Abstract
Endurance exercise (EE) leads to beneficial alterations in skeletal muscle lipid metabolism in overweight and obese individuals; however, the mechanisms of these improvements are poorly understood. The primary goal of the current investigation was to test the hypothesis that long-term EE training (6 mo) leads to alterations in the mRNA abundance of key lipid metabolism enzymes in skeletal muscle of overweight and obese middle-aged women and men. A secondary aim of this study was to investigate the hypothesis that exercise-mediated adaptations in mRNA levels differ between women and men. The mRNA abundance of representative lipogenic and lipolytic genes from major lipid metabolism pathways, as well as representative lipogenic and lipolytic transcription factors, were determined by real-time PCR from skeletal muscle biopsies collected before and ∼24 h after the final bout of 6 mo of EE. Six months of EE led to increases in muscle lipoprotein lipase, peroxisome proliferator-activated receptor-γ coactivator-1α, carnitine palmitoyltransferase-1 β, diacylglycerol acyltransferase-1, and acid ceramidase mRNA in women, but not men. In contrast, in men, EE led to reductions in the mRNA content of the lipogenic factors sterol regulatory element binding protein-1c and serine palmitoyl transferase. These data suggest that EE-mediated alterations in the abundance of the lipid metabolism genes studied here are fundamentally different between overweight and obese middle-aged women and men. Future studies should determine whether these adaptations in mRNA levels translate into changes in protein function.
Keywords: obesity, metabolic syndrome, diabetes, insulin resistance, cardiovascular disease
endurance exercise (EE) leads to beneficial alterations in skeletal muscle lipid metabolism in young, healthy subjects. Since modifications in the abundance of mRNA plays a fundamental role in skeletal muscle adaptations to EE (10), changes in skeletal muscle lipid metabolism following EE are likely due, at least in part, to changes in the quantity of lipid metabolism mRNAs. Indeed, numerous studies have noted EE-mediated alterations in the level of lipid metabolism mRNAs in skeletal muscle of young healthy individuals (21, 26, 27, 30). While EE also improves skeletal muscle lipid metabolism in obese/overweight subjects (3, 22, 36, 37), few studies have investigated the influence of EE on lipid metabolism mRNAs in this population.
Identifying the mechanisms of improved muscle lipid metabolism in obese/overweight populations has become clinically relevant since several recent studies have linked detrimental alterations in lipid metabolism and the accumulation of lipids and reactive lipid metabolites (i.e., diacylglyceride and ceramide) in skeletal muscle to insulin resistance (1, 6, 13, 35, 39). Furthermore, studies in cultured muscle cells provide convincing evidence of mechanistic links between altered lipid metabolism, elevated reactive lipid metabolites, and insulin resistance (4, 5, 28, 31). Therefore, the broad goal of the current investigation was to test the hypothesis that EE leads to adaptations in the mRNA level of representative lipogenic and lipolytic enzymes from key lipid metabolism pathways (Fig. 1), as well as important lipogenic/lipolytic transcription factors in the skeletal muscle of overweight and obese individuals. Given the relationship between muscle lipid metabolism and insulin sensitivity (Si), we also sought to determine whether changes in mRNA abundance correlated with changes in Si.
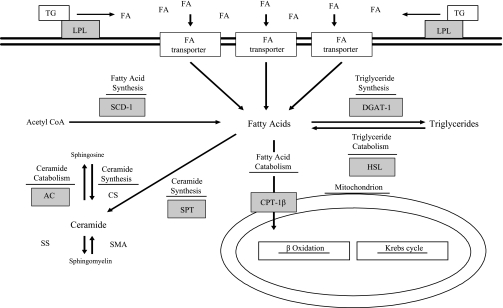
Fig. 1.
Schematic of lipogenic and lipolytic pathways. Genes assessed in the current investigation are denoted by gray boxes. TG, triglyceride; LPL, lipoprotein lipase; FA, fatty acid; SCD-1, stearoyl CoA desaturase 1; DGAT-1, diacylglycerol acyltransferase 1; HSL, hormone-sensitive lipase; CPT-1β, carnitine palmitoyltransferase-1β; SPT, serine palmitoyltransferase; AC, acid ceramidase; CS ceramide synthase; SS, sphingomyelin synthase; SMA, neutral and acidic sphingomyelinase. mRNA levels for the transcription factors sterol regulatory elements binding protein-1c (SREBP-1c) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) were also assessed but are not listed in this figure.
Studies of Targeted Risk Reduction Interventions through Defined Exercise (STRRIDE) is a randomized, controlled clinical trial designed to study the effects of three different EE training regimens on cardiovascular risk factors in middle-aged, overweight, and obese subjects. These three 6-mo EE regimens differed in quantity (kcal/wk) and/or intensity (relative VO2peak). Previous results from STRRIDE indicate that the amount and intensity of EE influence important clinical outcomes such as serum lipoprotein concentrations (33). Therefore, one specific aim of the current investigation was to determine whether the quantity and/or intensity of EE influence lipid metabolism mRNA levels. Another important issue is whether the quantity and/or intensity of EE affect the duration of mRNA changes following the cessation of exercise, as this may provide a better understanding of the duration of exercise training responses and allow potential mechanistic inferences to be made. We therefore assessed mRNA levels from skeletal muscle at four time points: 1) upon study entry, 2) after 6 mo of EE training (16–24 h after the last exercise bout), 3) 4 days after the last bout of exercise, and 4) 14 days after exercise cessation. Since we previously observed sexual dimorphism in skeletal muscle responses to EE in overweight/obese individuals (11), an additional aim of the present study was to explore the hypothesis that differences exist between women and men in EE-mediated adaptations in lipid metabolism mRNA levels.
RESEARCH DESIGN AND METHODS
Institutional review boards at Duke University and East Carolina University approved the investigation.
Subjects.
Detailed descriptions of the STRRIDE study design and methods are published elsewhere (19). In brief, subjects were 40–65 yr of age, sedentary, and overweight or obese (body mass index25–35 kg/m2) and were dyslipidemic (either high-density lipoprotein cholesterol <45 mg/dl for women or <40 mg/dl for men; or low-density lipoprotein cholesterol of 130–190 mg/dl). The subjects for the present study were selected from the ∼30 men and 30 women per group that completed the EE training and detraining time points according to availability of muscle tissue, and their characteristics are described in Table 1. The number of subjects per group is listed in the table and figure legends.
Table 1.
Subject characteristics
|
Women |
Men
|
|||||
|---|---|---|---|---|---|---|
| Pre- | Post- | Pre- | Post- | |||
| Inactive Control | Inactive Control | |||||
| Age | 55.1±1.2 | 50.4±3.1 | ||||
| % Body fat | 41.1±1.0 | 40.74±0.7 | 28.4±0.7 | 29.73±0.9 | ||
| VO2peak, ml·kg−1·min−1 | 22.79±0.9 | 23.26±0.8 | 33.06±1.2 | 32.23±1.2 | ||
| Low Volume Moderate Intensity | Low Volume Moderate Intensity | |||||
| Age | 57±1.5 | 50.71±1.3 | ||||
| % Body fat | 38.42±1.4 | 37.9±1.4 | 26.18±2.8 | 23.83±2.4 | ||
| VO2peak, ml·kg−1·min−1 | 23.26±1.2 | 24.46±1.1* | 32.14±2.2 | 34.9±1.7* | ||
| Low Volume Vigorous Intensity | Low Volume Vigorous Intensity | |||||
| Age | 56.5±1.8 | 51.25±2.7 | ||||
| % Body fat | 40.17±2.1 | 38.03±1.7 | 27.76±1.4 | 25.89±1.7 | ||
| VO2peak, ml·kg−1·min−1 | 22.54±1.0 | 25.29±1.1* | 35.16±1.0 | 38.75±1.3* | ||
| High Volume Vigorous Intensity | High Volume Vigorous Intensity | |||||
| Age | 52±1.4 | 48.78±1.2 | ||||
| % Body fat | 36.94±2.1 | 32.52±2.6 | 32.95±2.0 | 27.82±2.2* | ||
| VO2peak, ml·kg−1·min−1 | 25.89±1.3 | 29.9±1.2* | 31.3±1.2 | 38.9±1.5* | ||
Values are means ± SE. Pre- and Post-, pre- and postintervention; VO2peak, peak oxygen consumption.
Significantly different from preintervention (P < 0.05). Women: inactive control n = 10, LOW/MOD n = 9, LOW/VIG n = 9, HIGH/VIG n = 9; men: inactive control n = 9, LOW/MOD n = 7, LOW/VIG n = 7, HIGH/VIG n = 9.
Muscle biopsy.
A percutaneous needle biopsy technique was used to collect muscle biopsies from the vastus lateralis at the four time points described above. Muscle biopsies were flash frozen and stored at −80°C until use.
Exercise training.
In STRRIDE, subjects were randomly assigned to one of four different groups: 1) inactive control, 2) low volume/moderate intensity EE, 3) low volume/vigorous intensity EE, or 4) high volume/vigorous intensity exercise. Subjects participated in a 2–3 mo ramp-up period to avoid musculoskeletal injury, which was then followed by 6 mo of EE. The exercise volume/intensity of the training groups were: low volume/moderate intensity: aerobic exercise with the caloric equivalent of ∼12 mi/wk at 40–55% VO2peak; low amount/vigorous intensity: ∼12 mi/wk at 65–80% VO2peak; and high amount/vigorous intensity: ∼20 mi/wk at 65–85% VO2peak, where VO2peak was assessed after a 2 mo ramp period. The exercise regimens permitted a comparison between exercise amounts per week (low amount groups: ∼1,200 kcal/wk vs. high amount group: ∼2,000 kcal/wk) as well as exercise intensities (40–55% vs. 65–80% VO2peak). Three different modes of EE were available: cycle ergometers, treadmills, and elliptical trainers. All exercise sessions were verified by heart rate monitors that provided exercise heart rate data (Polar Electro, Woodbury, NY) and/or direct supervision.
Si.
Si was determined via intravenous glucose tolerance test as described previously (12). In brief, fasting blood samples were obtained, and 50% glucose was injected (0.3 g/kg body mass) into a catheter inserted in an antecubital vein. Blood samples were collected at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 25, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180 min, and insulin (0.025 IU/kg body mass) was injected at 20 min. The samples were centrifuged, and the plasma was frozen at −80°C. An immunoassay was used to measure insulin (Access Immunoassay System; Beckman Coulter, Fullerton, CA), and the minimal model (2) was used to calculate the insulin sensitivity index.
RNA isolation and cDNA synthesis.
Total RNA was extracted from ∼25 mg of skeletal muscle using the TRIzol method, according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). RNA quantity and quality were verified by means of spectrophotometry, and RNA was then DNase-treated (DNase I, Amplification Grade, Invitrogen) and reverse transcribed using oligo d(T) primers [High-Capacity cDNA Archive Kit; (ABI) Applied Biosystems, Foster City, CA] according to the manufacturer's specifications.
Real-time PCR and gene expression analysis.
Real-time PCR was performed using the Applied Biosystems TaqMan Two-Step protocol and the ABI Prism 7300 Sequence Detection System (ABI). ABI premade primer/probe assays were utilized for target genes (Table 2) and the 18S endogenous control. Experiments were carried out in duplicate or quadruplicate, and gene expression was calculated by the comparative CT method, using 18S as the internal control. 18S mRNA levels were unaffected by long-term EE (n = 30, data not shown).
Table 2.
Applied Biosystems primer/probe assays
| Gene | Assay ID |
|---|---|
| 18S | Hs99999901_s1 |
| CPT-1β | Hs00193219_m1 |
| SCD-1 | Hs00748952_s1 |
| DGAT-1 | Hs00201385_m1 |
| HSL (LIPE) | Hs00193510_m1 |
| AC | Hs00188842_m1 |
| SPT | Hs00272311_m1 |
| SREBP-1c | custom: SREBP 1C-1TO 2 |
| PGC-1α | Mm00447183_m1 |
| LPL | Hs00173425_m1 |
CPT, carnitine palmitoyltransferase; SCD, stearoyl CoA desaturase; DGAT, diacylglycerol acyltransferase; HSL, hormone-sensitive lipase; AC, acid ceramidase; SPT, serine palmitoyl transferase; SREBP, sterol regulatory element binding protein; PGC, peroxisome proliferator-activated receptor; LPL, lipoprotein lipase.
Statistical analysis.
To minimize skewedness, mRNA data were square root transformed prior to analysis. Inactive control group data (pre vs. post) were analyzed using paired t-tests, and exercise group data (four time points) were analyzed via one-way repeated-measures analysis of variance (ANOVA). Percent change for all four groups and percent change of control vs. “exercise” (i.e., combined exercise groups) were assessed via one-way ANOVA and unpaired t-tests, respectively. Post hoc analyses were carried out using Tukey's multiple comparison test. Sex differences for changes in gene expression were formally tested with two-way ANOVAs that included exercise or inactive group, sex, and a group × sex interaction term. However, based on our prior experiences of sex-specific skeletal muscle responses to exercise (11), we report separate analyses for women and men for all gene expression changes. Relationships between Si change and gene expression changes were analyzed with Spearman rank correlations. P < 0.05 was considered statistically significant, and data presented are means ± SE.
RESULTS
Only significant group differences and strong trends are presented here; however, all mRNA data are provided in Supplementary Tables S1–S5.1 Table 1 describes the mean age, percent body fat, and relative peak oxygen uptake (VO2peak) of the subjects, as well as changes in these measures following inactivity or EE. Importantly, EE improved VO2peak in all exercise groups (P < 0.05), indicating that EE training was effective.
Long-term EE training effects on lipid metabolism mRNA levels.
Changes in mRNA abundance are reported by sex and include relevant sex × gene expression change interaction terms for each gene.
Women.
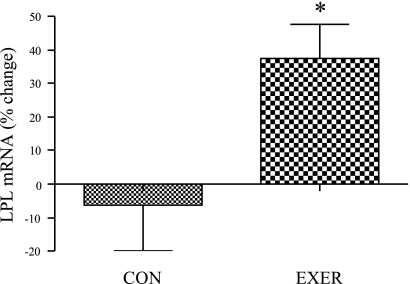
In general, EE increased the abundance of mRNAs involved in fatty acid transportation, storage, and lipolysis/oxidation in women. With respect to fatty acid transport, EE increased relative lipoprotein lipase (LPL) mRNA by ∼40% in grouped exercising women compared with inactive control women (Fig. 2, P < 0.05), and we observed a significant group × sex interaction for both absolute (P = 0.009) and relative change (P = 0.02) in LPL mRNA abundance.
Fig. 2.
Effect of endurance exercise (EE) on lipoprotein lipase (LPL) mRNA levels in women. Percent change in LPL mRNA levels in inactive control women (CON) compared with grouped exercise women (EXER) (inactive control n = 10, grouped exercise n = 30); *significantly different from inactive control women (P < 0.05). MOD, moderate exercise; VIG, vigorous exercise. Results are means ± SE.
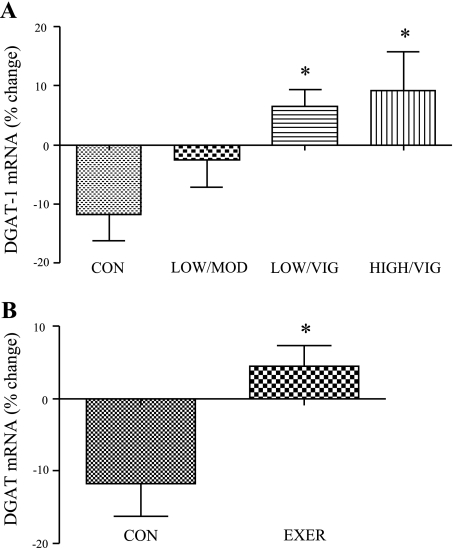
Diacylglycerol acyltransferase-1 (DGAT-1) gene expression, as a representative mRNA for fatty acid storage, was increased by ∼20 and 22% in the low/moderate and low/vigorous EE groups, respectively (P < 0.05, Fig. 3A), and DGAT-1 mRNA was significantly higher in the combined exercising women compared with the inactive control (Fig. 3B). In addition, there was a significant group × sex interaction for both absolute (P = 0.004) and relative (P = 0.003) change in DGAT-1 mRNA.
Fig. 3.
Effect of EE on DGAT-1 mRNA levels in women. A: percent change in DGAT-1 mRNA for all groups (n = 10 per group); *significantly different from inactive control women (P < 0.05). B: DGAT-1 mRNA levels in inactive control women compared with grouped exercise women (inactive control n = 10, grouped exercise n = 30); *significantly different from inactive control women (P < 0.05). Results are means ± SE.
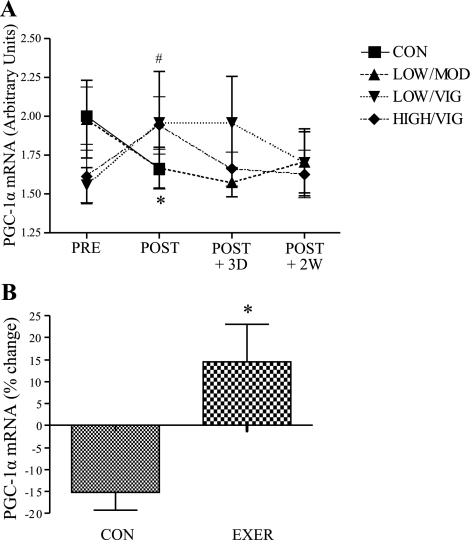
High amount/vigorous exercise increased absolute mRNA levels of a key transcription factor involved in promoting fatty acid oxidation, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), by ∼25% (P < 0.05, Fig. 4A), and there was approximately a 30% increase in PGC-1α mRNA in grouped exercising women compared with inactive control (P < 0.05, Fig. 4B). The group × sex interaction term was statistically significant for both absolute (P = 0.04) and relative (P = 0.007) change in PGC-1α gene expression.
Fig. 4.
Effect of EE on PGC-1α mRNA levels in women. A: absolute PGC-1α mRNA (inactive control n = 9, LOW/MOD n = 10, LOW/VIG n = 9, HIGH/VIG n = 8); *preintervention (PRE) significantly different from postintervention (POST) in inactive control women (P < 0.05), #preintervention significantly different from postintervention in HIGH/VIG EE women (P < 0.05). D, day; W, week. B: PGC-1α mRNA levels in inactive control women compared with grouped exercise women (inactive control n = 9, grouped exercise n = 27); *significantly different from inactive control women (P < 0.05). Results are means ± SE.
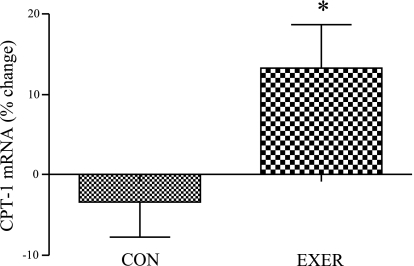
The relative mRNA abundance of carnitine palmitoyltransferase Iβ (CPT-Iβ), the rate-limiting enzyme for fatty acid transport into the mitochondria for oxidation, increased by ∼22% and 20% in low amount/vigorous intensity and high amount/vigorous intensity, respectively (P = 0.06), and CPT-Iβ gene expression was increased by ∼17% (P < 0.05, Fig. 5) in grouped exercisers vs. inactive control women. We found no statistically significant sex × group interaction for changes in expression of CPT-1β (P > 0.05).
Fig. 5.
Effect of EE on CPT-1β mRNA levels in women. CPT-1β mRNA levels in inactive control women compared with grouped exercise women (inactive control n = 10, grouped exercise n = 30); *significantly different from inactive control women (P < 0.05). Results are means ± SE.
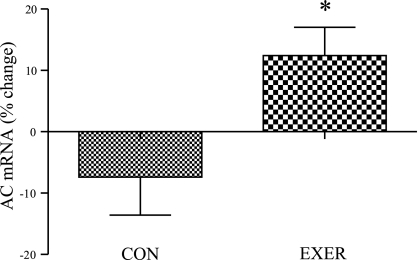
With respect to alterations in ceramide metabolism mRNAs, in grouped exercising women there was a 20% increase in acid ceramidase (AC) mRNA abundance compared with inactive control (P < 0.05, Fig. 6). We observed no significant group × sex interaction for change in AC gene expression (P > 0.05 for both relative and absolute change).
Fig. 6.
Effect of endurance exercise on AC mRNA levels in women. Percent change in AC mRNA levels in inactive control women compared with grouped exercise women (inactive control n = 10; grouped exercise n = 30); *significantly different from inactive control women (P < 0.05). Results are means ± SE.
Men.
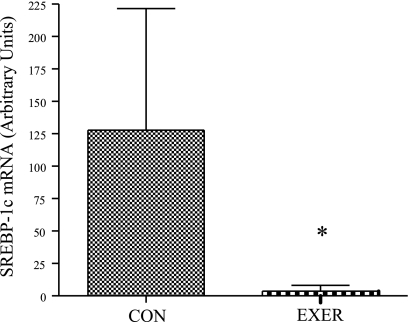
While EE-mediated alterations in mRNA content were geared toward upregulation of mRNAs encoding proteins involved in fatty acid transport, storage, and lipolysis in women, changes in obese/overweight middle-aged men favored reductions in the mRNA abundance of proteins involved in lipogenesis. The transcription factor sterol regulatory element-binding protein-1c (SREBP-1c) plays an important role in lipogenic gene expression, and absolute mRNA content for SREBP-1c increased by ∼125% in the inactive control men, though this change was not statistically significant (Supplementary Table S4). Conversely, there tended to be a reduction in SREBP-1c mRNA comparing percent change between inactive control and the three exercise groups of men (P = 0.13, Supplementary Table S4), and SREBP-1c mRNA was significantly reduced in the combined exercising men vs. inactive control men (P < 0.05, Fig. 7). There was a trend toward a significant sex × group interaction (P = 0.16).
Fig. 7.
Effect of EE on SREBP-1c mRNA levels in men. SREBP-1c mRNA levels in inactive control men compared with grouped exercise men (inactive control n = 7, grouped exercise n = 24); *significantly different from inactive control men (P < 0.05). Results are means ± SE.
There was a tendency for an increase in absolute DGAT-1 mRNA in the inactive control group (P = 0.09; Supplementary Table S3), and there was also a strong tendency for reduced DGAT-1 mRNA in the combined exercising men vs. the inactive control group (P = 0.06, Supplementary Table S3).
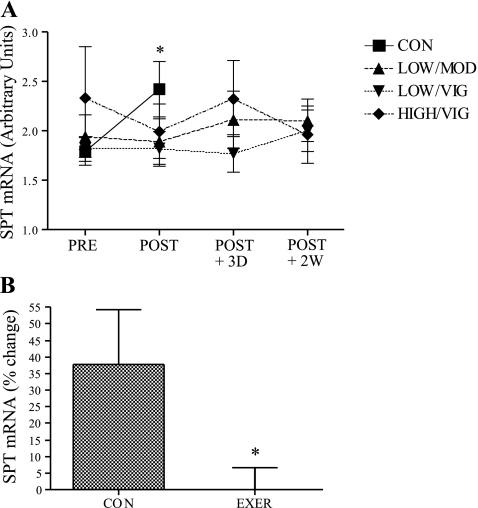
The absolute mRNA content of serine palmitoyl transferase (SPT), which plays a critical role in ceramide synthesis, was significantly increased in the inactive control men (∼ 37%) following 6 mo of inactivity (P < 0.05, Fig. 8A). Each exercise regimen appeared to prevent the increase in SPT with inactivity, although no statistical differences were observed between individual exercise groups and the inactive control group (Supplementary Table S1). When combined exercisers were compared with inactive controls, EE completely prevented the increase in SPT gene expression noted with inactivity (Fig. 8B). Additionally, a statistically significant group × sex interaction was observed for both absolute (P = 0.005) and relative (P = 0.006) change SPT gene expression.
Fig. 8.
Effect of EE on SPT mRNA levels in men. A: absolute change in SPT mRNA for all groups (inactive control n = 8, LOW/MOD n = 8, LOW/VIG n = 8, HIGH/VIG n = 9); *significantly different from preintervention (P < 0.05). B: percent change in SPT mRNA levels in inactive control men compared with grouped exercise men (inactive control n = 8, grouped exercise n = 25); *significantly different from inactive control men (P < 0.05). Results are means ± SE.
Intermuscular adipose tissue does not account for sex-specific changes in mRNA levels.
To rule out the possibility that differences in mRNA levels between women and men were due to alterations in intermuscular adipose tissue (IMAT) with EE or inactivity, we analyzed changes in mRNA abundance while controlling for IMAT change in 38 participants from the current study. We observed that controlling for IMAT change did not significantly alter our findings (data not shown). In addition, while we observed significant sex differences in intermuscular adiposity at baseline, men and women had similar reductions in intermuscular adipose tissue with exercise training (8).
Relationships between changes in mRNA levels for enzymes of lipid metabolism and changes in Si.
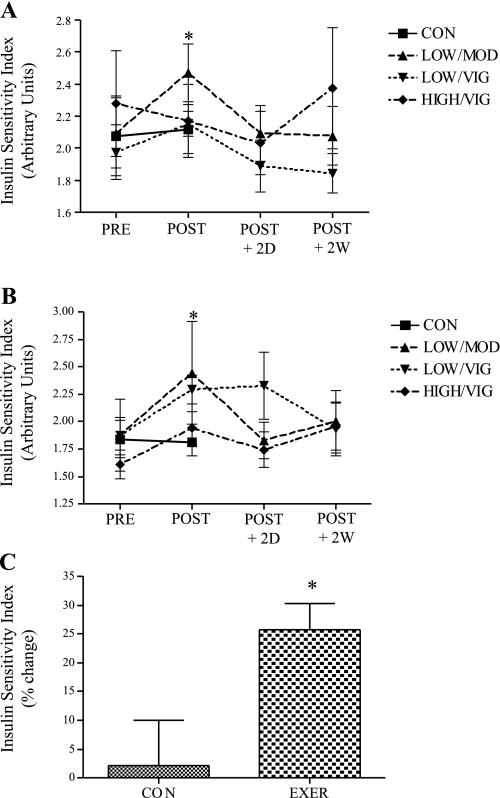
For the 74 individuals in this substudy, EE-mediated improvements in Si were similar to those previously observed in the larger STRRIDE cohort (12) with minor differences likely related to random selection of the subjects in this smaller subset. In women, low amount/moderate intensity EE improved absolute Si by ∼20% (P < 0.05, Fig. 9A). Additionally, EE reduced fasting insulin by ∼15% in grouped exercising women compared with inactive control women, and resulted in a strong trend toward group differences (P = 0.06, data not shown). As shown in Fig. 9B, in men, low amount/moderate intensity and low amount/vigorous intensity exercise improved Si by ∼30% (P < 0.05). Also, there was a strong tendency for enhanced absolute Si following high amount/vigorous intensity exercise (P = 0.051, Fig. 9B) in men. EE improved Si by ∼25% in grouped exercising men vs. inactive control men (P < 0.05, Fig. 9C). There was no significant exercise group × sex interaction for change in Si (P > 0.05).
Fig. 9.
Effect of EE on insulin sensitivity in women and men. A: absolute insulin sensitivity in women (inactive control n = 10, LOW/MOD n = 9, LOW/VIG n = 9, HIGH/VIG n = 9); *preintervention significantly different from postintervention in LOW/MOD EE women (P < 0.05). B: absolute insulin sensitivity in men (inactive control n = 9, LOW/MOD n = 6, LOW/VIG n = 7, HIGH/VIG n = 9); *significantly different from preintervention in LOW/MOD and LOW/VIG EE groups in men. C: insulin sensitivity percent change in inactive control men compared with grouped exercise men (inactive control n = 9, grouped exercise n = 22); *significantly different from inactive control men (P < 0.05). Results are means ± SE.
When evaluating relationships between change in mRNA levels and change in Si in women, we observed significant relationships for Si percent change and percent change in stearoyl CoA desaturase (SCD; P < 0.003, r = 0.47) and hormone-sensitive lipase (HSL; P < 0.048, r = 0.33). For men, we observed significant relationships for Si percent change and percent change in AC (P < 0.009, r = 0.46) and LPL (P < 0.046, r = 0.36).
DISCUSSION
To the best of our knowledge, this is the first study to comprehensively investigate the influence of long-term EE training on the abundance of important lipid metabolism mRNAs in skeletal muscle of overweight and obese middle-aged subjects who are at risk for insulin resistance and cardiovascular disease. In the present study, EE training produced adaptations in the mRNA abundance of lipid metabolism genes whose proteins products play critical roles in lipid metabolism. Most notably, the gene expression pattern induced by long-term EE training differed markedly between women and men. In general, EE training increased the mRNA content of proteins involved in fatty acid transport, storage, and oxidation in women, whereas in men EE reduced the mRNA level of important lipogenic genes. Similarly, exercise-mediated changes in the level of ceramide metabolism mRNAs differed between women and men.
Long-term EE training and changes in mRNA abundance in women.
In women, EE led to adaptations similar to those in young, healthy subjects, such as increased abundance of mRNAs for proteins involved in fatty acid transport, storage, and oxidation. EE increased LPL mRNA in women, but not in men. Skeletal muscle LPL plays an important role in muscle lipid metabolism as LPL hydrolyzes plasma triglycerides (TG) into free fatty acids, which are subsequently transported into skeletal muscle for oxidation or TG storage. Skeletal muscle-specific overexpression of LPL in rodents is associated with insulin resistance in some (9, 17), but not all studies (38). On the other hand, systematic LPL overexpression in rabbits attenuates high-fat diet-induced insulin resistance (18); and EE, which is consistently associated with enhanced Si, increases skeletal muscle LPL mRNA, protein content, and enzyme activity in healthy middle-aged men (32). The present observations suggest that EE-mediated induction of LPL protein content and enzyme activity is blunted in obese middle-aged men. However, it should be noted that LPL enzyme activity was similar in young healthy endurance-trained women and men despite greater LPL gene expression in trained women (15). Future studies should determine whether EE training leads to similar increases in muscle LPL protein and/or enzyme activity in middle-aged overweight/obese women and men.
Mitochondria from the skeletal muscle of obese individuals are smaller (14) and have reduced fatty acid oxidation capacity (16), and these perturbations are thought to contribute to the accumulation of muscle lipids and insulin resistance in the obese. Indeed, inhibition of CPT-1β, the rate-limiting enzyme in transportation of fatty acids into the mitochondria for β-oxidation, leads to insulin resistance in rodents (6), whereas CPT-1β overexpression in muscle cells prevents fatty acid-mediated insulin resistance (25). The increases in PGC-1α and CPT-1β mRNA expression in skeletal muscle of overweight and obese women in the present study are consistent with previous studies reporting enhanced muscle mitochondrial function and content following EE (3, 37). It is unclear why EE led to increased PGC-1α and CPT-1β gene expression in women, but not in men. Others have reported no difference in CPT-1β mRNA between endurance-trained and untrained men when mRNA levels were assessed 2 days after the cessation of EE (30). In contrast, PGC-1α mRNA was increased in young healthy untrained men (29) as well as middle-aged lean, obese, and type 2 diabetics (34) when assessed 1–2.5 h after EE. Thus, it is possible that the abundance of PGC-1α and/or CPT-1β mRNA were elevated at an earlier time point than measured here. Regardless, the present study identifies clear differences in EE training-mediated increases in PGC-1α and CPT-1β mRNA content between overweight/obese, middle-aged women and men, and additional studies should investigate the mechanisms and consequences of sexual dimorphisms in PGC-1α and CPT-1β gene expression in response to long-term EE training.
Recent studies indicate that skeletal muscle DGAT-1, which catalyzes TG synthesis from DAG and fatty acyl CoA, plays an important role in lipid partitioning and Si. DGAT-1 overexpression in myocytes reduces DAG (and ceramide) content, and overexpression of DGAT-1 in mouse skeletal muscle improves muscle Si and protects against high-fat diet-induced insulin resistance (20). In the present study EE increased DGAT-1 mRNA levels in women, but not in men, suggesting that the affects of EE on DGAT-1 mRNA may lead to beneficial alterations in lipid partitioning in the skeletal muscle of overweight and obese middle-aged women.
Skeletal muscle ceramide content is inversely related to Si (35), and it was recently reported that EE reduced muscle ceramide in obese subjects (3). The mechanisms of reduced ceramide have yet to be elucidated; however, experiments in muscle cells suggest that AC, which degrades ceramide to sphingosine and free fatty acid, may play an important role in regulating insulin action in muscle. For example, while lipid oversupply causes ceramide accumulation and inhibited insulin signaling in muscle cells, AC overexpression prevents lipid-mediated ceramide accretion and decreased insulin signaling (4). In the present study, long-term EE training significantly increased AC mRNA in women, suggesting that elevated AC expression may be one mechanism whereby EE reduces muscle ceramide in overweight/obese women. Additional studies are needed to further test the role of AC in the regulation of ceramide content in obese subjects.
Long-term EE training and changes in mRNA levels in men.
While many of the changes in mRNA abundance in women encode lipolytic proteins, in middle-aged obese/overweight men, EE reduced the mRNA content of key lipogenic factors. Six months of inactivity in men significantly increased skeletal muscle SPT mRNA content. Importantly, EE completely prevented elevated SPT gene expression in men. SPT, which participates in de novo ceramide synthesis (5), also appears to play an important role in muscle ceramide metabolism and insulin resistance. In cultured muscle cells, SPT inhibition prevents the lipid-mediated build-up of ceramide as well as lipid-mediated inhibition of insulin signaling (5, 28). Thus, it is reasonable to speculate that SPT may play an important role in ceramide metabolism, and potentially insulin resistance, in human subjects. These data also demonstrate that long-term EE training affects the mRNA abundance of important ceramide metabolism genes in a sex-specific manner in humans.
The transcription factor SREBP-1c stimulates fatty acid and TG synthesis via its downstream targets SCD-1, acyl-CoA carboxylase (ACC), and fatty acid synthase (FAS). The role of SREBP-1c in obesity/insulin resistance is controversial; some studies report no difference in basal levels of skeletal muscle SREBP-1c between lean control subjects and obese and diabetic subjects (7), yet others report reduced skeletal muscle SREBP-1c mRNA in morbidly obese subjects following bariatric surgery (23). In the present study, though not statistically significant, SREBP-1c mRNA increased by ∼125% in the skeletal muscle of inactive control men, and EE significantly reduced SREBP-1c gene expression in men. Interestingly, the mRNA level of another key TG synthesis gene followed a similar pattern in men, in that DGAT-1 tended to increase in control men (P = 0.09), and EE resulted in a strong tendency for reduced DGAT-1 mRNA expression (P = 0.06). Thus, elevated levels of mRNA for proteins participating in fatty acid and TG synthesis may contribute to detrimental partitioning of lipids in the direction of lipid synthesis/storage in inactive obese middle-aged men, and long-term EE prevents these perturbations.
Relationships between changes in lipid metabolism mRNAs and changes in Si.
We found limited evidence of relationships between mRNA changes and changes in Si. For women, we observed that increased expression of HSL and SCD-1 were related to improved Si. For men, we observed that increases in expression of AC and LPL were related to improvements in Si. These relationships suggest that part of the mechanism of exercise-mediated improvements in Si might be mediated through an alteration in abundance of these genes. However, we did not to observe a relationship between changes in Si and mRNA abundance for many of the genes that were altered by exercise. There are a number of potential explanations for the lack of such relationships. One possibility is that these genes may be important for lipid metabolism in skeletal muscle and that expression changes in response to exercise, but these changes have only a very small impact on Si. In light of the compelling previous data relating the impact of these gene products on Si, this seems improbable and certainly could not be proven in the context of this study. Alternatively, relationships between changes in expression of these genes and changes in Si might be confounded by other factors such as posttranscriptional regulation, genetics, and diet that could not be accounted for in this investigation. Similarly, perhaps there is a coordinated interplay of these genes, additional genes, and other contributing factors that impacts Si. Additional investigations that are designed to account for these many factors will be invaluable in continuing to better understand the mechanisms by which exercise training improves Si.
Effect of EE on gene expression 14 days after exercise cessation.
In the larger STRRIDE population we observed reduced Si 4 days after the cessation of EE training and a paradoxical increase in Si 14 days after the termination of EE (unpublished observations). To determine whether changes in lipid metabolism genes might play a role in post-EE improvements in Si, we assessed mRNA levels from muscle biopsies collected 4 and 14 days after the end of EE training. Three genes were significantly altered 14 days after the cessation of EE training in women (LPL, SPT and AC); however, none of these changes coincided with the alterations in Si observed in our previous study. Thus, it appears unlikely that the lipid metabolism genes studied herein play a role in post-EE adaptations in Si.
Study limitations.
It is important to note the limitations of the present study. First, limited muscle sample precluded the assessment of protein level and/or enzyme activity. Therefore, it is not known whether changes in mRNA abundance identified here translate into altered protein expression and/or function. However, correlations have been reported between changes in mRNA content and changes in protein content (24) or function (26) following EE. Thus, it seems plausible that the alterations in mRNA abundance identified here may contribute to important changes in muscle lipid metabolism. Second, recent research suggests that carbohydrate intake during the 24 h immediately following EE influences exercise-responsive genes (27). This factor was not controlled in the present investigation, and, in fact, subjects were instructed not to alter their dietary habits while participating in the study. However, this can also be viewed as a strength of the STRRIDE study because changes in gene expression were related to changes in exercise pattern in the absence of changes in dietary intake.
Additionally, it is worth noting that we did not detect a significant sex × group interaction for all gene expression changes presented as stratified analyses. Detecting statistically significant interaction terms requires significant power. Interestingly, despite this, we observed significant sex interactions for many of the findings presented above. While our analytic approach was based on our a priori hypothesis that responses would be unique for each sex, these significant sex interactions strengthen our findings. Most important, these observations highlight the emerging recognition that skeletal muscles response to exercise training differ by sex and emphasize the need to evaluate such sexual dimorphisms in future studies.
Conclusions
Long-term EE training influenced the mRNA abundance of key lipid metabolism genes in the skeletal muscle of middle-aged overweight and obese subjects. However, the pattern of change in gene expression differed considerably between women and men. In women, alterations in mRNA levels were consistent with adaptations observed in young, healthy subjects following EE, adaptations geared toward increased fatty acid transportation and oxidation, and TG synthesis. EE training also increased AC mRNA content in women, but not in men, suggesting that EE may play an important role in ceramide degradation in women. In sharp contrast, EE caused reductions in the expression of genes involved in lipogenesis in men, including decreased levels of SPT mRNA, which has been shown in muscle cells to play a key role in ceramide synthesis. These data suggest that long-term EE training leads to sex-specific adaptations in the expression of critical lipid metabolism genes in overweight and obese, middle-aged subjects, and future studies should determine whether changes in protein levels and/or function follow a similar pattern.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-057354 awarded to W. E. Kraus. I. J. Smith was supported by National Institute of Aging Grant 5T32 AG-000029-30.
Supplementary Material
Acknowledgments
Thanks to Milton Campbell for assistance isolating RNA and to Chris Miller with Applied Biosystems for excellent technical support.
Address for reprint requests and other correspondence: W. E. Kraus, Duke Univ. Medical Center, PO Box 3327, Durham, NC 27710 (e-mail: william.kraus@duke.edu).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Adams JM II, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev 6: 45–86, 1985. [DOI] [PubMed] [Google Scholar]
- 3.Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJF, Dyck DJ. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab 291: E99–E107, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem 280: 20148–20153, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem 278: 10297–10303, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes 50: 123–130, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Ducluzeau PH, Perretti N, Laville M, Andreelli F, Vega N, Riou JP, Vidal H. Regulation by insulin of gene expression in human skeletal muscle and adipose tissue: evidence for specific defects in type 2 diabetes. Diabetes 50: 1134–1142, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Durheim MT, Slentz CA, Bateman LA, Mabe SK, Kraus WE. Relationships between exercise-induced reductions in thigh intermuscular adipose tissue, changes in lipoprotein particle size, and visceral adiposity. Am J Physiol Endocrinol Metab 295: E407–E412, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira LD, Pulawa LK, Jensen DR, Eckel RH. Overexpressing human lipoprotein lipase in mouse skeletal muscle is associated with insulin resistance. Diabetes 50: 1064–1068, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Flück M Molecular mechanisms in muscle adaptation. Ther Umsch 60: 371–381, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Hittel DS, Kraus WE, Tanner CJ, Houmard JA, Hoffman EP. Exercise training increases electron and substrate shuttling proteins in muscle of overweight men and women with the metabolic syndrome. J Appl Physiol 98: 168–179, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol 96: 101–106, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51: 2005–2011, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Kiens B, Roepstorff C, Glatz JFC, Bonen A, Schjerling P, Knudsen J, Nielsen JN. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J Appl Physiol 97: 1209–1218, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279: E1039–E1044, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ, Breslow JL, Shulman GI. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA 98: 7522–7527, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitajima S, Morimoto M, Liu E, Koike T, Higaki Y, Taura Y, Mamba K, Itamoto K, Watanabe T, Tsutsumi K, Yamada N, Fan J. Overexpression of lipoprotein lipase improves insulin resistance induced by a high-fat diet in transgenic rabbits. Diabetologia 47: 1202–1209, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Kraus WETC, Duscha BD, Norris J, Brown SA, Cobb FR, Bales CW, Annex BH, Samsa GP, Houmard JA, Slentz CA. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE). Med Sci Sports Exerc 33: 1774–1784, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Liu LZY, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest 117: 1679–1689, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 19: 1498–1500, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Menshikova EV, Ritov VB, Ferrell RE, Azuma K, Goodpaster BH, Kelley DE. Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. J Appl Physiol 103: 21–27, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Mingrone GRG, Greco AV, Manco M, Vega N, Nanni G, Castagneto M, Vidal H. Intramyocitic lipid accumulation and SREBP-1c expression are related to insulin resistance and cardiovascular risk in morbid obesity. Atherosclerosis 170: 155–161, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Ort T, Gerwien R, Lindborg KA, Diehl CJ, Lemieux AM, Eisen A, Henriksen EJ. Alterations in soleus muscle gene expression associated with a metabolic endpoint following exercise training by lean and obese Zucker rats. Physiol Genomics 29: 302–311, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Perdomo G, Commerford SR, Richard AMT, Adams SH, Corkey BE, O'Doherty RM, Brown NF. Increased β-oxidation in muscle cells enhances insulin-stimulated glucose metabolism and protects against fatty acid-induced insulin resistance despite intramyocellular lipid accumulation. J Biol Chem 279: 27177–27186, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Pilegaard H, Saltin B, Neufer P. Exercise induces transient transcriptional activation of the PGC-1 gene in human skeletal muscle. J Physiol 546: 851–858, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pilegaard H, Osada T, Andersen L, Helge J, Saltin B, Neufer P. Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism 54: 1048–1055, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS. Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J 382: 619–629, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell AP, Hesselink MKC, Lo SK, Schrauwen P. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. FASEB J 04–3168fje, 2005. [DOI] [PubMed]
- 30.Schmitt BFM, Décombaz J, Kreis R, Boesch C, Wittwer M, Graber F, Vogt M, Howald H, Hoppeler H. Transcriptional adaptations of lipid metabolism in tibialis anterior muscle of endurance-trained athletes. Physiol Genomics 15: 148–157, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Sebastian D, Herrero L, Serra D, Asins G, Hegardt FG. CPT I overexpression protects L6E9 muscle cells from fatty acid-induced insulin resistance. Am J Physiol Endocrinol Metab 292: E677–E686, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Seip RL, Angelopoulos TJ, Semenkovich CF. Exercise induces human lipoprotein lipase gene expression in skeletal muscle but not adipose tissue. Am J Physiol Endocrinol Metab 268: E229–E236, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Slentz CA, Houmard JA, Johnson JL, Bateman LA, Tanner CJ, McCartney JS, Duscha BD, Kraus WE. Inactivity, exercise training and detraining, and plasma lipoproteins. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol 103: 432–442, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes 56: 836–848, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straczkowski MKI, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, Adamska A, Blachnio A, Gorski J, Gorska M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia 50: 2366–2373, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Toledo FGS, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship to glucose control in type 2 diabetes mellitus. Diabetes 56: 2142–2147, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Toledo FGS, Watkins S, Kelley DE. Changes induced by physical activity and weight loss in the morphology of intermyofibrillar mitochondria in obese men and women. J Clin Endocrinol Metab 91: 3224–3227, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Voshol PJ, Jong MC, Dahlmans VEH, Kratky D, Levak-Frank S, Zechner R, Romijn JA, Havekes LM. In muscle-specific lipoprotein lipase-overexpressing mice, muscle triglyceride content is increased without inhibition of insulin-stimulated whole-body and muscle-specific glucose uptake. Diabetes 50: 2585–2590, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230–50236, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.