SYNOPSIS
Objectives
Surveillance for incident asthma in the general population could provide timely information about asthma trends and new, emerging etiologic factors. We sought to determine the feasibility of an asthma incidence surveillance system using voluntary reporting of asthma by outpatient clinics and emergency departments (EDs).
Methods
Voluntary reporting occurred from July 2002 through June 2006. We classified reported asthma based on a case definition adapted from one developed by the Council of State and Territorial Epidemiologists. We validated the case definition by having pulmonologists review data from participant interviews, medical record abstractions, and pulmonary function test (PFT) results.
Results
The positive predictive value (PPV) of meeting any of the case definition criteria for asthma was 80% to 82%. The criterion of taking at least one rescue and one controller medication had the highest PPV (97% to 100%). Only 7% of people meeting the incident case definition had a PFT documented in their medical record, limiting the usefulness of PFT results for case classification. Compared with pediatric participants, adult participants were more likely to be uninsured and to obtain asthma care at EDs. The surveillance system cost $5,129 per enrolled person meeting the incident case definition and was difficult to implement in participating clinics and EDs because asthma reporting was not mandatory and informed consent was necessary.
Conclusions
The project was useful in evaluating the case definition's validity and in describing the participants' characteristics and health-care use patterns. However, without mandatory reporting laws, reporting of incident asthma in the general population by clinicians is not likely to be a feasible method for asthma surveillance.
Asthma is one of the most common chronic conditions in the United States, annually affecting an estimated 6.2 million children and 13.8 million adults during the three-year period 2001 through 2003.1 During that same period, asthma was annually responsible for an estimated 12.3 million physician office visits, 1.8 million emergency department (ED) visits, 504,000 hospitalizations, and 4,210 deaths.1 In 1998, asthma's direct and indirect costs were an estimated $12.7 billion.2
Public health surveillance involves the continuous collection, analysis, and dissemination of data, which are then used for prevention and control efforts.3 Several sources of surveillance data for prevalent asthma in the U.S. are available, such as the Behavioral Risk Factor Surveillance System (BRFSS) and the National Health Interview Survey. Furthermore, several states and communities have conducted surveys or used administrative data such as ED, hospitalization, and Medicaid claims data to determine the local burden of asthma.4–8 Although we found no reports of incident asthma surveillance in the general population, asthma incidence has been assessed in several population-based studies.9–15
Surveillance for incident asthma would offer several important advantages over surveillance for prevalent asthma. First, it would provide more current information about trends. Because of the chronic nature of asthma, a prevalent case of asthma could have been incident in the current year or as early as several decades ago. Second, with incident asthma surveillance, new, emerging etiologic factors and risk markers could be identified. Third, asthma clusters or outbreaks could potentially be identified. It has been clearly demonstrated that outbreaks of asthma symptoms occur and that their investigation can lead to the understanding of new etiologies16–19 and their control.20 Fourth, primary prevention measures could be more easily evaluated.
Asthma incidence surveillance could be conducted in several different ways. Administrative data such as pharmaceutical, outpatient, or hospital billing data have been used to identify cases of asthma.5–8,15,21,22 However, community-wide outpatient or pharmaceutical care systems are rare, and hospitalization and ED billing data may exclude milder cases of asthma. School-based surveillance for prevalent asthma has been successfully undertaken. School-based surveillance systems have an advantage in that school attendance is mandatory; thus, most children can be assessed.23 However, most incident cases occur before school age,24 and cases with adult onset would not be included in a school-based surveillance system.
The main public health surveillance system in the U.S. is based on a passive, notifiable disease surveillance system in which laboratories and health-care providers report cases of notifiable diseases to local or state health departments.25 This type of surveillance has several advantages. For example, it is usually more timely than systems involving administrative data and allows the collection of unusual cases or clusters of cases to be reported.26 Including childhood asthma as a notifiable disease or creating a childhood asthma registry has been proposed.27 However, registries are very costly, and both mandatory reporting systems and registries suffer from incomplete reporting. Furthermore, notifiable disease surveillance systems have historically been set up for diseases rarer than asthma.28
A method that is similar to a notifiable disease surveillance system is a sentinel surveillance system. The Sentinel Event Notification System for Occupational Risks (SENSOR) has been developed for occupational asthma. It involves reporting of selected health events by a group of sentinel health-care providers who are likely to encounter a case of occupational asthma, and it has been piloted in 10 U.S. states.26 SENSOR has led to the identification of new likely causes of occupational asthma.29 Although the SENSOR system provides valuable information about occupational asthma incidence, it does not provide information about childhood asthma or non-occupationally related adult-onset asthma.
To test the feasibility of an asthma incidence surveillance system using voluntary reporting, we piloted a system in southern Miami-Dade County, Florida. The Miami Asthma Incidence Surveillance System involved sentinel outpatient pediatric, allergy, and pulmonary medicine clinics and EDs.
METHODS
Eligible population and identification of eligible participants
The target population was residents of southern Miami-Dade County, a geographic area of 38 contiguous zip codes with 1,000,658 residents.30 The eligibility criteria included being aged 2 to 64 years and not institutionalized (e.g., not in a prison, jail, or nursing home). Nursing or physician staff asked patients if they would like to participate in the study if the patients had any of the following symptoms or signs: wheezing; chronic coughing lasting at least three weeks; nocturnal awakening at least once a week with dyspnea, cough, or wheezing; or a self-reported diagnosis of asthma. During the first two years of the study, which was conducted from July 2002 through June 2006, we asked that both prevalent and incident cases be reported. Subsequently, we requested only reports of incident cases with symptom onset within the last 12 months.
Enrollment procedures in participating clinics and EDs
We recruited volunteer hospital EDs and outpatient clinics to participate in this pilot project. Three hospital EDs and eight clinics initially volunteered. We reimbursed two of the eight outpatient clinics for the staff time to enroll patients. Because asthma is not a reportable condition in Florida, we enrolled people with possible asthma through a full informed consent process. If nursing or physician staff thought that a patient was likely to be eligible, they obtained informed consent at the clinic and had clients fill out a screening questionnaire that asked questions about symptoms to determine if the patient was likely to meet the case definition. Health department staff reviewed the screening questionnaire to determine if the participant was likely to have a case of incident asthma. Children aged 7 years and older completed an assent form, and their parents completed the consent form. During 2003, we revised all informed consent forms because the Institutional Review Boards (IRBs) of the Florida Department of Health, Baptist Hospital in Miami, and Miami Children's Hospital required additional specific wording on the informed consent forms as part of their response to requirements of the Health Insurance Portability and Accountability Act (HIPAA).
Because enrolling patients in the clinics proved to be time-consuming for clinic staff, we developed a second enrollment method in which potential participants were given a flyer explaining the study. If interested, the patient gave permission to be contacted by the health department. Health department staff then called the clients with more information and obtained informed consent from participants.
Case definition development
The case definition shown in Figure 1 was adapted from previous work by the Council of State and Territorial Epidemiologists (CSTE).31 Our definition was developed during two meetings attended by staff from the Miami-Dade County Health Department, Centers for Disease Control and Prevention (CDC), and Kaiser Permanente in Portland, Oregon, with CSTE representation at one meeting.
Figure 1.
Case definition for incident and prevalent asthma
FEV1 = forced expiratory volume in one second
FVC = forced vital capacity
Interviewing and chart abstraction
Health department staff attempted to interview the participants by telephone using a standardized questionnaire. Six phone attempts during different times of the day were made. If staff could not reach the participant by telephone, a letter was sent and up to three home visits were attempted. If staff were still unable to reach the participant, the participant was considered lost to follow-up. The health department staff answered any asthma-related questions from the participant or parent/guardian of the participant after the interview, and a follow-up educational mailing was sent. The participant was also given a thank-you gift for participation, which was a choice of a pillow cover or peak flow meter.
The questionnaire included questions to determine if the participant's illness met the case definition for incident asthma. It also had questions about participant demographics, severity of disease, health-care use, asthma management, knowledge about asthma, and risk factors.
Health department staff abstracted asthma-related information from the medical records of consenting participants who met the case definition for incident asthma. Abstracted information included hospitalization and outpatient visit history, any alternative diagnoses, pulmonary function test (PFT) results, and medication history.
If the patient had new onset of symptoms consistent with asthma but did not yet meet the case definition at the time of enrollment, we contacted the participant again three months later to find out if he/she met the criteria by then.
Tracking denominator data
Data about the total number of people captured in a surveillance system are necessary to calculate incidence rates. We ascertained the population for each zip code in the target area from census data. However, because not all outpatient clinics and EDs in the entire catchment area participated in the system, we attempted to get an estimate of the proportion of the denominator that is served by the participating clinics and hospitals. For EDs, this was estimated by the proportion of all hospitalized patients living in the targeted area who were hospitalized at participating hospitals. We requested the total number of unduplicated patients from each eligible clinic and compared the number to the total number of residents in the community. We also attempted to collect information about patients who declined to participate in the study to determine how representative participants were of patients with incident asthma and to assist with the estimates of incidence and prevalence rates. However, clinics did not have sufficient available staff to collect the necessary information.
Validation of case definition
Validation was performed using a combination of data from the surveillance questionnaire, abstracted medical record information, and PFTs, including measuring airway obstruction reversibility following administration of a short-acting bronchodilator. We asked all participants with incident cases (except those younger than age 5 years, nursing or pregnant mothers, or those with a history of adverse reaction to the bronchodilator) to participate in PFTs unless there was a recent result in the medical record. As a thank-you gift for their time, we gave patients a $30 gift certificate for a local supermarket. In addition, we gave the PFT results and their interpretation to the patients and sent the results to the patient's health-care provider with the patient's consent.
The validation process involved determining if the patient had asthma by having one of two pulmonologists review the information on the abstraction form, questionnaire, and pre- and post-bronchodilator PFTs. They used a standard form and classified the case as “asthma highly probable,” “asthma possible,” or “asthma unlikely.” For each case, one pulmonologist classified the case using two different methods. One method was the pulmonologist's overall impression of the case. The second method was using a score such that the more criteria the person met, the more likely the person was to have asthma.
Costs
We calculated the costs of the program from actual program expenditures and included all costs incurred by the health department. We excluded start-up costs for the first year because no participants were recruited that year.
Data management/analysis
We entered all data into a Microsoft® Access surveillance database. Frequencies were generated and compared using the Chi-square test. The positive predictive values (PPVs) were calculated by dividing the number of cases designated by a pulmonologist as “highly probable” by the total number meeting the case definition criteria. We used SAS Version 9.1.3 for statistical analysis.32
Reliability
We assessed reliability for three key questions that were crucial to the case definition: (1) had the person ever been told by a doctor or other health-care professional that he or she has “asthma or any other breathing problems such as reactive airway disease, hyperreactive airway disease, or wheezing-related respiratory illness” (for children, “chronic or recurrent bronchitis” was also included in the list); (2) what month and year were they given their diagnosis if they answered “yes” to question 1; and (3) when was the first time in their life that they had wheezing. Participants were given an option of answering “within the last 12 months” or “more than 12 months ago.” We assessed reliability by asking 31 participants the three questions three months after they were first interviewed.
Data dissemination
We distributed results on recruitment rates and characteristics of enrollees to each participating clinic/ED at least annually. We also presented the surveillance data along with asthma hospitalization and mortality data for the target area annually at a seminar for all participating health-care providers.
The IRBs of the Florida Department of Health, Florida International University in Miami, Baptist Hospital, and Miami Children's Hospital approved the study protocol. The CDC IRB deferred to the Florida Department of Health IRB.
RESULTS
Clinic participation
Enrollment began in August 2002 in two EDs and eight outpatient clinics. By the time the study ended in June 2006, one of the original two EDs and three of the eight original outpatient clinics remained. Ten additional clinics were added during the last three years of the study. The range of time of clinic participation was four months to five years. The total number of participants enrolled from each site ranged from two to 202.
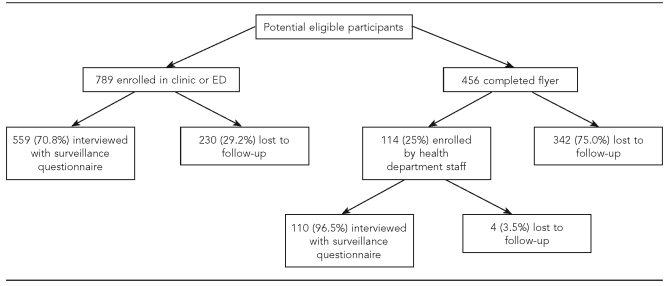
In 2003, all informed consent forms were revised to conform to each site's new HIPAA requirements. This meant increasing the length of the informed consent form by three pages; thus, potential participants had to read eight pages of paperwork to enroll. The new wording required by each IRB was also written at a higher reading level than that in the consent forms used prior to the HIPAA requirements. According to anecdotal information from clinics, this discouraged participation by potential subjects and led to clinic staff approaching fewer potential participants, especially when the clinics were busy. Beginning in the second year of enrollment, the flyer method of enrollment was begun at some of the sites. Although the flyer allowed some sites to participate that otherwise would not have been able to and made it simpler for potential participants to indicate their interest, only 114 (25%) of the 456 people who completed the flyer were ultimately enrolled (Figure 2). The primary reason for people not being enrolled after completing the flyer was that health department staff could not locate the potential participants due to incorrect or outdated contact information on the flyer. Additionally, some potential participants did not meet the eligibility criteria, and others did not complete and return the consent form that had been mailed to them.
Figure 2.
Diagram of the flow of participants, Miami Asthma Incidence Surveillance System, July 2002–June 2006
ED = emergency department
Patient participation
Between July 2002 and June 2006, 903 eligible patients were enrolled, 789 (87.4%) directly in the clinic or ED and 114 (12.6%) by health department staff after the patient completed the flyer (Figure 2). Of the 903 eligible patients, 579 (64.1%) were recruited by voluntary primary care, allergy, or pulmonology clinics; 307 (34.0%) were recruited by two federally funded community health centers; and 17 (1.9%) were recruited by EDs. Of the 789 people enrolled directly in a clinic or ED, 559 (70.8%) were interviewed with the surveillance questionnaire. Of the 114 who were recruited by flyer and were enrolled by health department staff, 110 (96.5%) were interviewed with the surveillance questionnaire.
Of the 669 enrolled patients who were interviewed, 197 (29.4%) met the case definition for an incident case of asthma, and 472 (70.6%) met the case definition for a prevalent case of asthma. Of the 197 incident case subjects, 89 (45.2%) had a PFT performed as part of the study and 57 (28.9%) did not have a PFT because they were too young to sufficiently cooperate (younger than age 5 years), leaving 51 (25.9%) eligible case subjects who chose not to have a PFT.
Demographic and health-care use characteristics of participants
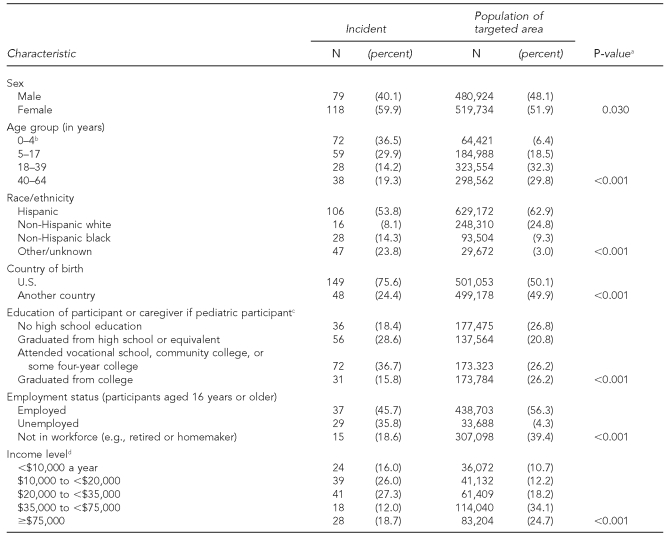
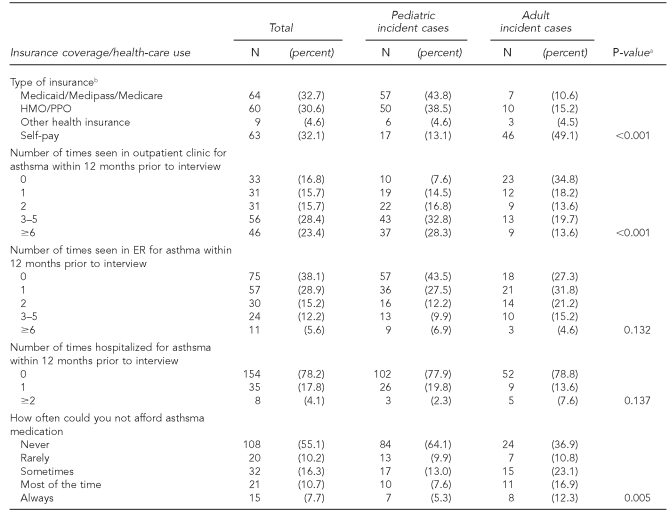
Compared with the targeted population, participants were more likely to earn less than $35,000 a year and to be female, younger than age 18, non-Hispanic black, U.S. born, and unemployed (Table 1). Compared with pediatric patients, adults were less likely to have health insurance, to be able to afford their asthma medications, and to have had any outpatient care for asthma, and adults were more likely to have been seen in the ED for asthma (Table 2). The numbers of hospitalizations were, however, similar between the two groups.
Table 1.
Demographic characteristics of interviewed participants with incident asthma cases and of targeted population, Miami Asthma Incidence Surveillance System, July 2002–June 2006 (n=197)
aP-values determined using Chi-square test
bParticipants had to be at least 2 years of age, but census data for the age distribution of the targeted area only allowed reporting for the age group 0–4 years.
cMissing two responses among incident cases
dMissing 47 responses among incident cases
Table 2.
Insurance coverage and health-care use of interviewed participants with incident asthma cases and of targeted population, Miami Asthma Incidence Surveillance System, July 2002–June 2006 (n=197)
aP-values determined using Chi-square test
bMissing one response among pediatric cases
HMO = health maintenance organization
PPO = preferred provider organization
ER = emergency room
How participants met the case definition
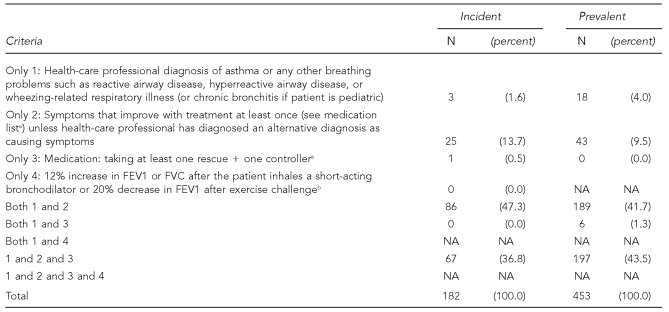
Of the 182 participants who met the incident case definition and for whom complete information was available regarding all elements of the case definition, the largest group—86, 47.3%—met the case definition by having both a diagnosis of an illness related to asthma or wheezing (which included reactive airway disease, hyperreactive airway disease, or wheezing-related respiratory illness and, for children only, chronic bronchitis) (Figure 1) and symptoms that improved with treatment (Table 3). The next largest group of participants meeting the case definition were those who had a diagnosis of an illness related to asthma or wheezing, had symptoms that improved with treatment, and were taking at least one rescue and one controller medication (67, 36.8%). There were only 12 incident case subjects who had a PFT result in their medical record. None of these met the case definition criteria of a 12% increase in the forced expiratory volume in one second (FEV1) or forced vital capacity (FVC) from baseline after inhaling a short-acting bronchodilator or a 20% decrease in FEV1 after exercise challenge. Twenty-six (14.3%) of the incident case subjects did not have a diagnosis of an illness related to asthma or wheezing. Among the 453 participants with prevalent cases and complete data regarding diagnoses, 43 (9.5%) did not have a health-care professional diagnosis of an illness related to asthma or wheezing, but did meet the criteria of symptoms improving with treatment.
Table 3.
How asthma cases met the case definition
- Controller
- Cromolyn
- Leukotriene
- Any steroid
- Theophyline
- Long-acting bronchodilator
- Rescue
- Short-acting bronchodilator
bOnly considers PFT result in medical record (n=12). Does not consider PFT results from testing done as part of study.
NOTE: Data missing for 15 incident and 19 prevalent cases
FEV1 = forced expiratory volume in one second
FVC = forced vital capacity
NA= not applicable
PFT = pulmonary function test
Validation of case definition
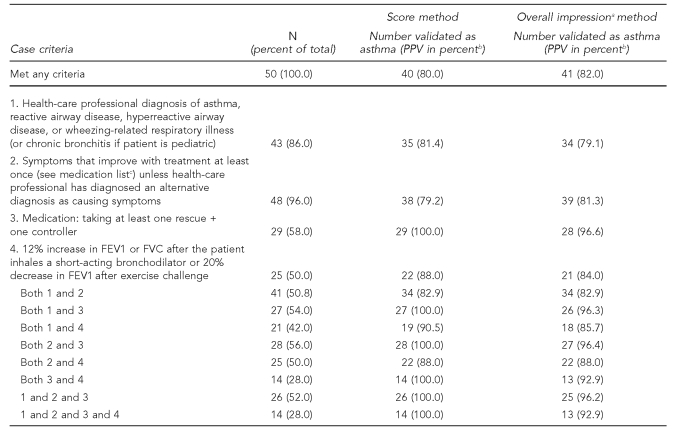
Using the score method of classification, 40 (80.0%) of the 50 validated incident cases were classified as “asthma highly probable,” six (12.0%) as “asthma possible,” and four (8.0%) as “asthma unlikely.” Using the overall impression method of classification, of the 50 cases that were assessed for validity, 41 (82.0%) were classified as “asthma highly probable,” five (10.0%) as “asthma possible,” and four (8.0%) as “asthma unlikely.” Agreement was high between the two methods, and in none of the seven cases in which there were classification discrepancies did one method classify the case as “highly probable” and the other method classify it as “unlikely.”
Considering “asthma highly probable” as the gold standard for asthma, the PPV of meeting any of the case definition criteria ranged from 80.0% to 82.0% depending on using the score or impression validation methods (Table 4). However, there was a difference by age group. Among the 36 pediatric cases validated, the PPV was 89.0% to 92.0%. Among the 14 adult cases validated, the PPV was 50.0% to 64.3% (data not shown). Among all cases validated, the PPV of a patient reporting a diagnosis of an illness related to asthma or wheezing was 79.1% to 81.4% (Table 4). The most common way the case definition was met was by improvement of symptoms with medication, and the PPV of this part of the case definition was similar to that of physician diagnosis (79.2% to 81.3%). The highest PPV was for the criterion “taking at least one rescue and one controller medication,” but this was met by only 58.0% of the validated cases. The next highest was a 12% increase in the FEV1 or FVC from baseline after inhaling a short-acting bronchodilator. However, all of the people in the validation group had their PFT as part of the project and did not have a result on record prior to enrollment.
Table 4.
Positive predictive value (PPV) of case definition among 50 validated cases by score and overall impression methods of classification, Miami Asthma Incidence Surveillance System, July 2002–June 2006
aThe pulmonologist's overall impression of whether the case was a case of asthma or not
bNumber classified as “highly probable”/number meeting criteria
- Controller
- Cromolyn
- Leukotriene
- Any steroid
- Theophyline
- Long-acting bronchodilator
- Rescue
- Short-acting bronchodilator
PPV = positive predictive value
FEV1 = forced expiratory volume in one second
FVC = forced vital capacity
Reliability of questions
At the second telephone interview to test the reliability of three of the questions, 29 (93.5%) of 31 participants gave the same answer regarding whether they had ever been told by a health-care provider that they had an illness related to asthma or wheezing. Eighteen (58.1%) gave the same month and year for the time their diagnosis was made, and 25 (80.6%) classified themselves the same way as to whether they first started wheezing more than or fewer than 12 months prior to enrollment.
Completeness of reporting
Because clinics were unable to monitor the number of patients who declined to participate in the study, the completeness of reporting is unknown. However, the total number of people seen who had a diagnosis of asthma (based on International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 493) during the various time frames at the two federally qualified health-care centers and the two EDs is known. The percentage of patients with an asthma diagnosis who were reported was 3.3% and 5.3% in the two hospital EDs and 20.8% and 50.0% in each of two community health-care centers. However, these are the percentage of all asthma cases and not necessarily incident cases, and we were encouraging incident cases to be reported.
Denominator data
The total number of unduplicated patients seen by each clinic was available from the community health centers but not for most of the private clinics. Because of the missing information, it was not possible to calculate the denominator for all of the participating clinics. However, it was estimated that one of the hospital EDs served about 25% of the target population and one of the federally qualified health-care centers served about 5% of the population.
Costs
Excluding the first-year start-up costs when no cases were enrolled, the cost per enrolled participant was about $1,119, and the cost per enrolled participant meeting the incident case definition was about $5,129. These costs exclude the costs incurred by the individual volunteer health-care providers.
DISCUSSION
Lessons learned
We learned three main lessons from our pilot surveillance system. The first lesson was regarding the validity of the case definition. The second was about the health-care use patterns of participants with incident asthma. The final and most important lesson concerned the feasibility of the system.
First, the case definition appeared to be reasonably specific, although the PPV was much higher for children than adults. This is likely due to adults being at higher risk for other chronic obstructive pulmonary diseases that have symptoms similar to asthma. Importantly, the PPV of a patient reporting a physician diagnosis of an illness related to asthma or wheezing was 79% to 81%. This would suggest that most patients who report a diagnosis of asthma do have asthma and that the more restrictive wording used by the BRFSS—“Did a doctor ever tell you that you had asthma?”33—would have an even higher PPV. The case definition may also be useful for those designing programs to improve asthma management; the criteria could be used to identify patients with asthma-like symptoms early and then follow those patients over time to provide better asthma management.
Even though the PFT criteria had an extremely high PPV, the PFT criterion turned out to be of little use for surveillance purposes because we found that relatively few participants had a PFT prior to their enrollment in the study. This finding is of concern given that in the National Heart, Lung, and Blood Institute's National Asthma Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma, pulmonary function testing is recommended at the time of the patient's first evaluation, after treatment is started, and at least every one to two years.34 Interestingly, about 14% of those who met the case definition for incident asthma did not report having been told they had an illness related to asthma or wheezing even though they were recruited from a clinic. This may be because the clinicians had not yet made the diagnosis (e.g., they wanted to wait and see if the symptoms were recurrent) or the patient was not aware of the diagnosis. Regardless, this result would suggest that studies that use a self-report of an asthma diagnosis or those that use asthma diagnosis from billing data to identify asthma cases miss some cases.
Second, we learned that among participants in our study who were uninsured, not being able to afford asthma medications at least sometimes and using the ED as the primary source of asthma care were common, particularly for adult participants. Pediatric participants had better health-care coverage, due to federal and state programs that are not available to most of the low-income adult participants, and thus were more likely to use outpatient care. It is possible that our findings were affected by how participants were recruited because adult participants were more likely to be recruited from community health centers than pediatric patients (82% of adult vs. 29% of pediatric participants).
The third lesson we learned was that conducting asthma incidence surveillance by requesting reports of new cases from outpatient clinics and EDs is not likely to provide usable incidence rate data unless incident asthma becomes a reportable condition. It might, however, be feasible in communities in which a high percentage of the population is covered by managed care or those communities with a more stable population and thus a stronger relationship between clinic staff and patients and their families facilitating enrollment. The system was difficult for providers to implement, as demonstrated by the high turnover of participating clinics and significant underreporting, especially from EDs. Furthermore, the system was costly, although costs may have decreased as the system became more established.
Barriers to asthma incidence surveillance
We identified three important barriers to a successful asthma incidence surveillance system. The first barrier was that asthma is not a reportable condition, which required clinics and health department staff to go through an informed consent process with potential participants. The process became even more difficult as the informed consent paperwork got longer after the IRBs responded to the new HIPAA requirements and likely discouraged enrollment. Furthermore, participation was voluntary, so some providers did not want to participate, and many eligible patients of participating providers did not enroll. In the United Kingdom, occupational asthma is reportable by pulmonologists and occupational medicine physicians. The United Kingdom surveillance system has provided useful national data, but it is a much simpler system that does not require patient consent.35 Similarly, in Finland, compulsory reporting of occupational asthma by physicians and insurance companies has resulted in relatively complete national data, which may at least be partly due to all Finnish employees being insured against occupational diseases.36 Furthermore, both systems only cover occupational asthma, and occupational asthma surveillance is different from a general population-based system.
The second, even more challenging barrier was the difficulty for staff to identify a potential case of incident asthma. The diagnosis of asthma is not always straightforward, and there is no simple laboratory result to identify a case of asthma. Furthermore, it is often very difficult to give an exact onset date for asthma because it is a chronic, recurring condition. In the small sample of patients in which we tested the reliability of selected questions, only 58% gave the same month and year of onset initially and during the second interview. Additionally, many participants with cases that were originally reported to the system as incident cases were found to be prevalent cases after review of the medical records. Either the patient had been diagnosed with asthma more than a year prior to enrollment or had symptoms consistent with asthma for many years. The pulmonary medicine and allergy specialists experienced an additional problem in that usually patients had had asthma longer than a year before they were referred to the specialists.
The final barrier was the difficulty in finding clients once they were enrolled. We recognized early on that it was very important to interview clients as soon as possible after they were enrolled, and in the case of the flyer, to enroll clients as soon as the clinic/ED site sent the completed flyer to the health department. Overall, 26% of participants who were enrolled were not interviewed because program staff could not locate them. This inability to locate participants occurred despite staff performing home visits and may have been partially due to the Miami-Dade County population being relatively transient, the targeted area having a high percentage of people living in poverty (17.1% compared with 12.7% for the country as a whole),37 and the targeted area including a substantial population of migrant farmers. Thus, this inability to locate participants may not be as large a problem in other communities.
Limitations
These study results have several limitations. First, we did not have information about potential eligible participants who were not identified or who chose not to participate. If these people were different from those who did participate, health-care use patterns may not be representative and the validity of the case definition may be affected. Another major limitation was that there was not complete coverage of EDs and clinics in the catchment area, and recruiting sites changed during the study period. Finally, our participating clinics were not representative of all clinics in the area because we had overrepresentation of community health centers and likely an overrepresentation of people in low socioeconomic status, which may explain why incident cases had lower income and higher unemployment than the general population of the targeted area.
Important findings
Despite the challenges of setting up the surveillance system, the program did yield some important findings in addition to learning about the functioning of the case definition and the demographic and health-care characteristics of those with incident asthma. First, it allowed the collection of detailed information about the participant, the participant's symptoms, the severity of the asthma case, medication use, impact on school and work, and barriers to obtaining medical care and medication. The surveillance data obtained through participant interviews provided information about the issues confronting patients with asthma in Miami-Dade County that was not previously known to health-care providers. For example, 18.4% of participants said that they were unable to obtain asthma medication most or all of the time because they could not afford the medication.
Second, the program provided an opportunity for health department staff to share information about the epidemiology of asthma and other public health issues in Miami-Dade County with collaborating health-care providers. Asthma mortality, hospitalization, and surveillance data were provided to participating and other providers at several meetings, and lively discussions ensued in the meetings concerning the extent to which the health-care community was meeting the challenges of asthma management.
Third, multiple outreach activities were associated with the project, such as an educational conference offered to all participants and others in the community. Also, asthma staff attended community events such as health fairs in the target area to explain the project and provide information about asthma.
CONCLUSIONS
In the absence of making incident asthma a reportable condition, we do not think that it is feasible to have an incident asthma surveillance system for the general population using the notifiable disease surveillance model. Even if the condition were reportable, it is likely that reporting would be incomplete, given the difficulty of clinic staff in identifying incident asthma, unless there was extensive training of clinic staff and some form of reimbursement for the time and cost of identifying cases. Furthermore, if there were such a system, the focus should be on primary care providers as reporters, as patients are often not referred to an allergist or pulmonologist for some time and thus may no longer have an incident case of asthma. Finally, such a system should include only providers that can provide denominator data electronically at little cost to themselves. Because of these potential problems, it would be important to pilot test a mandatory reporting system before widespread implementation. Because of the costs and barriers associated with the notifiable disease surveillance model for incident asthma surveillance, it would be prudent to test other models for incident asthma surveillance.
Acknowledgments
The authors acknowledge the following facilities and their staff for their participation in the study: Baptist Health System; Miami Children's Hospital; Helen B. Bentley Family Health Center, Inc.; Community Health Initiative of South Florida, Inc.; Pediatric Professional Assoc.; Pediatric Associates of Homestead; Pediatric Emergency Consultants, Inc.; Florida Center for Allergy and Asthma Care; Open Doors Health Center; Rosie Lee Wesley Community Health Center; South Florida Pediatric Partners; and the offices of F. Mauricio Tijerino, Gladys Lopez-Urizar, Felix Peñate, Mario Palomino, and Ramon Maldonado; all in Miami-Dade County, Florida. The authors also thank Dr. Michael Campos of the University of Miami in Miami, Florida, and the staff of Kaiser Permanente in Portland, Oregon, for assisting with the validation process.
Footnotes
This project was supported by grant no. U59/CCU420713-02 and contract 200-2004-10148 from the Centers for Disease Control and Prevention.
REFERENCES
- 1.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National surveillance for asthma—United States, 1980–2004. MMWR Surveill Summ. 2007;56(8):1–54. [PubMed] [Google Scholar]
- 2.Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol. 2001;107:3–8. doi: 10.1067/mai.2001.112262. [DOI] [PubMed] [Google Scholar]
- 3.Thacker SB. Historical development. In: Teutsch SM, Churchill RE, editors. Principles and practice of public health surveillance. 2nd ed. New York: Oxford University Press; 2000. pp. 1–14. [Google Scholar]
- 4.Brown CM, Anderson HA, Etzel RA. Asthma. The states' challenge. Public Health Rep. 1997;112:198–205. [PMC free article] [PubMed] [Google Scholar]
- 5.Deprez RD, Asdigian NL, Oliver LC, Anderson N, Caldwell E, Baggott LA. Development of a prototype system for statewide asthma surveillance. Am J Public Health. 2002;92:1946–51. doi: 10.2105/ajph.92.12.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dombkowski KJ, Wasilevich EA, Lyon-Callo SK. Pediatric asthma surveillance using Medicaid claims. Public Health Rep. 2005;120:515–24. doi: 10.1177/003335490512000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris RD, Naumova EN, Goldring J, Hersch M, Munasinghe RL, Anderson H. Childhood asthma surveillance using computerized billing records: a pilot study. Public Health Rep. 1997;112:506–12. [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts EM, English PB, Van den Eeden SK, Ray GT. Progress in pediatric asthma surveillance I: the application of health care use data in Alameda County, California. [cited 2008 Feb 7];Prev Chronic Dis. 2006 3:A91. Also available from: URL: http://www.cdc.gov/pcd/issues/2006/jul/05_0186.htm. [PMC free article] [PubMed] [Google Scholar]
- 9.De Marco R, Locatelli F, Cerveri I, Bugiani M, Marinoni A, Giammanco G. Incidence and remission of asthma: a retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002;110:228–35. doi: 10.1067/mai.2002.125600. [DOI] [PubMed] [Google Scholar]
- 10.Eagan TM, Bakke PS, Eide GE, Gulsvik A. Incidence of asthma and respiratory symptoms by sex, age and smoking in a community study. Eur Respir J. 2002;19:599–605. doi: 10.1183/09031936.02.00247302. [published erratum appears in Eur Respir J 2003;21:735] [DOI] [PubMed] [Google Scholar]
- 11.Huovinen E, Kaprio J, Laitinen LA, Koskenvuo M. Incidence and prevalence of asthma among adult Finnish men and women of the Finnish Twin Cohort from 1975 to 1990, and their relation to hay fever and chronic bronchitis. Chest. 1999;115:928–36. doi: 10.1378/chest.115.4.928. [DOI] [PubMed] [Google Scholar]
- 12.Rönmark E, Perzanowski M, Platts-Mills T, Lundbäck B. Incidence rates and risk factors for asthma among school children: a 2-year follow-up report from the obstructive lung disease in Northern Sweden (OLIN) studies. Respir Med. 2002;96:1006–13. doi: 10.1053/rmed.2002.1391. [DOI] [PubMed] [Google Scholar]
- 13.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in national British cohort. BMJ. 1996;312:1195–9. doi: 10.1136/bmj.312.7040.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torén K, Hermansson BA. Incidence rate of adult-onset asthma in relation to age, sex, atopy and smoking: a Swedish population-based study of 15,813 adults. Int J Tuberc Lung Dis. 1999;3:192–7. [PubMed] [Google Scholar]
- 15.Yunginger JW, Reed CE, O'Connell EJ, Melton LJ, 3rd, O'Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am Rev Respir Dis. 1992;146:888–94. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 16.Antó JM, Sunyer J, Rodriguez-Roisin R, Suarez-Cervera M, Vazquez L Toxicoepidemiological Committee. Community outbreaks of asthma associated with inhalation of soybean dust. N Engl J Med. 1989;320:1097–102. doi: 10.1056/NEJM198904273201701. [DOI] [PubMed] [Google Scholar]
- 17.Navarro C, Márquez M, Hernando L, Galvan F, Zapatero L, Caravaca F. Epidemic asthma in Cartagena, Spain, and its association with soybean sensitivity. Epidemiology. 1993;4:76–9. doi: 10.1097/00001648-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Wallis DN, Webb J, Brooke D, Brookes B, Brown R, Findlay A, et al. A major outbreak of asthma associated with a thunderstorm: experience of accident and emergency departments and patients' characteristics. [cited 2008 Oct 16];BMJ. 1996 312:601–4. doi: 10.1136/bmj.312.7031.601. Also available from: URL: http://www.bmj.com/cgi/content/full/312/7031/601?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&fulltext=major+outbreak+of+asthma&searchid=1&FIRSTINDEX=0&fdate=1/1/1996&tdate=10/31/1997&resourcetype=HWCIT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White MC, Etzel RA, Olson DR, Goldstein IF. Reexamination of epidemic asthma in New Orleans, Louisiana, in relation to the presence of soy at the harbor. Am J Epidemiol. 1997;145:432–8. doi: 10.1093/oxfordjournals.aje.a009125. [DOI] [PubMed] [Google Scholar]
- 20.Antó JM, Sunyer J, Reed CE, Sabrià J, Martínez F, Morell F, et al. Preventing asthma epidemics due to soybeans by dust-control measures. N Engl J Med. 1993;329:1760–3. doi: 10.1056/NEJM199312093292402. [DOI] [PubMed] [Google Scholar]
- 21.Gunderson EK, Garland CF, Gorham ED. Health surveillance for asthma in the US Navy: experience of 9,185,484 person-years. Ann Epidemiol. 2005;15:310–5. doi: 10.1016/j.annepidem.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Osborne ML, Vollmer WM, Johnson RE, Buist AS. Use of an automated prescription database to identify individuals with asthma. J Clin Epidemiol. 1995;48:1393–7. doi: 10.1016/0895-4356(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 23.Clark NM, Brown R, Joseph CL, Anderson EW, Liu M, Valerio M, et al. Issues in identifying asthma and estimating prevalence in an urban school population. J Clin Epidemiol. 2002;55:870–81. doi: 10.1016/s0895-4356(02)00451-1. [DOI] [PubMed] [Google Scholar]
- 24.Guilbert T, Krawiec M. Natural history of asthma. Pediatr Clin North Am. 2003;50:523–38. doi: 10.1016/s0031-3955(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 25.Chorba TL, Berkelman RL, Safford SK, Gibbs NP, Hull HF. Mandatory reporting of infectious diseases by clinicians. JAMA. 1989;262:3018–26. [PubMed] [Google Scholar]
- 26.Matte TD, Hoffman RE, Rosenman KD, Stanbury M. Surveillance of occupational asthma under the SENSOR model. Chest. 1990;98(5) Suppl:173S–8S. doi: 10.1378/chest.98.5_supplement.173s. [DOI] [PubMed] [Google Scholar]
- 27.Nolan P. Should childhood asthma be reportable? Med Health R I. 1999;82:235. [PubMed] [Google Scholar]
- 28.Koutsavlis AT, Kosatsky T, Cox J, Goyer E. Reporting childhood asthma: why? Why not? What else? J Public Health Policy. 2001;22:311–9. [PubMed] [Google Scholar]
- 29.Jajosky RA, Harrison R, Reinisch F, Flattery J, Chan J, Tumpowsky C, et al. Surveillance of work-related asthma in selected U.S. states using surveillance guidelines for state health departments—California, Massachusetts, Michigan, and New Jersey, 1993–1995. MMWR Surveill Summ. 1999;48(3):1–20. [PubMed] [Google Scholar]
- 30.Census Bureau (US) American FactFinder. [cited 2007 Jun 22]. Available from: URL: factfinder.census.gov.
- 31.Council of State and Territorial Epidemiologists. CSTE position statement 1998-EH/CD 1. Asthma surveillance and case definition. [cited 2007 Jun 19]. Available from: URL: www.cste.org/ps/1998/1998-eh-cd-01.htm.
- 32.SAS Institute Inc. SAS: Version 9.1.3. Cary (NC): SAS Institute Inc.; 2002. [Google Scholar]
- 33.Centers for Disease Control and Prevention (US) 1999 BRFSS asthma questions. [cited 2007 Jun 22]. Available from: URL: www.cdc.gov/asthma/pdfs/BRFSSasthmaquestions.pdf.
- 34.National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma, full report 2007. [cited 2008 Feb 22]. Available from: URL: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf.
- 35.McDonald JC, Keynes HL, Meredith SK. Reported incidence of occupational asthma in the United Kingdom, 1989–97. Occup Environ Med. 2000;57:823–9. doi: 10.1136/oem.57.12.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karjalainen A, Kurppa K, Virtanen S, Keskinen H, Nordman H. Incidence of occupational asthma by occupation and industry in Finland. Am J Ind Med. 2000;37:451–8. doi: 10.1002/(sici)1097-0274(200005)37:5<451::aid-ajim1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 37.Census Bureau (US) State and county QuickFacts. [cited 2007 Jun 22]. Available from: URL: quickfacts.census.gov.