Abstract
OBJECTIVES
Although EMR has been used for elimination of neoplasia in BE, the significance of positive carcinoma margins and depth of invasion on endoscopic resection pathology has not been assessed using a valid standard. The aim of this study was to assess the accuracy of tumor staging by EMR using esophagectomy as the standard.
METHODS
Medical records of patients, who underwent endoscopic resection for esophageal carcinoma or high-grade dysplasia in BE followed by esophagectomy, were reviewed. Data were abstracted from a prospectively maintained EMR database. Endosonography and endoscopic resection were performed by a single experienced endoscopist. Two experienced GI pathologists interpreted all histological results. Standard statistical tests were used to compare continuous and categorical variables.
RESULTS
Twenty-five patients were included in the study. Three patients had mucosal carcinoma and 16 had submucosal carcinoma following endoscopic resection. Surgical pathology staging was consistent with preoperative EMR staging in all patients. No patient with negative mucosal resection margins had residual tumor at the resection site at esophagectomy. In patients with submucosal carcinoma, 8 had residual carcinoma at the EMR site at surgery and 5 patients had metastatic lymphadenopathy.
CONCLUSIONS
Tumor staging using EMR pathology is accurate when compared with surgical pathology following esophagectomy. Negative margins on EMR pathology correlate with absence of residual disease at the EMR site at esophagectomy. Submucosal carcinoma on EMR specimens was associated with a high prevalence of residual disease at surgery (50%) and metastatic lymphadenopathy (31%).
INTRODUCTION
Endoscopic mucosal resection (EMR) is a technique initially introduced and popularized in Japan, for the treatment of early upper gastrointestinal (esophageal and gastric) carcinomas. The technique has been increasingly utilized in western countries. The significant morbidity and mortality of esophagectomy for esophageal malignancy makes endoscopic treatment an attractive alternative. EMR is an evolving technique with new technologies that are continuing to increase its potential for the treatment of malignancies. EMR is an ideal endoscopic technique to accurately stage neoplastic lesions. Since the depth of tumor invasion determines the risk of metastases, the precision with which this technology allows accurate histological staging is of critical importance in advancing endoscopic therapy of neoplastic lesions in the luminal GI tract. Most mucosal resections are not able to completely resect neoplastic lesions since the margins of these lesions are not endoscopically visible. However, there is very little information in the literature on the significance of neoplastic involvement of mucosal resection margins on histopathology as subsequently assessed by the traditional standard of esophagectomy. The significance of tumor margins in terms of lymph node involvement following esophagectomy has also not been determined.
METHODS
With the approval of our Institutional Review Board, 25 patients were identified from a prospectively maintained database who underwent EMR followed by esophagectomy for either high-grade dysplasia (HGD) or adenocarcinoma arising in a background of Barrett’s esophagus (BE) between June 1995 and September 2004 at the Barrett’s Esophagus Unit, Mayo Clinic, Rochester. Information obtained included basic demographics, pathology on referral, findings on upper endoscopy/endoscopic ultrasound, and pathology from EMR and operative specimens. Clinical course following EMR and esophagectomy was also extracted from our database.
All patients were initially evaluated with gastroscopy done using a standard video endoscope (GIF-Q140 or GIF-Q160 instrument; Olympus America Inc., Melville, NY). Four quadrant biopsies were obtained at 1-cm interval along the length of the BE segment. Endosonographic examination was performed in all patients using the radial scanning echoendoscope (GIF-UM20 or GIF-UM30 instrument; Olympus America Inc.) for tumor (T) staging of focal lesions. Lesions in five patients were also evaluated with high frequency ultrasound catheter probes (20 and 30 MHz). Suspicious lymph nodes (on the basis of size, shape, and echogenicity) were sampled with a linear array instrument using a 22-gauge Echo Tip needle (Wilson Cook Medical, Winston-Salem, NC).
Endoscopic Mucosal Resection
EMR was performed in all patients with neoplastic appearing nodular lesions to determine their histology and extent of involvement. EMR was performed by initially injecting 10–15 cc of diluted epinephrine (1:200,000) solution into the submucosa underneath the lesion. The initial technique used for EMR between 1996 and 2000 was a variceal ligation method with a Bard Six-Shooter (Bard Interventional Products, Billerica, MA). With this technique, suction was applied over a lesion of interest and a band was applied to create a pseudopolyp. The pseudopolyp was then resected using a standard snare and retrieved. Beginning in April 2000, EMR was performed using a commercially available EMR kit (EMR-001, Olympus America Inc.). A forward resecting cap was placed at the end of the endoscope. The distal end of the cap has a small ledge where a crescent snare can be placed around the circumference of the cap. The mucosal abnormality was suctioned into the cap, resected with the snare, and retrieved within the cap. A single experienced endoscopist (KKW) performed all mucosal resections.
Pathology Assessment
Two experienced gastrointestinal pathologists, with a focus in BE, assessed all resected specimens. All specimens were serially sectioned (“bread loaf” sectioning) and assessed for size, histology, tumor grade, extent of tumor penetration and margins (lateral and deep), and completeness of resection (1). Dysplasia within Barrett esophagus was classified according to previously published criteria, looking at surface maturation, gland architecture, and cytologic features (2). Low-grade dysplasia (LGD) was diagnosed if the nuclei were hyperchromatic with mild crowding and/or architectural changes, which extended at least focally to the surface. HGD was characterized by more significant alterations, including greater nuclear irregularities, prominent nucleoli, loss of nuclear polarity with respect to the basement membrane, and gland crowding. Intraluminal necrosis may or may not have been present. Intramucosal adenocarcinoma was diagnosed once there was invasion through the basement membrane into lamina propria or into muscularis mucosae. The former is often characterized by single cells or clusters of cells within the lamina propria. Invasive adenocarcinoma was defined as tumor, which invaded through muscularis mucosae into submucosa. All cases reviewed by the two GI pathologists and a consensus diagnosis was reached on all cases at a multiheaded microscope. Typically, when cases demonstrated a challenging morphology between the two above diagnoses, additional opinions were obtained from GI Pathologists.
Surgery
Patients were referred for esophagectomy when submucosal carcinoma was found on pathology following EMR. Patients with mucosal carcinoma or HGD on EMR underwent esophagectomy when it was determined that their disease could not be adequately managed endoscopically primarily due to extent of disease. All patients underwent esophagectomy at the Mayo Clinic, Rochester, Minnesota. Experienced members of the Thoracic Surgery Division, using either the transhiatal approach or the transthoracic approach, performed all esophagectomies. Following surgery, lesions of interest were carefully sectioned to determine the extent of tumor invasion. Lymph nodes were also dissected from the resection specimen and assessed for metastatic involvement. Final histopathology was reported in terms of tumor grade (if carcinoma was found), T staging, and lymph nodal involvement (N staging).
Statistical Methods
Following data extraction, statistical analysis was performed using the JMP statistical analysis package (JMP, Version 6, SAS Institute Inc., Cary, NC 1989–2002). Baseline continuous data were compared using the 2-sample t-test or the Wilcoxon’s rank sum tests depending on the data normality. Baseline categorical data were compared using the χ2 test (or Fisher Exact test when necessary because of small sample size).
RESULTS
Twenty-five patients underwent esophagectomy after EMR. Their mean age was 66 yr (SEM ± 1.9) with 19 patients (76%) being men. Fifteen patients were referred with a diagnosis of HGD (60%), 9 had adenocarcinoma (36%), and 1 patient had LGD (with a visible nodule suspicious for carcinoma). On initial endoscopy, 19 (76%) patients had a nodule, 4 (12.5%) had a mass (defined as a raised lesion > than 2 cm in size), and 2 (6.2%) had an ulcer. Five patients had tumor staging performed using high frequency (20 and 30 MHz) catheter EUS probes in addition to conventional EUS. On EUS examination, a focal lesion could not be identified (uT0) in 14 patients (56%). Table 1 describes the EUS staging of the 19 patients with carcinoma on EMR pathology. EUS did not identify 10 of 19 (51%) carcinomas, of which 7 were submucosal. EUS accurately staged 4 of 16 (25%) submucosal carcinomas and none of the 3 mucosal carcinomas. Six patients (24%) had EUS-guided lymph node aspirations, which were all negative on cytology. All lymph nodes aspirated were hypoechoic, periesophageal in location, measuring between 0.7 cm and 1.5 cm, and were aspirated using 23G needles (Wilson Cook, Winston Salem, NC), with multiple passes (3–5).
Table 1.
Comparison of EUS Stage and Endoscopic Mucosal Resection Stage in Patients With Carcinoma (N = 19)
| EUS Stage | EMR Pathology: Mucosal Ca | EMR Pathology: Submucosal Ca |
|---|---|---|
| U T0 (N = 9) | 2 | 7 |
| UT1 a (mucosal) (N = 4) | 0 | 4 |
| UT1b (submucosal) (N = 4) | 1 | 3 |
| UT2 (N = 2) | 0 | 2 |
The mean diameter of EMR specimens was 1.1 cm (SEM ± 0.1). Carcinoma was found on EMR pathology in 19 (76%) patients, HGD in 4 (16%) patients, LGD and nondysplastic BE in one patient each (these patients underwent esophagectomy as they had mucosal carcinoma in biopsies taken from the distal and proximal esophagus) (Table 2). Of the 19 patients with carcinoma on EMR pathology, 16 (84%) had submucosal carcinoma and 3 (20%) had mucosal carcinoma. Margins were positive (involved by carcinoma) in 13 patients (81%) with submucosal carcinoma and 1 patient (33%) with mucosal carcinoma. Both lateral and deep margins were positive in 12 patients with adenocarcinoma on EMR pathology. Three of four specimens with HGD had positive margins (involved with HGD). The patients with LGD and nondysplastic BE on EMR pathology had no residual carcinoma on esophagectomy (see Figs. 1–4).
Table 2.
Surgical Tumor Stage Compared to Endoscopic Mucosal Resection Tumor Stage
| EMR Pathology | EMR Margins | Surgical T Staging |
|---|---|---|
| Submucosal Ca (N = 16) | Positive (13) | T 0 = 5* |
| • deep (+)/lateral (+) : 12 | T 1b = 5 | |
| • deep (−)/lateral (+) : 1 | T 2 = 2 | |
| T 3 = 1 | ||
| Negative (3) | T 0 = 3 | |
| Mucosal Carcinoma (N = 3) | Positive (1) | T 0 = 1 |
| Deep (+)/lateral (+) : 1 | ||
| Negative (2) | T 0 = 1 | |
| T 1 a = 1† | ||
| HGD (N = 4) | Positive (3) | T 0‡ |
| Negative (1) | T 0‡ |
Two patients had synchronous intramucosal carcinomas at sites different from the EMR targeted lesion.
One patient had synchronous intramucosal carcinoma at a site different from the EMR targeted lesion.
Residual HGD present in all patients.
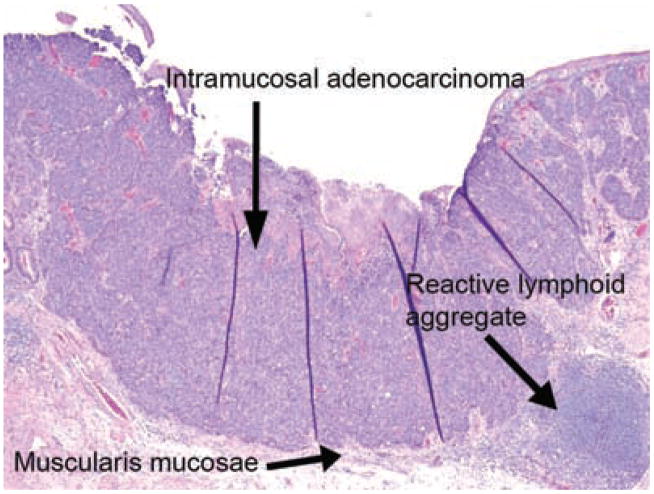
Figure 1.
Intramucosal adenocarinoma confined by muscularis mucosae. There is a lymphoid aggregate at the lower right corner of the field. No invasion into submucosa is present.
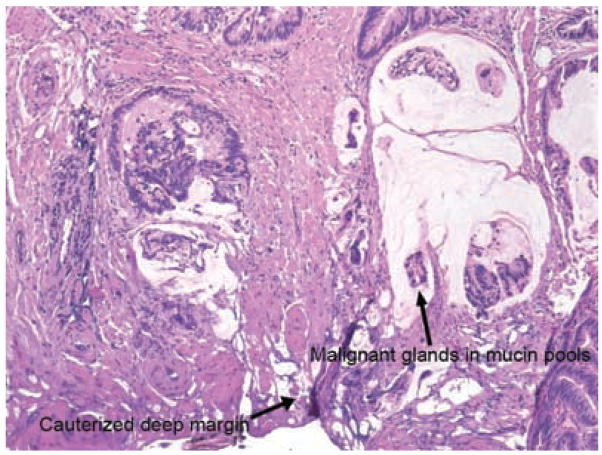
Figure 4.
Deep margin involved by carcinoma. There are malignant glands and single cells present within mucin pools which are present along the deep margin of the specimen.
Patients underwent esophagectomy after a median duration of 3.3 wk (IQR 2.4–4.5 wk) following EMR. Twenty patients were staged with a CT scan of the chest/abdomen/pelvis before surgery, with negative results in 17, periesophageal lymphadenopathy in 2, and a suspicion of a metastatic liver lesion in 1 patient. However, this lesion was not hypermetabolic on a PET scan and hence was thought to be benign. PET scans were done on 6 patients preoperatively and were negative in all cases. Esophagectomy was done by the transhiatal technique in 18 patients (72%) and the transthoracic technique in 7 patients (28%). A mean of 8.7 (SEM 1.0) lymph nodes were removed during surgery. Residual carcinoma was found at surgery in 10 of 16 patients with submucosal carcinoma, of which 2 were synchronous mucosal carcinomas. None of the 3 patients with negative margins had residual carcinoma at surgery. None of the patients with mucosal carcinoma had residual carcinoma at the EMR site on esophagectomy. One patient with mucosal carcinoma (33%) had synchronous intramucosal carcinoma on esophagectomy. Five patients with submucosal carcinoma on EMR (31%) had evidence of metastatic lymphadenopathy following esophagectomy. None of the 3 patients with mucosal carcinoma on EMR had evidence of metastatic lymphadenopathy following esophagectomy. Two patients had evidence of perigastric (lymph nodes along the lesser curvature of the stomach) lymph node involvement (1 of 9 and 2 of 10 lymph nodes dissected from the resection specimen). The remaining 3 had evidence of periesophageal lymph node involvement (2 of 5, 1 of 6, and 1 of 5 lymph nodes dissected from the resection specimen: the last patient had a 0.3 cm microscopic focus only). None of these patients had evidence of suspicious lymphadenopathy evident on preoperative EUS performed, except one patient who had evidence of a 5 mm hypoechoic node along the lesser curve of the stomach. This lymph node was aspirated using a 23G needle (Wilson Cook, Winston Salem, NC): 4 passes were made. Cytology was negative and surgical pathology revealed metastatic involvement in 1 of 6 periesophageal lymph node.
All 4 patients with HGD had residual HGD at esophagectomy and none had invasive carcinoma on surgical pathology. After a median follow up of 17.6 months (IQR 2.8, 37.8 months), 23 patients were alive and 2 had expired. Causes of death included recurrent esophageal carcinoma in one patient (9 months after surgery) and postoperative complications in one patient who expired 2 months after surgery for HGD.
DISCUSSION
This is the first large study from the United States assessing the accuracy of EMR pathology compared to surgical pathology in patients with esophageal adenocarcinoma arising in BE. We found that EMR is able to accurately stage neoplasia arising in BE, when compared to the gold standard of surgical pathology. No patient with mucosal carcinoma on EMR pathology had evidence of submucosal carcinoma at esophagectomy. Negative margins on EMR appear to reliably predict the absence of residual neoplasia at the EMR site at esophagectomy. Submucosal adenocarcinoma is associated with a significant risk of metastatic lymphadenopathy, precluding the use of endoscopic treatment alone as a curative therapy. The significant rate of mortality and morbidity associated with esophagectomy for HGD or esophageal carcinoma has been a driving force in the development of endoscopic techniques with lower morbidity rates. Cure of esophageal carcinoma with endoscopic therapy is biologically plausible given the low rates of lymph node involvement by metastatic disease in patients with mucosal esophageal carcinoma. Survival of patients, with early stage esophageal carcinoma treated with EMR alone or in combination with other techniques such as EMR and/or PDT, is comparable to those treated with surgery (3–5).
In contrast to other ablative modalities such as argon plasma coagulation (APC) and multipolar electrocoagulation, EMR provides mucosal and submucosal tissue for histopathologic staging. In addition, EMR can be therapeutic for smaller lesions. Correlation of EMR pathology with surgical pathology was reported by Maish et al. (6) in a small series of 7 patients with esophageal carcinoma. Five patients underwent vagal sparing esophagectomy and 2 underwent conventional esophagectomy following EMR. Two patients had submucosal carcinoma, 4 had mucosal carcinoma, and 1 patient had HGD on EMR pathology. Similar to their findings, we also found that EMR accurately confirmed the depth of tumor invasion in all patients, with the crucial differentiation between mucosal carcinoma (amenable to endoscopic therapy) and submucosal carcinoma (not amenable to endoscopic therapy) being made accurately in all patients. No focus of submucosal carcinoma was found in any patient found to have mucosal carcinoma on EMR. All patients with submucosal carcinoma had carcinoma extending either into the submucosa at esophagectomy (5 patients) or deeper (3 had invasion of the muscularis propria [T2], and 1 had invasion of the serosa [T3]). EMR led to complete resection of the tumor in 12 of 19 patients with esophageal carcinoma (63%).
Initial reports in the Japanese literature quoted a 1–3% prevalence of lymph node involvement in mucosal squamous esophageal carcinoma. This has been confirmed in studies from the United States (7–10). Carcinomas confined to the mucosa (m1), lamina propria (m2), and muscularis mucosa (m3) have much lower rates of LN involvement (0–3%) compared to those involving the submucosa (sm1–3, 20–50%) (11). In a retrospective review of 367 patients who underwent esophagectomy, Buskens et al. reported the absence of LN involvement in mucosal carcinoma and carcinoma involving the upper one-third of the submucosa (sm1). Patients with deeper invasion of the submucosa (sm2 and sm3) had higher rates of LN involvement (23% and 69%), identifying an important subgroup of patients who are inappropriate candidates for endoscopic treatments. Extensive lymph node dissection during esophagectomy is crucial to accurately determining the rates of metastatic lymphadenopathy. In this series, an average of nine lymph nodes was present in the surgical specimen. Lower rates reported in some series (6) may be due to small sample size or the lack of complete lymph node dissection. Our finding of a 31% rate of metastatic lymphadenopathy in patients with submucosal carcinoma is comparable to other reports in the literature.
At our institution, we do not routinely assess depth of submucosal invasion as EMR specimens do not contain the entire submucosa. It is well accepted that depth of tumor invasion correlates with the probability of metastatic lymphadenopathy in esophageal carcinoma (7). Patients with mucosal carcinoma have an extremely low risk (1–2%) compared to those with submucosal invasion (30–40%), due to the presence of lymphatics in the submucosa. More attention has been recently directed at superficial esophageal carcinoma to correlate depth of invasion in the mucosa and submucosa with risk of metastatic lymphadenopathy (12, 13). The results of these studies have been somewhat contradictory. Westerterp and colleagues (12) found that patients with carcinoma invading the superficial one-third of the submucosa (sm1) had no metastatic lymphadenopathy compared to those with deeper invasion (sm2 and sm3). More significantly, they found that this group of patients had survival comparable to those with mucosally confined disease (m1/m2/m3). In contrast, Liu et al. found that patients with invasion of the superficial 50% of the submucosa had an 8% rate of metastatic lymphadenopathy compared to 36% in those with deeper invasion of the submucosa. Of note however, they were unable to demonstrate a difference in survival between these groups. Patients with mucosal disease had a better overall and progression free survival compared to all those with submucosal disease. Hence, we believe that the observations of Westerterp et al. need to be confirmed by larger studies. It is our experience that nearly 90% of EMRs extend to at least the superficial submucosa, as demonstrated by the presence of large caliber vessels or submucosal mucous glands. We have also a found at least partial duplication of the muscularis mucosae in approximately 65% of EMRs. The significance of “intramucosal adenocarcinoma” which extends beyond the duplicated layer into the loose connective tissue between layers of muscularis mucosae is still under investigation. For purposes of this study, tumors are considered intramucosal until they extend beyond the muscularis mucosae (both layers) into submucosa. Therefore, an “intramucosal” carcinoma would be one that invades lamina propria, into or through duplicated muscularis mucosae, invades into the loose connective tissue layer between muscularis mucosae, or into but not through the original muscularis mucosae. This can at times be a difficult distinction to make owing to the tangential embedding which occasionally occurs.
Patients with positive margins had a statistically significant greater chance of residual carcinoma at the EMR site at surgery (Table 3). Eight of 14 patients (57%) had positive margins on EMR specimens in our series, but did not have residual tumor at surgery. Cautery applied at the time of resection may have eradicated residual tumor in the esophageal margin, leading to the absence of residual tumor at surgery. Negative margins on EMR pathology was a reliable predictor of the absence of residual tumor at the EMR site on esophagectomy, with none of the 5 patients with negative margins having residual tumor at the EMR site. Three patients had undetected synchronous mucosal carcinomas in the esophagectomy specimens. Sampling error and the well-known inadequacy of conventional endoscopy in identifying neoplasia in BE (8) probably contributed to this. The use of widespread EMR, which has been recently reported in the literature, may have helped to achieve negative lateral margins in a greater number of patients: this is a potential limitation of this study. However, this would not influence the proportion of patients with positive deep margins. This article included all patients seen at our center between 1994 and 2004 who underwent esophagectomy following EMR for either HGD and/or esophageal cancer, as we aimed to correlate EMR pathology results with surgical pathology results. It is recognized that patients with BE who have visible nodules/lesions are at higher risk for more advanced pathology such as HGD and/or cancer. It would be interesting indeed to see results in patients with flat lesions proceeding to surgery: the correlation of pathology between EMR specimens and surgical pathology may be different in this setting: assessment of this subset will require another study as this is the complete subset of patients seen at our center who underwent esophagectomy following EMR.
Table 3.
Association of Margin Status and Presence of Residual Tumor at the EMR Site (at Esophagectomy)
Residual tumor at site of EMR.
Fisher’s exact test: P = 0.03.
Currently, EUS is accepted as the principal tool in T (tumor) and N (lymph nodal) staging for patients with esophageal carcinoma. Initial reports (14) indicated that EUS was highly accurate in identifying submucosal invasion, with a sensitivity and specificity of greater than 90%. However, the limitations of this technique are being recognized. A systematic review of EUS accuracy concluded that EUS erroneously classifies T stage in 10–20% of cases (15). It is recognized that the greatest utility of EUS is in distinguishing T1 and T2 carcinomas from T3 and T4 carcinomas. The ability of conventional EUS (at 7.5 and 12 MHz) to distinguish between mucosal and submucosal carcinomas is limited. Rice et al. (16) reported an overall error rate of EUS tumor staging of 45%. The error rate fell to 16% on dichotomizing staging to T1–2 versus T3–4. Buskens et al. (11) were unable to distinguish between mucosal and submucosal carcinoma using EUS in 30 out of 75 (40%) patients. Furthermore, they found a false positive rate of 21% in identifying submucosal invasion in those where a distinction could be made. In the present study, overall T stage accuracy of EUS was only 24%. A recent report (17) reported a higher accuracy rate of 85% with EUS using high frequency catheter probes. Even though high frequency EUS has been reported to be more accurate in staging early esophageal carcinoma by investigators in Japan and Europe (18, 19), using ultrasound probes remains labor intensive and time consuming, with limited evidence from investigators in the United States on the accuracy and feasibility of using ultrasound probes to stage early esophageal carcinoma (20).
In conclusion, EMR appears to be an accurate technique in staging esophageal carcinoma arising in BE when compared to surgical pathology, making it a valuable adjunct to EUS in the management of patients with early esophageal carcinoma. EMR may be curative in some patients. Negative margins accurately predict the absence of tumor at the EMR site. Submucosal carcinomas are associated with a significantly higher rate of positive margins and a higher risk of lymph nodal spread compared with mucosal carcinomas and should not be treated with EMR alone.
STUDY HIGHLIGHTS
What Is Current Knowledge
Endoscopic mucosal resection (EMR) is currently accepted as a diagnostic and therapeutic tool in the management of early neoplasia arising in Barrett’s esophagus (BE).
Increasing depth of tumor invasion is associated with increasing risk of metastatic lymphadenopathy.
Limited data exists on the accuracy of neoplasia staging by EMR (when compared to surgical pathology following esophagectomy).
The significance of EMR margins in terms of residual tumor at esophagectomy and metastatic lymphadenopathy is also unknown.
What Is New Here
Tumor staging by EMR correlates well with tumor staging by surgical pathology following esophagectomy.
Negative EMR margins accurately predict the absence of residual tumor at the EMR site following esophagectomy.
Submucosal adenocarcinoma is associated with a substantial risk of metastatic lymphadenopathy.
Endosonographic staging of early neoplasia in BE correlates poorly with surgical pathology staging.
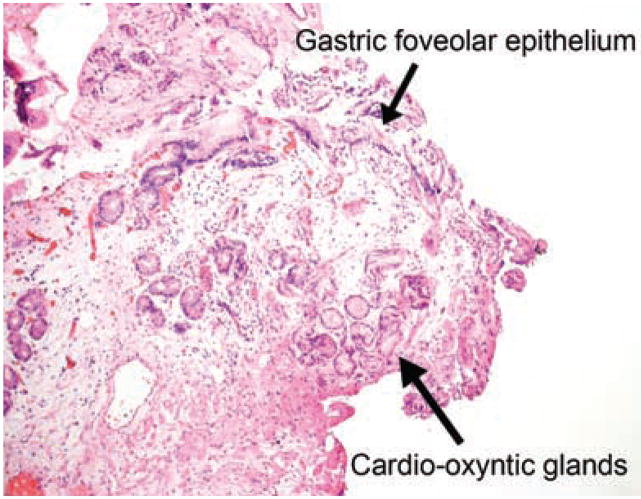
Figure 2.
Negative margin showing gastric cardio-oxyntic mucosa. No specialized Barrett mucosa is present.
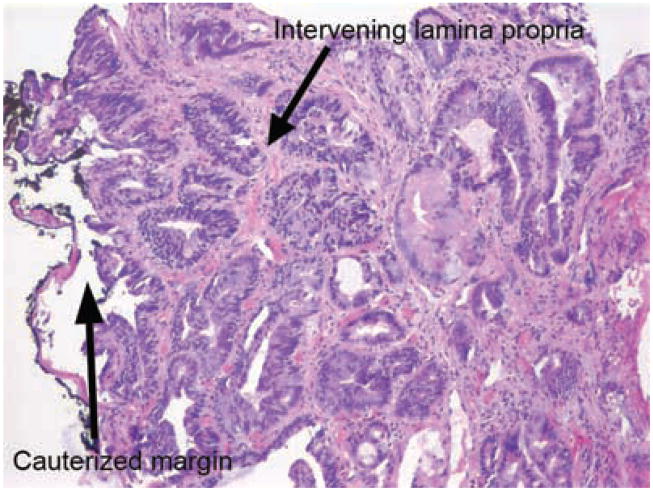
Figure 3.
High grade dysplasia at a lateral mucosal margin. While there is significant atypia present within these glands, there is no effacement of the lamina propria or significant gland fusion to qualify as carcinoma.
Acknowledgments
Financial support: Supported by NIH grants R01 CA111603–01A1 and R01CA097048.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Kenneth K. Wang, M.D.
Specific author contributions: G. A. Prasad: Manuscript preparation and revision, data extraction, statistical analysis; K. K. Wang: Concept, manuscript revision, guarantor, primary endoscopist, senior and corresponding author; N. S. Buttar: Manuscript revision, clinical care of patients; L. M. Wongkeesong: Manuscript revision, clinical care of patients; J. T. Lewis: Interpreration of pathology specimens; S. O. Sanderson: Interpretation of pathology specimens; L. S. Lutzke: Data extraction, clinical care of patients; L. S. Borkenhagen: Clinical care of patients, data extraction.
Potential competing interests: Dr. Wang: Research funding/equipment: Olympus Corp. Consulting fees: Wilson CooK.
References
- 1.Prasad GA, Wang KK, Lutzke LS, et al. Frozen section analysis of esophageal endoscopic mucosal resection specimens in the real-time management of Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:173–8. doi: 10.1016/j.cgh.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: A reaffirmation. Hum Pathol. 2001;32:368–78. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 3.Pacifico RJ, Wang KK, Wongkeesong LM, et al. Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett’s esophagus. [see comment] Clin Gastroenterol Hepatol. 2003;1:252–7. [PubMed] [Google Scholar]
- 4.Shimizu Y, Tsukagoshi H, Fujita M, et al. Long-term outcome after endoscopic mucosal resection in patients with esophageal squamous cell carcinoma invading the muscularis mucosae or deeper. Gastrointest Endosc. 2002;56:387–90. doi: 10.1016/s0016-5107(02)70043-6. [DOI] [PubMed] [Google Scholar]
- 5.Buttar NS, Wang KK, Lutzke LS, et al. Combined endoscopic mucosal resection and photodynamic therapy for esophageal neoplasia within Barrett’s esophagus. Gastroin-test Endosc. 2001;54:682–8. doi: 10.1067/gien.2001.0003. [DOI] [PubMed] [Google Scholar]
- 6.Maish MS, DeMeester SR. Endoscopic mucosal resection as a staging technique to determine the depth of invasion of esophageal adenocarcinoma. Ann Thorac Surg. 2004;78:1777–82. doi: 10.1016/j.athoracsur.2004.04.064. [DOI] [PubMed] [Google Scholar]
- 7.Rice TW, Zuccaro G, Jr, Adelstein DJ, et al. Esophageal carcinoma: Depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg. 1998;65:787–92. doi: 10.1016/s0003-4975(97)01387-8. [DOI] [PubMed] [Google Scholar]
- 8.Nigro JJ, Hagen JA, DeMeester TR, et al. Occult esophageal adenocarcinoma: Extent of disease and implications for effective therapy. Ann Surg. 1999;230:433–8. 438–40. doi: 10.1097/00000658-199909000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigro JJ, DeMeester SR, Hagen JA, et al. Node status in transmural esophageal adenocarcinoma and outcome after en bloc esophagectomy. J Thorac Cardiovasc Surg. 1999;117:960–8. doi: 10.1016/S0022-5223(99)70377-6. [DOI] [PubMed] [Google Scholar]
- 10.Nigro JJ, Hagen JA, DeMeester TR, et al. Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: Implications for therapy. [see comment] J Thorac Cardiovasc Surg. 1999;117:16–23. 23–5. doi: 10.1016/s0022-5223(99)70464-2. [DOI] [PubMed] [Google Scholar]
- 11.Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc. 2004;60:703–10. doi: 10.1016/s0016-5107(04)02017-6. [DOI] [PubMed] [Google Scholar]
- 12.Westerterp M, Koppert LB, Buskens CJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446:497–504. doi: 10.1007/s00428-005-1243-1. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Hofstetter WL, Rashid A, et al. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol. 2005;29:1079–85. [PubMed] [Google Scholar]
- 14.Scotiniotis IA, Kochman ML, Lewis JD, et al. Accuracy of EUS in the evaluation of Barrett’s esophagus and high-grade dysplasia or intramucosal carcinoma. Gastrointest Endosc. 2001;54:689–96. doi: 10.1067/mge.2001.119216. [DOI] [PubMed] [Google Scholar]
- 15.Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut. 2001;49:534–9. doi: 10.1136/gut.49.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice TW, Vargo JJ, Goldblum JR, et al. Endoscopic ultrasound errors in esophageal cancer. Am J Gastroenterol. 2005;100:601–6. doi: 10.1111/j.1572-0241.2005.41167.x. [DOI] [PubMed] [Google Scholar]
- 17.Larghi A, Lightdale CJ, Memeo L, et al. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett’s esophagus. Gastrointest Endosc. 2005;62:16–23. doi: 10.1016/s0016-5107(05)00319-6. [DOI] [PubMed] [Google Scholar]
- 18.May A, Gunter E, Roth F, et al. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: A comparative, prospective, and blinded trial. Gut. 2004;53:634–40. doi: 10.1136/gut.2003.029421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murata Y, Suzuki S, Ohta M, et al. Small ultrasonic probes for determination of the depth of superficial esophageal cancer. Gastrointest Endosc. 1996;44:23–8. doi: 10.1016/s0016-5107(96)70224-9. [DOI] [PubMed] [Google Scholar]
- 20.Chak A, Canto M, Stevens PD, et al. Clinical applications of a new through-the-scope ultrasound probe: Prospective comparison with an ultrasound endoscope. Gastrointest Endosc. 1997;45:291–5. doi: 10.1016/s0016-5107(97)70272-4. [DOI] [PubMed] [Google Scholar]