Abstract
Purpose
To report ocular findings in eyes with radiation retinopathy and visual acuity (VA) results of photocoagulation for macular edema and proliferative retinopathy.
Methods
This retrospective case series compared VA after photocoagulation treatment and nontreatment of radiation macular edema. Visual outcomes were assessed with regression analyses.
Results
Eighty-seven eyes (78 [89.7%] treated with external beam irradiation and 9 treated with local plaques) were studied. Fifty-nine (67.8%) and 28 (32.2%) eyes had nonproliferative and proliferative retinopathy, respectively; macular edema developed in 42 nonproliferative eyes (71.2%) and 24 proliferative eyes (85.7%). Initial VAs (median) were 20/40 and 20/65 for nonproliferative and proliferative eyes, respectively, and final VA was 20/80 and 20/400. Initial VA (median) in eyes with macular edema was 20/50 compared to 20/25 in eyes without edema; final VAs were 20/200 and 20/30.
Comparing treated (19 [45.2%]) and untreated (23[54.8%]) macular edema in 42 eyes with nonproliferative retinopathy, initial median VA (20/40 and 20/50) and final VA (20/100 and 20/200) were better in treated eyes. Regression analysis showed significant treatment effect (P = .003) when initial VA (logMAR) and months of follow-up were kept constant; treated eyes had mean final VA (logMAR) 0.36 (95% CI, 0.12–0.60) better than untreated eyes.
Conclusions
The presence of macular edema and proliferation indicates more severe retinopathy and worse visual prognosis than for eyes without macular edema and proliferation. Although these VA results suggest macular photocoagulation is beneficial, eyes with macular edema continue to lose vision despite treatment. Better prevention and treatment methods are needed for radiation retinopathy.
INTRODUCTION
In 1933, Stallard1 first described radiation retinopathy in a patient previously treated with radon seeds for retinoblastoma. Since that first description, radiation retinopathy has become a more frequently diagnosed treatment complication because of the increased use of radiotherapy for a multitude of diseases, many of which are life-threatening tumors of the eye, sinuses, and brain.2–4 Less commonly, radiation retinopathy occurs following radiation treatment for diseases that are not life-threatening (eg, Graves ophthalmopathy) but can be vision-threatening.5
In general, 3000 cGy or less has been considered a relatively safe total dose of radiation; most cases of radiation retinopathy have occurred following higher doses.4 The number of treatment fractions is also important.6 Reports of radiation retinopathy occurring at lower doses, however, periodically appear in the medical literature.7 Recognized risk factors that may account for the occurrence and severity of radiation retinopathy, especially in cases following low dosages, include diabetes mellitus and chemotherapy.8,9 Systemic hypertension may also be a risk factor in some cases. Each of these risk factors can cause injury to vascular endothelial cells, the primary site of radiation injury. In fact, retinopathy mimicking radiation retinopathy, in the absence of previous radiotherapy, can occur in patients that receive chemotherapy prior to bone marrow transplantation.10 Thus, any insult to the endothelial cells of the capillaries and other small retinal vessels may increase the risk and progression of radiation retinopathy. The resultant microvascular complications in the retina following radiotherapy, and subsequent vision loss in many affected patients, can occur anywhere from months to several years following the radiation exposure.11
Panretinal photocoagulation (PRP) for treatment of proliferative radiation retinopathy has been shown to be beneficial anatomically by decreasing neovascularization and subsequent vitreous hemorrhages, and macular photocoagulation has been successful in decreasing macular edema; however, the visual acuity (VA) results of treatment have been disappointing. Kinyoun and associates12 reported that PRP decreases the vascular proliferation of proliferative radiation retinopathy but that the VA continues to decrease following treatment; the investigators compared VA results in 14 eyes with proliferative radiation retinopathy followed for 8 to 145 months versus 27 eyes with nonproliferative radiation retinopathy followed for 3 to 204 months. Hykin and associates13 studied 19 patients with photocoagulation-treated macular edema and compared VA results with 23 untreated matched control patients; at 6 months, VA was improved in a higher percentage of treated as compared to untreated eyes, but at 2 years there was not a significant difference in VA between the 2 groups, both of which had been treated with radioactive scleral plaques.
This report presents the ocular findings in eyes with radiation retinopathy, primarily following external beam radiation, and the follow-up VA results of treatment with macular photocoagulation for eyes with macular edema and PRP for eyes with proliferative radiation retinopathy.
METHODS
After obtaining institutional review board approval for human studies, I reviewed the medical, fundus photograph, and fluorescein angiography records of all patients with the diagnosis of radiation retinopathy at one major referral center over 3 decades (1976–2007). Fifty-six patients with 87 affected eyes were identified. The Snellen VAs are converted to logMAR VAs for statistical analyses, and the geometric mean, median, and range of VAs are reported as Snellen fractions in the VA tables.14 For Snellen VA less than 20/400, counting fingers (CF; 0.025), hand motions (HM; 0.0125), light perception (LP; 0.006), and no light perception (NLP; 0.0) were used. Chart abstraction included 31 items of information for each patient and eye, as available; for those patients treated with PRP, focal and/or limited scatter macular photocoagulation, vitrectomy, penetrating keratoplasty, or cataract surgery; up to 14 additional data queries were made for one or both eyes from the medical record.
The analyzed radiation dosages are the total cGy recorded for each treatment indication. Radiation dosage at the level of affected retinas and number of radiation treatment fractions were analyzed when available but are not reported because these data were missing for more than 25% of the eyes.
The affected eyes were divided into 2 major categories, depending on whether new vessels had developed on the optic nerve, retina (NVE), or iris at the time of radiation retinopathy diagnosis or during follow-up. Eyes that had developed these new vessels at radiation retinopathy diagnosis or during follow-up were designated as proliferative radiation retinopathy, and eyes that had not developed these new vessels were designated as nonproliferative radiation retinopathy. Nonproliferative radiation retinopathy consisted of one or more retinal hemorrhages, microaneurysms, exudates, and/or nerve fiber layer infarcts.
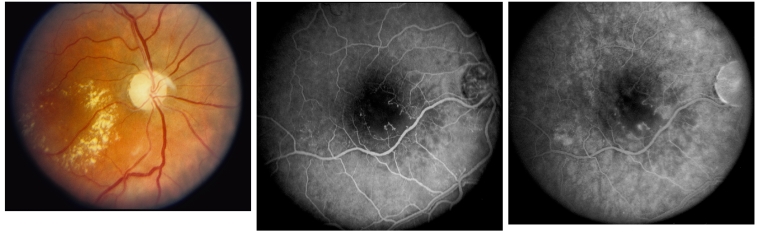
The eyes were further subdivided into eyes with and without macular edema. A diagnosis of macular edema was made if one or more of the following 3 criteria were met: retinal thickening at or within 500 μm of the center of the macula; hard exudates at or within 500 μm of the center of the macula if associated with adjacent retinal thickening; and a disc area of retinal thickening, any part of which was within 1 disc diameter (DD) of the center of the macula. Macular ischemia was judged to be present if there was capillary nonperfusion or retinal nerve fiber layer infarct within 2 DD of the center of the macula (Figure 1). Radiation optic neuropathy was diagnosed whenever there was optic disc edema or pallor.
FIGURE 1.
Left, Untreated clinically significant radiation macular edema. Fundus photograph of the right eye of a 42-year-old man 3 years following 7000 cGy of radiation for treatment of ethmoid sinus carcinoma. Visual acuity is 20/400. Exudates and retinal thickening are present in the center of the macula, and the optic disc is pale. Middle, Early venous phase of fluorescein angiography. Right eye angiogram shows capillary nonperfusion and irregular as well as enlarged capillary free zone. Right, Late-phase fluorescein angiography. Right eye angiogram shows leakage of fluorescein dye from the abnormal perifoveal capillaries and microaneurysms.
The photocoagulation treatment techniques were PRP for eyes with proliferative radiation retinopathy and macular photocoagulation for eyes with macular edema. Panretinal photocoagulation consisted of 500-μm burns scattered throughout the retinal periphery from the major vascular arcades (2 DD anterior to the center of the macula) to the equator and as far as the ora serrata when possible. Total number of burns ranged between 600 and 1200, and they were spaced 1 to 2 burn diameters apart and were not closer than 500 μm from the nasal disc border. Patches of new vessels on the retina but further than 1 DD from the optic nerve (NVE) were treated confluently with 500-μm burns; if the size of the NVE exceeded 2 disc areas, burns were scattered through the neovascularization rather than placed confluently. The burns were most commonly applied over 2 treatment sessions, but some eyes had more than 2 sessions.
The macular photocoagulation technique utilized 50- to 100-μm burns to focally treat leaking microaneurysms or limited scatter (1 to 2 burn diameters apart) throughout areas of capillary nonperfusion associated with the macular edema and within 2 DD of the center of the macula but not closer than 500 μm (Figure 2). If VA was less than 20/40, 50- to 75-μm burns were used to treat leaking microaneurysms as close as 300 μm from the center of the macula. Some eyes had more than one treatment session if the macular edema persisted or recurred.
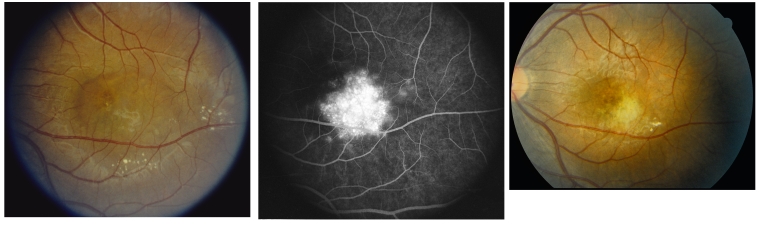
FIGURE 2.
Left, Clinically significant radiation macular edema. Fundus photograph of the left eye of a 16-year-old boy 6 years following 5400 cGy of radiation for treatment of a medulloblastoma. Vision is 20/100. The center and temporal macula are edematous with exudates along the temporal rim of the edema. Middle, Late-phase fluorescein angiography. There is leakage of fluorescein dye in the inferotemporal macula. Right, Resolved clinically significant radiation macular edema. Fundus photograph shows macular photocoagulation scars at the site of prior leakage, and the macula is not edematous 3 years following treatment. Vision is 20/70. A small clump of exudates remains in the inferotemporal macula.
Statistical analyses were performed and included summary statistics for continuous variables (eg, VA) and binary variables (eg, proliferative radiation retinopathy vs nonproliferative radiation retinopathy, macular edema vs no macular edema, and presence of confounders such as diabetes mellitus). The 87 eyes are treated independently in the statistical analyses. Two-sample t test was used to compare the means of VA and follow-up between eyes exposed to a radiation dose of less than 3000 cGy and eyes exposed to 3000 or more cGy.
To test for the effect of macular photocoagulation treatment of macular edema, regression analyses were performed with final VA (logMAR) as primary outcome. Since eyes with proliferative radiation retinopathy were frequently treated with both PRP and macular photocoagulation, only nonproliferative radiation retinopathy eyes with macular edema were included. One eye with VA less than 20/400 (0.05) was excluded from the analysis because the VA was obtained by extrapolation (eg, CF [0.025]) instead of actual measurement. The regression analyses are adjusted for initial VA (logMAR), months of follow-up since the initial diagnosis of radiation retinopathy, and confounders (eg, systemic hypertension, optic neuropathy, macular ischemia).
Model checking was performed. Residual plot was used to check curvature and heteroscedasticity; Q-Q plot was used for checking normality.15
RESULTS
Eighty-seven eyes of 56 patients with prior radiotherapy had findings of radiation retinopathy; 31 patients (55.4%) were affected bilaterally. The indications for radiotherapy included: sino-orbito-pharyngeal cancer in 16 patients (24 eyes), primary or metastatic eye tumor in 13 patients (13 eyes), primary or metastatic brain tumor in 12 patients (22 eyes), Graves ophthalmopathy in 9 patients (18 eyes), optic nerve tumor in 4 patients (6 eyes), and total body radiation for blood cancer in 2 patients (4 eyes) (Table 1). Possible associated risk factors for development of radiation retinopathy in these 87 eyes included chemotherapy in 40 (46%), systemic hypertension in 30 (34.5%), and diabetes mellitus in 10 (11.5%). The radiotherapy technique was external beam for 78 eyes (89.7%) and local plaque treatment for 9 eyes.
TABLE 1.
INDICATIONS FOR RADIOTHERAPY IN PATIENTS WITH RADIATION RETINOPATHY
| INDICATION | PATIENTS N (%) | EYES N (%) |
|---|---|---|
| Sino-orbito-pharyngeal cancer | 16 (28.6) | 24 (27.6) |
| Eye tumor (primary or metastatic) | 13 (23.2) | 13 (14.9) |
| Brain tumor (primary or metastatic) | 12 (21.4) | 22 (25.3) |
| Graves ophthalmopathy | 9 (16.1) | 18 (20.7) |
| Optic nerve tumor | 4 (7.1) | 6 (6.9) |
| Blood cancer (total body radiation) | 2 (3.6) | 4 (4.6) |
| Total | 56 (100) | 87 (100) |
The total radiation dose was available for 79 eyes; median total dose of radiation for each affected eye was 4000 cGy. The median time from radiation treatment to diagnosis of radiation retinopathy was 28 months, and the median follow-up after diagnosis of radiation retinopathy was 38 months (Table 2).
TABLE 2.
RADIATION DOSAGE RECEIVED AND FOLLOW-UP TIMES IN PATIENTS WITH RADIATION RETINOPATHY (RR)
| VARIABLE | EYES N (%) | AVERAGE* | MEDIAN | RANGE |
|---|---|---|---|---|
| Radiation dose (cGy†) | 79 (90.8) | 4776 | 4000 | 1440–11,314 |
| Radiotherapy to RR diagnosis (mo) | 87 (100) | 43 | 28 | 5–272 |
| Diagnosis of RR to final visit (mo) | 87 (100) | 76 | 38 | 0–351 |
Arithmetic mean.
cGy = rad (1 erg of ionizing radiation absorbed by 1 g of tissue).
At the time of radiation retinopathy diagnosis, the VA (median) of 21 eyes (26.6%) exposed to a total radiation dose of less than 3000 cGy was not significantly different than the VA of the 58 eyes (73.4%) exposed to 3000 or more cGy (20/50 and 20/50; P = .766; 95% confidence interval [CI] −0.22 to 0.14). At the final examination, the VA of the eyes exposed to less than 3000 cGy was also not significantly different than the VA of eyes exposed to 3000 or more cGy (20/200 and 20/178, respectively; P = .770; 95% CI −0.20 to 0.15). The follow-up (median months) was longer for the eyes exposed to less than 3000 cGy as compared to those exposed to 3000 or more cGy (120 and 30, respectively; P = .004, 95% CI 18.92 to 92.87).
There were 28 eyes that initially had or developed proliferative radiation retinopathy during follow-up and 59 eyes with nonproliferative radiation retinopathy. Sixty-six eyes initially had or developed macular edema; 42 (71.2%) were in eyes with nonproliferative radiation retinopathy and 24 (85.7%) were in eyes with proliferative radiation retinopathy. Other causes for vision loss that were present initially or developed during follow-up in these 87 eyes included macular ischemia (in 66 eyes), radiation optic neuropathy (48), cataract (45), vitreous hemorrhage (21), glaucoma (10), radiation keratopathy (9), and traction retinal detachment (4) (Table 3). Surgical procedures performed for treatment of complications causing vision loss in these 87 eyes included macular photocoagulation in 38 eyes (43.5%), PRP in 23 eyes (26.4%), cataract surgery in 24 eyes (27.6%), vitrectomy in 9 eyes, and penetrating keratoplasty in 1 eye.
TABLE 3.
FINDINGS IN 87 EYES WITH RADIATION RETINOPATHY
| FINDING | EYES N (%) |
|---|---|
| Nonproliferative radiation retinopathy | 59 (67.8) |
| Proliferative radiation retinopathy | 28 (32.2) |
| Clinically significant radiation macular edema | 66 (75.9) |
| Macular ischemia | 66 (75.9) |
| Radiation optic neuropathy | 48 (55.2) |
| Cataract | 45 (51.7) |
| Vitreous hemorrhage | 21 (24.1) |
| Glaucoma | 10 (11.5) |
| Radiation keratopathy | 9 (10.3) |
| Tractional retinal detachment | 4 (4.6) |
Preradiation VA was available for over half (46 [52.9%]) of the 87 study eyes with a median (geometric mean) VA of 20/20 (20/29) and range of 20/15 to CF. Visual acuity at the time of radiation retinopathy diagnosis was available for all 87 eyes with a median (geometric mean) VA of 20/50 (20/66) and range of 20/15 to HM. The final median (geometric mean) VA was 20/200 (20/156) with a range of 20/15 to NLP.
The median VA in the 28 eyes with proliferative radiation retinopathy was worse than in the 59 eyes with nonproliferative radiation retinopathy at the initial examination (20/65 vs 20/40) as well as the final examination (20/400 vs 20/80) (Table 4A). The median length of follow-up was longer for the eyes with proliferative radiation retinopathy than for the eyes with nonproliferative radiation retinopathy (109 vs 27 months, respectively) (Table 5).
TABLE 4A.
VISUAL ACUITIES IN EYES WITH PROLIFERATIVE AND NONPROLIFERATIVE RADIATION RETINOPATHY (RR)
| CATEGORY | EYES N (%) | VISUAL ACUITY
|
||
|---|---|---|---|---|
| MEAN* | MEDIAN | RANGE | ||
| Proliferative RR | 28 (32.2) | |||
| Initial VA | 20/155 | 20/65 | 20/20-HM | |
| Final VA | 20/440 | 20/400 | 20/25-NLP | |
| Nonproliferative RR | 59 (67.8) | |||
| Initial VA | 20/63 | 20/40 | 20/15–HM | |
| Final VA | 20/99 | 20/80 | 20/15–NLP | |
HM, hand motion; NLP, no light perception; VA, visual acuity.
Geometric mean converted to Snellen acuity.
TABLE 5.
LENGTH OF FOLLOW-UP IN EYES WITH RADIATION RETINOPATHY (RR)
| DIAGNOSIS | EYES N (%) | FOLLOW-UP (mos)
|
||
|---|---|---|---|---|
| AVERAGE* | MEDIAN | RANGE | ||
| RR | 87 (100) | 76 | 38 | 0–351 |
| PRR | 28 (32.2) | 113 | 109 | 5–291 |
| Treatment (PRP) | 23 (26.4) | 117 | 109 | 5–291 |
| No treatment (no PRP) | 5 (5.7) | 95 | 89 | 38–156 |
| NPRR | 59 (67.8) | 59 | 27 | 0–351 |
| CSRME | 66 (75.9) | 79 | 44 | 0–291 |
| Treatment (MP) | 38 (43.7) | 113 | 109 | 1–291 |
| No treatment (no MP) | 28 (32.2) | 34 | 7 | 0–139 |
| No CSRME | 21 (24.1) | 67 | 30 | 0–351 |
CSRME, clinically significant radiation macular edema; MP, macular photocoagulation; mos, months; NPRR, nonproliferative radiation retinopathy; PRP, panretinal photocoagulation; PRR, proliferative radiation retinopathy.
Arithmetic mean.
Initial VA (median) in the 66 eyes with macular edema was 20/50 compared to 20/25 for the 21 eyes without macular edema. At the final examination, the VAs (median) for eyes with macular edema vs eyes with no macular edema were 20/200 and 20/30, respectively (Table 4B). The median follow-up was 44 months for the eyes with macular edema and 30 months for the eyes without macular edema (Table 5).
TABLE 4B.
VISUAL ACUITIES IN EYES WITH RADIATION RETINOPATHY BY SUBCLASSIFICATION
| CATEGORY | EYES N (%) | VISUAL ACUITY
|
||
|---|---|---|---|---|
| MEAN* | MEDIAN | RANGE | ||
| CSRME | 66 (75.9) | |||
| Initial VA | 20/105 | 20/50 | 20/15-HM | |
| Final VA | 20/205 | 20/200 | 20/20-NLP | |
| No CSRME | ||||
| Initial VA | 21 (24.1) | 20/43 | 20/25 | 20/15–20/400 |
| Final VA | 20/64 | 20/30 | 20/15-NLP | |
CSRME, clinically significant radiation macular edema; HM, hand motion; NLP, no light perception; VA, visual acuity.
Geometric mean converted to Snellen acuity.
Thirty-eight of the eyes with macular edema were treated with macular photocoagulation. The initial and preoperative VAs (median) were 20/40 and 20/80 in these 38 eyes; the final median VA was 20/200. Twenty-eight eyes with macular edema that were not treated with macular photocoagulation had a median initial VA of 20/89 and final VA of 20/200 (Table 6). The median follow-up was 109 months for the treated eyes with macular edema and 7 months for the untreated eyes with macular edema (Table 5).
TABLE 6.
VISUAL ACUITIES IN EYES WITH CLINICALLY SIGNIFICANT RADIATION MACULAR EDEMA TREATED WITH MACULAR PHOTOCOAGULATION (MP) VS NO TREATMENT
| CATEGORY | EYES N (%) | VISUAL ACUITY
|
||
|---|---|---|---|---|
| MEAN* | MEDIAN | RANGE | ||
| Treatment (MP) | 38 (57.6) | |||
| Initial VA | 20/116 | 20/40 | 20/15-HM | |
| Preoperative VA | 20/150 | 20/80 | 20/30-CF | |
| Final VA | 20/185 | 20/200 | 20/20-NLP | |
| No treatment (no MP) | 28 (42.4) | |||
| Initial VA | 20/91 | 20/89 | 20/20-CF | |
| Final VA | 20/234 | 20/200 | 20/30-NLP | |
CF, counting fingers; HM, hand motion; NLP, no light perception; VA, visual acuity.
Geometric mean converted to Snellen acuity.
The 23 eyes with proliferative radiation retinopathy treated with PRP had initial and pretreatment median VAs of 20/60 and 20/200, respectively; final median VA was 20/400. The 5 eyes with proliferative radiation retinopathy that were not treated with PRP had initial and final median VAs of 20/200 and LP, respectively (Table 7). The median follow-up was 109 months for the treated proliferative radiation retinopathy eyes and 89 months for the untreated proliferative radiation retinopathy eyes (Table 5).
TABLE 7.
VISUAL ACUITIES IN EYES WITH PROLIFERATAIVE RADIATION RETINOPATHY TREATED WITH PANRETINAL PHOTOCOAGULATION (PRP) VS NO TREATMENT
| CATEGORY | EYES N (%) | VISUAL ACUITY
|
||
|---|---|---|---|---|
| MEAN* | MEDIAN | RANGE | ||
| Treatment (PRP) | 23 (82.1) | |||
| Initial VA | 20/152 | 20/60 | 20/20-HM | |
| Preoperative VA | 20/189 | 20/200 | 20/25-LP | |
| Final VA | 20/357 | 20/400 | 20/25-NLP | |
| No treatment (no PRP) | 5 (17.9) | |||
| Initial VA | 20/170 | 20/200 | 20/30–20/299 | |
| Final VA | 20/1371 | LP | 20/400-NLP | |
HM, hand motion; LP, light perception; NLP, no light perception; VA, visual acuity.
Geometric mean converted to Snellen acuity.
Of the 42 eyes with nonproliferative radiation retinopathy with macular edema, 19 were treated with macular photocoagulation and 23 were untreated. The median initial and final VAs of the treated eyes were 20/40 and 20/100, and the initial and final VAs for the untreated eyes were 20/50 and 20/200. Summary statistics for the 42 analyzed eyes are in Table 8. One untreated eye had less than 20/400 (0.05) VA at the initial diagnosis of radiation retinopathy and was excluded from the regression analysis.
TABLE 8.
INITIAL AND FINAL VISUAL ACUITIES AND MONTHS RR FOLLOW-UP FOR EYES WITH CSRME AND NPRR
| VARIABLE | N | MEAN* | AVERAGE† | SD | MIN | MEDIAN | MAX |
|---|---|---|---|---|---|---|---|
| Initial VA | 42 | 0.26 | — | 0.28 | 0.03 | 0.40 | 1.33 |
| Final VA | 42 | 0.15 | — | 0.27 | 0.01 | 0.11 | 1.00 |
| Months RR follow-up | 42 | — | 51.48 | 64.58 | 0 | 20.00 | 205.00 |
CSRME, clinically significant radiation macular edema; NPRR, nonproliferative radiation retinopathy; RR, radiation retinopathy; VA, visual acuity.
Geometric mean.
Arithmetic mean.
When using final VA (logMAR) as the primary outcome and adjusting for initial VA (logMAR) and months since diagnosis, regression analysis showed significant treatment effect (P = .003) (Table 9); treated eyes had mean final VA (logMAR) 0.36 (95% CI, 0.12 to 0.60) better than untreated eyes. The treated eyes had mean final VA 0.34 (95% CI, 0.07 to 0.60) better than untreated eyes (P = .013) when optic neuropathy, macular ischemia, diabetes mellitus, and systemic hypertension status were kept constant (Table 10).
TABLE 9.
REGRESSION ANALYSIS FOR EFFECT OF MP TREATMENT USING logMAR FINAL VA AS OUTCOME AND ADJUSTING FOR logMAR INITIAL VA AND MONTHS RR FOLLOW-UP
| VARIABLE | COEFFICIENT | SE | P VALUE | 95% CI |
|---|---|---|---|---|
| MP treatment | 0.36 | 0.11 | .003 | (0.12 to 0.60) |
| Initial VA | 0.62 | 0.16 | <.001 | (0.28 to 0.95) |
| Months RR follow-up | –0.0008 | 0.0007 | .443 | (–0.002 to 0.001) |
CI, confidence interval; MP, macular photocoagulation; RR, radiation retinopathy; SE, standard error; VA, visual acuity.
TABLE 10.
REGRESSION ANALYSIS FOR EFFECT OF MP TREATMENT USING logMAR FINAL VA AS OUTCOME AND ADJUSTING FOR logMAR INITIAL VA, MONTHS RR FOLLOW-UP, ON, MI, DM, AND SYSTEMIC HYPERTENSION
| VARIABLE | COEFFICIENT | SE | P VALUE | 95% CI |
|---|---|---|---|---|
| MP treatment | 0.34 | 0.12 | .013 | (0.07 to 0.60) |
| Initial VA | 0.69 | 0.14 | <.001 | (0.40 to 0.97) |
| Months RR follow-up | –0.001 | 0.001 | .243 | (–0.003 to 0.001) |
| ON | –0.10 | 0.15 | .503 | (–0.40 to 0.19) |
| MI | –0.44 | 0.22 | .845 | (–0.50 to 0.41) |
| DM | –0.29 | 0.53 | .594 | (–1.38 to 0.80) |
| Systemic hypertension | –0.28 | 0.17 | .112 | (–0.62 to 0.06) |
CI, confidence interval; DM, diabetes mellitus; MI, macular ischemia; MP, macular photocoagulation; ON, optic neuropathy; RR, radiation retinopathy; SE, standard error; VA, visual acuity.
Residual plot indicated neither curvature nor heteroscedasticity of the model. The Q-Q plot of the logMAR visual acuities showed no evidence for violating the normality assumption of the data. The coefficient of determination for the multivariate regression model, R2, is 0.63. The model checking procedure indicates that there is no violation of the linear model assumption, and a high R2 value indicates a good fit of the model to the data set.
DISCUSSION
Radiotherapy has been a great advance in the treatment of life- and sight-threatening diseases. Even when not curative, radiation treatment can prolong and improve quality of life, although not uniformly for all measured variables.16 When comparing mortality rates between 2 treatment methods for choroidal melanoma, the Collaborative Ocular Melanoma Treatment Trial showed that radiation treatment, following which the eye and vision can be maintained in most treated eyes, is equal (regarding death from metastases) to enucleation, following which the eye and vision are uniformly lost following treatment.17 Although radiotherapy is associated with risks of complications, patients usually are willing to accept the risks of radiotherapy because of its known benefits.
One of those risks is vision loss due to radiation retinopathy following treatment of ocular or periocular structures.18 Unlike cataract, a well-known risk of radiotherapy since the lens is more radiosensitive than the retina, radiation damage to the retina is not as easily nor as well treated as cataract.19-21 In addition, the radiation injury often is not limited to the retina but also involves the optic nerve, another ocular structure not presently amenable to replacement or other effective treatment following permanent damage.22,23 Patients frequently are not aware of the potentially serious complication of radiation retinopathy following exposure of the eye during radiation treatment of other vital structures; patients with blinding complications following radiotherapy for non-life-threatening diseases are often particularly surprised and devastated.
The VA results in this study are inconsistent with the known increased risk and severity of radiation retinopathy when total dosage of radiotherapy exceeds 3000 cGy4; there was not a significant difference in initial or final VA of eyes exposed to 3000 or more cGy when compared to those exposed to less than 3000 cGy. The association of higher radiotherapy doses with worse retinopathy and vision probably was not found in this study because the analyzed number of cGy dosages were for periocular structures rather than retinas. Only for the eye and optic nerve tumors (19 [22%] eyes) were the retinal dosages approximately the same as the analyzed dosages. The orbital dosages for treatment of Graves ophthalmopathy also approximate the retinal dosages; however, similar to at least one previous report,24 several of these patients likely were treated with a much higher dose, owing to probable errors in dosage calculations or techniques of radiation delivery, than the reported and analyzed 2000 to 2100 cGy.
Exposure to 2000 to 2100 cGy given over 10 fractions is generally safe and unlikely to result in sight-threatening complications of radiation retinopathy. Thus, total dosage is only one risk factor for radiation retinopathy. Others risk factors include errors in treatment technique and/or dosage calculations, number of fractions of radiotherapy, diabetes mellitus, and chemotherapy.25–27 The lowest dose of radiation in our patients was 1440 cGy total body radiation for acute lymphocytic leukemia prior to bone marrow transplantation; however, this patient also had previous chemotherapy as well as systemic hypertension. In addition, bone marrow transplant retinopathy can mimic radiation retinopathy, and it is not possible to distinguish the 2 retinopathies unless affected patients have a history of exposure to one (eg, radiation) and not the other (eg, bone marrow transplantation); the same predicament occurs with the coexistence of diabetic retinopathy and radiation retinopathy.8 Diseases and treatment complications that mimic radiation retinopathy may be additive, with resultant worse radiation retinopathy than would have been the case without the systemic disease (eg, diabetes mellitus) or treatment (eg, chemotherapy). In this study, however, regression analyses did not demonstrate a statistically significant effect on final VA for the commonly accepted radiation retinopathy risk factors (diabetes mellitus, chemotherapy [not reported], and systemic hypertension) and causes of vision loss in eyes with radiation retinopathy (eg, optic neuropathy and macular ischemia) for which treatment is not presently available (Table 10).
Multiple regression analyses in this study did show a statistically significant beneficial effect of macular photocoagulation treatment in 41 macular photocoagulation treated and untreated eyes with nonproliferative radiation retinopathy and macular edema. Although the design of this study differs from that of Hykin and associates,13 the results are similar following treatment in that VA is better in the treated eyes. The 2 studies differ, however, in that affected eyes were treated only once in Hykin and associates’ study, whereas retreatments were allowed in this study. The initial beneficial effect of macular photocoagulation treatment was no longer present after 2 years of follow-up in Hykin and associates’ study. Half of the 41 eyes analyzed in this study were followed for longer than 20 months, and the average follow-up was over 4 years. The regression analysis model also adjusted for many of the confounders so frequently present in eyes with radiation retinopathy. The results, however, are subject to the many problems associated with retrospective studies, such as selection bias regarding which eyes were and were not treated with macular photocoagulation and nonstandardized VA testing.28 Unless or until a prospective randomized trial is conducted, comparisons between treated and untreated groups in a nonrandomized retrospective study of eyes with macular edema, such as the present study, can be offered as tentative evidence that macular photocoagulation treatment of macular edema is effective in decreasing vision loss from macular edema.
The response of macular edema and proliferative radiation retinopathy to macular photocoagulation and PRP treatment, respectively, is similar to the response with macular photocoagulation treatment of clinically significant diabetic macular edema (CSME) and PRP treatment of proliferative diabetic retinopathy; this is not surprising, since radiation retinopathy and diabetic retinopathy have pathophysiologic features in common.29 Both entities are microvascular diseases associated with abnormally permeable retinal vessels and capillary nonperfusion with resultant retinal edema and ischemia as well as proliferation of new vessels with intraocular bleeding and rubeosis iridis. Macular photocoagulation treatment decreases leakage from the abnormal capillaries resulting in decreased macular edema, and PRP treatment decreases neovascularization resulting in decreased vitreous hemorrhages and neovascular glaucoma. Fewer burns, however, are used for PRP treatment of proliferative radiation retinopathy than are recommended for successful treatment of proliferative diabetic retinopathy.30 Six hundred to 1200 scatter burns are usually effective in proliferative radiation retinopathy, whereas 1200 to 1600 burns are recommended for maximum effect in proliferative diabetic retinopathy. Since optic atrophy commonly occurs following successful PRP, the optic neuropathy of radiation retinopathy may be worsened by PRP, and limiting the number of PRP burns to as few as possible to achieve involution of the proliferative radiation retinopathy is a rational objective.
Although the anatomic responses to photocoagulation treatment are similar, the VA results are not as good following photocoagulation treatment of radiation retinopathy as after photocoagulation treatment of diabetic retinopathy.31,32 Differences between these 2 sight-threatening complications that might explain decreased effectiveness of photocoagulation in preventing vision loss include the following: (1) There is more widespread capillary nonperfusion and resultant macular ischemia in radiation retinopathy as compared to diabetic retinopathy, perhaps due to the endothelial cells being the primary site of injury in radiation retinopathy, whereas the pericytes are primarily injured in diabetic retinopathy.18 It is generally accepted that the benefits of macular photocoagulation for CSME decrease with increasing capillary nonperfusion and macular ischemia33; there probably is a severity of capillary nonperfusion in the macula beyond which macular photocoagulation treatment for radiation macular edema and CSME is no longer beneficial. How much ischemia needs to be present to block any beneficial effect, however, has not been defined. Investigators in one study of CSME did not find a less favorable VA outcome of photocoagulation treatment when comparing eyes with varying areas of capillary nonperfusion in CSME.34 An example of macular ischemia in radiation macular edema that may be too severe to benefit from macular photocoagulation treatment is depicted in Figure 3. (2) Radiation retinopathy follows acute injury, whereas diabetic retinopathy is due to a long-term metabolic insult. (3) The optic nerve is affected more frequently and severely in radiation retinopathy than in diabetic retinopathy and, likely, contributes to more vision loss. The more widespread capillary nonperfusion in radiation retinopathy than in diabetic retinopathy probably also occurs in the optic nerve. (4) The damage to the retinal pigment epithelium and choroid is more severe following radiation than occurs in patients with diabetes mellitus.35–38
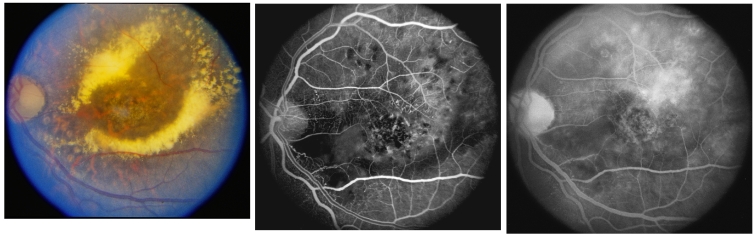
FIGURE 3.
Left, Unresolved clinically significant radiation macular edema. Fundus photograph of the left eye of 25-year-old woman 7 years following 5040 cGy of radiation for treatment of nasopharyngeal rhabdomyosarcoma. Vision is 20/200. Exudates and photocoagulation scars surround the center of the macula, which is edematous. The macular edema was retreated with limited scatter burns through areas of capillary nonperfusion but not closer than 500 μm from the center of the macula. Middle, Early venous phase fluorescein angiography. The left eye angiogram shows capillary nonperfusion in the papillomacular bundle and hyperfluorescence of microaneurysms and telangiectatic vessels. Prior photocoagulation burns are round and hypofluorescent. Right, Late-phase fluorescein angiography. Fluorescein dye has leaked into the superotemporal macula.
Attempts to improve VA results of photocoagulation treatment for radiation retinopathy would include development of a protocol to identify affected patients with radiation retinopathy as early as possible, so that treatment of macular edema and proliferative retinopathy can be instituted before vision loss due to either of these complications has occurred, similar to the practice pattern for diabetic retinopathy.39 The goal would be to intervene sooner, before vision is lost and certainly before a doubling or more of the visual angle has occurred, as was the case in this study (20/40 to 20/80 and 20/60 to 20/200, respectively, for eyes with treated [macular photocoagulation] macular edema and treated [PRP] proliferative retinopathy).
In this study, 57 eyes (65.5%) developed radiation retinopathy within 3 years of radiation treatment; 15 eyes (17.2%) were diagnosed with radiation retinopathy during the first year following radiotherapy; and 19 eyes (21.8%) were diagnosed 6 or more years following radiotherapy. On the basis of these data, eyes at risk for macular edema and proliferative radiation retinopathy (following radiotherapy of the eye, orbit, sinuses, or brain) could be detected early by performing dilated retinal examinations every 6 months for the first 3 years following exposure to more than 1000 cGy; the frequency of the retinal examinations could then be decreased to yearly for another 3 years, after which routine eye examinations appropriate for the patient’s age could be resumed. For patients with radiation retinopathy, the follow-up examinations would be more frequent (similar to every 3 to 4 months recommended for patients with diabetic retinopathy), depending on the severity of the radiation retinopathy (eg, area of retinal hemorrhages and microaneurysms, venous dilatation, intraretinal microvascular abnormalities, macular exudates, neovascularization).39
Along with increased surveillance of patients at risk for developing radiation retinopathy, more resources are needed to find better methods to prevent radiation retinopathy as well as better methods of treatment after radiation retinopathy develops. Elimination of human error, or at least decreasing to as low a risk as possible, in dosage calculations, as well as in delivery of the radiation treatment and improved shielding techniques, are desirable goals.
Affected patients in this study continued to lose vision despite photocoagulation treatment of radiation macular edema and proliferative radiation retinopathy; better treatment methods are clearly needed. Available treatments that may supplant or complement photocoagulation treatment for radiation macular edema and proliferative radiation retinopathy include intravitreal corticosteroids and anti–vascular endothelial growth factor (VEGF) compounds, both of which have shown benefit for other ocular diseases associated with leaky vessels and subsequent macular edema as well as neovascularization.40,41 These treatments have been, and are continuing to be, evaluated in clinical trials for CSME and other retinal vascular diseases; initial reports are encouraging.42 Even with additional preventive measures, the increasing use of radiotherapy for ocular and periocular diseases will likely increase the prevalence of radiation retinopathy. Well-designed clinical trials comparing laser treatment (macular photocoagulation and PRP) with intravitreal corticosteroid or anti-VEGF drugs, as well as combinations of photocoagulation with corticosteroid or anti-VEGF drugs, are needed to provide evidence for ongoing treatment decisions and, more important, to find better treatments than are presently available. In addition to the need for new and better treatments for proliferative radiation retinopathy and macular edema, however, is the need for effective treatments for optic neuropathy and macular ischemia, which so often accompany radiation retinopathy. The same new treatment for radiation macular edema is also showing promise for optic neuropathy.43 Patients with radiation retinopathy have reason to be hopeful as new effective treatments become available.
ACKNOWLEDGMENTS
Funding/Support: None.
Financial Disclosures: None.
Conformity With Author Information: Institutional review board approval was obtained from the University of Washington Human Subjects Review Committee.
Other Acknowledgments: Statistical consultation and assistance were provided by Nan Hu, MS, Department of Biostatistics, University of Washington, and Natalia Bajenova, MD, University of Washington School of Medicine.
REFERENCES
- 1.Stallard HB. Radiant energy as (a) a pathogenic and (b) a therapeutic agent in ophthalmic disorders. Br J Ophthalmol. 1933;1:1–79. [Google Scholar]
- 2.Puusaari I, Heikkonen J, Kivelä T. Ocular complications after iodine brachytherapy for large uveal melanomas. Ophthalmology. 2004;111:1768–1777. doi: 10.1016/j.ophtha.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Parsons JT, Mendenhall WM, Mancuso AA, Cassisi NJ, Million RR. Malignant tumors of the nasal cavity and ethmoid and sphenoid sinuses. Int J Radiat Oncol Biol Phys. 1987;14:11–22. doi: 10.1016/0360-3016(88)90044-2. [DOI] [PubMed] [Google Scholar]
- 4.Amoaku WMK, Archer DB. Cephalic radiation and retinal vasculopathy. Eye. 1990;4:195–203. doi: 10.1038/eye.1990.26. [DOI] [PubMed] [Google Scholar]
- 5.Miller ML, Goldberg SH, Bullock JD. Radiation retinopathy after standard radiotherapy for thyroid-related ophthalmopathy. Am J Ophthalmol. 1991;112:600–601. doi: 10.1016/s0002-9394(14)76869-2. [DOI] [PubMed] [Google Scholar]
- 6.Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Radiation retinopathy after external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30:765–773. doi: 10.1016/0360-3016(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 7.Quinn AG, Clemett RS. Retinopathy after low-dose retinal irradiation. Aust N Z J Ophthalmol. 1993;21:193–197. [PubMed] [Google Scholar]
- 8.Viebahn M, Barricks ME, Osterloh MD. Synergism between diabetic and radiation retinopathy: case report and review. Br J Ophthalmol. 1991;75:629–632. doi: 10.1136/bjo.75.10.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez PF, Sternberg P, Jr, Dabbs CK, Vogler WR, Crocker I, Kalin NS. Bone marrow transplant retinopathy. Am J Ophthalmol. 1991;112:635–646. doi: 10.1016/s0002-9394(14)77269-1. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham ET, Jr, Irvine AR, Rugo HS. Bone marrow transplantation retinopathy in the absence of radiation therapy. Am J Ophthalmol. 1996;122:268–270. doi: 10.1016/s0002-9394(14)72023-9. [DOI] [PubMed] [Google Scholar]
- 11.Brown GC, Shields JA, Sanborn G, Augsburger JJ, Savino PJ, Schatz NJ. Radiation retinopathy. Ophthalmology. 1982;89:1494–1501. doi: 10.1016/s0161-6420(82)34611-4. [DOI] [PubMed] [Google Scholar]
- 12.Kinyoun JL, Lawrence BS, Barlow WE. Proliferative radiation retinopathy. Arch Ophthalmol. 1996;114:1097–1100. doi: 10.1001/archopht.1996.01100140299007. [DOI] [PubMed] [Google Scholar]
- 13.Hykin PG, Shields CL, Shields JA, Arevalo F. The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology. 1998;105:1425–1429. doi: 10.1016/S0161-6420(98)98023-X. [DOI] [PubMed] [Google Scholar]
- 14.Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30:287–290. doi: 10.1016/j.jcrs.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Draper NR, Smith H. Applied Regression Analysis. Ed 2. New York: John Wiley &d Sons; 1981. [Google Scholar]
- 16.Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;16357:664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 17.Collaborative Ocular Melanoma Study (COMS) Group The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. Arch Ophthalmol. 2006;124:1684–1693. doi: 10.1001/archopht.124.12.1684. [DOI] [PubMed] [Google Scholar]
- 18.Maguire AM, Schachat AP. Radiation retinopathy. In: Ryan SJ, editor. Retina. Vol 2. St Louis, MO: Mosby; 2001. pp. 1509–1515. [Google Scholar]
- 19.Collaborative Ocular Melanoma Study Group Incidence of cataract and outcomes after cataract surgery in the past 5 years after iodine 125 brachytherapy. Ophthalmology. 2007;114:1363–1371. doi: 10.1016/j.ophtha.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Yanai D, Weiland JD, Mahadevappa M, Greenberg RJ, Fine I, Humayun MS. Visual performance using a retinal prosthesis in three subjects with retinitis pigmentosa. Am J Ophthalmol. 2007;143:820–827. doi: 10.1016/j.ajo.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Da Cruz L, Chen FK, Ahmado A, Greenwood J, Coffey P. RPE transplantation and its role in retinal disease. Prog Retin Eye Res. 2007;26:598–635. doi: 10.1016/j.preteyeres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Roden D, Bosley TM, Fowble B, et al. Delayed radiation injury to the retrobulbar optic nerves and chiasm. Ophthalmology. 1990;97:346–351. doi: 10.1016/s0161-6420(90)32582-4. [DOI] [PubMed] [Google Scholar]
- 23.Guy J, Schatz NJ. Hyperbaric oxygen in the treatment of radiation-induced optic neuropathy. Ophthalmology. 1986;93:1083–1088. doi: 10.1016/s0161-6420(86)33617-0. [DOI] [PubMed] [Google Scholar]
- 24.Kinyoun JL, Kalina RE, Brower SA, Mills RP, Johnson RH. Radiation retinopathy after orbital irradiation for Graves’ ophthalmopathy. Arch Ophthalmol. 1984;102:1473–1476. doi: 10.1001/archopht.1984.01040031193016. [DOI] [PubMed] [Google Scholar]
- 25.Monroe AT, Bhandare N, Morris CG, Mendenhall WM. Preventing radiation retinopathy with hyperfractionation. Int J Radiat Oncol Biol Phys. 2005;61:856–864. doi: 10.1016/j.ijrobp.2004.07.664. [DOI] [PubMed] [Google Scholar]
- 26.Gragoudas ES, Li W, Lane AM, Munzenrider J, Egan KM. Risk factors for radiation maculopathy and papillopathy after intraocular irradiation. Ophthalmology. 1999;106:1571–1578. doi: 10.1016/S0161-6420(99)90455-4. [DOI] [PubMed] [Google Scholar]
- 27.Khawly JA, Rubin P, Petros W, Peters WP, Jaffe GJ. Retinopathy and optic neuropathy in bone marrow transplantation for breast cancer. Ophthalmology. 1996;103:87–95. doi: 10.1016/s0161-6420(96)30728-8. [DOI] [PubMed] [Google Scholar]
- 28.Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology. 2007;114:1804–1809. doi: 10.1016/j.ophtha.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 29.Irvine AR, Alvarado JA, Wara WM, Morris BW, Wood IS. Radiation retinopathy: an experimental model for the ischemic-proliferative retinopathies. Trans Am Ophthalmol Soc. 1981;129:104–122. [PMC free article] [PubMed] [Google Scholar]
- 30.The Early Treatment Diabetic Retinopathy Study Research Group Techniques for scatter and local photocoagulation treatment of diabetic retinopathy. Int Ophthalmol Clin. 1988;28:254–272. doi: 10.1097/00004397-198702740-00005. [DOI] [PubMed] [Google Scholar]
- 31.The Diabetic Retinopathy Study Research Group Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology. 1978;85:82–106. doi: 10.1016/s0161-6420(78)35693-1. [DOI] [PubMed] [Google Scholar]
- 32.Early Treatment Diabetic Retinopathy Study Research Group Photocoagulation for diabetic macular edema. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- 33.McDonald HR, Schatz H. Grid photocoagulation for diffuse macular edema. Retina. 1985;5:65–72. doi: 10.1097/00006982-198500520-00001. [DOI] [PubMed] [Google Scholar]
- 34.Early Treatment Diabetic Retinopathy Study (ETDRS) Research Group Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline. Arch Ophthalmol. 1995;113:1144–1155. [PubMed] [Google Scholar]
- 35.Egbert PR, Fajardo LF, Donaldson SS, Moazed K. Posterior ocular abnormalities after irradiation for retinoblastoma: a histopathological study. Br J Ophthalmol. 1980;64:660–665. doi: 10.1136/bjo.64.9.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi K, Kishi S, Muraoka K, Tanaka T, Shimizu K. Radiation choroidopathy with remodeling of the choroidal venous system. Am J Ophthalmol. 1998;125:367–373. doi: 10.1016/s0002-9394(99)80148-2. [DOI] [PubMed] [Google Scholar]
- 37.Hidayat AA, Fine BS. Diabetic choroidopathy: light and electron microscopic observations of seven cases. Ophthalmology. 1985;92:512–522. [PubMed] [Google Scholar]
- 38.Flyczkowski AW, Hodes BL, Walker J. Diabetic choroidal and iris vasculature scanning electron microscopy findings. Int Ophthalmol. 1989;13:269–279. doi: 10.1007/BF02280087. [DOI] [PubMed] [Google Scholar]
- 39.AAO Retina Panel . San Francisco, CA: American Academy of Ophthalmology; 2003. Preferred practice pattern. Diabetic retinopathy. [Google Scholar]
- 40.Shields CL, Demirci H, Dai C, et al. Intravitreal triamcinolone acetonide for radiation maculopathy after plaque radiotherapy for choroidal melanoma. Retina. 2005;25:868–874. doi: 10.1097/00006982-200510000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Finger PT, Chin K. Anti-vascular endothelial growth factor bevacizumab (Avastin) for radiation retinopathy. Arch Ophthalmol. 2007;125:751–756. doi: 10.1001/archopht.125.6.751. [DOI] [PubMed] [Google Scholar]
- 42.Diabetic Retinopathy Clinical Research Network A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860–1867. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shields CL, Demirci H, Marr BP, et al. Intravitreal triamcinolone acetonide for acute radiation papillopathy. Retina. 2006;26:537–544. doi: 10.1097/00006982-200605000-00007. [DOI] [PubMed] [Google Scholar]