tailed Student’s t test. P values of <0.05 were considered significant.
Supplementary Material
Supplemental Data include one figure and can be found with this article online at http://www.cellstemcell.com/cgi/content/full/1/6/658/DC1/.
Acknowledgments
We thank Drs. A. Furuhata, N. Tada, T. Kuhara, H. Nishibeppu, and M. Muramatsu for excellent technical assistance. We thank Eisai Co., Ltd. (Tokyo, Japan) for kindly providing us with the recombinant tPA. This work was supported by grants from the JSPS (B.H.); the European Commission, qLGI-CT-2000-01131, LSHC-CT-2003-503297, and the Danish Cancer Society (to K.D., L.R.L., and J.R.); the National Institutes of Health (AG023218 to Z.W.), Grants-in-Aid for Scientific Research from MEXT (K.H. and B.H.); and in part by grants from Mitsui Life Social Welfare Foundation; Kirin Pharma Company, Ltd. (K.H); and Chugai Pharmaceutical Co., Ltd. (K.H.).
References
- Andersen HR, Nielsen TT, Rasmussen K, Thuesen L, Kelbaek H, Thayssen P, Abildgaard U, Pedersen F, Madsen JK, Grande P, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733–742. doi: 10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews RG, Bartelmez SH, Knitter GH, Myerson D, Bernstein ID, Appelbaum FR, Zsebo KM. A c-kit ligand, recombinant human stem cell factor, mediates reversible expansion of multiple CD34+ colony-forming cell types in blood and marrow of baboons. Blood. 1992;80:920–927. [PubMed] [Google Scholar]
- Barker JE. Sl/Sld hematopoietic progenitors are deficient in situ. Exp Hematol. 1994;22:174–177. [PubMed] [Google Scholar]
- Barker JE. Early transplantation to a normal microenvironment prevents the development of Steel hematopoietic stem cell defects. Exp Hematol. 1997;25:542–547. [PubMed] [Google Scholar]
- Bugge TH, Flick MJ, Daugherty CC, Degen JL. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- Camiolo SM, Thorsen S, Astrup T. Fibrinogenolysis and fibrinolysis with tissue plasminogen activator, urokinase, streptokinase-activated human globulin, and plasmin. Proc Soc Exp Biol Med. 1971;138:277–280. doi: 10.3181/00379727-138-35878. [DOI] [PubMed] [Google Scholar]
- Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernandez JA, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Friedrich M, Rafii S, Werb Z. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol. 2003;10:136–141. doi: 10.1097/00062752-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Heissig B, Ohki Y, Sato Y, Rafii S, Werb Z, Hattori K. A role for niches in hematopoietic cell development. Hematology. 2005a;10:247–253. doi: 10.1080/10245330500067249. [DOI] [PubMed] [Google Scholar]
- Heissig B, Rafii S, Akiyama H, Ohki Y, Sato Y, Rafael T, Zhu Z, Hicklin DJ, Okumura K, Ogawa H, et al. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J Exp Med. 2005b;202:739–750. doi: 10.1084/jem.20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman S, Grate HE. Haematopoietic stem cells: evidence for competing proliferative demands. Nature. 1967;216:65–66. doi: 10.1038/216065a0. [DOI] [PubMed] [Google Scholar]
- Herren T, Swaisgood C, Plow EF. Regulation of plasminogen receptors. Front Biosci. 2003;8:d1–d8. doi: 10.2741/916. [DOI] [PubMed] [Google Scholar]
- Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–2127. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- Huang E, Nocka K, Beier DR, Chu TY, Buck J, Lahm HW, Wellner D, Leder P, Besmer P. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, Sattler M, Johnson BE, Salgia R. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002;62:6304–6311. [PubMed] [Google Scholar]
- Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- Lijnen HR, Silence J, Lemmens G, Frederix L, Collen D. Regulation of gelatinase activity in mice with targeted inactivation of components of the plasminogen/plasmin system. Thromb Haemost. 1998a;79:1171–1176. [PubMed] [Google Scholar]
- Lijnen HR, Van Hoef B, Lupu F, Moons L, Carmeliet P, Collen D. Function of the plasminogen/plasmin and matrix metalloproteinase systems after vascular injury in mice with targeted inactivation of fibrinolytic system genes. Arterioscler Thromb Vasc Biol. 1998b;18:1035–1045. doi: 10.1161/01.atv.18.7.1035. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li N, Diaz LA, Shipley M, Senior RM, Werb Z. Synergy between a plasminogen cascade and MMP-9 in autoimmune disease. J Clin Invest. 2005;115:879–887. doi: 10.1172/JCI23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund LR, Green KA, Stoop AA, Ploug M, Almholt K, Lilla J, Nielsen BS, Christensen IJ, Craik CS, Werb Z, et al. Plasminogen activation independent of uPA and tPA maintains wound healing in gene-deficient mice. EMBO J. 2006;25:2686–2697. doi: 10.1038/sj.emboj.7601173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajan S, Lund LR, Takeuchi T, Craik CS, Werb Z. A plasma kallikrein-dependent plasminogen cascade required for adipocyte differentiation. Nat Cell Biol. 2001;3:267–275. doi: 10.1038/35060059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- Suelves M, Lopez-Alemany R, Lluis F, Aniorte G, Serrano E, Parra M, Carmeliet P, Munoz-Canoves P. Plasmin activity is required for myogenesis in vitro and skeletal muscle regeneration in vivo. Blood. 2002;99:2835–2844. doi: 10.1182/blood.v99.8.2835. [DOI] [PubMed] [Google Scholar]
- Wilcox RG. Clinical trials in thrombolytic therapy: what do they tell us? INJECT 6-month outcomes data. Am J Cardiol. 1996;78:20–23. doi: 10.1016/s0002-9149(96)00739-4. [DOI] [PubMed] [Google Scholar]
- Zou T, Ling C, Xiao Y, Tao X, Ma D, Chen ZL, Strickland S, Song H. Exogenous tissue plasminogen activator enhances peripheral nerve regeneration and functional recovery after injury in mice. J Neuropathol Exp Neurol. 2006;65:78–86. doi: 10.1097/01.jnen.0000195942.25163.f5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data include one figure and can be found with this article online at http://www.cellstemcell.com/cgi/content/full/1/6/658/DC1/.


