Abstract
Purpose
To determine the limits of the normal amount of interocular symmetry in retinal nerve fiber layer (RNFL) thickness measurements obtained with third-generation time domain optical coherence tomography (OCT3).
Methods
Both eyes of normal volunteers were scanned using the peripapillary standard and fast RNFL algorithms of OCT3.
Results
A total of 108 volunteers were included in the analysis. The mean ± standard deviation (SD) of age of the volunteers was 46.0 ± 15.0 years (range 20–82). Forty-two participants (39%) were male and 66 (61%) were female. Mean RNFL thickness correlated extremely well, with intraclass correlation coefficients of 0.89 for both algorithms (95% confidence interval [CI], 0.84–0.93). The mean RNFL thickness of the right eye measured 1.3 μm thicker than the left on the standard scan (SD 4.7, 95% CI 0.4–2.2, P = .004) and 1.2 μm on the fast scan (SD 5.2, 95% CI 0.1–2.2, P = .026). The 95% tolerance limits on the difference between the mean RNFL thicknesses of right minus left eye was −10.8 and +8.9 μm with the standard scan algorithm and −10.6 and +11.7 μm with the fast scan algorithm.
Conclusions
Mean RNFL thickness between the 2 eyes of normal individuals should not differ by more than approximately 9 to 12 μm, depending on which scanning algorithm of OCT3 is used and which eye measures thicker. Differences beyond this level suggest statistically abnormal asymmetry, which may represent early glaucomatous optic neuropathy.
INTRODUCTION
Glaucoma is a slowly progressive optic neuropathy characterized clinically by an increase in the size of the optic nerve cup and loss of peripheral, followed by central, vision. Pathologically, the retinal ganglion cells die by apoptosis and their axons disappear, over and above the normal apoptotic loss that occurs naturally with age. The retinal nerve fiber layer (RNFL) is the retinal layer that contains the axons of the retinal ganglion cells, connecting their cell bodies to the lateral geniculate nucleus. Quigley’s study of experimental glaucoma in nonhuman primates demonstrated that glaucoma causes progressive thinning and atrophy of the RNFL.1 As retinal nerve fiber axons die because of aging, glaucoma, or both, the RNFL becomes progressively thinner and the optic cup becomes progressively larger. Structural abnormalities of the RNFL and optic cup are often recognized several years before the accompanying loss of function is recognized with visual field testing. Pathologic studies have shown that 25% to 35% of retinal nerve fiber axons may be lost before any detectable defects appear with standard white-on-white automated perimetry.2–4 Clinically, this is manifested by optic nerve cupping that may precede detectable visual field loss in early glaucoma.5,6
In 1913, Vogt7 first observed that striations in the retina, representing the RNFL axon bundles, could be observed using the red-free light of a direct ophthalmoscope. Over 50 years later, Behrendt and Wilson8 noticed that RNFL visibility was affected by the color of the light with which the fundus is viewed. Capitalizing on this observation, Hoyt and colleagues9–11 are credited with making the association between clinical changes in the appearance of the RNFL with red-free light and early glaucoma. In their letter to The Lancet in 1972, they reported “slit-like defects among arcuate fibre bundles of the retina that correlate perimetrically with arcuate defects in the visual field.”9(p 693) In the actual published study that they refer to in that letter,11 Hoyt and colleagues demonstrated that abnormalities in the RNFL as viewed by red-free photographs may precede optic disc or visual field abnormalities. Subsequently, several investigators confirmed that defects in the RNFL can precede visual field loss and that these defects are best evaluated photographically, particularly with red-free photographs.12–15
Sommer and associates12 reviewed serial photographs of the optic discs and peripapillary retinas in patients who did and did not develop visual field defects. They found reproducible RNFL abnormalities an average of 1 to 1.5 years before the recognition of visual field defects on Goldmann perimetry, some as early as 5 years before. They also demonstrated that diffuse defects were more representative of damage than slit-like defects, which were more likely to be artifacts. Additionally, they showed that it was more difficult to appreciate RNFL changes in blacks and in older individuals, exactly those more likely to develop glaucoma. They discovered that it was difficult to teach RNFL appreciation and interpretation to general ophthalmologists. Quigley and colleagues13 performed a cross-sectional study of normal eyes, eyes with glaucoma, and eyes suspected of having glaucoma using red-free photographs and determined a much higher prevalence of RNFL defects in glaucoma patients (84%) compared to glaucoma suspects (13%) or normal subjects (3%). In that report, they speculate that the 13% of glaucoma suspects that had RNFL abnormalities might already have had glaucoma but that the glaucoma was below the threshold detectable with visual fields, at that time manual kinetic (Goldmann) perimetry. They also identified more localized than diffuse atrophy of the RNFL, although the latter is more difficult to recognize.
Airaksinen and associates14 reviewed stereo and red-free photographs of the optic disc and RNFL, respectively, in 25 ocular hypertensive patients with optic disc hemorrhages. They found that RNFL defects followed in the same location as optic disc hemorrhages in these patients. Airaksinen and Tuulonen15 subsequently reviewed sequential stereoscopic optic nerve and retinal red-free photographs from 191 presumably ocular hypertensive patients and found that an RNFL defect was the first observable sign of glaucomatous damage (ie, preceding optic disc or visual field changes with manual kinetic perimetry) in 46% of eyes with optic disc hemorrhages and 26% of eyes without disc hemorrhages. Changes in the appearance of the RNFL have also been shown to precede detection of visual field abnormality in glaucoma suspects followed longitudinally.16
Sommer and associates17 examined color photographs taken annually in 1344 ocular hypertensive eyes. Eighty-eight percent of eyes already had visible RNFL abnormalities at the time visual field loss was diagnosed, and 60% had visible abnormalities 6 years prior to the development of visual field defects. The prevalence of RNFL abnormalities was only 26% in ocular hypertensive eyes that did not develop visual field defects and 11% of normal eyes.
Airaksinen and colleagues18 examined RNFL photographs taken with a red-free filter in patients with glaucoma, ocular hypertension, and normal subjects in a masked fashion. They identified RNFL defects in 48 of 51 glaucoma patients, 27 of 52 patients with ocular hypertension, and only 5 of 29 normal subjects. This same group subsequently performed semiquantitative measurements of the RNFL using red-free photography and showed that structural changes in the RNFL precede functional changes on automated static perimetry.19 Tuulolen and Airaksinen20 followed 61 eyes of 61 ocular hypertensive patients using stereoscopic optic disc photography and red-free peripapillary RNFL photography for a minimum of 5 years. In 23 of these eyes, glaucoma developed during follow-up. The earliest changes in the RNFL of these eyes occurred in a diffuse manner without localized loss (12 of 23). Four patients had both diffuse and localized loss detected, and 7 had localized RNFL bundle defects.
In a cohort of 647 ocular hypertensive individuals, Quigley and associates21 found that the presence of RNFL atrophy, as judged by a reader masked to clinical and demographic characteristics and even the appearance of the optic disc, imparted a 7 to 8 times risk of developing glaucomatous defects on Goldmann perimetry. In another study, Quigley and Addicks22 injured the orbital portion of the optic nerve in monkeys by using direct mechanical trauma or by interrupting the blood supply to the central retinal artery. They found that 50% of the retinal nerve fiber axons needed to be destroyed before they were able to detect abnormalities in the RNFL photographically.
Despite the large body of evidence that RNFL abnormalities are an early sign of glaucomatous damage, widespread use of red-free photography and slit-lamp observation was never achieved for several reasons. First, the method is largely qualitative, despite some attempts to quantify the subjective observations. While the intraobserver variability of RNFL readings has been found to be fairly low,18 interobserver variability is high.23 Second, this technique requires highly skilled observers working from high-quality red-free photographs. And third, even skilled observers have a difficult time detecting diffuse thinning of the RNFL,14,24,25 which accounts for the majority of early changes in the RNFL appearance.13,18,20,23–25 Clearly, there was a need for more quantitative evaluation of the RNFL.
Caprioli and colleagues26 developed a more quantitative way of assessing the RNFL by measuring the contour of its surface using the Rodenstock Optic Disc Analyzer (Rodenstock Instrumente, Munich, Germany). This technique used red-free videographic images and made measurements of RNFL thickness based on a reference plane. The determination of a reference plane consistent from one individual to another or from one time to another in the same individual was a limitation of this technique. However, these investigators were able to document a generalized depression of RNFL thickness in glaucomatous eyes compared to normal eyes in a quantitative manner, and the differences were more marked superiorly and inferiorly.
Over the past 15 years, objective structural measurements of the optic disc and RNFL have been used more and more in the diagnosis of glaucoma. Specifically, scanning laser ophthalmoscopy (Heidelberg Retinal Tomography; Heidelberg Engineering, Heidelberg, Germany), laser polarimetry (GDx, Carl Zeiss Meditec, Dublin, California), and optical coherence tomography (OCT, Carl Zeiss Meditec, Dublin, California) are imaging devices that have been used to quantitatively measure the optic disc and RNFL in glaucoma. The potential advantage of these structural measurements is that they are objective, as opposed to current assessments of optic disc cupping that rely on subjective evaluation by a skilled observer. And, while visual fields often give an accurate representation of the functional status of the visual system in glaucoma, visual fields are highly subjective and may be subject to artifacts. Additionally, most studies have shown that structural changes precede functional changes in glaucoma, as mentioned above.
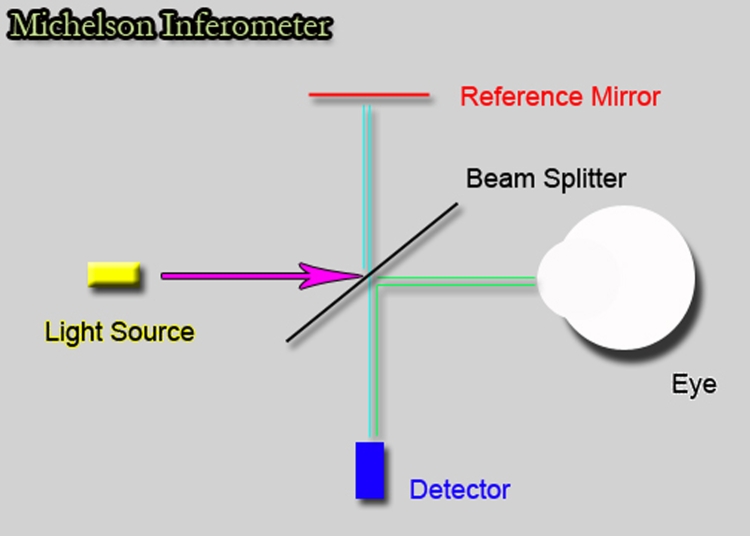
First described for ocular use by Huang and colleagues in 1991,27 OCT is a high-resolution, cross-sectional imaging technology that allows in vivo measurements of the RNFL. OCT uses reflectance data from interferometry to measure RNFL thickness. Near infrared light passing through the retina is compared with a reference beam at each level within the tissue. The strength of the interferometric signal depends on the optical reflectivity of each retinal interface, allowing construction of high-resolution cross-sectional images of the retinal layers. RNFL thickness is measured using algorithms based on reflectivity changes between adjacent structures. Figure 1 shows a simplified schematic of the OCT instrument. Measurements of RNFL thickness, even with first-generation OCT technology, have been found to have better correlation with functional status of the visual system in glaucoma patients than cup-disc (C:D) ratio or neuroretinal rim area.28 This is likely due to greater ease of measuring the retinal ganglion cell axons as they are spread out over the surface of the retina than when they are crowded together and mixed inseparable in measurements from the space occupied by the central retinal vessels within the optic disc.
FIGURE 1.
Schematic drawing of how interferometry works. A light source sends a light wave to a beam splitter that splits the beam in two, one going to the eye and one to a reference mirror. Each beam is reflected back to the detector, and the time delay between the arrival of the two waves is translated into distance between tissues.
The third-generation instrument, Stratus OCT (Carl Zeiss Meditec, Dublin, California), has an axial resolution in tissue of 8 to 10 μm (data on file, Carl Zeiss Meditec, Dublin, California), a significant improvement over the previous first- and second-generation instruments, with resolutions of only 15 to 20 μm.27 The normative database, incorporated into the instrument, allows comparison of an individual patient’s retinal or RNFL thickness measurements with that of age-matched controls for diagnostic purposes. This provides an objective tool to diagnose glaucomatous axonal loss, as reflected by thinning of the RNFL. There is a growing body of evidence suggesting that measurements of peripapillary RNFL using OCT have good sensitivity and specificity for the diagnosis of glaucoma when one uses the normative database provided by the manufacturer to compare a patient’s RNFL thickness to that of age-matched controls.29–41 Compared to current subjective techniques to diagnose glaucoma, such as the interpretation of optic disc appearance by the clinician and perimetry, objective measurement of the RNFL potentially provides an additional tool for the diagnosis of glaucoma and for following glaucomatous progression.
The present study was undertaken to determine normal tolerance limits for the amount of asymmetry of RNFL thickness when the values in each eye are within the range of normal. Once these tolerance limits are known, then patients exceeding these limits might be suspected of having early glaucomatous axonal loss. Such an approach has been used for the amount of optic disc cupping for many years. The presence of asymmetry between the optic nerve cup between the two eyes of an individual is considered an early sign of glaucomatous damage42 clinically and is a predictor of future damage in ocular hypertensive patients.21 All large epidemiologic studies on the prevalence of glaucoma have used C:D asymmetry of ≥0.2 or ≥0.3 in their case definition of glaucoma43–51 or for referral for definitive diagnosis.52 Clinical trials on ocular hypertensive patients, such as the Ocular Hypertension Treatment Study53 and the European Glaucoma Prevention Study,54 have used interocular asymmetry as an exclusion criteria, since subjects in these studies were supposed to be normal and be free of optic nerve damage at baseline. Now that there is an objective and noninvasive method to measure the thickness of the RNFL in vivo, it may be appropriate to apply the concept of asymmetry of the RNFL for the early diagnosis of glaucoma or suspected glaucoma. The case of a treated glaucoma suspect with mildly asymmetric intraocular pressures (IOPs), C:D ratios, visual fields, and RNFL thickness measurements performed by the Stratus OCT that demonstrates this concept is presented in Figures 2 through 4.
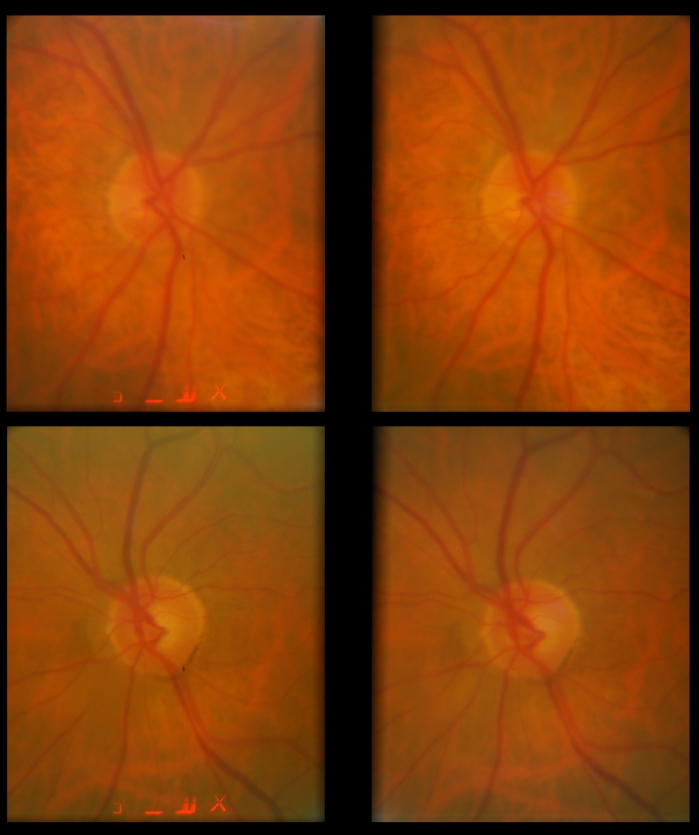
FIGURE 2.
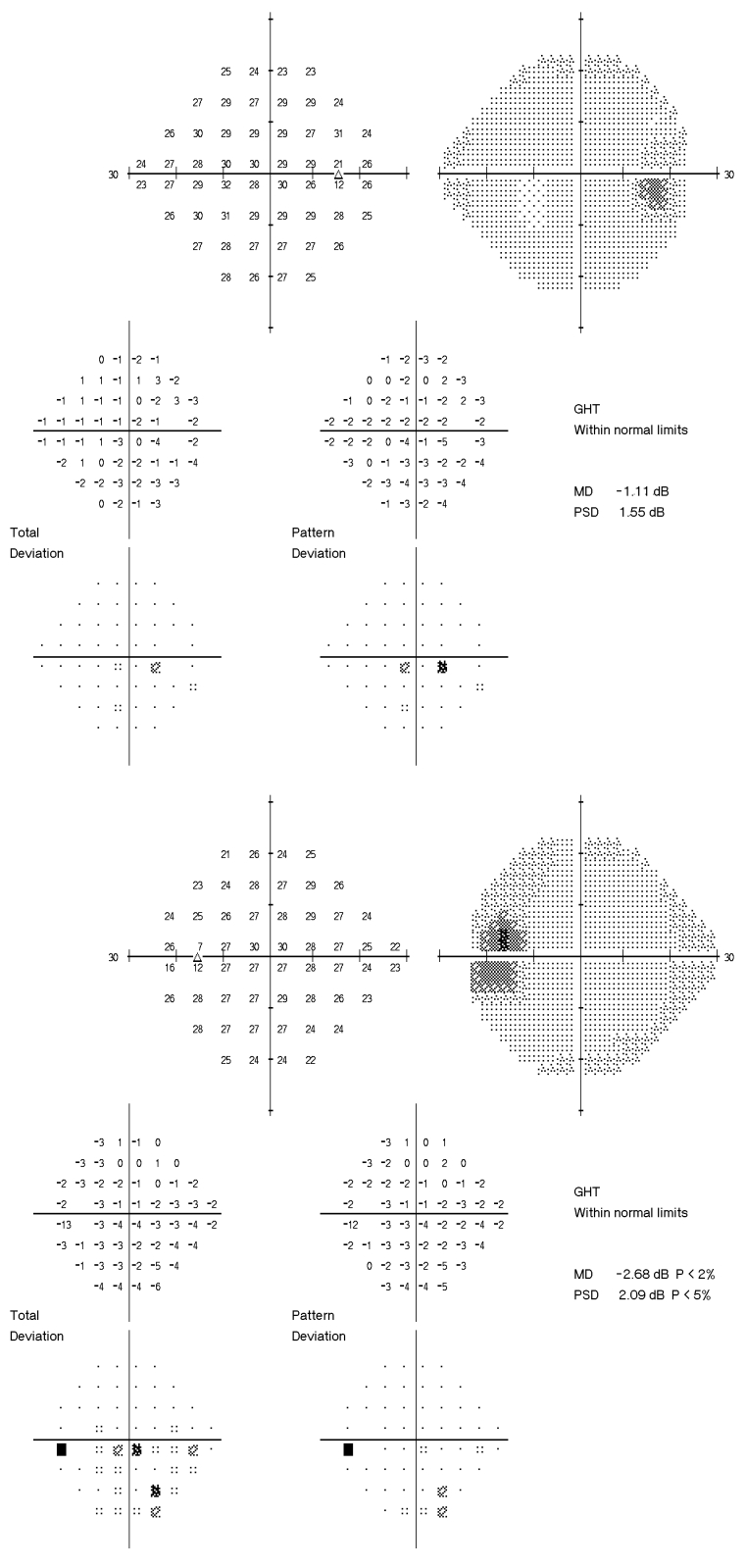
Stereoscopic optic disc photographs of the right (upper) and left (lower) eyes of a glaucoma suspect. Intraocular pressures ranged between 14 and 20 mm Hg in the right eye and 16 and 32 mm Hg in the left eye before treatment. On medical therapy, pressures ranged between 10 and 18 mm Hg in the right eye and 13 and 20 mm Hg in the left eye. The patient has been followed for more than 15 years while on therapy without progression. Note the mild cup-disc asymmetry with the left cup being slightly larger than the left despite equal optic disc sizes.
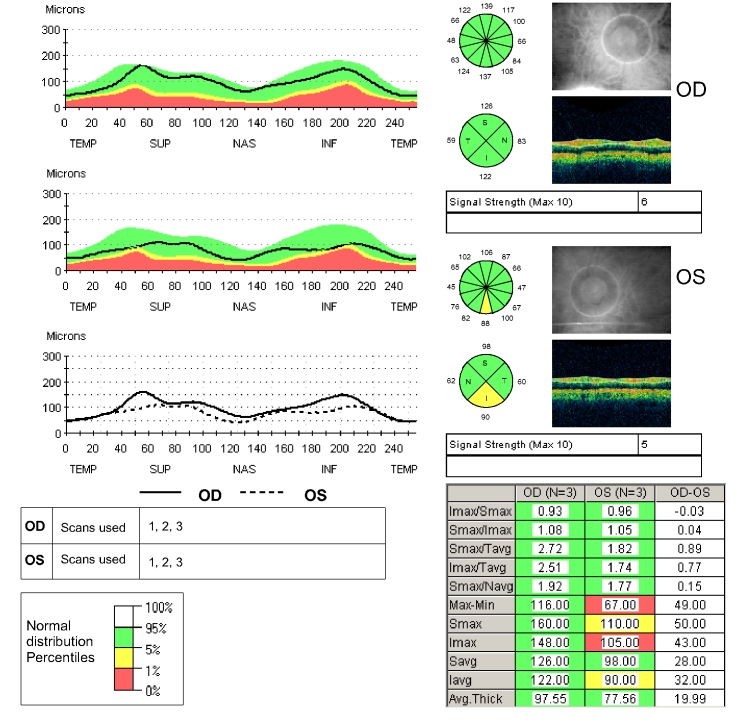
FIGURE 4.
Peripapillary fast Stratus OCT scan of the right and left eyes of the patient described in Figures 2 and 3. The Stratus OCT peripapillary retinal nerve fiber layer (RNFL) printout provides a number of representations of RNFL thickness measurements. The TSNIT plot (upper left) is a representation of the patient’s RNFL thickness (black line) in the 3.4-mm-diameter circle around the optic disc, starting in the temporal region (9:00 in the right eye and 3:00 in the left eye), then moving toward the superior region (12:00 in both eyes), then nasally (3:00 right eye, 9:00 left eye), then inferiorly (6:00 both eyes), and then winding up directly temporally. When the black line is in the green area of the graph, the RNFL thickness is normal in that location compared to normal age-matched controls. When the line dips into the yellow area, the patient’s RNFL thickness is considered thinner than the thinnest 5% of normal age-matched controls. When the black line dips into the red area, the patient’s RNFL thickness is considered thinner than the thinnest 1% of normal age-matched controls. Moving clockwise on the printout, the circle representation of the RNFL thickness is divided into clock-hour pictures, with RNFL averages for each of 12 clock-hours displayed in green, yellow, or red and interpreted in the same manner as compared to normal age-matched controls. Below the clock-hour circle is the quadrant circle, which is the average measurements in each of the 4 quadrants, color-coded and interpreted in the same manner. To the right of the circle representations is a black and white photograph of the optic disc and peripapillary region taken immediately after scanning is completed to document where around the optic disc the scan is actually being taken. The table of numbers in the lower right portion of the printout provides a variety of comparisons between upper and lower hemifields and between eyes, but the most interesting for our purposes are the mean RNFL values for right and left eyes given at the bottom of the table. And in the lower left are the TSNIT plots for both right and left eyes superimposed on each other. Note in this patient that the left mean retinal nerve fiber layer thickness is 20 μm thinner than the right and that the superimposed right/left TSNIT plots show mild thinning in the superior and inferior areas of the left eye. The 6:00 clock-hour in the left eye is shaded yellow, indicating that the patient’s average RNFL thickness measurement is thinner than 5% of that of age-matched controls. Also, the inferior quadrant of the left eye is shaded yellow. This corresponds well with the asymmetric intraocular pressures, mild C:D asymmetry, and 1-dB difference in mean deviation on the visual field between eyes. The OCT is the only test with a frankly abnormal result, the mild thinning inferiorly in the left eye.
To apply the principle of RNFL thickness asymmetry as a sign of disease, it is necessary to determine the degree of interocular symmetry that might normally be expected in normal individuals to develop parameters for statistically significant differences between eyes. The purpose of this study is to determine the normal amount of interocular asymmetry and to propose the use of interocular asymmetry as a way of identifying early glaucoma patients.
METHODS
SUBJECTS
This study was approved by the Human Subjects Committee of the Institutional Review Board (IRB) of the University of Miami, Miller School of Medicine, and followed the tenets of the treaty of Helsinki. Normal subjects at least 18 years of age, either employees or patients of the Bascom Palmer Eye Institute, were invited to participate. Each was informed of the nature of the study, and the subjects’ willingness to participate was documented with their signature on a consent form approved by the IRB. Both written and oral consent were obtained. Inclusion criteria included males or females aged 18 years or older who were able give consent and follow study instructions. Exclusion criteria included contraindication to dilation or intolerance to topical anesthetics or mydriatics; IOP ≥22 mm Hg or glaucoma in either eye; intraocular surgery in the study eye (except cataract or refractive surgery if performed more than 1 year prior to testing); best-corrected Snellen visual acuity worse than 20/40; evidence of diabetic retinopathy, diabetic macular edema, or other vitreoretinal disease in either eye; or evidence of optic nerve abnormality in either eye. Subjects who had C:D asymmetry of ≥0.2 were excluded, since this would have been suspicious for glaucoma. Subjects were also excluded by the principal investigator if their Stratus OCT scans showed algorithm failure, as evidenced by the lines that defined the boundaries of the RNFL being placed in the incorrect location by the instrument, or if either eye had a signal strength less than 6. Each subject had a complete ophthalmologic evaluation, including Snellen visual acuity, slit-lamp examination, IOP measurement, and dilated fundus examination. An assessment of optic disc size was made with the direct ophthalmoscope technique described by Gross and Drance55 and divided into small, medium, and large discs. This was done to later assess interocular differences in optic disc size, which may influence differences in RNFL thickness values. C:D ratios were estimated by an experienced examiner using a condensing lens at the slit lamp.
TESTING
The pupils of all subjects were dilated using mydriacyl 1% and Neo-Synephrine 2.5%. Testing was performed with the commercially available Stratus OCT-running software version 3.0. The same operator, who had over 1 year of experience performing Stratus OCT scans for clinical research studies, performed all scans. The same instrument was used for both eyes of the same subject, although a different OCT machine was used for a few subjects because the research OCT instrument required repairs for several weeks during the course of the study. Each subject was seated in front of the instrument with his or her chin in the chin rest. The OCT lens was adjusted for the patient’s refractive error. The subject was then instructed to fixate with the eye being measured on the internal fixation target to bring the optic nerve head within view of the examiner real-time. The Z-offset was adjusted to bring the OCT image into view, and polarization was optimized to maximize the reflective signal. The position of the aiming circle was adjusted by the operator to match the optic nerve head so that the nerve head scan would acquire an OCT image that was equidistant from the disc margins in all directions. Scan quality was monitored by continual assessment of the color coding of the scans with particular attention to maximizing the red-reflectivity of the retinal pigment epithelial cell layer. The exact same procedures for obtaining OCT measurements were followed for both eyes. The quality of a Stratus OCT scan is measured in part by the signal strength parameter, which is partly dependent on the amount of red reflectivity as described. The signal strength ranges between 0 (worst) and 10 (best) and is printed on the printout of the final results. Signal strength of 6 or higher is generally considered adequate for analysis of the results. Scans with signal strengths below 6 were excluded in this study, and any subject in whom a signal strength of 6 was not obtainable was excluded from the final analysis. Both standard and fast scans were obtained. The standard RNFL scan consists of a single peripapillary scan of 512 test points measured along a circle having a nominal diameter of 3.46 mm centered on the optic disc. The RNFL analysis then averages the peripapillary scan measurements and produces 17 averaged values for each scan set. These include the mean RNFL thickness, 4 quadrant averages (temporal, superior, nasal, and inferior), and 12 clock-hour averages. The fast RNFL scan consists of 3 sequential peripapillary scans, each consisting of 256 test points measured along a circle with a nominal diameter of 3.46 mm centered on the optic disc. The fast scan algorithm thus provides a total of 768 test points that are averaged into clock-hour, quadrant, and mean RNFL values. The RNFL analysis is thereafter identical to that of the standard scan algorithm. Figure 4 and its accompanying legend provide an overview of the OCT printout and its interpretation. By convention, subjects were tested in the right eye first and then the left eye in all cases used for the analysis.
To test the hypothesis that the order of scanning (right first vs left first) might affect the measurements, 8 measurements of RNFL thickness were made in 10 subjects tested right eye first then left eye, followed by left eye first then right eye and so forth, on the same day. The fast scan algorithm was used in this ancillary study. To test the hypothesis that variability between right and left eyes was due to scan placement by the operator, data was obtained from 47 unique normal subjects scanned with the fourth-generation OCT, Cirrus OCT (Carl Zeiss Meditec, Dublin, California) as part of the normative database collection for this new instrument. Separate IRB and informed consents were used for this ancillary study. The reason this was considered important is that, unlike Stratus OCT, Cirrus OCT extracts a scan circle of 3.46-mm diameter around the optic disc in the same location, each scan utilizing a geometric determination of the center of the optic disc and then extracting the equivalent scan circle’s worth of points. Thus the circle placement variation is not an issue with Cirrus OCT.
STATISTICAL METHODS AND DATA ANALYSIS
This study required enough individuals to estimate 2.5th and 97.5th percentiles; thus, 40 individuals might have been sufficient. However, with this sample size the percentiles could easily have been influenced by outliers, so the target sample size was increased to approximately 120 individuals. The data were exported with the XML export software made available by the manufacturer. The data were then imported into SPSS 15.0 (SPSS Inc, Chicago, Illinois); all analyses were performed with this software. Demographic and ocular characteristics of the study group were summarized with means and standard deviations (SDs) for interval level variables and with percentages for categorical variables. Normal ranges for interocular differences were established as the 2.5th and 97.5th percentiles; the Gaussian approximations of the mean ± 1.96 SDs were also calculated. The correlations between right and left eyes were expressed as intraclass correlation coefficients with 95% confidence intervals (CIs). By convention, intraclass correlation coefficients are classified as good to excellent if they are greater than or equal to 0.75, fair to good if between 0 .4 and 0.74, and poor if less than 0.4. Frequency distribution histograms of all subjects’ differences were constructed. The association between interocular differences and demographic/ocular characteristics was examined graphically, and the strengths of associations were quantified with Pearson correlation coefficients. To test the hypothesis that the symmetry of RNFL thickness measurements varies as a function of age, interocular differences in mean RNFL values were plotted against age and Pearson correlation coefficients and R2 values were calculated. The same analyses were performed to explore possible associations between signed differences in ocular characteristics, such as refractive error, IOP, and vertical C:D ratio and signed interocular RNFL thickness differences.
RESULTS
A total of 126 subjects were recruited for the study. Three subjects were excluded because of previously undetected ocular disease (one had glaucoma, one had an epiretinal membrane, and one had optic disc drusen), 2 were excluded because of algorithm failure in one or both eyes, and 13 were excluded because the signal strength in one or both eyes was below 6. Thus, a total of 108 subjects were included in the analysis. The mean age of subjects was 46.0 years (SD 15.0, range 20–82). There were 42 male (39%) and 66 female (61%) subjects. The sample included 48 Hispanics (44.4%), 39 non-Hispanic whites (36.1%), 11 African Americans (10.2%), and 10 Asians (9.3%).
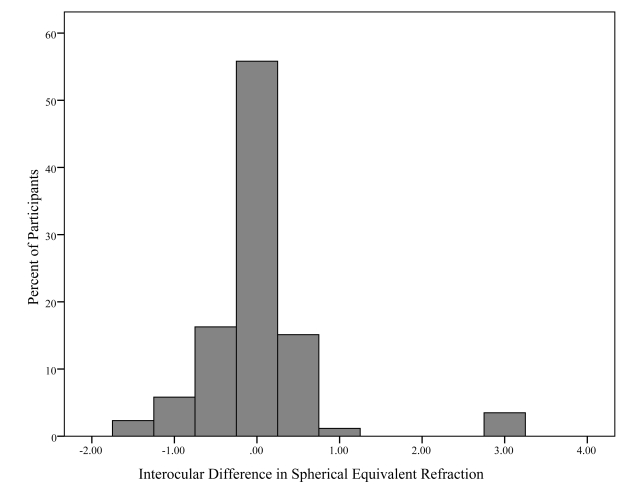
Table 1 summarizes the clinical features of the right and left eyes of subjects. There were no statistically significant differences between eyes in terms of visual acuity, refractive error, IOP, or C:D ratios. Table 2 is the frequency distribution of the difference in vertical C:D ratios demonstrating that the subjects did not often differ in disc size between eyes. Figure 5 is a histogram showing the interocular differences in spherical equivalent refractive error among participants. All but 6 participants varied in refractive error by 1 diopter (D) or less.
TABLE 1.
CLINICAL CHARACTERISTICS OF EYES MEASURED*
| VARIABLE | OD | OS | P VALUE |
|---|---|---|---|
| Median Snellen VA | 20/20 (20/15 to 20/40) | 20/20 (20/15 to 20/40) | |
| Mean spherical equivalent (diopters) | –0.7 (2.2, +4.75 to –7.75) | –0.8 (2.1, +4.50 to –7.75) | .22 |
| Mean intraocular pressure (mm Hg) | 15.1 (2.7, 10 to 21) | 16.1 (2.5, 10 to 21) | .72 |
| Mean vertical cup-disc | 0.31 (0.15, 0.1 to 0.8) | 0.30 (0.15, 0.1 to 0.8) | .85 |
| Mean horizontal cup-disc | 0.29 (0.14, 0.1 to 0.7) | 0.29 (0.14, 0.1 to 0.8) | .20 |
VA, visual acuity.
Standard deviation and range are given in parentheses where appropriate.
TABLE 2.
FREQUENCY DISTRIBUTION OF DIFFERENCES IN VERTICAL CUP-DISC RATIO FOR RIGHT MINUS LEFT EYES
| OD − OS | NUMBER (%)* |
|---|---|
| 0.10 | 12 (13%) |
| 0.05 | 3 ( 3%) |
| 0.00 | 70 (73%) |
| 0.10 | 10 (10%) |
| 0.15 | 1 ( 1%) |
Total number is 96 because of missing C:D ratios on 12 subjects.
FIGURE 5.
Histogram showing the frequency distribution of interocular differences (OD – OS) in spherical equivalent refractive error for each subject. Most subjects had less than 1 diopter difference between eyes.
Tables 3 and 4 show the average differences between right and left eyes for standard and fast scans, respectively. There was a small, but statistically significant, difference between mean RNFL thickness measurements of the 2 eyes. The right eye measured 1.3 μm thicker than the left on the standard scan (SD 4.7, 95% CI 0.4–2.2, P = .004) and 1.2 μm thicker on the fast scan (SD 5.2, 95% C.I. 0.1–2.2, P = .026). The quadrant and clock-hour differences were sometimes larger in the right eye and sometimes the left eye.
TABLE 3.
MEAN DIFFERENCE IN RETINAL NERVE FIBER LAYER THICKNESS (RIGHT MINUS LEFT) MEASURED WITH THE STANDARD SCAN ALGORITHM OF STRATUS OCT*
| VARIABLE | MEAN DIFFERENCE (μm) | STANDARD DEVIATION (μm) | 95% CONFIDENCE INTERVAL (μm) | P VALUE |
|---|---|---|---|---|
| Mean | 1.3 | 4.7 | 0.4 to 2.2 | .004 |
| Temporal | 1.6 | 8.9 | –0.1 to 3.3 | .066 |
| Superior | –0.6 | 13.1 | –3.1 to 2.0 | .662 |
| Nasal | 4.5 | 12.5 | 2.1 to 6.9 | <.001 |
| Inferior | –0.3 | 11.5 | –2.5 to 1.9 | .817 |
| 9:00/3:00 | 1.0 | 7.5 | –0.5 to 2.4 | .156 |
| 10:00/2:00 | 4.2 | 13.7 | 1.6 to 6.9 | .002 |
| 11:00/1:00 | 7.0 | 19.1 | 3.3 to 10.7 | <.001 |
| 12:00 | –1.1 | 19.9 | –4.9 to 2.7 | .564 |
| 1:00/11:00 | –7.6 | 17.7 | –11.0 to –4.2 | .000 |
| 2:00/10:00 | 3.3 | 20.4 | –0.6 to 7.2 | .102 |
| 3:00/9:00 | 4.2 | 14.4 | 1.4 to 6.9 | .003 |
| 4:00/8:00 | 6.0 | 13.6 | 3.4 to 8.6 | <.001 |
| 5:00/7:00 | 0.2 | 16.6 | –3.0 to 3.4 | .901 |
| 6:00 | 1.3 | 21.2 | –2.8 to 5.3 | .517 |
| 7:00/5:00 | –2.2 | 16.7 | –5.4 to 1.0 | .172 |
| 8:00/4:00 | –0.4 | 13.5 | –3.0 to 2.2 | .759 |
Clock-hour values given for right/left eyes.
TABLE 4.
MEAN DIFFERENCE IN RETINAL NERVE FIBER LAYER THICKNESS (RIGHT MINUS LEFT) MEASURED WITH THE FAST SCAN ALGORITHM OF STRATUS OCT*
| VARIABLE | MEAN DIFFERENCE (μm) | STANDARD DEVIATION (μm) | 95% CONFIDENCE INTERVAL (μm) | P VALUE |
|---|---|---|---|---|
| Mean | 1.2 | 5.2 | 0.1 to 2.1 | .026 |
| Temporal | 2.6 | 9.1 | 0.8 to 4.3 | .004 |
| Superior | –2.6 | 13.3 | –5.1 to –0.6 | .045 |
| Nasal | 3.8 | 12.5 | 1.4 to 6.2 | .002 |
| Inferior | 0.8 | 12.7 | –1.6 to 3.3 | .508 |
| 9:00/3:00 | 2.2 | 8.0 | 0.6 to 3.8 | .006 |
| 10:00/2:00 | 6.0 | 14.3 | 3.2 to 8.8 | <.001 |
| 11:00/1:00 | 6.2 | 18.6 | 2.6 to 9.8 | .001 |
| 12:00 | –5.0 | 19.2 | –8.7 to –1.3 | .009 |
| 1:00/11:00 | –9.1 | 16.6 | –12.3 to –5.9 | <.001 |
| 2:00/10:00 | 2.0 | 18.7 | –1.6 to 5.7 | .276 |
| 3:00/9:00 | 2.9 | 15.2 | –0.1 to 5.8 | .053 |
| 4:00/8:00 | 6.6 | 14.1 | 3.8 to 9.3 | <.001 |
| 5:00/7:00 | 1.9 | 17.9 | –1.5 to 5.4 | .279 |
| 6:00 | 2.7 | 22.4 | –1.7 to 7.0 | .219 |
| 7:00/5:00 | –2.1 | 17.7 | –5.6 to 1.3 | .226 |
| 8:00/4:00 | –0.5 | 12.4 | –2.9 to 1.9 | .680 |
Clock-hour values given for right/left eyes
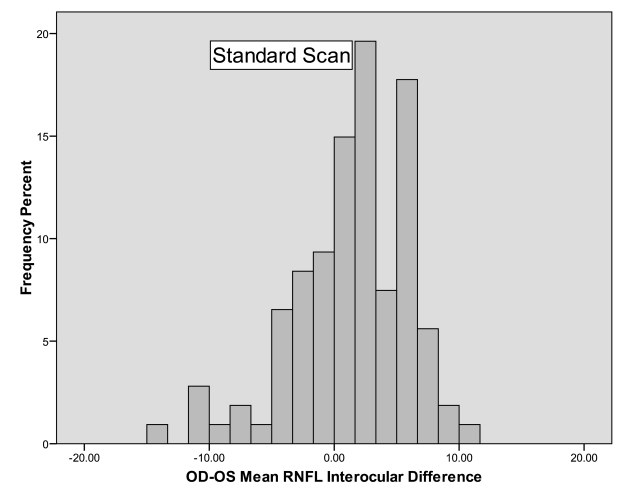
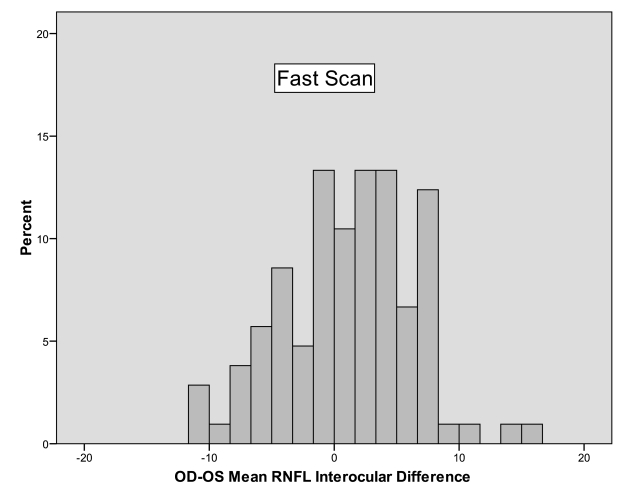
Tables 5 and 6 provide the percentile distributions of the differences between right and left eyes for the standard and fast scan algorithms, respectively. For standard mean RNFL measurements, 95% of normal subjects had a difference between right and left eyes less than 8.9 to 10.8 μm, depending on whether the right or left eye was thicker. If the right eye was thicker, then only 2.5% of subjects differed by more than 8.9 μm, and if the left eye was thicker, then only 2.5% of subjects differed by more than 10.8 μm. For fast mean RNFL measurements, 95% of normal individuals had a difference of less than 10.6 to 11.7 μm. If the right eye was thicker, then only 2.5% of subjects differed by more than 11.7 μm, and if the left eye was thicker, then only 2.5% of subjects differed by more than 10.6 μm. Interocular asymmetry for quadrant and clock-hour measurements was considerably higher.
TABLE 5.
PERCENTILE DISTRIBUTION OF RETINAL NERVE FIBER LAYER DIFFERENCES (RIGHT MINUS LEFT) MEASURED WITH THE STANDARD SCAN ALGORITHM OF STRATUS OCT*
| VARIABLE | 2.5 | 5 | 10 | 25 | 50 | 75 | 90 | 95 | 97.5 |
|---|---|---|---|---|---|---|---|---|---|
| Mean | –10.8 | –8.8 | –4.8 | –1.2 | 2.0 | 5.0 | 6.5 | 7.3 | 8.9 |
| Temporal | –11.7 | –10.9 | –9.6 | –5.3 | .8 | 7.8 | 11.5 | 16.9 | 25.2 |
| Superior | –30.0 | –23.4 | –16.8 | –7.9 | –.3 | 6.9 | 17.4 | 21.6 | 28.0 |
| Nasal | –24.4 | –17.5 | –9.9 | –1.9 | 4.7 | 12.6 | 18.4 | 24.4 | 28.1 |
| Inferior | –25.5 | –19.3 | –14.0 | –9.0 | .6 | 7.6 | 13.8 | 17.9 | 21.6 |
| 9:00/3:00 | –14.4 | –10.8 | –8.5 | –4.1 | 1.7 | 5.1 | 9.3 | 11.8 | 19.0 |
| 10:00/2:00 | –20.3 | –18.7 | –14.0 | –5.2 | 2.7 | 13.9 | 21.0 | 25.3 | 38.2 |
| 11:00/1:00 | –30.3 | –23.2 | –20.0 | –5.2 | 4.5 | 22.0 | 33.1 | 37.5 | 44.9 |
| 12:00 | –46.4 | –36.9 | –28.7 | –13.1 | –1.6 | 11.9 | 24.1 | 29.7 | 39.2 |
| 1:00/11:00 | –44.3 | –34.9 | –31.4 | –20.8 | –5.3 | 4.0 | 16.5 | 21.8 | 24.4 |
| 2:00/10:00 | –45.3 | –33.2 | –21.0 | –8.0 | 4.2 | 12.7 | 28.4 | 33.1 | 43.2 |
| 3:00/9:00 | –29.3 | –22.8 | –15.0 | –5.1 | 6.0 | 13.8 | 20.9 | 27.3 | 29.4 |
| 4:00/8:00 | –22.7 | –18.1 | –12.8 | –2.9 | 6.6 | 14.9 | 24.5 | 30.8 | 31.8 |
| 5:00/7:00 | –36.4 | –30.7 | –23.6 | –9.3 | 1.3 | 11.3 | 21.7 | 28.1 | 30.3 |
| 6:00 | –43.3 | –35.2 | –28.6 | –12.5 | 2.2 | 14.0 | 26.4 | 37.3 | 44.0 |
| 7:00/5:00 | –32.4 | –24.1 | –21.7 | –13.1 | –3.9 | 7.8 | 21.0 | 31.2 | 36.4 |
| 8:00/4:00 | –36.6 | –19.8 | –17.2 | –7.3 | –.4 | 7.0 | 15.5 | 17.5 | 29.8 |
Clock-hour values given for right/left eyes.
TABLE 6.
PERCENTILE DISTRIBUTION OF RETINAL NERVE FIBER LAYER DIFFERENCES (RIGHT MINUS LEFT) MEASURED WITH THE FAST ALGORITHM OF STRATUS OCT*
| VARIABLE | 2.5 | 5 | 10 | 25 | 50 | 75 | 90 | 95 | 97.5 |
|---|---|---|---|---|---|---|---|---|---|
| Mean | –10.6 | –7.9 | –6.3 | –2.4 | 1.4 | 4.9 | 7.7 | 8.1 | 11.7 |
| Temporal | –16.7 | –12.2 | –7.5 | –3.2 | 1.6 | 8.7 | 13.3 | 19.9 | 24.8 |
| Superior | –38.1 | –25.4 | –17.4 | –9.5 | –2.8 | 6.6 | 13.5 | 19.5 | 24.2 |
| Nasal | –24.4 | –18.4 | –14.9 | –3.7 | 6.2 | 12.8 | 18.2 | 21.1 | 24.2 |
| Inferior | –23.5 | –21.3 | –16.3 | –7.9 | 1.5 | 9.9 | 15.5 | 20.8 | 23.9 |
| 9:00/3:00 | –11.0 | –9.8 | –7.3 | –2.8 | 1.5 | 6.8 | 13.7 | 16.4 | 21.3 |
| 10:00/2:00 | –23.0 | –16.3 | –10.6 | –3.7 | 5.0 | 15.6 | 24.5 | 29.4 | 40.7 |
| 11:00/1:00 | –34.2 | –26.6 | –16.6 | –5.0 | 5.5 | 16.8 | 31.1 | 37.5 | 43.2 |
| 12:00 | –51.1 | –40.6 | –31.0 | –15.4 | –7.5 | 9.1 | 17.0 | 24.4 | 28.5 |
| 1:00/11:00 | –47.1 | –39.1 | –30.8 | –20.7 | –9.0 | 1.8 | 12.2 | 17.2 | 26.2 |
| 2:00/10:00 | –35.6 | –29.3 | –23.2 | –12.6 | 3.4 | 14.2 | 24.0 | 29.0 | 34.9 |
| 3:00/9:00 | –36.3 | –25.5 | –18.0 | –4.3 | 3.3 | 13.1 | 21.3 | 29.0 | 31.8 |
| 4:00/8:00 | –20.5 | –14.8 | –10.7 | –3.7 | 4.5 | 16.2 | 24.3 | 34.0 | 36.8 |
| 5:00/7:00 | –35.8 | –32.1 | –22.1 | –10.4 | 1.6 | 14.0 | 24.4 | 31.6 | 40.4 |
| 6:00 | –39.5 | –31.9 | –25.8 | –12.3 | 3.4 | 17.1 | 31.4 | 40.4 | 42.9 |
| 7:00/5:00 | –37.6 | –31.9 | –24.9 | –15.2 | –3.1 | 9.3 | 20.6 | 30.1 | 34.9 |
| 8:00/4:00 | –27.1 | –24.0 | –15.1 | –8.8 | –.1 | 6.8 | 14.7 | 19.6 | 26.2 |
Clock-hour values given for right/left eyes.
The 95% cutoff was between 11.7 and 30.0 μm for quadrant values in the standard scans and between 16.7 and 38.1 μm for quadrant values of the fast scans. Clock-hour asymmetry was even higher, with the 95% cutoff ranging from 14.4 to 49.9 μm for the standard scan algorithm and 11.0 to 51.1 μm for the fast scan algorithm. Figures 6 and 7 are histograms of the differences between right and left eyes for standard and fast mean RNFL measurements, respectively. These both show a tendency for right mean RNFL thicknesses to be greater than left, since there are more subjects on the positive side of zero than the negative side. This tendency prompted a secondary analysis, specifically, to examine whether right scan order, typically right followed by left, influenced thickness measurements in the same subjects. In the 10 subjects that were tested alternating right or left eye first, the right eye was still slightly thicker than the left, regardless of which eye was tested first, but the difference did not meet statistical significance, probably because of the small sample size. The average right eye/left eye difference was 2.1 μm (SD 3.9, P = .12). The magnitude of the right eye/left eye interocular difference in mean RNFL did not depend on the order in which the 2 eyes were tested. On average, the right eye/left eye difference was 0.62 μm larger when the right eye was measured first with a 95% CI ranging from −0.76 to +2.00 (P = .37, mixed model analysis of variance).
FIGURE 6.
Histogram showing the frequency distribution of the differences in retinal nerve fiber layer (RNFL) thickness between right and left eyes measured by the standard scan algorithm of the Stratus OCT. The histogram is slightly skewed to the left, with more subjects having a slight positive difference in the right minus left interocular differences than those having a negative difference.
FIGURE 7.
Histogram showing the frequency distribution of the differences in mean retinal nerve fiber layer (RNFL) thickness between right and left eyes measured by the fast scan algorithm of the Stratus OCT. Similar to Figure 4, there is a slight left skew to the curve owing to the fact that more subjects had a positive difference between right and left eyes, with more right eyes having a thicker RNFL than left.
Intraclass correlation coefficients between right and left eyes, with 95% CIs, are shown in Table 7 for standard scans and Table 8 for fast scans. Intraclass correlation coefficients were good to excellent for mean RNFL measurements, fair to good for quadrant measurements, and fair to good for clock-hour measurements with both scanning algorithms. The P values for all interocular correlations were <.001.
TABLE 7.
INTRACLASS CORRELATION COEFFICIENTS (ICC) COMPARING RETINAL NERVE FIBER LAYER THICKNESS USING THE STANDARD SCAN ALGORITHM OF STRATUS OCT*
| VARIABLE | ICC | 95% CONFIDENCE INTERVAL | P VALUE |
|---|---|---|---|
| Mean | 0.894 | 0.848 – 0.926 | <.001 |
| Temporal | 0.765 | 0.673 – 0.833 | <.001 |
| Superior | 0.740 | 0.640 – 0.815 | <.001 |
| Nasal | 0.646 | 0.521 – 0.744 | <.001 |
| Inferior | 0.798 | 0.717 – 0.857 | <.001 |
| 9:00/3:00 | 0.734 | 0.633 – 0.810 | <.001 |
| 10:00/2:00 | 0.693 | 0.581 – 0.780 | <.001 |
| 11:00/1:00 | 0.564 | 0.420 – 0.680 | <.001 |
| 12:00 | 0.709 | 0.601 – 0.792 | <.001 |
| 1:00/11:00 | 0.712 | 0.604 – 0.794 | <.001 |
| 2:00/10:00 | 0.565 | 0.421 – 0.681 | <.001 |
| 3:00/9:00 | 0.597 | 0.460 – 0.706 | <.001 |
| 4:00/8:00 | 0.564 | 0.420 – 0.680 | <.001 |
| 5:00/7:00 | 0.724 | 0.620 – 0.803 | <.001 |
| 6:00 | 0.694 | 0.582 – 0.781 | <.001 |
| 7:00/5:00 | 0.774 | 0.685 – 0.840 | <.001 |
| 8:00/4:00 | 0.645 | 0.519 – 0.743 | <.001 |
Clock-hour values given for right/left eyes.
TABLE 8.
INTRACLASS CORRELATION COEFFICIENTS (ICC) COMPARING RETINAL NERVE FIBER LAYER THICKNESS USING THE FAST SCAN ALGORITHM OF STRATUS OCT*
| VARIABLE | ICC | 95% CONFIDENCE INTERVAL | P VALUE |
|---|---|---|---|
| Mean | 0.890 | 0.843 – 0.924 | <.001 |
| Temporal | 0.706 | 0.596 – 0.790 | <.001 |
| Superior | 0.741 | 0.641 – 0.816 | <.001 |
| Nasal | 0.724 | 0.619 – 0.804 | <.001 |
| Inferior | 0.751 | 0.654 – 0.823 | <.001 |
| 9:00/3:00 | 0.608 | 0.473 – 0.716 | <.001 |
| 10:00/2:00 | 0.624 | 0.492 – 0.728 | <.001 |
| 11:00/1:00 | 0.600 | 0.462 – 0.709 | <.001 |
| 12:00 | 0.722 | 0.616 – 0.802 | <.001 |
| 1:00/11:00 | 0.720 | 0.614 – 0.801 | <.001 |
| 2:00/10:00 | 0.672 | 0.552 – 0.764 | <.001 |
| 3:00/9:00 | 0.621 | 0.488 – 0.725 | <.001 |
| 4:00/8:00 | 0.635 | 0.506 – 0.736 | <.001 |
| 5:00/7:00 | 0.740 | 0.639 – 0.815 | <.001 |
| 6:00 | 0.657 | 0.543 – 0.754 | <.001 |
| 7:00/5:00 | 0.724 | 0.619 – 0.804 | <.001 |
| 8:00/4:00 | 0.666 | 0.544 – 0.760 | <.001 |
Clock-hour values given for right/left eyes.
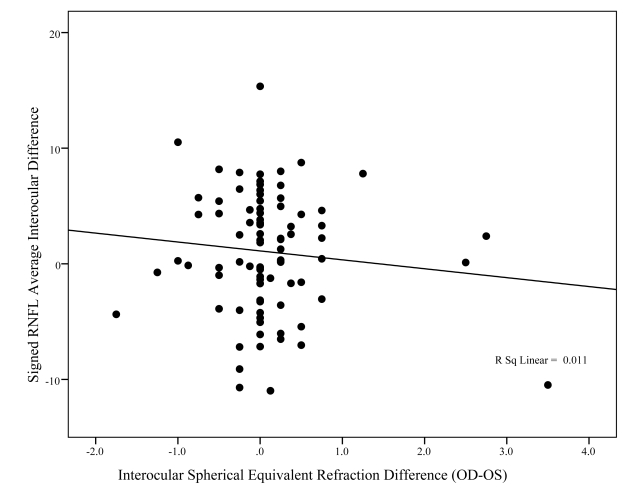
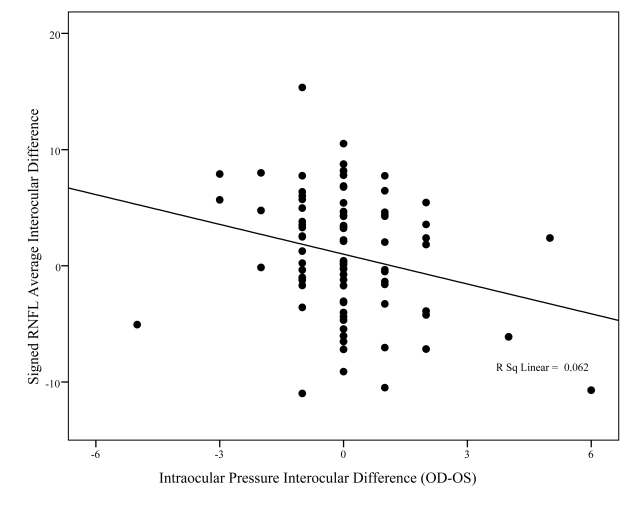
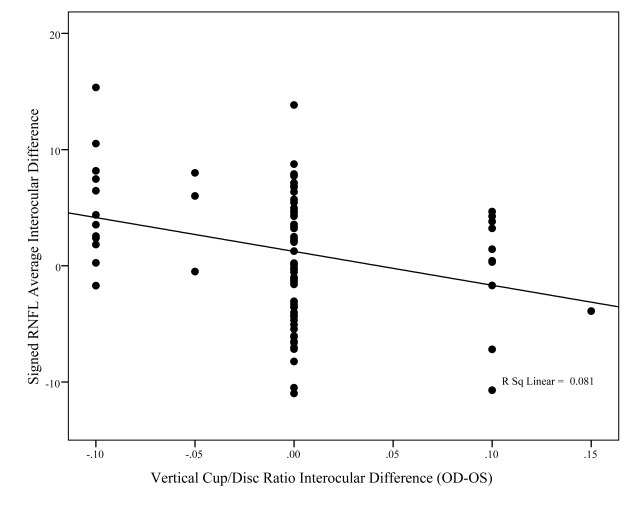
Figures 8, 9, and 10 show the association between signed differences in ocular characteristics (refractive error, IOP, and C:D ratio) and interocular RNFL thickness. The only significant correlations to emerge were with right minus left vertical C:D ratio (P = .005) and IOP (P = .021). However, while statistically significant, these relationships accounted for only a small percent of the variability in RNFL average interocular difference, respectively, 8% and 6%.
FIGURE 8.
This graph shows the relationship between interocular difference in fast mean retinal nerve fiber layer (RNFL) thickness (y-axis) and interocular difference in spherical equivalent of the refractive error (x-axis). The R2 value of 0.011 suggests that very little of the differences in interocular asymmetry between individuals can be explained by differences in refractive error.
FIGURE 9.
This graph shows the relationship between interocular difference in fast mean retinal nerve fiber layer (RNFL) thickness (y-axis) and interocular difference in intraocular pressure (x-axis). Although the relationship is statistically significant (P = .021), this relationship accounted for only 6% of the variability in interocular differences between individuals (R2 = 0.062).
FIGURE 10.
This graph shows the relationship between interocular difference in fast mean retinal nerve fiber layer (RNFL) thickness (y-axis) and interocular difference in vertical C:D ratio (x-axis). Although the relationship is statistically significant (P = .005), this relationship accounted for only 8% of the variability in interocular differences between individuals (R2 = 0.081).
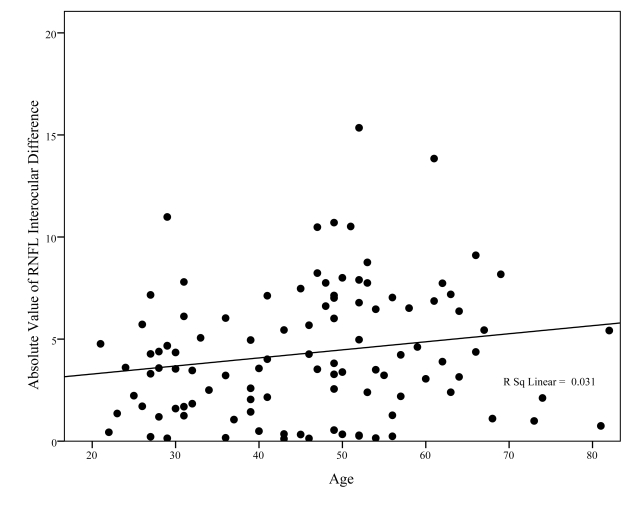
Figure 11 displays the relationship of Fast RNFL average interocular differences with age for absolute value of differences and the associated least-squares fits. There may be, if anything, a slight increase in the size of the absolute value of the interocular difference with age (P = .07), but the percent of variability in interocular difference accounted for by age is a clinically unsubstantial 3% (R2 = 0.031). These data do not suggest that accounting for age is desirable when evaluating interocular differences in RNFL thickness between the 2 eyes of the same person.
FIGURE 11.
This graph shows the relationship between interocular difference in fast retinal nerve fiber layer (RNFL) thickness (y-axis) and age (x-axis). Although the relationship approaches statistical significance (P = .07), this relationship accounted for only 3% of the variability in interocular differences between individuals (R2 = 0.031), suggesting that very little of the variability in interocular differences is attributable to increasing age.
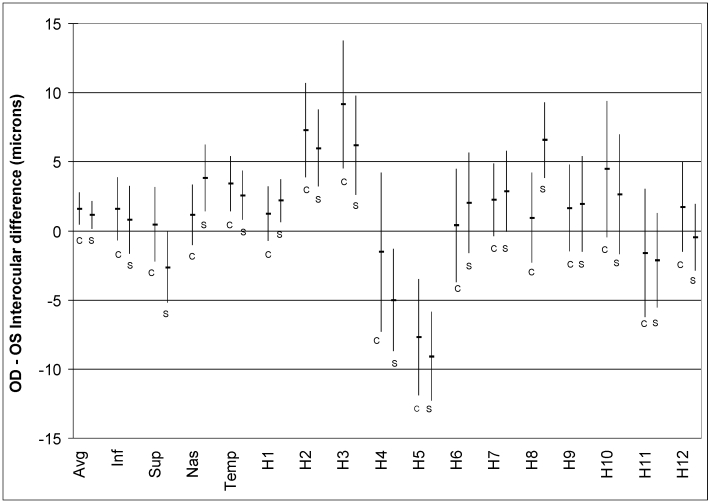
Data from the study of 47 unique normal subjects tested with Cirrus OCT, which is less subject to scan circle placement problems, showed the same results as the study using Stratus OCT. Figure 12 shows that, except for clock hour 8, mean interocular differences are similar between Stratus and Cirrus.
FIGURE 12.
Results of the analysis performed in 47 unique normal subjects using Cirrus OCT compared to the fast scan results of the Stratus OCT from the larger cohort that is the primary subject of this thesis. The graph shows similar interocular differences for mean RNFL, quadrants, and clock-hours, both in direction and magnitude. Since scan circle placement by the operator is not necessary in Cirrus OCT, it would appear that interocular differences are not a function of scan circle placement.
DISCUSSION
The early diagnosis of glaucoma is a critical step in the management of the disease. If treated early, the prognosis for vision is excellent. One of the first structures to be damaged in glaucoma is the RNFL, which is the retinal layer containing the axons of the retinal ganglion cells that are dying from glaucoma. The ability to quantify changes in RNFL thickness early in the course of glaucoma is one of the features that make OCT appealing as a diagnostic instrument for glaucoma. Since the majority of glaucoma patients present with damage in one eye before the other (glaucoma rarely developing at the same rate and time in both eyes), it may be valuable to explore the ability of RNFL measurements to detect the asymmetric development of glaucoma. This is analogous to using C:D ratio asymmetry in the early diagnosis of glaucoma. The upper limit of a 0.2 difference in C:D ratio observed in approximately 95% of the normal populations led to the use of this as a clinical sign for the early diagnosis of glaucoma.
The current study was undertaken to explore the hypothesis that one can combine the following 2 diagnostic concepts to enhance the ability of diagnosing early glaucomatous change. The first concept is that the RNFL, as one of the primary anatomic structures affected in glaucoma, can be accurately measured with OCT and that measurements showing the thinning of this structure may be used to diagnose glaucoma. The second concept is that, in normal eyes, the thickness of the RNFL, like the C:D ratio, differs by a small amount between the 2 eyes. If one can put statistical parameters around the expected difference, then perhaps differences exceeding this amount could be used to help differentiate early glaucoma from those who just have suspicious disc cupping or ocular hypertension, similar to the way in which we currently use asymmetry of C:D ratio. It may be that greater cupping or decreased RNFL thickness represents early glaucoma in the more affected eye that is not being detected with visual field testing. Of course, not all glaucoma patients lose axons in one eye at a different rate than the other, so the finding of significant RNFL asymmetry would be one of a number of signs of early glaucoma, similar to the way in which asymmetry of the C:D ratio is used clinically. In a similar way, the finding of symmetry of thickness may occur in the presence of glaucoma, so symmetry does not signify normalcy.
In medicine, by convention, “normal limits” are accepted as those including 95% of the healthy population.56 Although by this definition 5% of healthy individuals will have values outside this range, when a patient presents with a measurement outside of normal limits, it is prudent to suspect the possible presence of disease and manage the patient accordingly. If measurements of a diagnostic parameter follow a gaussian distribution in the healthy population, 95% normal limits may be calculated as the mean ± twice the standard deviation; however, when measurements do not conform to a Gaussian distribution, these limits are more appropriately obtained from the distribution’s empirical percentiles.56 In the current study, Figures 6 and 7 show a nongaussian distribution of interocular differences between right and left eye, supporting the latter type of analysis.
Thus, the current study demonstrates that the statistical normal upper limit of right eye/left eye differences in mean RNFL thickness measured with Stratus OCT is between approximately 9 and 12 μm, depending on which eye is thicker and which algorithm is used for testing. When one expands this to measurements of RNFL thicknesses in quadrants and clock hours, the differences between eyes are much higher and, perhaps, not as useful to quantify and use clinically. The larger differences in quadrant and clock-hour measurements may be explained by several factors. First, the test-retest variability of clock-hour and quadrant measurements, even in the same eye on the same day by the same operator on the same instrument, has been found to be higher in these smaller areas than the mean RNFL thickness over the entire circumference.57,58 Although we assume mirror image symmetry of RNFL thickness between eyes in these smaller areas, the test-retest variability in the measurements of these areas by the instrument is simply too great to measure accurately. Part of the reason for less accuracy is inclusion of fewer A-scans and thus less removal of random noise. Second, the position of the scan circle affects the measurement of mean RNFL thickness far less than individual quadrants and clock hours, and some degree of misalignment is inevitable. When measuring the mean RNFL thickness, scan position is less important because any decentering superiorly that would cause the superior RNFL quadrant measurement to be artificially thin (since RNFL thickness declines with increasing distance from the optic disc22,59,60) is offset by the increase in RNFL thickness that occurs inferiorly by virtue of being closer to the optic disc rim. Thus, the mean RNFL thickness would be expected to be very similar between eyes despite slight decentration. On the other hand, the quadrant and clock-hour measurements of RNFL thickness would be expected to be extremely dependent on scan circle position for the reason described above. If the scan circle is decentered superiorly in the right eye, causing the RNFL values to be artificially thin, comparison with the contralateral eye, which may be perfectly centered, would show relatively large differences. This phenomenon is even more pronounced when one gets down to the clock-hour level. However, the Cirrus OCT presumably gets around the problem of scan placement by finding the geometric center of the optic disc and extracting the same 3.46-mm scan circle every time. One would expect the differences between right and left eyes in the quadrant and clock-hour measurements to be less with Cirrus than Stratus, but Figure 12 shows these differences are about the same.
Similar results were recently reported by Huynh and colleagues61 in a population-based study of children in the Sydney Childhood Eye Study. They performed fast scans with the Stratus OCT on both eyes of 1172 6-year old children and found that 95% of them had a difference of less than 18 μm in mean RNFL thickness measurements. For quadrant measurements, the differences were much higher, with 95% of children having differences less than 48 μm. No data for clock hours were reported. They concluded that mean RNFL differences between eyes were less than quadrant or clock-hour measurements, similar to the current study results. It is possible that their greater difference in interocular symmetry is due to the age of their participants or differences in scanning technique. Perhaps the amount of asymmetry between eyes decreases with age, thus explaining greater differences found in their study than the current study. Or perhaps children may not fixate as well as adults, which would make measurements between right and left eyes of the same subject more variable due to variability in scan circle placement.
An unexpected result in the current study was that there was a small, but statistically significant difference in RNFL thickness between the right and left eyes of normal subjects. On average the right eye was thicker than the left eye by 1.2 to 1.3 μm. To investigate whether this was due to the common practice of always measuring the right eye before the left, a small study was performed alternating the order of testing in 10 subjects. The trend persisted and clearly was not related to scan order. There may be several explanations for this finding. First, the right eye may actually have a slightly thicker RNFL than the left eye. Second, it may be an error related to measuring RNFL thickness with the Stratus OCT. Possible sources of error include positioning of the subject in relation to the instrument, slight differences in the angle of measurement of RNFL between the 2 eyes, or some other systematic instrument or measurement error. Park and colleagues,62 using a similar study design, average age, and number of subjects, reported a difference of 3.27 μm between the right and left eyes of the same individuals measured with the fast scan algorithm of the Stratus OCT (right RNFL thicker than left). On the other hand, Huynh and associates,61 in their large study of 6-year-old children, did not find any difference in mean RNFL thickness between right and left eyes of the same individuals. When we tried to relate interocular differences in RNFL thickness with age (Figure 11), the contribution of increasing age to asymmetry was extremely small with an R2 value of 0.03. These data, combined with that of Park and Huynh, suggest that there may be a slight influence of age on interocular asymmetry and that, in their large study of 6-year old children, there were no differences, but the differences found in the 2 adult studies are picking up this effect of age on asymmetry. Studies of C:D asymmetry in children have found a lower percentage of subjects with asymmetry than those studies performed in older age-groups as well.
OCT IN GLAUCOMA DIAGNOSIS
It was not until the introduction of OCT that direct, noninvasive, in vivo, quantitative measurements of the RNFL were possible. Huang and colleagues27 demonstrated that measurements of the peripapillary RNFL could be made with OCT. OCT measures tissue thickness using the principle of interferometry. A low-coherence light wave produced by a diode laser is projected at biological tissue. The light wave either passes through or is reflected back by tissue interfaces. The waves reflected back are then matched with a wave sent to a reference mirror, and the principle of interferometry is used to determine the time delay of the reflected light, similar to ultrasound. The time delay is a function of the depth of the tissue being measured. If one wants to measure the thickness of the RNFL, the interface between the posterior vitreous face and the anterior surface of the RNFL is highly reflective, so the light waves are reflected back off of this surface. Also, sharp contrast between the RNFL and the retinal ganglion cell layer allows the posterior surface of the RNFL to be identified. The OCT is able to locate these 2 interfaces and then subtract the difference, which gives a measurement of the RNFL thickness. Multiple measurements, similar to echographic A-scans, can be made in various patterns determined by the operator. The 2 most commonly performed patterns consist of 6-mm line scans or 3.46-mm-diameter circle scans. The line scans have been used to measure retinal thickness in the macula and elsewhere, whereas the circle scans have been used to measure RNFL thickness in the peripapillary region. Early work on peripapillary RNFL measurements by Schuman and associates28 showed that these measurements had better correlation with visual field loss than C:D ratio or neuroretinal rim area. The early OCT instruments, OCT1 and OCT2, had an axial resolution of 17 μm in air27 and lower resolution in tissue. These early instruments were able to perform 100 A-scans per second. OCT3, or Stratus OCT, introduced by Carl Zeiss Meditec (Dublin, California) in 2002, was designed to have an axial resolution of approximately 10 μm in tissue and is able to generate approximately 400 A-scans per second. This has improved the measurements to the point that assessment of the peripapillary RNFL with OCT has been incorporated into glaucoma management. Taking advantage of this new technology, measurements of the RNFL have been shown to be highly reproducible57,58,63–67 and to have excellent sensitivity and specificity for glaucoma,29–41 particularly in glaucoma patients with visual field defects. Ideally, one would like to diagnose glaucoma and glaucomatous progression before visual field defects appear, since multiple histologic studies have shown that thousands of axons, which make up the RNFL itself, are lost before visual field defects appear,2–4 even when tested with automated static perimetry.
The way that glaucoma diagnosis is currently made with OCT is to determine whether the patient’s RNFL thickness differs from that of age-matched controls. It is important to compare an individual’s RNFL thickness to age-matched normal values, since RNFL thickness is known to decline a measurable amount with age. The OCT printout provides a color coding of the mean RNFL thickness values, 4 quadrant values, and 12 clock-hour values. If the patient’s value is in the highest fifth percentile compared to age-matched controls, the value is shaded in white. If the patient’s thickness value is in the middle 90% of age-matched individuals without disease, the value is displayed in green shading. If the patient’s thickness value is in the range of the thinnest fifth percentile of age-matched individuals without disease, the value is displayed in yellow shading. And if the patient’s value is in the range of the thinnest one percentile of RNFL for age-matched individuals without disease, the value is displayed in red shading. Figure 4 and its accompanying legend review the interpretation of peripapillary OCT in a glaucoma suspect.
The usefulness of any new test in medicine depends on its ability to distinguish people without disease from those with disease and/or its ability to detect disease progression. Demonstrating either of these in glaucoma is difficult. The diagnosis of glaucoma remains a clinical one, based on documented characteristic changes in the optic disc (a subjective determination requiring very obvious abnormalities or serial photographs over many years) and/or visual field abnormalities (a subjective test for the patient that lacks sensitivity for early glaucoma). Studies of the sensitivity and specificity of OCT for the diagnosis of glaucoma have primarily focused on cross-sectional studies of glaucoma patients who already have visual field defects. The reason for this is that there have simply not been enough longitudinal studies of patients converting from normal to glaucoma by serial optic disc photography who have also been measured using OCT. Understanding these limitations, measurements of the peripapillary RNFL with Stratus OCT have been shown to be sensitive and specific for glaucoma,29–41 especially in those patients who already have visual field defects. Ideally, we would like to have an objective test that is able to diagnose glaucoma and glaucomatous progression before visual field defects appear, since multiple histologic studies have shown that thousands of axons, which make up the RNFL, must be lost before visual field defects appear,2–4 even when tested with automated static perimetry. This level of sensitivity, especially if these “pre-perimetric” cases of glaucoma are to be included, has not yet been demonstrated with OCT. Perhaps the current proposed methodology, which may identify patients with asymmetric RNFL measurements before the development of frank abnormalities of the RNFL thickness compared to age-matched controls, can be used to improve the early detection of glaucoma. Another approach that we are working on is attempting to identify what would be considered statistically abnormal change in RNFL thickness over time within the normal range of thickness values. This method, like serial optic disc photography, also requires longitudinal measurements over many years, rather than a measurement at one point in time, which is one advantage of using asymmetry analysis.
There are a number of factors other than glaucoma, such as age, optic disc size, and axial length, that may affect RNFL thickness.66 Only age is accounted for in the normative database software, and since the same age correction applies to both eyes of the same patient, it is not a critical variable to consider. However, optic disc size is probably very important to consider when evaluating RNFL asymmetry in a glaucoma suspect. Budenz and associates68 found that using the fixed scan circle of 3.46-mm diameter around the optic disc, eyes with large optic discs have thicker RNFL values keeping everything else constant. Specifically, for every increase in optic disc area of 1 mm2, there is an increase in measured RNFL thickness of approximately 2 μm. This affects both the interpretation of results of an individual patient when trying to diagnose glaucoma (patients with larger optic nerves with thicker RNFL measurements would tend to fall into the normal range, perhaps incorrectly) and symmetry analysis (if the 2 eyes have different-sized optic nerves, this could produce asymmetry of RNFL thickness that is physiologic rather than pathologic). Carpineto and colleagues69 performed Stratus OCT measurements using the fixed 3.46-mm-diameter circle and a variable scan circle diameter designed to measure the RNFL 0.85 mm from the edge of the optic disc in the same group of 30 normal eyes. They showed that RNFL thickness is unrelated to vertical optic disc diameter if one measures RNFL thickness a fixed distance from the disc margin. In fact, they were able to eliminate the variability in RNFL thickness due to optic disc size by measuring a fixed distance from the optic disc rather than using a fixed circle around the optic disc. However, the current normative database for Stratus OCT is based on a fixed scan circle diameter around the optic disc rather than a variable scan circle taken a fixed distance from the optic disc margin. A new normative database would be needed to correct for differences in optic disc size. This would make assessment of RNFL asymmetry independent of disc size. In the meantime, the clinician would need to make an assessment of disc size asymmetry when evaluating RNFL asymmetry, just as he or she does now when assessing asymmetry of C:D ratios.
C:D ASYMMETRY AND GLAUCOMA
Now that we have collected data on asymmetry between the 2 eyes, a second concept, using asymmetry of the RNFL between the 2 eyes of the same individual for diagnosis, can be explored. The hope that this may be possible is based in part on the successful use of asymmetry of the optic nerve cup as an early sign of glaucoma for several decades. The amount of cupping of the optic nerve is, after all, dependent on the size of the optic nerve and the number of axons that make up the neuroretinal rim. The latter parameter is measurable as the RNFL thickness on OCT.
Most standard textbooks on the diagnosis and management of glaucoma identify C:D ratio asymmetry as an important sign in the early diagnosis of glaucoma. Chandler and Grant’s Glaucoma states, in bold, that “Asymmetry of the physiologic cups in the two eyes is seldom seen in normal eyes and, until proven otherwise, must be considered a sign of early glaucoma.”70 Spaeth, in describing findings consistent with glaucomatous optic nerve damage, has stated that asymmetry of the optic cup is “highly suggestive” of glaucoma in the absence of other causes, such as anisometropia, myopia, and obvious disc anomalies.71 This brings up an important point because, just as cup size can vary between eyes simply because of a difference in optic disc size (eg, larger optic discs may have larger cups that are physiologic rather than pathologic), one must be wary of differences in RNFL thickness due to differences in optic disc size. Larger optic discs have been shown to have thicker RNFL measurements with OCT.68 There are 2 possible explanations for this phenomenon. First, Stratus OCT measurements of RNFL thickness are made at a fixed radius of 3.47 mm from the center of the optic disc. RNFL thickness is known to decrease as one moves further from the optic disc margin. Varma and colleagues60 demonstrated this in a histologic study in human eyes. An alternate hypothesis is that large optic nerves simply have more axons. This has been shown in histologic studies of monkey eyes.72 Another important point brought up by Spaeth is the issue of anisometropia. Increasing myopia has been found to be associated with thinner nerve fiber layer thickness measurements in normal subjects,68,73,74 so patients with significant anisometropia would be expected to have differences in RNFL thickness due to that factor. However, in the absence of any significant asymmetry in optic disc size or refractive error between the 2 eyes, C:D ratio asymmetry has been found to be a useful early diagnostic sign of glaucoma in epidemiologic studies, clinical trials, and in clinical practice. In the current study, there were no significant differences in optic disc size, optic cup size, or refractive error between the eyes of the same individuals, so these variables would not be expected to affect the results.
A number of population-based cross-sectional surveys of C:D ratios have found that few normal subjects have asymmetry in C:D ratios greater than 0.2. In the Framingham Eye Study,52 a C:D ratio of ≥0.2 between eyes was used as a criterion for referral for complete evaluation for glaucoma. In the screening portion of the study, C:D ratio was assessed by direct ophthalmoscopy and compared to the C:D ratio assessed in standard stereoscopic photographs. Approximately 6.6% of their population had C:D ratios ≥0.2 in either the horizontal or vertical dimension.52 Interestingly, in subjects with C:D asymmetry defined this way, almost twice as many had a larger C:D ratio in the right eye.52 Disc size, however, was not factored into the analysis. An analysis of 3387 stereoscopic optic disc photographs taken as part of the Baltimore Eye Survey found that a difference in vertical C:D ratio of >0.2 occurred in only 2% of normal African Americans and 5% of normal Caucasians.75 A difference of >0.3 between the C:D ratios of the 2 eyes was found in fewer than 1% of normal African Americans and fewer than 2% of normal Caucasians.75 In the Rotterdam Study, 51 5.8% of the 6199 subjects examined by ophthalmoscopy had a vertical C:D ratio asymmetry ≥0.2 and 1.6% had a vertical C:D ratio ≥0.3. This number included individuals subsequently diagnosed as having glaucoma, so an estimate of the percentage of nonglaucomatous subjects with this much asymmetry would be even lower. In the Blue Mountains Eye Study,76 C:D asymmetry of 0.2 or more was found in only 6% of normal subjects compared to 24% of patients identified with glaucoma. When the difference in C:D ratio was expanded to 0.3 or more, 10% of glaucoma patients and only 1% of normal subjects were found to have this much asymmetry.
Several cross-sectional studies specifically designed to look at optic nerve cupping, but not performed as part of larger population-based eye surveys, have also shown that most normal individuals have very symmetric C:D ratios, particularly compared to glaucoma suspects, ocular hypertensive individuals, or glaucoma patients. Snydacker77 reviewed 500 randomly selected charts from normal patients in his ophthalmic practice and observed that “optic discs were the same in 485 out of 500 patients”77(p 961) when viewed with the direct ophthalmoscope. He concluded that “the optic disc is usually the same in each eye.”77(p 961) Armaly78 recorded the C:D ratios of 724 normal individuals as part of a study to determine the influence of genetics on this parameter. He found that only 8% of subjects had C:D ratios that differed by more than 0.1 and 1% by more than 0.2. Although not the main purpose of his study, he concluded, referring to C:D asymmetry, that “difference between the two eyes in this respect is more significant of possible pathology than that of the size of the optic cup alone.”78(p42) Richardson79 looked at the optic nerves of 468 newborn infants and found that only 11 (2.3%) had C:D asymmetry “of any degree”79(p138) and only 3 (0.6%) were judged to have “marked” asymmetry between the 2 eyes. Specific C:D ratio differences were not mentioned. In a cross-sectional study of optic disc asymmetry in 500 normal, 160 ocular hypertensive, and 53 established glaucoma patients, Fishman42 found significant C:D asymmetry in only 5.6% of normal individuals compared to 30% of those with ocular hypertension and 36% of glaucoma patients, supporting his conclusion that “any [italics added] asymmetry of disc cupping [is] an important alerting sign of possible glaucomatous change.”42(p590) Carpel and Engstrom80 examined 580 individuals with the direct ophthalmoscope and with the Hruby lens at the slit lamp primarily to compare the C:D estimates of the 2 methods. Only 14 of the 580 subjects (2.4%) had a difference of 0.2 or more. Jonas and colleagues81 performed magnification-corrected morphometry of 457 optic disc photographs of normal subjects. They found that the horizontal and vertical C:D ratio differed by more than 0.2 in only 4% of normal individuals. Carassa and associates82 compared the stereoscopic optic disc photographs of 75 ocular hypertensive patients to those of 57 control subjects and observed significant interocular asymmetry in the optic cup size of ocular hypertensive patients compared to controls, particularly in the inferior part of the cup.
Several longitudinal cohort studies of ocular hypertensive patients have identified C:D asymmetry as a significant risk factor for the development of glaucoma. Yablonski and associates83 followed 102 patients with ocular hypertension (elevated IOP without visual field loss on Goldmann perimetry) using stereoscopic optic disc photographs, among other methods. All 4 authors acted as masked readers in estimating C:D ratios and progressive cupping over the subsequent 5 years of the study. They found that baseline C:D ratio differed by 0.2 or more in 91% of ocular hypertensive subjects that progressed to glaucoma as determined by visual field loss. Moreover, baseline C:D ratio differed by 0.2 or more in 75% of subjects who progressed to glaucoma as shown by optic disc changes. Because of these findings, which suggest that C:D asymmetry may be a sign of early glaucoma, the Ocular Hypertension Treatment Study considered C:D asymmetry exceeding 0.2 an exclusionary criterion53 because this is a “sign of pre-existing glaucomatous damage” (Ocular Hypertension Treatment Study Group. Manual of Procedures, Ocular Hypertension Treatment Study, version 3.1, September 24, 2001:7–12). In a cohort study of 647 ocular hypertensive patients, Quigley and colleagues21 found that the top 2 risk factors among a number of baseline demographic and clinical characteristics were, first, the presence of nerve fiber layer atrophy (relative risk 3.67 to 8.89, depending on severity) and asymmetry in the C:D ratio (relative risk 2.20 to 2.70, depending on amount of asymmetry). In the European Glaucoma Prevention Study,54 individuals with ocular hypertension and a vertical C:D ratio difference between eyes of 0.4 or greater were excluded. In their report on the risk factors for conversion from ocular hypertension to primary open-angle glaucoma (POAG), these investigators84 found that vertical C:D asymmetry between eyes, even though baseline C:D ratio differences had to be within 0.3, increased the risk of developing POAG by 46%. This was one of only 5 risk factors for the development of POAG identified in the multivariate analysis.
ASYMMETRY USING OTHER GLAUCOMA IMAGING MODALITIES
Confocal scanning laser polarimetry, performed by the HRT, has been used to evaluate the neuroretinal rim and other optic disc parameters as a way of diagnosing and following glaucoma. Harasymowycz and colleagues85 looked at a unique HRT parameter known as RADAAR, or ratio of rim area to disc area asymmetry, in a group of 140 randomly selected glaucoma patients and found that asymmetry between the 2 eyes correlated with various measures of glaucomatous optic neuropathy. The ratio of rim area to disc area appears to be a surrogate for the amount of retinal ganglion cell axons anatomically, so it would seem that an actual measurement of the RNFL thickness would give a similar result. A difference in their measure of asymmetry, as compared to ours, is that they controlled for differences in optic disc area between the 2 eyes of the same patient, something that is not incorporated into the OCT software, even though disc size has been found to influence RNFL thickness.68 In a large population survey that measured HRT parameters in all subjects, Hawker and colleagues86 developed limits for the normal amount of symmetry in 459 of their normal subjects. They identified the rim-to-disc area ratio as the most useful parameter because it is less affected by differences in disc size, which occasionally occur in normal individuals.
Scanning laser polarimetry, performed by the GDx, provides an indirect measure of nerve fiber layer thickness calculated from the retardance of polarized light by the birefringent properties of axons. Shaikh and Salmon87 reviewed the GDx scans (with variable corneal compensator) in 43 glaucoma suspects. Asymmetry of the nerve fiber layer index was identified as the most useful parameter for decision making in these patients.
Measurements of total retinal thickness have not received as much attention clinically as have measurements of optic disc topography or retinal fiber layer analysis. However, the retinal ganglion cell layer and RNFL together make up a significant portion of the total retinal thickness; measurements of total retinal thickness would be expected to reflect loss of ganglion cells and their axons in glaucoma. Using the retinal thickness analyzer (RTA; Talia Technology Ltd, Mevaseret Zion, Israel), Zeimer and colleagues88 found a significant relationship between total retinal thickness asymmetry and visual field loss in glaucoma patients. This finding is most certainly due to the fact that asymmetry in the loss of retinal ganglion cells and their axons accounts for the change in total retinal thickness.
ASYMMETRY IN FUNCTIONAL TESTING FOR GLAUCOMA
The concept of using ocular symmetry to diagnose early glaucoma has also been applied to assessing the functional status of the visual system. Brenton and associates89 performed Humphrey (Carl Zeiss Meditec, Dublin, California) visual fields in 20 normal subjects and found that a difference in mean exceeding 1.4 dB occurred in fewer than 1% of normal subjects. As one compares individual points in the visual field, the differences get larger, analogous to looking at individual clock hours with OCT. This phenomenon has several explanations. First, since the mean deviation and mean RNFL thickness averages values from the entire test, signal averaging will create less variability in measurements by reducing noise, averaging out the overestimates and the underestimates. Second, positioning of the eye in perimetry and in OCT would likely create more variability in individual points than the mean value. Feuer and Anderson90 performed a similar study, in which they tested 20 normal subjects with Humphrey visual fields in both eyes and then tested one of the 2 eyes twice. They found that the average difference between the 2 eyes was very small (0.33 dB) and very similar to the test-retest variability when the same eye was tested twice. Their study supports the conclusion that most normal subjects (96%) have a difference in mean deviation of less than 1.5 dB between the 2 eyes and that a difference greater than this, particularly if confirmed in the same direction on repeat testing, is highly suggestive of glaucoma, especially in the presence of some other abnormality, such as asymmetry in IOPs or asymmetry in C:D ratio. Poinoosawmy and colleagues91 reviewed the visual fields at initial presentation of 403 patients with “normal-tension” glaucoma and 337 patients with “high-tension” glaucoma to determine the frequency of patients with visual field defects in one eye but none in the other. In the “normal-tension” glaucoma group, 25% presented with unilateral visual field loss, whereas 31% of patients in the “high-tension” glaucoma group had unilateral field loss. This is might be analogous to finding thinning of the RNFL in one eye compared to the other with OCT in patients with early glaucoma.
Multifocal visual evoked potentials (mVEPs) and pattern electroretinogram (PERG) are newer techniques for measuring the functional status of the visual system in glaucoma. The mVEP identifies localized functional deficiencies in the retina and, therefore, visual field, by stimulating the retina in 60 distinct locations and recording brain wave activity in the occipital cortex. In this way, one may obtain an objective, rather than subjective, assessment of visual field function at many locations. Although several investigators have shown that there is too much intersubject variation for this test to be useful in distinguishing glaucoma patients from normal individuals,92–94 the concept of intereye symmetry has been shown to be extremely useful in detecting early functional loss.94 The concept is similar to the one we are proposing: while both eyes with glaucoma may eventually develop visual field defects, these are unlikely to occur to the exact same degree in both eyes in the same individual at the same time. In a study of 6 normal subjects and 4 glaucoma patients, Hood and colleagues94(p1580) found that the multifocal VEP responses in the 2 eyes of the same subject were “essentially identical” in most regions of the visual field. Graham and colleagues95 confirmed the usefulness of asymmetry analysis with mVEP in a much larger study of 24 normal controls, 70 glaucoma patients, and 31 patients at high risk for glaucoma. Pattern ERG, on the other hand, is a functional measure of the visual system that works by presenting alternating high-contrast images (black and white checkerboard stripes) to each eye and measuring the electrical response with skin electrodes applied to the face.96 The PERG measures the global response of the central retina to this type of stimulus, and the response is dependent on healthy retinal ganglion cells. In glaucoma, of course, these are the cells that are lost in the retina, so the response of the retinal ganglion cells would be expected to correlate with the presence and severity of glaucoma. Especially because some sources of interocular variance is eliminated by recording from the 2 eyes simultaneously, asymmetry in PERG amplitude between eyes has been suggested as a way of diagnosing early glaucoma.97
ASYMMETRY IN INTRAOCULAR PRESSURE
Asymmetry in IOP is also rather unusual in the normal population. If one defines IOP asymmetry as a difference of 1 SD from the mean of the population, then, for instance, in the Blue Mountains Eye Study, approximately 5% of people over age 49 have a difference greater than or equal to 3 mm Hg between eyes.98 In this study, people who were identified with IOP asymmetry of ≥3 mm Hg had an odds ratio of 4.0 for developing glaucoma compared to those without significant asymmetry in a multivariate analysis. No attempt was made to correlate the eye with the higher IOP with glaucomatous damage in this brief report. Several studies of normal-tension glaucoma have demonstrated that even in the “normal” range of IOP, the eye with the higher IOP tends to be the one with the most glaucomatous damage.99–102 There is one recent study that disputes this, however. In the Low-Pressure Glaucoma Treatment Study,103 no relationship was found between IOP asymmetry and visual field asymmetry. In the Ocular Hypertension Treatment Study,104 the main analysis of risk factors for development of POAG was performed by averaging IOP values in the 2 eyes, making it impossible to analyze interocular pressure differences in the original multivariate analysis of risk factors for developing POAG. But a recent reanalysis of the Ocular Hypertension Treatment Study data in which data from the 2 eyes were analyzed separately found a 17% increased risk of developing POAG for every 1 mm Hg difference in symmetry between the 2 eyes of the same subject.105
CONCLUSIONS
In their early work in this area, Quigley and Sommer16 recommended using asymmetry in the appearance of the RNFL between the 2 eyes as a way to diagnose early glaucoma and compared this clinical sign to that of C:D asymmetry. Accepting that accurate and reproducible measurement of the RNFL can be made with OCT and that asymmetry of the RNFL thickness is an important clinical sign found in some early glaucoma patients, one can begin to put parameters on what constitutes the normal amount of variation in RNFL thickness between the right and left eyes. This is similar to what was done with C:D ratios, which uncommonly differ by more than 0.2. In the current study, it has been demonstrated that the normal individuals studied have mean RNFL thicknesses that differ by less than 8.9 to 10.8 μm with a standard OCT scan and 10.6 to 11.7 μm with a fast OCT scan, depending on which eye measures thicker. The current study also suggests that the symmetry of quadrant and clock-hour measurements of RNFL thickness is quite low and varies by location, making these parameters less useful clinically.
Two important variables that must be taken into account if one wants to use RNFL thickness variation between the 2 eyes as a sign of early glaucoma are symmetry of optic disc size and symmetry of refractive error. Just as in eyes with asymmetric discs, which may have asymmetric cups that are still normal (the larger disc usually having the larger cup and the smaller disc having the smaller cup), differences in RNFL thickness due to differences in disc size also exist.68 Thus one may expect thicker peripapillary RNFL measurements in the eye with the larger of the 2 discs and vice versa. Similarly, eyes with higher myopic refractive errors (greater axial length) will have thinner RNFL thickness measurements,68 so significant anisometropia would create asymmetry in RNFL measurements. So, when detected, these other factors must be taken into account. While these particular anatomic variations have been studied, it should be made clear that there may be other causes of asymmetry, in addition to glaucoma, that have not been studied. Amblyopia, especially if not associated with a history of misaligned eyes, may be due to undetected anatomic asymmetry of the 2 eyes and may affect RNFL thickness. Other diseases with clinically obvious or subclinical optic atrophy (such as multiple sclerosis) may have thinning of the RNFL that can be asymmetric. As with so many diagnostic tests, the results have to be interpreted in the context of other clinical signs and the reason that the clinical test was obtained. Thus, for example, if an RNFL scan is done as a matter of routine whenever the retina is examined (for example, for retinal vascular occlusive disease or diabetic retinal edema), abnormalities or asymmetries of the RNFL thickness may be part of the retinal disease, unless there are other reasons to suspect the presence of glaucoma.
Retinal nerve fiber layer asymmetry would not be expected in all cases of early glaucoma, just like not all early glaucoma cases have C:D asymmetry. There are clearly patients in whom both eyes progress at the same rate, in which case the optic disc cupping and RNFL thickness would stay symmetric but progressively worsen more or less at the same rate. But a finding of asymmetry of the RNFL greater than 9 to 12 μm may assist in identifying some early cases of glaucoma and, perhaps combined with other suspicious findings, may help push us into making the diagnosis of glaucoma earlier than we would have otherwise. Conversely, since we are using the 95th percentile of the normal difference between eyes, we would expect that 5% of normal individuals would, in fact, vary by more than the parameters defined above. Thus, we are not proposing the use of OCT RNFL asymmetry measurements as a screening tool in normal populations. Since our definition of abnormality is a statistical one, far too many people would screen positive for glaucoma using asymmetry alone as a diagnostic criteria. Rather, we are suggesting that OCT RNFL asymmetry might be used in conjunction with other clinical parameters, such as elevated IOP, C:D enlargement or asymmetry, or other clinical signs, in the diagnosis of early glaucoma. Specifically, the clinician would generally obtain OCT in patients with some other risk factor (such as elevated IOP) or clinical sign (such as large C:D ratio) of glaucoma. In such cases, adding the abnormal finding of significant RNFL asymmetry might provide additional evidence of early structural change consistent with glaucoma, thereby prompting the initiation of therapy at an earlier stage of the disease.
FIGURE 3.
Right (upper) and left (lower) visual fields of patient described in Figure 2. There is a 1-dB difference in mean deviation (MD), with the left eye being worse than the right, yet both within the normal range. The Glaucoma Hemifield Test (GHT) and Pattern Standard Deviation (PSD) are normal in the left eye. This same 1- to 1.5-dB asymmetry in mean deviation has been present throughout the patient’s course.
ACKNOWLEDGMENTS
Funding/Support: Supported by NIH center grant P30 EY014801, awarded by the National Eye Institute, Bethesda, Maryland, and an unrestricted grant from Research to Prevent Blindness, Inc, New York, New York.
Financial Disclosures: The Stratus OCT was on loan from Carl Zeiss Meditec, Dublin, California, for research purposes during the course of this study. The author has received research support, salary support for research, and lecture fees for speaking from Carl Zeiss Meditec.
Conformity With Author Information: This study was approved by the Human Subjects Committee of the Institutional Review Board (IRB) of the University of Miami, Miller School of Medicine, and followed the tenets of the treaty of Helsinki. The subjects’ willingness to participate was documented with their signature on a consent form approved by the IRB.
Other Acknowledgments: The author wishes to acknowledge Douglas R. Anderson, MD, for assistance in the study design; Navneet Sekhon, MD, and Alexei Moraczewski, MD, for assistance in the conduct of the study; and William J. Feuer, MS, and Giovanni Gregori, PhD, for statistical assistance. All are from the Department of Ophthalmology, University of Miami Miller School of Medicine.
REFERENCES
- 1.Quigley HA. Examination of the retinal nerve fiber layer in the recognition of early glaucoma damage. Trans Am Ophthalmol Soc. 1986;84:920–966. [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–464. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 3.Mikelberg FS, Yidegiligne HM, Schulzer M. Optic nerve axon count and axon diameter in patients with ocular hypertension and normal visual fields. Ophthalmology. 1995;102:342–348. doi: 10.1016/s0161-6420(95)31019-6. [DOI] [PubMed] [Google Scholar]
- 4.Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41:741–748. [PubMed] [Google Scholar]
- 5.Pederson JE, Anderson DR. The mode of progressive optic disc cupping in ocular hypertension and glaucoma. Arch Ophthalmol. 1980;98:490–495. doi: 10.1001/archopht.1980.01020030486010. [DOI] [PubMed] [Google Scholar]
- 6.Zeyen TG, Caprioli J. Progression of disc and field damage in early glaucoma. Arch Ophthalmol. 1993;111:62–65. doi: 10.1001/archopht.1993.01090010066028. [DOI] [PubMed] [Google Scholar]
- 7.Vogt A. Herstellung eines gelbblauen Lichtfiltrates, in welchem die Macula centralis in vivo in gelber Färbung erscheint, die Nervenfasern der Netzhaut und andere feine Einzelheiten derselben sichtbar werden, und der Grad der Gelbfärbung der Linse ophthalmoskopisch nachweisbar ist. Graefes Arch Ophthalmol. 1913;84:293–311. [Google Scholar]
- 8.Behrendt T, Wilson LA. Spectral reflectance photography of the retina. Am J Ophthalmol. 1965;59:1079–1088. [PubMed] [Google Scholar]
- 9.Hoyt WF, Newman NM. The earliest observable defect in glaucoma? Lancet. 1972;299:692–693. doi: 10.1016/s0140-6736(72)90500-4. [DOI] [PubMed] [Google Scholar]
- 10.Hoyt WF. Ophthalmoscopy of the retinal nerve fibre layer in neuro-ophthalmic diagnoses. Aust N Z J Ophthalmol. 1976;4:14–34. [Google Scholar]
- 11.Hoyt WF, Frisen L, Newman NM. Fundoscopy of nerve fiber layer defects in glaucoma. Invest Ophthalmol. 1973;12:814–829. [PubMed] [Google Scholar]
- 12.Sommer A, Miller NR, Pollack I, Maumanee AE, George T. The nerve fiber layer in the diagnosis of glaucoma. Arch Ophthalmol. 1977;95:2149–2156. doi: 10.1001/archopht.1977.04450120055003. [DOI] [PubMed] [Google Scholar]
- 13.Quigley HA, Miller NR, George T. Clinical evaluation of nerve fiber layer atrophy as an indicator of glaucomatous optic nerve damage. Arch Ophthalmol. 1980;98:1564–1571. doi: 10.1001/archopht.1980.01020040416003. [DOI] [PubMed] [Google Scholar]
- 14.Airaksinen PJ, Mustonen E, Alanko HI. Optic disc haemorrhages precede retinal nerve fibre layer defects in ocular hypertension. Acta Ophthalmol. 1981;59:627–641. doi: 10.1111/j.1755-3768.1981.tb08728.x. [DOI] [PubMed] [Google Scholar]
- 15.Airaksinen PJ, Tuulonen A. Early glaucoma changes in patients with and without an optic disc hemorrhage. Acta Ophthalmol. 1984;62:197–202. doi: 10.1111/j.1755-3768.1984.tb08394.x. [DOI] [PubMed] [Google Scholar]
- 16.Quigley HA, Sommer A. How to use nerve fiber layer examination in the management of glaucoma. Trans Am Ophthalmol Soc. 1987;85:254–272. [PMC free article] [PubMed] [Google Scholar]
- 17.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 18.Airaksinen PJ, Drance SM, Douglas GR, Mawson DK, Nieminen H. Diffuse and localized nerve fiber loss in glaucoma. Am J Ophthalmol. 1984;98:566–571. doi: 10.1016/0002-9394(84)90242-3. [DOI] [PubMed] [Google Scholar]
- 19.Airaksinen PJ, Drance SM, Douglas GR, Schulzer M, Wijsman K. Visual field and retinal nerve fiber layer comparisons in glaucoma. Arch Ophthalmol. 1985;103:205–207. doi: 10.1001/archopht.1985.01050020057019. [DOI] [PubMed] [Google Scholar]
- 20.Tuulonen A, Airaksinen PJ. Initial glaucomatous optic disk and retinal nerve fiber layer abnormalities and their progression. Am J Ophthalmol. 1991;111:485–490. doi: 10.1016/s0002-9394(14)72385-2. [DOI] [PubMed] [Google Scholar]
- 21.Quigley HA, Enger C, Katz J, Sommer R, Gilbert D. Risk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol. 1994;112:644–649. doi: 10.1001/archopht.1994.01090170088028. [DOI] [PubMed] [Google Scholar]
- 22.Quigley HA, Addicks EM. Quantitative studies of retinal nerve fiber layer defects. Arch Ophthalmol. 1982;100:807–814. doi: 10.1001/archopht.1982.01030030811018. [DOI] [PubMed] [Google Scholar]
- 23.Sommer A, Quigley HA, Robin AL, Miller NR, Katz J, Arkell S. Evaluation of nerve fiber layer assessment. Arch Ophthalmol. 1984;102:1766–1771. doi: 10.1001/archopht.1984.01040031430017. [DOI] [PubMed] [Google Scholar]
- 24.Airaksinen PJ, Tuulonen A, Werner EB. Clinical evaluation of the optic disc and retinal nerve fiber layer. In: Ritch R, Shields MB, Krupin T, editors. The Glaucomas. Ed 2. St Louis: Mosby-Year Book; 1996. pp. 617–657. [Google Scholar]
- 25.Stamper RL, Lieberman MF, Drake MV. Becker-Shaffer’s Diagnosis and Therapy of the Glaucomas. Ed 7. St Louis: Mosby; 1999. p. 201. [Google Scholar]
- 26.Caprioli J, Ortiz-Colberg R, Miller JM, Tressler C. Measurements of peripapillary nerve fiber layer contour in glaucoma. Am J Ophthalmol. 1989;108:404–413. doi: 10.1016/s0002-9394(14)73308-2. [DOI] [PubMed] [Google Scholar]
- 27.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113:586–596. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 29.Parikh RS, Parikh S, Sekhar GC, et al. Diagnostic capability of optical coherence tomography (Stratus OCT 3) in early glaucoma. Ophthalmology. 2007;114:2238–2243. doi: 10.1016/j.ophtha.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Hong S, Ahn H, Ha SJ, Yeom HY, Seong GJ, Hong YJ. Early glaucoma detection using the Humphrey matrix perimeter, GDx VCC, Stratus OCT, and retinal nerve fiber layer photography. Ophthalmology. 2007;114:210–215. doi: 10.1016/j.ophtha.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Pueyo V, Polo V, Larrosa JM, Ferreras A, Pablo LE, Honrubia FM. Diagnostic ability of the Heidelberg retina tomograph, optical coherence tomograph, and scanning laser polarimeter in open-angle glaucoma. J Glaucoma. 2007;16:173–177. doi: 10.1097/IJG.0b013e31802dfc1d. [DOI] [PubMed] [Google Scholar]
- 32.Hougaard JL, Heijl A, Bengtsson B. Glaucoma detection by Stratus OCT. J Glaucoma. 2007;16:302–306. doi: 10.1097/IJG.0b013e318032e4d4. [DOI] [PubMed] [Google Scholar]
- 33.Ojima T, Tanabe T, Hangai M, Yu S, Morishita S, Yoshimura N. Measurement of retinal nerve fiber layer thickness and macular volume for glaucoma detection using optical coherence tomography. Jpn J Ophthalmol. 2007;51:197–203. doi: 10.1007/s10384-006-0433-y. [DOI] [PubMed] [Google Scholar]
- 34.Shah NN, Bowd C, Medeiros FA, et al. Combining structural and functional testing for detection of glaucoma. Ophthalmology. 2006;113:1593–1602. doi: 10.1016/j.ophtha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Mastropasqua L, Brusini P, Carpineto P, et al. Humphrey matrix frequency doubling technology perimetry and optical coherence tomography measurement of the retinal nerve fiber layer thickness in both normal and ocular hypertensive subjects. J Glaucoma. 2006;15:328–335. doi: 10.1097/01.ijg.0000212230.65545.d3. [DOI] [PubMed] [Google Scholar]
- 36.Brusini P, Salvetat ML, Zeppieri M, Tosoni C, Parisi L, Filletti M. Comparison between GDx VCC scanning laser polarimetry and Stratus OCT optical coherence tomography in the diagnosis of chronic glaucoma. Acta Ophthalmol Scand. 2006;84:650–655. doi: 10.1111/j.1600-0420.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- 37.Medeiros FA, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Influence of disease severity and optic disc size on the diagnostic performance of imaging instruments in glaucoma. Invest Ophthalmol Vis Sci. 2006;47:1008–1015. doi: 10.1167/iovs.05-1133. [DOI] [PubMed] [Google Scholar]
- 38.Budenz DL, Michael A, Chang RT, McSoley J, Katz J. Sensitivity and specificity of the StratusOCT for perimetric glaucoma. Ophthalmology. 2005;112:3–9. doi: 10.1016/j.ophtha.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 39.Medeiros FA, Zangwill LM, Bowd C, Vessani RM, Susanna R, Weinreb RN. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139:44–55. doi: 10.1016/j.ajo.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 40.Kim TW, Park UC, Park KH, Kim DM. Ability of Stratus OCT to identify localized retinal nerve fiber layer defects in patients with normal standard automated perimetry results. Invest Ophthalmol Vis Sci. 2007;48:1635–1641. doi: 10.1167/iovs.06-0800. [DOI] [PubMed] [Google Scholar]
- 41.Hoh ST. Evaluating the optic nerve and retinal nerve fibre layer: the roles of Heidelberg retina tomography, scanning laser polarimetry and optical coherence tomography. Ann Acad Med Singapore. 2007;36:194–202. [PubMed] [Google Scholar]
- 42.Fishman RS. Optic disc asymmetry. Arch Ophthalmol. 1970;84:590–594. doi: 10.1001/archopht.1970.00990040592006. [DOI] [PubMed] [Google Scholar]
- 43.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. JAMA. 1991;266:369–374. [PubMed] [Google Scholar]
- 44.Klein BE, Klein R, Sponsel W, et al. Prevalence of glaucoma: The Beaver Dam Eye Study. Ophthalmology. 1992;99:1499–1504. doi: 10.1016/s0161-6420(92)31774-9. [DOI] [PubMed] [Google Scholar]
- 45.Leske MC, Connell AM, Schachat A, Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821–829. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell P, Smith W, Attebo K, Healy PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103:1661–1669. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 47.Cedrone C, Culasso F, Cesareo M, Zapelloni A, Cedrone P, Cerulli L. Prevalence of glaucoma in Ponza, Italy: a comparison with other studies. Ophthalmic Epidemiol. 1997;4:59–72. doi: 10.3109/09286589709057098. [DOI] [PubMed] [Google Scholar]
- 48.Bonomi L, Marchini G, Marraffa M, et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna-Neumarkt Study. Ophthalmology. 1998;105:209–215. doi: 10.1016/s0161-6420(98)92665-3. [DOI] [PubMed] [Google Scholar]
- 49.Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR. The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology. 1998;105:733–739. doi: 10.1016/S0161-6420(98)94031-3. [DOI] [PubMed] [Google Scholar]
- 50.Kahn HA, Milton RC. Alternative definitions of open-angle glaucoma. Effect on prevalence and associations in the Framingham eye study. Arch Ophthalmol. 1980;98:2172–2177. doi: 10.1001/archopht.1980.01020041024003. [DOI] [PubMed] [Google Scholar]
- 51.Wolfs RCW, Borger PH, Ramrattan RS, et al. Changing views on open-angle glaucoma: definitions and prevalences—The Rotterdam Study. Invest Ophthalmol Vis Sci. 2000;41:3309–3321. [PubMed] [Google Scholar]
- 52.Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24(suppl):335–610. [PubMed] [Google Scholar]
- 53.Gordon MO, Kass MA, Ocular Hypertension Treatment Study Group The ocular hypertension treatment study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–583. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 54.The European Glaucoma Prevention Study (EGPS) Group The European glaucoma prevention study design and baseline description of the participants. Ophthalmology. 2002;109:1612–1621. doi: 10.1016/s0161-6420(02)01167-3. [DOI] [PubMed] [Google Scholar]
- 55.Gross PG, Drance SM. Comparison of a simple ophthalmoscopic and planimetric measurement of glaucomatous neuro-retinal rim areas. J Glaucoma. 1995;4:314–316. [PubMed] [Google Scholar]
- 56.Colton T. Statistics in Medicine. Boston: Little Brown; 1974. p. 303. [Google Scholar]
- 57.Budenz DL, Chang RT, Huang X, Knighton RW, Tielsch JM. Reproducibility of retinal nerve fiber thickness measurements using the Stratus OCT in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2005;46:2440–2443. doi: 10.1167/iovs.04-1174. [DOI] [PubMed] [Google Scholar]
- 58.Budenz DL, Fredette M-J, Feuer WJ, Anderson DR. Reproducibility of peripapillary retinal nerve fiber thickness measurements with the Stratus™ OCT in glaucomatous eyes. Ophthalmology. 2008;115:661–666. doi: 10.1016/j.ophtha.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 59.Radius RL. Thickness of the retinal nerve fiber layer in primate eyes. Arch Ophthalmol. 1980;98:1625–1629. doi: 10.1001/archopht.1980.01020040477018. [DOI] [PubMed] [Google Scholar]
- 60.Varma R, Skaf M, Barron E. Retinal nerve fiber layer thickness in normal human eyes. Ophthalmology. 1996;103:2114–2119. doi: 10.1016/s0161-6420(96)30381-3. [DOI] [PubMed] [Google Scholar]
- 61.Huynh SC, Wang XY, Burlutsky G, Mitchell P. Symmetry of optical coherence tomography retinal measurements in young children. Am J Ophthalmol. 2007;143:518–520. doi: 10.1016/j.ajo.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 62.Park JJ, Oh DR, Hong SP, Lee KW. Asymmetry analysis of the retinal nerve fiber layer thickness in normal eyes using optical coherence tomography. Kor J Ophthalmol. 2005;19:281–287. doi: 10.3341/kjo.2005.19.4.281. [DOI] [PubMed] [Google Scholar]
- 63.Schuman JS, Pedut-Kloizman T, Hertzmark E, et al. Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology. 1996;103:1889–1898. doi: 10.1016/s0161-6420(96)30410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blumenthal EZ, Williams JM, Weinreb RN, Girkin CA, Berry CC, Zangwill LM. Reproducibility of nerve fiber layer thickness measurements by use of optical coherence tomography. Ophthalmology. 2000;107:2278–2282. doi: 10.1016/s0161-6420(00)00341-9. [DOI] [PubMed] [Google Scholar]
- 65.Jones AL, Sheen NJ, North RV, Morgan JE. The Humphrey optical coherence tomography scanner: quantitative analysis and reproducibility study of the normal human retinal nerve fibre layer. Br J Ophthalmol. 2001;85:673–677. doi: 10.1136/bjo.85.6.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carpineto P, Ciancaglini M, Zuppardi E, Falconio G, Doronzo E, Matropasqua L. Reliability of nerve fiber layer thickness measurements using optical coherence tomography in normal and glaucomatous eyes. Ophthalmology. 2003;110:190–195. doi: 10.1016/s0161-6420(02)01296-4. [DOI] [PubMed] [Google Scholar]
- 67.Paunescu LA, Schuman JS, Price LL, et al. Reproducibility of nerve fiber layer thickness, macular thickness, and optic nerve head measurements using Stratus OCT. Invest Ophthalmol Vis Sci. 2004;45:1716–1724. doi: 10.1167/iovs.03-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007;114:1046–1052. doi: 10.1016/j.ophtha.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carpineto P, Ciancaglini M, Aharrh-Gnama A, Cirone D, Mastropasqua L. Custom measurement of retinal nerve fiber layer thickness using STRATUS OCT in normal eyes. Eur J Ophthalmol. 2005;15:360–366. doi: 10.1177/112067210501500308. [DOI] [PubMed] [Google Scholar]
- 70.Epstein D. Examination of the optic nerve. In: Epstein DL, Allingham RR, Schuman JS, editors. Chandler and Grant’s Glaucoma. Ed 4. Baltimore: Williams & Wilkins; 1996. p. 87. [Google Scholar]
- 71.Spaeth GL. Development of glaucomatous changes of the optic nerve. In: Varma R, Spaeth GL, Parker KW, editors. The Optic Nerve in Glaucoma. Philadelphia: Lippincott; 1993. p. 66. [Google Scholar]
- 72.Quigley HA, Coleman AL, Dorman-Pease ME. Larger optic nerve heads have more nerve fibers in normal monkey eyes. Arch Ophthalmol. 1992;109:1441–1443. doi: 10.1001/archopht.1991.01080100121056. [DOI] [PubMed] [Google Scholar]
- 73.Choi SW, Lee SJ. Thickness changes in the fovea and peripapillary retinal nerve fiber layer depend on the degree of myopia. Korean J Ophthalmol. 2006;20:215–219. doi: 10.3341/kjo.2006.20.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leung CK, Mohamed S, Leung KS, et al. Retinal nerve fiber layer measurements in myopia: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2006;47:5171–5176. doi: 10.1167/iovs.06-0545. [DOI] [PubMed] [Google Scholar]
- 75.Varma R, Tielsch JM, Quigley HA, et al. Race-, age-, gender-, and refractive error-related differences in the normal optic disc. Arch Ophthalmol. 1994;112:1068–1076. doi: 10.1001/archopht.1994.01090200074026. [DOI] [PubMed] [Google Scholar]
- 76.Ong LS, Mitchell P, Healey PR, Cumming RG. Asymmetry in optic disc parameters: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 1999;40:849–857. [PubMed] [Google Scholar]
- 77.Snydacker D. The normal optic disc. Ophthalmoscopic and photographic studies. Am J Ophthalmol. 1964;58:958–964. [PubMed] [Google Scholar]
- 78.Armaly MF. Genetic determination of cup/disc ratio of the optic nerve. Arch Ophthalmol. 1967;78:35–43. doi: 10.1001/archopht.1967.00980030037007. [DOI] [PubMed] [Google Scholar]
- 79.Richardson KT. Optic cup symmetry in normal newborn infants. Invest Ophthalmol. 1968;7:137–140. [PubMed] [Google Scholar]
- 80.Carpel EF, Engstrom PF. The normal cup-disk ratio. Am J Ophthalmol. 1981;91:588–597. doi: 10.1016/0002-9394(81)90056-8. [DOI] [PubMed] [Google Scholar]
- 81.Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1988;29:1151–1158. [PubMed] [Google Scholar]
- 82.Carassa RG, Schwartz B, Takamoto T. Increased preferential optic disc asymmetry in ocular hypertensive patients compared with control subjects. Ophthalmology. 1991;98:681–691. doi: 10.1016/s0161-6420(91)32233-4. [DOI] [PubMed] [Google Scholar]
- 83.Yablonski ME, Zimmerman TJ, Kass MA, Becker B. Prognostic significance of optic disk cupping in ocular hypertensive patients. Am J Ophthalmol. 1980;89:585–592. doi: 10.1016/0002-9394(80)90071-9. [DOI] [PubMed] [Google Scholar]
- 84.European Glaucoma Prevention Study (EGPS) Group Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114:3–9. doi: 10.1016/j.ophtha.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 85.Harasymowycz P, Davis B, Xu G, Myers J, Bayer A, Spaeth GL. The use of RADAAR (ratio of rim area to disc area asymmetry) in detecting glaucoma and its severity. Can J Ophthalmol. 2004;39:240–244. doi: 10.1016/s0008-4182(04)80120-0. [DOI] [PubMed] [Google Scholar]
- 86.Hawker MJ, Vernon SA, Ainsworth G, Hillman JG, MacNab HK, Dua HS. Asymmetry in optic disc morphology as measured by Heidelberg Retinal Tomography in a normal elderly population: the Bridlington Eye Assessment Project. Invest Ophthalmol Vis Sci. 2005;46:4153–4158. doi: 10.1167/iovs.05-0423. [DOI] [PubMed] [Google Scholar]
- 87.Shaikh A, Salmon JF. The role of scanning laser polarimetry using the GDx variable corneal compensator in the management of glaucoma suspects. Br J Ophthalmol. 2006;90:1454–1457. doi: 10.1136/bjo.2006.099143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zeimer R, Asrani S, Zou S, Quigley H, Jampel H. Quantitative detection of glaucomatous damage at the posterior pole by retinal thickness mapping. Ophthalmology. 1998;105:224–231. doi: 10.1016/s0161-6420(98)92743-9. [DOI] [PubMed] [Google Scholar]
- 89.Brenton RS, Phelps CD, Rojas P, Woolson RF. Interocular differences of the visual field in normal subjects. Invest Ophthalmol Vis Sci. 1986;27:799–805. [PubMed] [Google Scholar]
- 90.Feuer WJ, Anderson DR. Static threshold asymmetry in early glaucomatous visual field loss. Ophthalmology. 1989;96:1285–1297. doi: 10.1016/s0161-6420(89)32724-2. [DOI] [PubMed] [Google Scholar]
- 91.Poinoosawmy D, Fontana L, Wu JX, Bunce CV, Hitchings RA. Frequency of asymmetric visual field defects in normal-tension and high-tension glaucoma. Ophthalmology. 1998;105:988–991. doi: 10.1016/S0161-6420(98)96049-3. [DOI] [PubMed] [Google Scholar]
- 92.Baseler HA, Sutter EE, Klein SA, Carney T. The topography of visual evoked response properties across the visual field. Electroenceph Clin Neurophysiol. 1994;90:65–81. doi: 10.1016/0013-4694(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 93.Klistorner AI, Graham SL, Grigg JR, Billson FA. Multifocal topographic visual evoked potential: improving objective detection of local visual field defects. Invest Ophthalmol Vis Sci. 1998;39:937–950. [PubMed] [Google Scholar]
- 94.Hood DC, Zhang X, Greenstein VC, et al. An interocular comparison of the multifocal VEP: a possible technique for detecting local damage to the optic nerve. Invest Ophthalmol Vis Sci. 2000;41:1580–1587. [PubMed] [Google Scholar]
- 95.Graham SL, Klistorner AI, Grigg JR, Billson FA. Objective VEP perimetry in glaucoma: asymmetry analysis to identify early defects. J Glaucoma. 2000;9:10–19. doi: 10.1097/00061198-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 96.Ventura LM, Porciatti V. Pattern electroretinogram in glaucoma. Curr Opin Ophthalmol. 2006;17:196–202. doi: 10.1097/01.icu.0000193082.44938.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Porciatti V, Ventura LM. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology. 2004;111:161–168. doi: 10.1016/j.ophtha.2003.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee AJ, Rochtchina E, Mitchell P. Intraocular pressure asymmetry and undiagnosed open-angle glaucoma in an older population. Am J Ophthalmol. 2004;137:380–382. doi: 10.1016/j.ajo.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 99.Cartwright MJ, Anderson DR. Correlation of asymmetric damage with asymmetric intraocular pressure in normal-tension glaucoma (low-tension glaucoma) Arch Ophthalmol. 1988;106:898–900. doi: 10.1001/archopht.1988.01060140044020. [DOI] [PubMed] [Google Scholar]
- 100.Crichton A, Drance SM, Douglas GR, Schulzer M. Unequal intraocular pressure and its relation to asymmetric visual field defects in low-tension glaucoma. Ophthalmology. 1989;96:1312–1314. doi: 10.1016/s0161-6420(89)32721-7. [DOI] [PubMed] [Google Scholar]
- 101.Haefliger IO, Hitchings RA. Relationship between asymmetry of visual field defects and intraocular pressure difference in an untreated normal (low) tension glaucoma population. Acta Ophthalmol. 1990;68:564–567. doi: 10.1111/j.1755-3768.1990.tb04788.x. [DOI] [PubMed] [Google Scholar]
- 102.Gugleta K, Orgül S, Flammer J. Asymmetry in intraocular pressure and retinal nerve fiber layer thickness in normal-tension glaucoma. Ophthalmologica. 1999;213:219–223. doi: 10.1159/000027425. [DOI] [PubMed] [Google Scholar]
- 103.Greenfield DS, Liebmann JM, Ritch R, Krupin T, Low-Pressure Glaucoma Study Group Visual field and intraocular pressure asymmetry in the Low-Pressure Glaucoma Treatment Study. Ophthalmology. 2007;114:460–465. doi: 10.1016/j.ophtha.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 104.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 105.Levine RA, Demirel S, Fan J, et al. Asymmetries and visual field summaries as predictors of glaucoma in the ocular hypertension treatment study. Invest Ophthalmol Vis Sci. 2006;47:3896–3903. doi: 10.1167/iovs.05-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]