Abstract
Purpose
Blepharitis and meibomian gland dysfunction (MGD) are common sources of complaints from patients. To evaluate the effect on ocular symptoms, ocular findings, and serum and meibomian gland contents, patients with blepharitis and MGD were prospectively evaluated to determine the effects of dietary supplementation with omega-3 fatty acids.
Methods
In a prospective randomized placebo-controlled masked trial, patients with simple obstructive MGD and blepharitis, who had discontinued all topical medications and tetracyclines, received oral omega-3 dietary supplementation consisting of two 1000-mg capsules 3 times a day. Patients were examined every 3 months for 1 year with the Ocular Surface Disease Index (OSDI) objective clinical measures, including tear production and stability, ocular surface and meibomian gland health, and biochemical plasma, red blood cell (RBC), and meibum evaluation. Primary outcome measures were change in tear breakup time (TBUT), meibum score, and overall OSDI score at 1 year.
Results
At 1 year, the omega-3 group had a 36% and 31% reduction in their omega-6 to omega-3 fatty acid ratios in RBCs and plasma, respectively (P = .3), whereas the placebo group demonstrated no change. At 12 months, the omega-3 group had an improvement in TBUT, OSDI score, and meibum score. Changes in meibum content were observed in the omega-3 group (P = .21); the level of meibum saturated fatty acids decreased.
Conclusions
This trial demonstrated a decrease in the RBC and plasma ratios of omega-6 to omega-3 in patients taking omega-3 dietary supplementation, as compared to controls, and improvements in their overall OSDI score, TBUT, and meibum score. This is the first demonstration of an induced change in the fatty acid saturation content in meibum as a result of dietary supplementation with omega-3 fatty acids.
INTRODUCTION
Up to 20% of adults over the age of 45 report some discomfort from blepharitis and meibomian gland dysfunction (MGD),1 making these conditions the most common sources of patient complaints in a comprehensive ophthalmology practice. It is often difficult to distinguish the cause of patient complaints,2 as there is considerable overlap between blepharitis, MGD, and dry eye. Recently, the International Dry Eye Workshop Research Subcommittee (DEWS) reviewed research on the basic mechanisms underlying dry eye disease. The DEWS classification separates the dry eye into aqueous-deficient and evaporative states.3 The evaporative dry eye may be due to evaporative loss from the tear film, which is directly affected by MGD, or posterior blepharitis. There is mounting evidence that dry eye discomfort results from ocular surface inflammation, which in turn stimulates immune-mediated inflammatory processes, further exacerbating the condition.4 It is thought that the initial inflammatory episode may stem from dysfunction of the meibomian glands, which results in changes in the tear film.5–7 Because the roles of the tear film and the meibomian glands are so intertwined in maintaining the health of the ocular surface, it is extremely difficult to separate blepharitis, MGD,6 and evaporative dry eye disease in terms of pathophysiology and clinical management. An ideal therapeutic approach would treat both the underlying etiology and signs and symptoms of blepharitis, dry eye disease, and MGD.
MEIBOMIAN GLANDS AND MEIBUM
The meibomian glands are tubuloacinar, holocrine glands that produce and secrete meibum,8,9 an oily substance that aids in tear-film stability. Embedded in the tarsal plates, there are normally 30 to 40 meibomian glands in the upper lid and 20 to 30 glands in the lower lid.7–9 Each meibomian gland consists of a main duct surrounded by grapelike acinar clusters. These ducts open into the lid margin just anterior to the mucocutaneous junction, delivering meibum to the tear film. As the eyelid closes, contraction of the orbicularis oculi muscles results in expression and spreading of the meibomian gland contents, over the preocular film, the latter of which occurs in the up-phase of each blink.10,11 The orifices of each meibomian gland are normally visible on the lid margin at the mucocutaneous junction. With mild lid pressure immediately below the lash line, the contents of the meibomian gland can easily be expressed.
Normal meibum is clear, fluid oil that easily spreads to become the outermost surface of the tear film. This outermost lipid layer, which is a combination of polar and nonpolar lipids, heavily influences the evaporation rate of the tear film. The polar lipids act as a surfactant to help the nonpolar lipids spread over the aqueous component of the tear film and provide a structure that supports the nonpolar phase.5,12,13 Meibum is approximately 77% wax and sterol esters,14 8% phospholipids, and 9% digylcerides and triglycerides and hydrocarbons in normal patients, but slight variations in this distribution do occur, accounting for observed variability in the meibum melting point.15 The relatively low melting point of the lipids (19°C to 32°C) facilitates delivery to the tear film by allowing meibum to remain fluid at body temperature (37°C).5,16 It is not clear how specific differences in lipid composition affect the stability or thickness of the lipid layer, despite the fact that a thicker tear film lipid layer correlates positively with better tear stability.5,17
Healthy meibum is essential for a healthy ocular surface, as the functions of the lipid layer include slowing evaporation of the aqueous tear components, enhancing tear film spreading and stability, preventing spillover of tears from the lid margin, preventing contamination of the tear film by sebum, and sealing the apposed lid margins during sleep.18–21 Additionally, meibum contributes to providing a smooth optical surface at the air-tear interface, allowing for optimal vision.15,22,23
MEIBOMIAN GLAND DYSFUNCTION
In most types of blepharitis, there is some involvement of the meibomian glands. The inherent lid margin inflammation, which is defined as part of blepharitis, affects the meibomian glands over time. MGD may be defined as progressive meibomian gland obstruction due to ductal hyperkeratinization or inspissation of secretions.7 MGD is synonymous with posterior blepharitis, particularly when chronic inflammation occurs,24 and may be obstructive, simple, or cicatricial in nature. Although MGD rarely threatens sight, it is a troublesome and symptomatic condition. Many patients move from doctor to doctor, seeking relief from their symptoms.7 There is general agreement that MGD is age-related, increasing in prevalence from nearly 0% in patients in the first decade of life to nearly 68% in patients over 60 years of age.5,25,26 MGD is one of the major causes of ocular discomfort, tear deficiency, and increased tear evaporation rates,18,27 perhaps because of the reduced lipid layer thickness.28 Increased evaporation of the tear film most commonly stems from meibomian gland disease, which leads to thickened secretions and reduced secretion volumes, both of which are detrimental to the formation and integrity of the protective lipid layer. In both aqueous tear–deficient and evaporative dry eye, hyperosmolarity has been shown to play a role in the pathological changes associated with dry eye.
Exactly what triggers the development of blepharitis is unknown; however, recent studies have linked changes in the tear film to the development of blepharitis. Numerous different etiologies have been considered for blepharitis, including staphylococcal infection, Demodex folliculorum infestation, and primary sebaceous gland disease, as in acne rosacea. Yet, the underlying pathophysiologic reaction is an inflammatory response in the lid area, including the eyelash follicles and meibomian glands. These include changes in the makeup of the tears and changes in tear film stability.5 It has also been suggested that defects in the ocular surface that occur independently from tear film changes are the underlying cause of blepharitis.20
In MGD, meibum is often abnormal, progressively changing in color from clear to yellow and in consistency from liquid to toothpaste-like. Additionally, ductal hyperkeratinization may result in blockage of the duct orifice, deterioration of acini clusters,29 and stagnation of the meibum within the gland. Obstructive MGD is hyposecretory in nature and results in an unstable tear film, increased evaporation, meibomian gland dropout, thickened lipid secretions, and low lipid volume.22 Simple obstructive MGD is typified by plugged meibomian gland orifices and cloudy or thickened secretions.8 Cicatricial MGD is characterized by scarred marginal and tarsal mucosa, which cause exposure and retraction of the meibomian ductules. The patients often present with posterior displacement and/or elongation of the orifices into the marginal or tarsal conjunctiva.8 In patients with MGD, we know that warm compresses will improve the tear stability.30 Evidence of alteration in the meibum melting point demonstrates that changes in the meibum content occur with MGD, and results in the production of toxic tear film destabilizing components such as fatty acids (FAs).12,31
Various studies have demonstrated the correlation between MGD and the evaporative dry eye. Patients with meibomian gland dropout generally have elevated tear osmolarity and low tear production.22 Even when tear production is moderately decreased in MGD, dry eye signs and symptoms can be severe because the evaporative rate of these patients is much higher than normal.22 Additionally, Korb and colleagues28 have shown that patients with severe symptoms have thinner lipid layers than patients with more moderate complaints. Long-term treatment is aimed at controlling symptoms through eyelid hygiene.16,17,32 Systemic antibiotics, such as minocycline,33 tetracycline,34 and doxycycline,35 and topical antibiotic/steroid combinations may be used for short-term therapy in patients with a significant amount of inflammation.32
ROLE OF INFLAMMATION IN MEIBOMIAN GLAND DYSFUNCTION AND DRY EYE DISEASE
Inflammation is an integral component of blepharitis, MGD, and the aqueous-deficient dry eye.4,36 In evaporative dry eye, increased expression of inflammatory mediators on the ocular surface and in tears of patients with ocular rosacea has been demonstrated.37 Increased expression of conjunctival markers of inflammation and proinflammatory mediators has also been demonstrated in the tear film of MGD patients.28 Topical anti-inflammatory therapies such as cyclosporin A and corticosteroids have been successful in the short-term management of MGD,4,37,38 with the ultimate goal to return the patient to exclusive lid hygiene therapy. These findings suggest that inflammation is not only a consequence of meibomian gland disease but also a potential target for treatment of MGD.
Alternative treatments, including dietary supplements, have become an explosive area of investigation. Numerous dietary supplements touted as revolutionary in their treatment of the dry eye and blepharitis have been marketed as over-the-counter treatments. Dietary supplementation with FAs has been recommended for MGD patients,39–41 but there is a lack of objective clinical studies published in the literature to demonstrate effectiveness. Miljanovic and associates39 reviewed the dietary diaries of patients enrolled in the Wonmen’s Health Study, and results suggest that a higher dietary intake of omega-3 FAs is associated with a decreased incidence of dry eye syndrome in women. Oxholm and colleagues41 treated 28 Sjogren syndrome patients with omega-6 FAs and found no documentable improvement when compared to placebo.40 Most recently,41 Creuzot reported that poluyunsaturated FA dietary supplementation improved subjective symptoms in dry eye patients.
OMEGA-3 FATTY ACIDS
Omega-3 FAs and omega-6 FAs are essential for normal growth and development. Omega-3 FAs and omega-6 FAs compete for the same enzyme to eventually be converted into anti-inflammatory prostaglandins (PGE3) and less inflammatory leukotrienes and into proinflammatory prostaglandins (PGE2) and more inflammatory leukotrienes, respectively (Figure 1). It is the ratio of omega-6 to omega-3 FAs that is important in influencing the overall inflammatory state of the body. Calder42 demonstrated that overproduction of proinflammatory PGE2 and underproduction of anti-inflammatory PGE1 and PGE3 occur when the omega-6 to omega-3 FA ratio is high. The ideal dietary intake of essential FAs is approximately 4:1, as is seen in the Mediterranean diet, rich in cold-water fish and natural oils.43 The typical American and Northern European diet is high in omega-6 FAs, with the average person having an omega-6 to omega-3 FA intake ratio between 15:1 and 18:1.44 These populations tend to overconsume red meat and underconsume unprocessed oils and cold-water fish.
FIGURE 1.
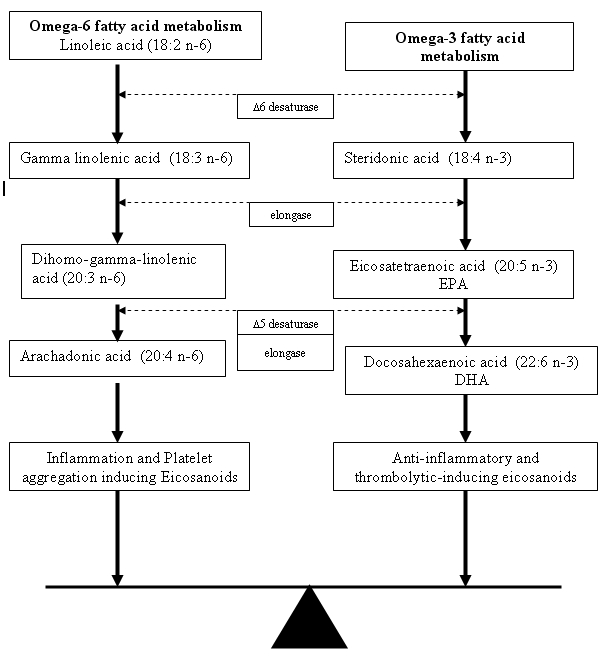
Metabolic pathways for omega-3 and omega-6 fatty acids.
Omega-3 FAs modulate prostaglandin metabolism toward anti-inflammatory prostaglandin (PGE3) synthesis due to competitive inhibition of the arachidonic acid pathway (Figure 1). In the early phase of inflammation, large amounts of interleukins and lipid mediators are released. Proinflammatory eicosanoids of arachidonic acid metabolism are released from membrane phospholipids in the course of inflammatory activation. Eicosapentaenoic acid (EPA), an omega-3 FA, competes with arachidonic acid, an omega-6 FA, for anti-inflammatory and proinflammatory prostaglandin and leukotriene synthesis at the cyclooxygenase and lipoxygenase level. Increasing systemic levels of EPA and docosahexaenoic acid (DHA) result in:
Decreased production of prostaglandin E2 (PGE2) metabolites;
Decreased thromboxane A2, a potent platelet aggregator and vasoconstrictor;
Decreased leukotriene B4 formation, a potent inducer of inflammation, leukocyte chemotaxis, and adherence;
Increased thromboxane A3, a weak platelet aggregator and a weak vasoconstrictor;
Increased prostacyclin PGI3, leading to an overall increase in total prostacyclin by increasing PGI3 without a decrease in PGI2 (both PGI2 and PGI3 are active vasodilators and inhibitors of platelet aggregation); and
Increased leukotriene B5, a weak inducer of inflammation and a weak chemotactic agent.45,46
The anti-inflammatory properties of omega-3 FAs, especially EPA, are due to competition with arachidonic acid as a substrate for cyclooxygenases and 5-lipoxygenase. The eicosanoids from the omega-6 and omega-3 FAs have opposing properties (Figure 1). In addition to their effects on prostaglandins, thromboxanes, and leukotrienes, omega-3 FAs suppress the production of interleukin 1 (IL-1ß) by suppressing the IL-1ß messenger RNA (mRNA) as well as the expression of COX2 (cytooxygenase) mRNA that is induced by IL-1ß. α-Linolenic acid (ALA), EPA, and DHA are involved in immune function. The precise effect of ALA depends on the level of linoleic acid and total polyunsaturated FA content of the diet. A high dose of ALA (about 15 g/day) will suppress human IL-1 and tumor necrosis factor (TNF). It is unclear whether ALA itself exerts these effects or whether they are the result of its conversion to EPA.47,48
DIETARY ROLE OF OMEGA-3 FATTY ACIDS
On the cellular level, omega-3 FA supplementation in healthy volunteers suppresses the capacity of monocytes to synthesize IL-1, through suppression of IL-1 mRNA49,50 and TNF.51 Studies in normal volunteers indicate that omega-3 FA supplementation reduced the ability of monocytes to produce IL-1ß upon stimulation with endotoxin. The effect was most pronounced 10 weeks after stopping the supplementation and suggests prolonged incorporation of omega-3 FAs into a pool of circulating monocytes.47 The capacity of the monocytes from these donors to synthesize IL-1ß returned to the presupplement level 20 weeks after ending dietary supplementation. This effect can explain the beneficial effects of omega-3 FAs in models of chronic inflammatory disease, as IL-1 and TNF influence a wide array of biologic functions.52–54 IL-1 potentiates procoagulent activity and increases production of endothelin and plasminogen activator inhibitor and the formation of eicosanoids. It also increases leukocyte adhesion by inducing the expression of adhesion molecules and promoting endothelial protein permeability. Omega-3 FAs suppress platelet activating factor, a potent platelet aggregator and leukocyte activator that strongly promotes arachidonic acid metabolism.55
The first evidence of the role of dietary intake of omega-3 polyunsaturated FAs in inflammation was derived from epidemiologic observations in 1979 identifying a lower incidence of autoimmune and inflammatory disorders, such as psoriasis, asthma, and type 1 diabetes, as well as the complete absence of multiple sclerosis, in a population of Greenland Eskimos compared with gender- and age-matched groups living in Denmark.56 Native Greenland Eskimos57 and the Japanese58 have a high dietary intake of long-chain omega-3 polyunsaturated FAs from the high amounts of seafood in the diet. These populations also had a low incidence of myocardial infarction and chronic inflammatory or autoimmune disorders compared to their Westernized counterparts of the same ethnic background. Diets high in omega-3 FAs have also been found to reduce the severity of experimental cerebral59 and myocardial infarction60 and autoimmune nephritis and to reduce the incidence of breast tumors in several animal models.61,62 Omega-3 FAs may play an important role in the prevention and treatment of asthma,63, 64 cardiac arrhythmias,65 cardiovascular diseases,66–68, adult-onset diabetes,69 rheumatoid arthritis,70, 71 rejection of transplanted organs, and various kidney diseases.72,73
Mounting evidence, accumulated over the past decade, shows a potential benefit of omega-3 FAs on the eye, particularly in macular disease74–77 and cataracts.78–82 These studies have been part of the evidence used to design and validate the need for multicenter longitudinal studies on the effect of omega-3 dietary supplementation in macular degeneration. Many of these studies have examined the dietary diaries of patient and correlated these with patient symptoms and findings.39–41 However, in none of these retrospective reviews was analysis of the effect of dietary supplements on the blood levels of FAs performed. Alteration of the omega-6 to omega-3 ratio in plasma and red blood cell (RBC) membranes is necessary to document that the changes in symptoms are attributable to dietary supplementation with oral omega-3 FAs.
As discussed above, it has been shown that shifting the dietary omega-6 to omega-3 FA ratio toward omega-3 FAs can lower the overall inflammatory state of the body.83 Anecdotal data suggest that omega-3 FA dietary supplementation can have a beneficial effect on blepharitis.84,85 In patients taking omega-3 FA supplements, reduced production of IL-1 and TNF plays a role in the reduction of inflammatory symptoms. Studies in normal volunteers indicate that omega-3 FA supplementation reduced the ability of monocytes to produce IL-1ß upon stimulation with endotoxin.47 Similar results were observed for IL-1 and TNF. Pharmacologic agents known to reduce the synthesis of IL-1 and TNF are corticosteroids and cyclosporine, both of which have been shown to be effective in managing posterior blepharitis flare-ups but have well-known adverse side effects, particularly during long-term administration. Because these omega-3 FAs act on these same biochemical pathways, speculation arose as to the effect of omega-3 FAs on blepharitis and MGD.
POTENTIAL MECHANISMS OF ACTION OF OMEGA-3 ON BLEPHARITIS AND MEIBOMIAN GLAND DYSFUNCTION
There are 2 hypotheses as to why dietary supplementation of omega-3 FAs may alleviate blepharitis and the resulting MGD and dry eye symptoms. The first hypothesis relies on the fact that the breakdown of omega-3 FAs results in the production of molecules that suppress inflammation, whereas the breakdown of omega-6 FAs results in the production of molecules that can lead to inflammation. Omega-3 and omega-6 FAs compete for the same enzymes for metabolism into usable components. These enzymes are part of the inflammatory pathway involving arachidonic acid, the pathway that is mediated by the anti-inflammatory agents aspirin and COX-2 inhibitors. The first hypothesis is that overwhelming the FA metabolic pathway with omega-3 FA molecules results in competitive inhibition of omega-6 FA metabolism, reducing the overall inflammatory state of the eyelid margin and the meibomian gland region. As blepharitis, MGD, and dry eye are thought to be inflammatory diseases, a reduction in the systemic inflammatory state may alleviate blepharitis, MGD, and dry eye–associated discomforts.
The second hypothesis regards the composition of the tear film. It has been suggested that an unstable tear film results from abnormal meibomian gland secretions and can result in the evaporative dry eye. Supplementing the diet with high amounts of omega-3 FAs is likely to change the FA composition and therefore the properties of meibomian gland secretions. This change may be beneficial in tear stabilization and may prevent the inflammation from blocking the meibomian gland ducts and meibum stagnation.
To further understand the effects of omega-3 FAs on blepharitis and MGD, we examined the effect of omega-3 FA dietary supplementation in a prospective randomized double-masked, placebo-controlled study in a group of 22 blepharitis patients with simple obstructive meibomian gland disease over a 12-month period. Dry eye symptoms and clinical presentation, meibomian gland health, meibum appearance and biochemical content, and blood FA levels were examined to elucidate the effects of omega-3 FA supplementation, in the form of flaxseed oil, on MGD.
METHODS
PATIENT ENROLLMENT
The Institutional Review Board of Evanston Northwestern Healthcare approved this study (including study design, data collection, analysis, and informed consent process and all forms), and all study conduct was performed within the guidelines of Good Clinical Practice. Health Insurance Portability and Accountability Act regulations were followed. All participants signed an IRB-approved informed consent form prior to enrollment.
PATIENT POPULATION
Thirty-eight patients with blepharitis and simple obstructive meibomian gland disease over the age of 18, not pregnant or nursing, and willing to comply with all study procedures were enrolled in this research study.
INCLUSION CRITERIA
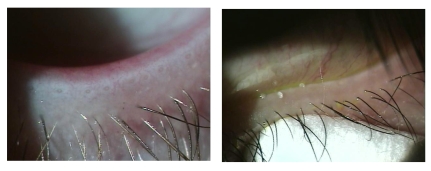
Clinical inclusion criteria required a diagnosis of moderate to severe chronic blepharitis and simple obstructive MGD (Figure 2), with onset and greater than 3 months duration of ocular symptoms consistent with blepharitis, dry eye, and meibomian gland disease. No entrance criteria were defined for vital dye staining, Schirmer testing, or Ocular Surface Disease Index (OSDI) score. All patients had not taken oral tetracycline drugs (including doxycycline and minocycline) or oral corticosteroids for at least 3 months and had discontinued all topical medications for at least 1 month prior to study enrollment. The use of artificial tears did not exclude participation and was allowed during study participation. All patients were performing daily eyelash cleansing with a nonirritating dilute baby shampoo prior to initiation of the study. Patients were instructed to continue their daily eyelash hygiene in the same manner for the duration of the study period, to attempt to avoid any change in routine that may represent a confounding factor.
FIGURE 2.
Left, Simple obstructive meibomian gland dysfunction typified by plugged meibomian gland orifices and lid margin telangiectasias. Right, Pressure is applied with a cotton-tipped applicator below the lashes to force the meibum out of the meibomian glands.
Because this study targets the arachidonic acid inflammatory pathway, patients on a regular course of aspirin or COX-2 inhibitors were not allowed to participate. Also, omega-3 FAs can inhibit platelet aggregation,86–96 and patients who were on anticoagulant therapies or who had blood disorders were also excluded from the study. Other exclusion criteria included preexisting ocular disease or pathology, systemic disease requiring anticoagulation, long-term use of nonsteroidal anti inflammatory agents or COX-2 inhibitors, and use of dietary FA supplementation, including omega-3 or omega-6 FAs, for 1 month prior to day 0 (baseline).
DIETARY INTAKE EVALUATION
Patients were asked to maintain their normal dietary habits during the course of the study; however, they were informed that 6 g of flaxseed oil would increase their daily caloric intake by 60 calories unless a slight caloric reduction of food was initiated. All patients were asked to keep a dietary intake journal to evaluate nutritional intake of foods rich in omega-3 FAs during the course of the study. The total amount (in grams) of omega-3 FAs per portion recorded in the patient diary was used to calculate the total dietary intake of omega-3 FAs. The total amount per serving for fish was derived from the amounts in salmon (1.4 g/serving), canned salmon (2.2 g/serving), and canned tuna (0.3 g/serving). These values were obtained from the Minnesota Nutrient Data Base 4.04, Tufts University School of Medicine, Boston, Massachusetts (Table 1).97
TABLE 1.
AMOUNTS OF OMEGA-3 FATTY ACIDS PER SERVING
| FOOD | OMEGA-3/SERVING (g) |
|---|---|
| Fish | 1.4 |
| Buffalo | n/a |
| Canola Oil | 1.3 |
| Walnuts | 2.6 |
| Greens | 0.05 |
| Eggs | 0.3 |
n/a, not available.
OMEGA-3 DIETARY SUPPLEMENTATION
Patients were given a 3-month supply (540 capsules) of either flaxseed oil capsules or placebo (olive oil)98–100 capsules. At the initiation of this study, there was no US Food and Drug Administration (FDA) recommended daily dose for omega-3 FAs or any of the essential FAs. The published proceedings of the National Heart, Lung and Blood Institute of the National Institutes of Health (1993) recommend that total doses of polyunsaturated FAs (including omega-3, omega-6, and omega-9 FAs) not exceed 10% of the total caloric intake. Further review of the safety considerations of dietary supplementation of polyunsaturated FAs70 resulted in the same conclusion. Based on a 2200-calorie diet, this would translate into a dose not exceeding 220 calories/day. Each 1-g flaxseed oil capsule contains 10 calories, so a dose of 22 capsules/day is theoretically acceptable, if this is the only source of dietary fat intake.
Manufacturers of flaxseed oil recommend taking between 1 and 6 1000-mg capsules daily. Based on literature from a variety of manufacturers, flaxseed oil is approximately 55% omega-3 FA (ALA), 15% omega-6 FA (linoleic acid), and 19% omega-9 FA (oleic acid). To observe the maximum effect, a dose of 6 1-g capsules of flaxseed each day was chosen. The total dose of omega-3 FAs given to subjects was 3.3g/day, well within the “moderate” dose range (2 to 5 g/day) of omega-3 FAs.67 Patients taking omega-3 FAs within this dose range have demonstrated no clinical evidence of an increased bleeding tendency54; however, for potential avoidance of increased risk of bleeding, patients requiring anticoagulation were excluded.
Both flaxseed and placebo (olive oil) capsules were donated by and specifically made for this study by Natrol (Chatsworth, California). Capsules were made to look alike as much as possible and were coded by content.
RANDOMIZATION AND ENSURING DOUBLE-MASK
Subject numbers were preassigned to the control or study group with the aid of the random number generator in Microsoft Excel, which randomly generated a number between 0 and 1. If the number generated for a subject number (eg, BLE-01, BLE-02) was less than 0.5, the study number was assigned to the “0” or placebo group; if the number generated for a subject number was greater than 0.5, the study number was assigned to the “1” or flaxseed group. The number “0.500000000” was never generated, and an even distribution between the groups occurred. This list was not incorporated into any documentation, and only research staff members not involved in patient care had access to these assignments.
Subjects were masked to the contents of the oil capsule. Both flaxseed and olive oil have similar properties in appearance and texture. As long as subjects did not bite open the capsules before swallowing the oil, the 2 capsules were indistinguishable from each other. Bottles were distributed to the subjects at the time of enrollment and at each visit. Subjects were instructed to begin taking study supplements the next morning so that the bottles were not opened while the subject was in the Eye Center. Subjects were instructed to return the bottles of study capsules at each 3-month visit, and any unused capsules were counted to determine patient compliance with the study protocol. Patients were also counseled to continue their daily eyelash hygiene, which involved daily eyelash shampoo with a dilute nonirritating baby shampoo on a washcloth in the shower followed by a thorough rinse with the eyes closed.
OUTCOME MEASURES
Primary outcome measures were change in tear breakup time (TBUT), meibum score, and patient symptoms (overall OSDI score) at 1 year. Remaining parameters studied are considered secondary measures.
CLINICAL EXAMINATION AND MEASURES
Patients were examined every 3 months for a total period of 1 year (Table 2). At each visit, subjects were asked to complete the OSDI, an established measure of patient dry eye symptoms,101–103 before any clinical measures were made. The OSDI was developed by Allergan Pharmaceuticals (Irvine, California) to reliably assess overall and categorized patient dry eye symptoms. Scores range from 0 to 100 for the overall score and in each category. A score of 0 to 12 indicates a normal eye, 13 to 22 mild dry eye, 23 to32 moderate dry eye, and over 33 indicates severe dry eye. It should be noted that a decrease in OSDI score indicates an improvement.
TABLE 2.
SCHEDULE OF EXAMINATION FREQUENCY AND SPECIMEN COLLECTION
| PROCEDURE | BASELINE (DAY 0) | 3 MONTHS | 6 MONTHS | 9 MONTHS | 12 MONTHS |
|---|---|---|---|---|---|
| Clinical exam | X | X | X | X | X |
| Meibum collection | X | X | X | ||
| Blood collection | X | X | X | X |
At the initial and final visit patients had a complete eye examination, including a dilated retinal and optic nerve examination. Objective clinical measures included tear production (Schirmer I with anesthesia); tear film stability (fluorescein TBUT), ocular surface health (fluorescein and rose bengal surface staining), and meibomian gland health (appearance and number of gland orifices, quality of meibum). The Schirmer I test was performed with anesthesia. At least 3 drops of topical anesthetic (Proparacaine Hydrochloride Ophthalmic Solution 0.5%; Akorn, Inc, Buffalo Grove, Illinois) were administered to the conjunctiva and both lid margins, to obtain the anesthesia of all the ocular structures. Schirmer strips were placed on the lower lid 2 mm lateral to the lateral canthus.104,105 Patients sat in the dark with both eyes closed for 5 minutes. The strips were removed and a measurement (in millimeters) of the wet area of the strip was made.
Fluorescein TBUT was determined following the procedure suggested by Lemp.103 A fluorescein strip (Haag-Streit AG, Köniz, Switzerland) was dampened with a drop of nonpreserved saline solution, and the strip was touched to the inferior palpebral conjunctiva. Patients were asked to blink several times to mix the fluorescein with the tear film. They were asked to open their eyes and not blink, and the time between the opening of the eyes and the appearance of the first dry spot was measured 3 times in seconds. The average of the 3 measurements was recorded as the final TBUT.
Fluorescein ocular surface staining was scored using the standardized methods recommended by the National Institutes of Health Symposium on Dry Eye (Figure 3) following the measurement of the TBUT.103 Briefly, the corneal fluorescein stain was evaluated 3 minutes after fluorescein instillation, by observing the cornea through a cobalt blue light. Corneal staining was graded using a scale of 0 to 3 (absent to diffuse) and recorded for the 5 corneal regions (Figure 3A). The final score was determined by totaling all individual scores for each eye.
FIGURE 3.
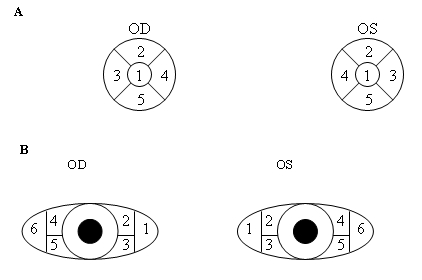
A, The 5 regions of the cornea evaluated for fluorescein staining. B, The 6 regions of conjunctiva evaluated for rose bengal staining.
Rose bengal vital staining was used to evaluate the health of the conjunctival epithelium. After all tests utilizing fluorescein, rose bengal staining was performed by using a Rosets Rose Bengal Ophthalmic Strip (Chauvin Pharmaceuticals Ltd, Essex, United Kingdom) wetted with sterile Proparacaine Hydrochloride Ophthalmic Solution 0.5% (Akorn, Inc, Buffalo Grove, Illinois) and allowed to sit for 3 minutes. The wetted strip was touched to the inferior bulbar conjunctiva while the patient looked up, and the lower lid was pulled away from the globe.106,107 Care was taken by the examiner to instill adequate dye (both fluorescein and rose bengal) while attempting to minimize reflex tearing.108 The conjunctival epithelium was assessed after patients were instructed to blink repeatedly to mix the rose bengal with the tear film.109 Six different areas were assessed in each eye for rose bengal staining (Figure 3B) and graded using a scale of 0 to 3 (absent to confluent areas of staining).
EXAMINATION AND CHARACTERIZATION OF MEIBUM AND MEIBOMIAN GLANDS
Meibomian gland health and meibum character were determined by assessing the percentage of gland blockage (orifice visible but no meibum expressible) and stenosis (orifice not visible, no meibum expressible) and of grading gross meibum character (Table 3). The lid margin, lashes, and meibomian glands were examined with a Haag Streit BQ Slit Lamp Biomicroscope BQ 900 (Koeniz, Switzerland). The presence or absence of collarettes, madarosis, and dystichiasis was noted. Lid margin telangiectasias were graded on a scale of 1 to 4. The number of meibomian glands were counted on both the upper and lower lid margin using transillumination (meiboscopy).7,110,111 The percentage of meibomian glands that were open, stenosed, or blocked was assessed at each visit. Following all lid assessments, meibum was expressed112 and grossly characterized as already described in Table 3. The meibum quality score was calculated by adding the meibum color (clear, yellow, or white) score and the meibum character (fluid, thickened, granular, or toothpaste like)113 score. A total score of 1.5 was considered healthy for analysis purposes. Normal values for all tests are summarized in Table 4.
TABLE 3.
DESCRIPTION OF MEIBUM CHARACTER AND COLOR SCORES*
| CHARACTER SCORE | DESCRIPTION |
|---|---|
| 0 | Fluid |
| 1 | Thickened |
| 2 | Granular (particulates visible) |
| 3 | Toothpaste-like (consistency of old toothpaste) |
| COLOR SCORE | |
| 0 | Clear |
| 0.5 | Yellow |
| 1 | White |
Total meibum score was obtained by adding the two component scores. A score of 1.5 was considered healthy for analysis purposes.
TABLE 4.
DESCRIPTION OF OBJECTIVE CLINICAL MEASURES OF MEIBUM AND MEIBOMIAN GLANDS
| MEASURE | DESCRIPTION | NORMAL VALUE |
|---|---|---|
| Schirmer test | Volume of aqueous tears produced (mm) | >10 mm |
| Tear breakup time | Time that tear film stays intact between blinks(s) | 10 seconds |
| Fluorescein | Staining on corneal surface indicates sick/dying cells (score 0–15) | 0 |
| Rose bengal | Staining on conjunctiva indicates sick/dying cells (score 0–18) | 0 |
| Meibum character | 0 = fluid, 1 = thickened, 2 = granular, 3 = toothpaste-like | 0 |
| Meibum color | 0 = clear, 0.5 = yellow, 1.0 = white | 0 |
| Meibum quality | Meibum character + meibum color (score 0–4) | 0 |
| Blocked meibomian glands | Glands visible on eyelid, but no meibum can be expressed (%) | 0 |
| Stenosed meibomian glands | Gland opening narrowed (%) | 0 |
| Visible ducts | Total number of visible ducts on the upper and lower eyelids | 50–70 ducts |
BLOOD AND MEIBUM SAMPLE COLLECTION AND ANALYSIS
Meibomian gland secretions were expressed and collected at baseline (day 0) and every 6 months (month 6, month 12) with a Kimura spatula (Bausch & Lomb, Rochester, New York) after digital pressure was applied to the eyelid margin. The Kimura spatula was gently applied to the lid margin to lift the meibum off the tear meniscus. Samples were immediately dissolved in 2:1 chloroform-to-methanol solution contained in clean borosilicate glass ampules to avoid sample contamination. Dissolved samples were immediately placed in a deep freezer (−80°F) and stored there until the time of shipping for sample analysis.
Blood samples (5 mL) were collected from the antecubital vein in an EDTA (lavender) topped tube at day 0 and months 3, 6, and 12, and immediately following collection, blood was centrifuged for 10 minutes at a speed of 2500 rpm to separate the plasma from the RBCs. Plasma was completely drawn off and pipetted into clean plastic 1-mL centrifuge tubes. Red blood cells were triple-washed with sterile isotonic saline solution (sodium chloride 0.9%; Hospira, Inc, Lake Forest, Illinois) and pipetted into clean plastic 1-mL centrifuge tubes. Immediately following preparation, samples were frozen and stored in a −80°F freezer until the time of shipping.
All meibum, plasma, and washed RBC samples were shipped on dry ice to the laboratory of Bruce A. Watkins, PhD, Purdue University, Department of Food Science, Lipid Chemistry and Molecular Biology Laboratory, where samples were analyzed by gas chromatography for lipid content. Chromatographs were interpreted for percent content by laboratory personnel at Purdue University and compiled into a summary table. Gas chromatography results were sent back to the Evanston Northwestern Healthcare Ophthalmology Research Center, where they were statistically analyzed for differences between groups and time points and examined for trends.
STATISTICAL ANALYSIS
Thirty-eight patients were enrolled in this study, with 30 reaching the primary end point of 1 year (12 months). Data were entered into a Microsoft Access database created specifically for this study and exported to Excel for analysis. Data are presented as mean ± standard deviation. Means within groups (eg, changes from baseline) were compared using paired Student t tests; means between groups (eg, flaxseed vs olive oil groups) were compared using unpaired Student t tests. Trends in data were tested for significance using single-variable regression analyses, and changes in frequency were examined using the Fisher exact test for small cell sizes. Owing to the limited amount of available data, statistical significance was defined as P≤.10.
RESULTS
Total study enrollment was 38 patients with 30 subjects reaching the primary end point of 12 months. Because blepharitis, MGD, and dry eye symptoms are often seasonal, it was important to have our primary end points at 1 year to reduce the influence of seasonal variation on results. Seven patients were lost to followup, and one patient was removed from the study because of a new diagnosis of Sjogren syndrome. All subjects reaching 1 year were extremely compliant with study medication, averaging a consumption of 5.1 ± 0.9 capsules/day (n = 14 patients, 85% daily compliance) and 4.4 ± 1.2 (n = 16 patients, 73% daily compliance), as determined through returned capsules and patient diaries. This difference was not statistically significant (P = .23).
PATIENT CHARACTERISTICS AT BASELINE
Patient characteristics were similar in both groups at baseline. Table 5 summarizes both the characteristics of study groups at baseline and the statistical comparison of both groups. Groups were statistically equal in all respects, with the exception that the flaxseed oil group had more visible meibomian gland orifices and patients in the flaxseed group were slightly younger at baseline compared to the olive oil group. Also, OSDI scores for the flaxseed group were in the severe range (>23), whereas the olive oil group were in the moderate range, although the differences in the mean scores between the groups were not significantly different. The average patient enrolled in this study was a white woman approximately 50 years of age, with low Schirmer scores, low TBUT, moderate surface staining, and significant blockage/stenosis of the meibomian glands. Meibum was thickened, generally rated as thickened or granular in consistency and yellow. OSDI symptoms were rated severe in the flaxseed oil group at baseline and moderate in the olive oil group; the other OSDI parameters were equal at baseline. All groups were statistically the same in terms of the clinical presentation of their blepharitis at baseline (Table 5). The distribution of healthy and unhealthy parameters (Table 6) was also examined at baseline in the group of patients reaching the 1-year end point.
TABLE 5.
PATIENT CHARACTERISTICS AND CLINICAL PRESENTATION AT BASELINE
| VARIABLE | FLAXSEED GROUP | OLIVE OIL GROUP |
|---|---|---|
| n (patients/eyes) | 18/36 | 20/40 |
| Patient age (yr) | 46.9 ± 8.6 | 54.5 ± 9.5 |
| Sex (M/F) | 4/14 | 2/18 |
| Intraocular pressure (mm Hg) | 13.7 ± 2.8 | 13.6 ± 3.8 |
| Schirmer score (mm) | 5.06 ± 3.76 | 7.14 ± 4.66 |
| Tear breakup time (s) | 5.6 ± 2.6 | 5.6 ± 3.4 |
| Fluorescein staining (0 to 15) | 2.2 ± 2.9 | 1.5 ± 1.5 |
| Rose bengal staining (0 to 18) | 2.3 ± 2.9 | 4.8 ± 6.3 |
| Meibum quality (score 0 to 4) | 2.5 ± 0.9 | 2.1 ± 1.0 |
| Meibomian gland blockage (%) | 48.3 ± 31.4 | 43.8 ± 32.8 |
| Meibomian gland stenosis (%) | 31.9 ± 32.9 | 25.5 ± 28.7 |
| Number visible ducts | 56.2 ± 6.1 | 47.9 ± 17.4 |
| OSDI overall score (0 to 100) | 26.2 ± 16.7 | 20.0 ± 12.5 |
| OSDI environmental trigger(0 to 100) | 33.3 ± 35.4 | 35.8 ± 26.7 |
| OSDI ocular symptoms (0 to 100) | 33.3 ± 23.2 | 21.7 ± 10.5 |
| OSDI visual symptoms (0 to 100) | 19.0 ± 17.9 | 11.3 ± 10.4 |
OSDI, Ocular Surface Disease Index.
TABLE 6.
DISTRIBUTION OF CLINICAL MEASURES CHARACTERIZED AS HEALTHY AND NONHEALTHY IN EACH STUDY GROUP AT BASELINE
| MEASURE | NUMBER OF HEALTHY EYES | NUMBER OF NONHEALTHY EYES | P VALUE* |
|---|---|---|---|
| Tear breakup time | .32 | ||
| Flaxseed oil | 6 | 22 | |
| Olive oil | 2 | 30 | |
| Schirmer test (mm) | .14 | ||
| Flaxseed oil | 6 | 22 | |
| Olive oil | 16 | 16 | |
| Fluorescein staining | 1.0 | ||
| Flaxseed oil | 22 | 6 | |
| Olive oil | 24 | 8 | |
| Rose bengal staining | .44 | ||
| Flaxseed oil | 22 | 6 | |
| Olive oil | 20 | 12 |
Fisher exact test.
DIETARY INTAKE EVALUATION
Dietary intake did not significantly change between baseline and 1 year in either the flaxseed or the olive oil group. Additionally, dietary intake was not significantly different between the groups at any time point measured (Table 7). One patient, who was lost to followup, refused to answer questions regarding dietary intake (n = 17 patients in flaxseed group in intent-to-treat analysis instead of 18). Our analysis included measurements of omega-3 dietary intake exclusively.
TABLE 7.
AVERAGE DIETARY INTAKE OF OMEGA-3 FATTY ACIDS
| MEASURE | INTENT-TO-TREAT ANALYSIS
|
MEASURE | ONLY PATIENTS REACHING 12 MONTHS
|
||||
|---|---|---|---|---|---|---|---|
| FLAXSEED OIL GROUP | OLIVE OIL GROUP | FLAXSEED VS OLIVE OIL GROUP P value | FLAXSEED OIL GROUP | OLIVE OIL GROUP | FLAXSEED VS OLIVE OIL GROUP P value | ||
| n (pts) | 17 | 20 | — | n (pts) | 14 | 16 | — |
| Baseline | 5.61 | 9.34 | .530 | Baseline | 4.84 | 8.57 | .581 |
| SD | 7.12 | 15.02 | — | SD | 7.32 | 15.94 | — |
| 1 year | 5.42 | 12.43 | .366 | 1 year | 4.62 | 12.43 | .392 |
| SD | 7.33 | 20.15 | — | SD | 7.53 | 22.13 | — |
| P value | 0.534 | 0.202 | — | P value | 0.540 | 0.206 | — |
| Change at 1 year | –0.19 | 1.04 | .894 | Change at 1 year | –0.22 | 12.43 | .156 |
| SD | 0.84 | 25.75 | — | SD | 0.90 | 22.13 | — |
| Significance from 0 | 0.534 | 0.901 | — | Significance from 0 | 0.540 | 0.134 | — |
SD, Standard deviation.
CHANGES IN SYSTEMIC FATTY ACIDS AT 1 YEAR
Patients in the flaxseed oil group had a significant increase in omega-3 FA levels at 1 year compared to baseline in both plasma (from 3.21 ± 0.96% to 4.88 ± 1.34%, n = 14 patients, P = .01) and RBCs (from 4.34 ± 1.43% to 6.08 ± 2.17%, n = 14 patients, P = .05). In the flaxseed oil group there was a 52% increase in the omega-3 FA level in the plasma and a 40% increase in the RBCs. Subjects in the olive oil group showed no significant changes in the omega-3 FA content in their plasma or RBCs at any time point studied. These results confirm that we were able to induce a systemic change in the omega-3 FA levels with dietary supplementation. Still, it is the omega-6 to omega-3 FA ratio that is biochemically important, because omega-6 FAs lead to higher levels of inflammation while omega-3 FAs lead to lower levels of inflammation due to competitive inhibition. At 1 year, the flaxseed group had significantly lower omega-6 to omega-3 FA ratios in both plasma (14.34 ± 4.96 vs 8.83 ± 2.79, P = .01) and RBCs (6.20 ± 2.52 vs 4.28 ± 1.82, P = .10) compared to baseline, whereas the olive oil group demonstrated no change (10.50 ± 4.51 vs 11.35 ± 3.27, P = .34). The omega-6 to omega-3 ratio decreased 36% in the plasma and 31% in the RBCs of the flaxseed oil group. No significant changes were observed in either group for saturated FA, total monounsaturated FA, or polyunsaturated FA content.
CHANGES IN OBJECTIVE CLINICAL MEASURES AT 1 YEAR
Following 1 year of supplementation with study capsules, the flaxseed group showed a marked improvement in fluorescein TBUT by 4.7 ± 3.7 seconds (n = 28 eyes, P = .01) to 7.0 ± 3.6 seconds, indicating an improvement in tear film stability. Schirmer test scores also improved by 1.82 ± 4.27 mm; however, this increase was not statistically significant (P = .14). Ocular surface fluorescein and rose bengal staining scores did not change. The olive oil group demonstrated similar results to the flaxseed group with an improvement in TBUT of 3.9 ± 4.6 seconds (n = 32 eyes, P = .1) to 9.2 ± 4.7 seconds, also indicating an improvement in tear film stability. Schirmer test scores improved by approximately 2 mm in each group; however, this increase was not statistically significant (P = .19). Interestingly, fluorescein scores did improve (P = .09) in the olive oil group. Rose bengal staining scores showed no significant change. Measures of lid hygiene moderately improved in the flaxseed group over the course of the study. The number of telangiectasias at the lid margin and collarettes decreased significantly in the flaxseed oil group (P = .01 and P = .03, respectively) and not in the olive oil group, as summarized in Table 8.
TABLE 8.
CHANGE FROM BASELINE IN OBJECTIVE CLINICAL MEASURES AT 1 YEAR FOR EACH STUDY GROUP
| MEASURE | FLAXSEED OIL GROUP | P VALUE* | OLIVE OIL GROUP | P VALUE* |
|---|---|---|---|---|
| n (eyes) | 28 | — | 32 | — |
| Schirmer score (mm) | 1.82 ± 4.27 | .14 | 2.16 ± 6.28 | .19 |
| Tear breakup time | 4.7 ± 3.7† | .01 | 3.9 ± 4.6† | .01 |
| Fluorescein staining (0 to 15) | 0.8 ± 2.0 | .80 | –0.9 ± 1.9 | .09 |
| Rose bengal staining (0 to 18) | –1.1 ± 2.8 | .15 | –1.4 ± 4.5 | .24 |
| Telangiectasias (0 to 4) ‡ | –1.1 ± 0.5† | .01 | –0.3 ± 1.0 | .33 |
| Collarettes (0 to 4) | –0.7 ± 1.1† | .03 | –0.6 ± 1.5 | .14 |
| Scurf (0 to 4) | 0.0 ± 0.0 | 1.0 | –0.1 ± 0.6 | .16 |
| Dystichiasis (No/Yes)§ | From 13/1 to 12/2 | .61 | From 14/2 to 13/3 | .61 |
| Madarosis (No/Yes) § | From 12/2 to 13/1 | 1.0 | From 14/2 to 15/1 | 1.0 |
P value obtained by comparison zero with paired t test for quantitative measures.
Statistically significant (P≤ .10).
Statistical significance between flaxseed and olive oil groups.
Yes/No distributions tested with Fisher exact test for small cell sizes.
CHANGES IN THE MEIBOMIAN GLANDS AND THEIR SECRETIONS AT 1 YEAR
The health of the meibomian glands and their secretions improved in both groups at the 1-year time point, as summarized in Table 9. In the flaxseed group, the average meibum quality significantly improved from granular at baseline to thickened or fluid at 1 year (n = 14 eyes, P = <.01), and meibomian gland orifice blockage decreased by 13.5 ± 29.7% (P = .10). Gland orifice stenosis tended to decrease in the flaxseed oil group, although this change was not statistically significant. Additionally, the number of visible meibomian gland ducts increased by 5.1 ± 5.7 (P < .01) in the flaxseed oil group. Results were similar in the olive oil group with a slightly smaller improvement in meibum quality and an identical improvement in gland orifice stenosis. The number of visible ducts also increased. Biochemical analysis of the meibum collected at all time points revealed no statistically significant changes in omega-3 FA, omega-6 FA, total monounsaturated FA, total polyunsaturated FA, or total saturated FA.
TABLE 9.
CHANGES IN THE MEIBOMIAN GLANDS AND MEIBUM AT 1 YEAR FOR EACH STUDY GROUP
| MEASURE | FLAXSEED OIL GROUP | P VALUE | OLIVE OIL GROUP | P VALUE |
|---|---|---|---|---|
| n (eyes) | 28 | — | 32 | — |
| Meibum quality (score 0 to 4) | –1.2 ± 1.2* | <.01 | –0.8 ± 1.2* | .02 |
| Meibomian gland blockage (%) | –13.5 ± 29.7* | .10 | –9.3 ± 23.8 | .13 |
| Meibomian gland stenosis (%) | –15.4 ± 44.2 | .21 | –15.2 ± 29.8* | .05 |
| Number visible ducts | 5.1 ± 5.7* | <.01 | 13.1 ± 21.0* | .02 |
Statistically significant changes (P ≤.10).
PATIENT SYMPTOMS AT 1 YEAR
Patients supplemented with flaxseed oil noted a decrease in their dry eye discomfort as compared to baseline. In the flaxseed oil group, the overall OSDI scores were significantly improved (P = .02), specifically those relating to ocular symptoms (P = .02) and environmental triggers (P = .04) (Table 10), at 1 year as compared to baseline. In the olive oil group, only the dry eye symptom scores showed an improvement (P < .01) at 1 year.
TABLE 10.
CHANGES IN OCULAR SURFACE DISEASE INDEX SCORES AT 1 YEAR FOR EACH STUDY GROUP
| MEASURE | FLAXSEED OIL GROUP | P VALUE | OLIVE OIL GROUP | P VALUE |
|---|---|---|---|---|
| Overall score | –11.6 ± 11.3* | .02 | –7.1 ± 12.8 | .17 |
| Environmental trigger | –16.7 ± 19.2* | .04 | –21.4 ± 42.2 | .20 |
| Ocular symptoms | –19.1 ± 19.1* | .02 | –9.5 ± 5.8* | <.01 |
| Visual symptoms | –5.4 ± 11.5 | .24 | 1.2 ± 8.6 | .72 |
Statistically significant (P < .10).
PRIMARY OUTCOME MEASURES
Primary outcome measures were change in TBUT, meibum score, and patient symptoms (overall OSDI score) at 1 year. Remaining parameters studied are considered secondary measures. An intent-to-treat analysis has been done by assuming that patients lost to follow-up had no change. For primary outcome measures (TBUT and meibum score at 1 year), Tables 11 and 12 show the 2 analyses side by side for convenience. The meibum scores in the flaxseed group changed significantly from baseline (P = .003), while a less significant change was found in the olive oil group (P = .026); however, the change in the flaxseed oil group was not significant when compared with the olive oil group (P = . 34) (Table 11). TBUT also had a significant improvement from baseline in the flaxseed oil group (P = .002), whereas a less significant change was found in the olive oil group (P = .011); however, the change in the flaxseed oil group was not significant when compared with the olive oil group (P = .79) (Table 12).
TABLE 11.
MEIBUM SCORES AT 1 YEAR
| MEASURE | INTENT-TO-TREAT ANALYSIS
|
MEASURE | ONLY EYES REACHING 12 MONTHS
|
||||
|---|---|---|---|---|---|---|---|
| FLAXSEED OIL GROUP | OLIVE OIL GROUP | FLAXSEED VS OLIVE OIL GROUP P VALUE | FLAXSEED OIL GROUP | OLIVE OIL GROUP | FLAXSEED VS OLIVE OIL GROUP P VALUE | ||
| n (eyes) | 36 | 40 | — | n (eyes) | 28 | 32 | |
| Baseline | 2.53 | 2.10 | .190 | Baseline | 2.82 | 2.13 | .035 |
| SD | 0.92 | 1.05 | — | SD | 0.82 | 0.89 | — |
| 1 year | 1.61 | 1.50 | .753 | 1 year | 1.64 | 1.38 | .496 |
| SD | 0.80 | 1.28 | — | SD | 0.91 | 1.18 | — |
| P value | 0.004 | 0.027 | — | P value | 0.002 | 0.058 | — |
| Change at 1 year | –0.92 | –0.60 | .401 | Change at 1 year | –1.18 | –0.75 | .343 |
| SD | 1.18 | 1.12 | — | SD | 1.22 | 1.21 | |
| Significance from 0 | 0.004 | 0.027 | — | Significance from 0 | 0.003 | 0.026 | |
SD, standard deviation.
TABLE 12.
TEAR BREAKUP TIME AT 1 YEAR
| MEASURE | INTENT-TO-TREAT ANALYSIS
|
MEASURE | ONLY EYES REACHING 12 MONTHS
|
||||
|---|---|---|---|---|---|---|---|
| FLAXSEED OIL GROUP | OLIVE OIL GROUP | FLAXSEED VS OLIVE OIL GROUP P VALUE | FLAXSEED OIL GROUP | OLIVE OIL GROUP | FLAXSEED VS OLIVE OIL GROUP P VALUE | ||
| n(eyes) | 36 | 40 | — | n (eyes) | 28 | 32 | |
| Baseline | 6.00 | 5.10 | .350 | Baseline | 6.00 | 4.44 | .149 |
| SD | 2.87 | 2.87 | — | SD | 3.04 | 2.73 | |
| 1 year | 9.22 | 8.05 | .579 | 1 year | 10.14 | 8.13 | .302 |
| SD | 4.93 | 4.88 | — | SD | 5.11 | 5.37 | |
| P value | 0.003 | 0.012 | — | P value | 0.002 | 0.011 | |
| Change at 1 year | 3.22 | 2.95 | .850 | Change at 1 year | 4.14 | 3.69 | .790 |
| SD | 3.95 | 4.76 | — | SD | 4.04 | 5.08 | |
| Significance from 0 | 0.003 | 0.012 | — | Significance from 0 | 0.002 | 0.011 | |
SD, standard deviation.
HEALTHY/NONHEALTHY DISTRIBUTIONS
The number of patients with healthy meibum (meibum score of ≤1.5, Table 3) in each group was examined at baseline and at 1 year. Six of 28 eyes (21%) in the flaxseed group had healthy meibum, compared to 20 of 32 eyes (62.5%) in the olive oil group at baseline. After 1 year of taking study dietary supplements, 20 of 28 eyes (71%) had healthy meibum in the flaxseed oil group, whereas in the olive oil group 24 of 32 eyes (75%) had healthy meibum. The change in the flaxseed group was statistically significant (P = .02), and the change in the olive oil group was not (P = .70). These results are summarized in Table 13.
TABLE 13.
NUMBER OF EYES WITH HEALTHY MEIBUM AT BASELINE AND 1 YEAR
| GROUP | BASELINE | 12 MONTHS | P VALUE |
|---|---|---|---|
| Flaxseed oil* | 6/28 (21%) | 20/28 (71%) | P = .02 |
| Olive oil | 20/32 (62.5%) | 24/32 (75%) | P = .70 |
Statistically significant, P < .10.
The percentage of patients with normal tear film stability (TBUT ≥10 seconds) significantly increased from 6 of 24 eyes (25%) to 20 of 28 eyes (71%) in the flaxseed group (P = .21) and 2 of 32 eyes (6%) to 12 of 32 eyes (38%) in the olive oil group (P = .04). The percentage of patients with normal tear production rates (Schirmer I) did not significantly change in either group. Also, the percentage of patients with a healthy ocular surface (as determined by fluorescein and rose bengal staining) did not significantly change, although all 4 patients in the olive oil group with an unhealthy corneal surface were deemed healthy at 1 year.
To elucidate the effect of omega-3 dietary supplementation on the meibomian glands, data were analyzed based on healthy meibum scores (≤1.5) and unhealthy meibum scores (>1.5). The characteristics between eyes, blood FA chemistry, and meibum FA chemistry were compared. At baseline, patients with healthy meibum had less meibomian gland orifice stenosis (18.3 ± 16.3% vs 38.7 ± 37.7%, P = .04), higher plasma omega-3 FA levels (4.3 ± 1.6% vs 3.4 ± 1.6, P = .08), lower plasma omega-6 to omega-3 ratios (10.8 ± 3.2 vs 14.0 ± 4.9, P = .03), and lower RBC omega-6 to omega-3 ratios (4.8 ± 1.3, vs 6.2 ± 2.4, P = .05). Meibum collected at baseline, before any interventions were made, was examined first. Interestingly, healthy meibum had a much lower saturated FA content than unhealthy meibum (5.5 ± 4.6% vs 11.0 10.6%, n = 19 samples form 19 patients, P = .04).
Data from all time points were then pooled by meibum status and again examined for differences between patients and clinical disease state. Again, healthy meibum samples were found to have a 28% lower saturated FA content than unhealthy meibum samples. Additionally, patients with healthy meibum tended to higher Schirmer scores, longer TBUT, less meibomian gland stenosis, and less surface staining. Additionally, patients with healthy meibum had lower omega-6 to omega-3 FA plasma ratios. Table 14 summarizes these results. Univariate regression analyses also indicated that meibum saturated FA levels (r2 = .06, P = .09), Schirmer scores (r2 = .09, P = .01), TBUT (r2 = .07, P = .10), meibomian gland stenosis (r2 = .11, P < .01), meibomian gland blockage (r2 = .20, P < .01), fluorescein staining (r2 = .09, P = .03), and rose bengal staining (r2 = .15, P < .01) were significantly correlated with meibum score. In all cases a better clinical test score predicted a lower (healthier) meibum score. When a multivariate regression analysis was performed, meibomian gland stenosis, meibomian gland blockage, Schirmer scores, and rose bengal surface staining were significantly correlated. Again, improvements in these tests predicted a lower (healthier) meibum score.
TABLE 14.
PATIENT CHARACTERISTICS ASSOCIATED WITH HEALTHY AND NONHEALTHY MEIBUM
| MEASURE | HEALTHY MEIBUM | NONHEALTHY MEIBUM | P VALUE |
|---|---|---|---|
| n (eyes)* | 124 | 72 | — |
| n (samples) | 66 | 40 | — |
| Plasma omega-6 to omega-3 ratio | 10.6 ± 2.9 | 11.9 ± 4.9 | .10 |
| Meibum saturated fatty acid (%) | 6.8 ± 5.0 | 9.3 ± 8.7 | .09 |
| Schirmer score (mm) | 9.0 ± 5.5 | 6.4 ± 4.2 | .01 |
| Tear breakup time (sec) | 8.1 ± 4.8 | 6.6 ± 4.0 | .10 |
| Meibomian gland stenosis (%) | 14.1 ± 11.9 | 28.9 ± 30.5 | <.01 |
| Meibomian gland blockage (%) | 30.4 ± 29.9 | 51.8 ± 33.1 | <.01 |
| Fluorescein staining | 1.1 ± 2.1 | 2.1 ± 2.3 | .03 |
| Rose bengal staining | 1.6 ± 2.6 | 4.7 ± 5.2 | <.01 |
At 3 patient visits, subjects had one eye fall into each category.
DISCUSSION
There has been increased interest over the past decade in the role dietary supplements play in the management of various disease states. Dietary supplementation with polyunsaturated FAs has been shown to affect the inflammatory pathways by shifting the balance of omega-6 to omega-3 FA ratio, thereby altering the inflammatory components of numerous diseases. The breakdown of omega-3 FAs results in products that suppress inflammation, whereas the breakdown of omega-6 FAs results in products that promote inflammation. Omega-3 and omega-6 FAs compete for the same enzymes (Figure 1) to be metabolized. Therefore, overloading the system with either omega-3 or omega-6 can shift the body’s inflammatory state through competitive inhibition, respectively.
To determine the effect of dietary supplementation with omega-3 FAs on blepharitis, MGD, and the evaporative dry eye, we conducted an institution-based, prospective randomized double-masked placebo-controlled trial where patients were randomly assigned to either the flaxseed oil (omega-3) or olive oil (placebo) group. Main outcome measures were changes in patient symptoms, clinical disease state, and meibum content at 1 year. The 1-year time-point was chosen to minimize the effects of seasonal variation on our measures, as the seasonal nature of dry eye disorders is well established.4 Plasma and RBC FAs were also measured to verify changes in systemic omega-6 to omega-3 FA ratios. By eliminating the use of other therapeutic options for blepharitis or dry eye, and having all enrolled patients perform a daily eyelash shampoo and use artificial tear supplements only on an as-needed basis, we were able to identify the effects of omega-3 FA dietary supplementation.
DIETARY SUPPLEMENTATION WITH OMEGA-3 FATTY ACIDS
At the initiation of this study, there had been no FDA recommended dose of omega-3 FAs or any of the essential FAs. The published proceedings of the National Heart, Lung, and Blood Institute of the National Institutes of Health (1993) recommend that total doses of polyunsaturated FAs (includes omega-3, omega-6, and omega-9 FAs) not exceed 10% of the total caloric intake. Review of the safety considerations of dietary supplementation of polyunsaturated FAs in the American Journal of Clinical Nutrition64 resulted in the same conclusion. Based on a 2200-calorie diet, this would translate into a dose not exceeding 220 calories/day. Each 1-g flaxseed oil capsule contains 10 calories, so a dose not exceeding 22 capsules/day would be acceptable if this were the only source of polyunsaturated fat intake.
Manufacturers of flaxseed oil recommend taking between 1 and 6 1000-mg capsules daily. Based on literature from a variety of manufacturers, flaxseed oil is approximately 55% omega-3 FA (ALA), 15% omega-6 FA (linoleic acid), and 19% omega-9 FA (oleic acid). Thus, we proposed that patients in this study take 6 1000-mg capsules of flaxseed oil each day. This would make their total dose of omega-3 FAs 3.3 g/day, well within the moderate dose range (2 to 5 g/day).67 Patients taking omega-3 FAs within this dose range have demonstrated no clinical evidence of an increased bleeding tendency.86, 87 Additionally, this dosage has affected disease changes in other groups of patients. A 1-year dietary supplementation trial with 6 g of fish oil daily in renal transplant patients demonstrated a beneficial effect on renal homodynamic, blood pressure, and improved graft survival.88 In patients with IgA nephropathy, treatment with fish oil for 2 years slowed the decrease of renal function.73 Omega-3 FAs lower plasma triglycerides and improve red cell flexibility in patients with lupus nephritis.72,89
There may be significant seasonal variation in signs and symptoms of blepharitis, MGD, and dry eyes. A 1-year supplementation was sufficient to reveal the long-term effects of flaxseed oil on MGD as dietary omega-3 polyunsaturated FAs are rapidly incorporated into the membrane phospholipids of circulating monocytes, suggesting that they are likely to have an effect on several aspects of cell function. Within 5 to 15 days, dietary supplementation with polyunsaturated FAs can be identified by changes in the concentrations in plasma and RBCs.90 Moderate dietary supplementation with omega-3 polyunsaturated FA significantly increases levels in monocytes within 2 weeks.91 The levels of EPA reached a maximum accumulation after 6 weeks of supplementation, and DHA reached a peak at 18 weeks.92 EPA returned rapidly to pretreatment levels in monocytes (although plasma levels remained significantly elevated from baseline after 24 weeks of washout), whereas DHA levels declined more slowly.89 In this study, the changes in plasma and RBC omega-3 levels were found by the first 3 months of blood collection and sustained at a steady state throughout the 12-month course.
FLAXSEED OIL, FISH OIL, AND OLIVE OIL
We elected to use flaxseed oil as a dietary supplement in this study in order to protect the subject component of the double-masking. Flaxseed oil was chosen as a supplement over fish oil because there is a perceived threat of contamination with heavy metals such as mercury in fish oil dietary supplementation.93,94 When fish oil is ingested, it is common for patients to complain of a fishy taste immediately or shortly after eructation. The limitation of using only flaxseed oil for dietary supplementation is evident in review of the omega-3 FA metabolic pathways. ALA, the only omega-3 FA contained in flaxseed oil, must first be metabolized into EPA to enter the anti-inflammatory cascade. Fish oil contains EPA and DHA, both of which can directly enter the anti-inflammatory cascade. Dietary supplementation with fish oil may therefore be more rapid and/or have a larger effect on inflammatory conditions than dietary supplementation with flaxseed oil alone. It may also be that a combination of both flaxseed and fish oils is ideal, and further testing with both supplements is necessary.
Olive oil was chosen as the placebo control because it contains no omega-3 FAs. Still, the health benefits of olive oil are well known and thought to stem from the richness of olive oil in polyphenols, powerful antioxidants and inhibitors of inflammation amplifiers. Polyphenols have been shown to be beneficial in diseases that are inflammatory in nature, such as rheumatoid arthritis.114 Olive oil contains large amounts of the omega-9 monounsaturated FA oleic acid. Oleic acid is converted to eicosatrienoic acid (ETA). ETA is converted to LTA3 which is a potent inhibitor of leukotriene B4, a powerful promoter of inflammation. Therefore, olive oil or ETA may exert an anti-inflammatory action through a mechanism that is similar to flaxseed oil, which is converted to EPA.114 In the current study, patients supplemented with olive oil also demonstrated significant improvements in their MGD and ocular-related discomfort. Tear-film stability increased, meibum quality improved, meibomian duct stenosis decreased, and the number of visible meibomian gland ducts increased.
Because olive oil is in part converted to ETA, which is more saturated than EPA, it may have greater chemical stability, which would be an advantage when using it as a dietary supplement. In addition, olive oil acts synergistically with fish oil by increasing the incorporation of omega-3 FAs into cell membranes.114 Given this knowledge, one might wonder why we chose to use olive oil as a placebo in this study. At the time of the design and execution of this study, this information was not yet available through the published medical literature. Moreover, there is no ideal placebo for inflammatory disease studies. Soy oil has a considerable amount of linolenic acid that could favor a proinflammatory state as well. Given the mechanism of action of olive oil and omega-3 FAs, it may be that the effects of the 2 oils are synergistic and that a combination therapy would be most effective, as has been demonstrated in patients with rheumatoid arthritis.114
MEN VERSUS WOMEN
Examination of the 2 study groups at baseline reveals remarkably similar characteristics; however, patients in the flaxseed oil group were slightly younger (average age, 47 years) than their counterparts in the olive oil group (55 years). Both groups contained mostly women, which is not surprising given that dry eye disorders are seen more often in women1 and that acne rosacea and keratoconjunctivitis sicca, both associated with MGD, are seen more commonly in women than in men. Though the biochemistry of inflammatory pathways of men and women is the same, the role and levels of androgens are different. As androgens play a role in both the meibomian and lacrimal gland,114,115 there may be a difference in the effect of omega-3 dietary supplementation in men vs women. Our study numbers were too low to segregate results based on sex, and further investigation is warranted.
PATIENT SYMPTOMS
At baseline, and at each study visit, all subjects were administered the OSDI. In general, the flaxseed oil group began study participation with more bothersome dry eye symptoms, although this difference was not statistically significant. The flaxseed oil group scored in the severe category for environmental triggers and ocular symptoms, in the moderate category overall, and in the mild category for visual symptoms. The olive oil group scored in the severe category for environmental triggers, in the mild category overall and for ocular symptoms, and in the normal category for visual symptoms.
At 12 months the flaxseed oil group had a statistically significant improvement in their overall OSDI scores (−11.6 points) and in both the environmental trigger (−19.2 points) and ocular symptom (−19.1 points) subcategories. We attribute these symptomatic improvements to the increased TBUT and the higher quality of meibum secretions. Interestingly, the olive oil group showed a statistically significant improvement only in ocular symptoms (−9.5 points), even though both TBUT and meibum quality also improved. It may therefore be that flaxseed oil supplementation induces ocular surface changes that were not measured here. It is not unreasonable to theorize that levels of inflammatory components of the tear film, such as prostaglandins (eg, PGE1), changed. Additionally, the actual number of inflammatory cells on the ocular surface may have changed, which is measurable by impression cytology. In future studies inclusion of these parameters may elucidate the etiology of the observed improvement in patient symptoms with omega-3 FA dietary supplementation.
At each visit during which the patient’s meibum was analyzed or characterized, the meibomian glands in all 4 lids were compressed to express the contents. This simple act of mechanical expression of the meibum every 3 months may play a role in the symptomatic relief experienced by patients in both groups. As this was part of the study protocol, this was not a variable for which we could adjust or analyze the data. In fact, it would be rather difficult to evaluate this effect in a masked fashion, as the patient is aware of the compression of the eyelid required to express the contents of the meibomian glands. As a result, it would be difficult to design a masked study in which expression of the meibomian gland is performed on one eye and not the other, since there is no substantially equivalent examination that can be performed.
Patients receiving flaxseed oil supplements noted a decrease in their dry eye discomfort as compared to baseline. In the flaxseed oil group, the overall OSDI scores were significantly improved (P = .02), specifically those relating to ocular symptoms (P = .02) and environmental triggers (P = .04) (Table 10) at 1 year as compared to baseline. In the olive oil group, only the dry eye symptom scores showed an improvement (P < .01) at 1 year.
PRIMARY OUTCOME MEASURES
Primary outcome measures were change in TBUT, meibum score, and patient symptoms (overall OSDI score) at 1 year. Remaining parameters studied were considered secondary measures. The meibum scores in the flaxseed group changed significantly from baseline (P = .003), and a less significant change was found in the olive oil group (P = .026); however, the change in the flaxseed oil group was not significant when compared with the olive oil group (P = .34). TBUT also had a significant improvement from baseline in the flaxseed oil group (P = .002), whereas a less significant change was found in the olive oil group (P = .011); however, the change in the flaxseed oil group was not significant when compared with the olive oil group (P = .79). Patients supplemented with flaxseed oil noted a decrease in their dry eye discomfort as compared to baseline. In the flaxseed oil group, the overall OSDI scores were significantly improved (P = .02), specifically, those relating to ocular symptoms (P = .02) and environmental triggers (P = .04) at 1 year as compared to baseline. In the olive oil group, only the dry eye symptom scores showed an improvement (P < .01) at 1 year .
AQUEOUS DEFICIENT VS EVAPORATIVE DISEASE IMPROVEMENTS
Given that inflammation is known to decrease aqueous tear production and that decreasing the inflammation with T-cell inhibitors (eg, cyclosporin A) increases Schirmer scores, it was surprising that we did not observe a statistically significant increase in Schirmer I scores in either study group; although each group, on average, did improve their Schirmer scores by approximately 2 mm. In both groups the Schirmer I scores were depressed at baseline (flaxseed group 5.06 ± 3.76, and olive oil group 7.14 ± 4.66), and, on average, all patients had components of both aqueous-deficient and evaporative etiology. It is bothersome to find a lack of correlation of the significant increase in the OSDI scores that describe the symptoms of the patients in the flaxseed oil group, in the face of no significant increase in the Schirmer I scores. This finding further clarifies the importance of not relying solely on one measurement in the assessment of the dry eye patient. Therefore, reliance on one dietary supplement may not be the ideal therapeutic approach.
In both groups (flaxseed and olive oil) there was a statistically significant improvement in the TBUT, meibum quality, and number of visible ducts as compared to baseline. Though we expected to find these changes in the flaxseed oil group, we were somewhat surprised to see them in the olive oil group, particularly given that no changes in systemic (plasma or RBC) FA levels were induced. As already discussed, olive oil contains relatively high amounts of ETA, which has been shown to be effective in reducing the effects of inflammatory diseases.113 If this is the case, flaxseed oil and olive oil should be synergistic therapies for dry eye disease, and combination therapies could be more effective than either oil alone.
Another interesting finding is the significant improvement in meibomian gland blockage in only the flaxseed oil group, while the improvement in meibomian gland stenosis was observed only in the olive oil group. This result again suggests that the individual benefits observed here with flaxseed and olive oil may occur through different mechanisms that both affect the inflammatory pathway. This change in the meibomian gland orifices and the ability to express the contents of the meibomian gland may indicate that the meibomian glands orifices have the ability to remodel at the lid margin. In cases of severe MGD, the goal of therapeutic intervention is to improve both the quality and quantity of the meibum present in the tear film. Initially, the meibomian gland orifices are blocked and the meibum is thickened, granular, or toothpaste-like. After effective therapeutic intervention, the meibum has changed in consistency and can be expressed from previously blocked meibomian gland orifices. Therefore, it is reasonable to theorize that there is remodeling of the meibomian glands during this process.
Our results therefore suggest that dietary supplementation with flaxseed oil (ALA) improves evaporative dry eye components. It is also possible that the long-standing inflammatory environment of these patients’ ocular surface may have caused irreversible lacrimal gland disease. A number of different measures, like the ones listed above, should be added to future trials investigating dietary supplementation and dry eye conditions. Again, further investigation with combination therapies, such as combining ALA and fish oil (EPA and DHA) or omega-3 FAs and cyclosporin A, as well as the synergism with olive oil, are warranted.
CHANGES IN SYSTEMIC FATTY ACID RATIO
Americans, in general, have higher-than-ideal omega-6 to omega-3 FA ratios. Patients in the flaxseed (omega-3) oil group had significant increases in both plasma (1.67%) and RBC (2.60%) omega-3 FA levels at 1 year compared to baseline. Still, it is the omega-6 to omega-3 FA ratio that is biochemically important, as omega-6 FAs lead to higher levels of inflammatory mediators and omega-3 FAs lead to lower levels of inflammatory mediators using the same pathway. Subjects in the olive oil group showed no significant changes. More important, this increase in omega-3 FAs in the plasma and RBCs resulted in a 36% and a 31% decrease in plasma omega-6 to omega-3 FA ratios, respectively. The fact that these ratios were lowered in conjunction with the observed improvement in dry eye symptoms and overall ocular health supports the role of systemic inflammation in the etiology of dry eye disease and MGD. These results also verify patient compliance, which was determined to be acceptable based on the tracking of study capsules
DIFFERENCES BETWEEN HEALTHY AND NONHEALTHY MEIBUM
When data were segmented into healthy meibum scores (≤1.5) and nonhealthy meibum scores (>1.5), interesting results were revealed. At baseline, patients with healthy meibum had less meibomian gland orifice stenosis (P = .04), higher plasma omega-3 FA levels (P = .08), lower plasma omega-6 to omega-3 ratios (P = .03), and lower RBC omega-6 to omega-3 ratios (P = .05). All of these differences further indicate inflammatory mediators in the etiology of MGD and the evaporative dry eye and the ability to alter the FA ratio in the serum and RBCs with dietary supplementation.
Meibum lipid content was also compared in healthy and unhealthy meibum. Lower meibum saturated FA levels (28%, P = .04) were found in the healthy meibum group. Saturated FAs, which are solid at body temperature, have higher melting points than their unsaturated FA counterparts, which are often liquid at body temperature. As meibum becomes less healthy, it also becomes more solid. Both saturated and unsaturated FAs are normally found in meibum; however, an increase in the amount of unsaturated lipids would theoretically result in a less fluid state at body temperature. Univariate regression analyses also indicated that meibum saturated FA levels (P = .09), Schirmer scores (P < .01), TBUT (P = .01), meibomian gland stenosis (P < .01), meibomian gland blockage (P < .01), fluorescein staining (P < .01), and rose bengal staining (P < .01) were significantly correlated with meibum score. In all cases a better clinical test score predicted a lower (healthier) meibum score. It is difficult to discern whether nonhealthy meibum is resulting from an unhealthy ocular surface microenvironment or the other way around. Our results do not confirm either theory. When a multivariate regression analysis was performed, meibomian gland stenosis, meibomian gland blockage, Schirmer scores, and rose bengal surface staining were significantly correlated with a lower (healthier) meibum score. The fact that the omega-6 to omega-3 ratio was not correlated with the meibum score may mean that there is a ratio threshold in which the ocular health begins to improve. In other words, there is not a graded effect as a significant correlation would indicate; rather, a threshold ratio is required for a clinical change to be identified. Further analysis of this threshold ratio is required.
In the initial study design, 2 hypotheses regarding why dietary supplementation of omega-3 FAs may alleviate blepharitis and dry eye symptoms were proposed. The first hypothesis was that overwhelming the FA metabolic pathway with omega-3 FA molecules results in competitive inhibition of omega-6 FA metabolism, reducing the overall inflammatory state of the eyelid margin and meibomian gland. As blepharitis and dry eye are thought to be inflammatory diseases, a reduction may alleviate blepharitis and dry eye–associated discomforts. The increase in the plasma and RBC concentrations of omega-3 and the decrease in the omega-6 to omega-3 ratio demonstrate that dietary supplementation with flaxseed oil, as a source of omega-3 FAs, can affect the FA metabolic pathway. The improvements found in the OSDI scores in the flaxseed oil group, as well as the decrease in lid margin telangiectasias and meibomian gland blockage, further support this hypothesis.
The second hypothesis proposed supplementing the diet with high amounts of omega-3 FAs to change the FA composition and therefore the properties of meibomian gland secretions. I theorized that this change may be beneficial in tear stabilization and may prevent the inflammation from blocking the meibomian gland ducts and meibum stagnation. There was a significant increase in the saturated FA content of the meibum in the flaxseed oil group at 1 year (P = .04); however, no change was found in the olive oil group. In both groups (flaxseed and olive oil) there was a statistically significant improvement in the TBUT, meibum quality, and number of visible ducts as compared to baseline. Though there was a slight change in the meibomian gland secretions detected, these were actually found in the meibum quality of both groups. Statistically significant findings were seen only when the data were segregated to examine the “healthy” meibum (score < 1.5)
CONCLUSION
In summary, the importance of omega-3 essential FAs in the diet is evident, as well as the need to return to a more physiologic omega-6 to omega-3 ratio of 4:1 rather than the ratio of 15–18:1 provided by current Western diets. To improve the ratio of omega-6 to omega-3 essential FAs, it is necessary to decrease the intake of omega-6 FAs from vegetable oils and to increase the intake of omega-3 FAs by using oils rich in omega-3 FAs. Experimental studies have provided evidence that dietary supplementation of omega-3 FAs modifies inflammatory and immune reactions, making omega-3 FAs potential therapeutic agents for inflammatory and autoimmune diseases. Their effects are brought about by modulation of the type and amount of eicosanoids and cytokines and by altering gene expression. To the best of my knowledge, this is the first demonstration of a change in the FA saturation content in meibum. There is a clear need for more carefully designed and controlled clinical trials in the therapeutic application of omega-3 FAs in blepharitis, MGD, and the evaporative dry eye.
My initial hypothesis was that overwhelming the FA metabolic pathway with omega-3 FA molecules results in competitive inhibition of omega-6 FA metabolism, reducing the overall inflammatory state of the eyelid margin and meibomian glands. As blepharitis, MGD, and dry eye are thought to be inflammatory diseases, a reduction in the omega-6 to omega-3 ratio may alleviate the associated discomforts patients experience from these chronic disease states. The increase in the plasma and RBC concentrations of omega-3 and the decrease in the omega-6 to omega-3 ratio that were measured in the study population demonstrate that dietary supplementation with flaxseed oil, as a source of omega-3 FAs, can affect the FA metabolic pathway. The improvements found in OSDI scores in the flaxseed oil group, as well as the decrease in lid margin telangiectasias and meibomian gland blockage, further support this hypothesis.
The second hypothesis assumed that supplementing the patients’ diets with high amounts of omega-3 FAs could change the FA composition and properties of meibomian gland secretions in patients with blepharitis and MGD. I theorized that this change may be beneficial in tear stabilization and may prevent inflammation of meibomian gland ducts and meibum stagnation. There was a significant increase in the saturated FA content of the meibum in the flaxseed oil group at 1 year (P = .04), but no change was found in the olive oil group. In both groups (flaxseed and olive oil) there was a statistically significant improvement in the TBUT, meibum quality, and number of visible ducts as compared to baseline. Though there was a slight change in the meibomian gland secretions detected, these were actually found in the meibum quality of both groups.
Statistically significant findings were seen only when the data were segregated to examine the “healthy” meibum (score < 1.5). Patients with healthy meibum had less meibomian gland orifice stenosis (P = .04), higher plasma omega-3 FA levels (P = .08), lower plasma omega-6 to omega-3 ratios (P = .03), and lower RBC omega-6 to omega-3 ratios (P = .05). Lower meibum saturated FAs levels (28%, P = .04) were found in the healthy meibum group. Saturated FAs, which are solid at body temperature, have higher melting points than their unsaturated FA counterparts, which are often liquid at body temperature. The fact that the omega-6 to omega-3 ratio was not correlated with the meibum score may mean that there is a ratio threshold in which the ocular health begins to improve. In other words, there is not a graded effect as a significant correlation would indicate; rather, a threshold ratio is required for a clinical change to be identified. Further analysis of this threshold ratio is required.
Current drug therapies have many side effects and do not treat both evaporative and aqueous deficient components of the dry eye. Nutritional supplementation with omega-3 FAs, either as an alternative or an adjunct therapy, holds great promise in the treatment of blepharitis, MGD, and the evaporative dry eye. In designing clinical interventions, genetic variation and sex-related differences in androgen levels should be taken into consideration, as the level of cytokines is, to a great extent, genetically determined, and the dose or amount of omega-3 FAs necessary to suppress the proinflammatory state may vary. This study supports the role of inflammation in the etiology of MGD and the resulting evaporative dry eye disease. It also demonstrates, for the first time scientifically, that omega-3 FA dietary supplementation can improve both ocular health and patient dry eye symptoms. Still, the number of patients examined in this study was small, and further work in a larger group of patients is warranted. Ultimately, the use flaxseed oil (ALA), fish oil (EPA and DHA), and potentially olive oil (polyphenols) may have an enhanced effect on ocular inflammation. Additional work is also needed to elucidate numerous treatment components, including the effects on meibum, inflammatory mediators in the tears, and the lacrimal gland based on sex, age, and supplement dose.
ACKNOWLEDGMENTS
Funding/Support: Supported by the Pearl Vision Foundation, Dallas, Texas; an unrestricted grant from Research for the Prevention of Blindness, Inc, to the Department of Ophthalmology, Northwestern University; and private contributions to the Ophthalmology Research Fund, Evanston Northwestern Healthcare, Division of Ophthalmology. The Natrol Corporation provided the flaxseed and olive oil capsules
Financial Disclosures: None.
Conformity With Author Information: The Institutional Review Board of Evanston Northwestern Healthcare approved this study (including study design, data collection, analysis, and informed consent process and all forms), and all study conduct was performed within the guidelines of Good Clinical Practice. Health Insurance Portability and Accountability Act regulations were followed.
Other Acknowledgments: The author wishes to acknowledge Lissa Padnik Silver, PhD, Evanston Northwestern Healthcare, for assistance in the design and conduct of the study and collection, management, analysis, and interpretation of data; Jennifer Smith, MD, Northwestern University, for assistance in design and conduct of the study and collection of the data; and Bruce Watkins, PhD, Purdue University, for assistance in management, analysis, and interpretation of the data.
REFERENCES
- 1.Brewitt H, Sistani F. Dry eye disease: the scale of the problem. Surv Ophthalmol. 2001;45(Suppl 2):S199–202. doi: 10.1016/s0039-6257(00)00202-2. [DOI] [PubMed] [Google Scholar]
- 2.Udell IJ, Nally L, Abelson M. An update on blepharitis treatment. Rev Ophthalmol. 2000:164–166. [Google Scholar]
- 3.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop Ocul Surf. 2007;2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 4.Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. 2000;19:644–649. doi: 10.1097/00003226-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Foulks GN. The correlation between the tear film lipid layer and dry eye disease. Surv Ophthalmol. 2007;52:369–374. doi: 10.1016/j.survophthal.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 6.McCulley JP, Dougherty JM, Deneau DG. Classification of chronic blepharitis. Ophthalmology. 1982;89:1173–1180. doi: 10.1016/s0161-6420(82)34669-2. [DOI] [PubMed] [Google Scholar]
- 7.Foulks GN, Bron AJ. A clinical description of meibomian gland dysfunction. Ocul Surf. 2003;1:107–126. doi: 10.1016/s1542-0124(12)70139-8. [DOI] [PubMed] [Google Scholar]
- 8.Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Cornea. 2004;2:149–164. doi: 10.1016/s1542-0124(12)70150-7. [DOI] [PubMed] [Google Scholar]
- 9.Bron AJ, Tiffany JM, Gouveia SM. Functional aspects of the tear film layer. Exp Eye Res. 2004;78:347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Ong BL, Larke JR. Meibomian gland dysfunction: some clinical, biochemical and physical observations. Opthalmic Physiol Opt. 1990;10:144–148. doi: 10.1111/j.1475-1313.1990.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 11.Doane J. Interactions of eyelids and tears in corneal wetting and the dynamics of the normal human eyeblink. Am J Ophthalmol. 1980;89:507–516. doi: 10.1016/0002-9394(80)90058-6. [DOI] [PubMed] [Google Scholar]
- 12.Shine WE, McCulley JP. Polar lipids in human meibomian gland secretions. Curr Eye Res. 2003;26:89–94. doi: 10.1076/ceyr.26.2.89.14515. [DOI] [PubMed] [Google Scholar]
- 13.Shine WE, McCulley JP. Meibomianitis: polar lipid abnormalities. Cornea. 2004;23:781–783. doi: 10.1097/01.ico.0000133995.99520.1f. [DOI] [PubMed] [Google Scholar]
- 14.Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:2272–2280. [PubMed] [Google Scholar]
- 15.McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003;1:97–106. doi: 10.1016/s1542-0124(12)70138-6. [DOI] [PubMed] [Google Scholar]
- 16.Nagymihalyi A, Dikstein S, Tiffany JM. The influence of eyelid temperature on the delivery of meibomian oil. Exp Eye Res. 2004;78:367–370. doi: 10.1016/s0014-4835(03)00197-0. [DOI] [PubMed] [Google Scholar]
- 17.Olson MC, Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens. 2003;29:96–99. doi: 10.1097/01.ICL.0000060998.20142.8D. [DOI] [PubMed] [Google Scholar]
- 18.Goto E, Endo K, Suzuki A. Tear evaporation dynamics in normal subjects and subjects with obstructive meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2003;44:533–539. doi: 10.1167/iovs.02-0170. [DOI] [PubMed] [Google Scholar]
- 19.Gilbard JP. Dry eye, blepharitis and chronic eye irritation: divide and conquer. J Ophthalmic Nurs Technol. 1999;18:109–115. [PubMed] [Google Scholar]
- 20.Gilbard JP. Dry eye disorders. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology. 2nd ed. Philidelphia: WB Saunders; 2000. [Google Scholar]
- 21.Gilbard JP, Rossi SR, Heyda GK. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology. 1989;96:1180–1186. doi: 10.1016/s0161-6420(89)32753-9. [DOI] [PubMed] [Google Scholar]
- 22.Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100:347–351. doi: 10.1016/s0161-6420(93)31643-x. [DOI] [PubMed] [Google Scholar]
- 23.Millar TJ, Tragoulias ST, Anderton PJ, et al. The surface activity of purified ocular mucin at the air-liquid interface and interactions with meibomian lipids. Cornea. 2006;25:91–100. doi: 10.1097/01.ico.0000164779.87795.3c. [DOI] [PubMed] [Google Scholar]
- 24.Foulks GN. Blepharitis: Lid Margin Disease and the Ocular Surface. New York: Spinger; 2001. pp. 39–48. [Google Scholar]
- 25.Norn M. Expressibility of meibomian secretion. Relation to age, lipid precorneal film, scales, foam, hair and pigmentation. Acta Ophthalmol (Copenh) 1987;65:137–142. doi: 10.1111/j.1755-3768.1987.tb06991.x. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch Ophthalmol. 2006;124:1286–1292. doi: 10.1001/archopht.124.9.1286. [DOI] [PubMed] [Google Scholar]
- 27.Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–1270. doi: 10.1001/archopht.1995.01100100054027. [DOI] [PubMed] [Google Scholar]
- 28.Korb DR, Scaffidi RC, Greiner JV, et al. The effect of two novel lubricant eye drops on tear film lipid layer thickness in subjects with dry eye symptoms. Optom Vis Sci. 2005;82:594–601. doi: 10.1097/01.opx.0000171818.01353.8c. [DOI] [PubMed] [Google Scholar]
- 29.Jester JV, Rajagopalan S, Rodrigues M. Meibomian gland changes in the rhino (hrrhhrrh) mouse. Invest Ophthalmol Vis Sci. 1988;29:1190–1194. [PubMed] [Google Scholar]
- 30.Goto E, Endo K, Suzuki A, et al. Improvement of tear stability following warm compression in patients with meibomian gland dysfunction. Adv Exp Med Biol. 2002;506:1149–1152. doi: 10.1007/978-1-4615-0717-8_161. [DOI] [PubMed] [Google Scholar]
- 31.Shine WE, McCulley JP. Role of wax ester fatty alcohols in chronic blepharitis. Invest Ophthalmol Vis Sci. 1993;34:3515–3521. [PubMed] [Google Scholar]
- 32.Driver P, Lemp MA. 1st ed. St Louis, MO: Mosby; 1997. Seborrhea and meibomian gland dysfunction; pp. 625–632. [Google Scholar]
- 33.Shine WE, McCulley JP, Pandya AG. Minocycline effect on meibomian gland lipids in meibomianitis patients. Exp Eye Res. 2003;76:417–420. doi: 10.1016/s0014-4835(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 34.Dougherty JM, McCulley JP, Silvany RE, Meyer DR. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Invest Ophthalmol Vis Sci. 1991;32:2970–2975. [PubMed] [Google Scholar]
- 35.Yoo SE, Lee DC, Chang MH. The effect of low-dose doxycycline therapy in chronic meibomian gland dysfunction. Korean J Ophthalmol. 2005;19:258–263. doi: 10.3341/kjo.2005.19.4.258. [DOI] [PubMed] [Google Scholar]
- 36.Solomon A, Dursun D, Liu Z. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2282–2292. [PubMed] [Google Scholar]
- 37.Pisella PJ, Brignole F, Debbasch C. Flow cytometric analysis of conjunctival epithelium in ocular rosacea and keratoconjunctivitis sicca. Ophthalmology. 2000;107:1841–1849. doi: 10.1016/s0161-6420(00)00347-x. [DOI] [PubMed] [Google Scholar]
- 38.Rubin M, Rao SN. Efficacy of topical cyclosporin 0.05% in the treatment of posterior blepharitis. J Occul Phamacol Ther. 2006;22:47–53. doi: 10.1089/jop.2006.22.47. [DOI] [PubMed] [Google Scholar]
- 39.Miljanovic B, Trivedi KA, Dana MR, et al. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–893. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oxholm P, Manthorpe R, Prause JU, et al. Patients with primary Sjögren's syndrome treated for two months with evening primrose oil. Scand J Rheumatol. 1986;15:103–108. doi: 10.3109/03009748609102073. [DOI] [PubMed] [Google Scholar]
- 41.Creuzot C, Passemard M, Viau S, et al. Improvement of dry eye symptoms with polyunsaturated fatty acids. J Fr Ophtalmol. 2006;29:868–873. doi: 10.1016/s0181-5512(06)70106-1. [DOI] [PubMed] [Google Scholar]
- 42.Calder PC. N-3 Polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–352. doi: 10.1007/s11745-003-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinna A, Piccinini P, Carta F. Effect of oral linoleic and gamma-linolenic acid on meibomian gland dysfunction. Cornea. 2007;26:260–264. doi: 10.1097/ICO.0b013e318033d79b. [DOI] [PubMed] [Google Scholar]
- 44.Simopoulos AP. The Mediterranean diets: what is so special about the diet of Greece? The scientific evidence. J Nutr. 2001;131:3065–3073S. doi: 10.1093/jn/131.11.3065S. [DOI] [PubMed] [Google Scholar]
- 45.Weber PC, Fischer S, von Schack C, et al. Orlando, FL: Academic Press; 1986. Dietary omega-3 polyunsaturated fatty acids and eicosanoid formation in man; pp. 49–60. [Google Scholar]
- 46.Lewis RA, Lee TH, Austen KF. Orlando, FL: Academic Press; 1986. Effects of omega-3 fatty acids on the generation of products of the 5-lipoxygenase pathway. [Google Scholar]
- 47.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 48.Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–1024. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- 49.Robinson DR, Urakaze M, Huang R, et al. Dietary marine lipids suppress continuous expression of interleukin-1 beta gene transcription. Lipids. 1996;32:S23–31. doi: 10.1007/BF02637046. [DOI] [PubMed] [Google Scholar]
- 50.Simopoulos AP. The role of fatty acids in gene expression: health implications. Ann Nutr Metab. 1996;40:303–311. doi: 10.1159/000177929. [DOI] [PubMed] [Google Scholar]
- 51.Weber PC, Leaf A. Vol. 66. Basel: Karger; 1991. Cardiovascular effects of omega 3 fatty acids: atherosclerosis risk factor modification by omega 3 fatty acids; pp. 218–232. [PubMed] [Google Scholar]
- 52.Drevon CA, Baksaas I, Krokan H. Basel, Boston: Birkhèauser Verlag; 1993. Omega-3 fatty acids: metabolism and biological effects; p. 389. [Google Scholar]
- 53.Vassalli P. The pathophysiology of tumor necrosis factors. Ann Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 54.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 55.Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland. incidence of some chronic diseases 1950–1974. Acta Med Scand. 1980;208:401–406. [PubMed] [Google Scholar]
- 56.Dyerberg J, Bang HO. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet. 1979;2:433–435. doi: 10.1016/s0140-6736(79)91490-9. [DOI] [PubMed] [Google Scholar]
- 57.Hirai A, Hamazaki T, Terano T, et al. Eicosapentaenoic acid and platelet function in Japanese. Lancet. 1982;2:1132. doi: 10.1016/s0140-6736(80)92558-1. [DOI] [PubMed] [Google Scholar]
- 58.Black KL, Culp B, Madison D, et al. The protective effects of dietary fish oil on focal cerebral infarction. Prostaglandins Med. 1979;3:257–268. doi: 10.1016/0161-4630(79)90067-3. [DOI] [PubMed] [Google Scholar]
- 59.Culp BR, Lands WE, Lucches BR, et al. The effect of dietary supplementation of fish oil on experimental myocardial infarction. Prostaglandins. 1980;20:1021–1031. doi: 10.1016/0090-6980(80)90056-8. [DOI] [PubMed] [Google Scholar]
- 60.Prickett JD, Robinson DR, Steinberg AD. Dietary enrichment with the polyunsaturated fatty acid eicosapentaenoic acid prevents proteinuria and prolongs survival in NZB x NZW F1 mice. J Clin Invest. 1981;68:556–559. doi: 10.1172/JCI110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prickett JD, Robinson DR, Steinberg AD. Effects of dietary enrichment with eicosapentaenoic acid upon autoimmune nephritis in female NZB x NZW F1 mice. Arthritis Rheum. 1983;26:133–139. doi: 10.1002/art.1780260203. [DOI] [PubMed] [Google Scholar]
- 62.Karmali RA, Marsh J, Fuchs C. Effect of omega-3 fatty acids on growth of a rat mammary tumor. J Natl Cancer Inst. 1984;73:457–461. doi: 10.1093/jnci/73.2.457. [DOI] [PubMed] [Google Scholar]
- 63.Villani F, Comazzi R, De Maria P, Galimberti M. Effect of dietary supplementation with polyunsaturated fatty acids on bronchial hyperreactivity in subjects with seasonal asthma. Respiration. 1998;65:265–269. doi: 10.1159/000029274. [DOI] [PubMed] [Google Scholar]
- 64.Broughton KS, Johnson CS, Pace BK, et al. Reduced asthma symptoms with n-3 fatty acid ingestion are related to 5-series leukotriene production. Am J Clin Nutrition. 1997;65:1011–1017. doi: 10.1093/ajcn/65.4.1011. [DOI] [PubMed] [Google Scholar]
- 65.Hallaq H, Smith TW, Leaf A. Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc Nat Acad Sci U S A. 1992;89:1760–1764. doi: 10.1073/pnas.89.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.United States Agency for Healthcare Research and Quality Tufts-New England Medical Center . Vol. 13. Rockville, MD: Agency for Healthcare Research and Quality, US Department of Health and Human Services; 2004. Evidence-based Practice Center. Effects of omega-3 fatty acids on cardiovascular risk factors and intermediate markers of cardiovascular disease; p. 169. [Google Scholar]
- 67.Eritsland J. Safety considerations of polyunsaturated fatty acids. Am J Clin Nutr. 2000;71(Suppl):197S–201S. doi: 10.1093/ajcn/71.1.197S. [DOI] [PubMed] [Google Scholar]
- 68.McLennan PL, Abeywardena MY, Charnock JS. Influence of dietary lipids on arrhythmias and infarction after coronary artery ligation in rats. Can J Physiol Pharmacol. 1985;63:1411–1417. doi: 10.1139/y85-232. [DOI] [PubMed] [Google Scholar]
- 69.Feskens EJ, Bowles CH, Kroumhout D. Inverse association between fish intake and risk of glucose intolerance in normoglycemic elderly men and women. Diabetes Care. 1991;14:935–941. doi: 10.2337/diacare.14.11.935. [DOI] [PubMed] [Google Scholar]
- 70.Cleland LG, James MJ. Rheumatoid arthritis and the balance of dietary N-6 and N-3 essential fatty acids. Br J Rheumatol. 1997;136:513–514. doi: 10.1093/rheumatology/36.5.513. [DOI] [PubMed] [Google Scholar]
- 71.Calder PC, Zurier RB. Polyunsatered fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4:115–121. doi: 10.1097/00075197-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Clark WF, Parbtani A, Naylor CD, et al. Fish oil in lupus nephritis: clinical findings and methodological implications. Kidney Int. 1993;44:75–86. doi: 10.1038/ki.1993.215. [DOI] [PubMed] [Google Scholar]
- 73.Donadio J, Jr, Bergstralh EJ, Offord KP, et al. A controlled trial of fish oil in IgA nephropathy. Mayo Nephrology Collaborative Group. N Engl J Med. 1994;442:1194–1199. doi: 10.1056/NEJM199411033311804. [DOI] [PubMed] [Google Scholar]
- 74.Robman L, Vu H, Hodge A, et al. Dietary lutein, zeaxanthin, and fats and the progression of age-related macular degeneration. Can J Ophthalmol. 2007;42:720–726. doi: 10.3129/i07-116. [DOI] [PubMed] [Google Scholar]
- 75.Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003;121:1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124:995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- 77.Smith W, Mitchell P, Leeder SR. Dietary fat and fish intake and age-related maculopathy. Arch Ophthalmol. 2000;118:401–404. doi: 10.1001/archopht.118.3.401. [DOI] [PubMed] [Google Scholar]
- 78.Iwig M, Glaesser D, Fass U, Struck HG. Fatty acid cytotoxicity to human lens epithelial cells. Exp Eye Res. 2004;79:689–704. doi: 10.1016/j.exer.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Jacques PF, Taylor A, Moeller S, et al. Long-term nutrient intake and 5-year change in nuclear lens opacities. Arch Ophthalmol. 2005;123:517–526. doi: 10.1001/archopht.123.4.517. [DOI] [PubMed] [Google Scholar]
- 80.Leske MC, Chylack LT, He Q, et al. Antioxidant vitamins and nuclear opacities: the longitudinal study of cataract. Ophthalmology. 1998;105:831–836. doi: 10.1016/s0161-6420(98)95021-7. [DOI] [PubMed] [Google Scholar]
- 81.Lu M, Taylor A, Chylack LT, et al. Dietary fat intake and early age-related lens opacities. Am J Clin Nutr. 2005;81:773–779. doi: 10.1093/ajcn/81.4.773. [DOI] [PubMed] [Google Scholar]
- 82.Lu M, Taylor A, Chylack LT, Jr, et al. Dietary linolenic acid intake is positively associated with five-year change in eye lens nuclear density. J Am Coll Nutr. 2007;26:133–140. doi: 10.1080/07315724.2007.10719594. [DOI] [PubMed] [Google Scholar]
- 83.Hwang D. Fatty acids and immune responses—a new perspective in searching for clues to mechanism. Ann Rev Nutr. 2000;20:431–456. doi: 10.1146/annurev.nutr.20.1.431. [DOI] [PubMed] [Google Scholar]
- 84.Boerner CF. Dry eye successfully treated with oral flaxseed oil. Ocular Surg News. 2000;15:147–148. [Google Scholar]
- 85.Ambrosia RJ, Jr, Stelzner SK, Boerner CF, et al. Nutrition and dry eye: the role of lipids. Rev Refract Surg. 2002;1:29–32. [Google Scholar]
- 86.Nelson GJ, Schmidt PC, Corash L. The effect of a salmon diet on blood clotting, platelet aggregation and fatty acids in normal adult men. Lipids. 1991;26:87–96. doi: 10.1007/BF02544000. [DOI] [PubMed] [Google Scholar]
- 87.Simopoulos AP. Health effects of [omega]3 polyunsaturated fatty acids in seafoods. World Rev Nutr Dietetics. 1991;66:592. [PubMed] [Google Scholar]
- 88.Homan van der Heide JJ, Bilo HJG, Donker JM, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1993;329:729–773. [Google Scholar]
- 89.Walton AJ, Snaith ML, Locniskar M, et al. Dietary fish oil and the severity of symptoms in patients with systemic lupus erythematosus. Ann Rheum Dis. 1991;50:463–466. doi: 10.1136/ard.50.7.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Skeaff CM, Hodson L, McKenzie JE. Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. J Nutr. 2006;136:565–569. doi: 10.1093/jn/136.3.565. [DOI] [PubMed] [Google Scholar]
- 91.Gibne MJ, Hunter B. The effects of short- and long-term supplementation with fish oil on the incorporation of n-3 polyunsaturated fatty acids into cells of the immune system in healthy volunteers. Eur J Clin Nutr. 1993;47:225–259. [PubMed] [Google Scholar]
- 92.Marangoni F, Angeli MT, Colli S, et al. Changes of n-3 and n-6 fatty acids in plasma and circulating cells of normal subjects, after prolonged administration of 20:5 (EPA) and 22:6 (DHA) ethyl esters and prolonged washout. Biochem Biophys Acta. 1993;1210:55–62. doi: 10.1016/0005-2760(93)90049-f. [DOI] [PubMed] [Google Scholar]
- 93.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 94.Costa LG. Contaminants in fish: risk-benefit considerations. Arh Hig Rada Toksikol. 2007;58:367–74. doi: 10.2478/v10004-007-0025-3. [DOI] [PubMed] [Google Scholar]
- 95.Jalili M, Dehpour A. Extremely prolonged INR associated with warfarin in combination with both trazodone and omega-3 fatty acids. Arch Med Res. 2007;38:901–904. doi: 10.1016/j.arcmed.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 96.Stoll AL, Severus WE, Freeman MP, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 97.Minnesota Nutrient Data Base 4.04 Available at: http://www.tufts.edu/med/nutrition-infection/hiv/health_omega3.html.
- 98.Hawthorne AB, Daneshmend TK, Hawkey CJ, et al. Treatment of ulcerative colitis with fish oil supplementation: a prospective 12 month randomized controlled trial. Gut. 1992;33:922–928. doi: 10.1136/gut.33.7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stenson WF, Cort D, Rodgers J. Dietary supplementation with fish oil in ulcerative colitis. Ann Intern Med. 1992;116:609–624. doi: 10.7326/0003-4819-116-8-609. [DOI] [PubMed] [Google Scholar]
- 100.Lorenz R, Weber PC, Szimnau P. Supplementation with n-3 fatty acids from fish oil in chronic inflammatory bowel disease: a randomized, placebo-controlled, double-blind cross-over trial. J Intern Med. 1989;225(suppl 1):225–232. doi: 10.1111/j.1365-2796.1989.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 101.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 102.Research in dry eye: report of the research subcommittee of the International Dry Eye Workshop Ocul Surf. 2007;2007;5:179–193. doi: 10.1016/s1542-0124(12)70086-1. [DOI] [PubMed] [Google Scholar]
- 103.Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eye. CLAO. 1995;21:221–232. [PubMed] [Google Scholar]
- 104.Aragona P, Tripodi G, Spinella R, et al. Test lacrimali nella diagnostica della sindrome di Sjögren. Boll Ocul. 1998;77:13–17. [Google Scholar]
- 105.Aragona P, Cannavo SP, Borgia F, Guarneri F. Utility of studying the ocular surface study in patients with acne vulgaris treated with oral isotretinoin: a randomized controlled trial. Br J Dermatol. 2005;152:576–578. doi: 10.1111/j.1365-2133.2005.06389.x. [DOI] [PubMed] [Google Scholar]
- 106.Eliason JA, Maurice DM. Staining of the conjunctiva and conjunctival tear film. Br J Ophthalmol. 1990;74:519–522. doi: 10.1136/bjo.74.9.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nichols KK, Nichols JJ, Lynn Mitchell G. The relation between tear film tests in patients with dry eye disease. Ophthalmic Physiol Opt. 2003;23:553–560. doi: 10.1046/j.1475-1313.2003.00153.x. [DOI] [PubMed] [Google Scholar]
- 108.Nichols KK, Mitchell GL, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23:272–285. doi: 10.1097/00003226-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 109.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 110.Tomlinson A, Khanal S. Assessment of tear film dynamics: quantification approach. Ocul Surf. 2005;3:81–95. doi: 10.1016/s1542-0124(12)70157-x. [DOI] [PubMed] [Google Scholar]
- 111.Robin JB, Jester JV, Nobe J, et al. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction. A clinical study. Ophthalmology. 1985;92:1423–1426. doi: 10.1016/s0161-6420(85)33848-4. [DOI] [PubMed] [Google Scholar]
- 112.Hom MM, Silverman MW. Displacement technique and meibomian gland expression. J Am Optom Assoc. 1987;58:223–226. [PubMed] [Google Scholar]
- 113.Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease. Classification and grading of lid changes. Eye. 1991;5:395–411. doi: 10.1038/eye.1991.65. [DOI] [PubMed] [Google Scholar]
- 114.Berbert AA, Kondo CR, Almendra CL, et al. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005;21:131–136. doi: 10.1016/j.nut.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 115.Sullivan DA, Sullivan BD, Ullman MD, et al. Androgen influence on the meibomian gland. Invest Ophthalmol Vis Sci. 2000;41:3732–3742. [PubMed] [Google Scholar]