Abstract
Perivascular adipose tissue (PAT) has been reported to blunt agonist-induced arterial tone via a relaxing factor acting in a paracrine manner. The purpose of this study was to test the hypothesis that PAT of porcine coronary artery blunts constriction similarly and that this anticontractile effect of PAT is altered by diet and/or exercise training.
Methods
Fourteen adult male pigs were fed a normal-fat (NF) diet, and 10 adult male pigs were fed a high-fat/cholesterol (HF) diet. Four weeks after the initiation of diet, pigs were exercised (EX) or remained sedentary (SED) for 16 wk, yielding four groups: 1) NF-SED, 2) NF-EX, 3) HF-SED, and 4) HF-EX. Left circumflex coronary artery (LCX) rings were prepared with PAT left intact or removed. LCX reactivity to acetylcholine (ACh), endothelin (ET-1), bradykinin (BK), and sodium nitroprusside (SNP) was assessed in vitro using standard techniques.
Results
The results demonstrate that both ACh and ET-1 elicited dose-dependent increases in tension from LCX rings from all groups. Removal of PAT had no significant effect on ACh-induced contractions in any group. In contrast, removal of PAT increased ET-1–induced tension in LCX from NF-SED, HF-SED, and HF-EX but not NF-EX. PAT had no significant effect on relaxation responses to BK except in HF-EX animals, where removal of PAT increased BK-induced relaxation. PAT removal decreased SNP-induced relaxation in HF-LCX, but not LCX from NF pigs, suggesting basal release of a relaxing factor LCX from HF pigs.
Conclusion
PAT blunts contractions induced by ET-1 in LCX from NF and HF pigs. Whereas EX abolished this effect of PAT in NF pigs, exercise did not alter the anticontractile effect in HF pigs.
Keywords: VASOCONTRACTION, VASORELAXATION, CORONARY, VASCULAR SMOOTH MUSCLE, ENDOTHELIN, ACETYLCHOLINE
Perivascular adipose tissue surrounds nearly all blood vessels and is commonly removed for in vitro studies of vasomotor function of arteries (11). Although perivascular adipose tissue was traditionally thought to provide structural support for arteries, increasing evidence suggests that perivascular adipose tissue also functions as an endocrine organ able to signal locally and/or systemically and as a paracrine tissue that can modulate the function of arterial vascular tone through release of a substance or substances that cause relaxation of vascular smooth muscle (2,11). The yet-to-be-identified substance(s), released by perivascular adipose tissue and referred to as adipocyte-derived relaxing factor (ADRF), seems to be a heat labile protein(s) (3). Available evidence indicates that ADRF causes smooth-muscle relaxation through opening K+ channels (1,18). Lohn et al. (11) have demonstrated that rat aortic rings with perivascular fat exhibited a blunted constriction in response to angiotensen II, serotonin, and phenylephrine compared with rings with perivascular adipose tissue removed. Similarly, Verlohren et al. (18) found that vessels with perivascular fat exhibited a blunted constriction in response to serotonin, endothelin-1, and phenylephrine. Furthermore, the anticontractile effect of perivascular fat was related to the amount of fat left on the ring preparation. Importantly, it is thought that differences in the function of perivascular adipose tissue in disease may contribute to vascular dysfunction (3).
On the basis of these observations, the experiments presented in this manuscript were designed to test three hypotheses. First, that coronary perivascular adipose tissue exerts anticontractile effect (i.e., releases ADRF) in a manner similar to that reported for rat aorta and visceral arteries (3,11,18). Second, we hypothesized that coronary arteries with perivascular adipose tissue isolated from pigs on a high-fat diet would exhibit greater vasoconstrictor responses and/or blunted vasodilator responses because of a decreased anticontractile effect of perivascular adipose tissue (5,6,12). Third, given our interest in the ability of physical activity/exercise training to have beneficial effects on coronary vasomotor function in health and disease (16), we hypothesized that sustained physical activity through treadmill exercise training for 5 d·wk−1 for 16 wk would increase the anticontractile effect of coronary perivascular adipose tissue in both normal-diet and high-fat-diet–fed pigs. The results reveal that porcine coronary perivascular adipose tissue blunts vasocontraction-stimulated by endothelin-1, but not contraction stimulated by acetylcholine, and that arteries from pigs on the high-fat diet did not exhibit greater vasoconstrictor responses and/or blunted vasodilator responses associated with the presence of perivascular adipose tissue. Finally, whereas exercise training seemed to decrease the anticontractile effects of perivascular adipose tissue on contractions induced by ET-1 in NF diet pigs, exercise had no apparent effect on the anticontractile effect of perivascular adipose tissue in coronary arteries from pigs on the high-fat diet.
METHODS
Experimental animals and design
Approval from the animal and use committee at the University of Missouri was obtained before the initiation of this study. The MU-ACUC requires that all approved studies follow policies and procedures detailed in the Guide for the Care and Use of Laboratory Animals published by the U.S. Department of Health and Human Services and proclaimed in the Animal Welfare Act (PL89-544, PL91-979, and PL94-279) and that rules, procedures, and recommendations for the care of laboratory animals as advocated by the American Association for Accreditation of Laboratory Animal Care are followed. Adult male Yucatan miniature swine (N = 24) used in this study were purchased from Sinclair Research Farm (Columbia, MO) and housed in the animal care facility at a temperature of 20°C with a 12-h:12-h light–dark cycle. Twenty pigs were assigned into four groups according to diet (normal fat or high fat) and exercise (sedentary or trained). The normal-fat (NF) diet consisted of Purina Lab Mini-Pig Chow, in which 8% of daily calories came from fat. The high-fat (HF) diet was composed of pig chow supplemented in the following manner: cholesterol (2%), coconut oil (17.1%), corn oil (2.3%), and sodium cholate (0.7%), such that 46% of daily calories came from fat. Four weeks after the initiation of either the NF or HF diets, pigs were either exercise trained (EX) or remained sedentary (SED) for 16 wk. Exercised pigs performed endurance running at approximately 75% of max capacity on a motorized treadmill, as reported previously (7–10,16). Sedentary pigs were restricted to their 2 × 4-m pens. After completion of the experiments with the first 20 pigs, we noted that variability within the NF-SED group required that we do additional experiments. Therefore, we added four NF-SED pigs to the study. This design yielded four experimental groups: NF, sedentary (NF-SED) (N = 9); NF, exercise (NF-EX) (N = 5); HF, sedentary (HF-SED) (N = 5); and HF, exercise (HF-EX) (N = 5). Before and at the conclusion of training/cage confinement programs, both EX and SED pigs performed a graded-intensity treadmill exercise test to exhaustion (7–10,16). The efficacy of the training protocol was evaluated from measurements of endurance time (from the treadmill exercise test) and heart weight/body weight ratio, and oxidative capacity of skeletal muscle tissue was estimated from measures of citrate synthase activity, as reported previously (9,13,16). The treadmill performance test consists of four stages of exercise: stage 1, pigs run 3.1 mph for 5 min on a 0% grade; stage 2, grade is increased to 10% and pigs run for 10 min at 3.1 mph; stage 3, grade remains at 10% but pigs increase speed to 4.3 mph for 10 min; and stage 4, pigs run on a 10% grade at 6 mph until exhaustion is reached. When a pig is exhausted (at any stage), the treadmill is stopped and the total run time is recorded.
Histology/immunohistochemistry methods
At the end of 20–24 wk, pigs were anesthetized with intramuscular atropine, ketamine, xylazine, and intravenous pentobarbital, and the chest was opened to achieve euthanasia. Hearts were removed and placed in iced Krebs–bicarbonate buffer for dissection of coronary artery samples. Samples of left circumflex branch of the left coronary artery were taken for examination of vasomotor function as described below. Samples of the epicardial left anterior descending branch of the coronary artery with periadventitial adipose tissue attached to the left ventricular myocardium were taken approximately midway between the base and apex of the left ventricle and were fixed in neutral buffered 10% formalin for a minimum of 24 h. Formalin-fixed samples were processed routinely through paraffin embedment, sectioned at five microns, and stained histochemically with hematoxylin and eosin, Verhoeff’s method for elastin, and immunohistochemically for scavenger receptor A (SRA) (http://exercisevascularcells.org/SOP_CoreB/43.htm); TNFα (http://exercisevascularcells.org/SOP_CoreB/48.htm), interleukin-6 (http://exercisevascularcells.org/SOP_CoreB/28.htm), and MCP-1 (http://exercisevascularcells.org/SOP_CoreB/34.htm). Diaminobenzidine was used as the chromogen with hematoxylin as the counterstain for IHC. Sections were examined using an Olympus BX40 photomicroscope and photographed with a Spot Insight digital camera. SRA is a marker of macrophage cells. TNFα, interleukin-6, and MCP-1 are markers of inflammation.
Citrate synthase activity
Skeletal muscle oxidative capacity was estimated with a citrate synthase assay. After euthanasia, samples of accessory, medial, long, and lateral heads of triceps brachii and of the deltoid muscle were isolated. Muscle samples were frozen in liquid N2 and were stored at −70°C until processed. Citrate synthase activity was measured from whole-muscle homogenate, using the spectrophotometric method of Srere (14).
In vitro assessment of vessel reactivity
Portions of 3- to 4-mm length of the left circumflex coronary arteries (LCX) from pigs in each group were dissected. Perivascular adipose tissue was either removed (−fat) or left intact (+fat). Vessel segments were then mounted onto a force transducer that would be able to measure grams of tension, and this was placed into a 20-mL bath containing Krebs–bicarbonate buffer maintained at 37°C with a gas mixture of 95% O2 and 5% CO2. Before dose–response curves, coronary rings were stretched to a length that produced maximal force stimulated by 80 mM KCl. Both acetylcholine (ACh; 10−9 to 10−4 M) and endothelin (ET-1; 10−10 to 10−8 M) were used to assess vasoconstriction in a dose–response manner. In all vessels, ACh dose–response was examined first, and ET-1 second. After completion of the ET-1 dose–response curve, the bathing solutions were exchanged several times during a period of 1 h. Then, the arteries were reconstricted with 10−8 M ET-1, and vasorelaxation curves were produced in a dose–response manner. Bradykinin (BK; 10−11 to 10−6 M) was used to assess endothelium-dependent relaxation, and sodium nitroprusside (SNP; 10−10 to 10−4 M) was used to assess endothelium-independent relaxation. After the SNP dose–response curve, the bathing solution was changed to a calcium-free buffer and then measured after 30 min of minimal tension. Change in force was measured from the force transducer in response to cumulatively increasing doses of agonist, with washouts occurring between each dose–response protocol.
Solutions and drugs
The Krebs–bicarbonate buffer solution contained 131.5 mM NaCl, 5.0 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 2.5 mM CaCl2, 11.2 mM glucose, 20.8 mM NaHCO3, 0.003 mM propranolol, and 0.025 mM EDTA. The solution was aerated with 95% O2 and 5% CO2 (pH 7.4) and maintained at 37°C. All drugs and chemicals were purchased from Sigma Chemical.
Statistical analysis
All values are means ± standard errors (SE). The vasomotor function experiments were in the form of a mixed linear model with fixed effects (diet, exercise, dose, fat) and random effects. The data analysis was done using the MIXED procedure in SAS V9 (SAS Institute Inc., Cary, NC). The animal was considered a random effect, and the GROUP option was used to model heterogeneous variances for different dose levels. In the analysis, we included all interaction terms in the model, and we noted that several of the pairwise interactions were significant. Consequently, we were not able to look only at the main effect of fat, because the effect of fat may differ depending on the level of the other factors. Therefore, we held each of the other factors constant and evaluated the effect of fat in each case by using least-squares means. For ACh, we evaluated each of the 2 × 2 × 6 = 24-factor combinations where diet, exercise, and dose were held fixed, and we asked whether there was a significant difference in the response with and without fat. Because BK and SNP had more levels, there were more factor combinations for them, but the data were analyzed as described above for ACh. In view of the large number of pairwise comparisons made in the dose–response curves, a significance level of 0.01 was used rather than the usual 0.05.
Differences between groups with respect to blood lipid data, heart weight, body weight, citrate synthase activity, treadmill performance data, ring characteristics, maximal responses, and half-maximal effective dose were determined via unpaired t-test. All data were analyzed in SuperANOVA.
RESULTS
Experimental animals
Plasma lipids were measured as described previously and were found to be similar to plasma lipids reported previously for Yucatan pigs (15). The HF diet induced elevated plasma cholesterol: NF-SED = 80 ± 5 mg·dL−1, NF-EX = 86 ± 3 mg·dL−1, HF-SED = 387 ± 28 mg·dL−1, HF-EX = 342 ± 59 mg·dL−1; but not triglyceride: NF-SED = 77 ± 3 mg·dL−1, NF-EX = 68 ± 3 mg·dL−1, HF-SED = 65 ± 2 mg·dL−1, HF-EX = 67 ± 3 mg·dL−1. Thus, the diet produced similar changes in plasma lipids as reported previously, and exercise training did not alter plasma lipids in NF or HF diet pigs (15).
There were no between-group differences in body weights. Also, average heart weight:body weight ratios were greater in both groups of trained pigs (NF-SED = 4.3 ± 0.2 g·kg−1, NF-EX = 5.1 ± 0.3 g·kg−1, HF-SED = 4.0 ± 0.2 g·kg−1, and HF-EX = 5.1 ± 0.1 g·kg−1). Further evidence of the effectiveness of the exercise training program is provided in that citrate synthase activity was increased in the muscles of both trained groups (i.e., the long head of the triceps brachii muscle: NF-SED = 16.6 ± 2.1 μmol·min−1·g−1, N-EX = 31.5 ± 3.5 μmol·min−1·g−1, HF-SED = 13.3 ± 1.8 μmol·min−1·g−1, and HF-EX = 21.5 ± 1.0 μmol·min−1·g−1). In the long head of the triceps brachii muscle, HF groups had significantly lower citrate synthase activity than NF (P = 0.01). However, there was no effect of diet on citrate synthase activity of the other muscles sampled (accessory, medical, lateral heads of the triceps and the deltoid muscle). Also consistent with the conclusion that the training program was effective is that the duration of running in the performance test was significantly increased in both training groups (NF-SED = 27.8 ± 2.4 min, NF-EX = 38.0 ± 0.5 min, HF-SED = 24.1 ± 2.3 min, and HF-EX = 34.1 ± 1.0 min). The duration of running in the performance test was significantly less in the HF-EX pigs than in the NF-EX pigs.
Coronary perivascular adipose tissue
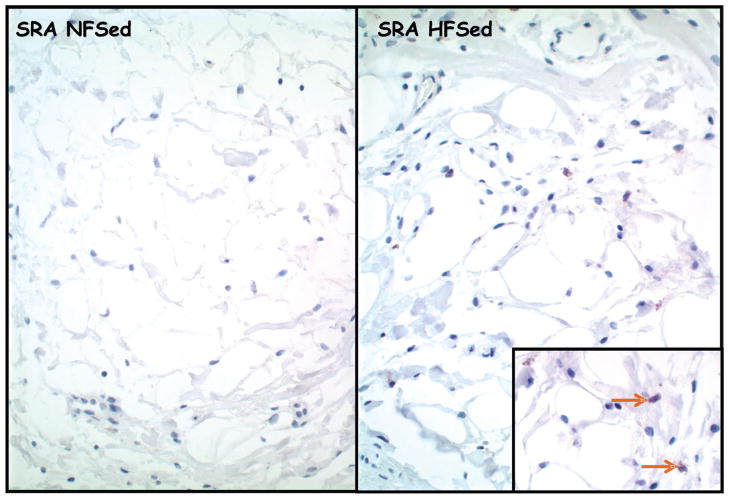
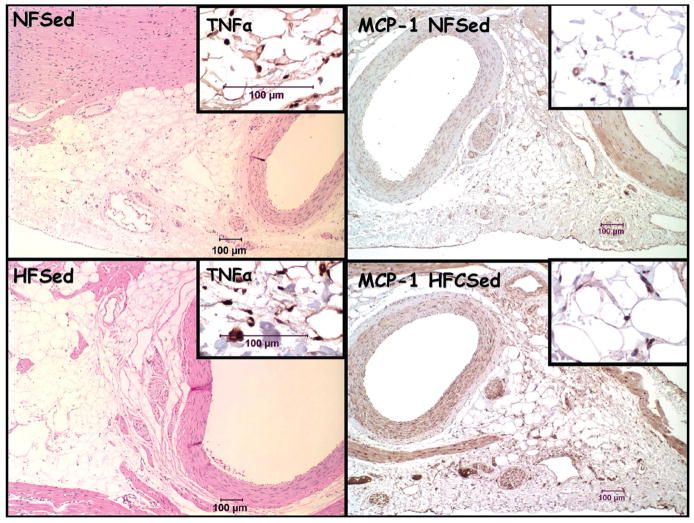
Histopathologic findings for coronary perivascular adipose tissue revealed that SRA-immunoreactive macrophages were numerous in HF-SED but not in coronary perivascular adipose tissue of other groups (Fig. 1). Immunoreactivity also documented TNFα (Fig. 2), IL-6, and MCP-1 in coronary perivascular adipose tissue. However, immunoreactivity for these markers was highly variable among the four groups.
Figure 1.
Representative photomicrographs of epicardial adipose tissue stained immunohistochemically for scavenger receptor-A (SRA). Immunoreactivity for macrophages in HFSed (right); the inset shows higher magnification of immunoreactive macrophages (brown arrows).
Figure 2.
Representative photomicrographs of epicardial adipose tissue stained with hematoxylin and eosin (top and bottom left). Inset: Immunohistochemistry for tumor necrosis factor-alpha (TNFα). Representative photomicrographs of epicardial adipose tissue stained immunohistochemically for monocyte chemoattractant protein-1 (MCP-1; top and bottom right). Inset: Higher magnification of immunoreactivity for MCP-1.
During preparation of the coronary artery rings for vasomotor responsiveness measurements, we estimated the amount of perivascular adipose tissue on each ring. The results reveal that there were no statistically significant differences among the four groups (NF-SED = 14.4 ± 3.5, NF-EX = 9.4 ± 3.6, HF-SED = 12.8 ± 3.1, and HF-EX = 14.9 ± 1.6; mm2). Also, there were no statistically significant differences among the measured wall thickness, artery area, % stretch at Lmax, length, outside diameter, or inside diameter of the artery segments isolated from the four groups of pigs used in these experiments. Thus, neither diet nor exercise significantly altered the structural characteristics of the circumflex branches of the coronary arteries, and the size of arteries isolated was similar among groups.
In vitro vasocontraction responses: NF diet
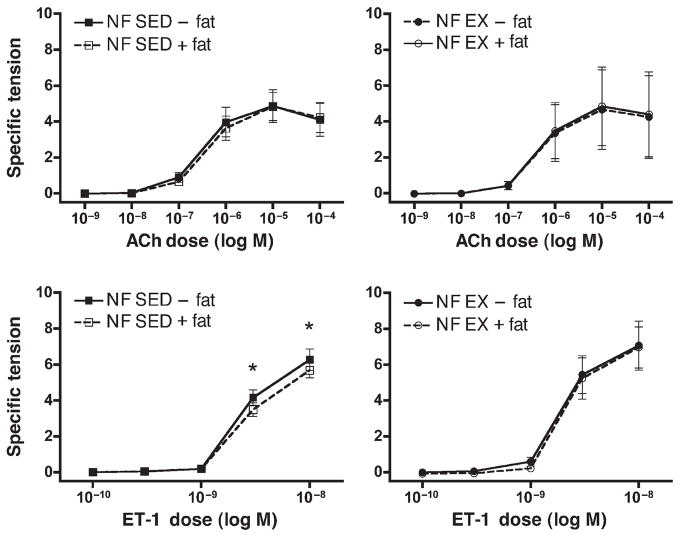
There were no differences among the outer or inner diameter or wall thicknesses of the left circumflex coronary artery segments used for these experiments (Table 1). However, the axial length of the segments with perivascular fat removed tended to be shorter than those segments with fat intact (Table 2). Resting tensions before vasoconstrictor experiments were not different among groups (Table 2). Both ACh and ET-1 elicited dose-dependent increases in tension from LCX rings from NF pigs (Fig. 3). Removal of perivascular fat from the LCX had no statistically significant effect on ACh-induced contractions, contrary to our hypothesis. In contrast, removal of perivascular fat resulted in increased contractile force produced in response to ET-1 in the NF-SED group at the two highest doses (Fig. 3B). This effect of perivascular fat is consistent with our hypothesis that predicted an increase in contractile force with fat removal. There was no significant difference between responses of SED and EX-LCX to ACh or ET-1. Exercise training did modify the effect of perivascular fat in that removal of perivascular fat had no effect on ET-1–induced contraction in LCX from exercise-trained NF pigs, whereas it did in SED.
TABLE 1.
Left circumflex coronary artery diameter and wall thickness.
| NF-SED | NF-EX | HF-SED | HF-EX | |
|---|---|---|---|---|
| (N = 9) | (N = 5) | (N = 5) | (N = 7) | |
| Outer diameter (mm) | 2.31 ± 0.11 | 2.08 ± 0.13 | 2.29 ± 0.13 | 2.47 ± 0.10 |
| Inner diameter (mm) | 1.51 ± 0.08 | 1.37 ± 0.11 | 1.43 ± 0.08 | 1.51 ± 0.08 |
| Wall thickness (mm) | 0.40 ± 0.02 | 0.36 ± 0.06 | 0.43 ± 0.03 | 0.49 ± 0.05 |
Means ± SEM are presented. NF, normal-fat diet; HF, high-fat diet; SED, sedentary; EX, exercise trained; N, number of pigs. There are no statistically significant differences among groups for these variables.
TABLE 2.
Ring characteristics and tensions at start of dose–response curves.
| NF-SED | NF-EX | HF-SED | HF-EX | |
|---|---|---|---|---|
| −fat | −fat | −fat | −fat | |
| (N = 9) | (N = 5) | (N = 5) | (N = 7) | |
| Axial length (mm) | 3.70 ± 0.22* | 3.68 ± 0.26 | 3.61 ± 0.107* | 3.47 ± 0.11* |
| Percent stretch to Lmax (%) | 165.00 ± 4.56 | 166.00 ± 2.45 | 162.00 ± 2.00 | 157.86 ± 4.06 |
| Resting tension pre ACh (ST) | 2.87 ± 0.23 | 2.63 ± 0.43 | 3.00 ± 0.37 | 2.71 ± 0.32 |
| Resting tension pre ET-1 (ST) | 3.16 ± 0.29 | 2.76 ± 0.56 | 2.74 ± 0.27 | 2.60 ± 0.25 |
| BK precontraction tension (ST) | 6.90 ± 0.76 | 6.40 ± 1.42 | 4.65 ± 1.06# | 5.33 ± 0.87# |
| SNP precontraction tension (ST) | 7.70 ± 0.65 | 6.23 ± 1.46 | 4.38 ± 1.06# | 5.44 ± 0.94# |
| NF-SED | NF-EX | HF-SED | HF-EX | |
|
|
||||
| +fat | +fat | +fat | +fat | |
|
|
||||
| (N = 9) | (N = 5) | (N = 5) | (N = 7) | |
|
| ||||
| Axial length (mm) | 4.09 ± 0.18 | 3.93 ± 0.34 | 4.05 ± 0.15 | 4.10 ± 0.15 |
| Percent stretch to Lmax (%) | 166.11 ± 4.47 | 164.00 ± 4.00 | 160.00 ± 0.00 | 151.43 ± 1.43 |
| Resting tension pre ACh (ST) | 3.37 ± 0.32 | 3.04 ± 0.73 | 3.04 ± 0.25 | 2.77 ± 0.46 |
| Resting tension pre ET-1 (ST) | 3.80 ± 0.37 | 3.29 ± 0.94 | 3.07 ± 0.32 | 2.78 ± 0.41 |
| BK precontraction tension (ST) | 6.92 ± 0.52 | 7.50 ± 1.31 | 4.55 ± 0.64# | 5.31 ± 0.36# |
| SNP precontraction tension (ST) | 6.931 ± 0.49 | 6.87 ± 1.19 | 4.51 ± 0.67# | 5.43 ± 0.40# |
Means ± SEM are presented. NF, normal-fat diet; HF, high-fat diet; SED, sedentary; EX, exercise trained; N, number of pigs; −fat, perivascular fat removed; +fat, with perivascular fat intact; Lmax, percent stretch of the artery ring that resulted in the greatest KCl-induced active tension; ST, specific tension in units of grams of tension per square millimeter of artery wall. Resting tension is the amount of tension present before the addition of vasoconstrictor agents (ACh, acetylcholine; ET-1, endothelin-1). Precontraction tension is the amount of tension (induced by 10−8 M ET-1) present before the addition of vasodilator agents (BK, bradykinin; SNP, sodium nitroprusside).
Indicates that the −fat value is significantly different from the +fat value for this variable;
indicates that the HF value is significantly less than the NF value for this variable.
Figure 3.
Acetylcholine (ACh)- and endothelin-1 (ET-1)–induced contractile responses of circumflex coronary arteries from sedentary (SED) and exercise-trained (EX) normal-fat diet (NF) pigs. Values represent means ± SE in grams of tension per square millimeter of artery wall. N = 5–9 pigs per group, as outlined in the Methods section. Top: Dose/response curve for ACh. Normal-fat (NF), sedentary (SED), and exercised (EX) pigs, with perivascular fat left intact (+) or removed (−). Bottom: Dose/response curve for ET-1. * Indicates a statistically significant difference between perivascular fat intact and removed.
In vitro vasocontraction responses: HF diet
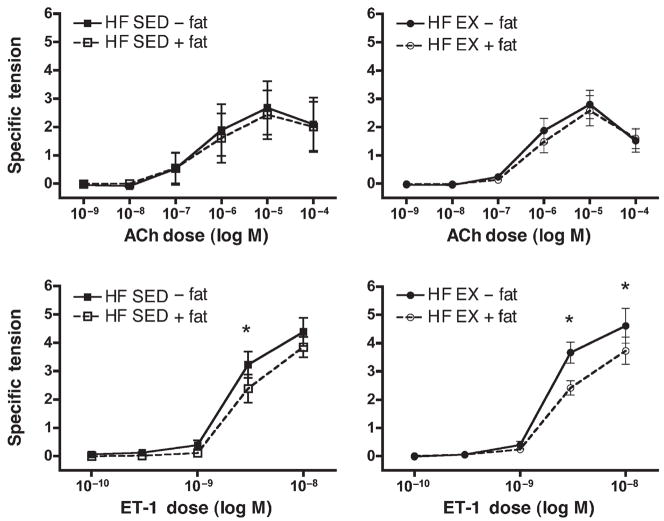
Both ACh and ET-1 elicited dose-dependent increases in tension from LCX rings from HF diet pigs, and these increases were similar to responses in arteries from NF pigs (Fig. 4). There was no statistically significant difference between the ACh dose–response curve of LCX from NF and HF diet pigs. In contrast, LCX from HF animals developed statistically significantly less specific tension than did arteries from NF animals. Also, there were no differences among groups for specific tension developed in response to 80 mM KCl (data not shown). As shown in Table 2, the ET-1–induced specific tension in arteries from NF pigs, which was greater than that found in arteries from HF pigs, was apparent in the ET-1–induced contractile tension measured before the BK and SNP vasorelaxation experiments.
Figure 4.
Acetylcholine (ACh)- and endothelin-1 (ET-1)–induced contractile responses of circumflex coronary arteries from sedentary (SED) and exercise-trained (EX) high-fat diet (HF) pigs. Values represent means ± SE in grams of tension per square millimeter of artery wall. N = 5–9 pigs per group, as outlined in the Methods section. Top: Dose/response curve for ACh. High-fat (HF), sedentary (SED), and exercised (EX) pigs, with perivascular fat left intact (+) or removed (−). Bottom: Dose/response curve for ET-1. * Indicates a statistically significant difference between perivascular fat intact and removed.
Removal of perivascular fat from LCX of HF animals had no statistically significant effect on ACh-induced contractions (Fig. 4). In LCX from HF-SED and HF-EX animals, removal of perivascular fat resulted in increased contractile force produced in response to ET-1 at higher doses. This effect of perivascular fat is consistent with our hypothesis that predicted an increase in contractile force with fat removal. Exercise training did not alter responses of LCX from HF diet pigs to ACh or ET-1. Thus, statistical analysis indicates that the removal of perivascular adipose tissue resulted in increased ET-1–induced contraction in LCX from sedentary and exercise-trained HF diet pigs.
In vitro vasorelaxation responses
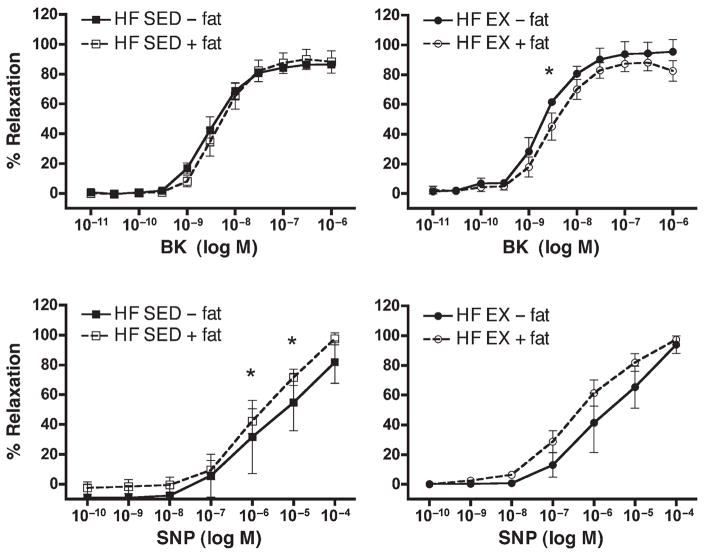
BK was used to assess endothelium-dependent relaxation. As expected, BK induced a concentration-dependent increase in relaxation in LCX rings from all groups (Figs. 5 and 6). Data in Figure 6 demonstrate that arteries from HF-EX pigs tended to exhibit greater BK-induced relaxation than HF-SED arteries, similar to the effects of exercise on coronary responses in male pigs on this diet as reported by Thompson et al. (16); however, differences between HF-EX and HF-SED were not statistically significant in the present study.
Figure 5.
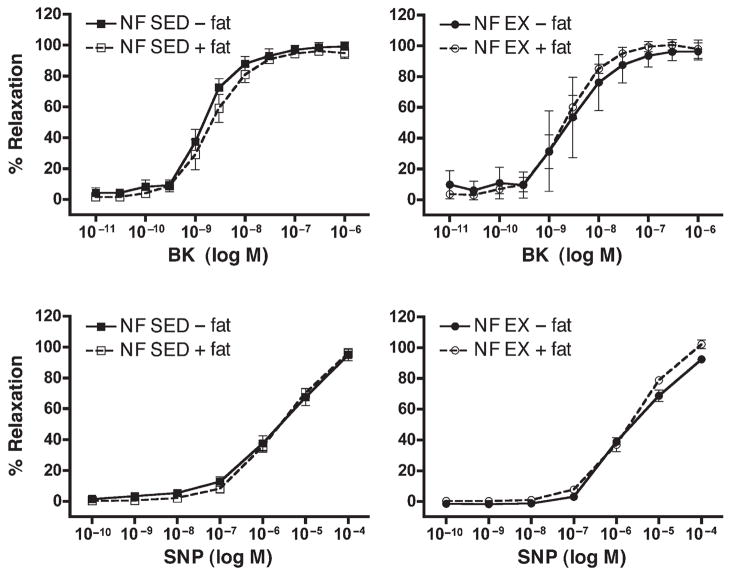
Bradykinin (BK)- and sodium nitroprusside (SNP)–induced relaxation responses of circumflex coronary arteries from sedentary (SED) and exercise-trained (EX) normal-fat diet (NF) pigs. Values represent means ± SE in % relaxation. Top: Dose/response curve for BK, N = 3–5 pigs per group. Normal-fat (NF), sedentary (SED), and exercised (EX) pigs, with perivascular fat left intact (+) or removed (−). Bottom: Dose/response curve for SNP, N = 5–7 pigs per group. Normal-fat (NF), sedentary (SED), and exercised (EX) pigs, with perivascular fat left intact (+) or removed (−). There were no between-group differences in responses among these groups.
Figure 6.
Bradykinin (BK)- and sodium nitroprusside (SNP)–induced relaxation responses of circumflex coronary arteries from sedentary (SED) and exercise-trained (EX) high-fat diet (HF) pigs. Values represent means ± SE in % relaxation. Top: Dose/response curve for BK, N = 3–5 pigs per group. High-fat (HF), sedentary (SED), and exercised (EX) pigs, with perivascular fat left intact (+) or removed (−). Bottom: Dose/response curve for SNP, N = 5–7 pigs per group. High-fat (HF), sedentary (SED), and exercised (EX) pigs, with perivascular fat left intact (+) or removed (−). * Indicates a statistically significant difference between perivascular fat intact and removed.
Removal of perivascular fat did not alter responses to BK in LCX from NF-SED, NF-EX, or HF-SED pigs (Figs. 5 and 6). However, in HF-EX pigs, removal of fat caused increased relaxation (statistically significant at only one dose) relative to LCX with fat (Fig. 6).
Sodium nitroprusside was used to assess endothelium-independent relaxation. SNP induced a concentration-dependent relaxation in LCX rings isolated from all groups of pigs. Direct smooth-muscle relaxation was similar in both NF groups (Fig. 5). In arteries from HF-SED and HF-EX pigs, we noted that removal of perivascular fat resulted in decreased relaxation at higher does (Fig. 6). This effect was not statistically significant in LCX from HF-EX pigs.
DISCUSSION
The purpose of this study was to test the hypothesis that coronary artery perivascular adipose tissue has an anticontractile effect comparable with that reported for rat aorta and visceral arteries (3,11,18). We further proposed that coronary perivascular adipose tissue of pigs fed an HF diet would exhibit less of a blunting effect on contraction (i.e., exhibit greater agonist-induced constriction) than would pigs on an NF diet, and that physically active pigs would exhibit increased anticontractile and/or enhanced dilator responses in coronary arteries from NF diet pigs as well as in pigs on an HF diet. The results indicate that porcine coronary perivascular adipose tissue exhibits blunting of vasoconstrictor responses to ET-1 similar to those reported previously in rat aorta and visceral arteries (Figs. 3 and 4) (3,11,18). Interestingly, porcine coronary perivascular adipose tissues did not seem to alter ACh-induced vasoconstrictor responses (Figs. 3 and 4). We chose to use ET-1 in these studies because the previous reports used this agent. We chose to examine responses to ACh because we know that ACh is a potent vasoconstrictor in porcine coronary arteries, and we routinely examine responses to 80 mM KCl as a standard part of characterizing vasoconstrictor properties of arteries in these types of experiments. Of further interest relative to the effects of perivascular fat, the present results indicate that the HF diet did not seem to alter the effects of coronary perivascular adipose tissue on contractions induced by ET-1. Sustained exercise training for 16 wk seemed to abolish the effect of perivascular adipose on ET-1 contraction in NF pigs, but it had no effect on the anticontractile effects of perivascular adipose tissue on ET-1 contractions in coronary arteries from HF diet–fed pigs.
We are surprised that our results indicate that perivascular adipose tissue of porcine coronary arteries has little to no effect on ACh-induced contraction, whereas coronary perivascular adipose tissue had anticontractile effects on ET-1–induced contractions similar to those reported by Lohn et al. (11) and Verlohren et al. (18) in rat aortic and rat mesenteric artery rings (11,18). Lohn et al. (11) have demonstrated that rat aortic rings with intact perivascular fat exhibited a blunted constriction in response to angiotensen II, serotonin, and phenylephrine compared with rings with the adipose removed. Verlohren et al. (18) found similar effects in rat mesenteric arteries in that arteries with perivascular fat left intact exhibited a blunted constriction in response to serotonin, ET-1, and phenylephrine. Of interest, Verlohren et al. (18) report that the anticontractile effect depended on the amount of fat left on the ring preparation. It is interesting that perivascular adipose has different effects on contractions induced by ACh and ET-1. Our results do not allow us to explain this differential response.
Our ET-1 results suggest that there are no major species differences (rats versus pigs) in the effects of perivascular adipose tissue on artery vasomotor function (11,18). We anticipated that our experiments might provide evidence of different results related to regional differences in the biochemical make-up/function of perivascular adipose tissue of different arteries (i.e., difference between coronary perivascular adipose tissue and perivascular adipose tissue of abdominal arteries) because both epidemiological and molecular characterizations of adipose tissue support the notion that regional fat depots have different phenotypes and functional effects. For example, epidemiological data suggest that central/visceral/intraabdominal adiposity is more predictive of negative health outcomes, such as coronary heart disease and diabetes, than total and subcutaneous adipose tissue (2,5,19)—so much so that increases in intraabdominal adipose tissue are now recognized as a component of the “metabolic syndrome” (4).
Regional differences in adipose tissue also exist at the molecular level. In patients with coronary artery disease, epicardial adipose tissue near the proximal right coronary artery was found to have higher expression of chemokine (monocyte chemotactic protein (MCP)-1) and inflammatory cytokines (IL-6, IL-1β, and TNF) compared with leg subcutaneous adipose (12). Our results indicate that porcine perivascular adipose tissue also expresses these inflammatory markers (Figs. 1 and 2). And, the results suggest that there may be more inflammation in perivascular fat of HF diet pigs (Fig. 1).
We expected to find that the perivascular adipose tissue in the pigs on the HF diet would have deleterious effects (i.e., greater constrictor responses) on vasomotor function of the coronary arteries. In contrast, the present results indicate that circumflex arteries from pigs on the HF diet developed less maximal tension in response to ACh and ET-1 than did arteries from the NF diet pigs. Somewhat consistent with present results indicating that the HF diet does not increase vasoconstrictor responses, Thompson et al. (16) report no effect of this HF diet on ACh-induced contractions of porcine coronary arteries. But Thompson et al. (18) also report that this HF diet increased contractile tension of left anterior descending arteries in response to KCl, serotonin (5-HT), and aggregating platelets. In the present study, we did not examine responses to 5-HT or aggregating platelets, but we did observe that responses to KCl were not different in coronary arteries from HF and NF pigs. Thus, in the present study, the HF diet did not result in increased vasoconstrictor responses. It is not clear at this time why the previous study saw increased vasocontraction responses in arteries from HF-fed pigs, whereas we did not see similar results in this study. Notwithstanding the lack of effect of the HF diet on contractile function, it is important to emphasize that the present results demonstrate that perivascular fat from HF-fed pigs exhibited a blunting effect on ET-1 contractions. Of equal importance to the purpose of this study, the anticontractile effect of perivascular fat was not significantly altered by exercise training.
The present results indicate that coronary perivascular adipose tissue has no effect on BK-induced, endothelium-dependent vasorelaxation in NF-SED, NF-EX, and HF-SED pigs; removal of vascular fat increased BK-induced relaxation in HF-EX pigs. Histopathology results indicate that some of the expected changes in inflammatory cytokines were present in the perivascular adipose tissue of the HF-fed pigs, but at this early stage of disease the changes are modest. This may also be related to the fact that obesity is not a feature of this model of coronary disease (17). It is important to report that there is immunoreactivity for SRA+ macrophages, TNFα, and MCP-1 in perivascular adipose tissue of coronary arteries from the Yucatan HF-SED model of early disease. Future work will be required to determine whether perivascular adipose tissue in advanced coronary disease would exhibit greater signs of inflammation than observed at this stage of disease (17) and whether, as a result of inflammation, the effects of perivascular adipose on vasomotor function are altered in more advanced coronary disease.
Finally, the effects of perivascular adipose tissue on vasorelaxation responses of coronary arteries from the HF pigs are interesting. As shown in Figure 5, removal of perivascular adipose tissue did not alter SNP-induced relaxation in NF pigs. In contrast, in HF pigs removal of the perivascular adipose tissue seemed to decrease SNP-induced relaxation in both HF-SED and HF-EX (this was only statistically significant in the HF-SED pigs; Fig. 6B). These results are consistent with the hypothesis that perivascular adipose tissue produces a vasorelaxing effect, perhaps through release of ADRF. If this interpretation is correct, it is not clear why this effect is not apparent in the responses of the HF arteries to BK. Whatever the explanation for this phenomenon, it is interesting that this effect of perivascular adipose tissue is greater in pigs on the atherogenic diet. This is contrary to our second hypothesis. Also, exercise training did not alter the effects of perivascular fat on coronary reactivity in the HF animals, which is contrary to our third hypothesis.
In summary, the results of this study indicate that porcine coronary perivascular adipose tissue blunts ET-1–induced vasocontraction responses of coronary arteries, but not ACh-induced contraction of these arteries. This effect of perivascular adipose tissue on ET-1 coronary vasomotor responsiveness was not influenced by diet. Exercise training seemed to abolish the effect of perivascular adipose tissue on ET-1–induced vasocontraction in pigs on the NF diet, but not in the pigs on the HF diet. Perivascular fat seemed to enhance direct, smooth-muscle responses to SNP in pigs on the HF diet, but not NF. Finally, perivascular adipose tissue seemed to blunt BK-induced relaxation only in LCX from HF-EX pigs, but not in NF pigs. Future studies should attempt to characterize regional differences in the biochemical make-up of perivascular adipose tissue to determine whether visceral perivascular adipose (aortic and mesenteric) tissue has effects on vasomotor reactivity that differ from those in coronary arteries. In addition, it is important in future work to determine whether coronary perivascular adipose tissue influences coronary vasoreactivity in obese subjects and/or in arteries with advanced coronary artery disease in contrast to the HF diet model used in this study, which results in very-early-stage disease (Stary stages 1–3).
Acknowledgments
We would like to thank Pam Thorne, Dave Harah, Ann Melloh, Robert Johnson, and Jennifer Casati for their technical assistance. The authors also acknowledge Dr. Richard Madsen for conducting statistical analysis of the data presented in this manuscript. This research was supported by National Institutes of Health Grants AR-048523, HL-36088, and HL-52490.
References
- 1.Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol. 2004;286:H1107–H1113. doi: 10.1152/ajpheart.00656.2003. [DOI] [PubMed] [Google Scholar]
- 2.Gao YJ, Takemori K, Su LY, et al. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci. 2004;25:647–653. doi: 10.1016/j.tips.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol. 1999;83:25F–29F. doi: 10.1016/s0002-9149(99)00211-8. [DOI] [PubMed] [Google Scholar]
- 5.Henrichot E, Juge-Aubry CE, Pernin A, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 6.Iacobellis G, Ribaudo MC, Assael F, et al. Epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 7.Laughlin MH, Cook J, Tremble R, Ingram D, Colleran PN, Turk JR. Exercise training produces nonuniform increases in arteriolar density of rat soleus and gastrocnemius muscle. Microcirculation. 2006;13:175–186. doi: 10.1080/10739680600556829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laughlin MH, Muller JM. Vasoconstrictor responses of coronary resistance arteries in exercise-trained pigs. J Appl Physiol. 1998;84:884–889. doi: 10.1152/jappl.1998.84.3.884. [DOI] [PubMed] [Google Scholar]
- 9.Laughlin MH, Overholser KA, Bhatte MJ. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol. 1989;67:1140–1149. doi: 10.1152/jappl.1989.67.3.1140. [DOI] [PubMed] [Google Scholar]
- 10.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol. 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- 11.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 12.Mazurek T, Zhang L, ZalewskI A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 13.Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation. 1994;89:2308–2314. doi: 10.1161/01.cir.89.5.2308. [DOI] [PubMed] [Google Scholar]
- 14.Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–5. [Google Scholar]
- 15.Thomas TR, Pellechia J, Rector RS, Sun GY, Sturek MS, Laughlin MH. Exercise training does not reduce hyperlipidemia in pigs fed a high-fat diet. Metabolism. 2002;51:1587–1595. doi: 10.1053/meta.2002.36313. [DOI] [PubMed] [Google Scholar]
- 16.Thompson MA, Henderson KK, Woodman CR, et al. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol. 2004;96:1114–1126. doi: 10.1152/japplphysiol.00768.2003. [DOI] [PubMed] [Google Scholar]
- 17.Turk JR, Henderson KK, Vanvickle GD, Watkins J, LAUGHLIN MH. Arterial endothelial function in a porcine model of early stage atherosclerotic vascular disease. Int J Exp Pathol. 2005;86:335–345. doi: 10.1111/j.0959-9673.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verlohren S, Dubrovska G, Tsang SY, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- 19.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]