Abstract
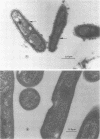
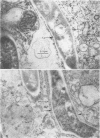
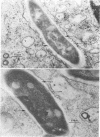
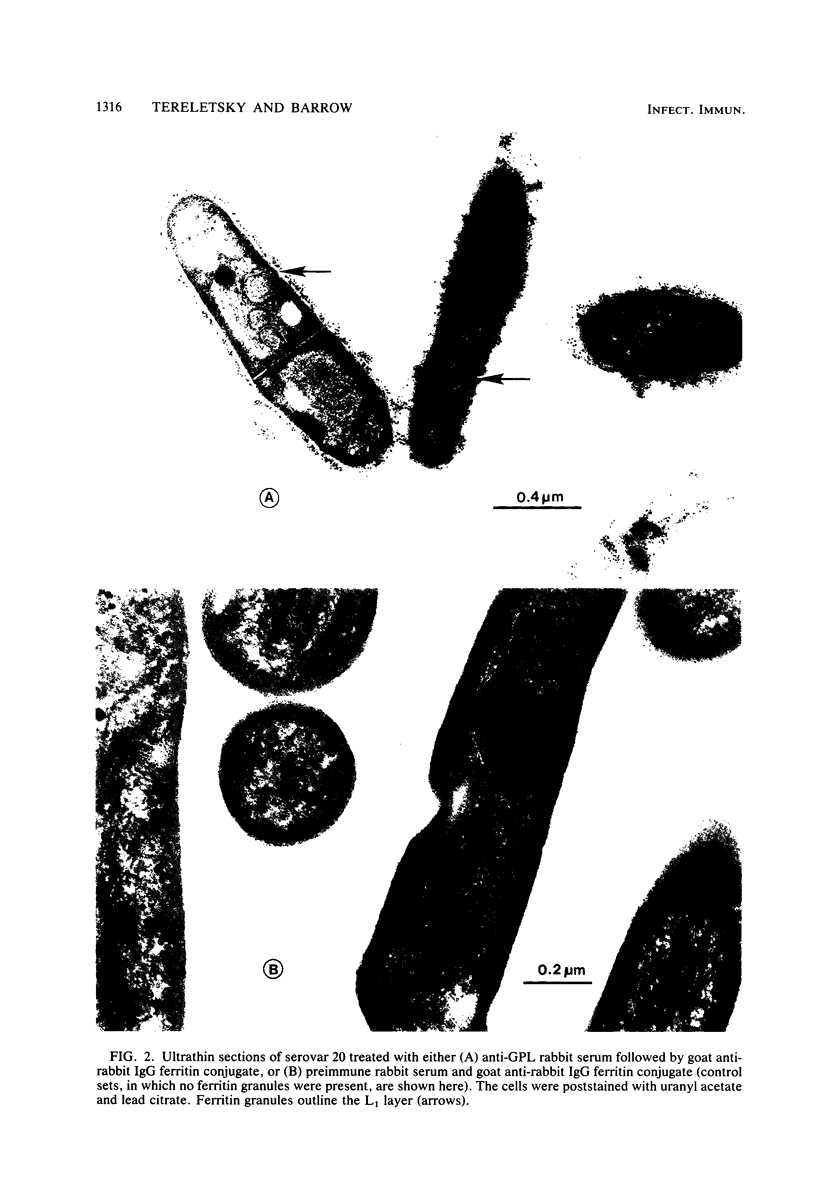
The superficial L1 layer of Mycobacterium intracellulare serovar 20 was detected by immunocytochemical procedures based on C-mycoside glycopeptidolipid (GPL) antigens. Specific immune serum was produced by injecting a GPL-methylated bovine serum albumin complex into rabbits with Freund incomplete adjuvant. The resulting antibodies were used to label serovar 20 with goat anti-rabbit immunoglobulin G (IgG) conjugated with either fluorescein or ferritin. The labeled GPL antigens were then detected by fluorescent and electron microscopy. With these procedures, it was possible to observe the superficial distribution of the GPL antigens on serovar 20 and to confirm their intraphagosomal location within the macrophages after phagocytosis. These immunocytochemical procedures now make it possible to monitor these mycobacterial antigens during phagocytosis and may be helpful in determining their role in pathogenesis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale L., Kim K. S. Mycobacterium. Bacteriol Rev. 1977 Mar;41(1):217–372. doi: 10.1128/br.41.1.217-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Brennan P. J. Immunogenicity of type-specific C-mycoside glycopeptidolipids of mycobacteria. Infect Immun. 1982 May;36(2):678–684. doi: 10.1128/iai.36.2.678-684.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Ullom B. P., Brennan P. J. Peptidoglycolipid nature of the superficial cell wall sheath of smooth-colony-forming mycobacteria. J Bacteriol. 1980 Nov;144(2):814–822. doi: 10.1128/jb.144.2.814-822.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Aspinall G. O., Shin J. E. Structure of the specific oligosaccharides from the glycopeptidolipid antigens of serovars in the Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex. J Biol Chem. 1981 Jul 10;256(13):6817–6822. [PubMed] [Google Scholar]
- Brennan P. J., Goren M. B. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979 May 25;254(10):4205–4211. [PubMed] [Google Scholar]
- Brennan P. J., Mayer H., Aspinall G. O., Nam Shin J. E. Structures of the glycopeptidolipid antigens from serovars in the Mycobacterium avium/Mycobacterium intracellulare/Mycobacterium scrofulaceum serocomplex. Eur J Biochem. 1981 Mar 16;115(1):7–15. doi: 10.1111/j.1432-1033.1981.tb06190.x. [DOI] [PubMed] [Google Scholar]
- CHANG Y. T. LONG-TERM CULTIVATION OF MOUSE PERITONEAL MACROPHAGES. J Natl Cancer Inst. 1964 Jan;32:19–35. [PubMed] [Google Scholar]
- Catanzaro P. J., Graham R. C., Jr, Schwartz H. J. Ultrastructural identification of possible sites of antigen processing in macrophages. J Immunol. 1969 Sep;103(3):618–621. [PubMed] [Google Scholar]
- Christensen E. E., Dietz G. W., Ahn C. H., Chapman J. S., Murry R. C., Anderson J., Hurst G. A. Pulmonary manifestations of Mycobacterium intracellularis. AJR Am J Roentgenol. 1979 Jul;133(1):59–66. doi: 10.2214/ajr.133.1.59. [DOI] [PubMed] [Google Scholar]
- Damsker B., Bottone E. J. Nontuberculous mycobacteria as unsuspected agents of dermatological infections: diagnosis through microbiological parameters. J Clin Microbiol. 1980 Jun;11(6):569–572. doi: 10.1128/jcm.11.6.569-572.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper P., Rees R. J. Electron-transparent zone of mycobacteria may be a defence mechanism. Nature. 1970 Nov 28;228(5274):860–861. doi: 10.1038/228860a0. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J Gen Microbiol. 1973 Jul;77(1):79–87. doi: 10.1099/00221287-77-1-79. [DOI] [PubMed] [Google Scholar]
- Edwards R. P. Electron-microscope illustrations of division in Mycobacterium leprae. J Med Microbiol. 1970 Aug;3(3):493–499. doi: 10.1099/00222615-3-3-493. [DOI] [PubMed] [Google Scholar]
- Kelsey D. S., Chambers R. T., Hudspeth A. S. Nontuberculous mycobacterial infection presenting as a mediastinal mass. J Pediatr. 1981 Mar;98(3):431–432. doi: 10.1016/s0022-3476(81)80713-5. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Salton M. R., Barksdale L. Ultrastructure of superficial mycosidic integuments of Mycobacterium sp. J Bacteriol. 1976 Feb;125(2):739–743. doi: 10.1128/jb.125.2.739-743.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus D. M., Janis R. Localization of glycosphingolipids in human tissues by immunofluorescence. J Immunol. 1970 Jun;104(6):1530–1539. [PubMed] [Google Scholar]
- Marcus D. M., Schwarting G. A. Immunochemical properties of glycolipids and phospholipids. Adv Immunol. 1976;23:203–240. [PubMed] [Google Scholar]
- Rausch P. G., Pryzwansky K. B., Spitznagel J. K. Immunocytochemical identification of azurophilic and specific granule markers in the giant granules of Chediak-Higashi neutrophils. N Engl J Med. 1978 Mar 30;298(13):693–698. doi: 10.1056/NEJM197803302981301. [DOI] [PubMed] [Google Scholar]
- SUTER E. The multiplication of tubercle bacilli within normal phagocytes in tissue culture. J Exp Med. 1952 Aug;96(2):137–150. doi: 10.1084/jem.96.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. B. Serologic identification and classification of the atypical mycobacteria by their agglutination. Am Rev Respir Dis. 1965 Dec;92(6):85–93. doi: 10.1164/arrd.1965.92.6P2.85. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Van Driel D., Wicken A. J., Dickson M. R., Knox K. W. Cellular location of the lipoteichoic acids of Lactobacillus fermenti NCTC 6991 and Lactobacillus casei NCTC 6375. J Ultrastruct Res. 1973 Jun;43(5):483–497. doi: 10.1016/s0022-5320(73)90025-7. [DOI] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., NISHIURA M., HARADA N., IMAEDA T. Electron microscopy of ultra-thin sections of lepra cells and Mycobacterium leprae. Int J Lepr. 1958 Jan-Mar;26(1):1–8. [PubMed] [Google Scholar]