Abstract
Clinical data indicate that secondary caries and restoration fracture are the most common problems facing tooth restorations. Our ultimate goal was to develop mechanically-strong and caries-inhibiting dental composites. The specific goal of this pilot study was to understand the relationships between composite properties and the ratio of reinforcement filler/releasing filler. Nanoparticles of monocalcium phosphate monohydrate (MCPM) were synthesized and incorporated into a dental resin for the first time. Silicon carbide whiskers were fused with silica nanoparticles and mixed with the MCPM particles at MCPM/whisker mass ratios of 1:0, 2:1, 1:1, 1:2, and 0:1. The composites were immersed for 1–56 days to measure Ca and PO4 release. When the MCPM/whisker ratio was changed from 0:1 to 1:2, the composite flexural strength (mean ± SD; n = 5) decreased from 174 ± 26 MPa to 138 ± 9 MPa (p < 0.05). A commercial nonreleasing composite had a strength of 112 ± 14 MPa. When the MCPM/whisker ratio was changed from 1:2 to 1:1, the Ca concentration at 56 days increased from 0.77 ± 0.04 mmol/L to 1.74 ± 0.06 mmol/L (p < 0.05). The corresponding PO4 concentration increased from 3.88 ± 0.21 mmol/L to 9.95 ± 0.69 mmol/L (p < 0.05). Relationships were established between the amount of release and the MCPM volume fraction vMCPM in the resin: [Ca]= 42.9 vMCPM2.7, and [PO4] = 48.7 vMCPM1.4. In summary, the method of combining nanosized releasing fillers with reinforcing fillers yielded Ca- and PO4-releasing composites with mechanical properties matching or exceeding a commercial stress-bearing, nonreleasing composite. This method may be applicable to the use of other Ca–PO4 fillers in developing composites with high stress-bearing and caries-preventing capabilities, a combination not yet available in any dental materials.
Keywords: dental composite, nanoparticles, whisker reinforcement, flexural strength, elastic modulus, Ca and PO4 release, tooth caries
INTRODUCTION
Dental resin composites are generally composed of reinforcing fillers in an acrylic monomer matrix that is polymerized to form a solid restoration, in which the particulate and fibrous fillers significantly affect the composite properties.1–4 The resin compositions and cure conditions in these materials have been improved, while polymerization shrinkages have been reduced.5–7 Nonetheless, recent reports indicate that secondary caries and restoration fracture remain the most common clinical problems that warrant further investigation.8,9 Secondary caries refers to the reoccurrence of tooth decay after the initial restoration, and is cited as the major reason for the replacement of existing restorations.10 To combat tooth caries, novel composites were developed that release calcium (Ca) and phosphate (PO4) ions, which formed hydroxyapatite [Ca10(PO4)6 (OH)2], the putative mineral in natural teeth.11,12 These Ca-and PO4-releasing composites were shown to effectively remineralize the decayed enamel and dentin in vitro by increasing the mineral content in the tooth lesions.11,12 However, their low mechanical strengths were “inadequate to make these composites acceptable as bulk restoratives.” 13
Recently, ceramic whiskers were used as fillers in dental resins.14 Nanometer-sized silica particles were fused onto the whiskers to facilitate silanization, minimize whisker entanglement, and enhance whisker retention in the resin matrix by roughening the whisker surfaces.14 These whisker composites demonstrated flexural strength and fracture toughness values nearly twofold those of currently available dental composites.15 They showed superior performance in thermal cycling between 5 and 60°C water baths up to 105 cycles,15 long-term water aging for 2 years,16 and three-body wear.17 An in vitro biocompatibility study showed that the whisker composites were noncytotoxic and supported cell attachment and proliferation.18
The overall objective of this research was to develop a suitable bulk restorative composite, with the high mechanical properties of a posterior composite and the capability of Ca and PO4 release to combat caries. It was postulated that this could be achieved by incorporating both whiskers and calcium phosphate particles into a dental resin. Nanosized monocalcium phosphate monohydrate (MCPM; Ca[H2-PO4]2·H2O) particles were synthesized via a spray-drying method. They were used as fillers for a dental resin because they could provide Ca and PO4 release,19 and because MCPM was used with dicalcium phosphate anhydrous (DCPA, CaHPO4) and tetracalcium phosphate (TTCP, Ca4 (PO4)2O) in a bone cement that precipitated nanosized hydroxyapatite crystals.20 Hence it would be desirable to incorporate MCPM with TTCP and DCPA into dental resins for caries inhibition. This pilot study focused on understanding the effects of incorporating one calcium phosphate compound, MCPM nanoparticles, on the composite mechanical properties and Ca and PO4 release. The following hypotheses were tested: (1) combining the releasing filler (nano-MCPM) with the reinforcing filler (whiskers) would result in a Ca- and PO4-releasing composite with mechanical strength and elastic modulus matching or exceeding those of a commercial stress-bearing, nonreleasing composite; (2) the composite mechanical properties and Ca and PO4 release could be tailored by controlling the ratio of releasing filler/reinforcing filler.
MATERIALS AND METHODS
Synthesis of MCPM Particles
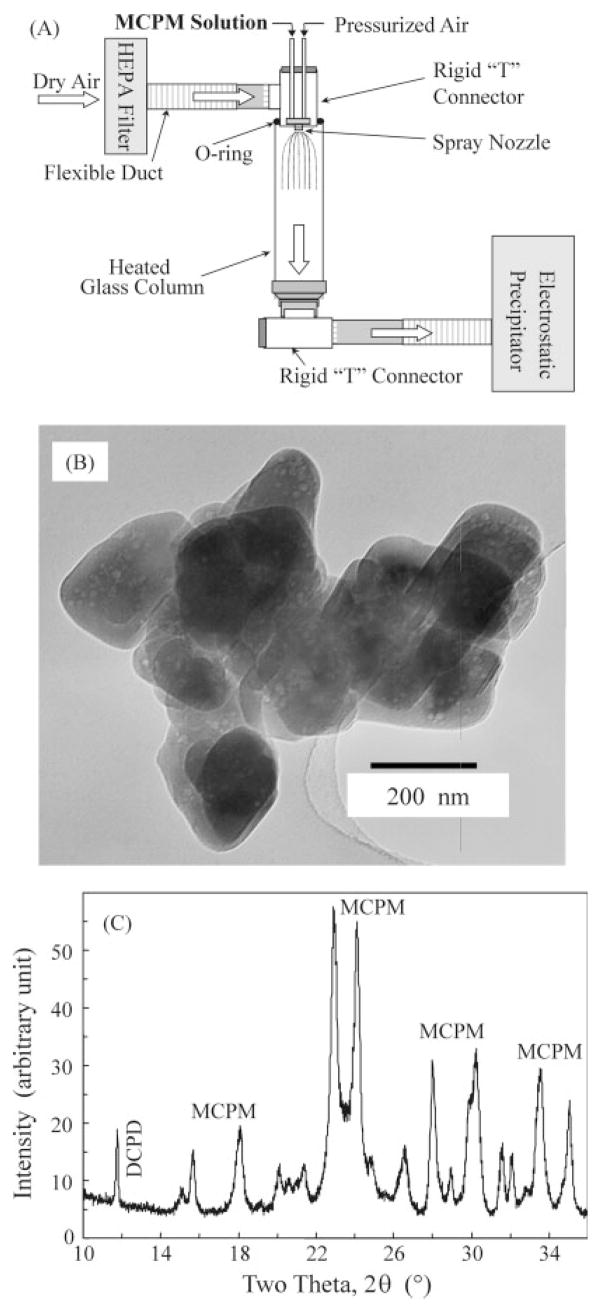
Although nanoforms of hydroxyapatite, α-tricalcium phosphate (α-TCP) and β-tricalcium phosphate (β-TCP) have been synthesized previously,21–23 nanosized monocalcium phosphate monohydrate (MCPM) has never been reported. The MCPM nanoparticles used in this study as fillers for the resin were synthesized for the first time, using a spray drying technique.24,25 A MCPM solution was prepared by dissolving CaCO3 (JT Baker Chemical, Phillipsburg, NJ) in a solution of H3PO4 (Sigma–Aldrich, St. Louis, MO), which was diluted with water to a Ca concentration of 8 mmol/L and a PO4 concentration of 16 mmol/L. As shown in Figure 1(A), the solution was sprayed through a nozzle (SUC1120, PNR America, Poughkeepsie, NY) that was situated on the top of a glass column (diameter = 152 mm, VM Glass, Vineland, NJ). The pressurized air had a pressure of 7 KPa, and the glass column was heated with electrical heating tapes (BIH 101100L, BH Thermal, Columbus, OH) and thermally insulated with a fiberglass tape. The temperature inside the tube ranged from 70 to 90°C. An electrostatic precipitator (MistBuster, Air Quality Engineering, Minneapolis, MN) was connected to the lower end of the column, creating a steady flow of air/mist through the column. The water in the Ca–PO4 solution was evaporated into the dry heated air in the column and expelled from the precipitator into a hood. The dried fine particles suspended in the flow were collected by the electrostatic precipitator. By using this method, the size of the nanoparticles could be controlled via two independent parameters: droplet size of the atomized solution, and concentrations of the ions in the solution. Droplets as small as 12-μm in diameter were produced by the above nozzle with a liquid feed rate of about 0.5 L/h. A nozzle with specialized design (e.g., Fujisaki, Tokushima, Japan) claimed droplet sizes as small as 1 μm. By adjusting these parameters, it was possible to vary the particle size over several orders of magnitude. However, preparation of very small particles, e.g., <20 nm in median size, in a laboratory scale apparatus was a slow process. For this reason, the present study focused on preparing nanoparticles of about 100–200 nm.
Figure 1.
(A) Schematic representation of the spray drying apparatus for synthesizing nanostructured MCPM particles. “HEPA” refers to high efficiency particulate air filter. (B) TEM image shows that some particles were agglomerated or on the top of each other, with the individual particles having a size of about 100–200 nm and agglomerate sizes of 300–800 nm. (C) XRD pattern shows that the powder was crystalline MCPM.
The collected powder was analyzed with a powder X-ray diffractometer (XRD, DMAX 2200, Rigaku Denki, Woodlands, TX). Transmission electron microscopy (TEM, 3010-HREM, JEOL, Peabody, MA) was used to examine the dispersed particles. To prevent the particles from agglomerating, a dilute acetone suspension of the particles was ultrasonicated and drops of the suspension were deposited onto the Cu grids that support a carbon film for the TEM image.
Preparation of Nano-Silica-Fused Whiskers
Silicon carbide whiskers with a mean diameter of approximately 0.9 μm and a length of 14 μm were obtained from Advanced Refractory Technologies (Buffalo, NY) as in a previous study.16 Nanosized silica particles with a size of about 40 nm (Degussa, Ridgefield, NJ) were mixed with whiskers at a whisker/silica mass ratio of 5:1.15 The dried powder was heated at 800°C for 30 min to coat the nano-silica particles onto the whiskers. As described in previous studies, the powder was silanized with 4% 3-methacryloxy-propyltrimethoxysilane and 2% n-propylamine (all amounts by mass in this article, unless specified otherwise).17 The silica-fused whiskers are referred to as whiskers.
Fabrication of MCPM-Whisker Composites
A resin consisting of 36.475% bisphenol glycidyl methac-rylate (Bis-GMA), 36.475% triethylene glycol dimethacrylate (TEGDMA), 25% 2-hydroxyethyl methacrylate (HEMA), 0.05% 2,6-di-tert-butyl-4-methylphenol (BHT), and 2% benzoyl peroxide (BPO) was used as part I, the initiator paste, of the two-part chemically-activated system. Part II, the accelerator, consisted of 37% Bis-GMA, 37% TEGDMA, 25% HEMA, and 1% N,N-dihydroxyethyl-p-toluidine (DHEPT). Because of the opacity of the whisker composite, two-part chemically-activated resins were used.14 The reason for incorporating hydrophilic monomer HEMA was to enhance the Ca and PO4 release.26
Five different batches of fillers were used, each consisting of a mixture of nano-MCPM particles and whiskers at one of the following MCPM/whisker mass ratios 1:0, 2:1, 1:1, 1:2, and 0:1. A combined filler level of 70% mass fraction was blended with the resin to form a cohesive paste. This filler level was selected because the filler-resin paste was readily mixable for MCPM/whisker ratios of 2:1, 1:1, 1:2, and 0:1. For MCPM/whisker = 1:0, the paste was too dry because of the small size of the nano-MCPM particles. Therefore, a filler level of 60% was used for MCPM/whisker = 1:0. At each MCPM/whisker ratio, equal masses of the two pastes (part I and part II) were manually blended with a spatula and filled into stainless steel molds of 2 × 2 × 25 mm3 to make specimens for mechanical testing. Each specimen was incubated at 37°C in air for 24 h, prior to testing. For measuring the release of Ca and PO4, disk molds of 15-mm diameter and 1-mm thickness were used.12
A hybrid composite (TPH, Caulk/Dentsply, Milford, DE) was used as a comparative control. It consisted of silicate particles about 0.8 μm in diameter at 78% filler level in a urethane-modified Bis-GMA and TEGDMA resin. The specimens were light-cured (Triad-2000, Dentsply, York, PA) for 1 min on each side of the specimen.
Mechanical Properties and Ca and PO4 Release
The flexural strength and elastic modulus of the specimens were measured using a three-point flexural test with a 20-mm span at a crosshead-speed of 1 mm/min on a computer-controlled Universal Testing Machine (5500R, MTS, Cary, NC). The load-displacement curve was recorded by computer. The flexural strength was calculated by S = 3PmaxL/(2bh2), where Pmax is the maximum load on the load-displacement curve, L is flexure span, b is specimen width, and h is specimen thickness.27 Elastic modulus was calculated by E = (P/d) × (L3/[4bh3]), where load P divided by the corresponding displacement d is the slope of the load-displacement curve in the linear elastic region.28
A method reported previously12,26,29 was used to measure the release of Ca and PO4. A disk specimen of 15-mm diameter and 1-mm thickness was immersed in 100 mL of NaCl solution (133 mmol/L) buffered with 50 mmol/L HEPES (Sigma–Aldrich, St. Louis, MO) to a pH of 7.4 at 37°C. The cured disk specimens were taken out of the molds and used without further polishing. The concentrations of Ca and PO4 released from the specimen were measured vs. immersion time at 1, 2, 4, 7, 14, 21, 28, 35, 42, 49, and 56 days. At each time period, an aliquot of 0.5 mL of the solution was removed and analyzed for Ca and PO4 concentrations with a spectrophotometer (DMS-80 UV-visible, Varian, Palo Alto, CA), using established standards and calibration methods.12,26,29 The standard uncertainty is estimated to be about 3% based on previous evaluations.12,26,29
Calculation of Remineralization Potential
The remineralization potential of a Ca- and PO4-releasing composite can be described by using a chemical potential diagram.12,30 For a solution saturated with respect to hydroxyapatite [Ca10(PO4)6(OH)2], the solubility constant relationship, , applies. Rear-ranging the equation leads to:
| (1) |
where KW = (H+)(OH−) is the dissociation constant of water. Taking the log of both sides of Eq. (1) and rearranging the equation leads to31:
| (2) |
where K = (1/6) × (log KSP + 9 log KW) is a constant at a fixed temperature. Equation (2) shows that for a solution saturated with respect to hydroxyapatite, the logarithms of the activities of the two components, H3PO4 and Ca(OH)2, are linearly related. For an experimental solution, the activities (Ca2+)(OH−)2 and can be calculated from the pH, the measured Ca and PO4 concentrations, and the ionic strength. Hence, a solution can be represented as a point in the potential diagram. Solutions located to the left of the hydroxyapatite line are undersaturated, and those to the right of the line are supersaturated, with respect to hydroxyapatite.
The Gibbs free energy was also used to quantify the thermodynamic driving force for remineralization12:
| (3) |
where R is the ideal gas constant, T is the absolute temperature, n is the number of ions in the IAP, and is the ion activity product for hydroxyapatite of the solution.30,31
One-way ANOVA was performed to detect the significant effects of the variables. Tukey’s multiple comparison test was used to compare the data at p of 0.05.
RESULTS
TEM image [Figure 1(B)] showed that the particles from the spray drying method were agglomerated with the individual particles having sizes of about 100–200 nm, and agglomerate sizes of 300–800 nm. Some of the particles beneath the top layer of particles were also visible, because the electron beam at 300 kV penetrated the top particles. Figure 1(C) shows the XRD pattern of the powder collected from the electrostatic precipitator. Most of the peaks corresponded with the patterns for crystalline MCPM. However, a minor peak of DCPD (dicalcium phosphate dihydrate, CaHPO4·2H2O) was also observed, as indicated at the left side of Figure 1(C).
Figure 2 plots the flexural strength and elastic modulus vs. nano-MCPM/whisker ratio for the composites. The lower x-axis shows the MCPM/whisker ratio, while the upper x-axis indicates the corresponding whisker/total filler mass fraction, WF. Increasing the whisker content significantly increased the flexural strength and elastic modulus. The composite at MCPM/whisker of 0:1 had a flexural strength (mean ± SD; n = 5) of 174 ± 26 MPa, significantly higher than 138 ± 9 MPa and 38 ± 11 MPa, at MCPM/whisker of 1:2 and 1:0, respectively (p < 0.05). The flexural strength for the hybrid control was 112 ± 14 MPa, not significantly different from those at MCPM/whisker of 1:2 and 1:1 (p > 0.1). Linear regression of the mean values yielded strength S = 29 + 146 WF, with correlation coefficient r = 0.98.
Figure 2.
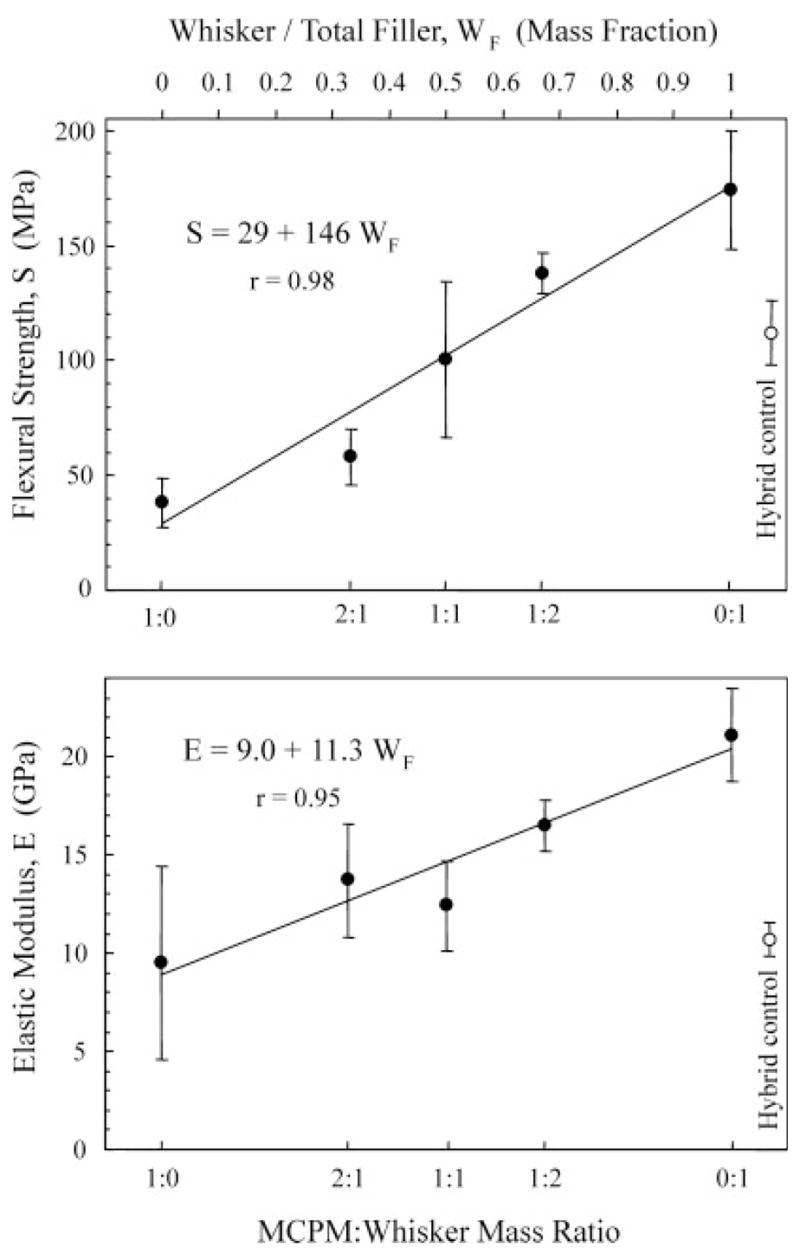
Flexural strength and elastic modulus. The lower x-axis shows the MCPM/whisker mass ratio, while the upper x-axis indicates the corresponding WF (whisker/total filler mass fraction). Total filler = whiskers + nano-MCPM in the composite. The straight line in each plot is a linear best fit to the data. For strength, S = 29 + 146 WF, with a correlation coefficient r = 0.98. For elastic modulus, E = 9.0 + 11.3 WF, with r = 0.95.
The elastic modulus of the MCPM-whisker composites ranged from 9.5 to 21.1 GPa. The modulus at MCPM/whisker of 0:1 was significantly higher than that of all the other materials (p < 0.05). The hybrid control had an elastic modulus of 10.9 ± 0.8 GPa, not significantly different from those at MCPM/whisker ratios of 1:1, 2:1, and 1:0 (p > 0.1). Linear regression yielded elastic modulus E = 9 + 11.3 WF, with correlation coefficient r = 0.95.
Figure 3 plots the concentration of Ca released from the composites vs. immersion time. The amount of Ca increased significantly with increasing the MCPM/whisker ratio from 0:1 to 1:0 (p < 0.05) [Figure 3(A)]. In Figure 3(B), for MCPM/whisker of 2:1, the Ca concentration decreased at 7 and 14 days because of precipitation in the solution. At MCPM/whisker = 1:0, precipitation in the solution became visibly more significant and could be readily seen as a whitish powdery deposit. No measurements were made after 14 days in this group, because the aliquots would not contain the precipitated deposit and hence would underestimate the actual ion release from the composite. At 56 days, the composite disk was removed from the solution, and the entire solution was acidified by adding 1.5 mL of HCl into 100 mL of the precipitated solution to turn it into a clear solution. The Ca concentration in the solution was then measured and is shown in Figure 3(B) with a “*”. Similarly, for MCPM/whisker of 2:1, the * indicates that the solution was acidified before the concentration was measured. The 56-day Ca concentrations at MCPM/whisker ratios of 1:2, 1:1, 2:1, and 1:0 were 0.77 ± 0.04 mmol/L, 1.74 ± 0.06 mmol/L, 3.27 ± 0.31 mmol/L, and 5.13 ± 0.49 mmol/L, respectively.
Figure 3.
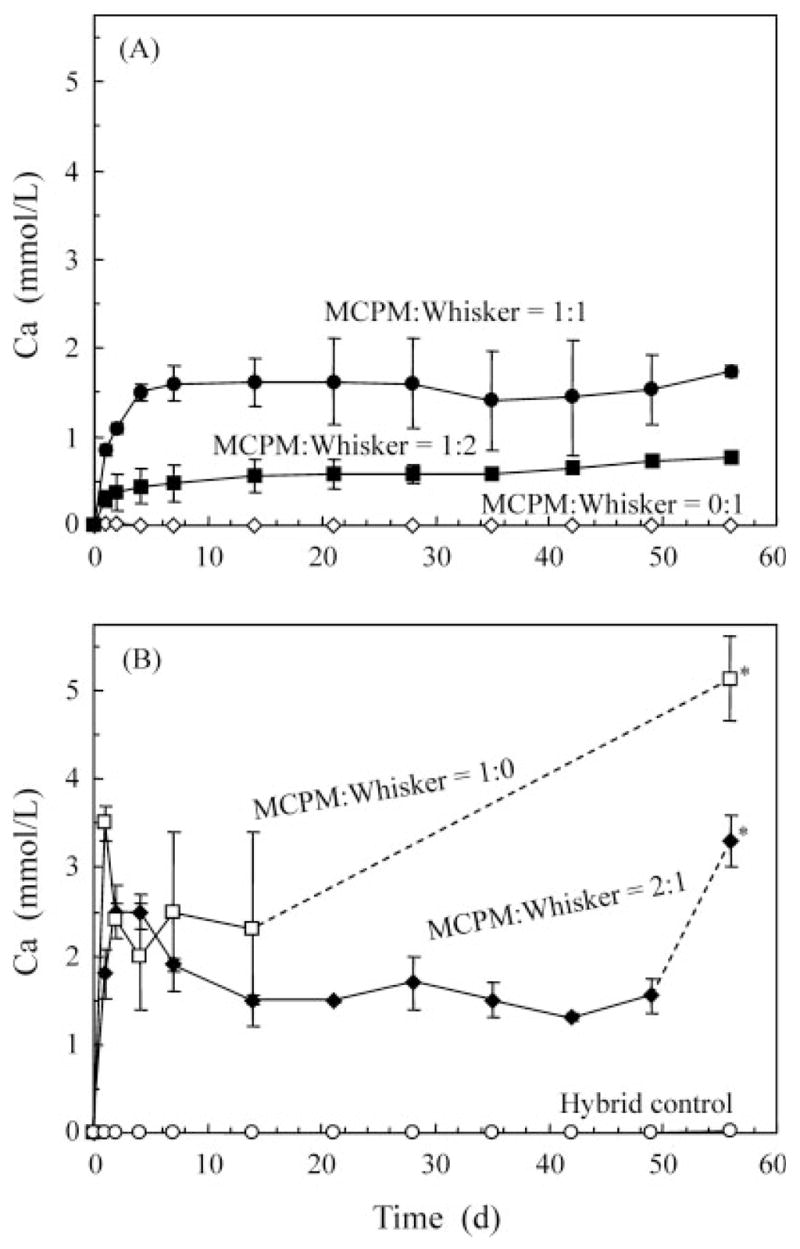
Release of Ca ions from the nano-MCPM–whisker composites vs. immersion time. The six materials were separated into two plots in A and B for clarity. Each value is mean ± SD; n = 3. The lines connect the data points to aid interpretation. The composite at MCPM/whisker ratio of 0:1 and the hybrid control had no detectable release.
Figure 4 plots the corresponding release of total ionic PO4. The composite at MCPM/whisker of 1:0 rapidly reached a steady state of concentration followed by precipitation. The composite at 2:1 ratio had slightly slower maximum concentration followed by precipitation. The composites at ratios of 1:1 and 1:2 had lower concentrations with no evidence of precipitation. In (B), the * at the 56-day data indicates that the precipitated solution was acidified as described above. The 56-day PO4 concentrations at MCPM/whisker ratios of 1:2, 1:1, 2:1, and 1:0 were 3.88 ± 0.21 mmol/L, 9.95 ± 0.69 mmol/L, 13.91 ± 1.07 mmol/L, and 14.84 ± 0.95 mmol/L, respectively.
Figure 4.
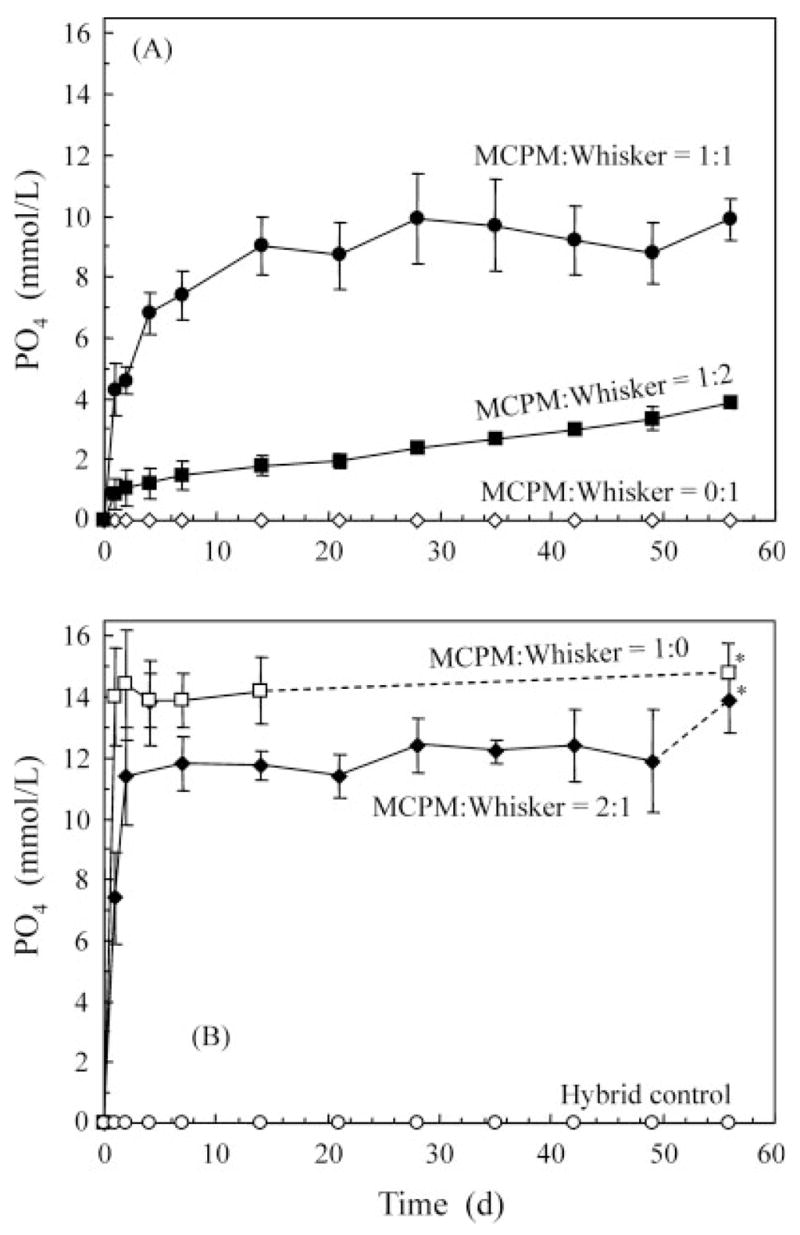
Total ionic PO4 release from MCPM–whisker composites. The six materials were separated into two plots for clarity. The lines connect the data points to aid interpretation.
Figure 5A shows the chemical potential diagram for MCPM/whisker of 1:2 and 1:1. The ratios of 2:1 and 1:0 are not included, because the precipitation and acidifying treatment to quantify the total releases into the solution have dissolved the precipitates and increased the ion concentrations. The ratios of 2:1 and 1:0 are also less important practically because of their lower mechanical properties (Figure 2). The solubility isotherm lines for hydroxyapatite and dicalcium phosphate dihydrate (DCPD; CaHPO4·2H2O) are also included in Figure 5(A). This diagram graphically illustrates that the mineral levels in the solutions of the composites at MCPM/whisker of 1:1 were supersaturated with respect to DCPD. The mineral levels in the solution of the composite for MCPM/whisker of 1:2 were undersaturated with respect to DCPD but supersaturated with respect to hydroxyapatite. It should be noted that the composite at MCPM/whisker of 1:2 also had the highest flexural strength and elastic modulus among the releasing composites, as shown in Figure 2. The Gibbs free energy ΔG0 values are plotted in Figure 5(B) for MCPM/whisker of 1:2 and 1:1. The ratios of 2:1 and 1:0 are not included for the reasons stated above. ΔG0 describes the thermodynamic driving force for mineral diffusion into a tooth lesion and the precipitation of the mineral within the lesion.12
Figure 5.
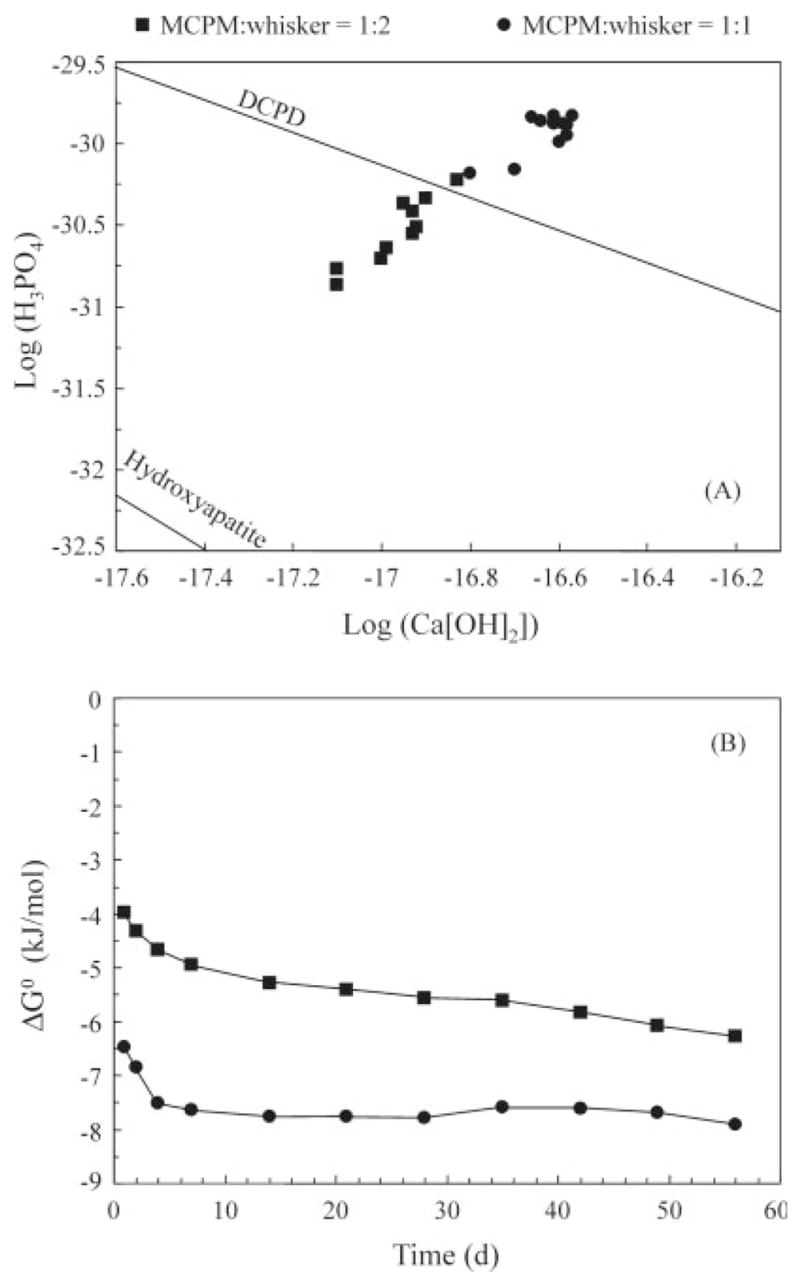
(A) Chemical potential diagram and (B) Gibbs free energy ΔG0 values for MCPM/whisker mass ratios of 1:2 and 1:1.
DISCUSSION
Ca and PO4 Release
In this study, nanosized Ca- and PO4-releasing fillers were combined with reinforcing whiskers in a dental resin. Previous studies showed that Ca and PO4 releasing composites were substantially weaker mechanically than stress-bearing dental composites.12,26 The nanocomposites of the present study had substantial Ca and PO4 release, while matching or exceeding the mechanical properties of a commercial stress-bearing, nonreleasing composite, for the first time. The nano-MCPM-whisker composite at an intermediate MCPM/whisker ratio of 1:1 (MCPM filler level of 35%) produced a Ca concentration of 1.74 mmol/L and a PO4 concentration of 9.95 mmol/L at 56 days. In a previous study, amorphous calcium phosphate (ACP) fillers were used to develop novel Ca- and PO4-releasing composites with remineralizing capabilities.26 At 40% ACP fillers, the composite exhibited a Ca release of ~1 mmol/L and a PO4 release of 0.7 mmol/L.26 Another study on remineralizing Ca–PO4 composites for pulp-capping/base applications reported a Ca release of 0.5 mmol/L and a PO4 release of 0.1 mmol/L in a buffered saline solution, using a Ca–PO4 filler level of 73% by mass.12 Hence, the nano-MCPM–whisker composite at an intermediate MCPM/whisker ratio of 1:1 produced much higher Ca and PO4 release. The ion concentrations in Figures 3 and 4 plateaued, likely because of the measurement method in which the solution was not changed. This study demonstrated that the high-strength composites released Ca and PO4 matching or exceeding the previous remineralizing composites measured using the same method, without attempting to simulate the in vivo saliva flow. Further studies should measure the concentration of the solution at certain time intervals and replenish with fresh solution.
There are two possible reasons for the higher Ca and PO4 release from the nano-MCPM–whisker composite. First, the high surface area of the nano-MCPM particles likely facilitated the Ca and PO4 release. Specific surface area a = 6/(dρ), where d is the equivalent spherical diameter of the particles, and ρ is density. For the purpose of illustration, use an estimated diameter d of 200 nm [Figure 1(B)]. For MCPM, ρ = 2.22 g/cm3. Hence a = 13.5 m2/g. In comparison, in a previous study, the dicalcium phosphate anhydrous (DCPA) particle size was 1.1 μm, and the tetracalcium phosphate (TTCP) particle size was 16 μm.12 The density is 2.89 g/cm3 for DCPA and 3.07 g/cm3 for TTCP.19 Hence a = 1.89 m2/g for DCPA, and a = 0.12 m2/g for TTCP. These values were 1–2 orders of magnitude less than the surface area of the nano-MCPM particles. Second, the solubility of the calcium phosphate compound likely affected the Ca and PO4 release as well. MCPM is known to be more soluble than DCPA and TTCP.19 Further studies are needed to systematically investigate the effects of filler particle size, surface area, composition, and solubility on the Ca and PO4 release.
Effect of MCPM Volume Fraction
This study used the same nano-MCPM particle size and varied the MCPM/whisker ratio, which in turn varied the MCPM content in the composite. Mass fractions were used for the fillers, because it was experimentally convenient to weigh the fillers. However, in composite analyses, it is important to use the volume fraction as the key parameter, because it describes how much volume of the composite is occupied by the fillers regardless of the filler density.3,32 Even at the same mass fraction, different types of fillers with different density values can occupy different amounts of volume in the resin. The MCPM filler volume fraction in the composite at different MCPM/whisker mass ratios is defined as vMCPM. vMCPM can be calculated via the masses of the components used in making the composites and their density values: dSiC whisker = 3.2 g/cm3 (manufacturer’s data), dNano silica = 2 g/cm3, and dMCPM = 2.22 g/cm3. With the whisker/silica ratio of 5:1, the density dwhisker–silica = 2.91 g/cm3. The density of the unfilled resin in the present study was measured (mean ± SD; n = 4) to be dresin = 1.19 ± 0.04 g/cm3. Therefore, at MCPM/whisker ratios of 0:1, 1:2, 1:1, 2:1 and 1:0, vMCPM was calculated to be: 0, 0.203, 0.297, 0.388, and 0.446, respectively (vMCPM of 0.446 and 44.6% are interchangeable in this article).
The composite with no MCPM fillers had, as expected, no release of Ca and PO4. When the MCPM content in the composite was increased, the amount of release also increased. This was because the source that provided the Ca and PO4 ions was increasing with increasing vMCPM. Furthermore, with increasing vMCPM, the MCPM–resin interfaces were also increasing. These interfaces possibly served as a relatively easy passageway for the diffusion of water and Ca and PO4 ions. Therefore, the release of Ca and PO4 ions may increase with increasing vMCPM at a faster rate than being simply linear. On the basis of these considerations, the following power-law relationships are proposed:
| (4) |
| (5) |
where Ca and PO4 are concentrations of ions released from the composite with the units of mmol/L, and k1, k2, α, and β are coefficients. Fitting the above equations to the measured data of Ca and PO4 in Figures 3 and 4 (at 56 days) yielded the equations in Figure 6. These empirical relationships developed from the bottle experiments are instructive because they (i) indicate that the volume fraction of the releasing filler can be used as a microstructural/processing parameter, which increases the amount of release at a rate faster than being linear; and (ii) provide a first approximation of the amount of releasing filler to be incorporated with reinforcement fillers in composites designed to provide Ca and PO4 ions to prevent demineralization. Further studies are needed to examine whether these relationships apply to other calcium phosphate fillers in dental resins.
Figure 6.
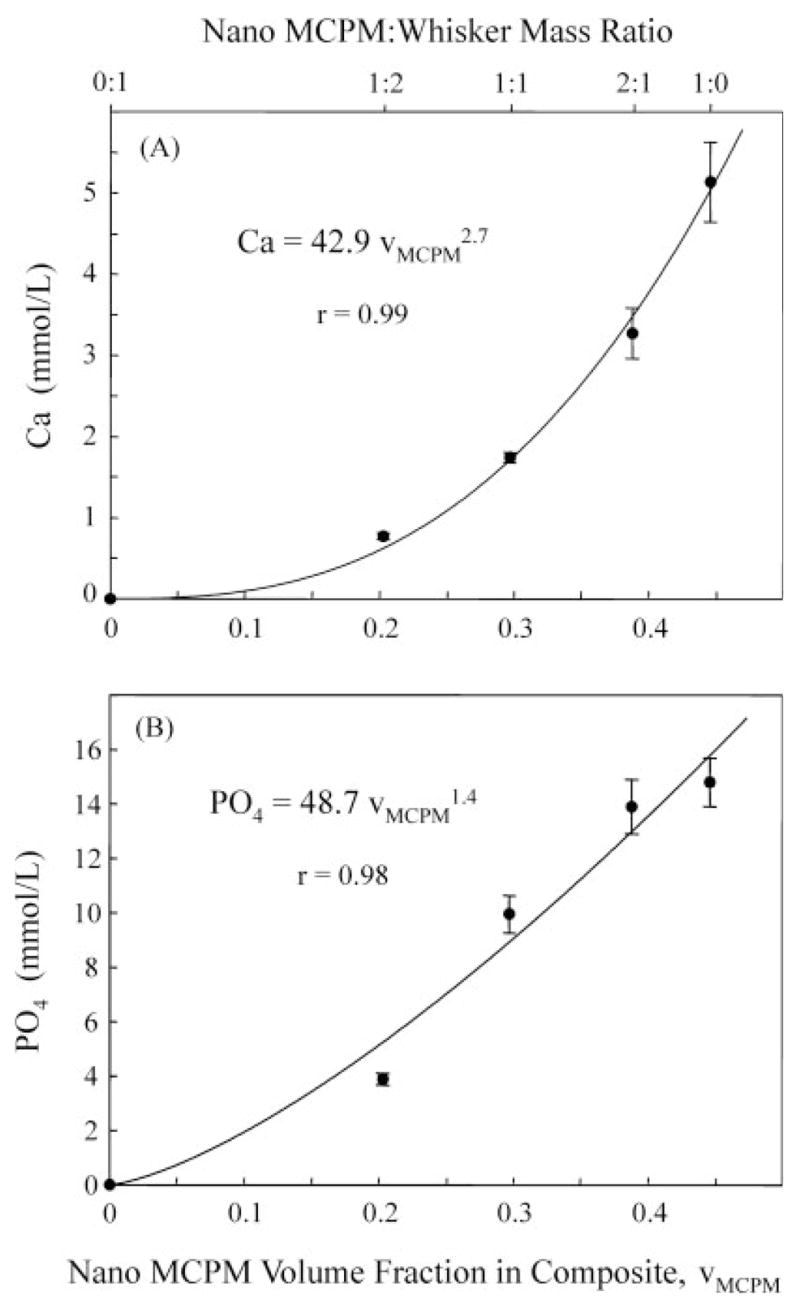
Relationships between MCPM volume fraction vMCPM and (A) Ca release, and (B) PO4 release (56 days). The curves are power-law best fit to the measured data, using Eqs. (4)–(6). Ca and PO4 in these equations have the units of mmol/L. vMCPM = 0.446 means a volume fraction of 44.6% (volume/volume).
Mechanical Properties
The reinforcement mechanisms for the whisker composites were discussed in previous studies to be whiskers pinning and bridging the cracks.14,15 The whiskers have a tensile strength of about 50 GPa, compared with 2.6 GPa of glass fibers. The fracture toughness of silicon carbide is above 2 MPa · m1/2, compared with 0.7 MPa · m1/2 for glass. Therefore, compared with the conventional glass fillers, the whiskers were more effective in resisting the cracks and less likely to be cut through by the cracks.14
As a result, the flexural strength of the MCPM–whisker composite reached 138 ± 9 MPa at MCPM/whisker of 1:2. To provide background and lend clinical perspective, comparisons are made with different dental composites. (1) Posterior composites had flexural strengths (measured using a similar three-point flexural test) of 65–133 MPa.33 (2) A heat/pressure-cured inlay/onlay composite had a flexural strength of approximately 60 MPa.34 (3) Core-buildup composites possessed flexural strengths of 97–120 MPa.35 (4) A glass fiber-reinforced composite had a flexural strength of 110 ± 10 MPa. (Jeneric/Pentron manufacturer’s data is available at www.jeneric.com.) These values are consistent with the flexural strength of 112 ± 14 MPa for the hybrid control of the present study. It should be noted that the MCPM–whisker composite specimens were not water-aged prior to the strength measurement, and water immersion will likely decrease their strengths. Further studies are needed to characterize the long-term water-aging behavior of these composites.
Furthermore, the elastic modulus at MCPM/whisker of 1:2 was 16.5 GPa (Figure 3), approaching the 18 GPa of dentin.36 In comparison, the elastic modulus of the MCPM composite without whiskers was 9.5 GPa, and that of the hybrid control was 10.9 GPa. Therefore, adding the whiskers increased the elastic modulus of the MCPM–whisker composite to more closely mimic the elastic modulus of natural dentin.
The ACP composite had a three-point flexural strength of 47 ± 5 MPa using unmilled ACP fillers and 56 ± 16 MPa using milled ACP fillers.37 The flexural strength of 138 ± 9 MPa for the nano-MCPM–whisker composites at MCPM/whisker of 1:2 was more than twofold higher. For the purpose of clarity, the above values are listed in Table I.
TABLE I.
Flexural Strength and Ca and PO4 Release of Several Dental Composites
| No. | Composite | Flexural Strength (MPa) | Ca Release (mmol/L) | PO4 Release (mmol/L) |
|---|---|---|---|---|
| 1 | Nano-MCPM:whisker composite at MCPM:whisker = 1:2 (This study) | 138 ± 9 | 0.77 | 3.88 |
| 2 | Hybrid composite control (this study) | 112 ± 14 | 0 | 0 |
| 3 | Core buildup composites33 | 97–120 | 0 | 0 |
| 4 | Posterior composites31 | 65–133 | 0 | 0 |
| 5 | Inlay/onlay composite32 | 60 | 0 | 0 |
| 6 | Fiber composite34 | 110 ± 10 | 0 | 0 |
| 7 | ACP composite37 | 56 ± 16 | 1.0 | 0.7 |
| 8 | Ca-PO4 composite38 | 40–60 | 0.5 | 0.1 |
Nos. 1 and 2 were measured in the present study. No. 3 was measured earlier in the same laboratory using the same test methods as the present study. Other information for the composites was described in the Discussion section. The purpose of this table was to compile available data on several relevant types of dental composites. As stated in the Disclaimer, the data of the present study should not be compared with data obtained in other laboratories under different conditions.
These comparisons demonstrate the potential of using both releasing fillers and reinforcing fillers simultaneously in the same composite. This can also be seen in Figure 2, where S = 29 + 146 WF and E = 9.0 + 11.3 WF. If WF =0, the properties became much lower than, for example, when WF = 0.5.
It should also be noted that this study provided a model composite via this approach: Composite = nano releasing fillers + reinforcing fillers + matrix resin. This method can be applied to other materials by using different releasing fillers (e.g., combining TTCP and DCPA with MCPM as described in a previous study,20 or using fluoride releasing fillers39) together with different reinforcing fillers (e.g., glass particles or glass fibers40 in combination with, or in place of, the whiskers). These reinforced Ca and PO4 releasing composites may be useful in treatments where complete removal of caries tissue is contraindicated; where carious lesions are beginning or are likely to occur; and for patients at high risk for dental caries (e.g, taking radiation treatments or certain medications, or with dry mouth). This may be especially so for patients of certain ethnicity and poverty levels with high incidence of untreated caries, for which the atraumatic restorative treatment (ART) can be widely and relatively easily performed.41,42 Many parts of the developing countries do not have electricity; ART does not require electricity, hence the two-part chemically-cured Ca–PO4 composites may be useful. ART requires no plumbed water nor anesthesia, but may not completely remove the carious tissue.41,42 Hence a Ca and PO4 releasing composite may be beneficial in remineralization and caries inhibition, while the opacity of the composite may be less of a concern for these patients. However, the opacity of these whisker composites will hinder restorations where a high degree of esthetics is desired. Further studies are needed to investigate the potential clinical applications of these composites.
SUMMARY
Nano-MCPM particles were synthesized and incorporated into a dental resin for the first time. The composite with nano-MCPM/whisker of 1:2 produced a Ca concentration of 0.77 mmol/L and a PO4 concentration of 3.88 mmol/L, matching or exceeding the reported Ca and PO4 concentrations of previous Ca- and PO4-releasing dental composites. However, the flexural strength of 138 ± 9 MPa for the composite with nano-MCPM/whisker of 1:2 is more than twofold those of previous Ca- and PO4-releasing composites.
The flexural strength of 138 MPa for the composite at nano-MCPM/whisker of 1:2 matched the 112 ± 14 MPa of a commercial stress-bearing hybrid composite without Ca and PO4 release. The elastic modulus of the nano-MCPM–whisker composite at 1:2 ratio was 16.5 ± 1.3 GPa, significantly higher than 10.9 ± 0.8 GPa of the hybrid control and approaching the 18 GPa of human dentin. This is the first time a releasing composite matched or exceeded the strength and elastic modulus of stress-bearing, nonreleasing dental composite.
Relationships were established between the amount of release and the releasing filler volume fraction in the resin: , and . This suggests that filler volume fraction is a key factor, and the release increases at a rate faster than being linear with increasing the volume fraction of the releasing fillers.
Acknowledgments
Contract grant sponsor: NIH/NIDCR; Contract grant number: R01 DE14190;
Contract grant sponsors: NIST and ADAF
We gratefully acknowledge Dr. Joseph M. Antonucci for providing the resin monomers. We also thank Dr. Frederick C. Eich-miller and Dr. Sabine H. Dickens for discussions.
Footnotes
Certain commercial materials and equipment are identified to specify the experimental procedure. This does not imply recommendation or endorsement by NIST or ADAF or that the material or equipment identified is necessarily the best available for the purpose. One standard deviation was used as the estimated standard uncertainty of the measurements. These values should not be compared with data obtained in other laboratories under different conditions. This is an official contribution of the National Institute of Standards and Technology and is not subjected to copyright in the United States.
References
- 1.Söderholm KJ, Zigan M, Ragan M, Fischlschweiger W, Bergman M. Hydrolytic degradation of dental composites. J Dent Res. 1984;63:1248–1254. doi: 10.1177/00220345840630101701. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg AJ, Burstone CJ, Hadjinikolaou I, Jancar J. Screening of matrices and fibers for reinforced thermoplastics intended for dental applications. J Biomed Mater Res. 1994;28:167–173. doi: 10.1002/jbm.820280205. [DOI] [PubMed] [Google Scholar]
- 3.Ferracane JL, Berge HX, Condon JR. In vitro aging of dental composites in water—Effect of degree of conversion, filler volume, and filler/matrix coupling. J Biomed Mater Res. 1998;42:465–472. doi: 10.1002/(sici)1097-4636(19981205)42:3<465::aid-jbm17>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Drummond JL, Bapna MS. Static and cyclic loading of fiber-reinforced dental resin. Dent Mater. 2003;19:226–231. doi: 10.1016/s0109-5641(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 5.Eick JD, Byerley TJ, Chappell RP, Chen GR, Bowles CQ, Chappelow CC. Properties of expanding SOC/epoxy copolymers for dental use in dental composites. Dent Mater. 1993;9:123–127. doi: 10.1016/0109-5641(93)90088-8. [DOI] [PubMed] [Google Scholar]
- 6.Bayne SC, Thompson JY, Swift EJ, Jr, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 7.Ferracane JL. Developing a more complete understanding of stresses produced in dental composites during polymerization. Dent Mater. 2005;21:36–42. doi: 10.1016/j.dental.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: Clinical, chemistry, and physical behavior considerations. Dent Mater. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Mjör IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J. 2000;50:361–366. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 11.Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J Dent Res. 1996;75:1679–1686. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- 12.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–566. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 13.Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physiological evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res. 2000;53:381–391. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Xu HHK, Martin TA, Antonucci JM, Eichmiller FC. Ceramic whisker reinforcement of dental composite resins. J Dent Res. 1999;78:706–712. doi: 10.1177/00220345990780021101. [DOI] [PubMed] [Google Scholar]
- 15.Xu HHK, Eichmiller FC, Smith DT, Schumacher GE, Giuseppetti AA, Antonucci JM. Effect of thermal cycling on whisker-reinforced dental resin composites. J Mater Sci: Mater Med. 2002;13:875–883. doi: 10.1023/a:1016504530133. [DOI] [PubMed] [Google Scholar]
- 16.Xu HHK. Long-term water aging of whisker-reinforced polymer-matrix composites. J Dent Res. 2003;82:48–52. doi: 10.1177/154405910308200111. [DOI] [PubMed] [Google Scholar]
- 17.Xu HHK, Quinn JB, Giuseppetti AA. Wear and mechanical properties of nano-silica-fused whisker composites. J Dent Res. 2004;83:930–935. doi: 10.1177/154405910408301208. [DOI] [PubMed] [Google Scholar]
- 18.Xu HHK, Smith DT, Simon CG. Strong and bioactive composites containing nano-silica-fused whiskers for bone repair. Biomaterials. 2004;25:4615–4626. doi: 10.1016/j.biomaterials.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 19.Chow LC, Markovic M, Takagi S. Calcium phosphate cements. In: Struble LJ, editor. Cements Research Progress. Westerville, OH: American Ceramic Society; 1999. pp. 2152–2238. [Google Scholar]
- 20.Carey LE, Xu HHK, Simon CG, Takagi S, Chow LC. Pre-mixed rapid-setting calcium phosphate composites for bone repair. Biomaterials. 2005;26:5002–5014. doi: 10.1016/j.biomaterials.2005.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Gonsalves KE. Preparation and characterization of thermally stable nanohydroxyapatite. J Mater Sci: Mater Med. 1997;8:25–28. doi: 10.1023/a:1018586128257. [DOI] [PubMed] [Google Scholar]
- 22.Sutorik AC, Paras MS, Lawrence D, Kennedy A, Hinklin T. Synthesis, characterization, and sintering behavior of calcium hydroxyapatite powders with average particle diameters of 150 nm. Ceram Trans. 2003;147:73–82. [Google Scholar]
- 23.Bow JS, Liou SC, Chen SY. Structural characterization of room-temperature synthesized nano-sized β-tricalcium phosphate. Biomaterials. 2004;25:3155–3161. doi: 10.1016/j.biomaterials.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 24.Chow LC, Sun L, Hockey B. Properties of nanostructured hydroxyapatite prepared by a spray drying technique. J Res Natl Inst Stand Technol. 2004;109:543–551. doi: 10.6028/jres.109.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu HHK, Sun L, Takagi S, Chow LC. Dental releasing materials. US Patent Application, Serial No. 11/138,182. filed on May 26, 2005.
- 26.Skrtic D, Antonucci JM, Eanes ED. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent Mater. 1996;12:295–301. doi: 10.1016/s0109-5641(96)80037-6. [DOI] [PubMed] [Google Scholar]
- 27.ISO/FDIS 4049. Dentistry—Polymer-based fillings, restorative and luting materials. 3. International Organization for Standardization; Geneva, Switzerland: 2000. [Google Scholar]
- 28.ASTM D 790–03. Standard test methods for flexural properties of unreinforced and reinforced plastic and electrical insulating materials. ASTM International; West Conshohocken, PA: 2004. [Google Scholar]
- 29.Vogel GL, Chow LC, Brown WE. A microanalytical procedure for the determination of calcium, phosphate and fluoride in enamel biopsy samples. Caries Res. 1983;17:23–31. doi: 10.1159/000260645. [DOI] [PubMed] [Google Scholar]
- 30.Chow LC, Brown WE. A physiochemical bench-scale caries model. J Dent Res. 1984;63:868–873. doi: 10.1177/00220345840630061101. [DOI] [PubMed] [Google Scholar]
- 31.Brown WE, Chow LC. Chemical properties of bone mineral. Ann Rev Mater Sci. 1976;6:213–236. [Google Scholar]
- 32.Agarwal BD, Broutman LJ. Analysis and Performance of Fiber Composites. 2. New York: Wiley; 1990. [Google Scholar]
- 33.Ferracane JL, Mitchem JC. Properties of posterior composites: Results of a round robin testing for a specification. Dent Mater. 1994;10:92–99. doi: 10.1016/0109-5641(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 34.Drummond JL, Savers EE. In vitro aging of a heat/pressure-cured composite. Dent Mater. 1993;9:214–216. doi: 10.1016/0109-5641(93)90123-8. [DOI] [PubMed] [Google Scholar]
- 35.Xu HHK, Smith DT, Schumacher GE, Eichmiller FC. Whisker-reinforced dental core buildup composites: Effect of filler level on mechanical properties. J Biomed Mater Res A. 2000;52:812–818. doi: 10.1002/1097-4636(20001215)52:4<812::aid-jbm26>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 36.Marshall GW., Jr Dentin: Microstructure and characterization. Quintessence Intl. 1993;24:606–617. [PubMed] [Google Scholar]
- 37.O’Donnell JNR, Antonucci JM, Skrtic D. Mechanical properties of amorphous calcium phosphate composites. J Dent Res. 2005:84. doi: 10.1177/0883911506064476. IADR Abstract No. 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickens SH, Flaim GM, Floyd CJE. Effect of resin composition on mechanical and physical properties of calcium phosphate filled bonding systems. Polym Prepr. 2004;45:329–330. [Google Scholar]
- 39.Xu HHK, Eichmiller FC, Antonucci JM, Flaim GM. Single-crystalline ceramic whisker-reinforced carboxylic acid-resin composites with fluoride release. Oper Dent. 2000;25:90–97. [PubMed] [Google Scholar]
- 40.Xu HHK, Schumacher GE, Eichmiller FC, Peterson RC, Antonucci JM, Mueller HJ. Continuous-fiber preform reinforcement of dental resin composite restorations. Dent Mater. 2003;19:523–530. doi: 10.1016/s0109-5641(02)00100-8. [DOI] [PubMed] [Google Scholar]
- 41.Mandari GJ, Frencken JE, van’t Hof MA. Six-year success rates of occlusal amalgam and glass-ionomer restorations placed using three minimally intervention approaches. Caries Res. 2002;37:246–253. doi: 10.1159/000070866. [DOI] [PubMed] [Google Scholar]
- 42.Frencken JE, van’t Hof MA, van Amerongen WE, Holmgren CJ. Effectiveness of single-surface ART restorations in the permanent dentition: A meta-analysis. J Dent Res. 2004;83:120–123. doi: 10.1177/154405910408300207. [DOI] [PubMed] [Google Scholar]