Abstract
Purpose
To compare the response of the cornea to laser in situ keratomileusis (LASIK) with flap creation using the IntraLase FS15, FS30, and FS60 femtosecond lasers.
Methods
A retrospective analysis of 55 patients (55 eyes) who underwent LASIK with flap creation using IntraLase was performed. Twelve FS15 patients (12 eyes), 14 FS30 patients (14 eyes), and 29 FS60 patients (29 eyes) were examined 3 months postoperatively by in vivo confocal microscopy. The accuracy of flap thickness, number of interface particles, interface backscatter, epithelial thickness, and activation of keratocytes were determined from the confocal data.
Results
Keratocyte activation was detected in 14 of 55 eyes. In general, keratocyte activation was limited to 1 or 2 cell layers adjacent to the interface. However, 2 eyes exhibited multiple layers of activation by confocal microscopy as well as significant clinical haze by slit-lamp examination. Keratocyte activation and interface backscatter were positively correlated with the raster energy used during surgery (R = 0.51, P < .01) and increased when the steroid treatment time was reduced. Overall, the difference between actual and intended flap thickness was 11.2 ± 8.6 μm, and the density of interface particles was 19.9 ± 12.1 particles/mm2.
Conclusions
LASIK with IntraLase provides more reproducible flap thickness and fewer interface particles than previously observed with use of mechanical microkeratomes. However, IntraLase can induce more significant keratocyte activation, which may underlie clinical observations of haze and transient light sensitivity syndrome in some patients. Activation can be avoided by using lower raster energies and an extended steroid treatment regimen.
INTRODUCTION
Femtosecond laser ablation for laser in situ keratomileusis (LASIK) flap creation (IntraLASIK) was introduced in late 2001 and is growing rapidly in use. Previous studies have shown that the IntraLase laser provides more consistent flap thickness with fewer complications than mechanical microkeratomes and results in better visual outcomes in most patients.1–6 One concern regarding IntraLASIK is that the procedure induces more damage than a microkeratome and therefore stimulates a more pronounced wound healing response, which can lead to transient light sensitivity syndrome, particularly when higher raster energies are used. To address this issue, IntraLASIK has evolved in recent years with the development of new lasers with higher pulse frequencies, which allow for faster procedure time, tighter spot placement, and the use of lower raster energies than first-generation lasers.
Recent studies using in vivo confocal microscopy, which allows assessment of wound healing at the cellular level, have identified increased keratocyte activation following LASIK with flap creation using IntraLase as compared to mechanical microkeratomes.7–12 However, because different confocal systems, time points, and analysis procedures were used, the key parameters that induce or modulate keratocyte activation have not been established quantitatively. In this study, we consolidate and reanalyze 3-D confocal data generated from 3 recent studies that used the same microscope and analysis software,10–12 in order to directly compare the influence of different IntraLase raster energies, pulse frequencies, and postoperative steroid treatments on the corneal response to IntraLASIK.
METHODS
A retrospective analysis of a subset of patients who underwent LASIK with flaps created using IntraLase from 3 previously published studies was performed.10–12 All data accumulation was in conformity with all Federal and State laws and specifically HIPAA (Health Insurance Portability and Accountability Act) guidelines. To allow direct comparisons between these 3 studies, only those patients with 3-month postoperative data were included. Furthermore, for patients in whom both eyes were examined, only one eye (chosen at random) was included for this analysis. Overall, we included clinical and confocal microscopy assessments from 55 eyes of 55 patients who underwent LASIK with flaps created by femtosecond laser ablation using the IntraLase FS15 laser (12 eyes), IntraLase FS30 laser (14 eyes), or IntraLase FS60 laser (29 eyes) between May 12, 2004, and March 16, 2007, at the University of Texas Southwestern Medical Center at Dallas. Five experienced surgeons performed the procedures. Corneas were examined clinically using a slit lamp and by confocal microscopy through focusing (CMTF) in order to assess interface particles, keratocyte activation, and epithelial and total flap thickness following surgery.13,14
LASIK WITH INTRALASE
Ten myopic eyes (attempted correction, –5.0 ± 2.1 diopters) and 2 hyperopic eyes (attempted correction, 2.8 ± 0.5 diopters) underwent LASIK with flap creation using the IntraLase FS15, which has a pulse frequency of 15 kHz. The planned flap diameter ranged from 8.9 to 9.4 mm, the planned flap thickness was 115 to 125 μm, the hinge angle was 50°, and the side cut energy was 3.0 to 3.1 μJ. For 5 eyes a raster energy of 2.6 μJ was used (raster line separation 9 μm, raster spot separation 12 μm), and for 7 eyes a raster energy of 1.9 to 2.0 μJ was used (raster line separation 8 to 9 μm, raster spot separation 9 to 10 μm).
Fourteen myopic eyes (attempted correction, –4.0 ± 1.8 diopters) underwent LASIK with flap creation using the IntraLase FS30, which has a pulse frequency of 30 kHz. The planned flap diameter ranged from 8.8 to 9.5 mm, the planned flap thickness was 115 to 120 μm, the hinge angle was 50°, the raster energy was 1.2 μJ, the raster line separation was 8 μm, the raster spot separation was 8 μm, and the side cut energy was 2.4 μJ.
Twenty-nine myopic eyes (attempted correction, –4.18 ± 3.37 diopters) underwent LASIK with flap creation using the IntraLase FS60, which has a pulse frequency of 60 kHz. The planned flap diameter ranged from 8.5 to 9.4 mm, the planned flap thickness was 110 to 120 μm, the hinge angle was 50°, the raster energy was 1.3 μJ, the raster line separation was 8 μm, the raster spot separation was 8 μm, and the side cut energy was 2.1 μJ.
All flaps had hinges placed superiorly. A standard intrastromal pocket extending outward from the flap’s hinge was also created to facilitate dispersion of the gas layer created by the IntraLase. After flap creation, all eyes were allowed to rest for 10 minutes to allow absorption of the gases from the microcavitation bubbles. The flap was then separated and reflected superiorly. For photoablation, patients were treated with either the Star S4 Custom View Excimer laser (VISX, Santa Ana, California), the LADARVision 4000 Custom Excimer Laser System (Alcon Laboratories, Fort Worth, Texas), or the Allegretto Wave Excimer Laser (Wavelight Laser Technologie AG, Germany).
POSTOPERATIVE TREATMENT
The protocol for steroid treatment for FS15 and FS30 patients was as follows: 1 drop of Econopred Plus (Alcon Laboratories, Fort Worth, Texas) every hour while awake for the first 24 hours, then 4 times a day for 4 weeks. The protocol for steroid treatment for FS60 patients was 1 drop of Econopred Plus every hour while awake for the first 24 hours, then 4 times a day for 7 days. All patients used 1 drop of Vigamox (Alcon Laboratories, Fort Worth, Texas) 4 times a day for the first week.
CONFOCAL MICROSCOPY THROUGH FOCUSING
In vivo confocal microscopy was performed using a previously described system consisting of a tandem scanning confocal microscope interfaced to a PC.14 CMTF scans were performed using a constant internal lens speed of 160 μm/sec, which corresponds to focal plane movement of approximately 64 μm/sec. Each scan started at the tip of the objective, went through the full thickness of the cornea, and ended in the anterior chamber of the eye. Two or 3 CMTF scans were performed on each eye. Scans in which significant eye movements were detected were not included in the quantitative analysis. Following each CMTF scan, a plot of image intensity vs depth (intensity curve), and the CMTF image stack, its 3-D projection, and side views were immediately displayed on the PC monitor and the data were saved. After the examination, the user could identify images of interest and record their exact z-axis position by moving a cursor along the intensity curve as corresponding images were displayed.
The thickness of the corneal epithelium and the LASIK flap were calculated from the intensity curve as previously described.15 Interface particles were also counted from each CMTF scan and converted to a density measure of particles per square millimeter.10 Individual images from the CMTF scans were evaluated for qualitative assessment of changes in keratocyte morphology and/or reflectivity. The area under the CMTF peak corresponding to the flap interface on the intensity curve was calculated as previously described16–18; this measurement incorporates all factors that might contribute to interface backscatter, such as keratocyte activation, edema, interface particles, and debris. The area is expressed in arbitrary confocal backscatter units, defined as μm × pixel intensity.16–18
STATISTICS
Normally distributed data are reported as mean ± standard deviation. Comparisons between 3 or more groups were performed using analysis of variance (ANOVA). Linear regression analysis was used to test for correlations between variables. SigmaStat for Windows (version 3.1; Systat Software, Inc, Point Richmond, California) was used to perform the statistical analysis.
RESULTS
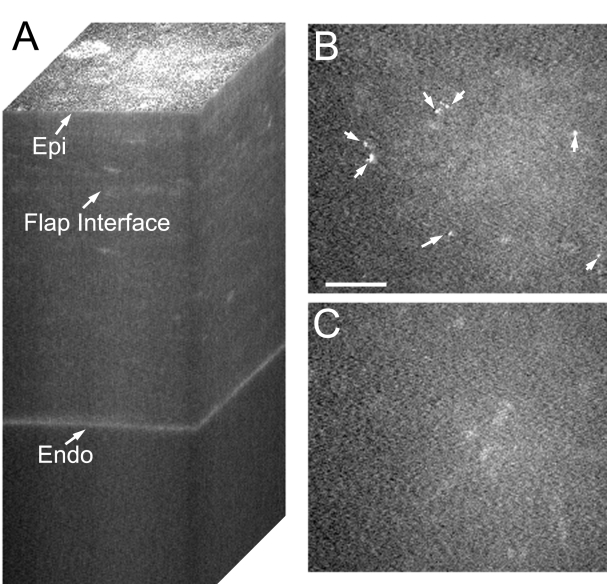
In most of the eyes examined (41 of 55), the corneal stroma appeared quiet by confocal microscopy, and only a subtle increase in reflectivity was detected at the interface. A typical CMTF 3-D reconstruction from such a patient is shown in Figure 1A. Examination of individual confocal images from the stack in these 41 eyes generally revealed a 2- to 9-μm-thick region of decreased cell density surrounding the flap interface (Figure 1B). However, a more normal keratocyte distribution was observed further above and below the interface (Figure 1C). Highly reflective particles were often detected at the interface (Figure 1B, arrows). No keratocyte activation, increased extracellular matrix (ECM) reflectivity (ECM haze), or fibrosis was detected by confocal microscopy in these 41 eyes. Furthermore, although clinical haze was not consistently graded on a scale, little or no interface haze was detected by slit-lamp examination in these eyes.
FIGURE 1.
Confocal images taken after IntraLASIK from a quiet eye. A, Reconstruction of confocal microscopy through focusing (CMTF) stack. Only a slight increase in reflectivity is detected at the interface. B, Single image taken from the CMTF stack in A, at the flap interface. No keratocyte activation is observed, and interface particles are present (arrows). Bar indicates 75 μm. C, Single image taken 20 μm above the interface showing normal quiescent keratocytes. Epi, epithelial surface; Endo, endothelium.
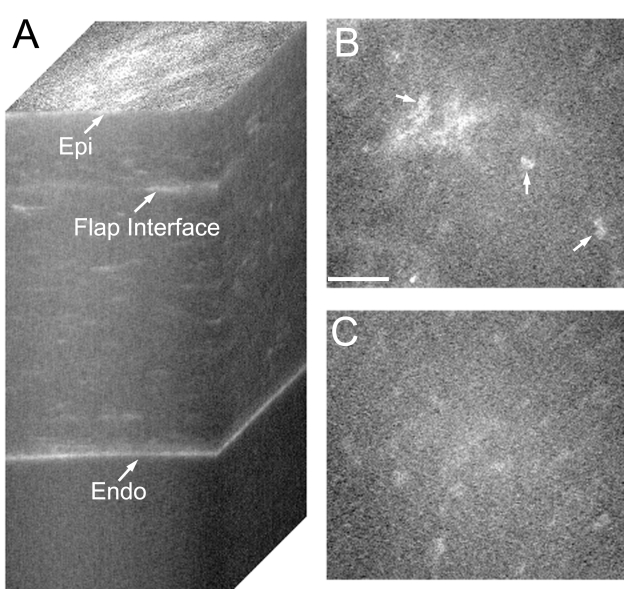
The other 14 eyes showed some degree of keratocyte activation by confocal microscopy. A 3-D stack from one such cornea is shown in Figure 2A. Note the areas of increased reflectivity at the flap interface (2A). Examination of individual images revealed keratocyte activation, as indicated by highly reflective nuclei (Figure 2B, arrows). An increase in ECM reflectivity (ECM haze) was also observed surrounding the cells in many cases. In addition, cell processes were sometimes visible, suggesting local stromal edema and/or fibroblast transformation of corneal keratocytes. Normal keratocytes were always observed above and below the region of activation (Figure 2C). In most cases, when present, the region of keratocyte activation was less than 20 μm thick.
FIGURE 2.
Confocal images taken after IntraLASIK from an eye with keratocyte activation. A, Reconstruction of confocal microscopy through focusing (CMTF) stack. Note the areas of increased reflectivity at the flap interface. B, Single image taken from the CMTF stack in A, at the level of the flap interface. Note highly reflective nuclei (arrows), indicating keratocyte activation. Bar indicates 75 μm. C, Single image from the CMTF stack taken 20 μm above the flap interface. Quiescent keratocytes are observed. Epi, epithelial surface; Endo, endothelium.
Overall, keratocyte activation appeared to be more pronounced at higher raster energies. Of the 14 eyes that showed some degree of keratocyte activation by confocal microscopy, most did not have significant haze detected by slit-lamp examination. However, 2 of the eyes in the FS15 group with the highest raster energy had significant clinical haze. These 2 eyes also had the thickest regions of keratocyte activation and ECM backscatter by confocal microscopy.
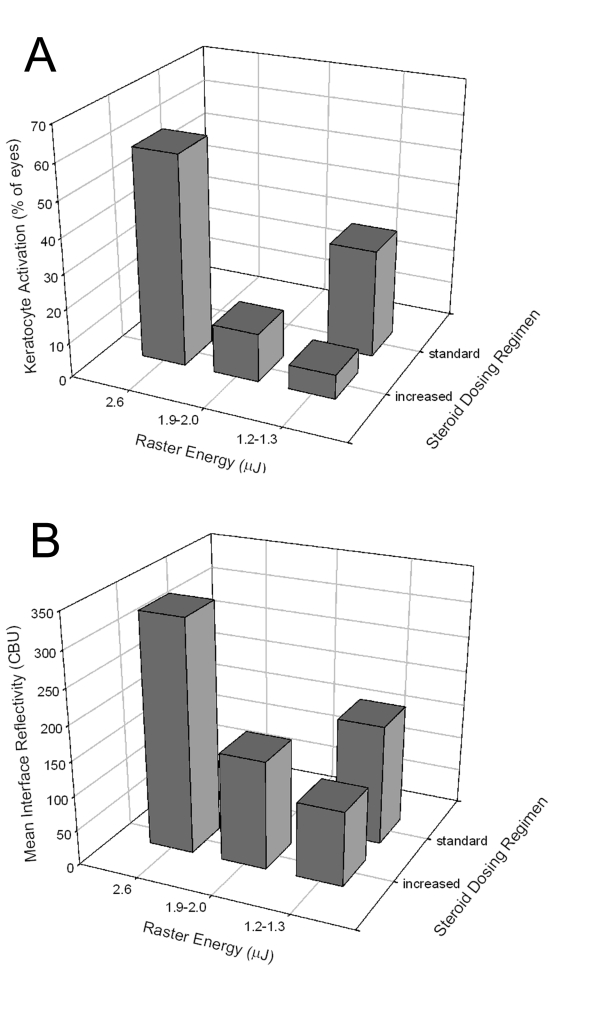
Quantitative confocal data (Table 1) confirmed that higher raster energies were associated with more keratocyte activation. For patients with increased steroid dosing, a decrease in the percent of eyes with keratocyte activation (from 60% to 7%) was found as the raster energy was reduced (Figure 3A). However, when the steroid treatment regimen was reduced to a more standard LASIK dosing (FS60 group), 30% of eyes showed activation even though the raster energy was low. A similar response pattern was observed for confocal measurements of interface backscatter (Figure 3B), which incorporates all factors that might contribute to interface backscatter, such as keratocyte activation, edema, interface particles, and debris. For patients with increased steroid dosing, a significant positive correlation was found between interface backscatter and raster energy (R = 0.51, P < .01, n = 26). However, backscatter also increased when steroids were reduced.
TABLE 1.
SUMMARY OF CONFOCAL DATA IN PATIENTS WHO UNDERWENT IntraLASIK
| VARIABLE | GROUP 1 | GROUP 2 | GROUP 3 | GROUP 4 |
|---|---|---|---|---|
| Laser | FS15 | FS15 | FS30 | FS60 |
| Raster energy (μJ) | 2.6 | 1.9–2.0 | 1.2 | 1.3 |
| Steroid treatment regimen | Increased* | Increased* | Increased* | Standard† |
| Total number of eyes | 5 | 7 | 14 | 29 |
| Number of eyes with keratocyte activation | 3 | 1 | 1 | 9 |
| % of eyes with keratocyte activation | 60 | 14 | 7 | 31 |
| Interface backscatter (CBU) | 331 ± 250 | 155 ± 96 | 107 ± 94 | 172 ± 139 |
| Interface particles (particles/mm2) | 22.4 ± 21.0 | 19.0 ± 15.3 | 11.4 ± 7.3 | 23.8 ± 9.5 |
| DIFT (μm) | 9.0 ± 2.8 | 19.5 ± 9.6 | 13.4 ± 7.1 | 8.8 ± 8.1 |
CBU, confocal backscatter units; DIFT, difference from intended flap thickness.
Every hour for 1 day, 4 times a day for 28 days.
Every hour for 1 day, 4 times a day for 7 days.
FIGURE 3.
Quantitative confocal analysis of cell activation and interface backscatter. A, Effects of raster energy and steroid dosage on the percentage of eyes with keratocyte activation in each group. B, Effects of raster energy and steroid dosage on the measured interface reflectivity in each group. CBU, confocal backscatter units.
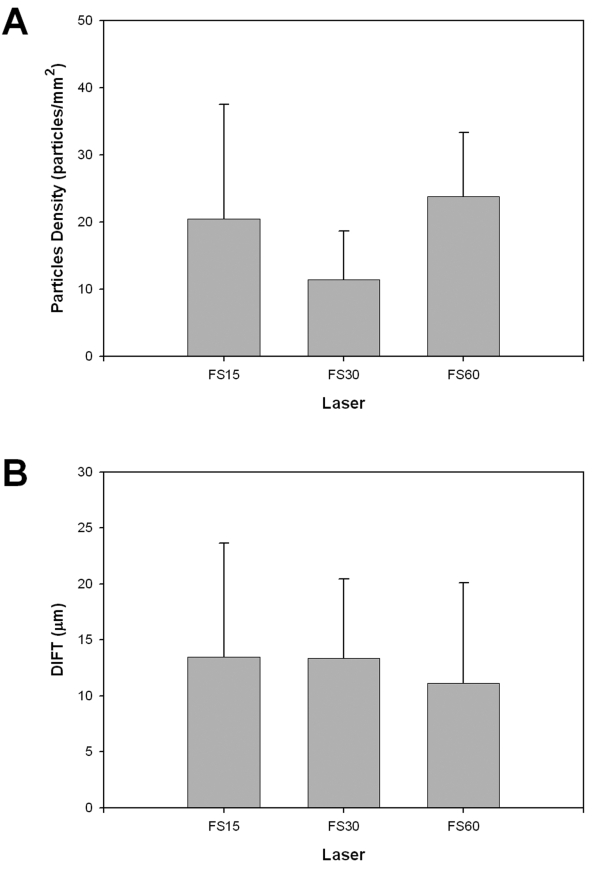
The number of interface particles detected in each cornea was also quantified. The particle density for all eyes combined was 19.9 ± 12.1 particles/mm2 and was lowest in the FS30 eyes (Table 1 and Figure 4A). Interestingly, the particle density was significantly higher in FS60 eyes than in FS30 eyes (P < .05, 1-way ANOVA on ranks), despite the fact that similar raster energies and raster line and spot spacings were used. A statistically significant correlation was found between the number of interface particles and the amount of interface backscatter, but the correlation coefficient was low (R = 0.27, P < .01, n = 56).
FIGURE 4.
Confocal assessment of interface particles and flap thickness. A, Measured flap interface particle density for different IntraLase pulse frequencies. B, Difference from intended flap thickness (DIFT) for different IntraLase pulse frequencies.
The overall difference from intended flap thickness (DIFT) was 11.2 ± 8.6 μm. Thus the flaps were slightly thicker on average at the time of observation than the intended IntraLase thickness setting used at the time of surgery. No correlation or pattern of association was apparent between raster energy and DIFT (Table 1). When grouped by laser type, the mean DIFT was lowest in the FS60 eyes (Figure 4B), although no statistically significant differences were identified. Epithelial thickness was also measured from the CMTF stacks. No significant differences in epithelial thickness were observed between groups. For all eyes combined, epithelial thickness was 51.6 ± 6.8 μm, which is close to previously established normal epithelial thickness in humans measured with the same microscope (51 μm).19
DISCUSSION
Confocal microscopy allows assessment of the cellular response of the cornea to infection, injury, and surgical procedures. Confocal microscopy following photorefractive keratectomy has demonstrated that the development of clinical corneal haze is correlated with the activation of corneal keratocytes and transformation to a fibroblast or myofibroblast phenotype.16–18,20 These activated cells are more reflective than quiescent corneal keratocytes and synthesize ECM components that also reduce corneal transparency. Keratocyte activation has also been identified by confocal microscopy following microkeratome-assisted LASIK.21,22 However, the degree of keratocyte activation and clinical haze is markedly less than that induced by PRK. Previous studies have shown that IntraLase provides more consistent flap thickness with fewer complications than traditional microkeratomes and results in better visual outcome in most patients.1–6 In this study, we compare the effects of different IntraLase raster energies, pulse frequencies, and postoperative steroid treatments on the corneal response to IntraLASIK.
One important concern regarding IntraLASIK is that the procedure induces more damage than a microkeratome and can therefore stimulate a more pronounced wound healing response. In this study, for patients with the same steroid dosing regimen, a decrease in the percent of eyes with keratocyte activation as well as amount of interface reflectivity was found as the raster energy was reduced. However, when the steroid treatment regimen was shortened (FS60 group), activation and reflectivity increased even though the raster energy was low. Most eyes with keratocyte activation had little or no haze detected by clinical examination. However, at the highest raster energy used, 2 eyes were found to have significant clinical haze by slit-lamp examination. Although they did not fit the criteria for our comparative analysis, we previously identified 2 patients with transient light sensitivity syndrome using similar raster energies.10 Overall, the data demonstrate that IntraLase can induce significant keratocyte activation, which may underlie clinical observations of haze in some patients. Activation can be avoided by using lower raster energies and an extended steroid treatment regimen.
Interface particles of varying sizes and reflectivity have been well documented using confocal microscopy following traditional LASIK.15,21,23–27 The particles observed following IntraLASIK in this study looked nearly identical to those reported following microkeratome-assisted LASIK, and both small and large particles were detected. However, the overall particle density (19.9 ± 12.1 particles/mm2) was much lower than that reported following microkeratome-assisted LASIK (135 to 400 particles/mm2).21,25,27 Potential sources of the particles include products of cell degradation, particles from the irrigation cannula, debris from sponges or drapes, and dust particles from the air.21,25,27 The ability to use smaller line and spot spacing with newer-generation IntraLase lasers reduces the amount of surgeon manipulation required to lift the flap, which might be expected to lead to a reduction in the number of interface particles. However, the particle density was significantly higher in FS60 eyes than in FS30 eyes, despite the fact that similar raster energies and raster line and spot separations were used. Thus the relationshsip between specific surgical parameters and interface particle density following IntraLASIK is still unknown.
Confocal microscopy was also used to measure flap thickness and epithelial thickness. The overall DIFT in this study was 11.2 ± 8.6 μm; thus IntraLase slightly overcut the flap on average, consistent with previous observations.1,5,28,29 The standard deviation was on the low end of previous confocal measurements following microkeratome-assisted LASIK, which ranged from 9.5 to 28 μm.15,21,24–26 No correlation or pattern of association was apparent between raster energy and DIFT. When grouped by laser type, the mean DIFT was lowest in the FS60 eyes, although no statistically significant differences were identified. All models stand in contrast to microkeratomes, which generally create flaps that are thinner than intended.15,21,24–26 It should be noted that since our flap thickness measurements were made 3 months after surgery, they could have been affected by any changes in epithelial thickness that occurred postoperatively. Epithelial hyperplasia has been reported following traditional LASIK in both the rabbit and human.15,26,30 However, mean epithelial thickness at 3 months was 51.6 ± 6.8 μm, which was close to previously established “normal” epithelial thickness in humans (~51 μm) measured using our instrument.16 Thus, significant changes in epithelial thickness did not appear to occur in these patients.
CONCLUSIONS
LASIK with IntraLase provides more reproducible flap thickness and fewer interface particles than previously observed using mechanical microkeratomes. However, IntraLase can induce more significant keratocyte activation, which may underlie clinical observations of haze in some patients. Activation can be avoided by using lower raster energies and an extended steroid treatment regimen. This observed keratocyte activation could potentially be of benefit in side cut keratoplasty surgery (IntraLase enabled keratoplasty).
PEER DISCUSSION
DR. REZA DANA
Femtosecond laser ablation (vs. the standard use of microkeratomes) for LASIK flap creation is increasingly in use. Different femtosecond laser systems have been developed, but the one with the most clinical experience is the IntraLase® laser, which has been in use since the early part of this decade. Laser-assisted flap creation has been reported to provide consistent flap thickness, and potentially a lower incidence of mechanical and optical complications, including aberrations, thereby leading to excellent visual outcomes.1 However, one of the concerns about this technology has been that it induces more damage to the corneal stroma, and thereby induces a more vigorous wound healing response with the ensuing keratocyte and extracellular matrix (ECM) changes that can lead to haze or photophobia. Femtosecond technology has evolved, however, with newer lasers allowing for higher frequency pulses that allow for tighter, smaller spots that receive lower (raster) energy.
McCulley and Petroll are to be congratulated for performing a systematic evaluation of several woundhealing parameters after IntraLase®-assisted flap creation for LASIK. The authors retrospectively evaluate the accuracy of flap thickness, interface particle density, interface backscatter, epithelial thickness, and “keratocyte activation” for three different IntraLase techniques using different pulse frequencies: IntraLase FS15, FS30, and FS60, using 15 kHz, 30 kHz, and 60 kHz pulse frequencies respectively. Their data derive from confocal microscopic evaluations of the patients’ eyes after the different IntraLase® protocols and result from consolidation and comparison of 3-month postoperative outcomes from 55 eyes of 55 patients in three previously conducted (and published; references 7–9 of the manuscript) studies.
Briefly, they report that a majority (41 of 55 [75%]) of the corneas are quiet at 3 months postoperatively, with only minimal changes in reflectivity. As expected, there is some keratocyte cell density decrease around the flap interface but a normal distribution above and below the interface. Overall, they note a mean difference between actual and intended flap thickness of ~11 microns (confirming previous reports of a slightly overcut flap with IntraLase®), and interface particle density of ~20 particles/mm2. While there was a higher particle density with the 60 KHz vs. the 30 KHz treatment, there was no significant overall trend between higher frequency IntraLase® (which allows for lower raster energy and closer spot separation) and particle density since the 15 KHz treatment was also associated with a relatively high interface particle density.
The central focus of this study is the effect of IntraLase® parameters, in particular raster energy, on “keratocyte activation”. The authors’ data suggest that among the 14 (of 55, or 25% of) eyes that demonstrated keratocyte activation, there was an association between raster energy and the confocal measures of heightened interface wound healing (including keratocyte activation). These data are important because they emphasize how the higher frequency lasers, which allow for tighter and lower energy spots, allow for a less robust wound healing response.
Several questions remain. First, the postoperative steroid regimen used for the eyes treated with the higher frequency (60 KHz FS60) IntraLase® was limited to one week as opposed to the four weeks provided for patients treated with the 15 KHz or 30 KHz treatments. While amongst the 4-week steroid treated eyes, there was an association (R=0.51, P<0.01) between higher raster energy and interface backscatter (which incorporates keratocyte activation), this association was not demonstrable for the 60 KHz-treated group that received low raster energy but demonstrated a more than four-fold increased incidence of keratocyte activation (31%) as compared to the 30 KHz-treated group (7%). The authors argue that in this case, this was due to the attenuated steroid regimen, and not the laser settings. While this certainly makes logical sense, it cannot be definitively supported, or refuted, based on the data provided alone. It would be valuable to address this issue in future studies focused on the higher frequency IntraLase® where the postoperative steroid regimen would be directly comparable to that used for the lower frequency treatments.
Second, while it is clear that femtosecond laser parameters can affect interface biology, as the authors’ previous work has demonstrated, so do factors related to the excimer photoablation performed under the flap as has been suggested by Sonigo et al.2 The authors of the current work used three different excimer systems for the photoablative treatments; the differential contribution of these lasers to the interface biology, if any, cannot be determined from the data presented.
Third, while the concept of “keratocyte activation” has been in the corneal wound healing literature for some time, it remains imprecisely defined. The definition is largely descriptive and morphological, rather than biological. The current authors define activated keratocytes as having reflective nuclei and associated variable cell processes and surrounding ECM haze. They interpret these changes as potentially signifying “fibroblastic transformation”. However, it is noteworthy that the corneal stroma also possesses a heterogeneous population of bone marrow-derived myeloid leukocytes, including CD11b+ macrophages and CD11c+ dendritic cells.3 These cells, whose identity, distribution, and biology has only been recently determined (until half a dozen years ago, the dogma held that the cornea was devoid of bone marrow-derived cells) are activated in response to the microenvironmental (e.g. cytokine) alterations as a result of inflammation, including those seen in refractive surgery (see excellent review by Netto et al4). These leukocytes contribute to wound healing as well as both innate and adaptive antigen-specific immunity in the cornea, as they do in all other tissues. When activated in the cornea in response to inflammation, they become hyperreflective and their processes change shape, morphologically almost indistinguishable at the magnifications afforded by tandem scanning confocal imaging from cells often labeled as “activated keratocytes” by many investigators. Since they also get recruited close to areas where there is keratocyte dropout (from apoptosis), it is easy to identify these cells as activated keratocytes, or vice versa, unless special staining or microscopic studies (such as intravital microscopy) are performed to distinguish these cells. This is not just of academic interest, since the molecular regulatory mechanisms of keratocyte and leukocyte activation are quite distinct; that is, pharmacological approaches needed to alter keratocyte activation are expected to differ from those that alter leukocyte activation and recruitment.
The current study by McCulley and Petroll makes a significant contribution to our understanding of the role of raster energy in affecting stromal wound healing responses, in particular those that lead to interface reflectivity. Future studies focusing on further characterizing the keratocyte and leukocyte activation patterns, as a result of various surgical parameters, would provide more information on the complex tissue responses to laser-assisted refractive procedures as well as clues to potential new therapeutics that can be used to suppress the complications associated with these surgical procedures.
REFERENCES
- 1.Tran DB, M AS, Bor Z. Randomized prospective clinical study comparing induced aberrations with IntraLase and Hansatome flap creation in fellow eyes. Potential impact on wavefront-guided laser in situ keratomileusis. J Cataract Refract Surg. 2005;31:97–105. doi: 10.1016/j.jcrs.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 2.Sonigo B, Iordanidou V, Chong-Sit D, et al. In vivo corneal confocal microscopy comparison of intralase femtosecond laser and mechanical microkeratome for laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2006;47:2803–2811. doi: 10.1167/iovs.05-1207. [DOI] [PubMed] [Google Scholar]
- 3.Hamrah P, Liu Y, Zhang Q, Dana R. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol. 2003;121:1132–1140. doi: 10.1001/archopht.121.8.1132. [DOI] [PubMed] [Google Scholar]
- 4.Netto MV, Mohan RR, Ambrosio R, Jr, Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
DR. JAMES CHODOSH
No conflicts of interest. The issue I had with this was really the very definition of keratocyte activation. I think that the term is used in a nebulous fashion and we do not really have the appropriate biochemical and molecular correlates for what we are seeing on confocal microscopy. Reza very nicely showed that we may not even be looking at keratocytes when we see these cells that are so hyper-reflective by confocal imaging. Thank you.
DR. DOUGLAS D. KOCH
I consult with Alcon and AMO. My question is whether there are clinical implications of these findings. Are these just interesting confocal findings or do they affect the rate of recovery of vision, algorithms because of differential healing at the edge of the flap, or patient symptoms related to the increased back scatter? Are there also implications for reducing the risk of developing ectasia? George Waring and Henry Edelhauser have shown that most LASIK flap healing occurs at the flap edge. Thus, a magnified healing response from the sidecut might reduce the risk of ectasia,
DR. STEVEN E. WILSON
I have no relevant conflicts. My fellow Marcelo Netto and I, the year before last, published a paper on the cell biology of the femtosecond laser compared with the microkeratome and we also looked at all three of these models, the 15, 30 and 16 kHz. The key finding in that study was that the femtosecond laser directly causes necrosis of the keratocytes along the lamellar cut, as compared to the microkeratome which primarily produces apoptosis along the cut. The difference that produces is a much greater inflammatory potential with the femtosecond laser. We showed that with the 15 kHz and 30 kHz lasers, where the energy levels were higher, that there was a much greater level of necrosis and, therefore, many more inflammatory cells. By the time we got to the 60 KHz, even though there was still necrosis, the level of energy delivered resulted in such a low level of necrosis that there was no clinical significance in inflammation or opacity producing cells, the myofibroblast cells, compared with the microkeratome. With 60 kHz there is no difference. We still have not studied the new 150 kilohertz laser yet, but there is no clinically significant difference in the wound-healing response between the microkeratome and the 60 kHz femtosecond laser. Thank you.
DR. Richard L. Lindstrom: I consult for AMO, VISX, IntraLase, and for their competitors Bausch & Lomb, and Alcon. I also practice lasik and use, the IntraLase, the Amadeus, and the Bausch & Lomb microkeratome, and charge a fee for what I do.
It has been interesting to me, as a long-term corneal surgeon, that there is no doubt that sutured wounds heal much better than non-sutured wounds. For example, the healing that occurs after an RK incision is much less than with a keratoplasty incision or a corneal laceration that is sutured. The question I would like to ask to Dr.McCulley is whether or not there is direct keratocyte activation, or whether it is really secondary to inflammation which has been mentioned earlier. There is no doubt that the femtosecond laser created flap heals much more strongly and has better biomechanical strength. It is much more difficult to lift when you do an enhancement and there are less surface microstriae if you scrape off the epithelium off when you do PRK enhancement, and there is less epithelial ingrowth. It has been my impression that this is at least partially related to inflammation. Inflammation is manageable both with the required energy and also with corticosteroids given postoperatively. If that is the case, we can probably learn as clinicians how to modulate the ideal amount of inflammation to create the ideal amount of wound healing. Is it inflammation that we can modulate or the direct effect of the femtosecond laser on the cornea that activates the keratocytes?
DR. JAMES E MCCULLEY
The cornea guys were hiding in the woods with all those retina papers, looking for an excuse to get up here. I thank all of you for your wonderful comments. Just to correct Reza, this was not really a retrospective study. All of the data presented was acquired from previous prospective studies. We combined data from these studies to perform the analysis of the four different phases presented today. It is important to note that the only real differences between the FS60 and the FS30 arms of the study were the hertz rate of the IntraLase and the corticosteroid regimen that we used. Our FS15 and FS30 results clearly demonstrate that it is the raster energy and not the hertz rate that determines the wound healing response. The raster energy and other parameters were nearly identical between FS60 and FS30, but we just simply reduced the corticosteroid use. The purpose was to determine if we could develop a more manageable and patient acceptable corticosteroid regimen with the new laser technologies that would allow us to reduce the energy delivered to the tissue. That was the principal change there.
Our clinical results are consistent with those of Dr. Wilson in the rabbit model, which demonstrated higher raster energies led to increased keratocyte activation and myofibroblast transformation. We also agree with Dr. Wilson that necrosis and subsequent active inflammation probably underlie these changes after IntraLase, just as they do in PRK. As for the issue of bone derived cells in the corneal stroma, this is certainly a topic of growing interest in the field of stromal biology. There is a large body of both in vitro and in vivo data that suggests the cells we observed are most likely keratocyte-derived. That being said, the issue certainly warrants further investigation as suggested by Dr. Dana. Whether there is a real difference in peripheral healing that might be of advantage to minimize secondary ectasia, I do not know. We did not assess that question. We have studied the visual outcomes and symptoms in these patients and have found no other significant changes to suggest that anything else is occurring in these eyes. I believe that addresses all of the comments. I appreciate all of the comments. I always learn more from presenting and hearing the comments from the members of the Society, than I do from virtually anything else. Thank you.
ACKNOWLEDGMENTS
Funding/Support: This study was supported in part by grants R01 EY013322 (W.M.P.) and Departmental infrastructure grant EY016664 from the National Institutes of Health, and a Senior Scientific Investigator Award (W.M.P.) and an unrestricted grant from Research to Prevent Blindness, Inc, New York, NY.
Financial Disclosures: None
Author Contributions: Design and conduct of the study (J.P.M., W.M.P.); Collection, management, analysis, and interpretation of data (J.P.M., W.M.P.); Preparation of the manuscript (W.M.P.); Review and approval of the manuscript (J.P.M.).
Conformity With Author Information: All data accumulation was in conformity with all Federal and State laws and specifically HIPAA guidelines.
REFERENCES
- 1.Binder PS. Flap dimensions created with the IntraLase FS laser. J Cataract Refract Surg. 2004;30:26–32. doi: 10.1016/S0886-3350(03)00578-9. [DOI] [PubMed] [Google Scholar]
- 2.Sugar A. Ultrafast (femtosecond) laser refractive surgery. Curr Opin Ophthalmol. 2002;13:4. doi: 10.1097/00055735-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Durrie DS, Kezirian GM. Femtosecond laser versus mechanical keratome flaps in wavefront-guided laser in situ keratomileusis: prospective contralateral eye study. J Cataract Refract Surg. 2005;31:120–126. doi: 10.1016/j.jcrs.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 4.Tran DB, Sarayba MA, Bor Z, et al. Randomized prospective clinical study comparing induced aberrations with IntraLase and Hansatome flap creation in fellow eyes: potential impact on wavefront-guided laser in situ keratomileusis. J Cataract Refract Surg. 2005;31:97–105. doi: 10.1016/j.jcrs.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Kezirian GM, Stonecipher KG. Comparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. J Cataract Refract Surg. 2004;30:804–811. doi: 10.1016/j.jcrs.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Nordan LT, Slade SG, Baker RN, Suarez C, Juhasz T, Kurtz R. Femtosecond laser flap creation for laser in situ keratomileusis: six-month follow-up of initial U.S. clinical series. J Refract Surg. 2003;19:8–14. doi: 10.3928/1081-597X-20030101-03. [DOI] [PubMed] [Google Scholar]
- 7.Patel SV, Maguire LJ, McLaren JW, Hodge DO, Bourne WM. Femtosecond laser versus mechanical microkeratome for LASIK: a randomized controlled study. Ophthalmology. 2007;114:1482–1490. doi: 10.1016/j.ophtha.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 8.Javaloy J, Vidal MT, Abdelrahman AM, Artola A, Alio JL. Confocal microscopy comparison of intralase femtosecond laser and Moria M2 microkeratome in LASIK. J Refract Surg. 2007;23:178–187. doi: 10.3928/1081-597X-20070201-10. [DOI] [PubMed] [Google Scholar]
- 9.Sonigo B, Iordanidou V, Chong-Sit D, et al. In vivo corneal confocal microscopy comparison of intralase femtosecond laser and mechanical microkeratome for laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2006;47:2803–2811. doi: 10.1167/iovs.05-1207. [DOI] [PubMed] [Google Scholar]
- 10.Petroll WM, Goldberg D, Lindsey SS, et al. Confocal assessment of the corneal response to intracorneal lens insertion and LASIK with flap creation using IntraLase. J Cataract Refract Surg. 2006;32:1119–1128. doi: 10.1016/j.jcrs.2006.01.093. [DOI] [PubMed] [Google Scholar]
- 11.Petroll WM, Bowman RW, Cavanagh HD, Verity SM, Mootha VV, McCulley JP. Assessment of Keratocyte Activation Following LASIK with Flap Creation using the IntraLase® FS60 Laser. J Refract Surg. 2008;24:847–849. doi: 10.3928/1081597x-20081001-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu MY, McCulley JP, Cavanagh HD, et al. Comparison of the corneal response to laser in situ keratomileusis with flap creation using the FS15 and FS30 femtosecond lasers: clinical and confocal findings. J Cataract Refract Surg. 2007;33:673–681. doi: 10.1016/j.jcrs.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Li HF, Petroll WM, Moller-Pedersen T, Maurer JK, Cavanagh HD, Jester JV. Epithelial and corneal thickness measurements by in vivo confocal microscopy through focusing (CMTF) Curr Eye Res. 1997;16:214–221. doi: 10.1076/ceyr.16.3.214.15412. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Jester JV, Cavanagh HD, Black TD, Petroll WM. On-line 3-dimensional confocal imaging in vivo. Invest Ophthalmol Vis Sci. 2000;41:2945–2953. [PubMed] [Google Scholar]
- 15.Gokmen F, Jester JV, Petroll WM, McCulley JP, Cavanagh HD. In vivo confocal microscopy through-focusing to measure corneal flap thickness after laser in situ keratomileusis. J Cataract Refract Surg. 2002;28:962–970. doi: 10.1016/s0886-3350(02)01275-0. [DOI] [PubMed] [Google Scholar]
- 16.Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: A 1-year confocal microscopic study. Ophthalmology. 2000;107:1235–1245. doi: 10.1016/s0161-6420(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 17.Moller-Pedersen T, Li H, Petroll WM, Cavanagh HD, Jester JV. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 1998;39:487–501. [PubMed] [Google Scholar]
- 18.Møller-Pedersen T, Vogel M, Li HF, Petroll WM, Cavanagh HD, Jester JV. Quantification of stromal thinning, epithelial thickness, and corneal haze after photorefractive keratectomy using in vivo confocal microscopy. Ophthalmology. 1997;104:360–368. doi: 10.1016/s0161-6420(97)30307-8. [DOI] [PubMed] [Google Scholar]
- 19.Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology. 2000;107:1235–1245. doi: 10.1016/s0161-6420(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 20.Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998;17:627–639. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Vesaluoma M, Perez-Santonja J, Petroll WM, Linna T, Alio J, Tervo T. Corneal stromal changes induced by myopic LASIK. Invest Ophthalmol Vis Sci. 2000;41:369–376. [PubMed] [Google Scholar]
- 22.Vesaluoma MD, Petroll WM, Perez-Santonja JJ, Valle TU, Alio JL, Tervo TM. LASIK flap margin: wound healing and complications imaged by in vivo confocal microscopy. Am J Ophthalmol. 2000;130:564–573. doi: 10.1016/s0002-9394(00)00540-7. [DOI] [PubMed] [Google Scholar]
- 23.Buhren J, Kohnen T. Stromal haze after laser in situ keratomileusis. Clinical and confocal microscopy findings. J Cataract Refract Surg. 2003;29:1718–1726. doi: 10.1016/s0886-3350(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 24.Avunduk AM, Senft CJ, Emerah S, Varnell ED, Kaufman HE. Corneal healing after uncomplicated LASIK and its relationship to refractive changes: a six-month prospective confocal study. Invest Ophthalmol Vis Sci. 2004;45:1334–1339. doi: 10.1167/iovs.03-1025. [DOI] [PubMed] [Google Scholar]
- 25.Pisella P-J, Auzerie O, Bokobza Y, Debbasch C, Baudouin C. Evaluation of corneal stromal changes in vivo after laser in situ keratomileusis with confocal microscopy. Ophthalmology. 2001;108:1744–1750. doi: 10.1016/s0161-6420(01)00771-0. [DOI] [PubMed] [Google Scholar]
- 26.Erie JC, Patel SV, McLaren JW, et al. Effect of myopic laser in situ keratomileusis on epithelial and stromal thickness: a confocal microscopic study. Ophthalmology. 2002;109:1447–1452. doi: 10.1016/s0161-6420(02)01106-5. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Gomez I, Efron N. Confocal microscopic evaluation of particles at the corneal flap interface after myopic laser in situ keratomileusis. J Cataract Refract Surg. 2003;29:1373–1377. doi: 10.1016/s0886-3350(03)00249-9. [DOI] [PubMed] [Google Scholar]
- 28.Binder PS. One thousand consecutive IntraLase laser in situ keratomileusis flaps. J Cataract Refract Surg. 2006;32:962–969. doi: 10.1016/j.jcrs.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 29.Talamo JH, Meltzer J, Gardner J. Reproducibility of flap thickness with IntraLase FS and Moria LSK-1 and M2 microkeratome. J Refract Surg. 2006;22:556–561. doi: 10.3928/1081-597X-20060601-07. [DOI] [PubMed] [Google Scholar]
- 30.Ivarsen A, Moller-Pedersen T. LASIK induces minimal regrowth and no haze development in rabbit corneas. Curr Eye Res. 2005;30:363–373. doi: 10.1080/02713680590964848. [DOI] [PubMed] [Google Scholar]