Abstract
Purpose
This study investigated the safety and potential retinal toxicity of intravitreally administered erythropoietin (EPO) in a rodent animal model.
Methods
Forty-two healthy Sprague-Dawley rats were divided into one of 7 groups (N = 6 per group): control, sham injection, vehicle injection, and EPO injections of 50 ng (5 U), 100 ng (10 U), 250 ng (25 U), and 625 ng (62.5 U). Only the right eye was treated in each animal. Standard full-field dark- and light-adapted electroretinography (ERG) was obtained at 1 day prior to injection and then on postinjection days 3, 7, 14, and 21. Intraocular pressure (IOP) was measured at the conclusion of each ERG recording. Animals were sacrificed and the eyes underwent histologic examination with light microscopy and hematoxylin-eosin staining.
Results
Rod peak, scotopic, and photopic responses (amplitude and latency) were not statistically different in the animals receiving 50 to 100 ng EPO. In the 250-ng group, the photopic b-wave amplitude at day 21 was elevated (P <.05), whereas in the 625-ng group, the scotopic OP3 latency ratio was higher at baseline (P <.05). No significant histologic abnormalities were noted except for one animal (625-ng group) with qualitative differences in retinal layer thickness and cellular density.
Conclusions
Intravitreal administration of EPO (at doses up to 625 ng) does not cause adverse effects on retinal function as assessed by ERG. Moreover, single intravitreal dosing does not appear to elicit retinal neovascularization. Further investigation is warranted to assess fully the potential of this neuroprotective cytokine as a treatment for glaucoma.
INTRODUCTION
The glaucomas constitute a large diverse group of optic neuropathies in which there is a characteristic optic disc cupping appearance with associated characteristic visual field defects. Primary open-angle glaucoma (POAG), the most common type of glaucoma in the United States, typically occurs after age 50 years and is frequently associated with elevated intraocular pressure (IOP). POAG affects more than 2.2 million Americans over the age of 40 with 130,000 functionally blind (as defined by central vision less than 20/200 or constricted visual field less than 10 degrees).1 Glaucoma is also the second leading cause of blindness worldwide. In 2010 there will be 60.5 million people with glaucoma worldwide, increasing to 79.6 million by 2020, of which 74% will have open-angle glaucoma (OAG).2
POAG should be viewed as a chronic neurodegenerative disease characterized by primary optic nerve injury and subsequent loss of retinal ganglion cells (RGCs).3 While initial experimental primate studies suggested a selective loss of magnocellular bodies and axons, recent contrast sensitivity testing of glaucoma patients suggests nonselective impairment of the low-spatial-frequency components of both magnocellular and parvocellular pathways, which are presumably mediated by cells with larger receptive fields.4 In addition to the atrophy and loss of RGCs, there is atrophy and loss of target neurons in the lateral geniculate nucleus of the brain. The first clinicopathological case of human glaucoma demonstrated neural degeneration in the brain involving the intracranial optic nerve, lateral geniculate nucleus, and visual cortex.5
RATIONALE FOR NEUROPROTECTION
Although glaucoma is a multifactorial, neurodegenerative disease, the only currently proven method of treatment involves reduction of IOP via medical, laser, or surgical therapies.6 While IOP lowering has been demonstrated to be beneficial across the entire spectrum of disease, the ideal IOP parameters remain unknown. In certain subgroups of patients, there appears to be a non-IOP-dependent component of the disease. For example, in the Collaborative Normal Tension Glaucoma Study,7 over 20% of patients continued to progress despite apparently adequate IOP reduction of greater than 30%. In addition, approximately 50% of untreated patients with normal-tension glaucoma did not progress over the 5-year period, whereas 5% progressed rapidly and 45% progressed slowly.8 Furthermore, a faster rate of progression occurs in women, in patients with migraine, and in patients with optic disc hemorrhages.9 Thus, this and other studies appear to demonstrate that OAG is a slowly progressing optic neuropathy (except for eyes with markedly elevated IOP) and that IOP lowering does not prevent visual loss in a significant subgroup of patients.
Although IOP lowering with conventional therapies is itself neuroprotective, the development of non-IOP-dependent neuroprotective therapies appears promising. Memantine, an uncompetitive, low-affinity, open-channel blocker of the NMDA-type glutamate receptor, has been shown to be effective in the treatment of both mild and moderate to severe Alzheimer disease.10 In an experimental glaucoma model in monkeys, memantine also protected against neuronal shrinkage in the lateral geniculate nucleus, the major target for RGCs, when compared to a vehicle-treated group.11 However, memantine treatment was recently reported ineffective in a large phase 3 clinical trial for glaucoma (Allergan press release, January 30, 2008), and this finding illustrates the limitations of any animal model to predict future successful outcomes in humans.
Significant effort has been made to explore possible neuroprotective agents to treat glaucoma.12–14 Potential therapeutic options have ranged from inhibitors of nitric oxide synthase (NOS)12 to T-cell–based vaccination.14 Neurotrophic factors show promise in retarding progression of neurodegenerative diseases. Current ongoing preclinical and clinical studies involve factors such as human ciliary neurotrophic factor (CNTF), brain-derived neurotrophic factor, pigment epithelium–derived factor, and others.15 A recently completed Phase 1 safety trial was reported involving CNTF delivered by cells transfected with the human CNTF gene and sequestered within surgically implanted capsules.16 The trial indicated that CNTF is safe for the human retina and may have application for retinal degenerative diseases, including glaucoma.
Triple combination therapy targeting the amyloid-beta pathway dramatically prevents RGC death in a rat model of glaucoma.17 Calpeptin, a calpain-specific inhibitor, provides functional neuroprotection by preventing calcium influx, proteolytic activities, and apoptosis of rat RGCs.18 Intraperitoneal injections of minocycline also enhance RGC survival in rats.19 The effects of minocycline-associated neuroprotection occur partly through an inducible NOS (iNOS)-suppressive mechanism.20 This is consistent with the finding that a selective inhibitor of iNOS, aminoguanidine, provides neuroprotection in a rat hypertensive model of glaucoma.21
ERYTHROPOIETIN
Background
Erythropoietin (EPO), a naturally occurring cytokine used to treat anemia by inhibiting apoptosis in erythrocyte progenitors, has been recently shown to have neuroprotective effects.22 EPO, first characterized as a hematopoietic factor produced by the fetal liver and adult kidney in response to hypoxia, stimulates differentiation and proliferation of erythroid progenitor cells.23 A 24-kDa glycoprotein that binds to targeted receptors on erythroid precursor cells in bone marrow tissue, EPO possesses cytokine functions as the main regulator of erythropoiesis by preventing apoptosis of the erythroid precursor cells.23 In addition, EPO can exert a multitude of biologic benefits, including antiapoptotic, neurotrophic, antioxidant, and angiogenic effects, in addition to up-regulation of oxidative metabolism pathways.24–26
Since EPO is able to cross the blood-brain barrier, it possesses the virtually essential property for any potential neuroprotective agent. EPO has been reported to be expressed by brain and retinal tissue, cultured neurons, and astrocytes.27 The neuroprotective effects of EPO have been characterized in numerous animal models of neural injury. In a mouse model of stroke, injection of EPO 24 hours before an ischemic injury was able to reduce the size of the cerebral infarct by 47%.28 In another mouse model of focal cerebral lesion leading to global degeneration, EPO was shown to improve behavioral abnormality, cognitive dysfunction, and brain atrophy up to 8 months after the insult.29 In a rat model of spinal cord injury, intraperitoneal injection of EPO was shown to be protective, possibly by the inhibition of lipid peroxidation.30
Systemic administration of EPO also appears to decrease apoptosis of dorsal root ganglion cells; in one study, treated rats had faster recovery rates compared to control rats, even though initial injuries were comparable.31 In response to nerve root crush injury in rats, EPO’s inhibition of spinal neuronal apoptosis was associated with functional improvement as rats showed quicker recovery in terms of pain behavior.32 In human beings, EPO improves the cognitive function of some patients undergoing chemotherapy, although whether or not this improvement is achieved through the correction of anemia vs neuroprotection or both is not delineated.33
The exact mechanism of this neuroprotective effect is not fully elucidated. There is emerging evidence that EPO prevents or retards apoptosis of neurons via its binding to a modified tissue-protective EPO receptor (EPO-R), a complex different in structure from the homodimer receptor associated with hematopoiesis.13,34 This tissue protective EPO-R has been reported to possess less binding affinity to EPO and a lower molecular weight than that of the hematopoietic homodimer EPO receptor.34,35 The neuroprotective effect induced by the EPO/EPO-R complex appears to involve cross-talk between the Jak2 and NF-kappaB signaling cascades, thereby leading to transcription of various neuroprotective factors.36 Activation of the Jak2 pathway may also inhibit the release of glutamate from surrounding cells.37 Activation of PI-3 kinase is also important in the process of EPO neuroprotection. The neuroprotective effect was abolished by Wortmannin, a PI-3 kinase inhibitor, in a rat focal ischemic model.38 PI-3 kinase activation may also deactivate Bcl-xL/Bcl-2-associated death promoter (BAD), a proapoptotic molecule, and/or caspase-3 to inhibit apoptosis.38–41
In light of the novel neuroprotective function of EPO, there has been recent interest in investigating the effects of exogenous administration in animal models of retinal degeneration (eg, glaucoma). As such, the retinal vascular system and histologic structure of the optic nerve head in the rat more closely resemble these ocular structures in humans when compared to other commonly employed experimental animals, such as the rabbit, dog, or cat.42 Systemic administration of EPO in rats has been shown to protect the retinal tissue, histologically and functionally, in an acute ischemia-reperfusion model.43 Intravitreal injections of EPO have been shown to rescue RGCs and prevent caspase-3 activation in axotomized rats, as well as retard against RGC loss in a rat model of ocular hypertension.38,44 The EPO-induced neuroprotection was shown to follow a bell-shaped dose-response curve in vitro and in vivo.38 A recent study22 summarized the literature on EPO and concluded that this cytokine is a potential candidate protein for neuroprotection treatment in glaucoma.
Potential Limitations as a Neuroprotective Agent
EPO has been administered systemically for many years in the treatment of patients with anemia secondary to kidney disease and chemotherapy. Its systemic side effects (eg, polycythemia vera, vascular thrombosis) are also well known.45,46 Earlier neuroprotection experiments with EPO were performed in hypoxic models of neuronal damage with demonstrable activation of hypoxia-inducible factor 1 (HIF-1).47 Therefore, the erythropoietic and angiogenic aspects of EPO administration were thought to be favorable. However, in the eye, angiogenesis leading to abnormal neovascularization could be a potentially serious complication of EPO treatment. In fact, a recent study48 described an increased level of EPO in the vitreous cavities of patients with proliferative diabetic retinopathy (compared to normal eyes). It is unclear whether the reported up-regulation in EPO levels was a primary causal factor for the vasoproliferative process or a secondary result of the proliferative signals.
Whether EPO induces intraocular neovascularization is unresolved. There are indeed studies suggesting an angiogenic role for EPO in various examples of tumor growth.49–52 In one study, 200 U of EPO 3 times a week for 4 weeks was shown to elicit polycythemia vera.53 In one in vitro study, human myocardial endothelial cells exposed to 2.5 U/mL of EPO increased capilliary outgrowth up to 220%; however, the cells were exposed to EPO in the medium for at least 12 days (up to 21 days).54 In a chemically induced murine hepatic tumor model, EPO concentration ranged from 6.1 to 97.8 mU/mL in the incited tumors with denser vascularization.55
On the other hand, some studies show that EPO does not cause angiogenesis. In patients with multiple myeloma, there is no correlation between EPO and other angiogenic factors.56 In ovarian cancer cells, EPO was actually inversely correlated with HIF-1 and vascular endothelial growth factor levels.57 Also, in a mouse model of various tumor cell lines, neither tumor growth nor angiogenensis was affected by EPO.58 The mice were injected with 2000 U/kg with 3 injections per week for 2 weeks. The researchers used 4 different tumor cell lines that all expressed EPO-R and had long-term exposure to EPO.58
Previous Studies in Experimental Glaucoma
Recent published studies support the neuroprotective potential of EPO in models of glaucoma and ocular hypertension. One study reconfirmed the neuroprotective effects of a single intravitreal injection of EPO in rats that had undergone optic nerve transection, while also demonstrating evidence of regeneration in a small proportion of the axons.59 Another study reported the use of EPO in the DBA/2J transgenic mouse model of glaucoma, wherein the animals developed iris pigment dispersion and increased IOP.60 Intraperitoneal injection of recombinant human EPO (rhEPO) in these animals inhibited RGC loss as effectively as the neuroprotective agent memantine.
Another study compared the systemic and intravitreal administration of rhEPO in a rat model of ocular hypertension.61 Two weeks after photocoagulation of the episcleral and limbal veins, both systemic and intravitreal injection of rhEPO were found to prevent RGC apoptosis. However, neutralization of endogenous EPO by injection of soluble EPO-R exacerbated ocular hypertensive injury, suggesting that the EPO/EPO-R system plays a role in protecting the viability of RGCs.
The episcleral vein cautery (EVC) protocol has been utilized in rats to generate a glaucoma model with elevated IOP and corresponding RGC loss.62,63 Briefly, experimental rats are anesthetized by intraperitoneal injection of a mixture of ketamine (50 mg/kg) and xylazine (5 mg/kg). A conjunctival incision is made approximately 2 mm posterior to the limbus in the right eye (the left eye serves as the unoperated control eye with regard to IOP). After surgical exposure, 2 dorsal and 1 temporal episcleral veins are identified. The veins are lifted with a fine curved, nonserrated tips forceps and cauterized. The conjunctival incision is then sutured and antibiotic ointment applied.
A previous study randomly subdivided experimental rats into 1 of 3 treatment groups: EVC only, EVC with intravitreal normal saline injection (EVC-NS), and EVC with intravitreal EPO treatment (EVC-EPO).44 Immediately following the EVC procedure, a 200-ng dose of EPO was injected intravitreally in the rats; the arbitrarily selected 200-ng dose was based on published in vivo evidence that EPO protects neurons from ischemia damage in a dosage range of 25 to 250 ng per day when administered via intracerebroventricular infusion.64 Quantitative RGC counts were obtained using the Axioplan II fluorescence microscope with Axiovision 4.1 and automated analysis with image-analysis software.44 After 21 days of elevated IOP, the mean RGC counts were decreased significantly (P < .005) in both the EVC (27.8% decrease from baseline) and EVC-NS (24.8% decrease from baseline) groups. In the EVC-EPO group, the mean RGC count was not significantly decreased when compared to the normal control value (11.1% decrease from baseline; P = .051).44 Moreover, rhodamine concanavalin-A (ConA lectin) labeling of the retinal vasculature in the treated right eye of EVC-EPO rats did not show any differences in either the superficial or deep vascular patterns when compared to the vasculature in the untreated left eye of the same rat, thereby suggesting no abnormal neovascularization process.44 On the basis of these results, the investigators concluded that EPO treatment has a beneficial role in preserving RGC survival following IOP elevation of at least 3 weeks duration. In addition, preservation of RGC viability was not seen in the EVC-placebo treatment group, thereby demonstrating that the intravitreal injection alone did not account for this neuroprotective effect.
One major limitation of previous experimental glaucoma studies is the lack of data in regard to EPO’s dose- and time-dependent effects. One study reported a peak protective effect at a total dose of 8 U (equivalent to 80 ng) in an axotomy-induced RGC apoptosis animal model.38 The dose-response curve showed no significant effect when a total dose of 16 U (160 ng) was administered, whereas another experiment utilizing an IOP-associated RGC apoptotic model demonstrated a protective effect at a higher intravitreal dose of 20 U (200 ng).44 It is possible that the variable dosage requirements for EPO neuroprotection may reflect differences in the animal models studied. The second study44 also involved a relatively short-term duration of treatment and studied a small number of animals.
Another limitation of the previous studies has been the inability to rule out selective RGC functional injury in the setting of normal histologic findings. Previous work has shown that in rats with experimental glaucoma, electroretinography (ERG) is able to detect selective RGC functional injury prior to the onset of structural damage, as assessed by light microscopy of optic nerve tissue.65,66 Recent studies investigating other potential neuroprotective compounds have used ERG measurements to demonstrate retinal safety/toxicity following administration of the agent.67,68
Rationale
Before EPO can be utilized safely for the treatment of glaucoma in humans, further data must be obtained to demonstrate its efficacy and safety in ocular tissues following in vivo administration. While a previous study44 utilized rhodamine concanavalin-A (ConA lectin) staining to demonstrate lack of gross vascular abnormalities in the EPO-treated eyes, systemic administration of EPO has known side effects, including polycythemia vera and risk of neovascularization.45,46 These complications of systemically administered EPO could be avoided by delivering the agent via the intraocular route. Thus, there is a great need to address safety and efficacy following the intraocular administration of EPO.
The hypothesis of this pilot study is that a single intravitreal injection of EPO does not cause ocular toxicity and selective RGC functional injury at dose ranges necessary for retinal neuroprotection. Rats were used, since there is substantial literature on experimental glaucoma models with this animal, as well as experience with ERG recordings.44,62,63,65,66 A single EPO dosing regimen was selected initially to decrease the variables inherent with multiple injections. The duration was set at 21 days, similar to that of a previous study44 demonstrating a significant neuroprotective benefit of a single 200-ng intravitreal dose of EPO. Varying doses of EPO up to 3 times the 200-ng figure were utilized. Potential retinal toxicity and untoward effects on retinal function were monitored utilizing histopathology and ERG measurements.
METHODS
All experimentation was conducted under approved institutional review board, biosafety, and animal care (IACUC) protocols of Columbia University College of Physicians and Surgeons. These protocols conformed in entirety with guidelines approved by the Association for Research in Vision and Ophthalmology (ARVO).
ANIMALS
Male Sprague-Dawley rats, 6 weeks old and weighing 250 to 300 g, were used for the experiments. The animals were housed in an approved temperature-controlled room with 12-hour dark and light cycles. Standard nutrition and water were provided ad libitum. The study protocol was approved by the Animal Research Committee and complied with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
HUMAN RECOMBINANT ERYTHROPOIETIN SOLUTION
Recombinant human erythropoietin (R&D Systems Inc, Minneapolis, Minnesota) was dissolved in sterile 0.9% saline solution to make the final concentrations of 10 ng/μL, 20 ng/μL, 50 ng/μL, and 125 ng/μL, such that 5 μL of the solution contained 50 ng, 100 ng, 250 ng, and 625 ng of EPO.
INTRAVITREAL RHEPO INJECTION
Forty-two rats were randomly divided into one of 7 treatment groups (N = 6 per group): control (ie, no injection), sham injection, saline vehicle injection, and rhEPO injections of 50 ng (5 U), 100 ng (10 U), 250 ng (25 U), and 625 ng (62.5 U). The sample sizes were chosen on the basis of prior work44 with intravitreal injections of EPO in rats that had assigned 5 to 10 animals to each subgroup. Prior to each injection, the rats were anesthetized with an intraperitoneal injection of ketamine (70 mg/kg) and xylazine (7 mg/kg) with additional ketamine/xylazine mixture as needed. Topical anesthesia was applied with proparacaine as well. Each rat (except control animals) received a single intravitreal injection (5 μL volume) of solution in the right eye; the left eye served as the contralateral control eye. The 5 μL volume was used in a prior study44 and did not appear to perturb IOP measurements in the rat eye. The eyes were examined with the surgical microscope for complications (eg, lens damage, vitreous hemorrhage) immediately following injection and at a regular interval afterwards.
ELECTRORETINOGRAPHY
In all rats, scotopic and photopic full-field ERGs were observed in a masked fashion from both eyes simultaneously 1 day prior to intravitreal injection (baseline), and then 3, 7, 14, and 21 days following injection. Prior to each ERG recording, the animals were dark-adapted for 2 hours and then anesthetized as described above. Topical proparacaine and tropicamide (1%)/phenylephrine (2.5%) were used for local anesthesia and pupillary dilation, respectively. The stimulus was delivered using a Ganzfeld dome.
The ERG protocol consisted of 3 steps: rod peak response, scotopic maximum response, and a phototopic response. Rod peak response and scotopic maximal response ERGs were elicited using stimulus intensities of 0.001 cd-s/m² and 3 cd-s/m², respectively, on a dark background. After light adaptation for 3 minutes, photopic ERGs were elicited using 3 cd-s/m² on a white background of 30 cd/m². The positive electrodes (Silver loop wires) were placed on both corneas, the negative electrode was placed in the mouth, and the ground electrode was placed in the tail. For each recording, 20 separate responses were averaged and notated using an ERG recording system (Espion, Diagnosys, LLC; Lowell, Massachusetts). The amplitudes and the latencies of rod peak wave, scotopic maximum response a-wave, b-wave, and the third wave of oscillatory potential waves (OP3), and photopic b-wave were evaluated. Interexperimental variabilities and fluctuations were adjusted by utilization of the values recorded from each contralateral untreated left eye. In particular, this was accomplished by calculating ratios of right eye response divided by left eye response for each study group, as described and analyzed previously.69
Of note, one animal in the sham injection group developed a cataract postinjection and was excluded from further analysis (N = 5 for this group only).
IOP MEASUREMENTS
All IOP measurements were obtained in a masked fashion with the Tonopen-XL (Mentor, Inc., Norwell, Massachusetts) while the rats were under anesthesia. Three IOP values were measured per eye and then averaged to obtain a mean IOP value. The data points are presented as mean ± standard deviation (mm Hg). The baseline IOP measurement was obtained prior to the intravitreal injection (eyes not dilated). Subsequent IOPs (starting at day 3) were then measured after each ERG recording while the animals were still under anesthesia and eyes dilated (eyes washed with sterile 0.9% saline solution prior to measurement). This was performed to minimize the amount of anesthesia for each animal.
HISTOLOGY
At 21 days following intravitreal injection of EPO, the rats were deeply anesthetized and sacrificed by means of transcardial perfusion of 0.01M PBS solution followed by 4% paraformaldehyde in 0.1 M phosphate buffer (4% FPB, pH 7.4). The eyes were then enucleated and immersed in 4% paraformaldehyde solution for at least 2 days. After processing in a standard histologic tissue preparation, they were embedded in paraffin. Five-μm-thick sections were cut in a sagittal/axial orientation. Retinal sections near the optic nerves were collected, stained with hematoxylin-eosin (×400), and examined under light microscopy (Leica Microsystems Inc, Allendale, New Jersey).
Sections from both eyes of all animals (experimental right eye and contraleral left eye) were examined in a masked fashion. The outer nuclear, inner nuclear, and ganglion cell retinal layers were evaluated for overall thickness and cellular density. Each section was also evaluated for evidence of new vessel formation.
STATISTICAL ANALYSIS
Group designations were unmasked after all ERGs were performed and interexperimental variability was corrected. Statistical analyses were performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, California). ERG and IOP data were compared between groups and time points using 2-way ANOVA. P values less than .05 (P < .05) were considered statistically significant. All data are presented as mean ± standard error of the mean.
RESULTS
ELECTRORETINOGRAPHY
The amplitude and latency of the scotopic rod peak waves are shown in Tables 1 and 2. Compared to the baseline and the control group, there was no significant difference in amplitudes and latencies of the waves with the intravitreal EPO injection group. Also, there was no significant downward trend with increasing doses of EPO. Furthermore, we did not observe any decrease in amplitude or delay in latency times up to 21 days postinjection. None of the left (contralateral) eyes showed unusual ERG responses in the animals receiving the EPO injections.
TABLE 1.
AMPLITUDE OF SCOTOPIC ROD PEAK WAVES IN μV*
| GROUP | BASELINE | DAY 3 | DAY 7 | DAY 14 | DAY 21 |
|---|---|---|---|---|---|
| Control | 241.7 ± 30.3 | 264.1 ± 65.0 | 214.7 ± 47.8 | 197.9 ± 41.0 | 191.7 ± 27.9 |
| Sham | 245.1 ± 34.8 | 236.5 ± 41.1 | 238.3 ± 89.9 | 224.7 ± 29.5 | 203.9 ± 50.4 |
| Vehicle | 253.3 ± 70.0 | 230.8 ± 55.4 | 215.1 ± 26.3 | 204.9 ± 35.0 | 231.2 ± 51.5 |
| 50 ng | 244.0 ± 64.0 | 238.6 ± 41.4 | 228.5 ± 41.7 | 213.7 ± 33.3 | 206.7 ± 13.2 |
| 100 ng | 265.0 ± 42.6 | 263.7 ± 51.0 | 244.8 ± 66.1 | 233.8 ± 29.0 | 210.3 ± 33.1 |
| 250 ng | 251.9 ± 41.3 | 234.0 ± 51.9 | 213.7 ± 29.4 | 207.2 ± 20.6 | 230.4 ± 31.0 |
| 625 ng | 234.2 ± 31.1 | 272.0 ± 72.4 | 232.2 ± 77.0 | 239.9 ± 49.7 | 208.4 ± 26.9 |
P > .05 for all values compared to control group.
TABLE 2.
LATENCY OF SCOTOPIC ROD PEAK WAVES IN msec*
| GROUP | BASELINE | DAY 3 | DAY 7 | DAY 14 | DAY 21 |
|---|---|---|---|---|---|
| Control | 92.0 ± 2.7 | 93.2 ± 3.1 | 92.1 ± 1.7 | 91.8 ± 1.1 | 91.5 ± 2.1 |
| Sham | 92.5 ± 3.0 | 91.2 ± 0.8 | 91.3 ± 1.5 | 91.3 ± 1.2 | 91.9 ± 2.0 |
| Vehicle | 92.7 ± 2.3 | 93.9 ± 3.9 | 91.9 ± 0.8 | 90.5 ± 1.6 | 91.7 ± 0.6 |
| 50 ng | 92.7 ± 2.1 | 92.0 ± 3.4 | 92.0 ± 2.8 | 93.0 ± 2.1 | 90.8 ± 0.8 |
| 100 ng | 91.6 ± 1.6 | 91.8 ± 1.9 | 91.3 ± 2.4 | 91.6 ± 1.4 | 92.0 ± 2.2 |
| 250 ng | 91.6 ± 1.8 | 92.1 ± 1.9 | 93.0 ± 1.2 | 92.1 ± 0.9 | 93.5 ± 2.5 |
| 625 ng | 93.9 ± 4.7 | 92.8 ± 3.3 | 91.6 ± 0.7 | 91.4 ± 1.0 | 91.8 ± 0.5 |
P > .05 for all values compared to control group.
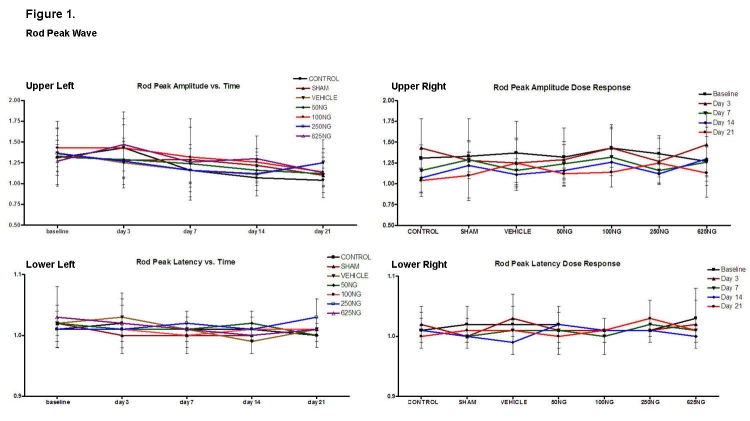
Figure 1 depicts rod peak wave amplitude and latencies as a function of time and EPO dosage. Compared to appropriate baseline and control group values, there was no significant difference in the ratios of the amplitudes and latencies as a function of time (upper and lower left) or as a function of intravitreal EPO dosage (upper and lower right), respectively (P > .05 for all values). All amplitude ratios remained greater than or equal to 1.0. In a similar fashion, the latency ratios did not rise significantly above 1.0 as a function of dosage.
FIGURE 1.
Mean ± SD of rod peak wave amplitude and latencies as a function of time (upper left and lower left) and as a function of EPO dosage (upper right and lower right). Compared to the baseline, none of the values were significantly different in the upper left and lower left figures (P > .05). Also, compared to the control group, none of the values were significantly different in the upper right and lower right graphs (P > .05).
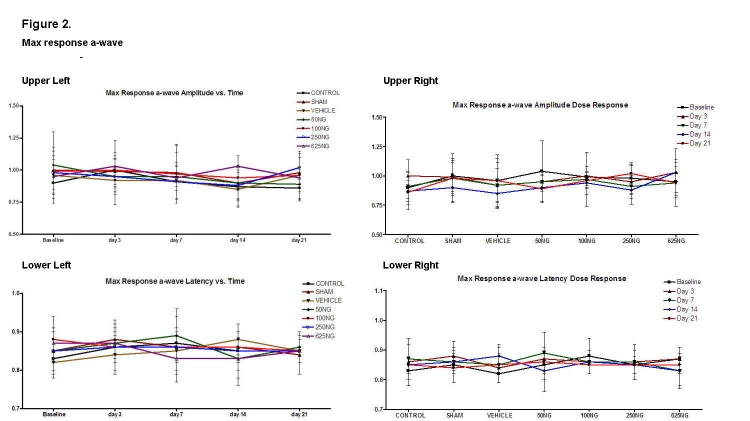
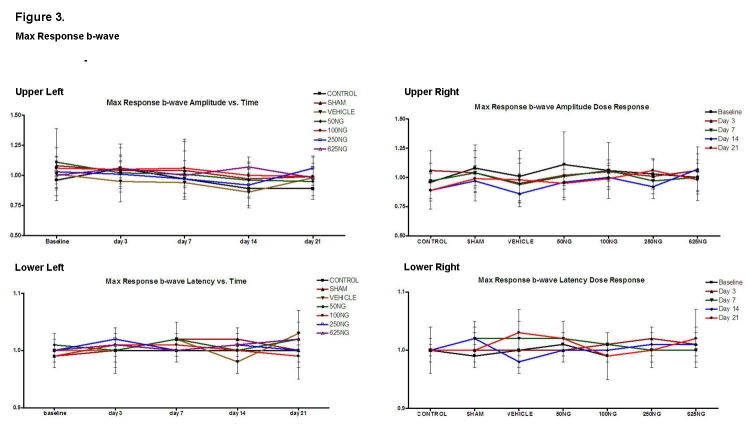
Computed ratios for the scotopic maximum response of the a- and b-waves are shown in Figures 2 and 3, respectively. Compared to appropriate baseline or control group values, the a-wave amplitude and latency ratios did not significantly change as a function of time (Figure 2, upper and lower left) or dosage (Figure 2, upper and lower right), respectively (P > .05 for all values). Similarly, the b-wave amplitude and latency ratios did not change (Figure 3; P > .05 for all values).
FIGURE 2.
Mean ± SD of scotopic maximum response a-wave amplitude and latencies as a function of time (upper left and lower left) and as a function of EPO dosage (upper right and lower right). Compared to the baseline, none of the values was significantly different in the upper left and lower left figures (P > .05). Also, compared to the control group, none of the values were significantly different in the upper right and lower right graphs (P > .05).
FIGURE 3.
Mean ± SD of scotopic maximum response b-wave amplitude and latencies as a function of time (upper left and lower left) and as a function of EPO dosage (upper right and lower right). Compared to the baseline, none of the values was significantly different in the upper left and lower left figures (P > .05). Also, compared to the control group, none of the values were significantly different in the upper right and lower right graphs (P > .05).
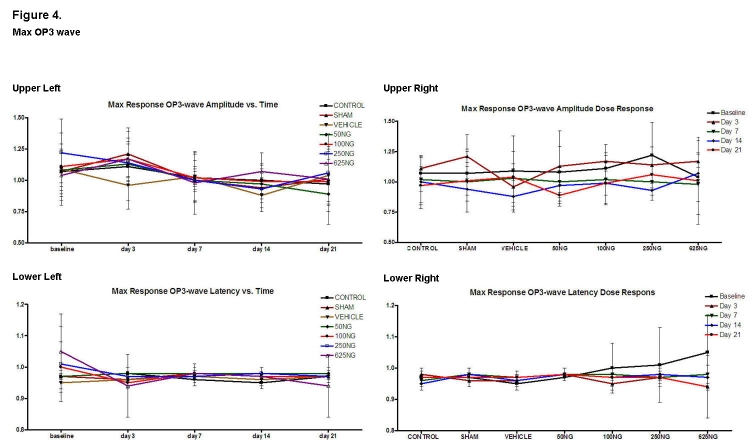
Corresponding ratios for the scotopic maximum response of the third OP wave (OP3) are presented in Figure 4. Again, compared to appropriate baseline and control group values, the amplitude and latency ratios were not significantly different with doses of 50 ng, 100 ng, and 250 ng. For the 625-ng group, the OP3 latency ratio was higher at baseline with decreased OP3 latency ratios noted at days 3, 14, and 21 (Figure 4, lower left; P < .05 for these 3 values). Furthermore, compared to the control group values, the baseline OP3 latency ratio was also higher in the 625-ng group (P < .05).
FIGURE 4.
Mean ± SD of scotopic maximum response OP3-wave amplitude and latencies as a function of time (upper left and lower left) and as a function of EPO dosage (upper right and lower right). Compared to the baseline, none of the values was significantly different in the upper left and lower left figures except for decreased latency ratios of 625-ng group at days 3, 14, and 21 when compared to baseline (P < .05 for these 3 values). Also, compared to the control group, none of the ratios were significantly different in the upper right and lower right graphs except for the 625-ng group at baseline (P < .05).
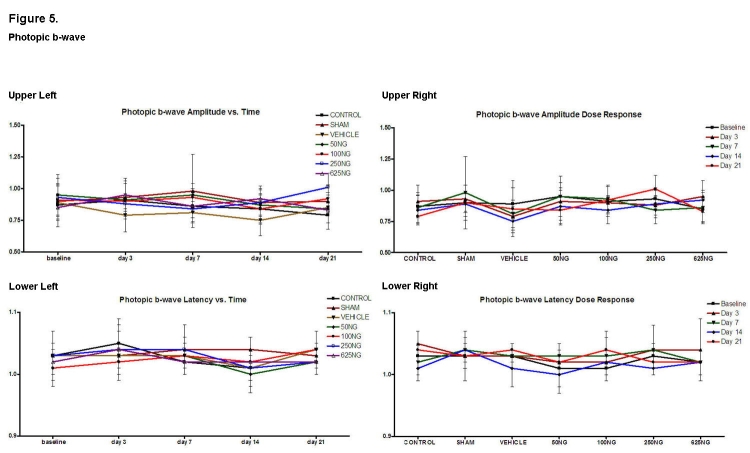
Photopic b-waves were recorded to isolate the cone response for the various groups (Figure 5). Compared to appropriate baseline or control group values, there was no significant difference in the amplitude and latency ratios for the 50-ng, 100-ng, and 625-ng groups. In the 250-ng group, the amplitude ratio was increased at day 21 (Figure 5, upper right; P < .05).
FIGURE 5.
Mean ± SD of photopic b-wave amplitude and latencies as a function of time (upper left and lower left) and as a function of EPO dosage (upper right and lower right). Compared to baseline, none of the values was significantly different in the upper left and lower left figures (P > .05). Also, compared to the control group, none of the values were significantly different in the upper right and lower right graphs except for the amplitude of the 250-ng group at day 21 (P < .05).
IOP MEASUREMENTS
For each animal group (including the control), IOP values were significantly greater starting at days 3, 7, and 14 compared to baseline (Figure 6, upper to lower middle; P < .05 for all values). The IOP values for the contralateral untreated eyes of each treatment group (including the control) were also equally elevated starting at days 3, 7, and 14 (Figure 6, upper to lower middle; P < .05 for all values). When compared to baseline, the IOP values for both eyes at day 21 were statistically similar (Figure 6, lower; P > .05 for both values).
FIGURE 6.
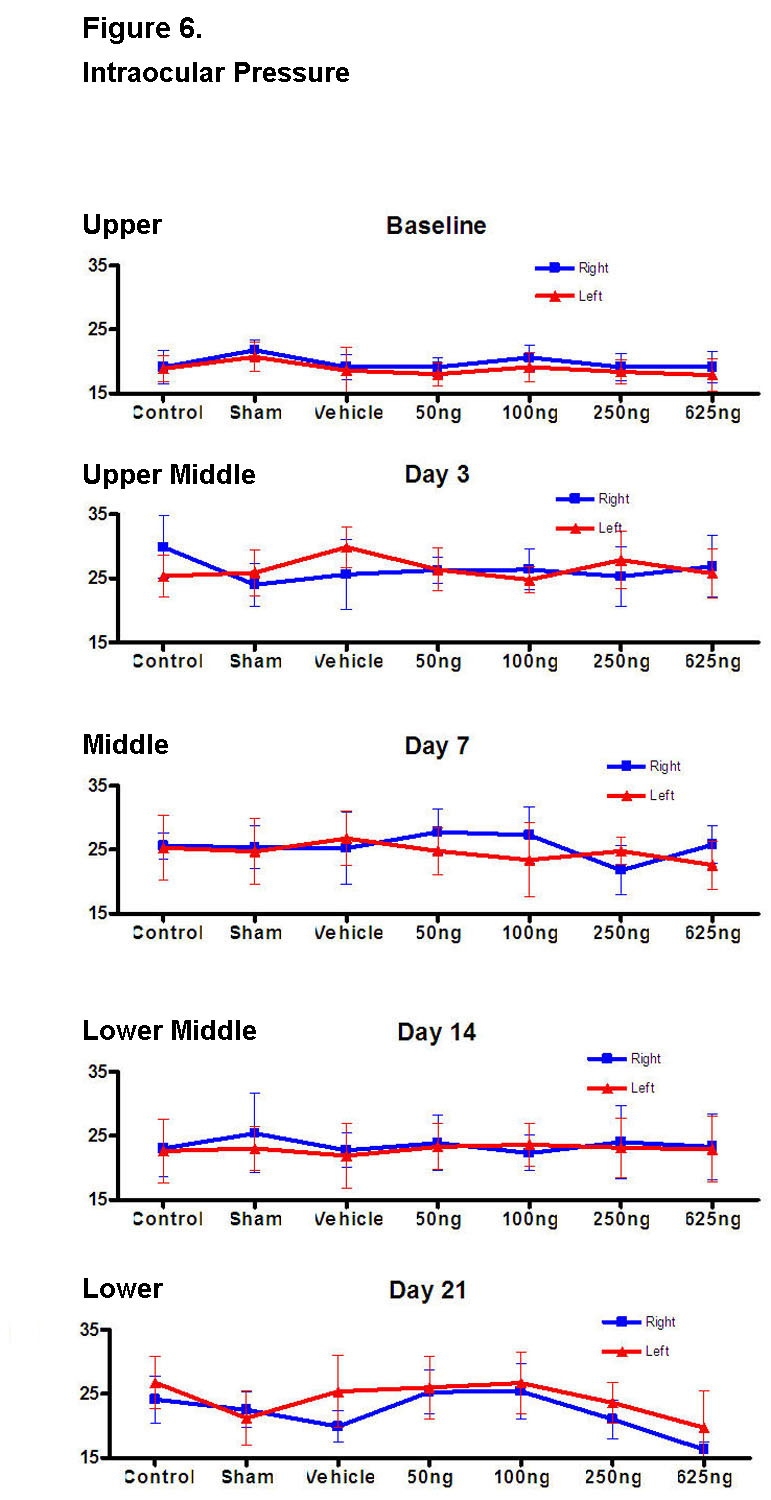
Baseline intraocular pressure (IOP) was measured prior to the intravitreal injection (upper figure). Starting at day 3, the IOPs were measured immediately after each ERG measurement while the rat was still under anesthesia with eyes dilated (upper middle through lower figures). This was done to minimize the amount of anesthesia for each animal. Compared to the control group, none of the IOPs was significantly different except for the right eye of the 625-ng group at day 21 (P < .05) and the left eye of the sham group at day 21 (P < .05).
HISTOLOGY
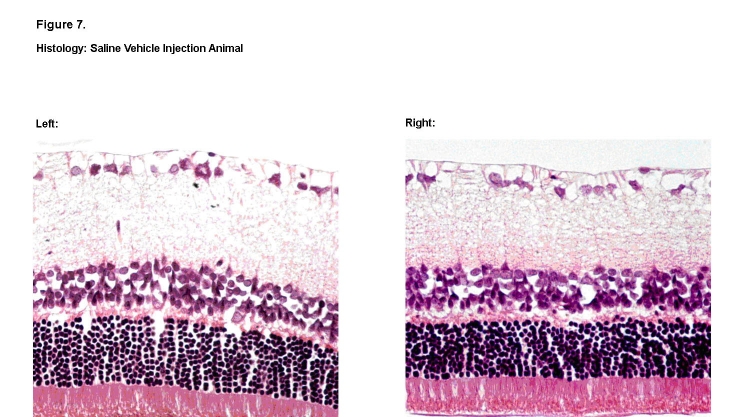
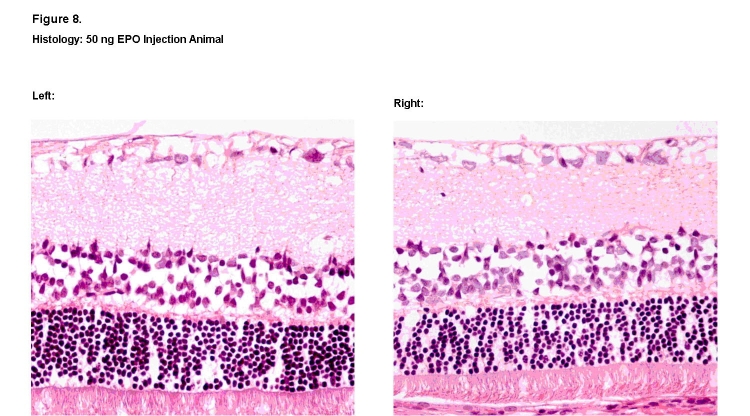
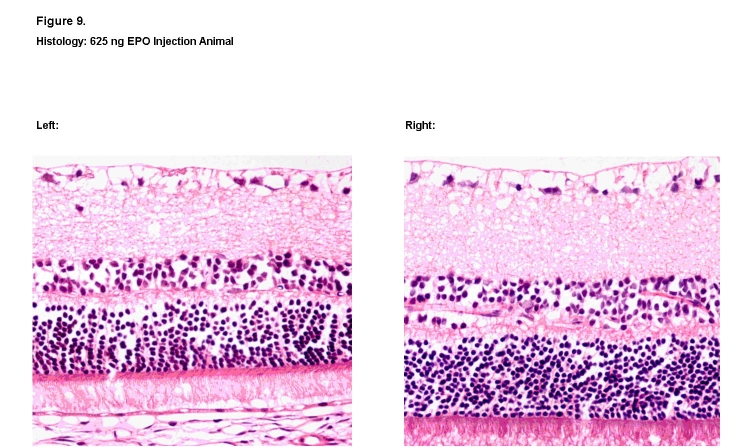
Overall, no qualitative changes were observed in the structure, morphology, and thickness of the individual retinal nerve layers among the animals exposed to dosages of 50 ng, 100 ng, and 250 ng. Representative histologic specimens are shown for the saline vehicle group (Figure 7) and the 50-ng rhEPO group (Figure 8). However, in retinal sections from one animal in the highest 625-ng rhEPO group, visible decreases were observed in both the thickness and cellular density of the outer nuclear and inner nuclear layers when compared to its contralateral control eye (Figure 9). No other animal in any of the groups showed this change. Finally, no sign of ocular neovascularization was observed in any of the groups.
FIGURE 7.
Histologic retinal sections of saline vehicle–treated right eye (Left) and contralateral left eye (Right). No observable differences in thickness and cellular density were noted among the various retinal layers (hematoxylin-eosin, ×400).
FIGURE 8.
Histologic retinal sections of 50-ng rhEPO–treated right eye (Left) and contralateral left eye (Right). No observable differences in thickness and cellular density were noted among the various retinal layers (hematoxylin-eosin, ×400).
FIGURE 9.
Histologic retinal sections of 625 ng rhEPO treated right eye (Left) and contralateral left eye (Right). Visible decreases in the thickness and cellular density of the outer nuclear and inner nuclear layers were noted in the treated eye (Left) when compared to the contralateral eye (Right) (hematoxylin-eosin, ×400).
DISCUSSION
An increasing multitude of studies have shown that EPO and EPO-R are expressed throughout the nervous system and demonstrate remarkable neurotrophic and neuroprotective effects in cell culture and animal models of nervous system disorders.23,70 This function has been shown to occur in different conditions of neuronal damage, such as spinal cord ischemia and injury, neonatal hypoxic ischemia brain injury, subarachnoid hemorrhage, retinal ischemia, and experimental models of glaucoma.23,34,38,44,70 Thus, EPO and EPO-R may play a beneficial role in protecting against the progression of neurologic and retinal degenerative diseases. However, the exact mechanism(s) by which EPO/EPO-R confers its neuroprotective effect is not fully understood. Current hypotheses include reduction of release of reactive oxygen species and glutamate, reversal of vasospasm, attenuation of apoptosis, modulation of inflammation, and recruitment of stem cells.70
Before intravitreal administration of EPO can be advocated for the treatment of human ocular disease, more detailed safety studies will need to be performed to rule out any structural, functional, or neovascularization side effects. Based on published data of vitreous body volume in rats (13.36 ± 0.64 μL),71 the calculated vitreous level (1497.0 U/mL) arising from a single intravitreal EPO dose of 200 ng (20 U) in rats (shown to be effective for neuroprotection)44 appears to be substantially higher than the median vitreous EPO levels of 0.464 U/mL (ie, 464 mU/mL) reported in patients with proliferative diabetic retinopathy.48 For comparison, the final concentrations of the doses injected in the current experiment were calculated at 374.3 to 4678.1 U/mL.
While ERG has been a “gold standard” test for central retinal function, a recent study in women treated with tamoxifen for breast cancer showed some degree of color vision loss and ocular toxic effects in a small subgroup of patients who had normal multifocal ERG amplitudes and latencies.72 Our experimental ERG data did not demonstrate any significant untoward effect of intravitreal injections of EPO (at the various dosages tested). The finding that the OP3-wave latency ratio was decreased for the 625-ng group at days 3, 14, and 21 (compared to baseline) is contrary to that predicted if EPO had an adverse effect, and is therefore presumed to be an artifact. Similarly, the photopic b-wave amplitude ratio was increased at day 21 for the 250-ng group, also the reverse of that predicted if EPO had an adverse effect.
Our qualitative analysis of the histologic specimens should be regarded as preliminary and limited in scope given the lack of quantitative review (eg, RGC counts) and lack of consistent orientation and uniform area sampling. The finding in one rat exposed to the highest dose of 625 ng (62.5 U) of visible reduction in retinal thickness and cell density raises the possibility of a dose-related adverse effect. While we are not aware of any published studies that have reported changes in retinal morphology with intraocular EPO administration, further histologic studies are needed to settle whether this finding is an artifact or a potential adverse effect of higher doses of rhEPO. A larger sample size or longer time points post-EPO injection may have elucidated whether this retinal change was isolated or the result of EPO retinal toxicity. Quantitative measures of retinal thickness and cell density would also have provided better assessment of retinal change. For example, quantitative analysis of RGC viability with a fluorogold neuron tracer44 would have been helpful for histologic examination. Recent technological advances may also allow for in vivo imaging of RGCs with fluorescent biomarkers, thus assisting in the clear identification of dying RGCs. A noninvasive real-time imaging technique utilizing confocal laser-scanning ophthalmoscopy and the biomarker Annexin-5-FITC has been devised for visualization of single nerve cell apoptosis in vivo, thus providing the opportunity to evaluate interventions with clinical applications.73
While in the short term, abnormal signs of neovascularization were not observed in any of the experimental animals, this theoretical concern still exists. In support of this concern, a recent publication demonstrated that EPO has a significant angiogenic effect in rat kidney with cyclosporine A–induced nephrotoxicity, similar to the effect of the classic angiogenic factor basic fibroblast growth factor.74 The current study did not employ more sophisticated staining and/or molecular techniques for identification of new vessel formation and/or angiogenic stimuli. For example, rhodamine-concanavalin-A (ConA lectin) staining, which has been used to screen for abnormal retinal neovascularization,44 was not used on these specimens.
Several factors may have caused the higher IOP levels noted during this study. In addition to the intravitreal injections, postinjection IOPs were taken immediately after each ERG recording in an attempt to minimize the duration of anesthesia needed. Therefore, the IOP levels may have been elevated as a result of the stress of ERG, the conductive medium used to place the electrodes, and/or pupillary dilation. Additional experiments are warranted to assess the possible prolonged effect of injections of EPO on IOP levels without these confounding factors.
This study did not address the potential consequences of multiple intravitreal injections of EPO (eg, longer-term implications of repeated dosing on angiogenesis stimulation) nor explore alternative means of cytokine delivery. While a previous study showed that a single intravitreal injection of EPO (200 ng) is protective against IOP-induced retinal ganglion cell damage,44 the author is not aware of any published study that has evaluated the neuroprotective efficacy of multiple intravitreal EPO administrations. We elected to define the dose-response toxicity of a single EPO injection before addressing that of multiple EPO administration. Furthermore, alternate methods of delivery will deserve consideration. Preliminary studies of the fluocinolone sustained-release intravitreal implant have proven promising for the treatment of posterior uveitis,75 thereby suggesting that this modality might be explored for the delivery of rhEPO. Moreover, the advent of nanotechnology will also allow for additional novel techniques for the effective delivery of EPO to the desired ocular tissues.76
In summary, a single intravitreal injection of rhEPO (at doses ranging from 50 to 625 ng) does not appear to adversely affect retinal function in rats. While ERG responses provide a relatively sensitive and noninvasive means to assess the safety of intravitreal injections, the absence of abnormal responses does not rule out subtle retinal toxicity undetectable by current technology. Nevertheless, the preliminary results warrant further investigation to evaluate fully the ocular safety and potential side effects of this neuroprotective cytokine. While our histologic analysis did not show any abnormal signs of neovascularization, this theoretical concern still exists. Furthermore, an unexpected finding of visible structural retinal change from one animal receiving the highest 625-ng (62.5 U) dose of rhEPO warrants further histologic analysis with quantitative methods. Subsequent studies are also needed to investigate EPO’s efficacy for neuroprotection in animal models of glaucoma. Additional study is also needed to address the efficacy and safety of different delivery methods of EPO, including multiple intravitreal injections and intravitreal sustained-release devices.
ACKNOWLEDGMENTS
Funding/Support: Supported by the Eye Bank for Sight Restoration, Inc, New York, New York; the Irving Hansen Memorial Foundation, New York, New York; and Research to Prevent Blindness, Inc, New York, New York.
Financial Disclosures: None.
Conformity With Author Information: All experimentation was conducted under approved institutional review board, biosafety, and animal care (IACUC) protocols of Columbia University College of Physicians and Surgeons. These protocols conformed in entirety with guidelines approved by the Association for Research in Vision and Ophthalmology.
Other Acknowledgments: I gratefully acknowledge the assistance provided by my colleagues at Columbia University College of Physicians and Surgeons, New York, New York: Max Forbes, MD, for manuscript review; Daniel Hwang, MA, for study conduct and data collection, management, analysis, and interpretation; and Li Wu, MD, for study design/conduct and data collection, management, analysis, and interpretation; and Vanderbilt University School of Medicine, Nashville, Tennessee: Denis O’Day, MD, for manuscript review.
REFERENCES
- 1.Friedman DS, Wolfs RC, O’Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allingham RR, Damji K, Freedman S, Moroi S, Shafranov . Shields’ Textbook of Glaucoma. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 73–115. [Google Scholar]
- 4.McKendrick AM, Sampson GP, Walland MJ, Badcock DR. Contrast sensitivity changes due to glaucoma and normal aging: low-spatial-frequency losses in both magnocellular and parvocellular pathways. Invest Ophthalmol Vis Sci. 2007;48:2115–2122. doi: 10.1167/iovs.06-1208. [DOI] [PubMed] [Google Scholar]
- 5.Gupta N, Ang LC, Noel de Tilly L, Bidaisee L, Yücel YH. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. 2006;90:674–678. doi: 10.1136/bjo.2005.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai JC, Forbes M. Medical Management of Glaucoma. 2nd ed. Caddo, OK: Professional Communications; 2004. pp. 79–112. [Google Scholar]
- 7.Collaborative Normal Tension Glaucoma Study Group Comparison of glaucomatous progression between untreated patients with normal tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DR, Normal Tension Glaucoma Study Group Collaborative normal tension glaucoma study. Curr Opin Ophthalmol. 2003;14:86–90. doi: 10.1097/00055735-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Drance S, Anderson DR, Schulzer M, Collaborative Normal-Tension Glaucoma Study Group Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 10.Lipton SA. Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation. Curr Drug Targets. 2007;8:621–632. doi: 10.2174/138945007780618472. [DOI] [PubMed] [Google Scholar]
- 11.Yücel YH, Gupta N, Zhang Q, Mizisin AP, Kalichman MW, Weinreb RN. Memantine protects neurons from shrinkage in the lateral geniculate nucleus in experimental glaucoma. Arch Ophthalmol. 2006;124:217–225. doi: 10.1001/archopht.124.2.217. [DOI] [PubMed] [Google Scholar]
- 12.Neufeld AH. Brain Res Bull. Vol. 62. 2004. Pharmacologic neuroprotection with an inhibitor of nitric oxide synthase for the treatment of glaucoma; pp. 455–459. [DOI] [PubMed] [Google Scholar]
- 13.Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- 14.Bakalash S, Shlomo GB, Aloni E, et al. T-cell-based vaccination for morphological and functional neuroprotection in a rat model of chronically elevated intraocular pressure. J Mol Med. 2005;83:904–916. doi: 10.1007/s00109-005-0689-6. [DOI] [PubMed] [Google Scholar]
- 15.Thanos C, Emerich D. Delivery of neurotrophic factors and therapeutic proteins for retinal diseases. Expert Opin Biol Ther. 2005;5:1443–1452. doi: 10.1517/14712598.5.11.1443. [DOI] [PubMed] [Google Scholar]
- 16.Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo L, Salt TE, Luong V, et al. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci U S A. 2007;104:13444–13449. doi: 10.1073/pnas.0703707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das A, Garner DP, Del Re AM, et al. Calpeptin provides functional neuroprotection to rat retinal ganglion cells following Ca2+ influx. Brain Res. 2006;1084:146–157. doi: 10.1016/j.brainres.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 19.Levkovitch-Verbin H, Kalev-Landoy M, Habot-Wilner Z, Melamed S. Minocycline delays death of retinal ganglion cells in experimental glaucoma and after optic nerve transaction. Arch Ophthalmol. 2006;124:520–526. doi: 10.1001/archopht.124.4.520. [DOI] [PubMed] [Google Scholar]
- 20.Yang LP, Li Y, Zhu XA, Tso MO. Minocycline delayed photoreceptor death in rds mice through iNOS-dependent mechanism. Mol Vis. 2007;13:1073–1082. [PMC free article] [PubMed] [Google Scholar]
- 21.Neufeld AH, Sawada A, Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc Natl Acad Sci U S A. 1999;96:9944–9948. doi: 10.1073/pnas.96.17.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai JC, Song BJ, Wu L, Forbes M. Erythropoietin: a candidate neuroprotective agent in the treatment of glaucoma. J Glaucoma. 2007;16:567–571. doi: 10.1097/IJG.0b013e318156a556. [DOI] [PubMed] [Google Scholar]
- 23.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–155. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Genc S, Akhisaroglu M, Kuralay F, Genc K. Erythropoietin restores glutathione peroxidase activity in 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine-induced neurotoxicity in C57BL mice and stimulates murine astroglial glutathione peroxidase production in vitro. Neurosci Lett. 2002;321:73–76. doi: 10.1016/s0304-3940(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 25.Maurer MH, Frietsch T, Waschke KF, Kuschinsky W, Gassmann M, Schneider A. Cerebral transcriptome analysis of transgenic mice overexpressing erythropoietin. Neurosci Lett. 2002;3:181–184. doi: 10.1016/s0304-3940(02)00425-1. [DOI] [PubMed] [Google Scholar]
- 26.Sirén AL, Ehrenreich H. Erythropoietin—a novel concept for neuroprotection. Eur Arch Psychiatry Clin Neurosci. 2001;251:179–184. doi: 10.1007/s004060170038. [DOI] [PubMed] [Google Scholar]
- 27.Farrell F, Lee A. The erythropoietin receptor and its expression in tumor cells and other tissues. Oncologist. 2004;9(Suppl 5):18–30. doi: 10.1634/theoncologist.9-90005-18. [DOI] [PubMed] [Google Scholar]
- 28.Bernaudin M, Marti HH, Roussel S, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metabol. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Sirén AL, Radyushkin K, Boretius S, et al. Global brain atrophy after unilateral parietal lesion and its prevention by erythropoietin. Brain. 2006;129:480–489. doi: 10.1093/brain/awh703. [DOI] [PubMed] [Google Scholar]
- 30.Kaptanoglu E, Solaroglu I, Okutan O, Surucu HS, Akbiyik F, Beskonakli E. Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: effect on lipid peroxidation and early ultrastructural findings. Neurosurg Rev. 2004;27:113–120. doi: 10.1007/s10143-003-0300-y. [DOI] [PubMed] [Google Scholar]
- 31.Campana WM, Myers RR. Exogenous erythropoietin protects against dorsal root ganglion apoptosis and pain following peripheral nerve injury. European Journal of Neuroscience. 2003;18:1497–506. doi: 10.1046/j.1460-9568.2003.02875.x. [DOI] [PubMed] [Google Scholar]
- 32.Sekiguchi Y, Kikuchi S, Myers RR, Campana WM. ISSLS prize winner: Erythropoietin inhibits spinal neuronal apoptosis and pain following nerve root crush. Spine. 2003;28:2577–84. doi: 10.1097/01.BRS.0000096674.12519.12. [DOI] [PubMed] [Google Scholar]
- 33.Smith RE., Jr Erythropoietic agents in the management of cancer patients. Part 2: studies on their role in neuroprotection and neurotherapy. Journal of Supportive Oncology. 2004;2:39. [PubMed] [Google Scholar]
- 34.Brines M, Grasso G, Fiordaliso F, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leist M, Ghezzi P, Grasso G, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 36.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 37.Kawakami M, Sekiguchi M, Sato K, Kozaki S, Takahashi M. Erythropoietin receptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. J Bio Chem. 2001;276:39469–39475. doi: 10.1074/jbc.M105832200. [DOI] [PubMed] [Google Scholar]
- 38.Weishaupt J, Rohde G, Pölking E, et al. Effect of erythropoietin axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:1514–1522. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- 39.Malhotra S, Savitz SI, Ocava L, Rosenbaum DM. Ischemic preconditioning is mediated by erythropoietin through PI-3 kinase signaling in an animal model of transient ischemic attack. J Neurosci Res. 2006;83:19–27. doi: 10.1002/jnr.20705. [DOI] [PubMed] [Google Scholar]
- 40.Digicaylioglu M, Garden G, Timberlake S, Fletcher L, Lipton SA. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I. Proc Natl Acad Sci U S A. 2004;101:9855–9860. doi: 10.1073/pnas.0403172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sättler MB, Merkler D, Maier K, et al. Neuroprotective effects and intracellular signaling pathways of erythropoietin in a rat model of multiple sclerosis. Cell Death Differ. 2004;11(Suppl 2):S181–S192. doi: 10.1038/sj.cdd.4401504. [DOI] [PubMed] [Google Scholar]
- 42.Morrison J, Farrell S, Johnson E, Deppmeier L, Moore CG, Grossmann E. Structure and composition of the rodent lamina cribrosa. Exp Eye Res. 1995;60:127–135. doi: 10.1016/s0014-4835(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 43.Junk AK, Mammis A, Savitz SI, et al. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2002;99:10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai JC, Wu L, Worgul B, Forbes M, Cai J. Intravitreal administration of erythropoietin and preservation of retinal ganglion cells in an experimental rat model of glaucoma. Curr Eye Res. 2005;30:1025–1031. doi: 10.1080/02713680500320729. [DOI] [PubMed] [Google Scholar]
- 45.van der Meer P, Voors A, Lipsic E, Smilde T, van Gilst W, van Veldhuisen D. Prognostic value of plasma erythropoietin on mortality in patients with chronic heart failure. J Am Coll Cardiol. 2004;44:63–67. doi: 10.1016/j.jacc.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 46.Henry D, Bowers P, Romano M, Provenzano R. Epoetin alfa: clinical evolution of a pleiotropic cytokine. Arch Intern Med. 2004;164:262–276. doi: 10.1001/archinte.164.3.262. [DOI] [PubMed] [Google Scholar]
- 47.Stockmann C, Fandrey J. Hypoxia-induced erythropoietin production: a paradigm for oxygen-regulated gene expression. Clin Exp Pharmacol Physiol. 2006;33:968–979. doi: 10.1111/j.1440-1681.2006.04474.x. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe D, Suzuma K, Matsui S, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 49.Ribatti D. Leukemia. Vol. 16. 2002. A potential role of erythropoietin in angiogenesis associated with myelodysplastic syndromes; p. 1890. author reply 1891. [DOI] [PubMed] [Google Scholar]
- 50.Ribatti D, Marzullo A, Nico B, Crivellato E, Ria R, Vacca A. Erythropoietin as an angiogenic factor in gastric carcinoma. Histopathology. 2003;42:246–250. doi: 10.1046/j.1365-2559.2003.01581.x. [DOI] [PubMed] [Google Scholar]
- 51.Ribatti D, Vacca A, Dammacco F, English D. Angiogenesis and anti-angiogenesis in hematological malignancies. J Hematother Stem Cell Res. 2003;12:11–22. doi: 10.1089/152581603321210091. [DOI] [PubMed] [Google Scholar]
- 52.Yasuda Y, Fujita Y, Matsuo T, et al. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis. 2003;24:1021–1029. doi: 10.1093/carcin/bgg060. [erratum appears in Carcinogenesis 2003;24:1567] [DOI] [PubMed] [Google Scholar]
- 53.Rakusan K, Cicutti N, Kolar F. Cardiac function, microvascular structure, and capillary hematocrit in hearts of polycythemic rats. Am J Physiol Heart Circ Physiol. 2001;281:H2425–H2431. doi: 10.1152/ajpheart.2001.281.6.H2425. [DOI] [PubMed] [Google Scholar]
- 54.Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, Kuck KH. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res. 2002;64:326–333. doi: 10.1006/mvre.2002.2426. [DOI] [PubMed] [Google Scholar]
- 55.Nakamatsu K, Nishimura Y, Suzuki M, Kanamori S, Maenishi O, Yasuda Y. Erythropoietin/erythropoietin-receptor system as an angiogenic factor in chemically induced murine hepatic tumors. Int J Clin Oncol. 2004;9:184–188. doi: 10.1007/s10147-004-0399-z. [DOI] [PubMed] [Google Scholar]
- 56.Di Raimondo F, Azzaro MP, Palumbo G, et al. Angiogenic factors in multiple myeloma: higher levels in bone marrow than in peripheral blood. Haematologica. 2000;85:800–805. [PubMed] [Google Scholar]
- 57.Hale SA, Wong C, Lounsbury KM. Erythropoietin disrupts hypoxia-inducible factor signaling in ovarian cancer cells. Gynecol Oncol. 2006;100:14–19. doi: 10.1016/j.ygyno.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 58.Hardee ME, Kirkpatrick JP, Shan S, et al. Human recombinant erythropoietin (rEpo) has no effect on tumour growth or angiogenesis. Br J Cancer. 2005;93:1350–1355. doi: 10.1038/sj.bjc.6602846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhong L, Bradley J, Schubert W, et al. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Invest Ophthalmol Vis Sci. 2007;48:1212–1218. doi: 10.1167/iovs.06-0757. [DOI] [PubMed] [Google Scholar]
- 60.King CE, Rodger J, Bartlett C, Esmaili T, Dunlop SA, Beazley LD. Erythropoietin is both neuroprotective and neuroregenerative following optic nerve transection. Exp Neurol. 2007;205:48–55. doi: 10.1016/j.expneurol.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 61.Fu QL, Wu W, Wang H, Li X, Lee VW, So KF. Cell Mol Neurobiol. Vol. 28. 2008. Up-regulated endogenous erythropoietin/erythropoietin receptor system and exogenous erythropoietin rescue retinal ganglion cells after chronic ocular hypertension; pp. 317–329. (Epub 2007 Jun 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garcia-Valenzuela E, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 63.Mittag TW, Danias J, Pohorenec G, et al. Retinal damage after 3 to 4 months of elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2000;41:3451–3459. [PubMed] [Google Scholar]
- 64.Sakanaka M, Wen T-C, Matsuda S, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fortune B, Bui BV, Morrison JC, et al. Selective ganglion cell functional loss in rats with experimental glaucoma. Invest Ophthalmol Vis Sci. 2004;45:1854–1862. doi: 10.1167/iovs.03-1411. [DOI] [PubMed] [Google Scholar]
- 66.Bui BV, Fortune B. Ganglion cell contributions to the rat full-field electroretinogram. J Physiol. 2004;555:153–173. doi: 10.1113/jphysiol.2003.052738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schuettauf F, Eibl K, Thaler S, et al. Toxicity study of erucylphosphocholine in a rat model. Curr Eye Res. 2005;30:813–820. doi: 10.1080/02713680591006093. [DOI] [PubMed] [Google Scholar]
- 68.Saito S, Ohashi M, Naito A, et al. Neuroprotective effect of the novel Na+/Ca2+ channel blocker NS-7 on rat retinal ganglion cells. Jpn J Ophthalmol. 2005;49:371–376. doi: 10.1007/s10384-005-0210-3. [DOI] [PubMed] [Google Scholar]
- 69.Bui BV, Edmunds B, Cioffi GA, Fortune B. The gradient of retinal functional changes during acute intraocular pressure elevation. Invest Ophthalmol Vis Sci. 2005;46:202–213. doi: 10.1167/iovs.04-0421. [DOI] [PubMed] [Google Scholar]
- 70.Buemi M, Cavallaro E, Floccari F, et al. Erythropoietin and the brain: from neurodevelopment to neuroprotection. Clin Sci (Lond) 2002;103:275–282. doi: 10.1042/cs1030275. [DOI] [PubMed] [Google Scholar]
- 71.Dureau P, Bonnel S, Menasche M, Dufier JL, Abitbol M. Quantitative analysis of intravitreal injections in the rat. Curr Eye Res. 2001;22:74–77. doi: 10.1076/ceyr.22.1.74.6974. [DOI] [PubMed] [Google Scholar]
- 72.Salomao SR, Watanabe SE, Berezovsky A, Motono M. Multifocal electretinography, color discrimination, and ocular toxicity in tamoxifen use. Curr Eye Res. 2007;32:345–352. doi: 10.1080/02713680701229638. [DOI] [PubMed] [Google Scholar]
- 73.Cordeiro MF, Guo L, Luong V, et al. Real-time imaging of single cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci U S A. 2004;101:13352–13356. doi: 10.1073/pnas.0405479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Efthimiadou A, Pagonopoulou O, Lambropoulou M, et al. Erythropoietin enhances angiogenesis in an experimental cyclosporine A-induced nephrotoxicity model in the rat. Clin Exp Pharmacol Physiol. 2007;34:866–869. doi: 10.1111/j.1440-1681.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- 75.Brumm MV, Nguyen QD. Fluocinolone acetonide intravitreal sustained release device—a new addition to the armamentarium of uveitic management. Int J Nanomed. 2007;2:55–64. doi: 10.2147/nano.2007.2.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar S. Nanoophthalmology: new frontier in fighting blindness? Eye. 2006;20:1455–1456. doi: 10.1038/sj.eye.6702333. [DOI] [PubMed] [Google Scholar]