Summary
Small RNA molecules have been known and utilized to suppress gene expression for more than a decade. The discovery that these small RNA molecules are endogenously expressed in many organisms and have a critical role in controlling gene expression have led to the arising of a whole new field of research. Termed small interfering RNA (siRNA) or microRNA (miRNA) these ~22 nt RNA molecules have the capability to suppress gene expression through various mechanisms once they are incorporated in the multi-protein RNA-Induced Silencing Complex (RISC) and interact with their target mRNA. This review introduces siRNAs and microRNAs in a historical perspective and focuses on the key molecules in RISC, structural properties and mechanisms underlying the process of small RNA regulated post-transcriptional suppression of gene expression.
A PubMed search for the terms RNA interference (RNAi), antisense RNA or short-interfering RNA (siRNA, also called small-interfering RNA) yields more than 10000 research papers going back to 1985. A similar search for microRNA (miRNA) yields about 2000 papers, of which 1500 have been published in the last two years, indicating a huge interest and effort in this field. Nevertheless, a search for Argonaute-2 (Ago2) or RNA-induced silencing complex” (RISC) yields 300 papers going back only to 1998 (http://www.ncbi.nlm.nih.gov/entrez). This indicates that while the RNAi phenomenon has been observed, investigated and even utilized for more than two decades and intensively for the past several years, only recently have the key molecules and mechanisms underlying the process been laboriously uncovered.
In 1985, a group of researchers stated that “The demonstration that a specific messenger RNA can be functionally inactivated in vivo by hybridization to complementary polynucleotide sequences suggests a direct approach to the study of gene function in cells of higher organisms” [1]. This gave rise to a new and very dynamic field of research which represents one of the most exciting topics nowadays. Known as RNA interference (RNAi) for its ability to interfere with protein expression at the mRNA level, researchers have used this technology for over two decades despite the underlying mechanism remaining mostly a mystery. Originally, antisense RNA technology was utilized, but the more efficient double-stranded RNA (dsRNA) techniques, maximizing RNAi efficiency, have progressively replaced it [2].
Ever since the discovery of RNAi, researchers have been in hot pursuit of the molecular machinery involved and the mechanisms by which that machinery works. Particular emphasis has been placed on understanding the RNA-Induced Silencing Complex, also known as RISC. This complex consists of several proteins and RNA molecules that altogether act as the key actors of RNAi, promoting mRNA degradation, repression of translation, and remodeling of chromatin structure.
Of the many proteins in RISC, only a few have been characterized at the functional level. Several have been identified after biochemical purification of the RISC from Drosophila melanogaster S2 cells extracts. These include Argonaute-2 (Ago2, also called eiF2C2), the Drosophila Fragile X-Related protein (dFXR, also called dFMR1), the Vasa Intronic Gene (VIG), and the micrococcal nuclease family member Tudor-SN [3–5].
Tudor-SN was first suggested to be the RNase responsible for the mRNA degrading ability of RISC [4], but has since been proven incorrect. Rather, a new function has been attributed to this protein as Tudor-SN binds to hyper-edited dsRNAs and promotes their degradation. Hyper-edited dsRNAs contain adenosine residues that have been converted to inosine through the activity of deaminases called “adenosine deaminases that act on RNA” or ADARs [6]. Shortly thereafter, the function of Tudor-SN has been extended to the degradation of miRNA precursors as it has been shown that pri-miR-142 was also hyper-edited by some ADARs, therefore promoting its degradation by Tudor-SN [7].
Tuschl and co-workers were the first to highlight the function of Argonaute2 or Ago2 as the core nuclease of the RISC machinery. They demonstrated that purified FLAG/HA-epitop-etagged Ago isoforms containing protein complexes from different human cell lines were only able to exhibit RISC-related endonuclease activity when they were associated with Ago2. This suggested that at least Ago2 is a key component of the RNAi machinery, and that it could also be the RNase directed by siRNA/miRNA towards mRNA [8]. This hypothesis was later confirmed, as mixing in vitro purified Ago2 with siRNAs and a target mRNA can reconstitute the RISC catalytic activity [9,10]. Further supporting evidence came from crystallographic analyses and directed mutagenesis studies, which revealed the organization of the core catalytic site as well as the structure of the complex formed between Ago2, the siRNA and the targeted mRNA [9]. Indeed, the research team of Joshua-Tor showed that the Ago2 catalytic site has a folding structure closely related to the RNase H family as an intact DDH (D597, D669, H807 in human Ago2) motif is absolutely required for catalytic activity. Moreover, they showed that the 5’end of the siRNA, described as being phosphorylated in vivo, is of major importance for siRNA recognition by Ago2. In contrast, in their purely in vitro system, Ago2 does not require ATP for the endonucleolytic cleavage [9]. Utilizing knockdown strategies of RISC proteins Crooke et al. showed Ago2 to be the rate limiting component for RISC mRNA silencing [11].
Knowing that the RNAi machinery targets specific mRNAs through Watson-Crick base-pair interactions between the target mRNAs and siRNAs or miRNAs, the biogenesis of siRNAs originating from the processing of dsRNA was elucidated and the role of Dicer in the maturation of dsRNAs into efficient ~22 nucleotides (nt) long siRNAs was uncovered [12]. Dicer is a ~200 kDa protein containing ATPase/RNA helicase and PAZ domains, two catalytic RNase III domains, and a C-terminal dsRNA binding domain (dsRBD). Interestingly, there are two different Dicers isoforms: Dcr-1 and Dcr-2 in the Drosophila melanogaster species whereas only one Dicer exists in vertebrates and Caenorhabditis elegans. In D. melanogaster, Dcr-1 is described as binding and processing miRNAs precursors whereas Dcr-2 prefers siRNA precursors. The specificity of these two isoforms reflects some preferences for two very different types of substrates as miRNA precursors are imperfectly paired stem loops, whereas siRNA precursors are typically long dsRNA helices [13].
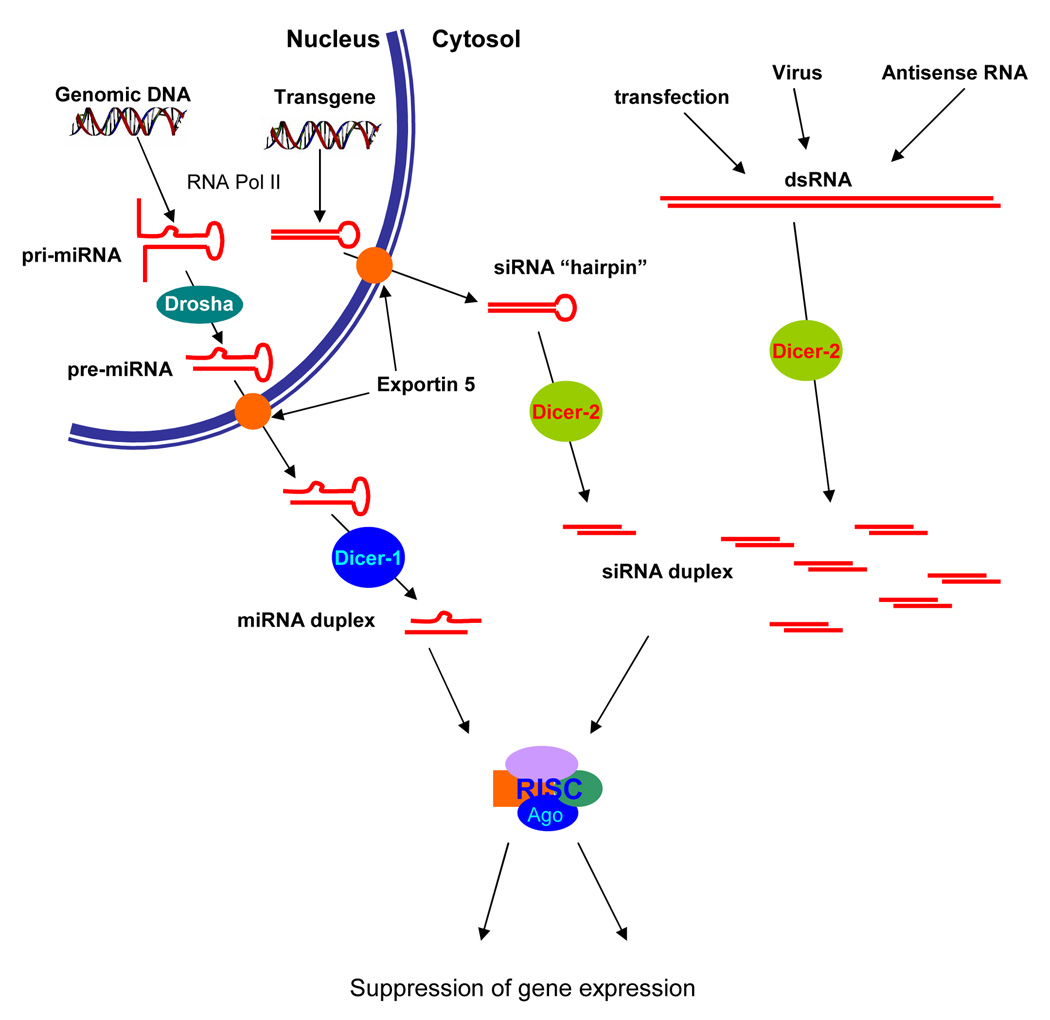
With the relationship between RNAi and the newly discovered world of miRNAs having been established, new biological functions for these small non-coding transcripts were progressively discovered. As already mentioned above, Dicer is required for the maturation of miRNAs. After transcription of miRNA genes, the nuclear RNase Drosha shortens the primary transcripts to generate pre-miRNAs of about 70 nt with an hairpin-like structure [14,15]. After translocation to the cytoplasm these pre-miRNAs are further processed by Dicer into mature miRNA duplexes of ~22 nt long [12,14]. See figure 1 for a comparison between the biogenesis of miRNAs and siRNAs in D. melanogaster.
FIGURE 1. miRNA and siRNA biogenesis in D. melanogaster.
microRNA genes are transcribed into precursors pri-miRNAs, which are first processed by Drosha in the nucleus to generate an imperfect hairpin-like pre-miRNA structure. By contrast, transgenes expressed to produce siRNAs do not require this first maturation step. Pre-miRNAs or pre-siRNAs are then transferred to the cytosol by the exportin-5, and then a second maturation step occurs by the Dicer proteins. In D. melanogaster, Dicer-2 processes perfectly matching helices typical of dsRNA whereas Dicer-1 prefers imperfectly matching hairpin-like structures. After Dicer cleavage, duplexes with 3’overhangs and phosphorylated 5’ termini are released, being either perfectly or imperfectly matching. After unwinding of these duplexes the guide strand is then incorporated into RISC.
Recently the relationship between miRNAs and RNAi has been further strengthened with the observation that some miRNAs, such as let-7 or miR16, can promote mRNA degradation like siRNAs.
RISC-Dependent mRNA Degradation
Once it was understood that RISC utilizes ~22 nt RNA duplexes of siRNAs or miRNAs as probes to target mRNAs for degradation in a sequence specific manner, the mechanisms underlying RNAi were explored and began to be uncovered. Since then a lot of effort has been directed at understanding the requirements for the siRNA strands to be uploaded into RISC and to be catalytically efficient.
Tuschl and co-workers used HeLa S100 cell lysates as a human system to replace the D. melanogaster embryo lysate system previously utilized and showed that RISC contains only one of the two strands of the siRNA duplex. Using UV-labile biotinylated siRNAs to precipitate RISC-bound complexes, they showed that only the 3’ biotinylated anti-sense siRNA strands were able to pull down an enzymatically active RISC [16]. They further observed that chemical modifications at the 5’ terminus of siRNAs abolish RISC activity whereas in cultured cells the presence of a phosphate group at this position allows efficient silencing of endogenous genes [16]. Using 13 to 29 nt siRNAs directed against Lamin A/C, they observed that siRNAs longer than 19 nt and phosphorylated at the 5’ terminus were significantly more efficient to knock-down Lamin A/C expression.
In a parallel study in cultured HeLa cells using the GFP fluorescence intensity as an indicator of knock-down efficiency, Chiu and Rana showed that the hydroxyl groups on the 5’ phosphate of the antisense strand of the siRNA duplex are required for RNAi, whereas blocking of the 3’ end has little effect on RNAi [17]. Unfortunately, this technical approach cannot address the question whether the silencing phenomenon could be a consequence of translational suppression, mRNA degradation or another process [17]. On the other hand, Tuschl and co-workers showed in their system that siRNA-targeted mRNAs are subjected to degradation by means of an early endonucleolytic cleavage [16], which is consistent with the activity described by Joshua-Tor and co-workers in an in vitro RISC reconstitution experiment [9].
The next major feature of the siRNA duplex to be uncovered was the structural asymmetry, which was unravelled by Zamore and co-workers in 2003. They observed that the two strands of an siRNA duplex are not equally eligible for assembly into RISC [18,19]. The thermodynamic stability of the extremities of the siRNA duplex determines the degree to which each strand participates in the RNAi pathway. This type of asymmetry is also observed for many miRNAs, and therefore the authors proposed a model in which both miRNA and siRNA duplexes are unwound by an ATP-dependent helicase, giving a rational explanation why only one siRNA strand is incorporated into RISC and why many miRNAs accumulate in vivo as single strands while the other strand is degraded.
Using firefly luciferase sense and antisense mRNA targets and corresponding siRNA duplexes in D. melanogaster embryo lysate, Zamore and co-workers showed that while both strands can direct mRNA degradation, certain structural features determine which strand will actually be incorporated into RISC. In duplexes not containing 3’overhangs both strands are equally eligible for RISC incorporation (albeit with a very low specific activity). By contrast, in duplexes containing 3’overhangs the strands display an asymmetric capacity to induce RNAi.
Zamore and co-workers utilized single nucleotide mismatches on either strand of the siRNA duplex but adjacent to the 3’ overhang to determine which strand is loaded into RISC. They showed that unwinding of the duplexes begins from the thermodynamically less stable extremity and that the strand incorporated into RISC has its 5’ end oriented to the less stable extremity of the duplex [19].
In 2004, the team of Zamore progressed further in explaining siRNA asymmetry. They showed in the D. melanogaster embryo lysate that the heterodimer between Dcr-2 and R2D2 functions as a polarized sensor of thermodynamic stability at the extremities of the siRNA duplex. Using 5-iodouracil groups located on the 20th nucleotide of the siRNA strands that photo-crosslinked to R2D2 and Dcr-2 [20], they showed that R2D2 binds more strongly to the 5-iodouracyl group closer to the more thermodynamically stable end of the siRNA duplex whereas Dcr-2 binds more strongly to the 5-iodouracyl group closer to the 5’ less stable extremity. As a result, Dcr-2 is in closer proximity to the 3’overhang of the strand meant to be destroyed (the passenger strand) and R2D2 crosslinks more easily to the 3’ overhang of the strand that directs the RNAi (the guide strand) [21]. This makes sense considering that Dcr-2 contains a helicase domain and has previously been shown to be the origin of the siRNA duplex unwinding [19].
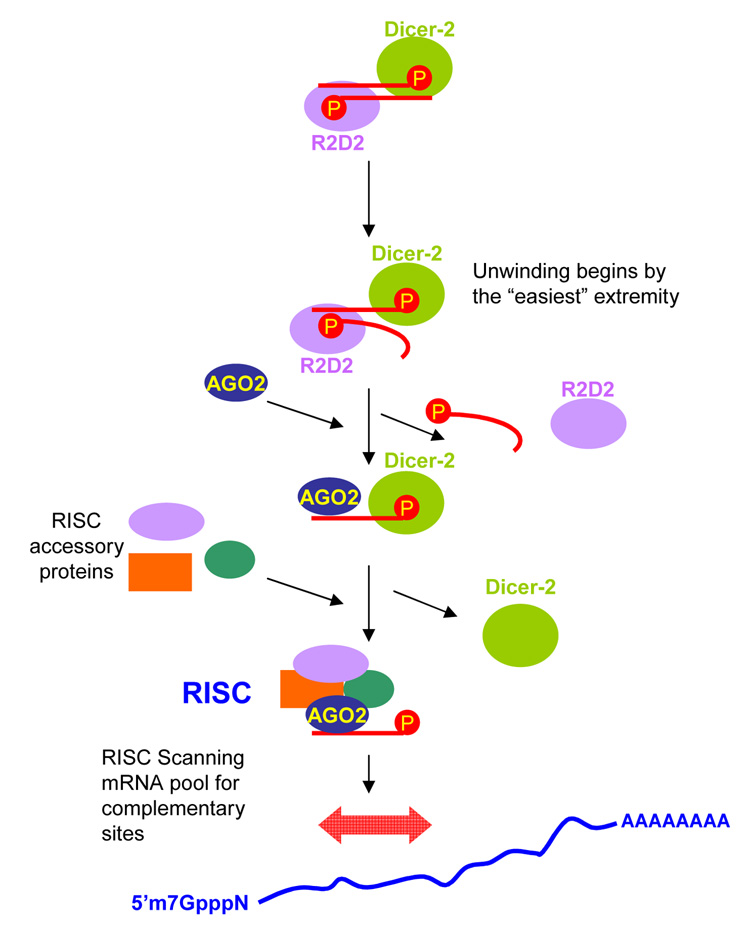
Zamore and co-workers also showed in the same photo-crosslinking experiment that when siRNAs are incubated in the D. melanogaster embryo lysate in the presence of ATP, the affinity of R2D2 for the guide strand decreases and progressively R2D2 is exchanged for Ago2. By contrast, the affinity of Dcr-2 for the siRNA duplex does not seem to be affected with time [22]. As a result, they suggest a model for RISC assembly in which R2D2 first orients the Dcr-2/R2D2 heterodimer on the siRNA within the RISC Loading Complex (RLC). Secondly, as siRNA unwinding proceeds, the heterodimer changes and R2D2 is exchanged for Ago2, the core component of RISC. Finally they hypothesized that unwinding occurs only when Ago2 is available, so siRNA duplexes in RLC are unwound only when RISC can be assembled [23]. See Figure 2 for a pictorial of the guide strand selection process.
FIGURE 2. The R2D2/Dicer-2 heterodimer directs which siRNA strand is incorporated into RISC.
The R2D2/Dicer-2 heterodimer binds to siRNA duplexes and acts as a sensor of the thermodynamic stability of the siRNA ends. R2D2 binds to the more stable extremity whereas Dicer-2 binds more strongly to the less stable extremity. Unwinding of the duplex starts from the thermodynamic less stable extremity in a mechanism remaining mostly unknown. The strand having its 5’ terminus located at this end is loaded in the RISC and directs RNAi. The first step in the assembly of a cleavage-competent RISC occurs with the exchange of R2D2 for Ago2. Subsequently, other accessory proteins are incorporated and Dicer-2 dissociates from the complex.
It should be noted though that for some miRNAs, there is no well defined polarity in terms of thermodynamic stability on either end of the diced duplex. Thus, in these cases both strands of the pre-miR stem loop may be incorporated in RISC. Following their thermodynamic stability rules of siRNA strand selection, Zamore and co-workers [19] predicted that a number of miRNAs would accumulate in both the guide (miRNA) and passenger (miRNA*) strand, including D. melanogaster miR-2-a-2, miR-4 and miR-10, which was confirmed by Tuschl and co-workers [24].
Similarly in mammalian cells, a screen for miRNAs in human embryonic stem cells revealed four novel miRNA genes (the miR-302 family and miR-373) yielding small RNAs from both strands in the pre-miR stem. Typically, but not in all cases, the expression level of the predicted passenger strand was lower than that of the predicted guide strand, indicating that the thermodynamic stability rule is generally valid though the mechanism of strand selection for miRNA is not as straightforward as that of siRNA and may involve additional mechanisms in mammalian cells [25].
This was particularly demonstrated more recently, when Ro et al. [26] reported that a large number (234 out of 969) of individually reported human and mouse miRNAs in the miRBase database were actually miRNA pairs. A miRNA pair is defined as miRNA and miRNA*, or miR-5p, derived from the 5’ end of the pre-miR stem loop, and miR-3p, derived from the 3’ end of the same stem loop.
Expression profiling analysis in mouse tissue showed that in some tissues, both strands of a given pre-miRNA accumulated whereas in other tissue only one strand of the same pre-miR could be detected. For example for miR-30e, both 5’ and 3’ strands accumulate in roughly equal amounts in liver tissue, whereas in heart tissue the 5’ strand is preferentially accumulated and in stomach tissue the 3’ strand dominates. This accumulation was apparently independent of the thermodynamic stability of the hairpin, which predicts the 3’ strand to accumulate. Furthermore, similar numbers of targets were predicted for the 5’-strand and the 3’-strand of a given predicted miRNA pair, and functionality of both strands was shown in the case of miR-30e. This led the authors to conclude that in the case of miRNAs, thermodynamic asymmetry is not the sole determining factor for which strand of the duplex accumulates and that yet unknown, tissue dependent mechanisms are involved in miRNA biogenesis and target selection.
Based on the results of this single report, the number of functional miRNAs and targets seems much larger than previously anticipated, though more studies are needed to strengthen this theory. If the accumulation of a miRNA is related to the presence of a target mRNA which would allow it to be incorporated and stabilized in RISC remains to be investigated.
In two recent publications [27,28], the team of Zamore showed that in flies, siRNAs and miRNAs are sorted into Ago1 or Ago2 complexes independent from their biogenesis pathway, but depending on the structural features of the short RNA molecule. Whereas a perfectly matching let-7 RNA duplex loaded mostly in Ago2-RISC, the introduction of a central mismatch on position 9 of the duplex shifted the balance towards Ago1-RISC loading. Addition of recombinant Dcr-2/R2D2 increased the amount of mismatched duplex into Ago2-RISC and decreased loading into Ago1-RISC, suggesting that a central mismatch reduces the affinity of the duplex to Dcr-2/R2D2 RISC Loading Complex and that there is competition between Ago-1 and Ago-2 pathways. Determination of the affinity of the Dcr-2/R2D2 complex for different small RNA duplexes confirmed that a central mismatch reduced the affinity of the complex for the RNA duplex. Even in the absence of the Ago2 loading machinery, Ago1 was preferentially loaded with mismatched RNA duplexes and largely rejected a perfectly matching duplex, indicating that both Ago1 and Ago2 pathways are selective. Furthermore, Ago1 was loaded more rapidly with its authentic let-7 substrate in the absence of the Ago2 loading machinery, which may suggest that miRNA duplexes transiently bind Dcr-2/R2D2 without leading to Ago-2 RISC formation [28].
In an accompanying paper, it was shown that D. melanogaster miRNAs are sorted in functionally distinct Argonaute complexes after cleavage by Dicer-1 [27]. The Drosophila miR-277 duplex is predicted to have a more double stranded character than a typical miRNA and thus resembles a siRNA. The authors investigated whether this miRNA resembling a siRNA would be loaded into Ago2 rather than Ago1, addressing the question whether the biogenesis of an miRNA is tightly coupled to its loading in Ago1. To that end, S2 cell lines were created expressing GFP reporter mRNA containing 2 perfect matching miR-277 sites or with 4 sites containing mismatches at positions 9, 10 and 11. Repression of the reporter bearing fully complementary miR-277 binding sites required Ago2 but not Ago1. Conversely, the repression of the reporter containing 4 mismatched miR-277 sites depended on Ago1.
Both papers indicate that in flies, the production of small RNA duplexes by Dicer is uncoupled from the consequent loading into Ago complexes. Whether differential loading of small RNA duplexes into functionally different Ago complexes also occurs in mammals in not yet known, but as discussed later, both miRNA and siRNA-mediated regulation of gene expression are dependent on Ago2.
Recently, several research teams have highlighted a possible role of miRNAs in mRNA degradation, which contrasts to most previous reports connecting miRNAs solely to translational repression. First, using microarray studies, Johnson and co-workers showed that some miRNAs downregulate a large number of mRNAs, possibly by directly targeting transcripts and reducing their cellular levels [29]. Even more intriguing, Han and co-workers showed that miR-16 is required for the fast decay of AU-rich containing mRNAs (the details of which are discussed below) [30]. Finally, another report stated that let-7 and lin-4 targeting results also in mRNA degradation [31], unveiling a new function of let-7 and challenging the previously held notion that it only functions by translational suppression [32–34].
mRNA Degradation : the AU-Rich Elements (ARE) Case Study
Some mRNAs display very short half-lives and are therefore referred to as unstable. Amongst those, AU-rich elements (ARE)-containing mRNAs have been widely studied for the past twenty years. cFos was one of the first proteins to be described as having an ARE in the 3’ untranslated region (3’UTR) of its mRNA, dictating its stability and therefore the expression of the corresponding protein [35,36].
The decay of cFos mRNA can be described as a two step process. First, the poly(A) tail undergoes deadenylation in an ARE independent manner, and secondly, mRNA degradation occurs. This latter requires an intact AUUUA pentamer sequence which is a key feature of most ARE [35]. Since the first observation of ARE in c-Fos mRNA, numerous other mRNAs have been studied and found to contain ARE. These include, mRNAs of Interleukins, Tumor Necrosis Factor Alpha (TNF-α), cyclooxygenase-2 (Cox-2) and inducible nitric oxide synthase (iNOS) [37–42].
Considering the major role of AREs in the regulation of gene expression, many teams have tried to identify ARE-binding proteins in order to assess their role in destabilizing ARE-containing mRNAs. The ubiquitously expressed Elav-like protein HuR was first shown to bind to a core element of 27 nucleotides that contain AUUUA, AUUUUA, and AUUUUUA motifs [43,44]. Later, it was shown that HuR is actually a mRNA stabilizing protein that in the nucleus may bind to ARE-containing mRNAs in order to provide protection against degradation that occurs in the cytoplasm after export [45].
By contrast, the TIA-1/TIAR proteins have also been shown to be able to bind uridylate-rich sequences and, particularly, ARE but their functionality has been linked primarily to translational suppression [46–48].
The lowly expressed but inducible cytosolic protein Tis11, also known as tristetraprolin (TTP), is a Zinc binding protein displaying three typical motifs made of four proline residues. TTP has been shown to be a key figure in the destabilization of TNF-α mRNA through direct binding to the ARE [49–51]. As a structural feature, TTP has a strong preference for the core sequence UUAUUUAUU [52]. Finally, TTP promotes the deadenylation of ARE-containing transcripts in a Mg2+ dependent manner [53].
Recently, a milestone discovery connected the ARE-dependent mRNA degradation to RNAi, and more specifically to miRNA-dependent mRNA degradation. Using a screening method in D. melanogaster S2 cells by the use of long dsRNA, Han and co-workers established which key molecules are required for the fast degradation of ARE-containing mRNAs. They showed that in addition to TTP, Dicer and Argonaute proteins are required for the decay of a chimeric rabbit globin mRNA reporter containing 3’UTR from different well known ARE-containing mRNAs. Furthermore, as the link between miRNAs and the instability of these reporter mRNAs was uncovered, they found in human HeLa cells, that the instability of their reporters was a consequence of miR-16 and of miR-289 in D. melanogaster S2 cells. Interestingly, these miRNAs are not fully complementary to their target 3’UTR with only the core sequence UAAAUAUU matching perfectly to the ARE [30]. Surprisingly, the complementary sequence of miR-16 to the ARE is not located in the seed region, and target mRNA degradation is dependent on the ARE binding protein TTP, though miR-16 does not directly bind to TTP. This poses the interesting concept that an RNA binding protein bound to the 3’ UTR of a mRNA is either able to modify RISC activity or enhance the affinity for a poor matching miRNA, thus triggering mRNA degradation.
Recently, Hua et al. described another ARE-containing mRNA encoding VEGF, to be repressed by miR-16 in a mammalian cell line. These authors showed that both VEGF mRNA levels as well as protein levels were downregulated under conditions of hypoxia. The exact mechanism by which miR-16 represses VEGF remains to be determined, but as VEGF mRNA levels were decreased the repression might partly be mediated by an increased VEGF mRNA degradation [54].
RISC-Dependent mRNA Translational Repression
During the earliest days of miRNA discovery, the miRNA let-7 was thought to regulate gene expression at the post-transcriptional level without affecting the stability of the targeted transcripts [32] This was later proved inaccurate as some miRNAs have been linked to a RISC-dependent mRNA destabilization effect [29–31,55] as already mentioned earlier. Nonetheless, most miRNAs are currently better known for their roles in translational suppression.
Coinciding with the link between miRNAs/siRNAs and translational suppression, several studies have shown that some core components of the RNAi machinery interact with protein expression by triggering translational suppression. Siomi and co-workers showed that a Drosophila Fragile X-Related protein (dFXR) interacts with components of RNAi and with ribosomal proteins [56]. In another study, Hammond and co-workers also co-purified both FXR and the Vasa Intronic Gene product (VIG) in association with RISC [3]. The fragile X syndrome is an inherited form of mental retardation caused by the loss of FXR expression, which is an RNA-binding protein associated with translating ribosomes and acting as a translational suppressor. Purification of the complex associated with D. melanogaster FXR (dFXR), revealed that the complex also includes the 5S RNA, two ribosomal proteins: L5 and L11 as well as Ago2 and a D. melanogaster homolog of p68 helicase (Dmp68), shown to be required for RNAi. Furthermore, dFXR is also associated with Dicer and miRNAs in vivo, supporting the idea that dFXR is involved in RNAi. Altogether, these results suggest that dFXR could link RNAi to the regulation of the translational machinery [3,56]. Historically, most of the data linking repression of translation and RISC come from direct studies of miRNA/siRNA functions in order to impair translation efficiency.
Sharp and co-workers were the first to highlight that siRNAs could also function as miRNAs [55]. Using siRNAs with a central mismatch, as compared to their mRNA targets, or bulged siRNAs they showed that a reporter luciferase expression system was repressed through a mechanism not affecting the intracellular concentration of luciferase mRNA and consequently related to a translational suppression mechanism. They further showed that while the sequence of the bulge was not important for translational repression, an increase in the number of binding sites for the bulged siRNAs improves the translational suppression efficiency when normalized per binding site. In comparison, increasing the number of binding sites for perfectly matching siRNAs does not increase the repression efficiency when normalized per binding site, demonstrating an interesting contrast between mRNA degradation-dependent and translational suppression-dependent RNAi processes [55].
These findings were later confirmed by Cullen and co-workers who demonstrated that an endogenously encoded human miRNA is able to cleave an mRNA bearing fully complementary target sites, whereas an exogenously supplied siRNA can inhibit the expression of an mRNA bearing partially complementary sequences without inducing detectable RNA cleavage. This suggests that miRNAs and siRNAs use similar mechanisms to trigger RNAi and that the choice between translational suppression or mRNA degradation is largely affected by the degree of complementarity with the mRNA target [57].
Further inquiries by the research teams of Sharp and Cullen demonstrated a translational repression affect when targeted mRNAs contained some repeats complementary to bulged siRNAs or miRNAs in their 3’UTR. By contrast, Dutta and co-workers showed that a single complementary target sequence to a bulged siRNA located inside of the coding sequence was solely able to induce translational suppression. This discovery is of particular importance as it raises concerns about off-target activity of siRNAs when utilized to specifically downregulate gene expression. If imperfectly matching siRNAs can repress translation, then the risk is quite high that siRNAs could target other sequences in the pool of mRNAs within the cell [58]. For further information regarding this specificity issue, please see ref [29,59–62].
Nonetheless, Sharp and Doench showed that repression of translation was mostly dependent on the free energy of the first eight nucleotides in the 5’ region of the miRNA but that G:U wobble base pairing in this region interferes with activity beyond that predicted on the basis of thermodynamic considerations [62].
Recently, a report by Filipowicz and co-workers [33] stated that let-7 represses gene expression through a mechanism involving suppression of translational initiation. Using luciferase reporters containing bulged let-7 complementary sites in the 3’UTR, they analyzed the polysomal distribution of these reporter mRNAs in a sucrose gradient. They found that three repeats of a bulged let-7 binding site were sufficient to shift their reporter mRNAs from the actively translated mRNA pool to the non-translated mRNA pool. This shift could be repressed by introducing 2’-o-methyl oligos specific for let-7. Furthermore, they showed that while cap-dependent translation was repressed by let-7, cap-independent translation was not affected by the presence of let-7, suggesting some involvement of the mRNA cap and its binding proteins.
Next, Filipowicz and co-workers analyzed the subcellular localization of miRNAs and translationally repressed mRNAs and found a colocalization of those RNA molecules with the decapping protein Dcp1a into cytoplasmic foci referred to as processing bodies or p-bodies [33].
Ago proteins, and more particularly Ago2, were then shown to localize in an RNA-dependent manner to those p-bodies, further strengthening the link between the RNAi machinery and translational suppression [63]. In a parallel study, Parker and co-workers also showed that some reporter mRNAs targeted for translational repression by endogenous or exogenous miRNAs colocalize in a miRNA-dependent manner in p-bodies with Ago proteins [64], confirming that the main RISC components (Ago, miRNAs/siRNAs and targeted mRNAs) are present in one single subcellular location.
Recent work from the group of Izaurralde showed that even in the absence of p-bodies, accomplished by depleting D. melanogaster S2 cells of LSm1 or LSm3 proteins, miRNAs still repressed their targets. This suggests that p-body formation is a consequence rather than a cause for RNA dependent gene silencing [65].
P-bodies are not only sites of non-translating mRNAs, but are also sites of some mRNA degradation. It has been described that mRNA degradation occurring in p-bodies is initiated by the shortening of the poly(A) tail in a process known as deadenylation, followed by the cleavage of the 5’m7GpppN cap (decapping), and finally by a 5’ to 3’ exonucleolytic mRNA degradation [66]. Interestingly, decapping and mRNA translation are two deeply intertwined processes. For example, while decreasing overall translation initiation increases the rate of decapping, inhibiting translation elongation decreases the rate of decapping [66]. Moreover, while the primary role of the cap binding protein eIF-4E is in translational initiation, it also inhibits decapping and therefore should inhibit translation elongation [67,68]. In sum, this evidence appears to suggest that mRNA degradation occurring in p-bodies is more related to a general mRNA turnover mechanism than to a specific mRNA degradation-dependent RNAi process. It also appears to suggest that the main RNAi pathway occurring in p-bodies is more related to translational suppression.
With the idea in mind that the translation machinery continuously scans the mRNA pool to initiate translation and therefore could facilitate RISC scanning and siRNA/miRNA base pairing to potential mRNA targets, Gu and Rossi used the Iron Responsive Elements (IRE) / Iron Regulatory Protein 1 (IRP-1) system to establish a link between RNAi and the translation machinery. When the intracellular iron concentration is low, IRP-1 binds to IREs, which are located in close proximity to the cap structure of the ferritin mRNA. This represses translation by blocking the recruitment of the small ribosomal subunit. Using this system, they showed that neither active translation nor unidirectional scanning is required for siRNA-mediated target mRNA degradation. Additionally, blocking translational scanning from both the 5’ and 3’ ends of mRNAs does not impede RNAi. In contrast, non-translated mRNAs are ten fold more susceptible to RNAi than mRNAs undergoing translation [34]. These observations were later confirmed by Blau and co-workers using the same IRE/IRP-1 system [69].
The mechanism of miRNA-mediated translational repression remains controversial. Recently, several laboratories have proposed different mechanisms for different or even similar miRNA targets. Some favor miRNA-directed translational repression at the initiation step, whereas others propose inhibitory mechanisms after initiation, such as premature ribosome drop off or cotranslational protein degradation.
Nottrott et al. [70] describe that in HeLa cells let-7a miRNA strongly repressed the translation of reporter mRNAs containing the C. Elegans lin-41 UTR. Yet, polysome profiling showed that most of the lin-41-UTR-containing reporter mRNA was associated with actively translating polysomes as observed before for other miRNA suppressed genes [71,72]. Further analysis using N-terminal myc-tagged reporters revealed that in the presence of let-7a miRNA target no reporter RNA could be immunoprecipitated with an anti-myc antibody. This indicated that the nascent polypeptide chain derived from reporter mRNA under let-7a control was either destroyed when it emerged from the ribosome or was masked by factors that direct the mature protein to be rapidly degraded.
Petersen et al. also report short RNA target repression in 293T cells at a step after initiation using a luciferase reporter construct bearing six bulged CXCR4 siRNA targets [73]. In this study, targeted mRNA is also associated with actively translating polyribosomes. Moreover, cap-independent translation was shown to be repressed as well, by means of bicistronic luciferase vectors with hepatitis C virus or cricket paralysis virus IRES sequences, the latter IRES bypassing all steps in normal translation initiation. In partial agreement with the study of Nottrott et al., neither full size reporter protein or nascent polypeptide was detected after a very short 3 minute 35S-methionine pulse labeling. However, the short duration of the pulse labeling argues against a post-translational degradation of completed protein, but indicates that repression occurs before completion of the nascent polypeptide chain. Next, a ‘read through’ assay, which is indicative of the frequency of translational termination, suggested that short RNAs may promote premature termination or early exit of ribosomes within the open reading frame. A ribosomal runoff assay using the eIF4A inhibitor hippuristanol showed that short RNA targeted mRNA is more rapidly lost from polysomes than untargeted mRNA upon initiation arrest, and thus the authors concluded that ribosomes exit prematurely from repressed mRNAs.
Evidence for regulation of translation initiation by RISC is also accumulating. Kiriakidou et al. identified a mRNA m7G cap binding-like motif in human Ago2, similar to that found in the cap binding protein eIF4E [74]. The domain, located in between the PAZ and PIWI domain contains two conserved phenylalanines at position 470 and 505. Mutation of these phenylalanines to valine residues abolished the ability of Ago2 to bind to m7G cap structures and also its ability to repress expression of a luciferase reporter, but did not affect its ability to cleave a perfectly matching RNA target. This led to the proposition that Ago2 competes with eIF4E for the mRNA m7G cap structure, thus inhibiting translation initiation of a targeted transcript.
Using a different approach, Mathonnet and co-workers also describe that in vitro translational inhibition by microRNA involves targeting the mRNA cap-binding complex [75]. Using in vitro transcribed let-7 targeted reporter mRNA and a mouse Krebs-2 cell extracts for in vitro translation, they found that translational inhibition requires a functional cap structure, and could be released by addition of purified eIF4F complex to cell extracts.
Yet another mechanism of silencing involving translation initiation was proposed by Chendrimada et al. [76]. Analysis of proteins associated with TRBP, which in mammals is thought to be involved in assembly of miRNAs into Ago2 containing complexes, revealed protein components of the 60S ribosomal subunit and eIF6 to be present in a RISC complex. eIF6 is known to prevent the formation of the 80S initiation complex by binding to the 60S ribosomal subunit [77]. Depletion of eIF6 by siRNA abolished the suppression of a reporter bearing a let-7b target site in its 3’ UTR, coinciding with a loss of free 60S ribosomal subunits in HeLa cells. The role for eIF6 in miRNA regulated gene expression was confirmed in developmentally regulated endogenous genes in C. elegans, pointing to an evolutionary conserved role for eIF6.
Therman and Hentze investigated miR-2-mediated translational control of a reporter target using a cell free system from D. melanogaster [78]. In this system, miR-2 inhibits 80S complex formation and induces heavy mRNP particles, referred to as ‘pseudopolysomes’. These pseudopolysomes contained miR-2 and still formed under conditions of blocked 60S subunit joining and blocked elongation. Furthermore, a functional cap structure was necessary for miR-2-induced translational repression, but not for the formation of pseudopolysomes. The biochemical composition of these large particles remains to be determined, but they might represent a p-body-like structure.
Taken together, diverse and sometimes contradicting mechanisms underlying miRNA-mediated translational silencing have been proposed. The controversies span the issues of whether the translational silencing is cap-dependent or not, and whether inhibition occurs at the inhibition step or at a later time point in protein synthesis. The diversity in proposed mechanisms may arise from differences in experimental approaches, cell types and from structural differences in miRNA target binding (see Table 1). For more insight in the current controversies the reader is referred to several excellent reviews that have recently been published [79–82].
Table 1.
Summary of the approaches utilized to study miRNA-mediated translational repression and the proposed mechanisms.
| Species | Cell type | miRNA | Reporter target | System | Proposed mechanism | Reference |
|---|---|---|---|---|---|---|
| Homo sapiens | HeLa | Let-7 | bulged let-7 target | Reporter RNA transfection | Cap dependent inhibition of translation initiation | 33 |
| Homo sapiens | 293T and HeLa | x | x | Tethering Ago to reporter | Cap dependent inhibition of translation initiation | 74 |
| Mus musculus | Krebs2 extract | Let-7 | bulged let-7 target | In vitro translation in cell extract | Cap dependent inhibition of translation initiation | 75 |
| Homo sapiens | HeLa | Let-7 | bulged let-7 target | Reporter DNA transfection | Inhibition of translation initiation by eIF-6 recruitment to RISC | 76 |
| D. melanogaster | Embryonic extract | miR-2 | miR-2 target | In vitro translation in cell extract | Cap dependent inhibition of translation after initiation | 77 |
| Homo sapiens | HeLa | let-7a | C. elegans lin-41 UTR | Reporter DNA transfection | Post-initiation: nascent protein degradation | 70 |
| Homo sapiens | 293T | artificia l | CXCR4 | Reporter DNA transfection | Post-initiation: ribosomal dropoff | 73 |
It is interesting to note that RNA binding proteins can affect miRNA-mediated translational repression. In liver cells cationic amino acid transporter 1 (CAT-1) repression by miR-122 can be relieved by binding of the ARE-binding protein HuR in cells subjected to stress. The stress induced binding of HuR to the ARE results in release of silenced CAT-1 mRNA from p-bodies and its recruitment into polysomes [83].
Even more intriguingly, the classical view of miRNAs as repressors of gene expression has recently been challenged. Vasudevan et al [84] demonstrated that in HEK293 cells translation of a reporter bearing the 3’UTR of TNF-α is upregulated in an ARE dependent way following serum starvation induced cell cycle arrest (in G1 phase). Surprisingly, the translational upregulation required FXR1 and Ago2, previously considered repressors of translation. The involvement of Ago2 led to the idea that miRNAs may be involved in the enhancement of translation upon cell cycle arrest. A bioinformatic screen revealed miR-369-3 to potentially bind the TNF-α-ARE at two sites, and the expression of miR-369-3 was increased in growth arrested cells. Knockdown and rescue experiments revealed that miR-396-3 is required for stimulating the translation of the TNF-α-ARE reporter gene. In contrast, in growing cells, miR-369-3 represses translation. Similarly, it was shown that repression of translation by the well known miRNA let-7 and the artificial CXCR miRNA switched to enhancing translation upon serum starvation induced cell cycle arrest [85]. Activation of translation by miRNA associated with Ago2 depended on the interaction with the RNA binding protein FXR1, which was shown to become more soluble and thus available upon serum starvation [84].
Thus, a given miRNA can switch from repression to activation of translation upon exiting of the cell cycle, apparently through modification of the miRNA-Ago2 complex by an RNA binding protein. Besides the questions these results raise on how miRNAs may function in developmental processes and cellular differentiation, they clearly demonstrate the dramatic effects of accessory proteins recruited to the RISC complex.
What Are the Key Requirements for siRNAs and miRNAs to Direct the RNAi Machinery Towards Translational Suppression or mRNA Degradation?
In our current understanding of miRNA-mediated regulation of gene repression, it is essentially unknown what exactly determines whether a targeted mRNA will be translationally repressed or directed to mRNA degradation. In vitro studies show that the degree of complementarity of the seed region is a structural key determinant in selecting the route for gene suppression. While perfectly matching siRNAs/miRNAs have been proven to direct mRNA cleavage, imperfectly matching and centrally bulged siRNAs/miRNAs have been shown to direct the RNAi machinery towards translational inhibition. Even though this applies to many of the studied miRNAs, exceptions have been documented. For example, imperfectly matching miRNAs like miR-16 targeting ARE are able to regulate mRNAs stability, despite the fact that from a thermodynamic point of view, their weak interactions with their target are more similar to the bulged siRNAs/miRNAs that trigger translational suppression. It is possible that this is because the ARE-containing mRNA degradation is a unique case of RISC activity that requires specific proteins (such as TTP, HuR, TIA-1, etc.) shown to bind to the ARE and either modulate RISC activity or enhance the binding capacity of miR-16 to the ARE. See Figure 3 for a graphical view of RISC-dependent translational suppression and RISC-dependent mRNA degradation.
FIGURE 3.
In general, perfectly matching siRNAs direct RISC-mediated mRNA degradation, whereas bulged siRNAs and miRNAs direct RISC-mediated translational suppression. Some miRNAs promote mRNA degradation which maybe directed by RNA-binding proteins.
Even more complex is the case of the miRNAs let-7 and lin-4, which have been shown to inhibit translational initiation and also to target mRNA degradation [31–33,86]. Pasquinelli and colleagues utilized the nematode C. elegans to study the regulation of lin-41, lin-14 and lin-28 gene expression at the mRNA level during developmental stages. The corresponding transcripts of these genes are targets for the miRNAs let-7 (lin-41) and lin-4 (lin-14 and lin-28). They concluded that lin-41, lin-14 and lin-28 mRNAs were degraded in a mechanism involving these two miRNAs [31].
Also using C. elegans, Ruvkun and co-workers showed that lin-14 expression is temporally regulated by let-7 via the mRNA 3’UTR but without affecting the transcript stability. They also showed that lin-4 has 7 potential hybridization sites that are not fully complementary to the 3’UTR (bulged structure) of lin-14 mRNA. As a consequence of these interactions, the protein level of lin-14 decreases by a factor of 10 between early and late stages of development. By contrast, a lin-4 deficient mutant showed only a 2.3-fold decrease and using a RNase protection assay, the authors finally showed that the level of lin-14 mRNA was not altered enough to explain such a dramatic change in protein expression [86].
Filipowicz and co-workers showed in an elegant biochemical approach using human cultured cells that let-7 represses translation initiation. They found that mRNAs containing three bulged let-7 targeting sites were observed in the non-actively translated fractions of the polysomal RNA distribution and that a treatment of the cells with 2’o-Me oligos targeting let-7 resulted in the restoration of active translation of those mRNAs [33].
At this stage in our understanding of RNAi, it is still difficult to predict the molecular requirements directing an mRNA toward its degradation or its translational suppression. It is known from the work of Tuschl and colleagues that siRNAs matching perfectly to reporter mRNAs caused cleavage between the 10th and 11th nucleotides (counting from the 5’ end) [16]. It is also known from Sharp and co-workers that in cases of imperfect miRNA-mRNA binding, the bulged structure between the miRNA and its target starts between the 9th and the 11th nucleotide (of the miRNA, counting from the 5’end), and this induces translational suppression [55]. Consequently, translational suppression is observed when the bulged structure of the miRNA is located at the site at which a perfectly matching siRNA would direct mRNA cleavage. While this may be a rule regulating the fate of many RISC-targeted mRNAs studied, it seems that this rule can be overcome by the presence of RNA binding proteins on a targeted mRNA.
Concluding remarks
Suppression of gene functions can be achieved by complete or partial base-pairing between siRNA/miRNA and a transcript. This suppression can be the result of very complex and different mechanisms that nonetheless require some common key molecules.
siRNAs have been shown to trigger mRNA degradation in a way that is dependent on Ago2 and the upstream enzyme Dicer, which is required for the maturation of siRNAs and their polarized uploading into RISC. Many miRNAs are also loaded into RISC in a polarized fashion, although the mechanism of strand selection in mammalian cells is not as clear cut as for siRNAs and seems to involve additional factors.
miRNAs have also been shown to trigger mRNA degradation even if they only hybridize partially to their target mRNAs, which seems to depend on RNA binding proteins tethered to the 3’ UTR of the target mRNA. Additionally, miRNAs have been widely observed in mammals suppressing translation, and this biological function has been attributed to the fact that they only match imperfectly with their target mRNA. Imperfectly matching siRNAs have also been shown to repress translation in a similar way. Interestingly, ARE binding proteins have been shown to dramatically affect miRNA-mediated translational repression, from relieving repression to even upregulating translation of a targeted mRNA. It is tempting to speculate that the interaction between RNA binding proteins and the RISC complex is a more common mechanism by which the cell can fine tune its gene expression according to its specific needs. Besides the post-transcriptional effects of small RNA molecules on gene expression reviewed here, it should be noted that siRNAs complementary to promoter sequences can also modulate gene expression by either inhibiting [87–89] or activating [90] gene transcription through chromatin remodeling, vastly expanding the possibilities of small RNA molecules to attenuate gene expression (for review see [91]).
Taken together, the assiduous effort undertaken in the last decade to unravel the mechanism by which small RNA molecules modulate gene expression has provided valuable insights and raised intriguing questions. Can the multiple mechanisms proposed to explain miRNA-mediated translational suppression be unified? Do RNA binding proteins play a common role in fine tuning miRNA-meditated gene regulation? What factors besides thermodynamic asymmetry are decisive for miRNA strand selection in mammals? Can a single miRNA simultaneously affect transcriptional and post-transcriptional gene expression of a single gene?
The intricate role that small RNA molecules have been observed to play in countless, often critical cellular events warrant our continued effort in trying to elucidate these and many more questions.
Acknowledgements
The authors would like to thank Dr. Isaac R. Mehl for critical reading of the manuscript. This work was supported by grants from National Institutes of Health grants AI41637 and AI54696.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenberg UB, Preiss A, Seifert E, Jackle H, Knipple DC. Production of phenocopies by Kruppel antisense RNA injection into Drosophila embryos. Nature. 1985;313:703. doi: 10.1038/313703a0. [DOI] [PubMed] [Google Scholar]
- 2.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 3.Caudy AA, Myers M, Hannon GJ, Hammond SM. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 2002;16:2491. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caudy AA, Ketting RF, Hammond SM, Denli AM, Bathoorn AM, Tops BB, Silva JM, Myers MM, Hannon GJ, Plasterk RH. A micrococcal nuclease homologue in RNAi effector complexes. Nature. 2003;425:411. doi: 10.1038/nature01956. [DOI] [PubMed] [Google Scholar]
- 5.Caudy AA, Hannon GJ. Induction and biochemical purification of RNA-induced silencing complex from Drosophila S2 cells. Methods Mol. Biol. 2004;265:59. doi: 10.1385/1-59259-775-0:059. [DOI] [PubMed] [Google Scholar]
- 6.Scadden AD. The RISC subunit Tudor-SN binds to hyper-edited double-stranded RNA and promotes its cleavage. Nat. Struct. Mol. Biol. 2005;12:489. doi: 10.1038/nsmb936. [DOI] [PubMed] [Google Scholar]
- 7.Yang W, Chendrimada TP, Wang Q, Higuchi M, Seeburg PH, Shiekhattar R, Nishikura K. Modulation of microRNA processing and expression through RNA editing by ADAR deaminases. Nat. Struct. Mol. Biol. 2006;13:13. doi: 10.1038/nsmb1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell. 2004;15:185. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Rivas FV, Tolia NH, Song JJ, Aragon JP, Liu J, Hannon GJ, Joshua-Tor L. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat. Struct. Mol. Biol. 2005;12:340. doi: 10.1038/nsmb918. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 11.Vickers TA, Lima WF, Nichols JG, Crooke ST. Reduced levels of Ago2 expression result in increased siRNA competition in mammalian cells. Nucleic Acids Res. 2007;35:6598. doi: 10.1093/nar/gkm663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 13.Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 16.Martinez J, Patkaniowska A, Urlaub H, Luhrmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 17.Chiu YL, Rana TM. RNAi in human cells: basic structural and functional features of small interfering RNA. Mol. Cell. 2002;10:549. doi: 10.1016/s1097-2765(02)00652-4. [DOI] [PubMed] [Google Scholar]
- 18.Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD. A protein sensor for siRNA asymmetry. Science. 2004;306:1377. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 20.Tomari Y, Du T, Haley B, Schwarz DS, Bennett R, Cook HA, Koppetsch BS, Theurkauf WE, Zamore PD. RISC assembly defects in the Drosophila RNAi mutant armitage. Cell. 2004;116:831. doi: 10.1016/s0092-8674(04)00218-1. [DOI] [PubMed] [Google Scholar]
- 21.Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD. A protein sensor for siRNA asymmetry. Science. 2004;306:1377. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 22.Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD. A protein sensor for siRNA asymmetry. Science. 2004;306:1377. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 23.Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD. A protein sensor for siRNA asymmetry. Science. 2004;306:1377. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 24.Aravin AA, Lagos-Quintana M, Yalcin A, Zavolan M, Marks D, Snyder B, Gaasterland T, Meyer J, Tuschl T. The small RNA profile during Drosophila melanogaster development. Dev. Cell. 2003;5:337. doi: 10.1016/s1534-5807(03)00228-4. [DOI] [PubMed] [Google Scholar]
- 25.Suh MR, Lee Y, Kim JY, Kim SK, Moon SH, Lee JY, Cha KY, Chung HM, Yoon HS, Moon SY, Kim VN, Kim KS. Human embryonic stem cells express a unique set of microRNAs. Dev. Biol. 2004;270:488. doi: 10.1016/j.ydbio.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Ro S, Park C, Young D, Sanders KM, Yan W. Tissue-dependent paired expression of miRNAs. Nucleic Acids Res. 2007;35:5944. doi: 10.1093/nar/gkm641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forstemann K, Horwich MD, Wee L, Tomari Y, Zamore PD. Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1. Cell. 2007;130:287. doi: 10.1016/j.cell.2007.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomari Y, Du T, Zamore PD. Sorting of Drosophila small silencing RNAs. Cell. 2007;130:299. doi: 10.1016/j.cell.2007.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 30.Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di PF, Lin SC, Gram H, Han J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120:623. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 31.Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 32.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 33.Pillai RS, Bhattacharyya SN, Artus CG, Zoller T, Cougot N, Basyuk E, Bertrand E, Filipowicz W. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science. 2005;309:1573. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 34.Gu S, Rossi JJ. Uncoupling of RNAi from active translation in mammalian cells. RNA. 2005;11:38. doi: 10.1261/rna.7158605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shyu AB, Belasco JG, Greenberg ME. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991;5:221. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg ME, Shyu AB, Belasco JG. Deadenylylation: a mechanism controlling c-fos mRNA decay. Enzyme. 1990;44:181. doi: 10.1159/000468756. [DOI] [PubMed] [Google Scholar]
- 37.Wang E, Ma WJ, Aghajanian C, Spriggs DR. Posttranscriptional regulation of protein expression in human epithelial carcinoma cells by adenine-uridine-rich elements in the 3'-untranslated region of tumor necrosis factor-alpha messenger RNA. Cancer Res. 1997;57:5426. [PubMed] [Google Scholar]
- 38.Stoecklin G, Hahn S, Moroni C. Functional hierarchy of AUUUA motifs in mediating rapid interleukin-3 mRNA decay. J. Biol. Chem. 1994;269:28591. [PubMed] [Google Scholar]
- 39.Ristimaki A, Narko K, Hla T. Down-regulation of cytokine-induced cyclooxygenase-2 transcript isoforms by dexamethasone: evidence for post-transcriptional regulation. Biochem. J. 1996;318(Pt 1):325. doi: 10.1042/bj3180325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linker K, Pautz A, Fechir M, Hubrich T, Greeve J, Kleinert H. Involvement of KSRP in the post-transcriptional regulation of human iNOS expression-complex interplay of KSRP with TTP and HuR. Nucleic Acids Res. 2005;33:4813. doi: 10.1093/nar/gki797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacob CO, Tashman NB. Disruption in the AU motif of the mouse TNF-alpha 3' UTR correlates with reduced TNF production by macrophages in vitro. Nucleic Acids Res. 1993;21:2761. doi: 10.1093/nar/21.11.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cok SJ, Morrison AR. The 3'-untranslated region of murine cyclooxygenase-2 contains multiple regulatory elements that alter message stability and translational efficiency. J. Biol. Chem. 2001;276:23179. doi: 10.1074/jbc.M008461200. [DOI] [PubMed] [Google Scholar]
- 43.Myer VE, Fan XC, Steitz JA. Identification of HuR as a protein implicated in AUUUA-mediated mRNA decay. EMBO J. 1997;16:2130. doi: 10.1093/emboj/16.8.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma WJ, Cheng S, Campbell C, Wright A, Furneaux H. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J. Biol. Chem. 1996;271:8144. doi: 10.1074/jbc.271.14.8144. [DOI] [PubMed] [Google Scholar]
- 45.Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein,increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, Chen S, Gueydan C, Kruys V, Streuli M, Anderson P. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 2000;19:4154. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gueydan C, Droogmans L, Chalon P, Huez G, Caput D, Kruys V. Identification of TIAR as a protein binding to the translational regulatory AU-rich element of tumor necrosis factor alpha mRNA. J. Biol. Chem. 1999;274:2322. doi: 10.1074/jbc.274.4.2322. [DOI] [PubMed] [Google Scholar]
- 48.Dember LM, Kim ND, Liu KQ, Anderson P. Individual RNA recognition motifs of TIA-1 and TIAR have different RNA binding specificities. J. Biol. Chem. 1996;271:2783. doi: 10.1074/jbc.271.5.2783. [DOI] [PubMed] [Google Scholar]
- 49.Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol. Cell Biol. 1999;19:4311. doi: 10.1128/mcb.19.6.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai WS, Carballo E, Thorn JM, Kennington EA, Blackshear PJ. Interactions of CCCH zinc finger proteins with mRNA. Binding of tristetraprolin-related zinc finger proteins to Au-rich elements and destabilization of mRNA. J. Biol. Chem. 2000;275:17827. doi: 10.1074/jbc.M001696200. [DOI] [PubMed] [Google Scholar]
- 51.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 52.Worthington MT, Pelo JW, Sachedina MA, Applegate JL, Arseneau KO, Pizarro TT. RNA binding properties of the AU-rich element-binding recombinant Nup475/TIS11/tristetraprolin protein. J. Biol. Chem. 2002;277:48558. doi: 10.1074/jbc.M206505200. [DOI] [PubMed] [Google Scholar]
- 53.Lai WS, Kennington EA, Blackshear PJ. Tristetraprolin and its family members can promote the cell-free deadenylation of AU-rich element-containing mRNAs by poly(A) ribonuclease. Mol. Cell Biol. 2003;23:3798. doi: 10.1128/MCB.23.11.3798-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB, Zhang Y. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS. ONE. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes Dev. 2003;17:438. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishizuka A, Siomi MC, Siomi H. A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 2002;16:2497. doi: 10.1101/gad.1022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9779. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saxena S, Jonsson ZO, Dutta A. Small RNAs with imperfect match to endogenous mRNA repress translation. Implications for off-target activity of small inhibitory RNA in mammalian cells. J. Biol. Chem. 2003;278:44312. doi: 10.1074/jbc.M307089200. [DOI] [PubMed] [Google Scholar]
- 59.Snove O, Jr, Holen T. Many commonly used siRNAs risk off-target activity. Biochem. Biophys. Res. Commun. 2004;319:256. doi: 10.1016/j.bbrc.2004.04.175. [DOI] [PubMed] [Google Scholar]
- 60.Qiu S, Adema CM, Lane T. A computational study of off-target effects of RNA interference. Nucleic Acids Res. 2005;33:1834. doi: 10.1093/nar/gki324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holen T, Moe SE, Sorbo JG, Meza TJ, Ottersen OP, Klungland A. Tolerated wobble mutations in siRNAs decrease specificity, but can enhance activity in vivo. Nucleic Acids Res. 2005;33:4704. doi: 10.1093/nar/gki785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sen GL, Blau HM. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 2005;7:633. doi: 10.1038/ncb1265. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat. Cell Biol. 2005;7:719. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eulalio A, Behm-Ansmant I, Schweizer D, Izaurralde E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol. Cell Biol. 2007;27:3970. doi: 10.1128/MCB.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coller J, Parker R. Eukaryotic mRNA decapping. Annu. Rev. Biochem. 2004;73:861. doi: 10.1146/annurev.biochem.73.011303.074032. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz DC, Parker R. Mutations in translation initiation factors lead to increased rates of deadenylation and decapping of mRNAs in Saccharomyces cerevisiae. Mol. Cell Biol. 1999;19:5247. doi: 10.1128/mcb.19.8.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwartz DC, Parker R. mRNA decapping in yeast requires dissociation of the cap binding protein, eukaryotic translation initiation factor 4E. Mol. Cell Biol. 2000;20:7933. doi: 10.1128/mcb.20.21.7933-7942.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sen GL, Wehrman TS, Blau HM. mRNA translation is not a prerequisite for small interfering RNA-mediated mRNA cleavage. Differentiation. 2005;73:287. doi: 10.1111/j.1432-0436.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 70.Nottrott S, Simard MJ, Richter JD. Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nat. Struct. Mol. Biol. 2006;13:1108. doi: 10.1038/nsmb1173. [DOI] [PubMed] [Google Scholar]
- 71.Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev. Biol. 1999;216:671. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- 72.Seggerson K, Tang L, Moss EG. Two genetic circuits repress the Caenorhabditis elegans heterochronic gene lin-28 after translation initiation. Dev. Biol. 2002;243:215. doi: 10.1006/dbio.2001.0563. [DOI] [PubMed] [Google Scholar]
- 73.Petersen CP, Bordeleau ME, Pelletier J, Sharp PA. Short RNAs repress translation after initiation in mammalian cells. Mol. Cell. 2006;21:533. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 74.Kiriakidou M, Tan GS, Lamprinaki S, De Planell-Saguer M, Nelson PT, Mourelatos Z. An mRNA m7G cap binding-like motif within human Ago2 represses translation. Cell. 2007;129:1141. doi: 10.1016/j.cell.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 75.Mathonnet G, Fabian MR, Svitkin YV, Parsyan A, Huck L, Murata T, Biffo S, Merrick WC, Darzynkiewicz E, Pillai RS, Filipowicz W, Duchaine TF, Sonenberg N. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science. 2007;317:1764. doi: 10.1126/science.1146067. [DOI] [PubMed] [Google Scholar]
- 76.Chendrimada TP, Finn KJ, Ji X, Baillat D, Gregory RI, Liebhaber SA, Pasquinelli AE, Shiekhattar R. MicroRNA silencing through RISC recruitment of eIF6. Nature. 2007;447:823. doi: 10.1038/nature05841. [DOI] [PubMed] [Google Scholar]
- 77.Ceci M, Gaviraghi C, Gorrini C, Sala LA, Offenhauser N, Marchisio PC, Biffo S. Release of eIF6 (p27BBP) from the 60S subunit allows 80S ribosome assembly. Nature. 2003;426:579. doi: 10.1038/nature02160. [DOI] [PubMed] [Google Scholar]
- 78.Thermann R, Hentze MW. Drosophila miR2 induces pseudo-polysomes and inhibits translation initiation. Nature. 2007;447:875. doi: 10.1038/nature05878. [DOI] [PubMed] [Google Scholar]
- 79.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 2008;9:102. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 80.Wu L, Belasco JG. Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol. Cell. 2008;29:1. doi: 10.1016/j.molcel.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 81.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 82.Meister G. miRNAs get an early start on translational silencing. Cell. 2007;131:25. doi: 10.1016/j.cell.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 83.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 84.Vasudevan S, Steitz JA. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell. 2007;128:1105. doi: 10.1016/j.cell.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 86.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 87.Han J, Kim D, Morris KV. Promoter-associated RNA is required for RNA-directed transcriptional gene silencing in human cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12422. doi: 10.1073/pnas.0701635104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Janowski BA, Huffman KE, Schwartz JC, Ram R, Nordsell R, Shames DS, Minna JD, Corey DR. Involvement of AGO1 and AGO2 in mammalian transcriptional silencing. Nat. Struct. Mol. Biol. 2006;13:787. doi: 10.1038/nsmb1140. [DOI] [PubMed] [Google Scholar]
- 89.Zhang MX, Ou H, Shen YH, Wang J, Wang J, Coselli J, Wang XL. Regulation of endothelial nitric oxide synthase by small RNA. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16967. doi: 10.1073/pnas.0503853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR. Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat. Chem. Biol. 2007;3:166. doi: 10.1038/nchembio860. [DOI] [PubMed] [Google Scholar]
- 91.Morris KV. RNA-mediated transcriptional gene silencing in human cells. Curr. Top. Microbiol. Immunol. 2008;320:211. doi: 10.1007/978-3-540-75157-1_10. [DOI] [PubMed] [Google Scholar]