Abstract
The consequences of viral infection during pregnancy include impact on fetal and maternal immune responses and on fetal development. Transplacental infection in cattle with noncytopathic bovine viral diarrhea virus (ncpBVDV) during early gestation results in persistently infected (PI) fetuses with life-long viremia and susceptibility to infections. Infection of the fetus during the third trimester or after birth leads to a transient infection cleared by a competent immune system. We hypothesized that ncpBVDV infection and presence of an infected fetus would alter immune response and lead to downregulation of proinflammatory processes in pregnant dams. Naïve pregnant heifers were challenged with ncpBVDV2 on day 75 (PI fetus) and day 175 [transiently infected (TI) fetus] or kept uninfected (healthy control fetus). Maternal blood samples were collected up to day 190 of gestation. Genome-wide microarray analysis of gene expression in maternal peripheral white blood cells, performed on days 160 and 190 of gestation, revealed multiple signal transduction pathways affected by ncpBVDV infection. Acute infection and presence of a TI fetus caused upregulation of the type I interferon (IFN) pathway genes, including dsRNA sensors and IFN-stimulated genes. The presence of a PI fetus caused prolonged downregulation of chemokine receptor 4 (CXCR4) and T cell receptor (TCR) signaling in maternal blood cells. We conclude that: 1) infection with ncpBVDV induces a vigorous type I IFN response, and 2) presence of a PI fetus causes downregulation of important signaling pathways in the blood of the dam, which could have deleterious consequences on fetal development and the immune response.
Keywords: interferon, CXCL12/CXCR4, T cell receptor, gene expression
bovine viral diarrhea virus (BVDV), along with classical swine fever virus and border disease virus of sheep, belongs to the genus Pestivirus in the family Flaviviridae (35, 60). Pestiviruses are small enveloped viruses with a positive single-stranded RNA genome of ∼12.5 kb in length, which encodes a single viral polyprotein, processed co- and posttranslationally by host cell- and virus-derived proteases to the mature viral proteins. BVDV strains, genotypically divided into two distinct types (type 1 and 2), are also classified by two biotypes based on their lytic activity in cell culture: cytopathic (cp) and noncytopathic (ncp) (34). Both cp and ncp BVDV strains cause similar clinical symptoms in postnatal and adult animals, including diarrhea, pyrexia, anorexia, enteric disease (13), as well as reproductive failure (36). NcpBVDV strains, in contrast to cpBVDV, are able to cause persistent infection in fetuses of cows infected during early (before day 120–150) stages of gestation (5, 10). Development of persistent infection is one of the strategies utilized by viruses to evade the immune response of the host and to establish a reservoir of infection in a population. Animals persistently infected (PI) with BVDV serve as a continuous source of the virus due to life-long shedding (24). Clinical detection of PI calves is complicated, because some PI animals may survive for years in a relatively healthy state (19), while others may succumb to superimposed infections during the first year of life. This is often caused by immunosuppression (7) or development of a fatal mucosal disease after superinfection with a homologous strain of cpBVDV (9, 49). Multiple birth defects in PI calves have also been reported (18, 62). Early diagnosis of pregnant cows carrying PI fetuses and timely elimination of the PI animal before birth would greatly benefit the programs of BVDV control. Currently, available methods of BVDV diagnostics do not distinguish cows with PI fetuses from pregnant cows that seroconverted in response to vaccination or BVDV infection.
Mechanisms of establishment of persistent infection in fetuses infected before the development of a competent immune system are not completely elucidated. Failure of ncpBVDV to induce type I interferon (IFN) and the ability of these viruses to interfere with the induction of the type I IFN response and downstream pathways, which serve as the major innate immune defense mechanism, were considered to be among the mechanisms employed by the virus to develop persistent infection (2, 16). Recent studies by our group and by others have demonstrated presence of bioactive type I IFN in serum of cows and calves transiently infected (TI) with ncpBVDV (15, 53). Dramatic upregulation of IFN-stimulated gene 15 (ISG15), a downstream member of the type I IFN signaling pathway and a marker of its upregulation, was detected in blood of ncpBVDV-infected cows during acute infection (53). We have also shown that TI fetuses of cows infected in late gestation are able to mount a robust response of bioactive type I IFN and upregulation of IFN stimulated genes (ISGs), such as ISG15, protein kinase R (PKR), 2′,5′-olygoadenylate synthetase 1 (OAS-1), and myxovirus resistance factor 2 (MX2), while PI fetuses and PI steers infected early in gestation demonstrated mild chronic upregulation of the type I IFN response (51, 53). Cytosolic dsRNA sensors retinoic acid-induced gene I (RIG-I) and melanoma differentiation-associated protein 5 (MDA-5), recently implicated in type I IFN gene regulation upon cytoplasmic dsRNA stimulation (1, 66), showed the same trend in blood of TI fetuses, PI fetuses, and PI steers (51). Delineation of other genes in the type I IFN pathway and related immunoregulatory pathways may help clarify responses to persistent vs. transient BVDV infection.
Infection with both cp and ncp biotypes of BVDV induces immunosuppression in infected cattle and results in predisposition to other pathogens (43). Multiple immunosuppressive processes have been described in BVDV infection: decreased lymphocyte proliferation (8), decreased chemokine production and chemotaxis (31, 65), decreased mononuclear cell proliferation (45), and stimulation of prostaglandin E2 production (63). However, a complete genomic screen of different signaling pathways associated with immunosuppression has not been described.
In this study we have sought to analyze immune response processes affected by ncpBVDV infection in pregnant heifers at different stages of gestation. By using a genome-wide microarray analysis we tested the hypothesis that ncpBVDV infection would alter immune response and lead to downregulation of proinflammatory processes in pregnant dams. A genome-wide microarray screen was used to compare gene expression in white blood cells of pregnant heifers carrying TI, PI, or healthy uninfected fetuses and to analyze signaling pathways affected by: 1) transient ncpBVDV2 infection of the pregnant heifer and presence of the TI fetus, 2) long-term presence of a PI fetus in the pregnant dam after seroconversion and clearance of the maternal ncpBVDV2 infection. Based on the data presented in this paper we conclude that: 1) infection with ncpBVDV induces a vigorous type I IFN response and 2) presence of a PI fetus causes downregulation of important signaling pathways in the blood of the dam, which could have deleterious consequences on fetal development and immune responses.
MATERIALS AND METHODS
Animals
All experiments using cattle were approved by the Animal Care and Use Committees at the University of Wyoming and Colorado State University. Eighteen weaned Hereford heifers not vaccinated against BVDV were screened for anti-BVDV antibodies twice and were found to be seronegative for BVDV1 and BVDV2 in standard serum neutralization assay. No BVDV virus was found in ear notch extracts using commercial ELISA (IDEXX Laboratories, Westbrook, ME). Prior to the start of the experiment all heifers were confirmed to be seronegative to bovine herpesvirus type 1 and to bovine respiratory syncytial virus. Estrous cycles were synchronized at ∼12 mo of age, and the heifers were artificially inseminated and assigned to one of the three viral treatment groups.
Viral Challenge
The heifers (n = 6 per each group) were inoculated with ncp BVDV2 strain 96B2222 (61) on day 75 (PI group) or day 175 (TI group) or kept uninfected (control) to generate PI, TI, or control uninfected fetuses, respectively, as previously described (53). In brief, each heifer of the PI and TI groups received 2 ml of viral stock aliquot at 4.4 log10 TCID50/ml intranasally. Blood samples were drawn for RNA isolation on multiple days of infection. Fetuses were recovered by cesarean section on day 190 of gestation.
RNA Isolation for Gene Microarray and RT-PCR
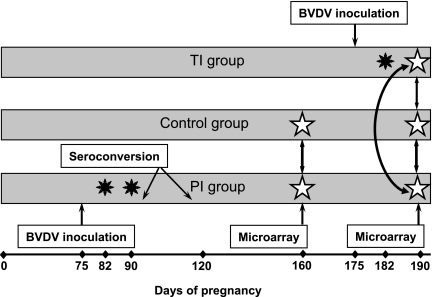
Blood samples for genome-wide microarray analysis were collected into EDTA-containing vacutainers. Red blood cells were lysed with erythrocyte lysis buffer to prevent contamination of RNA with abundant red blood cell proteins; remaining total white blood cells were collected by centrifugation and processed to yield total RNA using QIA shredder spin column and QIAmp procedures and reagents (Qiagen, Valencia, CA) following the manufacturer's instructions. After DNase treatment and clean-up using QIAmp columns RNA was eluted in 30 μl of RNase-free water and used for the genome-wide microarray analysis by hybridization and screening of the Affymetrix bovine DNA chip at the Montana State University INBRE Functional Genomics Core Facility (Bozeman, MT). Genome-wide microarray analysis on day 160 of gestation was completed on blood samples of four control heifers and five blood samples of heifers of the PI group. Three heifers from each experimental group (TI, PI, and control) were used for the genome-wide microarray analysis on day 190 of gestation. Blood samples from heifers for both microarray experiments were selected at random from groups of six per treatment. For microarray screens, time points of infection (Fig. 1) were chosen in a way that would allow us to study pathways in blood cells of pregnant heifers affected by the presence of a PI fetus after clearance of maternal infection (day 160) and identify pathways affected by transient infection of heifers and their fetuses compared with control heifers and heifers carrying PI fetuses (day 190).
Fig. 1.
Experimental design. Heifers (n = 6 per group) were inoculated with BVDV on day 75 (PI group) and day 175 (TI group) of gestation. Control group was kept uninfected throughout the experiment. Black stars show days of viremia detected by qRT-PCR. Genome-wide microarray analysis, shown with white stars, was completed on day 160 of gestation [2-way analysis, comparing control heifers (n = 4) and heifers of the PI group, (n = 5)] and day 190 of gestation [3-way analysis, comparing heifers (n = 3 per group) of the control, TI, and PI groups] using Affymetrix bovine chips. Blood samples for RNA isolation were collected from all heifers on days 75, 78, 82, 90, 120, 160, 175, 178, 182, and 190 of gestation. BVDV, bovine viral diarrhea virus; PI, persistently infected; TI, transiently infected.
RNA concentrations and purity were determined by measuring absorbances at 260 and 280 nm on a GeneQuant II spectrophotometer (Pharmacia Biotech). RNA quality was evaluated using the RNA 6000 NanoChip assay on a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). RNA samples with 18S/28S ratio of 1.7 and A260/A280 ratio of 1.8+/−0.2 were used for the arrays. Total RNA was amplified, biotin-labeled, and hybridized to Affymetrix GeneChip Bovine Genome Arrays (#900562; Affymetrix, Santa Clara, CA) as described in the users' manual (Affymetrix GeneChip Expression Analysis Technical Manual, November 2004), using the GeneChip Expression 3′ Amplification One-Cycle Target Labeling and Control Reagents kit (#900493). Briefly, total RNA (∼3.5 μg per sample) was reverse transcribed to cDNA using a T7-oligo(dT) primer. Following second-strand cDNA synthesis, the double-stranded cDNA was purified as a template for the subsequent in vitro transcription (IVT) reaction. Linearly amplified biotin-labeled complementary RNA (cRNA) was synthesized in the presence of a biotinylated nucleotide analog/ribonucleic acid mix. The labeled cRNA was purified, fragmented, and hybridized to the arrays at 45°C for 16 h with constant rotational mixing at 60 rpm. Washing and staining of the arrays were performed using the Affymetrix GeneChip Fluidics Station 450. Arrays were scanned using an Affymetrix GeneChip Scanner 7G and GCOS software version 1.4. The Bovine Genome gene chip contains 24,027 probe sets representing >23,000 transcripts. The array was designed based on content from Bovine UniGene Build 57 (2004) and GenBank mRNAs. Each probe set on the array is represented by 11 pairs of perfect match and mismatch probes.
Total RNA for semiquantitative real-time PCR (qRT-PCR) was isolated from samples of whole blood preserved with Tri reagent BD for Blood Derivatives (Sigma-Aldrich, St. Louis, MO) as previously described (53). In brief, total RNA was isolated with Tri reagent BD and purified using RNeasy MinElute Cleanup Kit (Qiagen). Synthesis of cDNA was performed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Synthesized cDNA was diluted fivefold with RNase-free water and used for the qRT-PCR reaction.
Microarray Analysis
Overview of genome-wide microarray analysis.
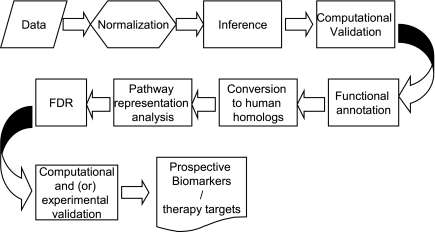
The general overview of the analysis pipeline is given in Fig. 2. Our pipeline includes most of the standard analysis steps but has a few important differences, allowing us to maximize the advantage of pathway analysis. Standard normalization procedure was used. Inference of potentially differential genes was performed using relaxed criteria. The genes important for understanding the biological processes involved in immune response were selected not solely by the difference in signal emitted by microarray probes. Instead, we concentrated on the “group behavior” of genes, their ability to interact, and preexisting annotation placing the genes into the same biological pathway and linking to the same cellular function. Thus, the inference was done with very liberal selection criteria and not adjusted for multiple testing. We selected a large list of potentially differential genes, which may contain a large number of false positives. We then selected biological pathways, molecular function, and Gene Ontology (GO) terms, which were overrepresented in the initial intensity-based list. The benefits of using pathways and ontological analyses of microarray data have been presented previously (20, 33). The significance of biological pathways was estimated through a variation of Fisher's exact test as implemented in GeneGo Metacore and adjusted for multiple testing using Benjamini-Hochberg false discovery rate (FDR) analysis (a built-in function of GeneGo Metacore software). Single genes that did not map into any statistically significant pathway (i.e., missing all regulators, downstream targets, ligands, and other components necessary for a functional molecular mechanism) may still be considered significant if reproducible and independently validated in additional experiments. Our approach relies on collective effects of the groups of genes interlinked by functional relationships, which may be inapplicable to some genes lacking information on function, regulation, and interaction with other genes. Specific biomarkers are selected among the members of statistically significant pathways and independently verified by additional RT-PCR experiments.
Fig. 2.
Overview of the microarray analysis pipeline used for the analysis of the genome-wide microarray data. FDR, Benjamini-Hochberg false discovery rate analysis.
Normalization.
The data were normalized using a quantile algorithm similar to one described by Bolstad et al. (6). We applied our own C++ software for normalization (available from Dr. Ptitsyn upon request). Box-plots for prenormalized and normalized expression value distributions are shown in Supplemental Fig. S1A.1
Preliminary selection of differentially expressed genes.
A set of differentially expressed genes was selected using University of Pittsburgh Gene Expression Data Analysis suite (GEDA, http://bioinformatics.upmc.edu/GE2/GEDA.html). For selection, the standard J5 metric with threshold 4 and optional 4 iteration of Jackknife procedure was applied to reduce the number of false-positive differential genes (42). Both J5 metric and threshold parameter are standard preset values recommended by the developers. We did not attempt to estimate the confidence level of individual genes and used J5 not as a statistical test, but as a selection procedure providing a short list of genes deviating from the expected average value and enriched with differential genes. The MA plot showing selected differential genes is presented in Supplemental Fig. S1B. Notably, the plot shows a balanced representation of moderately and highly expressed genes, i.e., the categories most appropriate for selection of diagnostic biomarkers. Application of selection procedures biased away from highly expressed genes may reveal truly differential genes, but fewer suitable biomarker candidates. DAVID web-based tools were applied to perform functional annotation of all potentially differential genes selected by GEDA. The complete annotated lists for analyzed data sets are given in the GEO database (provisional accession number GSE11835).
Conversion to the nearest human homolog.
Current versions of commercial pathway analysis software do not support bovine transcriptome data. We presume that human biological pathways are sufficiently similar to those of Bos taurus. The Affymetrix support website (http://www.affymetrix.com/support) offers tables delineating the nearest homologs between target sequences used to derive sets of probes for different expression arrays. The table of nearest homologs between bovine and human expression arrays was used to convert the bovine list of genes to the list of human homologous genes. Since expression arrays are biased toward less evolutionary conservative 3′-untranslated regions of expressed genes the conversion procedure loses a considerable number of genes lacking strong similarity between corresponding human and bovine target sequences. However, the remaining list of genes was sufficient to identify statistically overrepresented pathways.
Functional annotation and pathway analysis.
Analysis of biological pathways was performed using MetaCore software (GeneGo) licensed through the Colorado State University Center for Bioinformatics and free DAVID tools (25).
qRT-PCR Procedure and Primers
qRT-PCR reactions were performed with IQ SYBR green supermix (Bio-Rad) on the LightCycler480 (Roche, Basel, Switzerland) using the 384-well plate format. qRT-PCR reaction, performed in duplicate, contained 2 μl of cDNA, 5 μl of Supermix, and 1.5 μl of 7.5 nM solution of each primer. The qRT-PCR reaction was conducted at 95°C for 3 min, followed by 40 cycles of 95°C for 30 s, 61°C for 30 s, and 72°C for 15 s. Upon completion of qRT-PCR amplification, melting curve analysis was performed to evaluate the quality of amplification as described previously (53). The qRT-PCR results were analyzed with the Comparative Ct (ΔΔCt) method, and data were presented as a relative expression. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as endogenous control gene for normalization of data. Target genes and primers for qRT-PCR are listed in Supplemental Table S1. Primers were designed using Primer3 software to generate 90–110 bp amplicons and optimized for melting point as well as lack of self-annealing or folding at high temperatures. All amplicons were sequenced to confirm identity to the target genes.
Statistical Analysis
Comparison of the gene expression in TI and PI groups of heifers vs. control group and comparison of the gene expression for each group of heifers between multiple time points (days 75–190 of gestation) was performed by applying the unstructured covariance matrix design for longitudinal raw data for relative expression for all time points. Differences of P < 0.05 were considered statistically significant.
RESULTS
Genome-wide Microarray Analysis
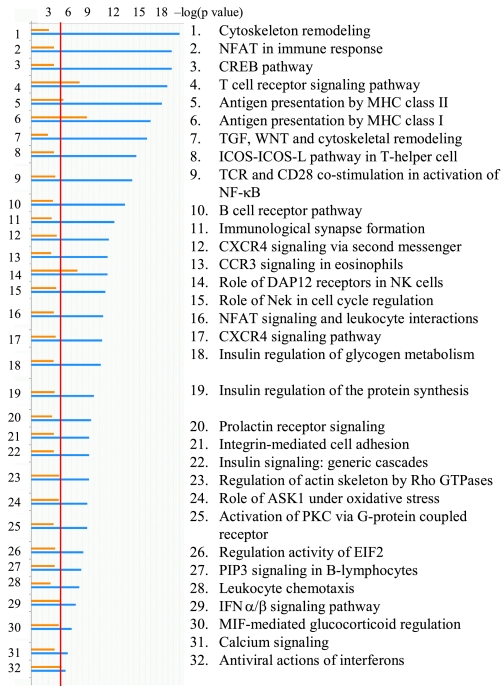
Genome-wide microarray analysis of the gene expression in white blood cells of heifers was completed twice during the experimental infection: on day 160 and day 190 of gestation. For the first analysis on day 160 of gestation total white blood cells mRNA of control heifers was compared with mRNA of heifers carrying PI fetuses. The second microarray experiment using blood of heifers from all three experimental groups was completed on day 190 of gestation. Supplemental Tables S3–S6 contain full lists of differentially expressed genes for both day 160 and 190 microarray screens. The multiple-way comparison of gene expression in all groups at separate time points, along with the comparison of the microarray data sets between two analyzed time points of infection, revealed that multiple signaling pathways are affected by transient BVDV infection and by the presence of TI fetuses in heifers of the TI group, as well as by the presence of PI fetuses in pregnant heifers of the PI group. The most affected pathways are shown in Fig. 3. While there is a difference in the amplitude of changes in gene expression in microarray data sets of day 160 and day 190, it is evident that major pathways mediating the innate and adaptive immune responses are among the most affected by BVDV infection at both time points: antiviral actions of IFNα/β, antigen presentation both by MHC class I and class II, immunological synapse formation, T cell receptor (TCR) signaling, and TCR-CD28 costimulation of NF-κB, B-cell receptor (BCR) pathway, nuclear factor of activated T cells (NFAT) in immune response and leukocyte interactions, DAP12 receptor signaling in NK cells, and leukocyte chemotaxis.
Fig. 3.
Diagram of canonic pathways affected by noncytopathic (ncp) BVDV2 infection in blood of pregnant heifers. Orange (top) bars represent microarray data for day 190 of gestation, blue (bottom) bars represent microarray data for day 160 of gestation. X-axis represents significance estimated for the groups of genes known to be co-regulated in the context of specific cellular function (biological pathways); P values are shown in a log format. The red line corresponds to P = 0.05 significance threshold for biological pathways.
Molecular processes changed by the presence of a BVDV PI fetus in the pregnant dam on day 160 of pregnancy are listed in Table 1. The ratio of the differentially expressed genes represents the “severity” of the impact on the process: antigen processing and presentation, regulation of T cell differentiation, positive regulation of lymphocyte differentiation and activation, response to oxidative stress, and immune response showed the highest ratio of the affected genes out of all currently known.
Table 1.
Molecular processes affected by BVDV infection in blood of heifers carrying PI fetuses, day 160
| Process | Affected Genes | All Genes | Ratio, % | P Value |
|---|---|---|---|---|
| Immune response | 99 | 586 | 16.90% | 8.62E-16 |
| Immune system process | 126 | 859 | 14.70% | 5.33E-15 |
| Response to stimulus | 221 | 2,107 | 10.50% | 1.00E-09 |
| Cytoskeleton organization and biogenesis | 65 | 472 | 13.80% | 4.89E-07 |
| Antigen processing; presentation of peptide antigen (MHC class II) | 8 | 13 | 61.50% | 7.78E-07 |
| Intracellular transport | 74 | 578 | 12.80% | 1.36E-06 |
| Positive regulation of T cell differentiation | 8 | 14 | 57.10% | 1.70E-06 |
| Protein transport | 54 | 381 | 14.20% | 1.98E-06 |
| Antigen processing and presentation | 13 | 42 | 31.00% | 5.88E-06 |
| Regulation of T cell differentiation | 8 | 17 | 47.10% | 1.13E-05 |
| Positive regulation of lymphocyte differentiation | 8 | 18 | 27.80% | 1.90E-05 |
| Positive regulation of lymphocyte activation | 15 | 60 | 25.00% | 2.08E-05 |
| Metabolic process | 405 | 4,830 | 8.40% | 3.59E-05 |
| Protein import | 22 | 120 | 18.30% | 5.60E-05 |
| Cellular biosynthetic process | 88 | 800 | 11.00% | 6.43E-05 |
| Response to oxidative stress | 20 | 106 | 18.90% | 7.95E-05 |
| Nucleocytoplasmic transport | 28 | 178 | 14.00% | 1.02E-04 |
| Sequestering of calcium ion | 7 | 17 | 41.20% | 1.17E-04 |
BVDV, bovine viral diarrhea virus; PI, persistently infected.
Upregulation of the Type I Interferon Pathway in Transient BVDV Infection
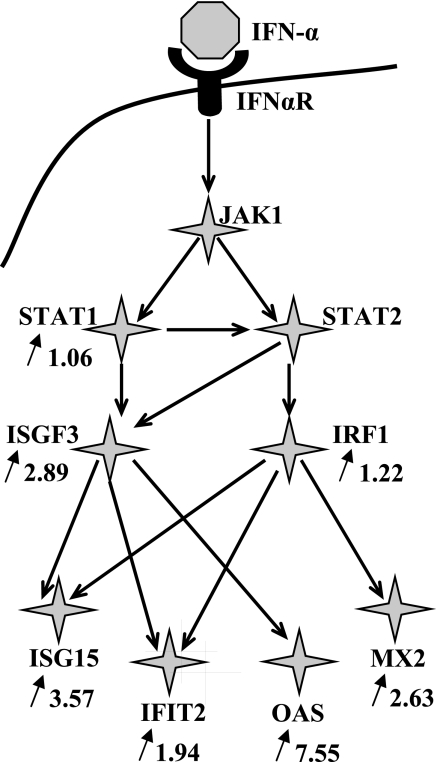
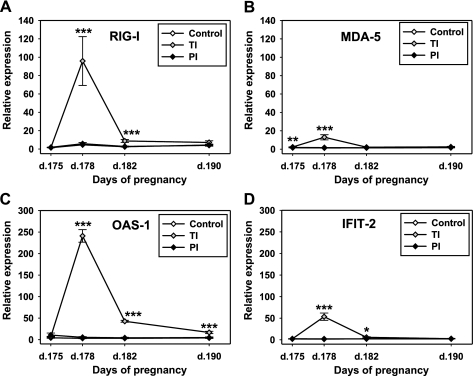
Analysis of the microarray data set for day 190 of gestation revealed strong and evident upregulation of the type I IFN pathway in blood of heifers of the TI group. Expression of cytosolic sensors for dsRNA (RIG-I and MDA-5) was significantly (2.19 and 1.46 times, respectively) higher in TI heifers compared with control heifers (data not shown). Multiple genes, including the STAT1/STAT2, IRF1, and ISGF3, as well as several ISGs, have been upregulated in blood of heifers of the TI group compared with both control and PI group 15 days post-TI challenge. Figure 4 shows some of the significantly upregulated genes within the type I IFN pathway. Both cytosolic sensors (RIG-I and MDA-5) and three ISGs [OAS-1, IFN-induced protein with tetratricopeptide repeats (IFIT2) from the ISG56 gene family, and ISG15] were selected for the confirmation of the differential expression with the qRT-PCR approach. Figure 5 shows the relative expression of RIG-I, MDA-5, OAS-1, and IFIT2 in blood of heifers of the PI, TI, and control groups of heifers. RIG-I expression (Fig. 5A) in blood of heifers of the PI and TI groups on day 175 (prior to BVDV challenge) did not differ from the control heifers, while baseline MDA-5 (Fig. 5B) expression was slightly (1.3 times) higher in blood of TI heifers compared with both control and PI groups. Three days post-BVDV challenge of the TI heifers (day 178 of gestation) both RIG-I and MDA-5 demonstrated upregulation, although with different amplitude. MDA-5 was not upregulated as much as RIG-I and returned to the baseline level by day 7 postinfection (p.i.) (day 182 of gestation). Upregulation of RIG-I was more pronounced and lasted longer, with the return to the baseline by 15 days p.i. (day 190 of gestation).
Fig. 4.
Type I IFN pathway genes affected by acute ncpBVDV infection and by the presence of a TI fetus in pregnant heifers of the TI group, as detected with the microarray screen on day 190 of gestation. Arrows show direction of the change in gene expression, number shows the ratio of mRNA abundance in blood of heifers of the TI group when compared with the blood of heifers of the control group (microarray data, day 190, P < 0.01). Accession numbers for the differentially expressed genes are listed in Supplemental Table S2.
Fig. 5.
Relative expression of mRNA for cytosolic dsRNA sensors: RIG-I (A) and MDA-5 (B), and for ISGs: OAS-1 (C) and IFIT2 (D) in blood of heifers carrying control, TI, or PI fetuses, detected with qRT-PCR. Data are presented as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001. Please note the scale differences between top panel (A, B) and bottom panel (C, D) graphs. RIG-I, retinoic acid-induced gene I; MDA-5, melanoma differentiation-associated protein 5; OAS-1, 2′,5′-olygoadenylate synthetase 1; IFIT-2, IFN-induced protein with tetratricopeptide repeats; d, day.
qRT-PCR also confirmed rapid and very significant upregulation of OAS-1 and IFIT-2 by 3 and 7 days p.i. (days 178 and 182 of gestation, respectively; Fig. 5, C and D). IFIT-2 expression returned to baseline level by day 15 p.i., while OAS-1 expression remained greater compared with the control but was evidently returning to the baseline. The complete profile of ISG15 relative expression during the course of the infection (days 75–190) has been published previously (53). Briefly, we saw a rapid and dramatic upregulation of ISG15 during acute BVDV infection (days 3–7 p.i.) followed by a return to baseline levels 15 days after both PI and TI challenge of heifers.
A summary of processes affected by changes in the type I IFN signaling in BVDV infected heifers is presented in Table 2. The greatest differences in gene expression were observed in a group of genes that mediate response to the virus, followed by cell surface receptor-linked signal transduction genes, caspase activation, and negative regulation of virion penetration into host.
Table 2.
Processes affected by changes in type I IFN signaling in maternal blood from heifers acutely infected with ncpBVDV and carrying TI fetuses, day 190
| Processes Affected by Changes in Type I IFN Signaling | % | P Value |
|---|---|---|
| Response to virus | 20 | 6.74E-09 |
| Cell surface receptor linked signal transduction | 13.33 | 2.98E-03 |
| Negative regulation of virion penetration into host | 3.33 | 7.05E-03 |
| DNA damage response | 3.33 | 7.05E-03 |
| Regulation of MHC class I biosynthetic process | 3.33 | 7.05E-03 |
| Peptydil-arginine methylation | 3.33 | 7.05E-03 |
| Positive regulation of histone deacetylation | 3.33 | 7.05E-03 |
| Peptydil-arginine methylation | 3.33 | 7.05E-03 |
| Caspase activation | 6.67 | 8.45E-03 |
npc, noncytopathic; TI, transiently infected.
Downregulation of CXCR4 and TCR Signaling Pathways in Heifers With PI Fetuses
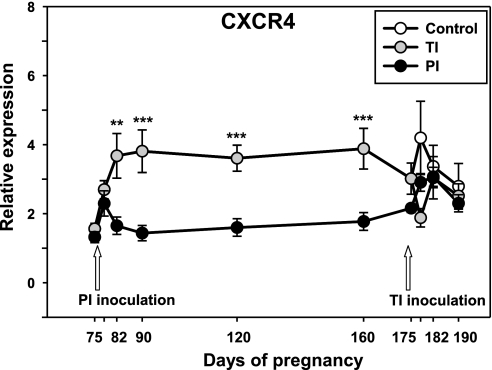
J5 statistical analysis was applied to the Affymetrix microarray screen of white blood cell mRNA from pregnant heifers carrying PI and uninfected fetuses on day 160 and revealed differential expression of 997 genes from numerous pathways. Several members of the type I IFN pathway and members of the chemokine family and chemokine receptors were differentially expressed in blood of PI heifers (for a full list of differentially expressed genes for day 160 microarray dataset see Supplemental Table S3). One notable differentially expressed gene was chemokine C-X-C motif receptor 4 (CXCR4), which was approximately twofold downregulated in blood of PI mothers. qRT-PCR of the total maternal blood cell mRNA not only confirmed decreased CXCR4 expression on day 160 of gestation (Fig. 6) but also demonstrated that CXCR4 downregulation occurred early in infection at the time of viremia in infected heifers (day 7 p.i.), and remained at this level for ∼3 mo, returning to the baseline level only by day 175 of gestation. Notably, while TI inoculation on day 175 caused a brief CXCR4 downregulation in TI heifers by day 3 p.i., the difference between TI and control heifers did not reach statistical significance.
Fig. 6.
Relative expression of CXCR4 mRNA, detected with qRT-PCR in blood of pregnant heifers carrying control, TI, or PI fetuses. Data are presented as means ± SE. **P < 0.01, ***P < 0.001.
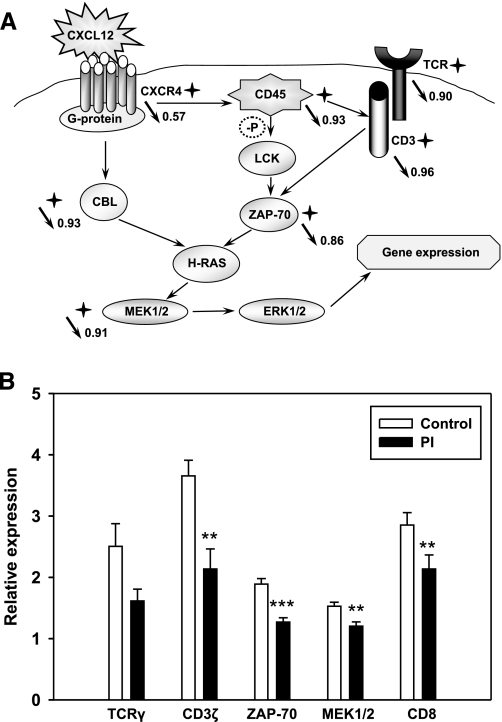
Genome-wide microarray analysis demonstrated that expression of CXCR4 in addition to several genes encoding components of the CXCL12/CXCR4 signaling pathway were downregulated in heifers carrying PI fetuses compared with control uninfected pregnant heifers (Fig. 7A): guanine nucleotide binding protein (G protein); leukocyte common antigen precursor, tyrosine phosphatase CD45; proto-oncogene tyrosine-protein kinase Lck; E3 ubiquitin-protein ligase CBL; dual specificity protein kinases MEK1/2; mitogen-activated protein kinase 1 ERK2; zeta-associated protein 70 (ZAP-70). Direction and ratio of the changes in gene expression on microarray screen (Fig. 7A) are shown for the genes used for the confirmation with qRT-PCR (Fig. 7B). qRT-PCR confirmed significant downregulation of expression of ZAP-70 and MEK1/2 in blood of heifers of the PI group compared with the control group. Expression of CBL and CD45 mRNA was not different in experimental groups in qRT-PCR (data not shown). Processes affected by changes in CXCR4 signaling are summarized in Table 3. Protein amino acid phosphorylation, intracellular signaling cascade, Ras protein signaling transduction, integrin-mediated signaling pathway, protein kinase cascade, and T cell proliferation and differentiation show the highest changes in gene expression.
Fig. 7.
Schematic of CXCL12/CXCR4 and T cell receptor (TCR) pathways (A) and relative expression of differentially expressed genes (B) in blood of pregnant heifers carrying control or PI fetuses. A: stars show differentially expressed genes on microarray screen, completed on day 160 of gestation. Arrows show direction of the change in gene expression, number shows the ratio of mRNA amount in blood of heifers of the PI group when compared with the blood of heifers of the control group for the genes tested with microarray and qRT-PCR (microarray data, day 160, P < 0.01). Circled −P shows dephosphorylation. B: relative expression of mRNA for differentially expressed genes of CXCL12/CXCR4 pathways in blood of heifers carrying PI or control fetuses, detected with qRT-PCR on day 160 of gestation. Data are presented as means ± SE. **P < 0.01, ***P < 0.001. Accession numbers for the differentially expressed genes are listed in Supplemental Table S2.
Table 3.
Processes affected by changes in CXCR4 and TCR signaling in blood of heifers carrying PI fetuses, day 160
| Processes Affected by Changes in CXCR4 Signaling | % | P Value |
|---|---|---|
| Ras protein signal transduction | 15.38 | 1.62E-07 |
| Integrin-mediated signaling pathway | 12.82 | 6.98E-07 |
| Cell motility | 17.95 | 7.76E-06 |
| Signal complex formation | 7.69 | 1.40E-05 |
| T cell proliferation | 7.69 | 2.43E-05 |
| Protein amino acid phosphorylation | 23.08 | 2.87E-05 |
| Intracellular signaling cascade | 15.38 | 2.88E-05 |
| T cell differentiation | 7.69 | 5.77E-05 |
| Nerve growth factor receptor signaling pathway | 5.13 | 8.20E-05 |
| Protein kinase cascade | 12.82 | 1.04E-04 |
| Processes Affected by Changes in TCR Signaling | % | P Value |
|---|---|---|
| Positive regulation of T cell activation | 13.79 | 4.53E-30 |
| TCR signaling pathway | 14.66 | 7.95E-27 |
| Regulation of TCR signaling pathway | 7.76 | 3.58E-19 |
| Activation of NF-κB transcription factor | 13.79 | 7.37E-19 |
| T cell differentiation | 10.34 | 2.25E-16 |
| Intracellular signaling cascade | 24.14 | 3.16E-14 |
| Positive regulation of calcium-mediated signaling | 7.76 | 1.63E-13 |
| Ras protein signal transduction | 10.34 | 1.02E-12 |
| I-κB kinase/NF-κB cascade | 7.76 | 1.55E-10 |
| Response to molecule of bacterial origin | 5.17 | 2.43E-10 |
| Regulation of cell cycle | 12.93 | 2.43E-10 |
| Positive regulation of T cell proliferation | 6.90 | 1.15E-08 |
| Cell surface receptor-linked signal transduction | 12.93 | 4.03E-08 |
| Cellular defense response | 7.76 | 8.21E-08 |
| Immune response | 15.52 | 1.55E-05 |
| Protein modification process | 7.76 | 1.69E-05 |
| MAPK kinase cascade | 5.17 | 5.20E-05 |
| Caspase activation | 5.17 | 8.77E-05 |
TCR, T cell receptor.
TCR and CD3 zeta (CD3ζ) chain, the main members of the TCR pathway, were also downregulated on the microarray (Fig. 7A). qRT-PCR confirmed downregulation of CD3ζ chain expression (Fig. 7B) and revealed a tendency to downregulation of TCRγ cluster expression (P = 0.058). Expression of surface marker of cytotoxic T cells CD8 was significantly lower in heifers of the PI group both on microarray (ratio 0.79) and in qRT-PCR (Fig. 7B). Processes affected by the TCR signaling changes in heifers of the PI group are presented in Table 3. Intracellular signaling cascade, T cell differentiation and activation, regulation of TCR signaling pathway, IκB kinase/NF-κB cascade, MAPK kinase cascade, and immune response, specifically cellular defense response and response to molecule of bacterial origin, were affected the most.
DISCUSSION
Type I IFNs are the first line of defense against many viruses due to their ability to induce apoptosis in the infected cells and to stimulate signaling cascades aimed at developing the cellular resistance to viral infection, as well as their role in activation of the adaptive immune system (reviewed in Refs. 54, 57, 58). Evasion of the type I IFN response by ncpBVDV viruses was considered to be one of the strategies used by these viruses to establish persistent infection (3, 16). Despite all the controversy of the studies reporting an absence of the type I IFN response to ncpBVDV infection in vivo and in vitro (17, 43), several groups recently confirmed the ability of ncpBVDV viral strains to induce a strong type I IFN response in ncpBVDV infected cells or animals (15, 31, 53). We have previously detected bioactive type I IFN in blood of pregnant heifers infected with ncpBVDV2 and their TI fetuses (53), as well as upregulation of ISG15 in heifers and of numerous genes - members of the type I IFN pathway - in blood of TI fetuses (51). Results of genome-wide microarray analysis conducted on the blood of the same experimental heifers are consistent with the previous findings by our group. Multiple genes from different levels of the type I IFN pathway were significantly upregulated based on our microarray data.
Type I IFN signaling starts with the detection of the virus. Viruses have conserved structural moieties, known as pathogen-associated molecular patterns (PAMPs), which are recognized by pattern recognition receptors, such as Toll-like receptors (TLRs) and RIG-I-like RNA helicases RIG-I and MDA-5 (59). TLRs represent an extracytoplasmic pathogen sensing, while RNA helicases mediate intracellular (cytosolic) recognition of viruses (23). Genome-wide microarray analysis did not reveal upregulation of the TLRs following transient infection of pregnant heifers but did detect strong upregulation of both cytosolic dsRNA sensors, RIG-I and MDA-5, which was confirmed by qRT-PCR. Previously, we showed upregulation of RIG-I and MDA-5 in TI and PI fetuses and in PI steers (51). RIG-I and MDA-5 distinguish different RNA viruses, even belonging to the same family. Within the Flaviviridae, RIG-I, but not MDA-5, is essential for the production of the type I IFN in response to Japanese encephalitis and hepatitis C viruses (26, 55), while dengue and West Nile viruses are recognized by a combination of RIG-I and MDA-5 (22, 32). Our data indicate that both RIG-I and MDA-5 sensors can play a role in the recognition of ncpBVDV. Further studies are needed to determine if TLR recognition of BVDV plays a role in the type I IFN response to ncpBVDV viruses.
A type I IFN response induces expression of numerous ISGs, including OAS-1, PKR, ISG15 and members of the ISG56 (IFIT1) gene family (47, 48). Functions of OAS-1 in antiviral defense are well described as aimed at degradation of viral RNA through activation of ribonuclease L (RNase L) (46), while functions of ISG15 and IFIT2 are not clear yet. The ability of ISG15 to conjugate with proteins is suggested to be one of the important mechanisms in defense against viral infection (67), while IFIT2 (ISG54) functions are not clearly elucidated (48). We detected upregulation of OAS-1, ISG15 and IFIT2 in transient ncpBVDV infection in pregnant heifers utilizing genome-wide microarray screen and qRT-PCR, in addition to the previously reported detection of bioactive type I IFN in blood of these heifers (53). These data complement existing knowledge of the ability of ncpBVDV to induce the type I IFN response.
Type I IFNs acting as cytokines extend their antiviral activity to other immune response components (reviewed in Ref. 23), activating cytotoxicity of natural killer (NK) cells (30) and regulating cytotoxic T cell (CTL) responses by induction of cytokines, positively regulating CTL numbers and activities, and chemokines, recruiting CTLs to the site of infection (39, 68). Microarray data, showing T cell receptor signaling, NFAT in immune response and leukocyte interactions, DAP12 receptor signaling in NK cells, and leukocyte chemotaxis among the most affected pathways by BVDV infection, are in concordance with the dramatic type I IFN response detected in TI heifers. This is not considered to be the selective influence of the type I IFN response, since multiple interacting and cross-linking pathways were affected by ncpBVDV infection. However, the developing type I IFN response may be a major inducer of these events.
The most notable and intriguing finding from the genome-wide microarray comparison between heifers carrying PI vs. healthy fetuses was the very significant downregulation of the CXCL12/CXCR4 signaling pathway by the time of established viremia, which lasted for ∼3 mo post-BVDV challenge. While CXCR4 expression was shown to be decreased in response to several viral infections as reviewed in Ref. 64, there are no indications in the literature for a possible role of the CXCL12/CXCR4 pathway in BVDV pathogenesis. CXCR4, a G protein-coupled 7-transmembrane receptor, expressed on the leukocytes and on cells of the central nervous system, has a unique specific endogenous ligand - stromal derived factor 1 (SDF-1), in current nomenclature chemokine C-X-C-motif ligand 12 (CXCL12). CXCR4 is expressed by most T cell subsets, serves as the receptor for CXCL12, and mediates signaling that results in T cell adhesion, chemotaxis, and expression of genes regulating cell-cycle progression and apoptosis (56). CXCL12/CXCR4 signaling regulates the development of T and B lymphocytes and contributes to the survival of mature lymphocytes and to the generation of memory T cells (27). Disruption of CXCL12/CXCR4 interaction has been shown to affect multiple biological processes, such as hematopoiesis, cardiogenesis, vasculogenesis, neuronal development, immune cell trafficking, and to cause embryonic lethality due to the severe developmental defects, such as defective B cell lymphopoeisis and bone marrow colonization, as well as deficient cardiac septum formation (37, 38, 69). Due to the similarity of the symptoms observed in PI animals to the symptoms characteristic for CXCL12/CXCR4 signaling disruption, we chose this pathway for further study as one of the possible reasons of immunosuppression and fetal defects in PI animals.
Genome-wide microarray analysis did not reveal differential expression of CXCL12 in blood of heifers with PI fetuses compared with control animals. Since CXCL12 is constitutively expressed in numerous tissues, while CXCR4 mRNA is subject to a rapid turnover and has a short half-life (about 2 h), the CXCL12/CXCR4 signaling is likely to be regulated at the level of CXCR4 expression (12). Genome-wide microarray analysis detected downregulation of several downstream genes in CXCL12/CXCR4 pathway, including guanine nucleotide binding protein (G protein) (28); leukocyte common antigen precursor, tyrosine phosphatase CD45, required for T-cell activation through the antigen receptor (21); proto-oncogene tyrosine-protein kinase Lck, essential for the selection and maturation of developing T-cell in the thymus and in mature T-cell function and playing a key role in TCR-linked signaling pathways (40); negative regulator of signaling pathways E3 ubiquitin-protein ligase CBL (44); dual specificity protein kinases MEK1/2, playing a critical role in mitogen growth factor signal transduction (14); mitogen-activated protein kinase 1 ERK2, required for the initiation of translation (41); and ZAP-70, serving as a cross-link between CXCL12/CXCR4 and TCR pathways (29). While qRT-PCR approach confirmed downregulation of CXCR4, ZAP-70, and MEK1/2, it did not confirm downregulation of CBL and CD45 expression. Different sensitivity of the microarray screen and qRT-PCR techniques, as well as possible fine attenuation of CD45 and CBL activity through phosphorylation (21), are suggested as possible reasons underlying the discrepancy between microarray and qRT-PCR data.
All mentioned genes are necessary for the successful CXCL12/CXCR4 signaling, with ZAP-70 being of special interest because it serves as a cross-link between CXCR4 and TCR signaling pathways, both of which were significantly downregulated in heifers with PI fetuses. In response to CXCL12, CXCR4 physically associates with TCR via reorganization of the actin cytoskeleton, which permits CXCR4 to signal via TCR-ZAP-70 complexes, leading to a prolonged ERK2 activation (29). Cytoskeletal remodeling is listed as a highly affected process in BVDV infection (Fig. 3), and ERK2-activator MEK1/2 kinase was downregulated both on microarray and in qRT-PCR.
CXCR4 was considered to be a unique receptor for CXCL12 until a recent discovery that CXCR7 (previously known as RDC1) can serve as another CXCL12 receptor (4). Although CXCR7 and CXCR4 mediate CXCL12 signaling, they have both common and distinct features, e.g., both receptors can serve as coreceptors for certain human immunodeficiency virus strains (50) and are essential for heart valve formation (38, 52) and angiogenesis (11), but CXCR7 in contrast with CXCR4 appears to not play significant role in hematopoietic or nervous system development (52). Because CXCR7 is not represented on the Affymetrix bovine microarray, we were unable to compare its expression in our experimental animals, but this gene might be of interest for future BVDV studies.
We confirmed downregulation of several TCR pathway members in blood of heifers carrying PI fetuses with both microarray screen and qRT-PCR: CD3ζ chain, ZAP-70, and MEK1/2 were significantly downregulated, while TCRγ showed a trend to downregulation. Decreased expression of CD8 surface marker in blood of heifers carrying PI fetuses, detected by both approaches, might be considered a consequence of a significant downregulation of TCR pathway and reflective of a deficiency of cellular defense response in pregnant dams.
Downregulation of the two major pathways, CXCR4/CXCL12 and TCR, in heifers with PI fetuses has not been described before. The reasons for a prolonged CXCR4 and TCR downregulation after clearance of the infection need to be clarified by further studies. Very rapid downregulation of the CXCR4 receptor after virus challenge might suggest some direct influence of the viral presence on CXCR4 transcription. However, the fact that the receptor remains downregulated even after BVDV clearance and seroconversion in the dam, which happened by day 120 of gestation in heifers of PI group (53), supports the idea that a PI fetus might be releasing a factor(s) that circulates in maternal blood and causes inhibition of the CXCR4 receptor. The long-lasting downregulation of CXCL12/CXCR4 signaling could have deleterious consequences for fetal development and postnatal responses to infections or inflammatory responses.
Genome-wide microarray approach confirmed the hypothesis that presence of the PI fetus causes downregulation of proinflammatory pathways in blood of the pregnant dam. It also provides justification for further study of biomarkers in maternal blood with a potential to distinguish the PI fetus carrying cow prior to the birth of the PI calf. For example, CXCR4 can be considered as one of the candidate biomarkers due to its quick and prolonged downregulation in cows carrying PI fetus. However, comparatively low fold change for the CXCR4 expression might be a limiting factor in practical applications. Further studies in this direction can lead to the development of the diagnostics of the cows that are carrying PI fetuses.
GRANTS
This study was supported by the National Research Initiative (NRI) of the USDA Cooperative State Research, Education and Extension Service (CSREES), grants no. 2004-35204-17005 and 2008-35204-04652. Montana State University INBRE Functional Genomics Core Facility is supported by NIH grant P20-RR16455.
Supplementary Material
Acknowledgments
We are grateful to Dr. D. L. Montgomery for help with the fetal necropsies. We thank Kate MacInnerney for technical assistance with microarray.
Present addresses: H. Han, Dept. of Animal Sciences, Colorado State Univ., 1171 Campus Delivery, Ft. Collins, CO 80523-1171; A. L. van Olphen, Dept. of Global Health, College of Public Health, Univ. of So. Florida, 3720 Spectrum Blvd., IDRB 304, Tampa, FL 33612; H. Bielefeldt-Ohmann, School of Veterinary Science, Univ. of Queensland, St. Lucia, Qld 4072, Australia.
Address for reprint requests and other correspondence: T. R. Hansen, Animal Reproduction and Biotechnology Lab., Dept. of Biomedical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State Univ., 1683 Campus Delivery, Ft. Collins, CO 80523-1683 (e-mail: thomas.hansen@colostate.edu).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci USA 101: 17264–17269, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baigent SJ, Goodbourn S, McCauley JW. Differential activation of interferon regulatory factors-3 and -7 by non-cytopathogenic and cytopathogenic bovine viral diarrhoea virus. Vet Immunol Immunopathol 100: 135–144, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Baigent SJ, Zhang G, Fray MD, Flick-Smith H, Goodbourn S, McCauley JW. Inhibition of beta interferon transcription by noncytopathogenic bovine viral diarrhea virus is through an interferon regulatory factor 3-dependent mechanism. J Virol 76: 8979–8988, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem 280: 35760–35766, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bielefeldt-Ohmann H The pathologies of bovine viral diarrhea virus infection. A window on the pathogenesis. Vet Clin North Am 11: 447–476, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Brackenbury LS, Carr BV, Charleston B. Aspects of the innate and adaptive immune responses to acute infections with BVDV. Vet Microbiol 96: 337–344, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Brown GB, Bolin SR, Frank DE, Roth JA. Defective function of leukocytes from cattle persistently infected with bovine viral diarrhea virus, and the influence of recombinant cytokines. Am J Vet Res 52: 381–387, 1991. [PubMed] [Google Scholar]
- 9.Brownlie J The pathways for bovine virus diarrhoea virus biotypes in the pathogenesis of disease. Arch Virol 3: 79–96, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Brownlie J, Clarke MC, Howard CJ. Experimental production of fatal mucosal disease in cattle. Vet Rec 114: 535–536, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med 203: 2201–2213, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta 1768: 952–963, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carman S, van Dreumel T, Ridpath J, Hazlett M, Alves D, Dubovi E, Tremblay R, Bolin S, Godkin A, Anderson N. Severe acute bovine viral diarrhea in Ontario, 1993–1995. J Vet Diagn Invest 10: 27–35, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 17: 1263–1293, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Charleston B, Brackenbury LS, Carr BV, Fray MD, Hope JC, Howard CJ, Morrison WI. Alpha/beta and gamma interferons are induced by infection with noncytopathic bovine viral diarrhea virus in vivo. J Virol 76: 923–927, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charleston B, Fray MD, Baigent S, Carr BV, Morrison WI. Establishment of persistent infection with non-cytopathic bovine viral diarrhoea virus in cattle is associated with a failure to induce type I interferon. J Gen Virol 82: 1893–1897, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Charleston B, Hope JC, Carr BV, Howard CJ. Masking of two in vitro immunological assays for Mycobacterium bovis (BCG) in calves acutely infected with non-cytopathic bovine viral diarrhoea virus. Vet Rec 149: 481–484, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Constable PD, Hull BL, Wicks JR, Myer W. Femoral and tibial fractures in a newborn calf after transplacental infection with bovine viral diarrhoea virus. Vet Rec 132: 383–385, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Coria MF, McClurkin AW. Specific immune tolerance in an apparently healthy bull persistently infected with bovine viral diarrhea virus. J Am Vet Med Assoc 172: 449–451, 1978. [PubMed] [Google Scholar]
- 20.Draghici S, Khatri P, Martins RP, Ostermeier GC, Krawetz SA. Global functional profiling of gene expression. Genomics 81: 98–104, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Fernandis AZ, Cherla RP, Ganju RK. Differential regulation of CXCR4-mediated T-cell chemotaxis and mitogen-activated protein kinase activation by the membrane tyrosine phosphatase, CD45. J Biol Chem 278: 9536–9543, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M Jr. Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J Virol 82: 609–616, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312: 879–882, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Houe H Epidemiology of bovine viral diarrhea virus. Vet Clin North Am 11: 521–547, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Huang da W, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC, Lempicki RA. The DAVID gene functional classification tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol 8: R183, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441: 101–105, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Klein RS, Rubin JB. Immune and nervous system CXCL12 and CXCR4: parallel roles in patterning and plasticity. Trends Immunol 25: 306–314, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol 35: 233–245, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, Hedin KE. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity 25: 213–224, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Lee CK, Rao DT, Gertner R, Gimeno R, Frey AB, Levy DE. Distinct requirements for IFNs and STAT1 in NK cell function. J Immunol 165: 3571–3577, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Lehmkuhl HD, Kaeberle ML. Synergistic effects of bovine respiratory syncytial virus and non-cytopathic bovine viral diarrhea virus infection on selected bovine alveolar macrophage functions. Can J Vet Res 63: 41–48, 1999. [PMC free article] [PubMed] [Google Scholar]
- 32.Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, Garcia-Sastre A, Katze MG, Gale M Jr. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol 82: 335–345, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manoli T, Gretz N, Grone HJ, Kenzelmann M, Eils R, Brors B. Group testing for pathway analysis improves comparability of different microarray datasets. Bioinformatics (Oxford) 22: 2500–2506, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Meyers G, Thiel HJ. Molecular characterization of pestiviruses. Adv Virus Res 47: 53–118, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Moerman A, Straver PJ, de Jong MC, Quak J, Baanvinger T, van Oirschot JT. Clinical consequences of a bovine virus diarrhoea virus infection in a dairy herd: a longitudinal study. Vet Q 16: 115–119, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Murray RD A field investigation of causes of abortion in dairy cattle. Vet Rec 127: 543–547, 1990. [PubMed] [Google Scholar]
- 37.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382: 635–638, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Sem Immunol 10: 179–185, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol 169: 4279–4287, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Okabe S, Fukuda S, Kim YJ, Niki M, Pelus LM, Ohyashiki K, Pandolfi PP, Broxmeyer HE. Stromal cell-derived factor-1alpha/CXCL12-induced chemotaxis of T cells involves activation of the RasGAP-associated docking protein p62Dok-1. Blood 105: 474–480, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Park JI, Strock CJ, Ball DW, Nelkin BD. Interleukin-1beta can mediate growth arrest and differentiation via the leukemia inhibitory factor/JAK/STAT pathway in medullary thyroid carcinoma cells. Cytokine 29: 125–134, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Patel S, Lyons-Weiler J. caGEDA: a web application for the integrated analysis of global gene expression patterns in cancer. Appl Bioinformatics 3: 49–62, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Peterhans E, Jungi TW, Schweizer M. BVDV and innate immunity. Biologicals 31: 107–112, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Qiao G, Li Z, Molinero L, Alegre ML, Ying H, Sun Z, Penninger JM, Zhang J. T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b. Mol Cell Biol 28: 2470–2480, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roth JA, Bolin SR, Frank DE. Lymphocyte blastogenesis and neutrophil function in cattle persistently infected with bovine viral diarrhea virus. Am J Vet Res 47: 1139–1141, 1986. [PubMed] [Google Scholar]
- 46.Samuel CE Antiviral actions of interferons. Clin Microbiol Rev 14: 778–809, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarkar SN, Sen GC. Novel functions of proteins encoded by viral stress-inducible genes. Pharmacol Therapeut 103: 245–259, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Sen GC, Sarkar SN. The interferon-stimulated genes: targets of direct signaling by interferons, double-stranded RNA, and viruses. Curr Top Microbiol Immunol 316: 233–250, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Sentsui H, Nishimori T, Kirisawa R, Morooka A. Mucosal disease induced in cattle persistently infected with bovine viral diarrhea virus by antigenically different cytopathic virus. Arch Virol 146: 993–1006, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu N, Soda Y, Kanbe K, Liu HY, Mukai R, Kitamura T, Hoshino H. A putative G protein-coupled receptor, RDC1, is a novel coreceptor for human and simian immunodeficiency viruses. J Virol 74: 619–626, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shoemaker M, Smirnova NP, Bielefeldt-Ohmann H, Austin K, van Olphen A, Clapper J, Hansen T. Differential expression of the type I interferon pathway during persistent and transient bovine viral diarrhea virus infection. J Interferon Cytokine Res 29: 23–36, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, Harvey RP, Martinez AC, Mackay CR, Mackay F. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci USA 104: 14759–14764, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smirnova NP, Bielefeldt-Ohmann H, Van Campen H, Austin KJ, Han H, Montgomery DL, Shoemaker ML, van Olphen AL, Hansen TR. Acute non-cytopathic bovine viral diarrhea virus infection induces pronounced type I interferon response in pregnant cows and fetuses. Virus Res 132: 49–58, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity 25: 373–381, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Sumpter R Jr, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M Jr. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol 79: 2689–2699, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki Y, Rahman M, Mitsuya H. Diverse transcriptional response of CD4(+) T cells to stromal cell-derived factor (SDF)-1: cell survival promotion and priming effects of SDF-1 on CD4(+) T cells. J Immunol 167: 3064–3073, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol 20: 17–22, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev 220: 214–224, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Thompson AJ, Locarnini SA. Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol Cell Biol 85: 435–445, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Valle PS, Skjerve E, Martin SW, Larssen RB, Osteras O, Nyberg O. Ten years of bovine virus diarrhoea virus (BVDV) control in Norway: a cost-benefit analysis. Prev Vet Med 72: 189–207; discussion 215–189, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Van Campen H, Vorpahl P, Huzurbazar S, Edwards J, Cavender J. A case report: evidence for type 2 bovine viral diarrhea virus (BVDV)-associated disease in beef herds vaccinated with a modified-live type 1 BVDV vaccine. J Vet Diagn Invest 12: 263–265, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Van Oirschot JT Congenital infections with nonarbo togaviruses. Vet Microbiol 8: 321–361, 1983. [DOI] [PubMed] [Google Scholar]
- 63.Van Reeth K, Adair B. Macrophages and respiratory viruses. Pathologie-Biologie 45: 184–192, 1997. [PubMed] [Google Scholar]
- 64.Von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res 100: 27–40, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Welsh MD, Adair BM, Foster JC. Effect of BVD virus infection on alveolar macrophage functions. Vet Immunol Immunopathol 46: 195–210, 1995. [DOI] [PubMed] [Google Scholar]
- 66.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunol 5: 730–737, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Yuan W, Krug RM. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. EMBO J 20: 362–371, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8: 591–599, 1998. [DOI] [PubMed] [Google Scholar]
- 69.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393: 595–599, 1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.