Abstract
Purpose
To compare long-term keratometric changes after penetrating keratoplasty (PK) for keratoconus and Fuchs endothelial dystrophy.
Methods
We retrospectively analyzed 168 corneas after PK for keratoconus (85 eyes of 63 subjects) and Fuchs dystrophy (83 eyes of 60 subjects). Patients were examined after final suture removal at 12 months after PK to 30 years after surgery. Operations were performed by one surgeon using the same suturing technique in all cases. Eyes were excluded from further analysis after regrafting or after relaxing incisions. Mean keratometric corneal power and astigmatism were measured by manual keratometry. Data were assessed by using generalized estimating equation models to determine change over time.
Results
Mean keratometric corneal power and keratometric astigmatism increased through 30 years after PK for keratoconus (P < .001 and P < .001) but did not change through 20 years after PK for Fuchs dystrophy (P = .55 and P = .55). The change in keratometric corneal power and astigmatism after PK in keratoconus patients differed from the change in Fuchs dystrophy patients only at 10 or more years after PK (P = .002 and P = .003).
Conclusions
Corneal curvature and regular astigmatism increase progressively after PK for keratoconus but remain stable after PK for Fuchs dystrophy. This keratometric instability after PK for keratoconus may lead to delayed corneal ectasia.
INTRODUCTION
Keratoconus is a progressive, noninflammatory ectatic corneal disorder characterized by protrusion of the cornea and thinning of the corneal stroma, without neovascularization.1 The estimated prevalence of keratoconus ranges from 0.04% to 0.22%.2,3 Keratoconus typically progresses during the second and third decades of life, although the etiology and pathogenesis of keratoconus are unclear.4
The probability of recurrent ectasia after penetrating keratoplasty (PK) for keratoconus is estimated to be 6% to 11% at 20 to 25 years after surgery, based on clinical diagnosis and histopathologic examination.5–10 The mean latency to recurrent ectasia is generally greater than 10 years, paralleling the natural evolution of keratoconus in adolescence.8–13 Keratometric studies after PK for keratoconus are mixed, some reporting progression of astigmatism and corneal steepening14,15 and others unable to detect a change.16–20
The purpose of this retrospective study was to measure and compare long-term keratometric changes after PK for keratoconus and Fuchs dystrophy performed by one surgeon between 1976 and 1986 by using the same surgical technique.
METHODS
The study protocol was reviewed and approved by the Institutional Review Board of the Mayo Clinic. The cohort of our retrospective, comparative case series consisted of 181 consecutive subjects (216 eyes) who had PKs for keratoconus and Fuchs dystrophy performed by one surgeon (W.M.B.) between 1976 and 1986. Excluded from the study were 9 subjects who withdrew research authorization, 8 subjects who did not have all sutures removed, and 31 subjects who did not have keratometry recorded or did not have adequate follow-up, leaving 168 eyes of 123 subjects having a PK for keratoconus (85 eyes of 63 subjects) and Fuchs dystrophy (83 eyes of 60 subjects). In the keratoconus group there were 40 men (63%) and 23 women (36%), and age at PK was 36 ± 13 years (mean ± SD, range 16–80 years). In the Fuchs dystrophy group there were 6 men (10%) and 54 women (90%), and the mean age at PK was 69 ± 8 years (range, 52–92 years). Nine subjects (10 eyes) were followed until regrafting (6 keratoconus eyes, 1 Fuchs dystrophy eye) or relaxing incisions (3 keratoconus eyes) and thereafter were excluded from analysis.
The surgical technique has been described in detail previously.21–23 Briefly, a manual trephine of Castroviejo was used to cut the host and donor tissue, with the donor tissue cut from the endothelial side. Donor diameter was 7.9 ± 0.4 mm (mean ± SD; range, 7.25–9.5 mm) for keratoconus and 7.9 ± 0.2 mm (range, 7.5–8.0 mm) for Fuchs dystrophy. Recipient diameter was 7.6 ± 0.4 mm (range, 7.0–9.0 mm) for keratoconus and 7.5 ± 0.1 mm (range, 7.25–7.5 mm) for Fuchs dystrophy. Donor buttons were sutured into the recipient by using a double-running technique, which consisted of 12 bites of a single 10-0 nylon suture and 12 bites of a single 11-0 nylon suture. All grafts were centered on the pupil. The diagnosis of keratoconus and Fuchs dystrophy was confirmed after PK by histopathology. Postoperatively, prednisolone acetate 1% was administered topically by the patient from the time of epithelial healing for 3 to 6 months, but rarely more than once daily after the second month. Suture removal for all patients was scheduled at 2 months (10-0 nylon) and 12 months (11-0) after surgery.
Mean keratometric corneal power and astigmatism were measured by using a Bausch & Lomb keratometer (Bausch & Lomb, Rochester, New York). Follow-up examinations were scheduled at 13 months (1 month after final suture removal), and at 5, 10, 15, 20, 25, and 30 years after PK. Follow-up examinations at other visits were included in the analysis if keratometry was recorded. Contact lenses, if worn, were removed immediately before examination. Central corneal thickness was measured by the same contact specular microscope at each examination.21–23
All statistical analyses were performed with SAS software (SAS Institute Inc, Cary, North Carolina). Trends over time were investigated by using linear regression models. All models were completed by using generalized estimating equation models to account for the potential correlation within eyes over time and between fellow eyes of the same subject. These models were used to establish trends over time within each of the groups (keratoconus and Fuchs dystrophy) and for testing for differences between groups. Within each group, a linear model that related time to each of the measured parameters was completed. To test for differences between groups, a model was fit with a parameter for time and group, as well as an interaction between the time and group parameters. This modeling investigated keratometry vs time for differences in slope as well as intercept between the groups. Keratometric changes in astigmatism were evaluated by vector analysis and presented as doubled-angle plots.24
RESULTS
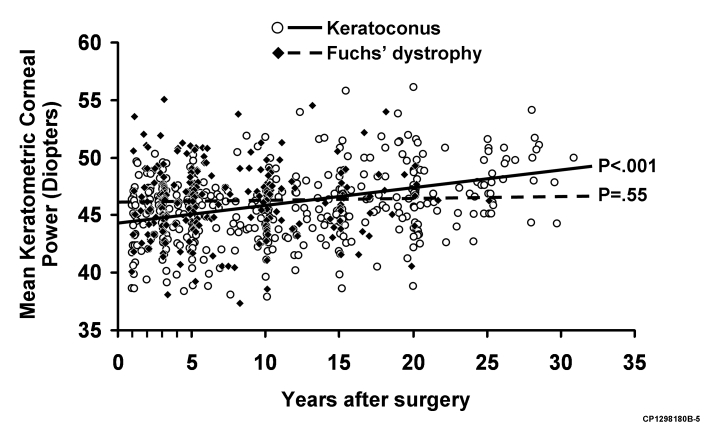
The Table shows the change in mean keratometric corneal power and astigmatism after PK for keratoconus and Fuchs dystrophy from baseline at 13 months (1 month after final suture removal) up to 30 years after PK. After PK for keratoconus, mean keratometric corneal power and astigmatism increased over the entire study period (P < .001 and P < .001; Figures 1 through 4). By contrast, after PK for Fuchs dystrophy, the mean keratometric corneal power and astigmatism did not change for up to 20 years (P = .55; Figures 1 through 4). The increase in mean keratometric corneal power and astigmatism after PK for keratoconus became significantly different than after PK for Fuchs dystrophy only beyond the first decade after surgery (P = .002 and P = .003; Figures 1 and 3).
TABLE. MEAN KERATOMETRIC CORNEAL POWER AND ASTIGMATISM (DIOPTERS, MEAN ± STANDARD DEVIATION) AFTER PENETRATING KERATOPLASTY FOR KERATOCONUS AND FUCHS DYSTROPHY
| TIME AFTER PENETRATING KERATOPLASTY | ||||||||
|---|---|---|---|---|---|---|---|---|
| 13 mo | 5 yr | 10 yr | 15 yr | 20 yr | 25 yr | 30 yr | P* | |
| Mean keratometry | ||||||||
| Keratoconus | 44.8 ± 2.8 | 44.9 ± 2.7 | 45.4 ± 2.9 | 46.2 ± 3.3 | 47.1 ± 3.6 | 47.6 ± 2.3 | 49.4 ± 3.1 | <.001 |
| (n = 46) | (n = 57) | (n = 67) | (n = 53) | (n = 50) | (n = 24) | (n = 10) | ||
| Fuchs dystrophy | 46.1 ± 4.1 | 46.7 ± 2.9 | 46.3 ± 3.2 | 46.7 ± 2.9 | 46.6 ± 3.3 | NA | NA | .55 |
| (n = 39) | (n = 58) | (n = 52) | (n = 28) | (n = 23) | ||||
| Keratometric cylinder | ||||||||
| Keratoconus | 4.7 ± 2.9 | 3.7 ± 2.4 | 5.3 ± 3.5 | 6.1 ± 4.2 | 7.3 ± 4.8 | 6.9 ± 3.6 | 8.0 ± 5.3 | <.001 |
| (n = 46) | (n = 57) | (n = 67) | (n = 53) | (n = 50) | (n = 24) | (n = 10) | ||
| Fuchs dystrophy | 5.6 ± 2.9 | 4.3 ± 2.6 | 4.4 ± 3.0 | 5.3 ± 3.4 | 4.2 ± 2.2 | NA | NA | .55 |
| (n = 39) | (n = 58) | (n = 52) | (n = 28) | (n = 23) | ||||
1. NA, not applicable.
2. Over entire study period, generalized estimating equation model.
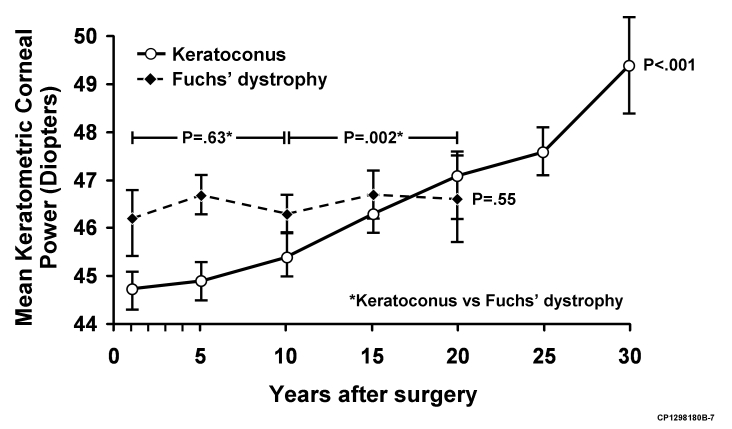
FIGURE 1.
Change in mean keratometric power (± standard error) after penetrating keratoplasty (PK) for keratoconus and Fuchs' dystrophy. Over the study period, mean keratometric corneal power increased after PK for keratoconus (P < .001) but was unchanged after PK for Fuchs' dystrophy (P = .55). When comparing keratoconus to Fuchs' dystrophy (indicated by asterisk), the change in mean keratometric corneal power was not different before 10 years after PK (P = .63), but was greater in keratoconus beyond 10 years after PK (P = .002).
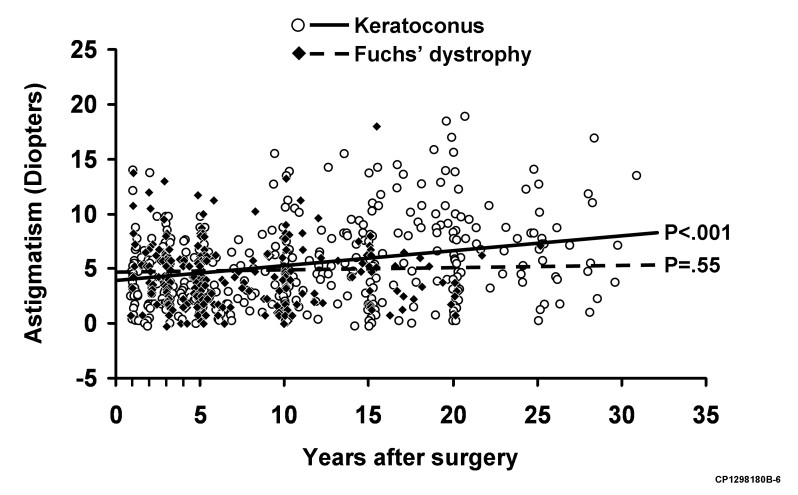
FIGURE 4.
Scatter-plot of mean keratometric astigmatism in all cases at all examinations after penetrating keratoplasty (PK) for keratoconus and Fuchs' dystrophy. Keratometric astigmatism increased after PK for keratoconus (P < .001) but was unchanged after PK for Fuchs dystrophy (P = .55).
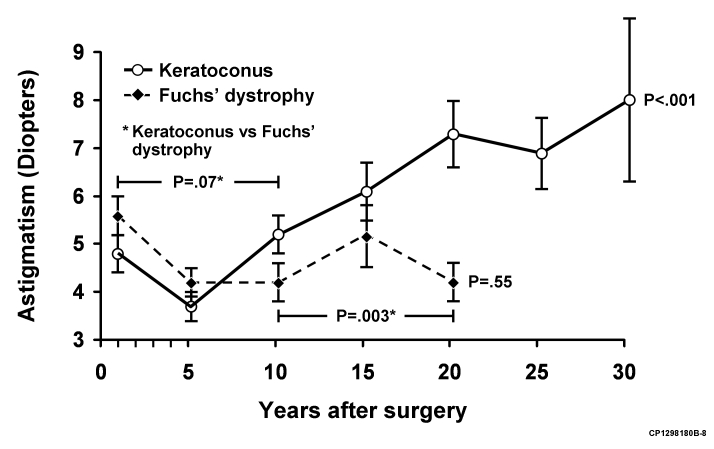
FIGURE 3.
Change in mean keratometric astigmatism (± standard error) after penetrating keratoplasty (PK) for keratoconus and Fuchs' dystrophy. Mean keratometric astigmatism over the study period increased after PK for keratoconus (P < .001) but was unchanged after PK for Fuchs' dystrophy (P = .55). When comparing keratoconus to Fuchs' dystrophy (indicated by asterisk), the change in mean keratometric astigmatism was not different before 10 years after PK (P = .07), but was greater in keratoconus beyond 10 years after surgery (P = .003).
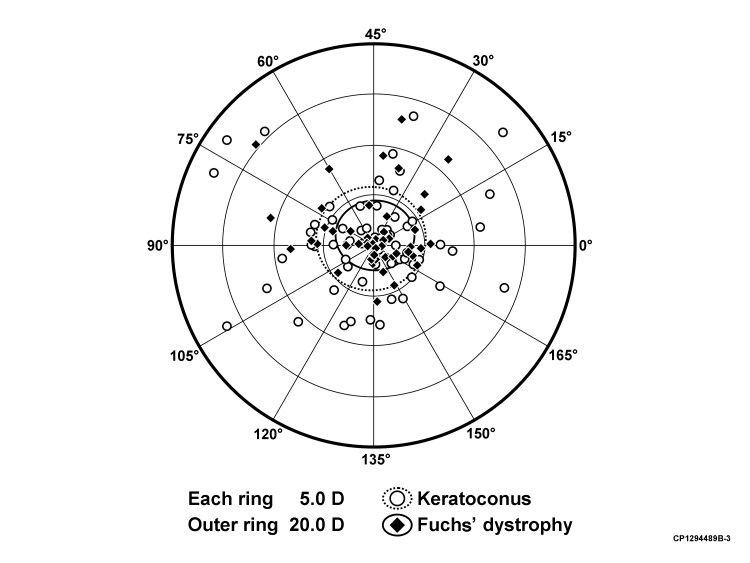
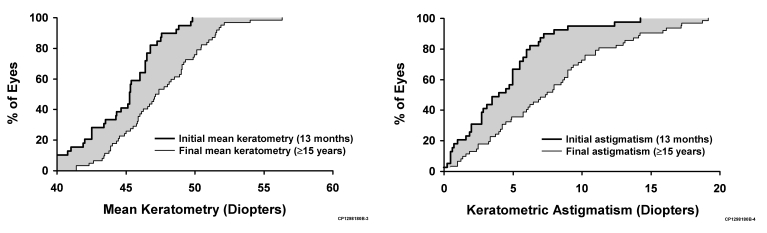
Figure 5 shows the doubled-angle plot of the vectoral change in magnitude and axis of astigmatism for each subject between 13 months (1 month after final suture removal) and the last follow-up (if at least 15 years after surgery) after PK for keratoconus and Fuchs dystrophy. The cumulative distribution plot in Figure 6 shows the increase in mean keratometric corneal power and astigmatism after PK for keratoconus over the study period.
FIGURE 5.
Doubled-angle plot of the vectoral change (difference vector) observed in the astigmatism magnitude and axis for each subject between 13 months and last follow-up (if at least 15 years) after penetrating keratoplasty for keratoconus and Fuchs' dystrophy. The refractive centroid was 0.9 D × 51 ± 5.5 D, and the shape factor was ρ = 0.84 for keratoconus patients. The refractive centroid was 1.0 D × 44 ± 3.5 D, and the shape factor was ρ = 0.83 for Fuchs dystrophy patients. This suggests no difference in astigmatic shift in any given axis direction when comparing PK for keratoconus with PK for Fuchs' dystrophy.
FIGURE 6.
Cumulative frequency distribution of (left) mean keratometric corneal power and (right) astigmatism at 1 month after suture removal (13 months after PK) and at last follow-up (if at least 15 years) after penetrating keratoplasty for keratoconus. The gray area between the 2 stepped lines represents the increase in postoperative corneal steepening or astigmatism.
The mean difference between donor graft diameter and recipient diameter was 0.26 ± 0.09 mm in the keratoconus cohort and 0.43 ± 0.12 mm in the Fuchs dystrophy cohort. This difference was significant (P > .001), suggesting that more oversized grafts were present in the Fuchs dystrophy cohort.
Mean central corneal thickness was 0.53 ± 0.05 mm in keratoconus subjects and 0.53 ± 0.05 mm in Fuchs dystrophy subjects at 1 year after PK. By 20 years after PK, central corneal thickness increased to 0.60 ± 0.05 mm in keratoconus subjects (P < .001) and 0.67 ± 0.07 mm in Fuchs dystrophy subjects (P < .001). The increase in central corneal thickness at 20 years after PK was not different between the two cohorts (P = .32)
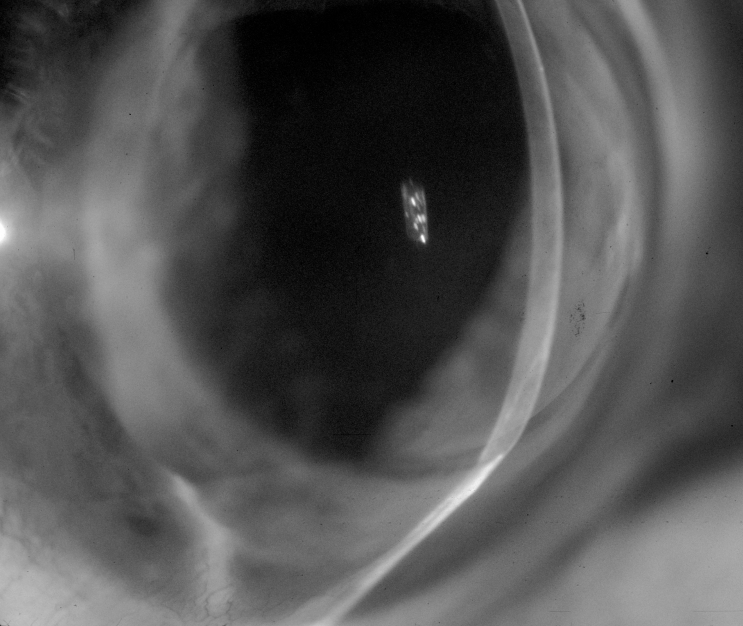
Five (6 %) of the 85 PKs for keratoconus were eventually diagnosed with recurrent ectasia. The mean latency to diagnosis was 18 years (range, 16–19 years). Four (80%) of the 5 PKs had thinning of the recipient rim at the graft-host junction (Figure 7). Four of these 5 eyes had a second PK. Histologic characteristics consistent with keratoconus were not detected in any of the 4 donor buttons.
FIGURE 7.
Slit-lamp photograph showing thinning of the recipient rim at the inferior graft-host junction 18 years after penetrating keratoplasty for keratoconus.
DISCUSSION
Our retrospective, comparative case series suggests a mechanism of delayed but progressive corneal steepening and increasing regular astigmatism after PK for keratoconus that continues for up to 30 years. When compared to PK for Fuchs dystrophy, the keratometric instability after PK for keratoconus did not become statistically evident until 10 years after surgery. After PK for Fuchs dystrophy, we detected no significant keratometric changes through 20 years after surgery.
Recurrence of ectasia or keratoconus-like characteristics after PK for keratoconus has been reported in several studies.5–15 Many provide histologic documentation of keratoconus in the donor button.5–13 The latency to recurrence is generally not until 10 or more years after surgery.8–15 It is hypothesized that subtle progression of corneal ectasia after PK for keratoconus may manifest itself as change in astigmatism and corneal curvature over time. Clinical studies after PK for keratoconus with follow-up limited to less than 12 years have failed to detect evidence of progressive astigmatism.16–20 With longer follow-up, increasing regular myopic astigmatism beginning 7 or more years after final suture removal and progressing up to 25 years after surgery has been reported by de Toledo and coworkers.15 De Toledo attributed their findings to longer follow-up, which allowed the natural slow progression of keratoconus in the host corneal rim to progress into significant thinning and keratometric instability. Our long-term findings of keratometric instability are consistent with these previous studies.15–20 We were unable to detect a significant difference in keratometric change in the first 10 years when comparing PK for keratoconus with PK for Fuchs dystrophy. Beyond 10 years after surgery, corneal steepening and regular astigmatism progressively increased in the keratoconus cohort, remaining unchanged in the control Fuchs dystrophy cohort.
Although the etiology of late-onset keratometric instability after PK for keratoconus is unclear, our findings provide some insight. We detected no keratometric change after PK for Fuchs dystrophy, which served as the control group. Fuchs dystrophy was treated by PK during the same time period, by the same surgeon, and with the same trephination and suturing techniques as were used for PK to treat keratoconus. Therefore, it is unlikely that a normal age-related structural change in the corneal graft or the double-running suturing technique accounted for the delayed keratometric changes after PK for keratoconus. After final suture removal (13 months after PK), mean central corneal curvature was flatter in keratoconus patients than in Fuchs dystrophy patients. This might be explained by our finding of more oversized grafts in the Fuchs dystrophy cohort. Alternatively, bulging of the presumably thinner keratoconus recipient rim allows the graft-host junction to steepen, as compared to corneas transplanted in Fuchs dystrophy patients, thus leaving the central cornea less curved.25
The etiology of recurrent keratoconus-like characteristics includes recurrence of keratoconus in the donor graft, progression of the original disease in the host rim, or grafting with forme fruste keratoconus donor corneas. Histopathologic findings in removed donor corneas many years after PK for keratoconus are sometimes identical to those in primary keratoconus corneas.7–9,11 There is evidence that host keratocytes replace donor keratocytes after PK,26 and some investigators have postulated that graft repopulation by recipient keratocytes is responsible for recurrent keratoconus characteristics.9 Also, host epithelium eventually covers the donor cornea, and chronic epithelial-stromal interactions may contribute to the changes in Bowman’s layer that lead to histopathologic findings that are consistent with recurrence of keratoconus in the grafted cornea.
A continued natural progression of keratoconus in the host rim may induce thinning of both the host and donor stroma at the graft-host interface, leading to wound slippage and progressive keratometric steepening and astigmatism. Lim27 and de Toledo15 found thinning of the host cornea 14 and 20 years after PK, and that thinning preceded signs of recurrent ectasia. Histopathologic evidence shows that keratoconus changes exist in the peripheral cornea as well as the central cornea.28 Reports of recurrent keratoconus after PK for keratoconus generally do not describe central stromal thinning or cone-like steepening in the graft cornea, as seen in primary keratoconus.9,14 In our study, mean central corneal thickness increased over the entire study period in grafts for both keratoconus and Fuchs dystrophy. This finding is likely the result of chronic stromal thickening due to progressive endothelial cell loss.21,23
It has been suggested that some patients with apparent recurrent keratoconus received donor corneas having forme fruste keratoconus.29 Current estimates of the prevalence of keratoconus in the general population range from 0.04% to 0.22%,2,3 although we recognize that the prevalence of forme fruste may be higher. The chances of patients receiving donor corneas with forme fruste keratoconus are very small, making it unlikely to account for the estimated 7% to 11% recurrence rate after PK for keratoconus.10 Additional evidence against the donor cornea having forme fruste keratoconus is the lack of reports of recurrent ectasia after PK for nonectatic corneal disorders. If cases are followed long enough, one would expect a small proportion of PKs for Fuchs dystrophy to develop ectasia if forme fruste corneas were used for transplantation.
Although recurrence of keratoconus in the donor button after PK for keratoconus is well described, our findings are best explained by progression of the disease in the host rim with thinning and secondary structural instability at the graft-host junction. This is corroborated by the absence of clinical features of central keratoconus in the patients in our series and no histopathologic evidence of keratoconus in donor buttons that required a second PK.
In summary, our long-term data support previous observations that after PK for keratoconus, there is delayed-onset keratometric instability that becomes manifest approximately 10 years after surgery and continues to progress for up to 30 years. Delayed refractive instability or recurrent ectasia should be discussed preoperatively, as young grafted patients with keratoconus will live long enough to be at risk for this problem, whether they receive a PK or deep anterior lamellar keratoplasty. These findings should also be considered when contemplating further surgical refractive procedures in patients who previously have had PK for keratoconus.
PEER DISCUSSION
DR. HENRY GELENDER
The authors are to be complimented for their retrospective analysis of cornea transplant patients. They studied the long-term keratometric changes after penetrating keratoplasty by comparing the results of keratoconus with Fuchs’ dystrophy. What they found was rather interesting. They discovered that for keratoconus patients, there was a late onset of keratometric instability, which was manifest by an increase in mean corneal power and astigmatism. This was detectable greater than 10 years after surgery and progressed up to 30 years. However, this was in contrast to the long term, relative keratometric stability for Fuchs’ patients. They also identified the wound margin thinning of the keratoconus grafts as a sign of corneal ectasia. This study confirms previous observations that keratoconus as a disease process is not halted by corneal transplantation and may recur in the graft as a delayed progression of the disease.1
There are several areas of data analysis that merit reflection. First, the authors mention the size of the grafts performed, which for the recipient cornea was 7.6 + 0.4 mm for keratoconus grafts and 7.5 + 0.1 mm for Fuchs’ grafts. These grafts are somewhat small and leave more peripheral recipient corneal tissue. The question may be asked, would a larger keratoconus graft improve the long term stability of the graft? However, it has been suggested that infiltration of the graft by host keratocytes that produce abnormal collagen is really the cause of keratoconus recurrence in the graft, much like the disease process that led to keratoconus originally.2 It would be interesting to look at a subgroup of patients who had larger grafts to see if the findings are consistent. If this is the case, this would support the evidence that graft size is not a factor in keratoconus recurrence and that other stromal factors lead to keratometric instability.
Second, keratometric data was collected immediately after contact lens removal. However, it is well known that contact lenses, and especially gas permeable contacts, can induce corneal warpage. Moreover, discontinuing contact lens wear allows the cornea to normalize. As a result, measuring the keratometry of the cornea immediately after lens removal may represent an artifact induced by the contacts. It would be helpful to compare the keratoconus and Fuchs’ dystrophy groups looking at the number of patients wearing contacts and the type of contacts worn to determine if contact lenses could account for some of the disparity between these groups. Would contact lens withdrawal in the keratoconus group have affected the observed keratometric instability noted in this study?
Third, it has been shown that LASIK and PRK are useful means of correcting the refractive error, which may result from corneal transplantation.3 However, this study’s finding of the late onset of instability for the keratoconus group would suggest that laser refractive surgery would be of limited and at best temporary benefit for these patients.
Finally, the recent introduction of the femtosecond laser for penetrating keratoplasty offers the hope of greater control of postoperative astigmatism through uniquely designed wound construction.4 Moreover, collagen cross linking of corneal stroma has recently been introduced as a novel approach in stabilizing the keratoconus cornea.5 Could these exciting new technologies be harnessed to improve the surgical outcome and long-term stability of PKP for keratoconus patients?
I wish to thank the authors for providing an interesting manuscript in a timely fashion and the American Ophthalmological Society for the opportunity to discuss this presentation.
ACKNOWLEDGMENTS
Funding/Support: None
Financial Disclosures: None.
REFERENCES:
- 1.de Toledo JA, de la Paz MF, Barraquer RI, Barraquer J. Long-term progression of astigmatism after penetrating keratoplasty for keratoconus: evidence of later recurrence. Cornea. 2003;22:317–23. doi: 10.1097/00003226-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Cannon DJ, Foster CS. Collagen cross-linking in keratoconus. Invest Ophthalmol Vis Sci. 1978;17:63–5. [PubMed] [Google Scholar]
- 3.Donnenfeld EF, Kornstein HS, Amin A, et al. Laser in situ keratomileusis for correction of myopia and astigmatism after penetrating keratoplasty. Ophthalmol. 1999;106:1966–75. doi: 10.1016/S0161-6420(99)90410-4. [DOI] [PubMed] [Google Scholar]
- 4.Buratto L, Bohm E. The use of the femtosecond laser in penetrating keratoplasty. Am J Ophthalmol. 2007;143:732–42. doi: 10.1016/j.ajo.2007.01.056. [DOI] [PubMed] [Google Scholar]
- 5.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen cross-linking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
DR. PETER R. LAIBSON
No financial interest in any companies. I also want to congratulate the authors and the discussant for an excellent paper and discussion. This has been a very interesting topic for many years. I have been doing corneal transplants since 1965 and I certainly have noticed the ectasia that was brought out in this paper, particularly the inferior ectasia which starts below, persists, and progresses. It is very interesting to note that the ectasia almost invariably occurs in the inferior location. We know that keratoconus is mostly inferior, or inferonasal, but even when the keratoconus is central the ectasia that develops after keratoplasty is located inferiorly. I wondered in the past whether pressure from the upper lid might cause some of these changes inferiorly. Recurrent keratoconus in the graft is rare, as Ralph Eagle has pointed out, and does not cause inferior ectasia. It is also interesting that in Fuchs’ corneal dystrophy, the long-term grafts do not show inferior ectasia. Perhaps it is pressure from the upper lid, or a continuing inferior ectasia from the keratoconus still existing in the host cornea that contributes to the development of this condition. To improve vision, we have reopened the keratoplasty wound and resutured it, as well as doing wedge resections to help minimize the ectasia. Elisabeth Cohen at Wills Eye Hospital has fitted these patients with contact lenses, but when the ectasia is significant, surgical repair or repeat corneal transplantation is necessary. Thank you.
DR. JAMES CHODOSH
I have nothing to disclose. In my limited experience, it seems to me that keratoconus is not really a disease of the central cornea. When I operated on these eyes they often seem to lack the typical rigidity and have something that I like to call “floppy eye”. I wonder if there is a relationship between the status of the eye as a whole and the location and development of postoperative keratoconus-like changes. We are operating on the central cornea, but are failing to address the structural changes that are present in the eye around the new cornea. I would also like Jay to know that it was a beautiful paper. I am wondering if there might be some other data to study, specifically regarding the axis of preoperative astigmatism and how that relates to the axis postoperatively. It seems to me that if I could identify some characteristic of the preoperative cornea, then I might be able to predict the postoperative astigmatism in a way that would permit me to alter my surgical technique. I believe that you might have the data to examine that relationship.
DR. JAMES C. BOBROW
I have no financial conflicts to disclose. In the last year, I have had the occasion to perform cataract surgery on three individuals, all of whom had corneal transplants for keratoconus more than 30 years ago. One of them had undergone keratoplasty by Bob Drews, one by Ramon Castroviejo, and one by Max Fine. The patient of Max Fine actually had a square graft. Because of my belief that patients with this disorder do not have stable corneas, I followed each of them for periods up to three yeas before consenting or agreeing to do their surgery. During this time their keratometric readings were actually quite stable. I wonder if the author would comment on whether he has cared for a similar group of patients whose keratoconus progressed to a certain point and then stabilized. Thank you.
DR. ALAN SUGAR
No financial interest. Thank you, Jay, for this excellent paper. As you know, we reviewed a similar series of patients and presented them at ARVO a few weeks ago, and we have discussed our findings with Sanjay Patel and Bill Bourne. We did determine a cumulative recurrence of keratoconus of about 10% of the patients after 20 years by Kaplan-Meier analysis. Victor Elner did find histologic evidence in the repeated grafts of recurrent keratoconus. I believe that one of the problems we had was the definition of recurrent keratoconus. By considering astigmatism as a continuous variable, I believe that you partly abrogated that problem. All of this discussion points out that we really have a poor understanding of keratoconus. We need to define the condition at a molecular level diagnosis and to better understand the underlying chemistry. I wonder if you have any ideas in that direction. Thank you.
DR. DOUGLAS D. KOCH
No financial conflicts relevant to this discussion. Great paper, Jay. We now have some new tools that will help sort this out. Those tools fall into two categories. First, there are those that measure corneal thickness. Examples include the Pentacam and Galilei, which use the Scheimpflug principle, and secondly, is the anterior segment OCT. They will enable us to learn where thinning begins and how it progresses. We also have devices, such as the Ocular Response Analyzer, which could enable us to better understand the biomechanical features of the disease process.
DR. ALLAN J. FLACH
It just struck me that historically, it was the interaction between the pathologist and the microbiologist, and the correlation with the clinical ophthalmic findings of the disease that led to the knowledge we have today. Pathology appears to be a disappearing subspecialty in the opinion of some people. Perhaps to evaluate all of these cases there should be a paid pathologist who studies every single specimen and is paid by the minute for his time and for putting his thoughts into what you are all seeing. Maybe then we could make greater strides in understanding what is really going on with this condition. I would like you to comment on that.
DR. JAY C. ERIE
Thank you everybody. Henry brought up an issue regarding donor recipient disparity. When we looked at these groups, there were more oversized grafts in the Fuchs’ dystrophy group than in the keratoconus group. We felt that this contributed to the keratoconus grafts being initially flatter than the grafts for Fuchs dystrophy. We recognize the limitations of contact lens wear and, unfortunately, Henry, I do not have the numbers in hand for that, although we do have the data, so we can examine that issue. Now that we have recognized the condition of host rim thinning, we are suddenly seeing more of it in our grafts after 20 or more years. I think once you are clued into looking for it, you start to detect it, although you did not realize it was there before.
James Chodosh asked for information about the preoperative axis of astigmatism and how this related to axis postoperatively. This is an excellent question. We should have that data, but again, we did not analyze it for this study. In response to Alan Sugar, I cannot shed any light on the molecular basis for keratoconus. Regarding the comments of Doug Koch, I appreciated them very much. He raised the issue of corneal thickness, and the central cornea significantly thickened over the study period in both groups, although there was no statistical difference between them. Thank you very much.
FIGURE 2.
Scatter-plot of mean keratometric corneal power in all cases at all examinations after penetrating keratoplasty (PK) for keratoconus and Fuchs' dystrophy. Mean keratometric corneal power increased after PK for keratoconus (P < .001) but was unchanged after PK for Fuchs' dystrophy (P = .55).
ACKNOWLEDGMENTS
Funding/Support: Supported in part by grant EY02037 from the National Institutes of Health; Research to Prevent Blindness, Inc (S.V.P. as Olga Keith Weiss Special Scholar and an unrestricted departmental grant), New York, New York; and the Mayo Foundation, Rochester, Minnesota.
Financial Disclosures: None.
Author Contributions: Design and conduct of the study (M.E.R., J.C.E., S.V.P., W.M.B.); Collection, management, and analysis, and interpretation of the data (M.E.R., J.C.E., S.V.P., W.M.B.); Preparation, review, and approval of the manuscript (M.E.R., J.C.E., S.V.P., W.M.B.).
Conformity With Author Information: The study protocol was reviewed and approved by the Institutional Review Board of the Mayo Clinic, Rochester, Minnesota.
Other Acknowledgments: The authors acknowledge David O. Hodge, MS, Department of Biostatistics, Mayo Clinic, Rochester, Minnesota, for statistical consultation and assistance.
REFERENCES
- 1.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267–273. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- 3.Pearson A, Soneji B, Sarvananthan N, Sandford-Smith JH. Does ethnic origin influence the incidence or severity of keratoconus? Eye. 2000;14:625–628. doi: 10.1038/eye.2000.154. [DOI] [PubMed] [Google Scholar]
- 4.Maguire L. Ectatic corneal degenerations. In: Kaufman HS, Barron BA, McDonald MB, editors. The Cornea. 2nd ed. Boston: Butterworth-Heineman; 1998. [Google Scholar]
- 5.Rubinfeld RS, Traboulsi EI, Arensten J, Eagle RC., Jr Keratoconus after penetrating keratoplasty. Ophthalmic Surg. 1990;21:420–422. [PubMed] [Google Scholar]
- 6.Belmont SC, Muller JW, Draga A, Lawless M, Troutman RC. Keratoconus in a donor cornea. J Refract Corneal Surg. 1994;10:658. [PubMed] [Google Scholar]
- 7.Kremer I, Eagle RE, Rapuano CJ, Laibson PR. Histologic evidence of recurrent keratoconus seven years after keratoplasty. Am J Ophthalmol. 1995;119:511–512. doi: 10.1016/s0002-9394(14)71239-5. [DOI] [PubMed] [Google Scholar]
- 8.Bechrakis N, Blom ML, Stark WJ, Green WR. Recurrent keratoconus. Cornea. 1994;13:73–77. doi: 10.1097/00003226-199401000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Bourges J-L, Savoldelli M, Dighiero P, et al. Recurrence of keratoconus characteristics. Ophthalmology. 2003;110:1920–1925. doi: 10.1016/S0161-6420(03)00617-1. [DOI] [PubMed] [Google Scholar]
- 10.Pramanik S, Musch DC, Sutphin JE, Farjo AA. Extended long-term outcomes of penetrating keratoplasty for keratoconus. Ophthalmology. 2006;113:1633–1638. doi: 10.1016/j.ophtha.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 11.Abelson MB, Collin B, Gillette TE, Dohlman CH. Recurrent keratoconus after keratoplasty. Am J Ophthalmol. 1980;90:672–676. doi: 10.1016/s0002-9394(14)75135-9. [DOI] [PubMed] [Google Scholar]
- 12.Nirankari VS, Karesh J, Bastion F, Lakhanpal V, Billings E. Recurrence of keratoconus in donor cornea 22 years after successful keratoplasty. Br J Ophthalmol. 1983;67:23–28. doi: 10.1136/bjo.67.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thalasselis A, Etchepareborda J. Recurrent keratoconus 40 years after keratoplasty. Ophthalmic Physiol Opt. 2002;22:330–332. doi: 10.1046/j.1475-1313.2002.00046.x. [DOI] [PubMed] [Google Scholar]
- 14.Szczotka-Flynn L, McMahon TT, Lass JH, et al. Late-stage progressive corneal astigmatism after penetrating keratoplasty for keratoconus. Eye Contact Lens. 2004;30:105–110. doi: 10.1097/01.icl.00000118526.35929.0f. [DOI] [PubMed] [Google Scholar]
- 15.de Toledo JA, de La Paz MF, Barraquer RI, Barraquer J. Long-term progression of astigmatism after penetrating keratoplasty for keratoconus: evidence of late recurrence. Cornea. 2003;22:317–323. doi: 10.1097/00003226-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Ruben M, Colebrook E. Keratoconus keratoplasty curvatures and contact lens wear. Br J Ophthalmol. 1979;63:268–273. doi: 10.1136/bjo.63.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suveges I, Nagy Z, Alberth B. Refraktions Bestimmungen nach der Keratoplasik bei Keratoconus: Fallen ist ein Keratoconus-rezidiv möglich? Fortschr Ophthalmol. 1983;80:228–229. [PubMed] [Google Scholar]
- 18.Tuft SJ, Gregory W. Long-term refraction and keratometry after penetrating keratoplasty for keratoconus. Cornea. 1995;14:614–617. [PubMed] [Google Scholar]
- 19.Langenbucher A, Naumann GOH, Seitz B. Spontaneous long-term changes of corneal power and astigmatism after suture removal after penetrating keratoplasty using a regression model. Am J Ophthalmol. 2005;140:29–34. doi: 10.1016/j.ajo.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Seitz B, Langenbucher A, Szentmary N, Naumann GOH. Corneal curvature after penetrating keratoplasty before and after suture removal: a comparison between keratoconus and Fuchs’ dystrophy. Ophthalmologica. 2006;220:302–306. doi: 10.1159/000094619. [DOI] [PubMed] [Google Scholar]
- 21.Bourne WM, Hodge DO, Nelson LR. Corneal endothelium five years after transplantation. Am J Ophthalmol. 1994;118:185–196. doi: 10.1016/s0002-9394(14)72898-3. [DOI] [PubMed] [Google Scholar]
- 22.Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998;105:1855–1865. doi: 10.1016/S0161-6420(98)91030-2. [DOI] [PubMed] [Google Scholar]
- 23.Patel SV, Hodge DO, Bourne WM. Corneal endothelium and postoperative outcomes 15 years after penetrating keratoplasty. Am J Ophthalmol. 2005;139:311–319. doi: 10.1016/j.ajo.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 24.Holladay JT, Moran JR, Kezirian GM. Analysis of aggregate surgically induced refractive change, prediction error, and intraocular astigmatism. J Cataract Refract Surg. 2001;27:61–79. doi: 10.1016/s0886-3350(00)00796-3. [DOI] [PubMed] [Google Scholar]
- 25.Meyer HJ, Cordesmeyer H. Postoperative astigmatism following keratoplasty in keratoconus. Fortschr Ophthalmol. 1987;84:427–428. [PubMed] [Google Scholar]
- 26.Wollensak G, Green WR. Analysis of sex-mismatched human corneal transplants by fluorescence in situ hybridization of the sex-chromosomes. Exp Eye Res. 1999;68:341–346. doi: 10.1006/exer.1998.0611. [DOI] [PubMed] [Google Scholar]
- 27.Lim L, Pesudovs K, Goggin M, Coster DJ. Late onset post-keratoplasty astigmatism in patients with keratoconus. Br J Ophthalmol. 2004;88:371–376. doi: 10.1136/bjo.2003.027037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherwin T, Brookes NH, Loh I-P, Poole CA, Clover GM. Cellular incursion into Bowman’s membrane in the peripheral cone of the keratoconic cornea. Exp Eye Res. 2002;74:473–482. doi: 10.1006/exer.2001.1157. [DOI] [PubMed] [Google Scholar]
- 29.Eiferman RA. Recurrence of keratoconus [letter] Br J Ophthalmol. 1984;68:289–290. doi: 10.1136/bjo.68.4.289-a. [DOI] [PMC free article] [PubMed] [Google Scholar]