Abstract
Estrogen exposure and metabolism may play an important role in the development of estrogen-sensitive cancers in postmenopausal women. In this study we investigated whether past oral contraceptive (OC) administration or current dietary isoflavonoids (IF) affected expression and/or activity of steroid hormone-metabolizing cytochrome P450 (CYP) enzymes using complementary primate and cell culture models. One-hundred-eighty-one female cynomolgus macaques were randomized to receive OC or nothing for 26 months premenopausally, then ovariectomized and randomized to one of three diets for 36 months: an IF-depleted soy protein isolate (Soy−) diet, a Soy diet with IF (Soy+), or a Soy− diet supplemented with conjugated equine estrogens (CEE). Prior OC-treatment significantly reduced CYP gene expression in the mammary gland (≤60% of OC−). Dietary IFs had no effect on CYP expression, while CEE-treatment decreased CYP1A1 and increased CYP3A4 mRNA in a tissue-specific manner. For in vitro studies, we measured effects of the isoflavonoids genistein, daidzein and equol on CYP activity using intact V79 cells stably transfected to express CYP1A1, CYP1B1, or CYP3A4. All three IFs significantly altered CYP activity in a dose-dependent and isoform-specific manner (20–95% inhibition vs. controls). These results suggest potential mechanisms for prior OC and dietary IF effects on cancer risk in estrogen-responsive tissues.
Keywords: estrogen metabolism, cytochrome P450, oral contraceptive, isoflavonoids, mammary gland, Macaca fascicularis
1. Introduction
In postmenopausal women, cumulative estrogen exposure (endogenous and exogenous) and altered metabolism of those estrogens may play an important role in the development and progression of estrogen-sensitive cancers. Early menarche, late menopause, and use of exogenous hormones (e.g., postmenopausal hormone replacement therapy) are associated with increased risk of developing breast and endometrial cancers through prolonged exposure to estrogens (1–2). Early hypotheses regarding estrogen involvement in carcinogenesis emphasized increases in estrogen receptor (ER)-mediated proliferation that could lead to propagation of genetic damage and subsequent tumor formation, but more recent hypotheses incorporate estrogens and their metabolites as potential genotoxic agents (3). Estrogens have been shown to induce mammary, pituitary, cervical, and uterine tumors in rodent models through metabolism-dependent oxidative DNA damage and covalent adduct formation between estrogen species and DNA (4–5). However, little is known about how non-genetic, modifiable factors such as dietary soy isoflavones or oral contraceptives (OCs) might alter exposure to genotoxic estrogen metabolites.
Decreased risk of breast cancer has been demonstrated in women who regularly consume significant amounts of soy foods (6–7). The mechanism behind this association has not been explained, but some studies suggest soy isoflavonoid (IF)-mediated alterations in estrogen metabolism (8–9). A few studies have found that IFs can inhibit CYP-mediated metabolism of carcinogens (10–13), although effects of dietary isoflavonoids on estrogen metabolizing CYP enzymes in healthy individuals have not been investigated. These prior investigations have relied primarily on carcinogen-induced expression of CYPs in carcinoma-derived cell lines and exposures to isoflavonoids at supraphysiologic concentrations (≥ 25μM).
Epidemiologic studies reporting associations between OC use and breast cancer risk have been inconsistent (14–16), while other epidemiologic evidence suggests that prior OC use up to 20 years earlier is associated with significant reductions in endometrial and ovarian cancer risk (17). Recently Chan and colleagues described a reduction in urinary estrogen concentrations in postmenopausal women who were previous users of oral contraceptives (18) suggesting a potential role for OCs in influencing the long-term estrogen burden. Although these changes were not large, they suggest that OC-associated reductions in estrogen exposure may partially explain the lowered risk of cancer in estrogen-sensitive tissues.
We investigated whether long-term past OC use or current dietary isoflavones affected expression of steroid hormone metabolizing CYP enzymes. Based on previous unpublished data from our lab1, we hypothesized that dietary IF treatments could affect mRNA expression of estrogen-metabolizing CYP enzymes and that past OC treatment might be associated with reduced CYP gene expression. This work was initially planned as an animal study only, but in vitro experiments were added to further explore IF effects on the activity of individual CYP enzymes. We postulated that IFs could further alter CYP enzyme activity directly due to their structural similarities to endogenous estrogens. We have also explored the possibility that CYPs may be regulated by DNA methylation.
2. Materials and Methods
2.1. Animals and study design
One hundred and eighty-one mature, premenopausal adult cynomolgus monkeys (Macaca fascicularis) (average age, 5.7 years, estimated from dentition) were obtained from the Institut Pertanian Bogor, Bogor, Indonesia. These monkeys were utilized in a large-scale, multifaceted, randomized, controlled study designed to investigate the effects of premenopausal OCs and postmenopausal soy IFs or conjugated equine estrogens (CEE) on cardiovascular, bone, and cancer-risk marker endpoints. Data on many of these endpoints as well as diet composition and study design have been reported previously (19–24). In brief, over a 26-month premenopausal period half of the monkeys (OC+) were given an oral contraceptive (Triphasil, Wyeth Pharmaceuticals, Madison, NJ), consisting of cyclic administration of ethinyl estradiol and levonorgestrel at doses equivalent to those used by women. At the end of this premenopausal phase of the study, the animals were ovariectomized to make them surgically menopausal. The postmenopausal phase of this study was a three-group, parallel-arm design, with the treatments lasting for 36 months. A stratified randomization scheme, taking into account premenopausal social group and oral contraceptive exposure, was used to establish the groups. The treatment diet (Soy+) included soy protein isolate containing IF at a dose approximately equivalent to 129 mg/day for women, expressed as aglycone units. Isoflavones included genistein (71%), daidzein (24%), and glycitein (5%). The control diet (Soy−) contained isolated soy protein that had been alcohol washed to remove the IF, so that the protein source remained consistent between the groups. A third diet group was fed IF-depleted soy protein supplemented with conjugated equine estrogens (CEE+) at a human equivalent dose of 0.625 mg/day. Doses were scaled on a metabolic basis and produced serum concentrations similar to those observed in women. The CEE group was used as a positive control for the effect of estrogen therapy; the specific formulation was chosen because it is the most commonly prescribed postmenopausal hormone therapy in the US. The isolated soy proteins used for this study were generously provided by The Solae Company (St. Louis, MO). All diets met National Research Council requirements for non-human primates. We had complete tissue sample sets and treatment information for 161 macaques; subsequent analyses include only this number of animals. All procedures involving animals were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by the Wake Forest University Animal Care and Use Committee.
2.2. Design of CYP mRNA primers
We chose three CYP enzymes (CYP1A1, CYP1B1, and CYP3A4) expressed within the liver and breast that are key enzymes in the formation of the 2-hydroxy-, 4-hydroxy-, and 16α-hydroxyestrogens respectively (25). To measure CYP expression, macaque-specific real-time RT-PCR primer-probe sets were generated through the Applied Biosystems (ABI, Foster City, CA) Taqman Assay-by-Design service using segments of the Macaca fascicularis CYP mRNA sequences available through Genbank (Genbank accession numbers 263692 (CYP3A), 440370 (CYP1A1), and 67971435 (CYP1B1)). The cynomolgus CYP3A mRNA sequence is most identical (94%) to human CYP3A4 mRNA making it a suitable substitute as there is no publically available macaque CYP3A4 mRNA sequence. The following CYP primer-probe sets were used: CYP3A-Forward (CACACCTTTGCCTTTATTGGGAAAT), CYP3A-Reverse (AGCCCCACACTTTTCCATACTTTT), CYP3A-Probe (CAAACGTCCAAAAGCC); CYP1A1-Forward (CCACAGCCACCTCCAAGATC), CYP1A1-Reverse (TGGCCGACATGGAGATTCG), CYP1A1-Probe (CCTACACTGATCATGCTTTT); CYP1B1-Forward (CCGCGCTGCAGTGG), CYP1B1-Reverse (CACTCGAGCCTGCACATCA), CYP1B1-Probe (CTCCTCTTCATCAGGTATCC). These sequences were subject to Basic Local Alignment and Search Tool (BLAST) analysis (26) to determine homology to human CYP sequences for identification of exon-intron boundaries, and against other CYP isoform sequences to ensure specificity of probe product for the selected enzyme of interest. Probes that hybridized across exon junctions (denoted with bold underlined text) were selected when possible to eliminate genomic DNA amplification.
2.3. RNA isolation and quantitation of mammary gland and liver CYP mRNA levels
Total RNA was isolated from frozen mammary and liver samples using Tri Reagent (Molecular Research Center, Cincinnati, OH) and quantitated at A260 nm using a Beckman DU650 spectrophotometer (Beckman Coulter, Fullerton, CA). Reverse transcription and quantitative real-time PCR were performed as described previously (17). Relative expression amounts (R0) of mRNA were calculated using a Data Analysis for Real Time PCR (DART-PCR) worksheet (27) with the change in raw fluorescence (ΔRn) data generated by the ABI Prism software (version 1.1). The liver data were derived from a random subset (n=48) of all animals within the study with all treatment groups equally represented. For each sample we then normalized the R0 values to a constitutively expressed control (GAPDH). For mammary samples an external plate control (mammary tumor sample) was also used to control for inter-plate differences in reaction efficiency.
2.4. Cell culture and assay of isoflavonoid effects on CYP activity
Our next aim was to measure IF-associated changes in CYP protein and activity levels in macaque mammary gland and liver, but insufficient amounts of tissue sample prevented this. So to complement our studies in monkeys and investigate specific IF effects on individual CYP isoforms, we measured the effect of IF on CYP activity using a VIVID® fluorogenic CYP activity assay (Invitrogen, Carlsbad, CA) in intact V79 cells stably transfected to express CYP1A1, CYP1B1, or CYP3A4. Using an intact cell model system for studying the effects of a single isoflavonoid species on individual CYP isozymes is a simple, yet straightforward alternative and provides novel information about IF-effects on CYP-catalyzed metabolism. In the intact V79-hCYP cell system, the IF must cross the cell membrane to interact with the CYP enzyme, which is more physiologically relevant than a broken cell homogenate or microsomal assay. Additionally, the use of tissue homogenate introduces unnecessary variables into the analysis, e.g. varied amounts of IF isolated with the protein for the assay mixture and combinations of different cell types, each with varied Phase I enzyme expression profiles. Because V79 cells express no endogenous CYPs, they are ideal for transfection studies. The parental V79-MZ (University of Mainz strain) Chinese Hamster lung fibroblast cell line and the stable transfection of the V79-MZ cell line to express human CYP enzymes have been described (28–30). All cell lines were grown and maintained in DMEM medium supplemented with 5% FBS (both from Gibco/BRL; Grand Island, NY) at 37°C in 5% CO2 atmosphere and maintained under selection with 0.250 mg/ml G418. Cells were subcultured at 1:20 dilution every 2–3 days.
We chose three isoflavonoids for cell culture studies; genistein, the most abundant soy isoflavone; daidzein, the second-most abundant; and equol, a major metabolite of daidzein. Equol may have particular relevance for cancer prevention by modulation of estrogen metabolism through alteration of catechol estrogens (31). Concentrations of isoflavonoids used in our cell culture studies ranged from a physiologic concentration (1 and 3 micromolar) to 10 times that amount. For each experiment, 1×105 cells in 6-well tissue culture plates were preincubated with nothing, solvent vehicle (0.3% EtOH as a control for genistein and equol or 0.3% DMSO as a control for daidzein), or 1, 3, 10, or 30μM genistein, daidzein (Sigma Chemical, St. Louis, MO), or equol (a major metabolite of daidzein) (PKC Pharmaceuticals, Woburn, MA), which were added to the medium thirty minutes prior to addition of substrate for assay of activity. Cells were then incubated for an additional thirty minutes after addition of the substrate. Vehicle-treated cells exhibited the same responses as untreated cells. One ml of medium was collected and fluorescence of product read at 460nm on a fluorimeter. Cells were then lysed and protein dissolved in 1N NaOH overnight. Picomoles of product formed per milliliter of medium were calculated using a standard curve, and total protein concentrations were measured using Bradford reagent (Sigma-Aldrich, St. Louis MO). CYP activity was calculated as pmol of product/min/mg total protein and is presented as percentage of vehicle treated control cells expressing the same CYP isoform. At least 4 replicate experiments were performed to test the effects of each isoflavonoid on CYP activity.
2.5. Prediction of macaque CYP CpG islands
To provide preliminary support for potential regulation of CYP enzymes by DNA methylation we scanned CYP genomic DNA sequences for CpG islands using a publicly available computer algorithm. Ensembl DNA sequences from rhesus macaque (Macaca mulatta) CYP1B1 and CYP3A64 spanning from −2.5kbp upstream of transcription start site to +100 bp relative to the translation start codon (Ensembl transcript IDs: ENSMMUT00000019573 and ENSMMUT00000031623, respectively) were examined. We used only the available −191bp of the 5′ flanking region to +100bp for the macaque CYP1A1 gene (ENSMMUT00000031615) CpG island prediction. The macaque CYP3A64 sequence was used as it is structurally and enzymatically similar to human CYP3A4 (32) and the macaque CYP3A4 DNA sequence is not publically available. These sequences were analyzed using the European Molecular Biology Open Software Suite (EMBOSS) CpGReport program (33). CpG islands were designated according to criteria set by Gardiner-Garden and Frommer (34): length > 200bp, Percent GC > 50.0%, Obs/Exp GC> 0.60.
2.6. Statistical analysis
Gene expression data were log-transformed prior to statistical analysis to ensure homoscedasticity. Statistical significance was determined using factorial two-way ANOVA by treatment (OC treatment or postmenopausal diet as main effects). OC treatment effect compares OC treated (OC+; n=78) versus not treated (OC−; n=83) regardless of postmenopausal treatment and postmenopausal diet effect examines CEE+ (n=54) or Soy+ (n=55) diet versus Soy− (n=52) diet regardless of prior OC treatment. Expression data represented are from analyses including all treatment groups unless otherwise indicated. Interaction between main effects was not significant, and therefore only main effects are presented.
Statistical significance for CYP activity assays was determined using a mixed general linear model analysis to control for inter-experimental variation and intra-experimental correlation, and to increase certainty that the calculated changes in activity were due to addition of various concentrations of isoflavones. Variances produced by experiment sets and the interaction between experiment sets and isoflavone concentration were designated as random effects and isoflavone concentration alone was designated a fixed effect. P-values adjusted for multiple comparisons with the control are reported. Analyses were performed using the SAS statistical analysis software (version 9.1.3; SAS Institute, Cary, NC).
3. Results
3.1. Dietary and hormonal intervention effects on liver and mammary CYP mRNA expression
Long-term IF consumption did not significantly alter postmenopausal mammary CYP mRNA levels, while premenopausal OC administration (3 years prior) was surprisingly associated with lower postmenopausal mRNA levels of all three enzymes assayed. Figure 1 illustrates the 40% reduction in both CYP1A1 and CYP1B1 mRNA and 80% reduction of CYP3A mRNA associated with premenopausal OC use. Postmenopausal estrogen treatment (CEE+) altered mammary CYP mRNA expression in an isoform-specific manner, reducing CYP1A1 levels by 54% and increasing CYP3A by 178% relative to controls (p<0.01, Figure 1). Neither current dietary IFs nor past OC use altered any CYP mRNA level within the liver, while CEE treatment produced a 50% reduction in liver CYP1A1 mRNA, comparable to that seen in the mammary tissue (p<0.05) (Figure 2).
Figure 1. OC and IF treatment effects on mammary CYP mRNA expression.
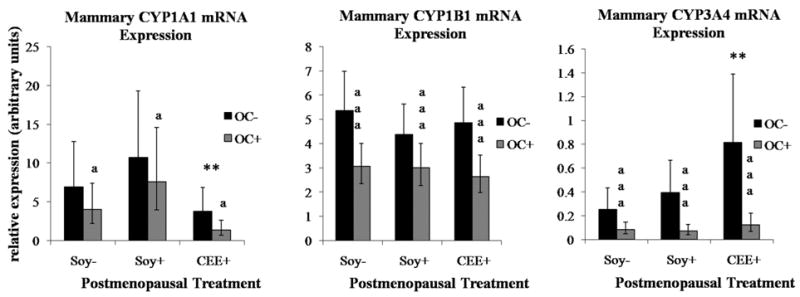
Values represent geometric means with 95% confidence intervals (95% CI). Significant differences are indicated as: **CEE effect relative to Soy− groups (p < 0.01), aaa OC effect across all postmenopausal groups (p < 0.0001), a OC effect across all postmenopausal groups (p < 0.05)
Figure 2. OC and IF treatment effects on liver CYP mRNA expression.
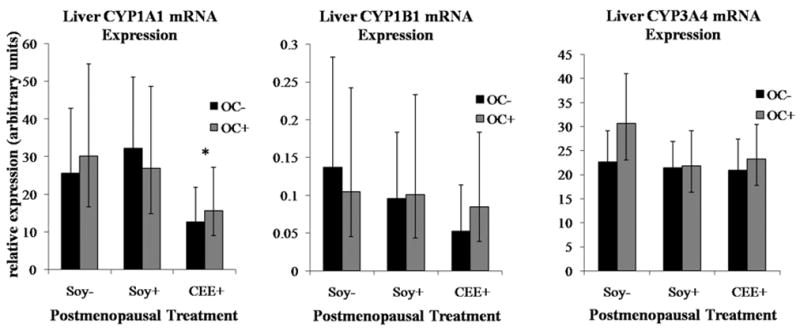
Values represent geometric means with 95% confidence intervals (95% CI). Significant differences are indicated as: *CEE effect relative to Soy− groups (p < 0.05)
3.2. In vitro isoflavonoid effects on CYP activity
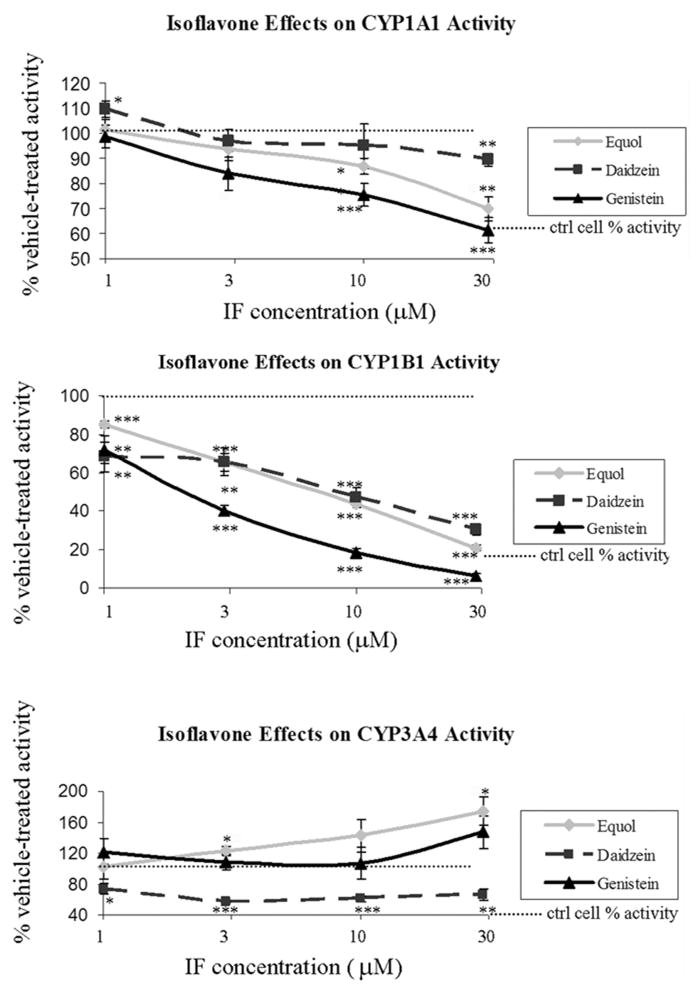
In the V79 cells, CYP1B1 was most strongly inhibited by all isoflavones, with greater than 50% reduction in activity at less than 10μM concentrations (approximate IC50 values of 2.1, 5.6, and 7.9μM for genistein, equol, and daidzein respectively) (p≤0.002, Figure 3). CYP1A1 was mildly inhibited by all three isoflavones with 20–40% inhibition occurring at 10 and 30uM concentrations. Daidzein was also weakly but significantly stimulatory of CYP1A1 activity at 1μM (10% increase over vehicle-treated cells, p<0.05). Daidzein and its major metabolite, equol, had opposing effects on CYP3A4 activity with daidzein inducing 30–40% reductions in activity and equol stimulating CYP3A4 activity by 20–40% relative to vehicle-treated controls. Genistein was the most potent inhibitor of the CYP1 gene family of enzymes tested producing statistically significant inhibition of CYP1B1 activity at concentrations as low as 1μM (40% inhibition) and culminating with 95% inhibition of activity at the 30uM concentration. Genistein also produced an interesting U-shaped curve for CYP3A4 activity with a tendency toward increased activity at 1μM, no effect at 3 and 10μM, and a trend toward significant induction of activity at 30μM (all p≥0.09).
Figure 3. Isoflavone effects on activity of specific human CYP isozymes expressed in V79-MZ cells.
Plots represent data as a percentage of the activity within the vehicle treated cells (set at 100%) as represented by the dashed black line. Whiskers representing standard errors of the means are plotted for all treatments, but some are smaller than the associated markers and therefore not visible. Significant differences are indicated as: * isoflavone effect relative to vehicle treated cells at p < 0.05, ** isoflavone effect relative to vehicle treated cells at p ≤ 0.002, *** isoflavone effect relative to vehicle treated cells at p < 0.0001
3.3. Predicted CpG islands 5′ upstream of macaque CYP genes
On the negative strands, only CYP1B1 had a CpG island, which extended from position 2756 to 3026 (Exon 1) of the 3329bp sequence (Table 1). The positive strands of each sequence however contained at least 2 CpG islands. The CpG islands were present in the 5′ flanking region and exon 1 of the CYP1B1 gene, spanning exon 1/intron 1 junction and solely within intron 1 of CYP1A1, and within the 5′ flanking region as well as crossing the transcription start site of the CYP3A64 gene. Several potential transcription factor binding sequences were also identified within the CpG islands for each CYP gene (data not shown).
Table 1.
Predicted CpG islands within macaque CYP genomic sequences
| Ensembl ID | Gene | Start pos. | End pos. | 5′/Exon/Intron | Strand |
|---|---|---|---|---|---|
| ENSMMUT00000019573 | CYP1B1 | 2756 | 3026 | Exon 1 | − |
| ENSMMUT00000019573 | CYP1B1 | 669 | 922 | 5′ | + |
| ENSMMUT00000019573 | CYP1B1 | 2518 | 3058 | Exon 1 | + |
|
| |||||
| ENSMMUT00000031615 | CYP1A1 | ND | ND | ND | − |
| ENSMMUT00000031615 | CYP1A1 | 193 | 446 | Exon 1/Intron 1 | + |
| ENSMMUT00000031615 | CYP1A1 | 457 | 852 | Intron 1 | + |
|
| |||||
| ENSMMUT00000031623 | CYP3A64 | ND | ND | ND | − |
| ENSMMUT00000031623 | CYP3A64 | 726 | 979 | 5′ | + |
| ENSMMUT00000031623 | CYP3A64 | 1158 | 1499 | 5′ | + |
| ENSMMUT00000031623 | CYP3A64 | 1564 | 1768 | 5′ | + |
| ENSMMUT00000031623 | CYP3A64 | 2345 | 2594 | 5′/Exon 1 | + |
ND – none detected
4. Discussion
In this study we investigated whether long-term past OC use or current dietary isoflavones affected expression of steroid hormone metabolizing CYP enzymes. We further tested the effect of IFs on activity of recombinant expressed CYPs in a V79 cell culture model. In our monkey studies, we found that CEE was capable of affecting CYP mRNA expression in both the mammary gland and liver, while past OC use lowered CYP expression in mammary gland and soy IFs had no significant effect on CYP mRNA expression at either site. With our cell culture experiments we observed dose-dependent, isozyme- and isoflavone-specific effects on CYP activity independent of any potential effects on expression.
Our data on dietary soy isoflavone exposure in monkeys are consistent with findings from Kishida and colleagues who reported no effect of dietary soy isoflavones on hepatic CYP mRNA expression in rats (35). These results do not however rule out a direct effect of isoflavones on CYP enzyme activity as opposed to an estrogen receptor or aryl-hydrocarbon receptor-mediated transcriptional mechanism, which explains most variation observed in CYP activity (36). Our cell culture studies supported this possibility, indicating that soy IF altered CYP activity acutely at concentrations achievable in the tissues of individuals on a diet including significant amounts of soy isoflavones. Non-fasting serum isoflavone concentrations of greater than 4μM have been detected in Asian populations consuming soy foods regularly (37), but mean serum IF concentrations following a high-soy meal are more commonly reported to be around 0.5–2μM (38). The IF concentrations that target tissues encounter over an extended period of exposure are not clear, but it is probable that the isoflavonoid concentrations at the low end of our examined range are attained in tissues of humans consistently consuming significant amounts of soy foods.
The strong reduction of CYP1B1 activity in vitro by IFs, even at low concentrations, could translate to reduced amounts of 4-OHE1, a precursor to mutagenic catechol estrogen quinones, produced in the tissue, thereby reducing the risk of estrogen-induced cancers. As equol, genistein, and daidzein were all present in the serum of IF-fed monkeys in significant amounts (total peak serum IF concentrations >1μM at 4–6hr post feeding, data not shown), it is plausible that all three IFs might act in concert to markedly diminish CYP1B1 activity in vivo. However, more work is needed to confirm this idea. The end metabolic activity for CYP1A1 and CYP3A4 would be highly variable with all three IFs present since they may have different effects on a particular isozyme at different concentrations, e.g. CYP3A4 activity in presence of 1μM daidzein and 3μM equol concentrations. Further investigation into the potential additive, synergistic, and/or opposing effects of IF combinations is thus warranted.
The CEE effect on CYP gene expression in the liver and mammary gland may be unique to this treatment. ERα transactivation by estradiol has been shown to induce transcription of CYP1B1 (39–40) and increase translation of CYP1A1 protein (40).The equine estrogens equilin, 17β-dihydroequilin, 17α-dihydroequilin, equilenin, 17β-dihydroequilenin and Δ8,9-estrone are biologically active and contribute significantly to the total estrogenicity in CEE-treated animals (41). In addition, there are many other estrogens in CEE including estrone, which have ER binding capacities and may act in concert with or compete against the equine estrogens, thus creating a CYP expression outcome that is different from pure estradiol. The implications of CEE-induced CYP3A4 and repressed CYP1A1 expression are also unclear as it has been shown that both of these enzymes have 2-hydroxylase activity, depending on the substrate (42). CYP3A4 also catalyzes production of 16αOHE1, so even though its expression has been increased it is uncertain what product would be formed in vivo. The data presented here do not provide information on any potential changes in product formation of these enzymes associated with CEE treatment, which would be important in determining the benefit or detriment of its effect on enzyme expression.
As previously mentioned CYP3A was used to approximate CYP3A4 expression in our macaque model of human exposure to isoflavonoids and/or oral contraceptives. For the sake of simplicity and our intent in approximation we report our results in the context of CYP3A4, but we recognize that macaques also express CYP3A8 (cynomolgus) and CYP3A64 (rhesus), both of which are similar to human CYP3A4 in sequence, function and expression patterns (32), but not completely identical. Thus we acknowledge the inherent uncertainty in interpreting our results on cynomolgus macaque CYP3A gene expression. Our interest was in the effects of isoflavonoid and oral contraceptive exposures on CYP-mediated metabolism of estrogens. Previous findings from our lab demonstrate that these exposures are associated with altered endogenous estrogen metabolism. Our current results suggest that these effects may be due in part to changes in expression and/or activity of CYP enzymes involved in estrogen metabolism.
Our results also suggest that OCs may be able to lower the baseline transcriptional activation levels of estrogen-metabolizing CYP enzymes in estrogen-responsive tissues. Epigenetic changes such as DNA methylation and chromatin remodeling are potential mechanisms for long-term downregulation of CYP enzymes. It has been previously reported that nuclear receptor-mediated activation of transcription of both CYP1A1 and CYP3A4 can be strongly influenced by histone deacetylase (HDAC) and DNA methyltransferase (DNMT) activities (43–44). Additionally, DNA hyper- and hypomethylation have been correlated with alterations in CYP1A1, CYP1B1, and CYP3A4 expression levels in various cell types (45–49). Epigenetic modifications have been cited as a mechanism behind persistent changes in gene expression associated with exposure to native and synthetic steroid hormone analogs (50–53). The CpG island locations identified from our screening of the macaque CYP genes further supports the potential for epigenetic regulation of their expression.
5. Conclusion
Cumulatively, these findings imply that oral contraceptives may be another example of synthetic steroid hormones that can modulate long-term gene expression by altering DNA methylation patterns. Additional research is required to verify this supposition however. The lower levels of CYP mRNA expression in the previously OC treated animals implies that this OC formulation may actually attenuate the risk thought to be associated with metabolism of estrogens to catechols in this pathway. Depending on exposure to certain concentrations of genistein, daidzein and equol and potential interactive effects on CYP activity in vivo, these findings also suggest that IFs could reduce production of carcinogenic estrogen metabolites and/or increase formation of non-carcinogenic metabolites in accordance with previous human and non-human primate studies (54, 20).Since CYP-mediated metabolism of exogenous or endogenous pro-carcinogens is considered an important factor in carcinogenesis, it follows that agents that modify these processes are likely to be significant modulators of cancer risk. These data suggest a potential mechanism for OC and IF-associated reductions in cancer risk for estrogen-responsive tissues through modulation of estrogen-metabolizing CYP enzymes.
Acknowledgments
We are grateful to the National Center for Complementary and Alternative Medicine, National Institutes of Health for financial support (RO1 AT00639).
Footnotes
L.M. Scott, X. Xu, T.D. Veenstra, J.A. Tooze, C.E. Wood, T.C. Register, N.D. Kock, J.M. Cline, Past oral contraceptive use and current dietary soy isoflavones influence estrogen metabolism in postmenopausal monkeys (Macaca fascicularis), Cancer Epidemiol. Biomarkers Prev. (in press).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dumitrescu RG, Cotarla I. Understanding breast cancer risk -- where do we stand in 2005? J Cell Mol Med. 2005;9(1):208–221. doi: 10.1111/j.1582-4934.2005.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 3.Okobia MN, Bunker CH. Estrogen metabolism and breast cancer risk--a review. Afr J Reprod Health. 2006;10(1):13–25. [PubMed] [Google Scholar]
- 4.Liehr JG. Genotoxic effects of estrogens. Mutat Res. 1990;238(3):269–276. doi: 10.1016/0165-1110(90)90018-7. [DOI] [PubMed] [Google Scholar]
- 5.Liehr JG. Genotoxicity of the steroidal oestrogens oestrone and oestradiol: possible mechanism of uterine and mammary cancer development. Hum Reprod Update. 2001;7(3):273–281. doi: 10.1093/humupd/7.3.273. [DOI] [PubMed] [Google Scholar]
- 6.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98(7):459–471. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 7.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98(1):9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerber B, Müller H, Reimer T, Krause A, Friese K. Nutrition and lifestyle factors on the risk of developing breast cancer. Breast Cancer Res Treat. 2003;79(2):265–276. doi: 10.1023/a:1023959818513. [DOI] [PubMed] [Google Scholar]
- 9.Kendall A, Folkerd EJ, Dowsett M. Influences on circulating oestrogens in postmenopausal women: relationship with breast cancer. J Steroid Biochem Mol Biol. 2007;103(2):99–109. doi: 10.1016/j.jsbmb.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Khan TH, Prasad L, Sultana A, Sultana S. Soy isoflavones inhibits the genotoxicity of benzo(a)pyrene in Swiss albino mice. Hum Exp Toxicol. 2005;24(3):149–155. doi: 10.1191/0960327105ht504oa. [DOI] [PubMed] [Google Scholar]
- 11.Shertzer HG, Puga A, Chang C, Smith P, Nebert DW, Setchell KD, Dalton TP. Inhibition of CYP1A1 enzyme activity in mouse hepatoma cell culture by soybean isoflavones. Chem Biol Interact. 1999;123(1):31–49. doi: 10.1016/s0009-2797(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 12.Chan HY, Leung LK. A potential protective mechanism of soya isoflavones against 7,12-dimethylbenz[a]anthracene tumour initiation. Br J Nutr. 2003;90(2):457–465. doi: 10.1079/bjn2003913. [DOI] [PubMed] [Google Scholar]
- 13.Shon YH, Park SD, Nam KS. Effective chemopreventive activity of genistein against human breast cancer cells. J Biochem Mol Biol. 2006;39(4):448–451. doi: 10.5483/bmbrep.2006.39.4.448. [DOI] [PubMed] [Google Scholar]
- 14.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- 15.Kumle M, Weiderpass E, Braaten T, Persson I, Adami HO, Lund E. Use of oral contraceptives and breast cancer risk: The Norwegian-Swedish Women’s Lifestyle and Health Cohort Study. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1375–1381. [PubMed] [Google Scholar]
- 16.Marchbanks PA, McDonald JA, Wilson HG, Folger SG, Mandel MG, Daling JR, Bernstein L, Malone KE, Ursin G, Strom BL, Norman SA, Wingo PA, Burkman RT, Berlin JA, Simon MS, Spirtas R, Weiss LK. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346(26):2025–2032. doi: 10.1056/NEJMoa013202. [DOI] [PubMed] [Google Scholar]
- 17.Deligeoroglou E, Michailidis E, Creatsas G. Oral contraceptives and reproductive system cancer. Ann N Y Acad Sci. 2003;997:199–208. doi: 10.1196/annals.1290.023. [DOI] [PubMed] [Google Scholar]
- 18.Chan MF, Dowsett M, Folkerd E, Wareham N, Luben R, Welch A, Bingham S, Khaw KT. Past oral contraceptive and hormone therapy use and endogenous hormone concentrations in postmenopausal women. Menopause. 2008;15(2):332–339. doi: 10.1097/gme.0b013e31806458d9. [DOI] [PubMed] [Google Scholar]
- 19.Wood CE, Register TC, Anthony MS, Kock ND, Cline JM. Breast and uterine effects of soy isoflavones and conjugated equine estrogens in postmenopausal female monkeys. J Clin Endocrinol Metab. 2004;89(7):3462–3468. doi: 10.1210/jc.2003-032067. [DOI] [PubMed] [Google Scholar]
- 20.Wood CE, Register TC, Cline JM. Soy isoflavonoid effects on endogenous estrogen metabolism in postmenopausal female monkeys. Carcinogenesis. 2007;28(4):801–808. doi: 10.1093/carcin/bgl163. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86(1):41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 22.Register TC, Jayo MJ, Anthony MS. Soy phytoestrogens do not prevent bone loss in postmenopausal monkeys. J Clin Endocrinol Metab. 2003;88(9):4362–4370. doi: 10.1210/jc.2003-030493. [DOI] [PubMed] [Google Scholar]
- 23.Register TC, Cann JA, Kaplan JR, Williams JK, Adams MR, Morgan TM, Anthony MS, Blair RM, Wagner JD, Clarkson TB. Effects of soy isoflavones and conjugated equine estrogens on inflammatory markers in atherosclerotic, ovariectomized monkeys. J Clin Endocrinol Metab. 2005;90(3):1734–1740. doi: 10.1210/jc.2004-0939. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan JR, Manuck SB, Anthony MS, Clarkson TB. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstet Gynecol. 2002;99(3):381–388. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhu BT, Lee AJ. NADPH-dependent metabolism of 17beta-estradiol and estrone to polar and nonpolar metabolites by human tissues and cytochrome P450 isoforms. Steroids. 2005;70(4):225–244. doi: 10.1016/j.steroids.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 26.National Library of Medicine. [Accessed 2005 Aug 2];2005 doi: 10.1080/15360280801989377. [online] Available from: http://www.ncbi.nlm.nih.gov/blast/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome". [DOI] [PubMed]
- 27.Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31(14):e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmalix WA, Mäser H, Kiefer F, Reen R, Wiebel FJ, Gonzalez F, Seidel A, Glatt H, Greim H, Doehmer J. Stable expression of human cytochrome P450 1A1 cDNA in V79 Chinese hamster cells and metabolic activation of benzo[a]pyrene. Eur J Pharmacol. 1993;248(3):251–261. doi: 10.1016/0926-6917(93)90052-r. [DOI] [PubMed] [Google Scholar]
- 29.Schneider A, Schmalix WA, Siruguri V, de Groene EM, Horbach GJ, Kleingeist B, Lang D, Böcker R, Belloc C, Beaune P, Greim H, Doehmer J. Stable expression of human cytochrome P450 3A4 in conjunction with human NADPH-cytochrome P450 oxidoreductase in V79 Chinese hamster cells. Arch Biochem Biophys. 1996;332(2):295–304. doi: 10.1006/abbi.1996.0345. [DOI] [PubMed] [Google Scholar]
- 30.Luch A, Coffing SL, Tang YM, Schneider A, Soballa V, Greim H, Jefcoate CR, Seidel A, Greenlee WF, Baird WM, Doehmer J. Stable expression of human cytochrome P450 1B1 in V79 Chinese hamster cells and metabolically catalyzed DNA adduct formation of dibenzo[a,l]pyrene. Chem Res Toxicol. 1998;11(6):686–695. doi: 10.1021/tx970236p. [DOI] [PubMed] [Google Scholar]
- 31.Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. The effect of soy consumption on the urinary 2:16-hydroxyestrone ratio in postmenopausal women depends on equol production status but is not influenced by probiotic consumption. J Nutr. 2005;135(3):603–608. doi: 10.1093/jn/135.3.603. [DOI] [PubMed] [Google Scholar]
- 32.Carr B, Norcross R, Fang Y, Lu P, Rodrigues AD, Shou M, Rushmore T, Booth-Genthe C. Characterization of the rhesus monkey CYP3A64 enzyme: species comparisons of CYP3A substrate specificity and kinetics using baculovirus-expressed recombinant enzymes. Drug Metab Dispos. 2006;34(10):1703–1712. doi: 10.1124/dmd.106.009977. [DOI] [PubMed] [Google Scholar]
- 33.Rice P, Longden I, Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 2000;16(6):276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 34.Gardiner-Garden M, Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987;196(2):261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- 35.Kishida T, Nagamoto M, Ohtsu Y, Watakabe M, Ohshima D, Nashiki K, Mizushige T, Izumi T, Obata A, Ebihara K. Lack of an inducible effect of dietary soy isoflavones on the mRNA abundance of hepatic cytochrome P-450 isozymes in rats. Biosci Biotechnol Biochem. 2004;68(3):508–515. doi: 10.1271/bbb.68.508. [DOI] [PubMed] [Google Scholar]
- 36.Lin JH, Lu AY. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet. 1998;35(5):361–390. doi: 10.2165/00003088-199835050-00003. [DOI] [PubMed] [Google Scholar]
- 37.Morton MS, Arisaka O, Miyake N, Morgan LD, Evans BA. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr. 2002;132(10):3168–3171. doi: 10.1093/jn/131.10.3168. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen IL, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer. 2007;57(1):1–10. doi: 10.1080/01635580701267677. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchiya Y, Nakajima M, Kyo S, Kanaya T, Inoue M, Yokoi T. Human CYP1B1 is regulated by estradiol via estrogen receptor. Cancer Res. 2004;64(9):3119–3125. doi: 10.1158/0008-5472.can-04-0166. [DOI] [PubMed] [Google Scholar]
- 40.Han W, Pentecost BT, Pietropaolo RL, Fasco MJ, Spivack SD. Estrogen receptor alpha increases basal and cigarette smoke extract-induced expression of CYP1A1 and CYP1B1, but not GSTP1, in normal human bronchial epithelial cells. Mol Carcinog. 2005;44(3):202–211. doi: 10.1002/mc.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhavnani BR. Pharmacokinetics and pharmacodynamics of conjugated equine estrogens: chemistry and metabolism. Proc Soc Exp Biol Med. 1998;217(1):6–16. doi: 10.3181/00379727-217-44199. [DOI] [PubMed] [Google Scholar]
- 42.Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227(2):115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Schnekenburger M, Peng L, Puga A. HDAC1 bound to the Cyp1a1 promoter blocks histone acetylation associated with Ah receptor-mediated trans-activation. Biochim Biophys Acta. 2007;1769(9–10):569–578. doi: 10.1016/j.bbaexp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JY, Ahn MR, Kim DK, Sheen YY. Histone deacetylase inhibitor stimulate CYP3A4 proximal promoter activity in HepG2 cells. Arch Pharm Res. 2004;27(4):407–414. doi: 10.1007/BF02980082. [DOI] [PubMed] [Google Scholar]
- 45.Okino ST, Pookot D, Li LC, Zhao H, Urakami S, Shiina H, Igawa M, Dahiya R. Epigenetic inactivation of the dioxin-responsive cytochrome P4501A1 gene in human prostate cancer. Cancer Res. 2006;66(15):7420–7428. doi: 10.1158/0008-5472.CAN-06-0504. [DOI] [PubMed] [Google Scholar]
- 46.Anttila S, Hakkola J, Tuominen P, Elovaara E, Husgafvel-Pursiainen K, Karjalainen A, Hirvonen A, Nurminen T. Methylation of cytochrome P4501A1 promoter in the lung is associated with tobacco smoking. Cancer Res. 2003;63(24):8623–8628. [PubMed] [Google Scholar]
- 47.Takahashi Y, Suzuki C, Kamataki T. Silencing of CYP1A1 expression in rabbits by DNA methylation. Biochem Biophys Res Commun. 1998;247(2):383–386. doi: 10.1006/bbrc.1998.8791. [DOI] [PubMed] [Google Scholar]
- 48.Tokizane T, Shiina H, Igawa M, Enokida H, Urakami S, Kawakami T, Ogishima T, Okino ST, Li LC, Tanaka Y, Nonomura N, Okuyama A, Dahiya R. Cytochrome P450 1B1 is overexpressed and regulated by hypomethylation in prostate cancer. Clin Cancer Res. 2005;11(16):5793–5801. doi: 10.1158/1078-0432.CCR-04-2545. [DOI] [PubMed] [Google Scholar]
- 49.Dannenberg LO, Edenberg HJ. Epigenetics of gene expression in human hepatoma cells: expression profiling the response to inhibition of DNA methylation and histone deacetylation, B.M.C. Genomics. 2006;7:181. doi: 10.1186/1471-2164-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilks A, Seldran M, Jost JP. An estrogen-dependent demethylation at the 5′ end of the chicken vitellogenin gene is independent of DNA synthesis. Nucl Acids Res. 1984;12(2):1163–1177. doi: 10.1093/nar/12.2.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S, Washburn KA, Moore R, Uno T, Teng C, Newbold RR, McLachlan JA, Negishi M. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 1997;57(19):4356–4359. [PubMed] [Google Scholar]
- 52.Contractor RG, Foran CM, Li S, Willett KL. Evidence of gender-and tissue-specific promoter methylation and the potential for ethinylestradiol-induced changes in Japanese medaka (Oryzias latipes) estrogen receptor and aromatase genes. J Toxicol Environ Health A. 2004;67(1):1–22. doi: 10.1080/15287390490253633. [DOI] [PubMed] [Google Scholar]
- 53.Ginger MR, Gonzalez-Rimbau MF, Gay JP, Rosen JM. Persistent changes in gene expression induced by estrogen and progesterone in the rat mammary gland. Mol Endocrinol. 2001;15(11):1993–2009. doi: 10.1210/mend.15.11.0724. [DOI] [PubMed] [Google Scholar]
- 54.Kurzer MS. Hormonal effects of soy in premenopausal women and men. J Nutr. 2002;132(3):570S–573S. doi: 10.1093/jn/132.3.570S. [DOI] [PubMed] [Google Scholar]