Abstract
Nano-particles of dicalcium phosphate anhydrous (DCPA) were synthesized for the first time. The objectives of this study were to incorporate DCPA nano-particles into resin for Ca-PO4 release to combat dental caries, and to investigate the filler level effects. Nano-DCPA and nano-silica-fused silicon nitride whiskers at a 1:1 ratio were used at filler mass fractions of 0–75%. The flexural strengths in MPa (mean ± SD; n = 6) of DCPA-whisker composites ranged from (106 ± 39) at 0% fillers to (114 ± 23) at 75% fillers, similar to (112 ± 22) of a non-releasing composite (TPH) (p > 0.1). The composite with 75% fillers in a NaCl solution (133 mmol/L, pH = 7.4, 37°C) yielded a Ca concentration of (0.65 ± 0.02) mmol/L and PO4 of (2.29 ± 0.07) mmol/L. Relationships were established between ion-release and DCPA volume fraction VDCPA: Ca = 4.46 VDCPA1.6, and PO4 = 66.9 VDCPA2.6. Nano-DCPA-whisker composites had high strength and released high levels of Ca-PO4 requisite for remineralization. These new nano-composites could provide the needed combination of stress-bearing and caries-inhibiting capabilities.
Keywords: dental composite, nano-particles, whisker reinforcement, tooth caries inhibition, Ca and PO4 ion release
INTRODUCTION
Secondary caries at the tooth-restoration margins is the most-frequent reason for replacement of restorations (Sakaguchi, 2005; Sarrett, 2005). Replacement dentistry accounts for 70% of all operative work and costs $5 billion/year in the U.S. (Jokstad et al., 2001; CDC, 2005). Several calcium-phosphate phases are regarded as biological precursors that form initially and then transform to apatites (LeGeros, 1991). Recent studies showed that methacrylate-based composites containing calcium-phosphate fillers released calcium (Ca) and phosphate (PO4) ions to supersaturated levels for apatite precipitation, and effectively remineralized tooth lesions in vitro (Skrtic et al., 1996a, 2000; Dickens et al., 2003).
However, the Ca-PO4 fillers did not reinforce the resin as do glass fillers (Söderholm et al., 1984; Goldberg et al., 1994; Bayne et al., 1998; Ferracane et al., 1998; Drummond and Bapna, 2003). Ca-PO4 composites had flexural strengths half that of unfilled resin (Skrtic et al., 1996b). Such low strengths were “inadequate to make these composites acceptable as bulk restoratives” (Skrtic et al., 2000).
Whiskers were used as fillers to reinforce dental composites (Xu, 1999). Silica nano-particles were fused onto the whiskers to facilitate silanization and enhance retention in the resin. These composites possessed strength and toughness nearly two-fold greater than those of several commercial composites (Xu et al., 2002a).
Calcium phosphate nano-particles were recently developed and incorporated into resins (Chow et al., 2004; Xu et al., 2006). A recent study investigated the effects of different resins/cure conditions with a single filler level (Xu et al., 2006). In the present study, two hypotheses were tested: (1) DCPA-whisker composites with filler levels from 0–75% would possess strengths matching/exceeding those of a commercial non-releasing, stress-bearing composite; and (2) the Ca-PO4 release would be proportional to the DCPA nano-particle filler level in the resin. The first hypothesis was tested because low filler levels could be envisioned for Ca-PO4-releasing sealant applications, medium filler levels for Ca-PO4 flowable composites, and high filler levels for stress-bearing and caries-inhibiting restorations. The purpose of the second hypothesis was to establish a relationship between release and volume fraction, to guide the tailoring/processing of composites.
MATERIALS & METHODS
Materials
Nano-particles of DCPA (CaHPO4) were synthesized via a spray-drying technique for the first time (Chow et al., 2004; Xu et al., 2006). X-ray diffraction indicated that the powder was DCPA. Transmission electron microscopy showed particles having diameters around 50 nm. Due to agglomeration, the Brunauer, Emmet, and Teller (BET) surface-area measurement yielded an equivalent particle diameter of 112 nm (Xu et al., 2006).
Silicon-nitride whiskers (β-Si3N4, UBE, New York, NY, USA) with diameters of 0.1–2 μm (mean = 0.4 μm) and lengths of 2–30 μm (mean = 5 μm) were mixed with silica (Aerosil-OX50, Degussa, Ridgefield, NJ, USA; diameter = 40 nm) at a whisker:silica mass ratio of 5:1. The mixture was heated in a furnace at 800°C for 30 min to fuse the silica onto the whiskers. The powder was silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine (mass fractions) (Xu, 1999).
A monomer consisting of 48.975% Bis-GMA (bisphenol glycidyl methacrylate), 48.975% TEGDMA (triethylene glycol dimethacrylate), 0.050% 2,6-di-tert-butyl-4-methylphenol, and 2.000% benzoyl peroxide formed part I, the initiator, of a two-part chemically activated resin. Part II, the accelerator resin, consisted of 49.5% Bis-GMA, 49.5% TEGDMA, and 1.0% N,N-dihydroxyethyl-p-toluidine.
The fillers consisted of nano-silica-fused whiskers and nano-DCPA at a DCPA:whisker mass ratio of 1:1 (Xu et al., 2006). The (DCPA+whiskers)/(DCPA+whiskers+resin) mass fractions were: 0%, 30%, 40%, 50%, 60%, 65%, 70%, and 75%. Filler levels ≥80% resulted in a dry paste. Equal masses of parts I and II were mixed and filled into 2×2×25 mm3 molds. The specimens were incubated at 37°C and 50% humidity for 24 hrs prior to being tested.
A hybrid composite (TPH, Caulk/Dentsply, Milford, DE, USA) was used as a non-releasing control. It consisted of 0.8-μm silicate fillers at 78% level in a urethane-modified Bis-GMA-TEGDMA resin. The specimens were photo-cured (Triad-2000, Dentsply, York, PA, USA).
Flexural Strength and Nano-indentation
Flexural strength was measured by a three-point test with a 10-mm span at a crosshead speed of 1 mm/min on a Universal Testing Machine (5500R, MTS, Cary, NC, USA) at approximately 25°C and 50% relative humidity. Nano-indentation (Nano-Instruments, Knoxville, TN, USA) was used to measure elastic modulus and hardness at a peak-load of 1 N (Xu et al., 2002a).
Ca and PO4 Release
A NaCl solution (133 mmol/L) buffered with 50 mmol/L HEPES (pH = 7.4; 37 C) was used. Following a previous study (Xu et al., 2006), we immersed 3 specimens of approximately 2×2×12 mm3 in 50 mL of solution, yielding specimen volume/solution = 2.9 mm3/mL. This was similar to the specimen volume/solution of approximately 3.0 mm3/mL in a previous study (Skrtic et al., 1996b). The concentrations of Ca and PO4 released from the specimens were measured vs. immersion time (in days): 1, 2, 4, 7, 14, 21, 28, 35, 42, 49, and 56. At each time period, aliquots of 0.5 mL were removed and replaced by fresh solution. The aliquots were analyzed with a spectrophotometer (DMS-80 UV-visible, Varian, Palo Alto, CA, USA) according to established standards and calibration methods (Skrtic et al., 1996b; Dickens et al., 2003).
Potential Diagram
The remineralization potential of a Ca- and PO4-releasing composite can be described via a potential diagram (Dickens et al., 2003). For a solution saturated with respect to hydroxyapatite [Ca10(PO4)6(OH)2], the solubility constant relationship, KSP = (Ca2+)10(PO43−)6(OH−)2, applies (Chow and Brown, 1984). Rearranging the equation leads to:
| (1) |
where KW = (H+)(OH−) is the dissociation constant of water. Taking log of both sides and rearranging the equation lead to:
| (2) |
where K = (1/6)(logKSP+9logKW) is a constant. Eq. (2) shows that, for a solution saturated with respect to hydroxyapatite, the logarithms of the activities of H3PO4 and Ca(OH)2 are linearly related. The activities (H+)3(PO43−) and (Ca2+)(OH−)2 for a solution can be calculated from the pH, the measured Ca-PO4 concentrations, and the ionic strength. Hence, a solution can be represented as a point in the potential diagram. Solutions located to the left of the hydroxyapatite line (e.g., Fig. 8, Dickens et al., 2003) are undersaturated, and those to the right are supersaturated, with respect to hydroxyapatite.
The extent of this supersaturation can be quantified via the saturation ratio. The saturation index is (Margolis et al., 1999):
| (3) |
where q is the number of ions in the ion activity product expression (for hydroxyapatite, q = 10+6+2 = 18). IAP = (Ca2+)10(PO43−)6(OH−)2 is the ion activity product, which can be calculated from the measured concentrations with specific software (Chemist, Micromath Research, St. Louis, MO, USA). The saturation ratio
| (4) |
where SR < 1 means that the solution is undersaturated, and SR > 1 means that the solution is supersaturated, with respect to hydroxyapatite (Margolis et al., 1999).
Furthermore, the Gibbs free energy can be used to quantify the thermodynamic driving force for remineralization (Dickens et al., 2003):
| (5) |
where R is the ideal gas constant, and T is absolute temperature.
We performed one- and two-way ANOVA to detect the significant effects of the variables. We used Tukey’s multiple-comparison test to compare the measured data at a p value of 0.05.
RESULTS
Mechanical Properties
Increasing the filler level did not significantly change the strength (p > 0.1) (Fig. 1A). Flexural strength in MPa (mean ± SD; n = 6) at 60% and 75% fillers were (117 ± 17) and (114 ± 23), respectively, not significantly different from (106 ± 39) of the unfilled resin and (112 ± 22) of the hybrid control (p > 0.1).
Figure 1.

Mechanical properties. (A) Flexural strength, (B) elastic modulus, and (C) hardness, vs. filler level for the nano-DCPA-whisker composites. Values for the stress-bearing, non-releasing hybrid control (TPH, Caulk/Dentsply) are also included. Each value is the mean of 6 measurements for strength (mean ± SD; n = 6), and 10 measurements for elastic modulus and hardness (mean ± SD; n = 10), with each error bar showing the standard deviation (SD). Line connects the datapoints for visual clarity.
Elastic modulus (Fig. 1B) and hardness (Fig. 1C) increased with filler level. Modulus in GPa (mean ± SD; n = 10) at 75% fillers was (14.9 ± 0.7), higher than (11.7 ± 0.4) for the hybrid control and (3.9 ± 0.1) for the unfilled resin (p < 0.05).
Ca and PO4 Release
The release increased with time and then started to plateau (Figs. 2A, 2B). At 56 days, the Ca concentration in mmol/L (mean ± SD; n = 3) was (0.65 ± 0.02) with 75% fillers, significantly higher than (0.59 ± 0.02) with 70% fillers, and (0.39 ± 0.03) with 65% fillers (p < 0.05). The corresponding PO4 concentrations were (2.29 ± 0.07), (1.92 ± 0.14), and (1.26 ± 0.09), significantly different from each other (p < 0.05).
Figure 2.

Ca and PO4 release. (A) Ca ion release and (B) total ionic PO4 release, from the nano-DCPA-whisker composites vs. filler level and immersion time. The symbols on the x-axis represent the hybrid control (TPH, Caulk/Dentsply) and the unfilled resin that had no Ca or PO4 release. Each value is the mean ± SD; n = 3. Lines connect the datapoints for visual clarity.
Potential Diagram
In the chemical potential diagram (Fig. 3A), the solution of the composite with 30% fillers was undersaturated, that with 40–50% fillers was supersaturated after 2 days, and those of all other composites were supersaturated from 1 day, with respect to hydroxyapatite. The saturation ratio increased with increasing filler levels (Fig. 3B). The larger the negative value of ΔG0 (Fig. 3C), the lower the energy state for hydroxyapatite precipitation, and the higher the remineralization potential (Dickens et al., 2003).
Figure 3.

Remineralization potential. (A) Diagram demonstrating the potential of DCPA-whisker composites to form hydroxyapatite. The straight line in the middle represents the hydroxyapatite solubility isotherm line. The line at the upper right corner represents the solubility isotherm for DCPD (dicalcium phosphate dihydrate, CaHPO4·2H2O). The dots represent log([Ca2+][OH−]2) vs. log([H+]3[PO4]3−) from the measured Ca and PO4 concentrations. (B) Degree of saturation SR < 1 means that the solution is undersaturated, and SR > 1 means supersaturated, with respect to hydroxyapatite. (C) Thermodynamic driving force for remineralization. The larger the negative value of the Gibbs free energy ΔG0, the lower the energy state for hydroxyapatite precipitation, and the higher the remineralization potential. Values near the right axis are filler level mass fractions.
DISCUSSION
Synergistic Effects of Nano-particles/Whiskers
Previous studies have measured fluoride release from dental materials (Geurtsen et al., 1999; Anusavice et al., 2005). Other studies developed Ca-PO4 composites with flexural strengths of about 55 MPa (Skrtic et al., 1996b). This led to the observation that “all the amorphous calcium phosphate fillers yielded polymerized materials weaker than unfilled polymers” (Skrtic et al., 1996b). Another composite, with micron-sized DCPA, had a flexural strength of 40–50 MPa (Dickens et al., 2004). In the present study, the nano-DCPA-whisker composites had flexural strengths of about 110 MPa. The DCPA-whisker composite at 75% fillers had an elastic modulus of 14.9 GPa. It was lower than the 18 GPa of dentin, but higher than the 11.7 GPa of the commercial stress-bearing, non-releasing composite control.
Previous Ca-PO4 composites released to PO4 concentrations of 0.1–0.7 mmol/L, and Ca to 0.3–1.0 mmol/L, measured with a similar method (Skrtic et al., 1996b; Dickens et al., 2003). These composites remineralized tooth lesions in vitro (Skrtic et al., 1996a; Dickens et al., 2003). The nano-DCPA-whisker composites released PO4 with concentrations up to 2.2 mmol/L, and Ca up to 0.65 mmol/L (at 75% total fillers), even when half of the fillers were non-releasing whiskers. This was likely because the DCPA nano-particles had a high surface area, A = 18.6 m2/g (Xu et al., 2006).
In a previous study (Dickens et al., 2003), the DCPA particle size, d, was 1.1 μm and the TTCP (tetracalcium phosphate) particle size was 16 μm. The density, ρ, is 2.89 g/cm3 for DCPA and 3.07 g/cm3 for TTCP. Hence, A = 6/(ρd) = 1.9 m2/g for DCPA, and A = 0.12 m2/g for TTCP. These traditional particles had surface areas 1–2 orders of magnitude less than the new DCPA nano-particles. As a result, these traditional composites needed to be fully filled with Ca-PO4 fillers to have significant release. Replacing part of these Ca-PO4 fillers with reinforcing fillers would substantially reduce the release. Even if only 10% of the ACP fillers had been replaced by reinforcing fillers, the Ca-PO4 release would have been decreased from about 0.75 to only 0.1 mmol/L (Skrtic et al., 1996b). Therefore, there was little room left in traditional Ca-PO4 composites for reinforcement fillers without diminishing the ion release capability.
In contrast, with nano-DCPA, high release could be achieved with less filler, thus making room available in the resin for reinforcement fillers. This synergistic releasing-filler/reinforcing-filler approach helped achieve a flexural strength of 110 MPa for the Ca-PO4-releasing composites, matching that of a commercial stress-bearing, non-releasing composite.
Effect of Nano-DCPA Volume Fraction
The masses used in making the composites and the density were used to calculate the volume fraction of DCPA in the composite. The density of DCPA, dDCPA, is 2.89 g/cm3. The density of the unfilled resin was measured to be dresin = (1.19 ± 0.04) g/cm3. The density of silicon nitride whiskers is 3.34 g/cm3(manufacturer’s data), and dfumed-silica = 2 g/cm3. Hence, dsilica-fused-whisker = 2.97 g/cm3 at a whisker:silica ratio of 5:1. At total filler mass fractions of 30%, 40%, 50%, 60%, 65%, 70%, and 75%, the volume fraction VDCPA was calculated to be 0.075, 0.108, 0.147, 0.192, 0.218, 0.247, and 0.279, respectively.
There appear to be two main factors influencing Ca-PO4 release: (1) the amount of the source of release, VDCPA, with the amount of release expected to increase with increasing VDCPA; and (2) the resin polymerization conversion. Increasing the filler level usually decreases the polymerization conversion (Xu, 1999), because a higher concentration of air in the heavily filled composite may adversely affect the conversion. In addition, the fillers may partially absorb the heat of polymerization, thereby moderating the exotherm of polymerization. Therefore, with higher VDCPA in the composite, there is not only more DCPA for release, but also the diffusion of water and ions through the resin may be somewhat enhanced, due to the decreased polymerization conversion. If only factor (1) had been operative, the relationship between VDCPA and Ca-PO4 release might have been simply linear. However, these two factors may both be operative. Hence, the release may increase with increasing VDCPA at a rate faster than linear. Based on these reasons, the following empirical relationships are proposed:
| (6) |
| (7) |
| (8) |
where Ca and PO4 (mmol/L) are concentrations, and k and α-γ are coefficients. Fitting the above equations to the measured data (at 56 days) yielded the equations in Fig. 4.
Figure 4.
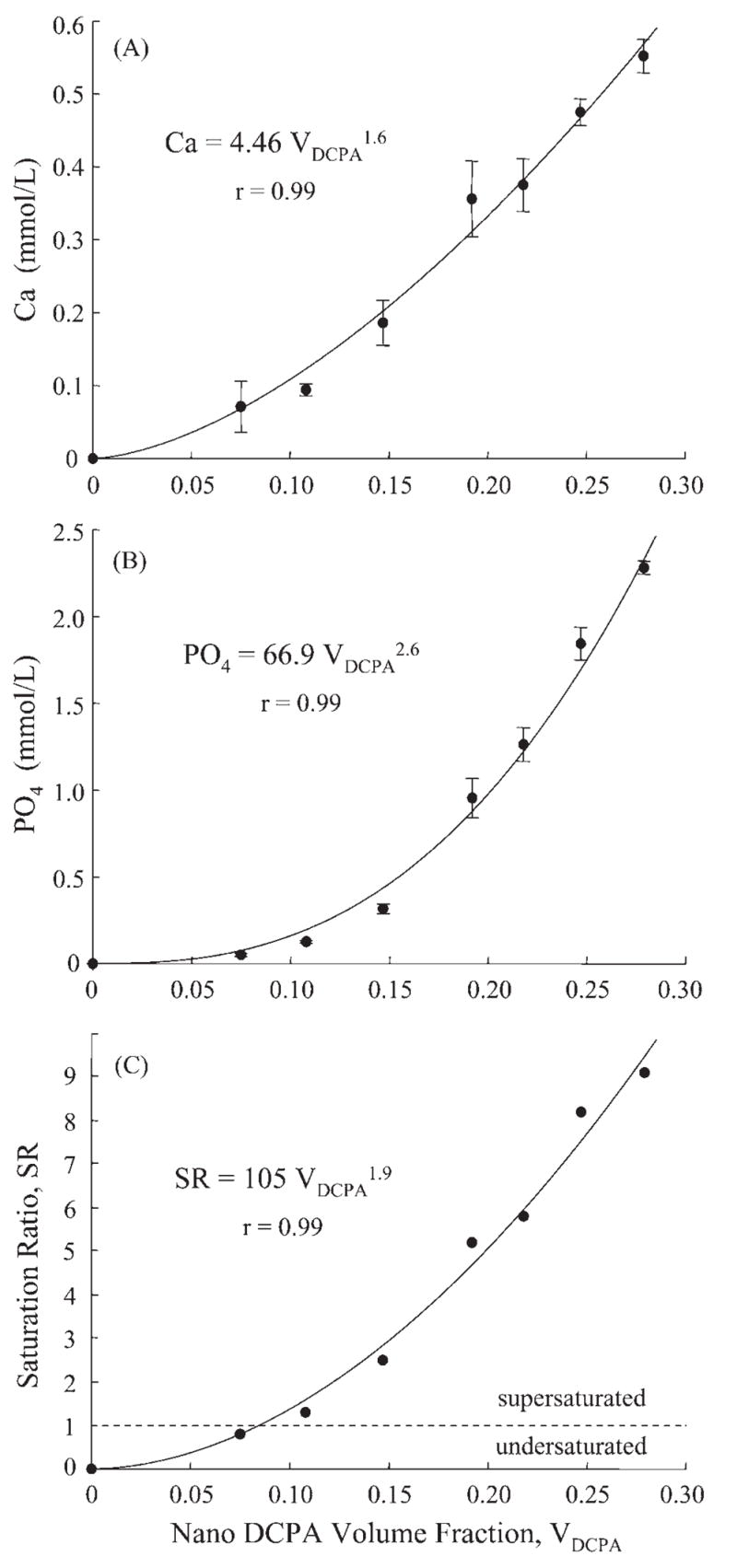
Relationships between (A) Ca and (B) PO4 release and nano-DCPA volume fraction VDCPA. The curves are best fits to the measured data by Eqs. 6–8, yielding Ca = 4.46 VDCPA1.6, PO4 = 66.9 VDCPA2.6, and SR = 105 VDCPA1.9. The correlation coefficient r = 0.99 for all three equations. Ca and PO4 in these equations are ion concentrations and have the units of mmol/L. VDCPA is unitless; e.g., VDCPA = 0.25 means a volume fraction (volume of DCPA/volume of composite) of 25% (0.25 is equivalent to 25%).
Regarding potential applications, nano-DCPA-whisker composites with 30–50% fillers may be suitable for use as Ca-PO4-releasing tooth cavity liners, adhesives, and pit-and-fissure sealants. Flowable DCPA-whisker composites with 50–60% fillers may be used as crown cements and orthodontic bracket cements, and to repair defective margins. Composites with 70–75% fillers may be useful in stress-bearing and caries-inhibiting restorations. The Ca-PO4 release from the DCPA-whisker composites matched/exceeded those of previous composites known to remineralize tooth lesions (Skrtic et al., 1996a; Dickens et al., 2003). Further research is needed to investigate their potential applications.
The dimensional change of the composite with time is another issue that needs to be investigated. Previous studies on a Ca- and F-releasing composite (Ariston pHc) for buffering the local pH in plaque retention areas showed considerable enamel cracks after 2 yrs, which were related to the water-expansion of the restorations (Braun et al., 2001; van Dijken, 2002; Krämer et al., 2005). Ariston was completely covered with cracks after 24 mos (Frankenberger et al., 2005). It exhibited the highest dimensional expansion among the materials immersed for 2 mos (Martin et al., 2003), and a high wear-rate (Manhart et al., 2000). These failure phenomena may be related to its hydrophilic monomer (Table I, Manhart et al., 2000) and the formation of calcium carbonates with an expanding effect (van Dijken, 2002). One advantage of the new nano-composite was that it released high levels of Ca-PO4 without the use of a hydrophilic monomer. The Bis-GMA/TEGDMA resin for the nano-composite was similar to the resin in previous composites, showing no significant degradation in thermal-cycling and water-aging for 2 yrs (Xu et al., 2002b; Xu, 2003). However, studies are needed to evaluate the long-term performance of the new nano-composites in vitro and in vivo.
In summary, we developed novel nano-composites using DCPA nano-particles with Ca-PO4 release to combat dental caries. The effects of nano-DCPA filler level were systematically investigated and correlated with Ca-PO4 release for the first time. The Ca-PO4 release from DCPA-whisker composites matched/exceeded those of previous composites known to remineralize tooth lesions, while the strengths of the DCPA-whisker composites were two-fold those of previous Ca-PO4 composites. Relationships between Ca-PO4 release and nano-DCPA volume fraction, VDCPA, were established: Ca = 4.46 VDCPA 1.6, and PO4 = 66.9 VDCPA 2.6. This suggests that the filler volume fraction is a key factor, and the release increases with VDCPA at a rate greater than linear. The new nano-composites, with substantial Ca and PO4 release, possessed mechanical properties matching those of a commercial stress-bearing, non-releasing composite. Hence, the nano-DCPA-whisker composites may have both stress-bearing and caries-inhibiting capabilities, a combination not yet available in current dental materials.
Acknowledgments
We gratefully thank Dr. J.M. Antonucci for providing the resin monomers, and Dr. F.C. Eichmiller, Dr. S.H. Dickens, and Dr. D. Skrtic for discussions. This study was supported by NIDCR grant R01 DE14190 (to Xu), NIST, and the ADAF.
Certain commercial materials and equipment are identified to specify the experimental procedure. This does not imply recommendation or endorsement by NIST or ADAF, or that the material or equipment identified is necessarily the best available for the purpose. The standard uncertainty of the flexural strength measurement was estimated to be 1%. The standard uncertainty for the Ca and PO4 release measurements was estimated to be 3%. Unless otherwise specified in the paper, one standard deviation was used as the estimated standard uncertainty of the measurements.
References
- Anusavice KJ, Zhang NZ, Shen C. Effect of CaF2 content on rate of fluoride release from filled resins. J Dent Res. 2005;84:440–444. doi: 10.1177/154405910508400508. [DOI] [PubMed] [Google Scholar]
- Bayne SC, Thompson JY, Swift EJ, Jr, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- Braun AR, Frankenberger R, Krämer N. Clinical performance and margin analysis of Ariston pHc versus Solitaire I as posterior restorations after 1 year. Clin Oral Investig. 2001;5:139–147. doi: 10.1007/s007840100116. [DOI] [PubMed] [Google Scholar]
- CDC (Center for Disease Control) December 2005; www.cdc.gov/OralHealth/factsheets, Dental amalgam use and benefits.
- Chow LC, Brown WE. A physicochemical bench-scale caries model. J Dent Res. 1984;63:868–873. doi: 10.1177/00220345840630061101. [DOI] [PubMed] [Google Scholar]
- Chow LC, Sun L, Hockey B. Properties of nanostructured hydroxyapatite prepared by a spray drying technique. J Res Natl Inst Stand Technol. 2004;109:543–551. doi: 10.6028/jres.109.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–566. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- Dickens SH, Flaim GM, Floyd CJE. Effect of resin composition on mechanical and physical properties of calcium phosphate filled bonding systems. Polymer Prepr. 2004;45:329–330. [Google Scholar]
- Drummond JL, Bapna MS. Static and cyclic loading of fiber-reinforced dental resin. Dent Mater. 2003;19:226–231. doi: 10.1016/s0109-5641(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Berge HX, Condon JR. In vitro aging of dental composites in water—effect of degree of conversion, filler volume, and filler/matrix coupling. J Biomed Mater Res. 1998;42:465–472. doi: 10.1002/(sici)1097-4636(19981205)42:3<465::aid-jbm17>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Frankenberger R, García-Godoy F, Lohbauer U, Petschelt A, Krämer N. Evaluation of resin composite materials. Part I: in vitro investigations. Am J Dent. 2005;18:23–27. [PubMed] [Google Scholar]
- Geurtsen W, Leyhausen G, García-Godoy F. Effect of storage media on the fluoride release and surface microhardness of four polyacid-modified composite resins (“compomers”) Dent Mater. 1999;15:196–201. doi: 10.1016/s0109-5641(99)00034-2. [DOI] [PubMed] [Google Scholar]
- Goldberg AJ, Burstone CJ, Hadjinikolaou I, Jancar J. Screening of matrices and fibers for reinforced thermoplastics intended for dental applications. J Biomed Mater Res. 1994;28:167–173. doi: 10.1002/jbm.820280205. [DOI] [PubMed] [Google Scholar]
- Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations. FDI Commision Project 2–95. Int Dent J. 2001;51:117–158. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Krämer N, García-Godoy F, Frankenberger R. Evaluation of resin composite materials. Part II: In vivo investigations. Am J Dent. 2005;18:75–81. [PubMed] [Google Scholar]
- LeGeros RZ. Chapters 3–4. In: Myers HM, editor. Calcium phosphates in oral biology and medicine. Basel, Switzerland: S. Karger; 1991. [PubMed] [Google Scholar]
- Manhart J, Kunzelmann KH, Chen HY, Hickel R. Mechanical properties of new composite restorative materials. J Biomed Mater Res. 2000;53:353–361. doi: 10.1002/1097-4636(2000)53:4<353::aid-jbm9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Margolis HC, Zhang YP, Lee CY, Kent RL, Jr, Moreno EC. Kinetics of enamel demineralization in vitro. J Dent Res. 1999;78:1326–1335. doi: 10.1177/00220345990780070701. [DOI] [PubMed] [Google Scholar]
- Martin N, Jedynakiewicz NM, Fisher AC. Hygroscopic expansion and solubility of composite restoratives. Dent Mater. 2003;19:77–86. doi: 10.1016/s0109-5641(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dent Mater; Summary of discussion from the Portland Composites Symposium (POCOS); June 17–19, 2004; Oregon Health and Science University, Portland, Oregon. 2005. pp. 3–6. [DOI] [PubMed] [Google Scholar]
- Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J Dent Res. 1996a;75:1679–1686. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- Skrtic D, Antonucci JM, Eanes ED. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent Mater. 1996b;12:295–301. doi: 10.1016/s0109-5641(96)80037-6. [DOI] [PubMed] [Google Scholar]
- Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physicochemical evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res. 2000;53:381–391. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Söderholm KJ, Zigan M, Ragan M, Fischlschweiger W, Bergman M. Hydrolytic degradation of dental composites. J Dent Res. 1984;63:1248–1254. doi: 10.1177/00220345840630101701. [DOI] [PubMed] [Google Scholar]
- van Dijken JW. Three-year performance of a calcium-, fluoride-, and hydroxyl-ions-releasing resin composite. Acta Odontol Scand. 2002;60:155–159. doi: 10.1080/000163502753740179. [DOI] [PubMed] [Google Scholar]
- Xu HH. Dental composite resins containing silica-fused ceramic single-crystalline whiskers with various filler levels. J Dent Res. 1999;78:1304–1311. doi: 10.1177/00220345990780070401. [DOI] [PubMed] [Google Scholar]
- Xu HH. Long-term water aging of whisker-reinforced polymer-matrix composites. J Dent Res. 2003;82:48–52. doi: 10.1177/154405910308200111. [DOI] [PubMed] [Google Scholar]
- Xu HH, Quinn JB, Smith DT, Antonucci JM, Schumacher GE, Eichmiller FC. Dental resin composites containing silica-fused whiskers—effects of whisker-to-silica ratio on fracture toughness and indentation properties. Biomaterials. 2002a;23:735–742. doi: 10.1016/s0142-9612(01)00178-8. [DOI] [PubMed] [Google Scholar]
- Xu HH, Eichmiller FC, Smith DT, Schumacher GE, Giuseppetti AA, Antonucci JM. Effect of thermal cycling on whisker-reinforced dental resin composites. J Mater Sci Mater Med. 2002b;13:875–883. doi: 10.1023/a:1016504530133. [DOI] [PubMed] [Google Scholar]
- Xu HH, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC, et al. Nano DCPA-whisker composites with high strength and Ca and PO(4) release. J Dent Res. 2006;85:722–727. doi: 10.1177/154405910608500807. [DOI] [PMC free article] [PubMed] [Google Scholar]


