Conclusive evidence shows that maternal tobacco consumption increases the risk of infertility, subfertility, ectopic pregnancy, preterm birth, low birthweight, and stillbirth.1 Nevertheless, evidence with respect to the risk of spontaneous abortion (SA) (i.e., miscarriage) is inconsistent.2 In a case-control study, Kline et al. identified an increase in miscarriages in women who were smokers.3 In Canadian women, Armstrong et al. found a 20% increase in the risk of SA for every 10 cigarettes consumed daily.4 Other authors have also shown a positive association between cotinine levels in urine and SA.5,6 On the other hand, in Rasch's study, after adjusting for other risk factors, women who smoked 10 to 19 cigarettes and 20 or more cigarettes per day did not have significantly increased odds ratios (ORs) for having SAs.7
The role of paternal tobacco exposure in adverse reproductive effects has been scarcely evaluated. Some reports in the literature assert that tobacco consumption in men is associated with morphological alterations in sperm,8 a decrease in sperm density and motility,9,10 and a reduction in semen volume,11 which could have implications for male fertility and embryo viability. However, the scarce studies that to date have evaluated the association between exposure to paternal tobacco and SA are also contradictory.
Contrast that with the prospective study of Venners et al.,12 in which information on paternal tobacco consumption was collected directly from the father, and an 80% increase was observed in the risk of SA in people who smoked 20 or more cigarettes per day. In the case-control studies13–15 in which exposure to paternal tobacco was reported by the wife, no significant association was found between this exposure and the risk of SA.
According to the 2002 National Survey of Addictions (ENA 2002) in Mexico,16 70% of men between the ages of 18 and 65 reported having consumed tobacco in varying amounts, while in women this frequency was 29%. So, in case there was some effect of paternal tobacco consumption on the risk of SA, even if this effect was small, it could be important in absolute terms because the frequency of exposure in men is much higher than in women.
We evaluated the effect of maternal and paternal tobacco consumption on the risk of SA, through a nested case-control study within a cohort of pregnant women in four municipalities of the state of Morelos, Mexico.
METHODS
To evaluate the effect of organochlorines' environmental exposure on child neurodevelopment, in January 2001 we formed a cohort study of women of reproductive age who were not breastfeeding and who did not have a history of chronic diseases (e.g., hepatitis, renal, and/or thyroid disease). During the state's obligatory prenuptial marriage counseling before the civil wedding ceremony, 1,585 eligible women were identified. The response rate was 63%. Each participant, once informed of the study's objectives, signed a letter of informed consent. This study had the approval of the Ethics Committee of the National Institute of Public Health.17
At the time of entering the cohort, each participant and her partner was given a basal questionnaire that included sociodemographic and dietary characteristics, reproductive history, and tobacco and alcohol consumption. The couple was asked about tobacco, alcohol, and work history in the present and during the three years prior to their participation in the study.
Information on maternal and paternal alcohol consumption and maternal coffee consumption was obtained through a semi-quantitative questionnaire of food consumption. For each beverage item, there was a defined portion, and women and men were asked to choose from 10 consumption frequency options, which ranged from never to six times per day.
After this first interview, and taking into account the date of the last (menstrual) period reported in the basal questionnaire, each woman was contacted every six weeks to conduct an early detection of a possible gestation. Those who reported menstrual delays of 10 days or more were asked to take a pregnancy test and/or have a clinical exam to determine whether or not she was pregnant. If the pregnancy was confirmed, a visit was scheduled to learn about the evolution of the pregnancy and possible changes in the pregnant woman's habits during each trimester. The attrition rate during this stage of the study was 28%. For women who remained in the study and those who were lost to follow-up, no significant difference (p>0.05) was found among age (21.9 vs. 21.7 years), education level (10.7 vs. 10.4 years), occupation (45.6% vs. 44 %), and parity >1 (18.9% vs. 14.7%).
From the beginning of the study until July 1, 2004, 456 women in the cohort became pregnant, among whom 23 cases of SA were identified (defined as the loss of pregnancy during the first 20 weeks). For each case, up to four women whose pregnancies survived more than 20 weeks were randomly selected; cases and controls were matched by date of initiation of the last menstrual period (± 15 days), resulting in 84 controls, as it was not possible to find four controls that fulfilled the pairing criteria for all cases.
For the analysis, the maternal and paternal periconceptional consumption of tobacco was classified in four groups as follows: nonsmoking mother and nonsmoking father, smoking mother and nonsmoking father, nonsmoking mother and smoking father, and smoking mother and smoking father.
The paternal occupation was classified as “risky” and “not risky” according to the reported work activities and in view of the fact that, in previous studies, certain occupations have been associated with the risk of miscarriage.18 Occupations that were considered to be risky included poultry farmer, farmer, bricklayer, painter, maintenance worker, floriculturist (flower grower), fumigator (pesticide applier), and potter.
To estimate the association between maternal and paternal tobacco consumption, taking into account potential confounders that significantly contributed to explaining the event being studied, we constructed multiple conditional logistic regression models. To evaluate the presence of an exposure gradient, as a function of whether only the mother or the father or both parents smoked, the variable previously described was included as continuous in the final model. All analyses were conducted using Stata 9.2.19
RESULTS
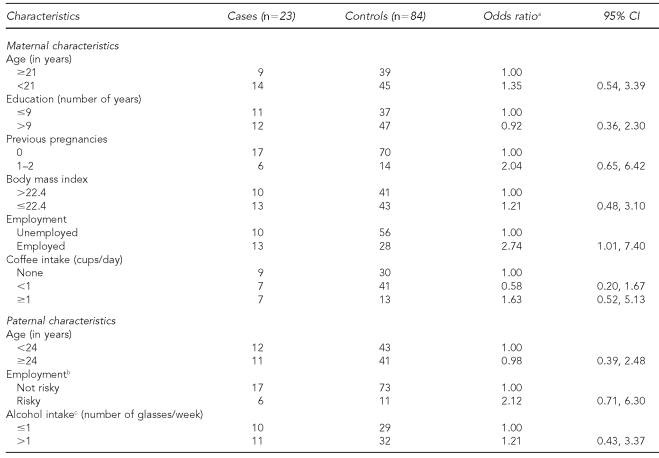
The frequency of SA among the cohort was 5%. The greatest number of SAs (90%) occurred before week 13; the median gestational age at which SA occurred was 10 weeks. Table 1 shows the risk of SA associated with some maternal and paternal characteristics of the study population. Only employed women were significantly associated with an increase in the risk of SA (OR=2.74, 95% confidence interval [CI] 1.01, 7.40).
Table 1. Risk of spontaneous abortion according to selected maternal and paternal characteristics.
aConditional regression models
bPoultry farmer, bricklayer, painter, maintenance worker, farmer, potter, flower grower, pesticide applier, or blacksmith were occupations considered to be risky.
cDue to missing values, numbers for these variables do not equal the total of cases and controls.
CI = confidence interval
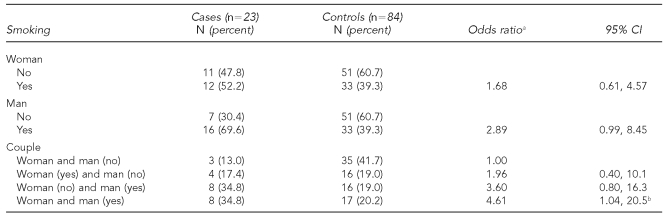
In 87% of the cases, one or both members of the couple reported being smokers, while for the controls this proportion was marginally lower (58%, p<0.05). Paternal or maternal tobacco consumption, considered separately, was associated in a nonsignificant manner with an increase in the risk of SA. However, when the combined exposure of both members was analyzed, it was observed that independently of age, occupation, and maternal caffeine consumption, as well as the father's occupation, those couples in which only the man was exposed or both members were exposed showed a risk of SA that was four times greater (p=0.03) compared with nonsmoking couples (Table 2).
Table 2. Adjusted effect of maternal and/or paternal smoking on spontaneous abortion risk.
aConditional regression model adjusted by: maternal age, maternal and paternal occupation, and maternal intake of coffee.
bp for trend=0.03
CI = confidence interval
DISCUSSION
To our knowledge, this is the first study to establish the presence of a dose-response relationship between maternal and/or paternal tobacco consumption and the risk of SA. Our results suggest that paternal tobacco consumption plays a relevant role in the increase of this risk, independently of the tobacco consumption reported by the woman.
In contrast with reports by other authors,3,4,6 we did not find a significant association between maternal tobacco consumption and SA, which could be partially explained by the difference in the methodology used in the study, the reduced sample size, and the fact that few women were in the category of high tobacco consumption.
Most of the studies have explained the adverse effects of tobacco on the evolution of pregnancy as a function of maternal exposure. Tobacco contains several components that are toxic for the developing embryo: nicotine, carbon monoxide, and mutagens.20 Nicotine has a vasoconstricting effect that reduces the flow to the placenta.21 Carbon monoxide attaches to hemoglobin and displaces oxygen, which causes maternal as well as fetal hypoxia. Tobacco has also been associated with inhibition of the aromatization of granulose cells and, as a consequence, with insufficiency of the corpus luteum.22 All of these mechanisms could decrease the viability of the product and increase the risk of SA.
In our study, although active maternal tobacco consumption was not significantly associated with the risk of SA, it is reasonable to consider that women who are smokers, and whose partners also smoke, are more intensely exposed to tobacco smoke, which would explain the association observed when both partners smoke.
An alternative hypothesis to explain the observed effect would be through paternal exposure to tobacco. As stated previously, some studies have found that tobacco consumption in men is associated with dyspermia, a decrease in sperm density, reduced sperm motility, or reduction in semen volume.10,11 Also, studies conducted with infertile couples who seek fertility treatment have found that the probability of carrying the pregnancy to term decreases in couples in which the man is a smoker.23 In a prospective study, Venners et al.12 found an independent association between paternal tobacco consumption and early SA; however, other authors have not found this association once they control for maternal tobacco consumption.13–15 It is worth noting that these last studies were of the case-control type in which the measurement of paternal tobacco consumption was conducted retrospectively through the mother, which could partially explain the differences in the results.
Limitations
With respect to the existence of biases that could provide alternative explanations to our results, we discard the presence of a selection bias among the controls because these were selected within the cohort, which guarantees that both cases and controls come from the same population. Also, there could be selection bias among the cases if the distribution of the tobacco habit were smaller among pregnancies that were lost early and which, as a consequence, could not be detected, than in losses that occurred at later stages of the pregnancy. However, the studies conducted in infertile couples—where there is strict monitoring to identify losses before the pregnancy is clinically diagnosed—reveal that tobacco consumption in both parents increases the probability of losses.24 In the same way, the study by Venners et al., in a cohort of women who provided a daily urine sample for early detection of conception, showed a significant association between paternal tobacco consumption and very early miscarriage.12 So, if there were a selection bias, it would be underestimating the observed OR.
On the other hand, we consider that the possibility of a selection bias in relation to smoking habits is low. The study population consisted of mainly urban and suburban residents and, according to the ENA 2002,16 about 34% of Mexican women from urban areas smoke or have smoked. This proportion is similar to our results (39%) among women in the control group.
The prospective study design allowed for the evaluation of exposure to be conducted prior to the occurrence of miscarriage, which decreased the possibility of a differential information bias. Nevertheless, due to the knowledge that the population possesses regarding tobacco's harmful effects on health, some smokers could have been classified as nonsmokers, and the association that was found in our study could be an underestimation of the real one.
Another strength of the study is the fact that our results were adjusted by variables that acted as potential confounders: maternal age, maternal coffee consumption, and paternal and maternal occupation. In addition, in the design, pairs were formed by gestational age according to what was suggested by Olsen and Skov25 for case-control studies in pregnant women.
The frequency of miscarriages in our population was less than what was generally reported in the literature (10% to 15%)26 and similar to what was found in other studies conducted with women in Mexico City.27 The differences found may be partially due to differences in the methodology used in the study: the definition of miscarriage as a function of gestational age (e.g., 20, 24, or 28 weeks), the studied population (e.g., primiparous or multiparous), and the study design.
Finally, we must take into account that the main limitations of this study were the reduced sample size and the scarce number of smokers in high exposure categories. These factors had an effect on the power of the study, as well as on our ability to evaluate the existence of a dose-response relationship between the number of cigarettes smoked by both members of a couple and the risk of SA. Also, the reduced number of cases did not allow us to evaluate whether maternal and/or paternal tobacco consumption is mainly associated with early or late SA.
CONCLUSION
Our results provide additional evidence on the deleterious effect of maternal and/or paternal tobacco consumption on gestation and the need to maintain and intensify campaigns to reduce tobacco consumption in the population. Larger prospective studies that evaluate active and secondhand smoking exposure in both parents, including biomarkers of exposure like cotinine before and during pregnancy, are needed to detect the potential mechanism (female or male gamete damage) and to identify the true window of tobacco exposure for early and later miscarriage risk.
Footnotes
This study was supported by Consejo Nacional de Ciencia y Tecnología de México, Conacyt (31034-M, 41708, 13915); Mount Sinai School of Medicine International Training and Research in Environmental and Occupational Health Program (D43TW00640); and International Exchange Program (T37MD001452).
REFERENCES
- 1.Rogers JM. Tobacco and pregnancy: overview of exposures and effects. Birth Defects Res C Embryo Today. 2008;84:1–15. doi: 10.1002/bdrc.20119. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health and Human Services (US) The health consequences of smoking: a report of the Surgeon General. Rockville (MD): DHHS, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health (US); 2004. [Google Scholar]
- 3.Kline J, Stein ZA, Susser M, Warburton D. Smoking: a risk factor for spontaneous abortion. N Engl J Med. 1977;297:793–6. doi: 10.1056/NEJM197710132971501. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong BG, McDonald AD, Sloan M. Cigarette, alcohol, and coffee consumption and spontaneous abortion. Am J Public Health. 1992;82:85–7. doi: 10.2105/ajph.82.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George L, Granath F, Johansson AL, Anneren G, Cnattingius S. Environmental tobacco smoke and risk of spontaneous abortion. Epidemiology. 2006;17:500–5. doi: 10.1097/01.ede.0000229984.53726.33. [DOI] [PubMed] [Google Scholar]
- 6.Ness RB, Grisso JA, Hirschinger N, Markovic N, Shaw LM, Day NL, et al. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med. 1999;340:333–9. doi: 10.1056/NEJM199902043400501. [DOI] [PubMed] [Google Scholar]
- 7.Rasch V. Cigarette, alcohol, and caffeine consumption: risk factors for spontaneous abortion. Acta Obstet Gynecol Scand. 2003;82:182–8. doi: 10.1034/j.1600-0412.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 8.Ozgur K, Isikoglu M, Seleker M, Donmez L. Semen quality of smoking and non-smoking men in infertile couples in a Turkish population. Arch Gynecol Obstet. 2005;271:109–12. doi: 10.1007/s00404-003-0572-z. [DOI] [PubMed] [Google Scholar]
- 9.Sofikitis N, Miyagawa I, Dimitriadis D, Zavos P, Sikka S, Hellstrom W. Effects of smoking on testicular function, semen quality and sperm fertilizing capacity. J Urol. 1995;154:1030–4. [PubMed] [Google Scholar]
- 10.Zinaman MJ, Brown CC, Selevan SG, Clegg ED. Semen quality and human fertility: a prospective study with healthy couples. J Androl. 2000;21:145–53. [PubMed] [Google Scholar]
- 11.Pasqualotto FF, Sobreiro BP, Hallak J, Pasqualotto EB, Lucon AM. Cigarette smoking is related to a decrease in semen volume in a population of fertile men. BJU Int. 2006;97:324–6. doi: 10.1111/j.1464-410X.2005.05906.x. [DOI] [PubMed] [Google Scholar]
- 12.Venners SA, Wang X, Chen C, Wang L, Chen D, Guang W, et al. Paternal smoking and pregnancy loss: a prospective study using a biomarker of pregnancy. Am J Epidemiol. 2004;159:993–1001. doi: 10.1093/aje/kwh128. [DOI] [PubMed] [Google Scholar]
- 13.Chatenoud L, Parazzini F, di Cintio E, Zanconato G, Benzi G, Bortolus R, et al. Paternal and maternal smoking habits before conception and during the first trimester: relation to spontaneous abortion. Ann Epidemiol. 1998;8:520–6. doi: 10.1016/s1047-2797(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 14.Windham GC, Swan SH, Fenster L. Parental cigarette smoking and the risk of spontaneous abortion. Am J Epidemiol. 1992;135:1394–403. doi: 10.1093/oxfordjournals.aje.a116250. [DOI] [PubMed] [Google Scholar]
- 15.Windham GC, Von Behren J, Waller K, Fenster L. Exposure to environmental and mainstream tobacco smoke and risk of spontaneous abortion. Am J Epidemiol. 1999;149:243–7. doi: 10.1093/oxfordjournals.aje.a009798. [DOI] [PubMed] [Google Scholar]
- 16.Instituto Nacional de Estadistica Geografia e Informatica. Encuesta Nacional de Adicciones 2002. 2004. [cited 2008 May 12]. Available from: URL: http://www.inegi.gob.mx/prod_serv/contenidos/espanol/bvinegi/productos/continuas/sociales/salud/2004/Ena02.pdf.
- 17.Torres-Sanchez L, Rothenberg SJ, Schnaas L, Cebrian ME, Osorio E, Del Carmen Hernandez M, et al. In utero p,p′-DDE exposure and infant neurodevelopment: a perinatal cohort in Mexico. Environ Health Perspect. 2007;115:435–9. doi: 10.1289/ehp.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savitz DA, Sonnenfeld NL, Olshan AF. Review of epidemiologic studies of paternal occupational exposure and spontaneous abortion. Am J Ind Med. 1994;25:361–83. doi: 10.1002/ajim.4700250306. [DOI] [PubMed] [Google Scholar]
- 19.StataCorp. Stata: Release 9.2. College Station (TX): StataCorp.; 2005. [Google Scholar]
- 20.Stillman RJ, Rosenberg MJ, Sachs BP. Smoking and reproduction. Fertil Steril. 1986;46:545–66. doi: 10.1016/s0015-0282(16)49628-7. [DOI] [PubMed] [Google Scholar]
- 21.Economides D, Braithwaite J. Smoking, pregnancy and the fetus. J R Soc Health. 1994;114:198–201. doi: 10.1177/146642409411400406. [DOI] [PubMed] [Google Scholar]
- 22.Gocze PM, Szabo I, Freeman DA. Influence of nicotine, cotinine, anabasine and cigarette smoke extract on human granulosa cell progesterone and estradiol synthesis. Gynecol Endocrinol. 1999;13:266–72. doi: 10.3109/09513599909167565. [DOI] [PubMed] [Google Scholar]
- 23.Joesbury KA, Edirisinghe WR, Phillips MR, Yovich JL. Evidence that male smoking affects the likelihood of a pregnancy following IVF treatment: application of the modified cumulative embryo score. Hum Reprod. 1998;13:1506–13. doi: 10.1093/humrep/13.6.1506. [DOI] [PubMed] [Google Scholar]
- 24.Pattinson HA, Taylor PJ, Pattinson MH. The effect of cigarette smoking on ovarian function and early pregnancy outcome of in vitro fertilization treatment. Fertil Steril. 1991;55:780–3. doi: 10.1016/s0015-0282(16)54248-4. [DOI] [PubMed] [Google Scholar]
- 25.Olsen J, Skov T. Design options and methodological fallacies in the studies of reproductive failures. Environ Health Perspect. 1993;2(101) Suppl:145–52. doi: 10.1289/ehp.93101s2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:839–54. doi: 10.1053/beog.2000.0123. [DOI] [PubMed] [Google Scholar]
- 27.Borja-Aburto VH, Hertz-Picciotto I, Rojas Lopez M, Farias P, Rios C, Blanco J. Blood lead levels measured prospectively and risk of spontaneous abortion. Am J Epidemiol. 1999;150:590–7. doi: 10.1093/oxfordjournals.aje.a010057. [DOI] [PubMed] [Google Scholar]