Abstract
Purpose
To establish that increased autofluorescence of mitochondrial flavoproteins, an indicator of mitochondrial oxidative stress, correlates with retinal cell dysfunction.
Methods
Retinal flavoprotein autofluorescence (FA) was imaged in humans with a fundus camera modified with 467DF8-nm excitation and 535-nm emission filters and a back-illuminated, electron-multiplying, charge-coupled device camera interfaced with a computer equipped with customized image capture software. Multiple digital images, centered on the fovea, were obtained from each eye. Histograms of pixel intensities in grayscale units were analyzed for average intensity and average curve width. Adults with diabetes mellitus, age-related macular degeneration (ARMD), central serous retinopathy, and retinal dystrophies, as well as healthy control volunteers, were imaged. Monolayers of cultured human retinal pigment epithelial (HRPE) cells, HRPE cells exposed to sublethal doses of H2O2, and HRPE cells exposed to H2O2 in the presence of antioxidants were imaged for FA using fluorescent photomicroscopy.
Results
Control patients demonstrated low levels of retinal FA, which increased progressively with age. Diabetics without visible retinopathy demonstrated increased FA levels compared to control volunteers (P < .001). Diabetics with retinopathy demonstrated significantly higher FA values than those without retinopathy (P < .04). Patients with ARMD, central serous retinopathy, or retinal dystrophies also demonstrated significantly increased FA. Compared to control RPE cells, cells oxidatively stressed with H2O2 had significantly elevated FA (P < .05), which was prevented by antioxidants (P < .05).
Conclusions
Retinal FA is significantly increased with age and diseases known to be mediated by oxidative stress. Retinal FA imaging may provide a novel, noninvasive method of assessing retinal health and retinal dysfunction prior to retinal cell death.
INTRODUCTION
Mitochondria are critical cell organelles whose primary function is to provide energy, in the form of adenosine triphosphate, to power essential cellular processes. Mitochondria are also recognized to play a crucial role in the process of programmed cell death, or apoptosis.1 Apoptosis plays a role in physiologic cell turnover in tissues, but inappropriate activation of the apoptotic pathways in mitochondria in disease leads to premature cell loss and tissue dysfunction. Apoptosis occurs via a careful interplay of mitochondrial membrane permeability, which results in uncoupling of the respiratory chain, cessation of adenosine triphosphate production, induction of the apoptotic cascade, and ultimately cell death.2 Preapoptotic cells, or those with stressed mitochondria, develop impaired electron transport by the energy-generating enzymes in the respiratory chain, which cause increased percentages of flavoprotein in the chain to be oxidized.3,4 These oxidized, or electron-poor, flavoproteins are capable of absorbing blue light and emitting green autofluorescence.5–7
Flavoprotein autofluorescence (FA) has been recognized as a measure of mitochondrial dysfunction or cell stress in nonocular tissues.8–12 Skeletal muscle, liver, and heart muscle were among the first tissues in which ex vivo flavoprotein was studied, owing to the high metabolic rates and greater numbers of mitochondria in these tissues.13–15 Subsequent studies in vivo have indicated that FA is elevated in apoptosis-prone regions of ischemia-reperfusion injury in heart and brain tissue and in chondrocytes prone to apoptosis.16–18 Flavoprotein autofluorescence elevations in these studies correlated well with other markers of apoptosis, such as Bcl-2 depletion and mitochondrial transmembrane potential (Ψ) instability.18 Flow cytometric studies have also been adapted to examine FA to detect mononuclear cell mitochondrial dysfunction in patients with chronic progressive external ophthalmoplegia.19 Thus, in tissues outside of the eye, detection of FA is well accepted as a key method in assessing mitochondrial health.
We recently described a novel method for the clinical detection of early metabolic dysfunction in human ocular fundus via measurement of retinal FA.20 Using this method, we reported the FA in 6 women with newly diagnosed pseudotumor cerebri, which can result in visual loss from retinal ganglion cell dysfunction. Flavoprotein autofluorescence values were found to be elevated in the eye more affected by pseudotumor cerebri and when compared to FA in age-matched controls.20 Additionally, we recently reported for the first time the association of increased retinal FA and diabetes in a study evaluating patients in the fourth through sixth decades of life.21
In this study, we examined FA in older patients with diabetes mellitus and other patients with retinal-specific diseases to determine the clinical sensitivity of this technique in detecting disease-associated retinal metabolic stress. To show that preapoptotic conditions induce FA elevations in retinal cells, we compared in vitro FA measurements. Cultures of normal human retinal pigment epithelial (HRPE) cells, HRPE cells exposed to sublethal doses of ceramide or H2O2 (agents known to induce apoptosis in HRPE), and HRPE cells exposed to ceramide or H2O2 in the presence of either antioxidants or inhibitors of these agents were measured and compared.
METHODS
HUMAN SUBJECTS
Thirty-six patients total, ranging in age from 23 to 77 years, were imaged for FA. Fourteen patients (aged 54 to 68) with a history of diabetes mellitus, along with 7 age-matched control volunteers (aged 57 to 67), were imaged to examine the effects of diabetes and diabetic retinopathy on FA. One patient with age-related macular degeneration (ARMD), one patient with central serous retinopathy, and one patient with retinitis pigmentosa, along with an age-matched control for each, were also imaged. Nine additional healthy volunteers without a history of diabetes mellitus or ocular disease were imaged to examine the effects of age on retinal FA. This study was approved by the institutional review board at the University of Michigan. All patients signed a written informed consent prior to inclusion in the study.
RETINAL FA IMAGING IN HUMANS
To measure FA in humans, a Zeiss FF4 fundus camera (Carl Zeiss Corporation, Oberkochen, Germany) underwent more than 50 modifications as previously described.20,21 These included inserting special 467-nm excitation and 535-nm emission filters, attaching a back-illuminated electron-multiplying, charge-coupled device (EMCCD) camera, and connecting to computers with custom software. The EMCCD chip was cooled to −30°C to reduce noise. Additional modifications included optimizing light transmission to improve signal and placing optical baffles to reduce noise due to light reflections.
Each patient’s/volunteer’s pupils were dilated with tropicamide 1%/phenylephrine 2.5%, and the EMCCD camera was used to capture four 535-nm FA readings, each induced by a 1-millisecond, 467-nm incident flash. Imaging required 5 minutes per individual. The depth of focus of the instrument results in capture of FA from all retinal layers. The images were stored as 512×512-pixel 16-bit grayscale TIFF files.
RETINAL FA IMAGE FILE ANALYSIS
Histogram curves of pixel intensities in grayscale units (gsu) captured by each well of a 512×512 CCD chip (262,144 pixels) were extracted from the TIFF files using Metavue, Adobe Photoshop CS2 (Adobe Systems, San Jose, California) and Lispix (National Institute of Standards and Technology, Gaithersburg, Maryland). The histograms of pixel intensities, ranging from 0 to 256 gsu, were plotted for each eye to yield average intensity (AI) and average curve width (ACW) of retinal FA.
MATERIALS
N-acetylcysteine, hydrogen peroxide, C2-ceramide, and dihydroceramide C2 were purchased from Sigma-Aldrich (St Louis, Missouri). Costar tissue culture 96-well assay plates (black walls with clear bottoms) were purchased from Fisher Scientific (Pittsburgh, Pennsylvania).
HPRE CELL CULTURE
The HRPE cells were isolated from donor eyes by enzymatic digestion as previously described.22,23 Briefly, the sensory retina was separated gently from the HRPE monolayer, and the HRPE cells were removed from Bruch’s membrane using 1-hour incubation with papain. Isolated HRPE cells were grown into Falcon Primaria flasks in DMEM/F12 containing 10% fetal bovine serum (FBS), penicillin G (100 U mL–1), streptomycin sulfate (100 μg mL–1), and amphotericin B (0.25 μg mL–1) at 37°C in a humidified incubator under 5% CO2. In all experiments, parallel assays were performed on the second to fourth passaged HRPE cells. HRPE cells were seeded into tissue culture 96-well assay plates (black walls with clear bottoms [Costar]) at the same time and density from the same parent cultures and grown in phenol red–free complete DMEM/F12 for at least 7 days. All experiments were repeated at least 3 times on different cell lines.
HRPE CELL INCUBATIONS
The HRPE cells in 96-well plates were washed with Hanks balanced salt solution (HBSS), containing Ca2+ and Mg2+, without phenol red. The HRPE cells were preincubated for 30 minutes with or without N-acetylcysteine (NAC; 1 mM), followed by stimulation with hydrogen peroxide (H2O2; 0.2 mM) for 3 hours in the presence and absence of the same inhibitor used during preincubation. Other HRPE cells in 96-well plates were preincubated for 30 minutes with or without dihydroceramide C2 (50 μM), followed by stimulation with C2-ceramide (50 μM) for 2 hours in the presence and absence of the same inhibitor used during preincubation.
HRPE CELL FA MEASUREMENT
Flavoprotein autofluorescence was measured with a FlexStation fluorescence plate reader (Molecular Devices, Sunnyvale, CA). Excitation and emission wavelengths were set to 450 nm and 520 nm, respectively.
RESULTS
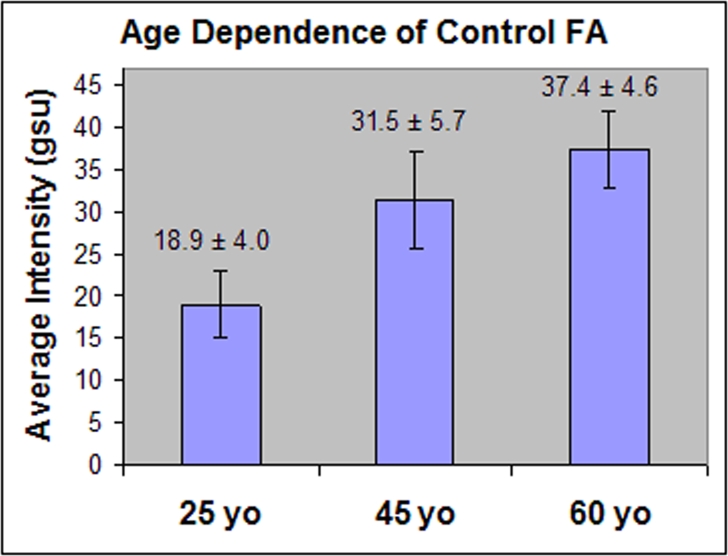
Nine control volunteers without evidence of diabetes mellitus or ocular disease were imaged for retinal FA to examine the potential changes in retinal FA that may occur with age. Three control volunteers in each of 3 age groupings were studied, and the average retinal FA pixel intensity for each is presented in Figure 1. Average age groupings of 25, 45, and 60 years were studied, with each volunteer within an age group being within 1 to 2 years of the average for that group. Control volunteers in the 25-year-old group showed substantially less FA average pixel intensity (AI = 18.9 ± 4 gsu) than either the 45-year-old group (AI = 31.5 ± 5.7 gsu, P = .04) or the 60-year-old group (AI = 37.4 ± 4.6 gsu, P = .01). Although the 45-year-old group showed less retinal FA than the 60-year-old group, this was not significant (P = .24). The ACW also increased progressively with increasing age, with 25-year-olds having narrower curves (ACW = 25.3 ± 3.1 gsu) than either the 45-year-old (ACW = 31.0 ± 5.3 gsu) or 60-year-old (ACW = 36.7 ± 5.7 gsu; P = .05) group.
FIGURE 1.
Bar graph of average pixel intensities for retinal flavoprotein autofluorescence (FA) from control volunteers in 3 age groupings. Three volunteers were included in each age group, with all volunteers within 1 to 2 years of the group’s average age. Control volunteers in the 25-year-old group had significantly less retinal FA than either the 45-year-old group (P = .04 ) or the 60-year-old group (P = .01). gsu, grayscale units.
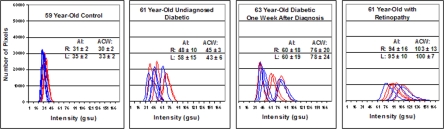
Representative histograms of numbers of pixel counts at each of 256 pixel intensities for retinal FA for diabetic patients without retinopathy, those with retinopathy, and age-matched control volunteers are shown in Figure 2. Four histograms, corresponding to the 4 images taken in each eye, are graphed for each patient. Curves shifted to the right indicate greater intensity of FA. Broader curves indicate a greater variation in FA intensity in retinal cells within the area of the retina imaged. Since the retinal imaging only subtends 3° on the retina, nonoverlapping histograms seen for a single eye may result from small changes in the patient’s/volunteer’s fixation during imaging, resulting in slightly different areas of the macula being imaged.
FIGURE 2.
Histograms of retinal flavoprotein autofluorescence (FA) pixel intensities from 4 age-matched patients demonstrating increased pixel intensity in diabetes mellitus and particularly in diabetic retinopathy. Four histograms from each eye (right = blue, left = red) are shown. Far left panel, 59-year-old control volunteer without ocular disease or history of diabetes (control). Middle left panel, 61-year-old patient without ocular disease or known history of diabetes mellitus. Increased retinal FA, however, prompted serum glucose testing, which was found to be abnormal. Middle right panel, 63-year-old patient with diabetes mellitus without retinopathy. Far right panel, 61-year-old patient with bilateral nonproliferative diabetic retinopathy. Right shift of histograms denotes increased retinal FA intensity. Broader curve width denotes greater cellular variability in FA intensity within the 3° field that is imaged. AI, average pixel intensity, ACW, average curve width.
The retinal FA histograms for a 59-year-old nondiabetic control volunteer demonstrated lower AIs and narrower curves (ACW), compared to any of the patients with diabetes mellitus (Figure 2). Retinal FA histograms for the 61-year-old diabetic patient with retinopathy demonstrated broader curves with higher AIs compared to the 2 representative diabetic patients without retinopathy (Figure 2).
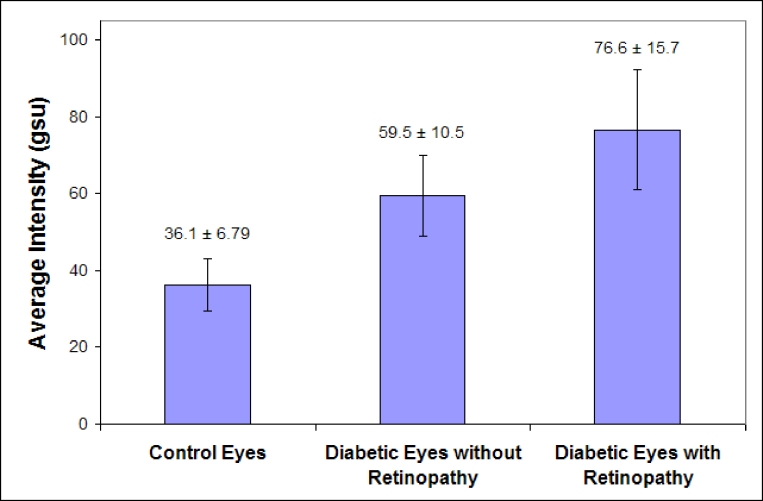
Fourteen total patients, aged 54 to 68 years, with diabetes mellitus were imaged for retinal FA. All but one of the patients had type 2 diabetes mellitus. Seven of the 14 patients had evidence of diabetic retinopathy on examination, with the retinal disease bilateral in all but one patient. Patients with retinopathy had an average HbA1c level of 7.7 ± 1.7%, and patients without retinopathy had an average HbA1c level of 6.8 ± 8%. All diabetic eyes had visual acuity of 20/40 or better, except one eye with 20/200 acuity in a patient without retinopathy. Seven healthy age-matched control patients, aged 57 to 67 years, were also imaged for retinal FA and compared to the diabetics. Patients with diabetes, but without retinopathy, had significantly greater average retinal FA intensity (AI = 59.5 ± 10.5 gsu) compared to the average retinal FA intensity in age-matched control volunteers (AI = 36.1 ± 6.79 gsu); (P = .001) (Figure 3). Diabetic patients with retinopathy showed increased average retinal FA intensities (AI = 76.6 ± 15.7 gsu) compared to eyes of diabetics without retinopathy (P = .04) (Figure 3). The ACW was increased in diabetics without retinopathy (ACW, 58.7 ± 9.1 gsu), compared to controls (ACW, 35.7 ± 6.8 gsu; P = .001). Diabetics with retinopathy had broader curves than diabetics without retinopathy (ACW, 72.1 ± 15.9 gsu; P = .08). These findings suggest that retinal FA is not only a reliable indicator of diabetes mellitus, but also an indicator of the severity of disease.
FIGURE 3.
Bar graph of average pixel intensities from patients with diabetic retinopathy (aged 56 to 68, n = 7), diabetics without retinopathy (aged 54 to 67, n = 7), and age-matched nondiabetic controls (aged 57 to 67, n = 7). Patients with diabetes demonstrated significantly increased retinal flavoprotein autofluorescence (FA) pixel intensity compared to nondiabetic control volunteers (P = .001). Diabetics with retinopathy demonstrated yet higher intensities of retinal FA compared to diabetics without retinopathy (P = .04). gsu, grayscale units.
Other retinal diseases were screened for retinal FA and compared to age-matched control patients. A 77-year-old patient with nonexudative ARMD, extensive central geographic atrophy and visual acuity of 20/400 OS, and focal exudative ARMD with visual acuity of 20/50 OD, recently treated with bevacizumab injections, was imaged for retinal FA. This ARMD patient was compared to retinal FA of our oldest control volunteer, aged 67 (Figure 4). Four retinal histograms from each eye in each individual are presented. Both eyes in the patient with ARMD had greater AI (OD = 53 ± 27 gsu, OS = 91 ± 7 gsu) than the control volunteer (OD = 39 ± 6 gsu, OS = 43 ± 9 gsu). The left eye of the ARMD patient, which had more clinically diffuse nonexudative disease, showed greater AI and ACW than the right eye (ACW: OD = 49 ± 22 gsu, OS = 74 ± 3 gsu), which had more focal disease clinically. The variability in the AI and ACW in the right eye of the ARMD patient may represent slight changes in the patient’s fixation during imaging, resulting in slightly different areas of the macula being imaged (Figure 4).
FIGURE 4.
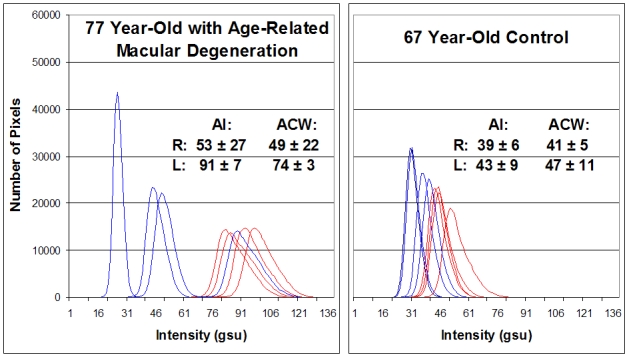
Retinal flavoprotein autofluorescence (FA) pixel intensity histograms from 77-year-old patient with age-related macular degeneration (ARMD) (left) and 67-year-old control patient without visible clinical findings of ARMD (right). ARMD patient had history of resolved exudative ARMD with VA 20/50 (best corrected) OD and dry ARMD with geographic atrophy and VA 20/400 (best corrected) OS. Four histograms of the right (blue) and left (red) eye from each patient are presented. Right shift of histograms denotes increased retinal FA intensity. Broader curve width denotes greater cellular variability in FA intensity within the 3° field that is imaged. AI, average pixel intensity; ACW, average curve width; gsu, grayscale units.
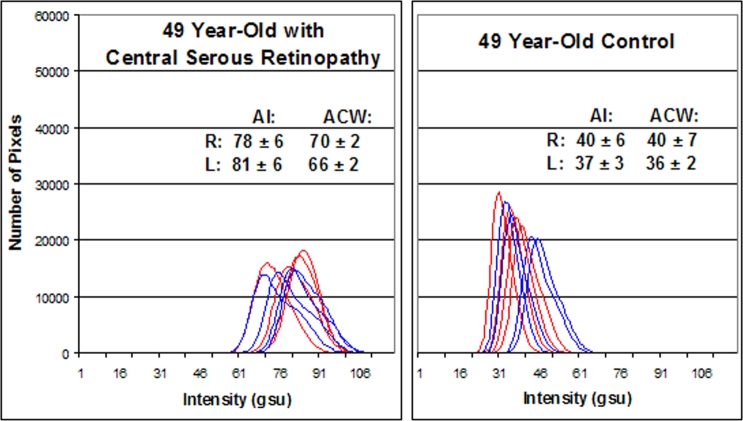
A 49-year-old patient with a history of bilateral central serous retinopathy OU was imaged for FA. This patient noted small bilateral paracentral scotomata on Amsler grid testing. Visual acuity was 20/20 OU, and fundus examination revealed parafoveal pigment disruption corresponding to the Amsler changes. Ophthalmic coherence tomography demonstrated no subretinal or sub-RPE fluid OU, but fluorescein angiography demonstrated areas of mild hyperfluorescence corresponding to the areas of Amsler grid abnormality OU. Flavoprotein autofluorescence imaging demonstrated a significant increase in AI and ACW in both eyes (AI: OD = 78 ± 6 gsu, OS = 81 ± 6 gsu; ACW: OD = 70 ± 2 gsu, OS = 66 ± 2 gsu) compared to an age-matched control volunteer (AI: OD = 40 ± 6 gsu, OS = 37 ± 3 gsu; ACW: OD = 40 ± 7 gsu, OS = 36 ± 2 gsu) (Figure 5).
FIGURE 5.
Flavoprotein autofluorescence (FA) pixel intensity histograms from 49-year-old patient with bilateral central serous retinopathy (left) and age-matched control (right). Four histograms of the right (blue) and left (red) eye from each patient are presented. Right shift of histograms denotes increased retinal FA intensity. AI, average pixel intensity; ACW, average curve width; gsu, grayscale units.
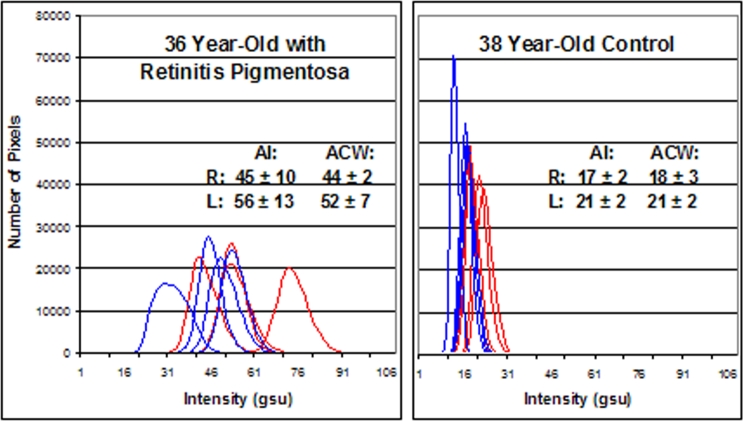
Retinal FA imaging was also performed on a 36-year-old with a diagnosis of retinitis pigmentosa. The patient had VA of 20/20 OU, optic disc pallor, retinal vessel attenuation, mild bone-spicule pigmentary deposits, and a ring scotoma on Goldmann visual field testing in both eyes. The photopic electroretinogram (ERG) was decreased, and the rod-isolated ERG was barely recordable in both eyes. Retinal FA imaging demonstrated a marked increase in the AI and ACW in both eyes (AI: OD = 45 ± 10 gsu, OS = 56 ± 13 gsu; ACW: OD = 44 ± 2 gsu, O = 52 ± 7 gsu) compared to an age-matched control volunteer (AI: OD = 17 ± 2 gsu, OS = 21 ± 2 gsu; ACW: OD = 183 gsu, OS = 21 ± 2 gsu) (Figure 6).
FIGURE 6.
Flavoprotein autofluorescence (FA) pixel intensity histograms from 36-year-old patient with retinitis pigmentosa (left) and age-matched control (right). Four histograms of the right (blue) and left (red) eye from each patient are presented. Right shift of histograms denotes increased retinal FA intensity. AI, average pixel intensity; ACW, average curve width.
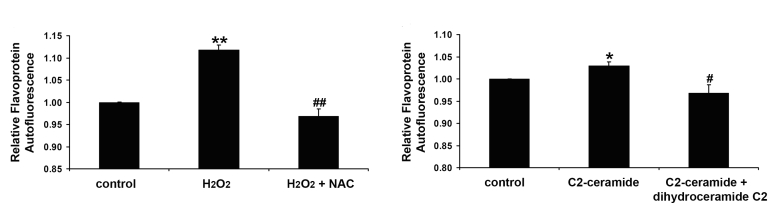
To show that preapoptotic conditions induce FA elevations in retinal cells, cultured retinal cells were subjected to sublethal concentrations of hydrogen peroxide and ceramide, both of which are known to induce apoptosis of these cells.24,25 As shown in Figure 7, exposure to each agent for 2 hours resulted in significantly elevated FA. In the case of hydrogen peroxide, the antioxidant, N-acetylcysteine, completely blocked FA elevations. For ceramide, its competitive antagonist, dihydroceramide, also completely blocked FA increases. In fact, the inhibitors tended to reduce FA, compared to control cells, suggesting improved redox status of the cells’ mitochondria compared to control cells. As both agents were present in sublethal concentrations, no morphologic evidence of apoptosis by TUNEL or Annexin-V assays was found (data not shown). These data suggest that as has been observed in cells of the heart, brain, and cartilage, retinal cells under apoptotic conditions exhibit enhanced FA.16,18
FIGURE 7.
Flavoprotein autofluorescence (FA) production induced by hydrogen peroxide (H2O2) (left) and C2-ceramide (right) in human retinal pigment epithelial (RPE) cells. Left, Effect of ROS scavenger on H2O2-induced FA production. RPE cells were preincubated with or without N-acetylcysteine (NAC; 1 mM) for 30 minutes and then treated with H2O2 (0.2 mM) in the presence and absence of NAC for 3 hours. Right, Effect of inactive form of C2-ceramide, dihydroceramide C2 on C2-ceramide-induced FA production. RPE cells were preincubated with or without dihydroceramide C2 (50 μM) for 30 minutes and then treated with C2-ceramide (50 μM) in the presence and absence of dihydroceramide C2 for 2 hours. Flavoprotein autofluorescence was quantitated with a FlexStation fluorescence plate reader with excitation and emission wavelengths set to 450 nm and 520 nm, respectively. Data are presented as mean ± SEM. N = 3 cell lines. **P < .001, compared with control; ##P < .01, compared with H2O2-stimulated cells. *P < .05, compared with control; #P < .05, compared with C2-ceramide-stimulated cells.
DISCUSSION
Flavoprotein autofluorescence of human and animal tissues has been known for almost 40 years and has been used to study metabolic dysfunction of skeletal muscle, liver, heart, and other tissues.7–9,11 Because of inherent low signal-to-noise ratios, most descriptions of FA monitoring have been performed on cells and tissues ex vivo using fluorescence microscopy.7–9,11,18 These studies have shown the dependence of FA fluorescence on the redox status of NAD(P)H and flavin-adenine dinucleotide (FAD) in a reciprocal relationship.2,26 Under physiologic conditions in which electron transport is efficient, FAD is found in a reduced state. Reduced FAD molecules in the respiratory chain embedded in the mitochondrial membranes do not fluoresce when exposed to blue light. However, when electron transport is deficient, as occurs when the mitochondrial membrane potential is unstable or lost during proapoptotic conditions, the FAD molecules become oxidized and their electrons assume maximum resonance, becoming prone to brief excitation to a higher energy state by blue light, followed by reversion to lower energy orbitals with the emission of green light.
Our results using instrumentation customized to detect FA of retinal cells in vitro demonstrate enhanced signals when HRPE cells are exposed briefly to H2O2 and ceramide at minimal concentrations that have been shown to induce apoptosis.24,25 Since our cells were passaged 5 days before experiments were performed, it is unlikely that other fluorophores such as lipofuscin, advanced glycated end products (AGEs), or collagen were responsible for the FA signal we detected. Furthermore, when the proapoptotic effects of H2O2 and ceramide were antagonized by specific inhibitors, enhanced FA signal was not detected. These data support the contention that FA oxidation due to instability of the mitochondrial membrane potential (Ψ) is responsible for the autofluorescence at 535 nm upon excitation with 467-nm light, as performed in our study.
Retinal cell apoptosis is a key pathophysiologic mechanism in many systemic and retinal diseases, including diabetes, ARMD, and inherited retinal degenerations. In animal models of diabetic retinopathy, for example, hyperglycemia has been shown to lead to oxidative damage of the retinal mitochondria by increased mitochondrial production of superoxide, leading to translocation of proapoptotic Bax into mitochondria, activation of capase 3, and apoptosis of retinal pericytes and endothelial cells.3,27 These effects have been shown to be mitigated by antioxidants, superoxide dismutase, vitamin E, and alpha-lipoic acid, which reduce levels of superoxide produced in the mitochondria, leading to better preservation of retinal capillaries in diabetic rats.3,28 Although the initiating insults to retinal cells by systemic and retinal diseases are diverse, affecting either the intrinsic or extrinsic apoptotic pathways, all ultimately affect mitochondrial membrane stability and lead to the opening of the mitochondrial membrane pore, causing loss of membrane potential and disruption of electron transport.29 As an essential component of electron transport, FAD oxidation is an early indication of mitochondrial dysfunction before significant disease-associated cell loss occurs.29
Recent advances in camera technology, narrow band-pass filter technology, and computer hardware and software now permit detection of FA even in vivo despite the inherent low signal-to-noise ratio of oxidized FA. In recent in vivo studies on ischemic-reperfusion injury of heart muscle in experimental animals, FA was shown to increase during reperfusion-induced cellular injury.17 The FA signal intensity correlated well with measures of apoptosis. Other studies using inhibitors of mitochondrial membrane pore opening have shown that increases in FA signal during proapoptotic conditions are dependent on pore opening leading to FAD oxidation.17 These studies give strong support to the use of FA as a marker for metabolic instability, if the technical problems of signal-to-noise ratio and confounding fluorophores can be overcome.
We believe that our method does address these 2 issues. First, our use of high-efficiency photomultiplying CCD chips and photomultiplier detectors improves the ability to detect the weak signals emitted by oxidized FAD when stimulated by blue light. Second, our use of narrow-band high-technology filters enables selection of a small bandwidth at the extreme shoulder of the lipofuscin emission curve, permitting separation of the FA signal from the emission of the dominant retinal fluorophore, lipofuscin. Computer processing by specialized hardware and custom software allows further enhancement of the FA signal to permit separating control from diseased tissue in vivo with more sensitivity and specificity.
Flavoprotein autofluorescence imaging of control individuals showed significant age-dependent increases that were greatest during the fourth to fifth decades of life, likely due to ongoing, physiologic apoptosis, but possibly due to some degree of contamination resulting from the age-dependent increases in lipofuscin, which may contribute to the baseline noise in the FA signal we detect. Nevertheless, at each age, patients with diabetes or retinal-specific diseases could be distinguished as having metabolic dysfunction, based on higher FA intensity, when compared to age-matched controls. Furthermore, diabetics with retinopathy had significantly higher FA signals than those without retinopathy, indicating that FA may be useful in monitoring the severity of metabolic tissue dysfunction. The higher FA signals in those with retinopathy also make it unlikely that the FA signal is due to the accumulation of lipofuscin, a fluorophore that has not been found to be increased in diabetes. In fact, Hammer and coworkers,30 in a recent study using a broad excitation bandwidth, found the ratio of green to red fluorescence to be higher in diabetics than controls. As red is the main wavelength emitted by lipofuscin, the investigators postulated that lipofuscin was not increased, but that the green signal resulted from “a hint of AGEs” autofluorescence. We believe that this is unlikely, as the bandwidth used for their in vivo imaging did not include the low-wavelength blue/ultraviolet light needed to stimulate AGEs.31 As previously reported in a study on patients with pseudotumor cerebri,20 we found that the eyes of patients with systemic or retinal disease have also shown considerable asymmetry in FA AI and ACW when compared to control individuals, who showed highly symmetric FA values. Thus, FA asymmetry appears to be an important indicator of retinal metabolic dysfunction and is unlikely due to asymmetric accumulation of other fluorophores. Our in vivo data, taken together with the clear emission of FA by cultured retinal cells, support FAD and not collagen, AGEs, or lipofuscin as the origin of the signal we detect.
Flavoprotein autofluorescence imaging in vivo is sensitive but not specific to a disease entity. It does, however, permit detection of metabolic dysfunction before any current clinical method can. The recognition of cell or mitochondrial stress before the onset of apoptosis is potentially very beneficial in recognizing a disease state before irreversible damage has occurred. Automated visual field testing, multifocal ERG, and new magnetic resonance imaging technologies may also detect functional abnormalities in eyes that do not yet display morphologic abnormalities. However, these methods are time-intensive and require trained technicians. As the tests take time to perform, they may not be suitable for children or adults unable to cooperate and maintain fixation for the duration of the study. Currently, FA requires less than 5 minutes to perform, obtaining the data as 4 rapid snapshots of each eye. Future development of the technology promises to reduce time with the screening instrument, readings of which are used with clinical data obtained by the eye care professional to arrive at a diagnosis or monitor the severity of the disease. Future development of FA imaging will be necessary to produce instruments capable of rendering spatial resolution of FA to particular retinal regions or cell layers, thereby permitting disease-specific data.
PEER DISCUSSION
DR. HANS E. GROSSNIKLAUS
Various methodologies have been utilized to detect changes indicative of early retinal disease, in particular, age-related macular degeneration. These techniques have included psychophysical tests (heterochromatic flicker photometry or HF, color matching), signal-based tests (Raman spectroscopy) and image-based tests (fundus reflectometry, autofluorescence).1 The relationship between fundus autofluorescence generated by lipofuscin and early age-related macular degeneration (ARMD) is currently being evaluated at several centers.2
Dr. Elner and co-workers have added a novel image-based test for evaluating retinal health. Their instrument utilizes the biologic principal that under the physiologic conditions, flavin adenine dinucleotide (FAD) is in the reduced state. Under pro-apototic conditions, FAD molecules become oxidized and their most highly resonating electrons are excited to a higher energy state by blue light followed by return to lower energy orbits, thus resulting in green light emission. This property has been coupled with flavin-binding blue-light photoreceptors in various organisms, such as photosynthetic bacteria, resulting in transcription or repression of photosynthesis genes under various light and oxygen tension conditions. Dr. Elner and coworkers’ instrument includes a modified Zeiss FF4 fundus camera, 467 nm excitation and 535 nm emission filters, a back-illuminated EMCCD camera, and computers with custom software. Their image analysis results in average intensity (AI) and average curve width (ACW) measurements. They found that AIs and ACWs were significantly increased in non-proliferative diabetic retinopathy (PDR) diabetic versus control patients, PDR versus non-PDR diabetic patients, ARMD versus control, central serous retinopathy versus control, and retinitis pigmentosa versus control patients. Their in vitro studies showed increased AI and ACW in retinal pigment epithelium (RPE) stressed by H2O2 versus control RPE, with abrogation of this effect when the RPE was treated with anti-oxidants. This is important work for evaluation of this new instrument.
The main question regarding this instrument, as for any similar instrument, is the biologic significance of this test. For instance, there is some evidence that the macular oxycarotenoid free radical scavengers, lutein and zeaxanthine, may be protective against the development of ARMD.1 These macular pigments absorb autofluorescent wavelengths emitted from RPE lipofuscin and this property is used in determining their concentration in the macula. Do these macular pigments absorb 467nm or 535 nm light, as used in retinal flavoprotein autofluorescence? Is there any spectral bleeding of the 500 nm or 524 nm emission wavelengths of autofluorescent RPE lipufuscin that will interfere with the test? These questions may be answered theoretically or empirically with in vitro experiments, although the biologic significance of the test must be evaluated clinically. That is precisely what Dr. Elner and colleagues have done in this study and in a previous study.3 Longitudinal studies using larger patient populations are needed to adequately assess the utility of this novel, exciting, and potentially very important instrument. I congratulate Dr. Elner and colleagues for their innovative technique and instrument.
ACKNOWLEDGMENTS
Funding/Support: None
Financial Disclosures: None.
REFERENCES
- 1.Beatty S, van Kuijk FJGM, Chakravarthy U. Macular pigment and age-related macular degeneration: longitudinal data and better techniques of measurement are needed. Inv Ophthalmol Vis Sci. 2008;49:843–845. doi: 10.1167/iovs.07-1276. [DOI] [PubMed] [Google Scholar]
- 2.Bindewald A, Bird AC, Dandekar SS, et al. Classification of fundus autofluroescence patterns in early age-related macular disease. Inv Ophthalmol Vis Sci. 2005;46:3309–3314. doi: 10.1167/iovs.04-0430. [DOI] [PubMed] [Google Scholar]
- 3.Elner VM, Park S, Cornblath W, et al. Flavoprotein autofluroescence detection of early ocular dysfunction. Arch Ophthalmology. 2008;126:259–260. doi: 10.1001/archophthalmol.2007.44. [DOI] [PubMed] [Google Scholar]
DR. MALCOLM R. ING
I have no conflicts of interest. I would like to congratulate the Elners on another brilliant presentation. I always feel that as a non-retinal person, prevention for us becomes a personal interest as we get older, number one, and for the public in general. If we can get at the root of how our cells deteriorate in the retina, we may not have to use all those hydrogels later, and so the question. As I recall, Victor Elner’s thesis showed that the inclusion of omega 3 fatty acids in rodent diets prevented or delayed the deposit of lipofuscin, which is the number one microscopic sign of macular degeneration, as far as I know. This elegant study demonstrated that the ingestion of omega 3 fatty acids could affect this process. Do you postulate that omega 3 fatty acids could also interact and interface with the flavoprotein autofluorescence (FA)? If you do, then should we do FA tests on people who are predisposed to macular degeneration, namely all of us, because perhaps omega 3 fatty acids might play a contributing role? Other antioxidants, such as xeozanthin levels have been associated with delayed macular degeneration in the POLA study, so that is another therapeutic possibility. With respect to antioxidants, could they be recommended if the FA studies showed this predisposition or could the antioxidants actually delay the FA in everyone?
DR. THOMAS W. GARDNER
I have no relevant interest. Certainly, I want to congratulate you. It has always been ironic that astronomers could tell more about the composition of stars light years away, than we can tell about the metabolism of the retina an inch away. I have a couple of specific questions. The first relates to really what is being measured here, because obviously the pathology of diabetes and macular degeneration are quite different. I would be very interested to know what cellular layers are being analyzed by this technique. My second question is whether you have any data on any reversal of these changes with the treatment of diabetic retinopathy. This might also be very interesting to study, if you have not already done so. I appreciate your comments about looking at different areas of the retina within the same eye, such as areas of perfusion or nonperfusion in diabetic retinopathy. Keep up the good work.
DR. LEONARD A. LEVIN
My only interests are intellectual, in the subject. I have a couple of specific questions. You mentioned the fact that the fluorescence characteristics of some other molecules might be abrogated or not implied in your studies because you use very narrow band filters. It is very possible with any kind of molecule that has a large spectrum emission or excitation spectrum, that they may be overlapped somewhat, as alluded to by Dr. Grossniklaus. Have you done any studies where you have more or less done spectrophotometry or used different filters across the range, to see if this signal you are seeing that differs in groups has a peak in the area that corresponds to oxidized versus FADH?
The other question is, you showed the data from the RPE cells exposed to hydrogen peroxide, and that is a huge oxidative stimulus. I think you used a 0.2 mM or more and it looked like you only had a 10% increase, which is relatively small compared to what I perceive as a huge increase in the patient. At one end, you have humans where the cells are dying fairly slowly and you get a large increase, and you get a large increase in this specific fluorescence you are seeing, while in a culture where you are inducing almost a maximal stimulus, which is without the presence, as far as you know, of other dyes, and you are not seeing very much change. How do you put those things together? I love the presentation.
DR. SUSAN G. ELNER
Thank you very much. I appreciate Han’s careful review of our paper and his kind comments. First, I would like to address his question, regarding the role of different fluorophors, particularly the carotenoids, which are also present in the macula. I would like to concentrate on lipofuscin and the AGEs. Lutein and xeozanthin absorb at 460 nm peak absorption, which is very close to the absorption wave length of flavoprotein. However, they emitted a quite different wavelength, 650 nm, quite different from 535 nm for flavoprotein. The amount of light associated with the flash to induce fluorescence is enough to saturate both, the carotenoids and flavoproteins, so we do not feel that the lutein and zeoxanthin are probably absorbing enough to influence the flavoprotein autofluorescence. In addition, at the narrow band width excitation wavelengths that we are looking at, we are probable not imaging fluorescence from the carotenoids.
There was also a question, if there was any spectral bleeding of the fluorescence that we are measuring with that of lipofuscin autofluorescence. Because of the narrow band width filters used, we feel that we are far enough away from the 524 nm for lipofuscin and there is no spectral bleeding.
Dr. Ing, I am very impressed that you remembered my husband’s thesis from a number of years ago. The question that you raised about the potential study of omega 3 fatty acids and their effects on flavoprotein autofluorescence is one of the things that Vic has been planning to analyze in the future. We are very interested in using some of this technology to further find out which things are beneficial in preventing apoptosis and damage to cells before cell death has occurred.
Dr. Gardner had also asked about which cell layers are imaged in the retina. We are assuming we are getting fluorescence from all of the retinal cells, including the retinal pigment epithelium. We really have not sorted it out, except by the cell culture studies that we have done on the retinal pigment epithelium, showing that we can get fluorescence from the pigment epithelial cells themselves. We also assume that retinal cells are also contributing to the fluorescence that we are seeing. The current prototype camera that we have only takes a three degree retinal image. Future versions of this camera, that we are hoping to develop, may image larger areas. We really have not imaged anything outside of the macula itself. That will be an obvious area of future study. The technology has not been such that we could really differentiate between non-ischemic and ischemic retina, but that would be another area of interest. I would anticipate that in areas of ischemic retina, where the cells have died, we would see less flavoprotein autofluorescence.
There was a question regarding the difference in fluorescence intensities that we were seeing clinically and in our in vitro studies of pigment epithelium. First of all, the amount of fluorescence that is coming back out of the eye is actually weak, but the images are enhanced by the camera and the computer. Therefore, we are actually doing very fine measurements. So although we are showing significant differences, we are actually measuring very small absolute differences in these studies. These differences may be a little bit more in line with the differences that we are measuring in cell culture. The clinical images are imaging multiple cell layers, and not just the pigment epithelium that we measure in culture. Thank you.
ACKNOWLEDGMENTS
Funding/Support: This research was supported by grants EY09441 (V.M.E.) and EY07003 (Core) from the National Institutes of Health. Dr V. M. Elner is a recipient of the Senior Scientific Investigator Award from Research to Prevent Blindness, Inc, New York, New York.
Financial Disclosure: Dr V. M. Elner and Dr Petty have a financial interest in the presented material by having founded OcuSciences, Inc, to commercialize the technology. All other authors have no conflict of interest.
Author Contributions: Design and conduct of the study (V.M.E., H.R.P., M.G.F, S.P.); Collection, management, analysis, and interpretation of the data (V.M.E., M.G.F., S.P., S.G.E., H.R.P.); Preparation, review, or approval of the manuscript (V.M.E., S.G.E., J.R.H.).
Conformity With Author Information: This study was approved by the institutional review board at the University of Michigan, Ann Arbor.
REFERENCES
- 1.Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol. 2001;2:63–67. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- 2.Lemasters JJ. Modulation of mitochondrial membrane permeability in pathogenesis, autophagy and control of metabolism. J Gastroenterol Hepatol. 2006;22:S31–S37. doi: 10.1111/j.1440-1746.2006.04643.x. [DOI] [PubMed] [Google Scholar]
- 3.Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Rad Biol Med. 2003;35:1491–1499. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Ning X, Baoyu Q, Yuzhen L, Shuli S, Reed E, Li QQ. Neuro-optic cell apoptosis and microangiopathy in KKAY mouse retina. Int J Mol Med. 2004;13:87–92. [PubMed] [Google Scholar]
- 5.Benson RC, Meyer RA, Zaruba ME, McKhann GM. Cellular autofluorescence—is it due to flavins? J Histochem Cytochem. 1979;27:44–48. doi: 10.1177/27.1.438504. [DOI] [PubMed] [Google Scholar]
- 6.Kindzelskii A, Petty HR. Fluorescence spectroscopic detection of mitochondrial flavoprotein redox oscillations and transient reduction of the NADPH oxidase-associated flavoprotein in leukocytes. Eur Biophys J. 2004;33:291–299. doi: 10.1007/s00249-003-0361-4. [DOI] [PubMed] [Google Scholar]
- 7.Reinert KC, Dunbar RL, Wangcai G, Gang C, Ebner TJ. Flavoprotein autofluorescence imaging of neuronal activation in the cerebellar cortex in vivo. J Neurophysiol. 2004;92:199–211. doi: 10.1152/jn.01275.2003. [DOI] [PubMed] [Google Scholar]
- 8.Kuznetsov AV, Mayboroda O, Kunz D, Winkler K, Schubert W, Kunz WS. Functional imaging of mitochondria in saponin-permeabilized mice muscle fibers. J Cell Biol. 1998;140:1091–1099. doi: 10.1083/jcb.140.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunz WS. Impairment of mitochondrial function in skeletal muscle of patients with amyotrophic lateral sclerosis. J Neurol Sci. 1998;156:65–72. doi: 10.1016/s0022-510x(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 10.Kunz D, Luley C, Winkler K, Lins H, Kunz WS. Flow cytometric detection of mitochondrial dysfunction in subpopulations of human mononuclear cells. Anal Biochem. 1997;246:218–224. doi: 10.1006/abio.1997.2007. [DOI] [PubMed] [Google Scholar]
- 11.Kunz WS, Gellerick FN. Quantification of the content of fluorescent flavoproteins in mitochondria from liver, kidney, cortex, skeletal muscle, and brain. Biochem Med Metabol Biol. 1993;50:103–110. doi: 10.1006/bmmb.1993.1051. [DOI] [PubMed] [Google Scholar]
- 12.Romashko DN, Marban E, O’Rourke B. Subcellular metabolic transients and mitochondrial redox waves in heart muscle. Proc Natl Acad Sci U S A. 1998;95:1618–1623. doi: 10.1073/pnas.95.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace DC. Diseases of the mitochondrial DNA. Ann Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- 14.Morgan-Hughes JA. Mitochondrial diseases of muscle. Curr Opin Neurol. 1994;7:457–462. doi: 10.1097/00019052-199410000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Koke JR, Wylie W, Wills M. Sensitivity of flavoprotein fluorescence to oxidative state in single isolated heart cells. Cytobios. 1981;32:139–145. [PubMed] [Google Scholar]
- 16.Shino A, Matsuda M, Handa J, Chance B. Poor recovery of mitochondrial redox state in CA1 after transient forebrain ischemia in gerbils. Stroke. 1998;29:2421–2425. doi: 10.1161/01.str.29.11.2421. [DOI] [PubMed] [Google Scholar]
- 17.Ranji M, Kanemoto S, Matsubara M, et al. Fluorescence spectroscopy and imaging of myocardial apoptosis. J Biomed Optics. 2006;11:064036-1–064036-4. doi: 10.1117/1.2400701. [DOI] [PubMed] [Google Scholar]
- 18.Rajpurohit R, Mansfield K, Ohyama K, Ewert D, Shapiro IM. Chondrocyte death is linked to development of a mitochondrial membrane permeability transition in the growth plate. J Cell Physiol. 1999;179:287–296. doi: 10.1002/(SICI)1097-4652(199906)179:3<287::AID-JCP6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 19.Winkler K, Kuznetsov AV, Lins H, et al. Laser-excited fluorescence studies of mitochondrial function in saponin-skinned skeletal muscle fibers of patients with chronic progressive external ophthalmolplegia. Biochim Biophys Acta. 1995;1272:181–184. doi: 10.1016/0925-4439(95)00085-2. [DOI] [PubMed] [Google Scholar]
- 20.Elner VM, Park S, Cornblath W, Hackel R, Petty HR. Flavoprotein autofluorescence detection of early ocular dysfunction. Arch Ophthalmol. 2008;126:259–260. doi: 10.1001/archophthalmol.2007.44. [DOI] [PubMed] [Google Scholar]
- 21.Field MG, Elner VM, Puro DG, et al. Rapid, noninvasive detection of diabetes-induced retinal metabolic stress. Arch Ophthalmol. 2008;126:934–938. doi: 10.1001/archopht.126.7.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elner SG, Strieter RM, Elner VM, Rollins BJ, Del Monte MA, Kunkel SL. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab Invest. 1991;64:819–825. [PubMed] [Google Scholar]
- 23.Yang D, Elner SG, Bian Z-M, Till GO, Petty HR, Elner VM. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res. 2007;85:462–472. doi: 10.1016/j.exer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin GF, Hurst JS, Godley BF. Hydrogen peroxide stimulates apoptosis in cultured human retinal pigment epithelial cells. Curr Eye Res. 2001;22:165–173. doi: 10.1076/ceyr.22.3.165.5517. [DOI] [PubMed] [Google Scholar]
- 25.Barak A, Morse LS, Goldkorn T. Ceramide: a potential mediator of apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:247–254. [PubMed] [Google Scholar]
- 26.Chance B, Salkovitz IA, Kovach AG. Kinetics of mitochondrial flavoprotein and pyridine nucleotide in perfused heart. Am J Physiol. 1972;223:207–218. doi: 10.1152/ajplegacy.1972.223.1.207. [DOI] [PubMed] [Google Scholar]
- 27.Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci. 2003;44:5327–5334. doi: 10.1167/iovs.03-0353. [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Bierhaus A, Bugert P, et al. Effect of R-(+)-alpha-lipoic acid on experimental diabetic retinopathy. Diabetologia. 2006;49:1089–1096. doi: 10.1007/s00125-006-0174-y. [DOI] [PubMed] [Google Scholar]
- 29.Duchen MR. Section III: Mitochondria, β-cell function and type 2 diabetes. Diabetes. 2004;53:S96–S102. [Google Scholar]
- 30.Hammer M, Konigsdorffer E, Lieberman C, et al. Ocular fundus auto-fluorescence observations at different wavelengths in patients with age-related macular degeneration and diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:105–114. doi: 10.1007/s00417-007-0639-9. [DOI] [PubMed] [Google Scholar]
- 31.Gopalkrishnapillai B, Nadanathangam V, Karmakar N, Anand S, Misra A. Evaluation of autofluorescent property of hemoglobin-advanced glycation end product as a long-term glycemic index of diabetes. Diabetes. 2003;52:1041–1046. doi: 10.2337/diabetes.52.4.1041. [DOI] [PubMed] [Google Scholar]