Abstract
Background
We investigated the role of calcium-activated potassium (KCa) channel activity in human skeletal muscle microvascular function in the setting of cardiopulmonary bypass (CPB).
Methods and Results
Human skeletal muscle arterioles (80- to 180 μm in diameter) were dissected from tissue harvested before and after CPB. In vitro relaxation responses of precontracted arterioles in a pressurized no-flow state were examined in the presence of KCa channel activators/blockers and several other vasodilators. Post-CPB responses to the activator of intermediate (IKCa) and small conductance (SKCa) KCa channels, NS309, to the endothelium-dependent vasodilator adenosine 5′-diphosphate (ADP), and to substance P were reduced compared with pre-CPB responses (P < .05), respectively, whereas responses to the activator of large conductance (BKCa) KCa channels, NS1619, and to the endothelium-independent vasodilator, sodium nitroprusside (SNP) were unchanged. Endothelial denudation decreased NS309-induced relaxation and abolished that induced by ADP or substance P (P< .05), but had no effect on relaxation induced by either NS1619 or SNP. Polypeptide levels of BKCa, IKCa, and SK3Ca were not altered post-CPB.
Conclusion
IK/SK-mediated relaxation is predominantly endothelium dependent, whereas BK-mediated relaxation seems to be largely independent of endothelial function in human skeletal muscle microvasculature. CPB-associated microvascular dysfunction likely arises in part from impaired function of endothelial SK and IK channels in the peripheral microvasculature.
Cardiopulmonary bypass (CPB) induces a systemic inflammatory response, resulting in various degrees of organ dysfunction in multiple systems.1 We have shown previously that CPB elicits complex, multifactorial vasomotor disturbances that vary according to the affected organ beds.1-4 Specifically, CPB was associated with reduced vascular resistances in the skeletal muscle and peripheral circulation, and increased propensity to spasm in the cardiac, pulmonary, mesenteric, and cerebral vascular beds. Smooth muscle, but not endothelium, has been found to be responsible for this CPB-mediated vasomotor dysfunction.1-4 In contrast, previous studies have shown that CPB-injured endothelium and impaired microvascular endothelial function in heart and other peripheral organs/tissues by showing decreased responses to endothelial-dependent relaxation.1-3 Therefore, many procedures, such as heparin-coated circuits, systemic cooling, complement inhibition, and neutrophil depletion have been used in the CPB circuit to abrogate CPB-mediated inflammatory responses and peripheral endothelial dysfunction.
Endothelium has been found to release 3 major endothelium-dependent relaxing factors, namely, nitric oxide, prostacylin, and endothelium-dependent hyperpolarization factor.5 Endothelium-dependent hyperpolarization factor dilates arteries via opening of calcium-activated potassium channels in endothelial and smooth muscle cells5; however, the role of KCa channel activation in CPB-related microvascular endothelial dysfunction in human peripheral resistance arterioles and the regulatory properties of these KCa channels in this vascular bed have been little investigated. This study was designed to examine the effect of CPB on vascular responses of human skeletal muscle microvessels to KCa channel openers and other vasoactive substances and to correlate these responses to possible alterations in expression of KCa polypeptides in human skeletal muscle tissue.
MATERIALS AND METHODS
Human subjects and tissue harvesting
Samples of skeletal muscle from the left internal mammary artery bed were harvested before and after CPB. The pre-CPB specimen was taken after cannulation, and the post-CPB specimen was collected from a different location in the left internal mammary artery bed after removal of the aortic cross-clamp and weaning from CPB. Tissue samples for immunoblot analysis assay were immediately frozen in liquid nitrogen. Tissue for microvascular studies was placed in cold (5°C to 10°C) Krebs buffer solution. All procedures were approved by the Institutional Review Board of Beth Israel Deaconess Medical Center, Harvard Medical School, and informed consent was obtained from all enrolled patients as required by the Institutional Review Board.
Microvessel relaxation studies
Skeletal muscle arterioles (80 to 180 μm internal diameters) from the left internal mammary artery bed were dissected before and after CPB. Microvessel studies were performed by in vitro organ bath videomicroscopy as described previously.6 After vessel precontraction with U46619, endothelium-independent and -dependent relaxation to sodium nitroprusside (SNP), adenosine 59-diphosphate (ADP), and substance P were examined.
Immunoblot
Small arteries were dissected, cleaned of connective tissues, and solubilized in SDS-PAGE buffer. Total protein (40 μg) was fractionated on an 8% to 16% SDS-PAGE, then transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corporation, Bedford, Mass) as described previously.3 Membranes were incubated for 1 hour at room temperature with 1:200 dilutions of individual rabbit polyclonal primary antibodies to BKCa-β1 (Santa Cruz Biotechnology, Santa Cruz, Calif.), IKCa or SK3Ca (Alomone Labs Ltd, Jerusalem, Israel). The membranes were then incubated for 1 hour with horse-radish peroxidase-conjugated secondary anti-immunoglobulin washed 3 times in Tris saline buffer, and processed for chemiluminescent detection (Pierce, Rockford, Ill) on x-ray film (Kodak, Rochester, NY). Band intensity was measured by densitometric analysis of autoradiograph films using NIH Image J 1.33.
Chemicals
NS309, NS1619, U46619, SNP, ADP, and substance P were obtained from Sigma. SNP, ADP, and substance P were dissolved in ultrapure distilled water and prepared on the day of the study. U46619 was dissolved in ethanol to make a stock solution. NS309 and NS1619 were dissolved in dimethylsulfoxide to make a stock solution. All stock solutions were stored at -20°C. All dilutions were prepared daily.
Data analysis
Data are presented as mean values and standard error of the mean. The relaxation responses were expressed as the percentage of relaxation of the U46619-preconstricted diameter of the microvessels. Repeated-measures ANOVA and the Student t test were used to compare variables among or between vessels. The treatment effects were statistically examined by the paired or independent 2-tailed Student t test. Statistical significance was taken at a probability value of <.05.
RESULTS
Patient characteristics
Tissue samples from 27 patients were studied. Twenty-seven patients (mean age, 67 ± 6 years) underwent coronary artery bypass grafting with duration of cardioplegic arrest of 58 ± 4 minutes and CPB time of 71.0 ± 18 minutes. Twenty-one patients were male and 6 were female. Of the 27 patients, 22 carried a preoperative diagnosis of hypertension. All patients with preoperative hypertension were on medication (β-blocker, calcium channel blocker, or angiotensin-converting enzyme inhibitor), and received perioperative β-blockade. Diabetes mellitus (type 1 or 2) was present in 5 of 27 patients.
Vessel characteristics
There were no significant differences in the values of vessel diameters among groups (Table). Similar U46619 concentrations were required to produce adequate precontraction in both groups.
Table.
Vessel diameters (μm, mean ± SEM)
| Prebypass | Postbypass | |
|---|---|---|
| NS309 | 146 ± 10 | 135 ± 10 |
| TRAM34/apamin + NS309 | 157 ± 9 | |
| Endothelium-denudation + NS309 | 150 ± 15 | |
| NS1619 | 162 ± 16 | 148 ± 12 |
| Iberiotoxin + NS1619 | 168 ± 6 | |
| Endothelium-denudation + NS 1619 | 167 ± 5 | |
| ADP | 146 ± 11 | 151 ± 8 |
| Endothelium-denudation + ADP | 158 ± 8 | |
| Substance P | 149 ± 9 | 157 ± 6 |
| Endothelium-denudation + substance P | 158 ± 8 | |
| SNP | 137 ± 14 | 145 ± 9 |
| Endothelium-denudation + ADP | 163 ± 7 |
ADP, adenosine 5′-diphosphate; SNP, sodium nitroprusside.
Responses to NS309 and NS1619
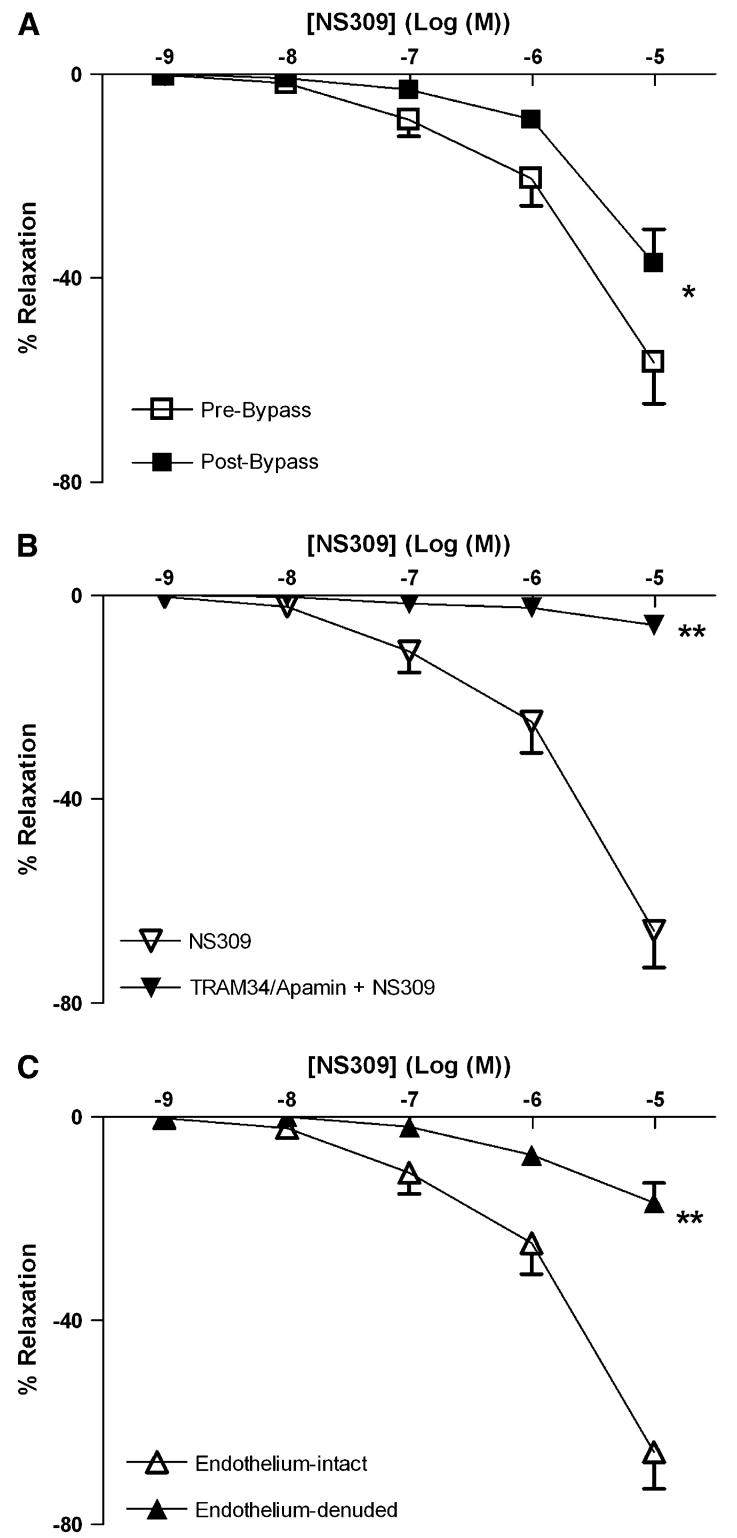
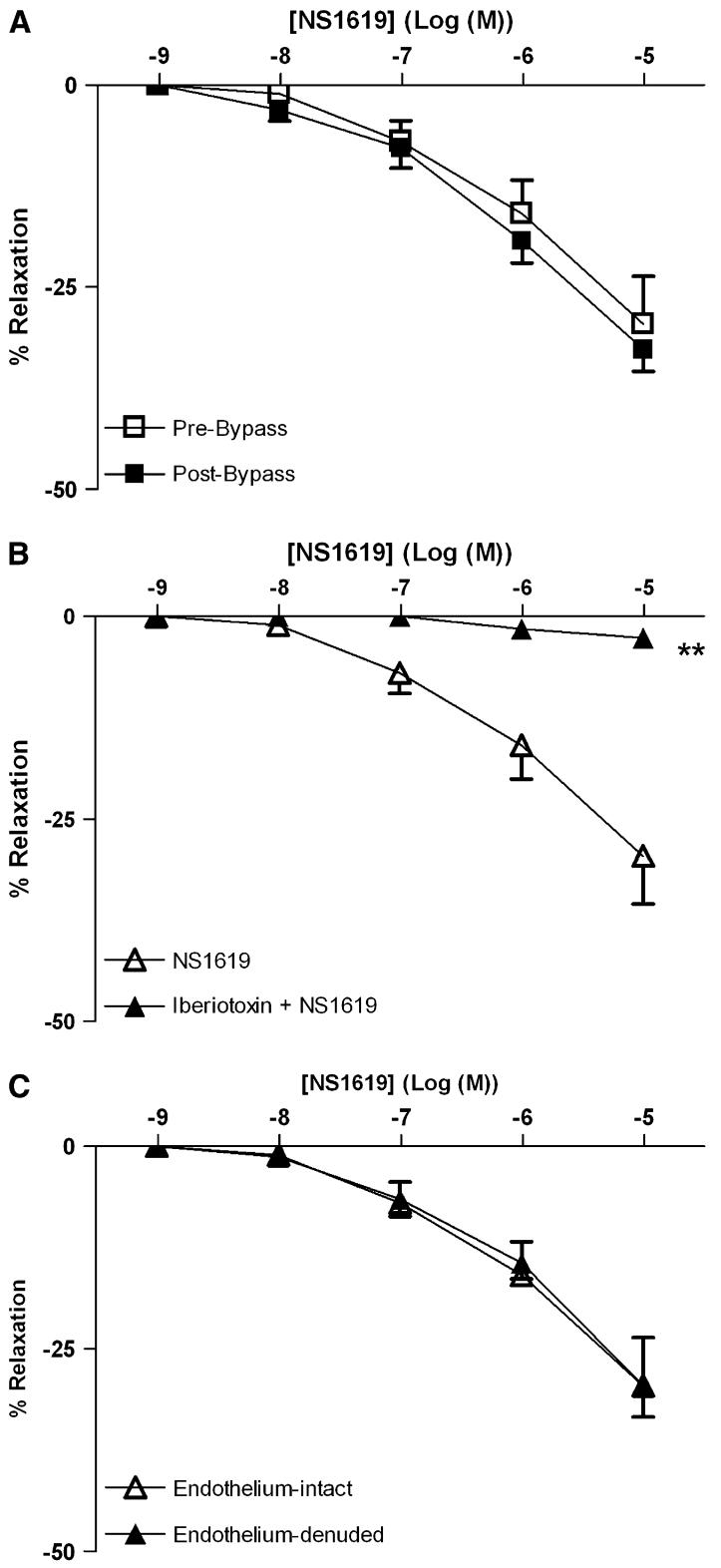
Both NS309 (Fig 1, A) and NS1619 (Fig 2, A) induced dose-dependent relaxation of skeletal muscle arterioles. CPB impaired the relaxation response to NS 309, compared with pre-CPB responses (P < .05; Fig 1, A). In contrast, the responses to NS1619 were unchanged pre- and post-CPB (Fig 2, A). Pretreatment with TRAM34/apamin abolished vessel relaxation induced by NS309 (P < .001; Fig 1, B), and NS1619-induced relaxation was inhibited by pretreatment with iberiotoxin (P < .001; Fig 2, B). Furthermore, NS309-mediated vasorelaxation of the skeletal muscle arterioles was decreased by removal of the endothelium (P < .001; Fig 1, C), whereas responses to NS1619 were unaffected (Fig 2, C).
Fig 1.
Vasoreactivity of human skeletal muscle arterioles to the IK/SK channel activator, NS309. A, Dose-dependent relaxation of human skeletal muscle arterioles (n = 8) in response to NS309 pre- and post-CPB. *P < .05. B, Dose-dependent, NS309-mediated relaxation of pre-CPB human skeletal muscle arterioles pretreated in the absence or presence of TRAM34 and apamin (n = 6). **P < .001. C, Dose-dependent, NS309-mediated relaxation of pre-CPB human skeletal muscle arterioles (n = 6) with intact or denuded endothelium. **P < .001. Data are presented as percent relaxation after preconstriction with U46619.
Fig 2.
Vasoreactivity of human skeletal muscle arterioles to the BK channel activator NS1619. A, Dose-dependent relaxation of human skeletal muscle arterioles in response to NS1619 either pre- or post-CPB (n = 8 per group). B, Dose-dependent, NS1619-mediated relaxation of pre-CPB human skeletal muscle arterioles pretreated in the absence or presence of iberiotoxin (n =6; **P < .001). C, Dose-dependent, NS1619-induced relaxation of pre-CPB human skeletal muscle arterioles with intact or denuded endothelium (n = 6 per group). Data are presented as percent relaxation after preconstriction with U46619.
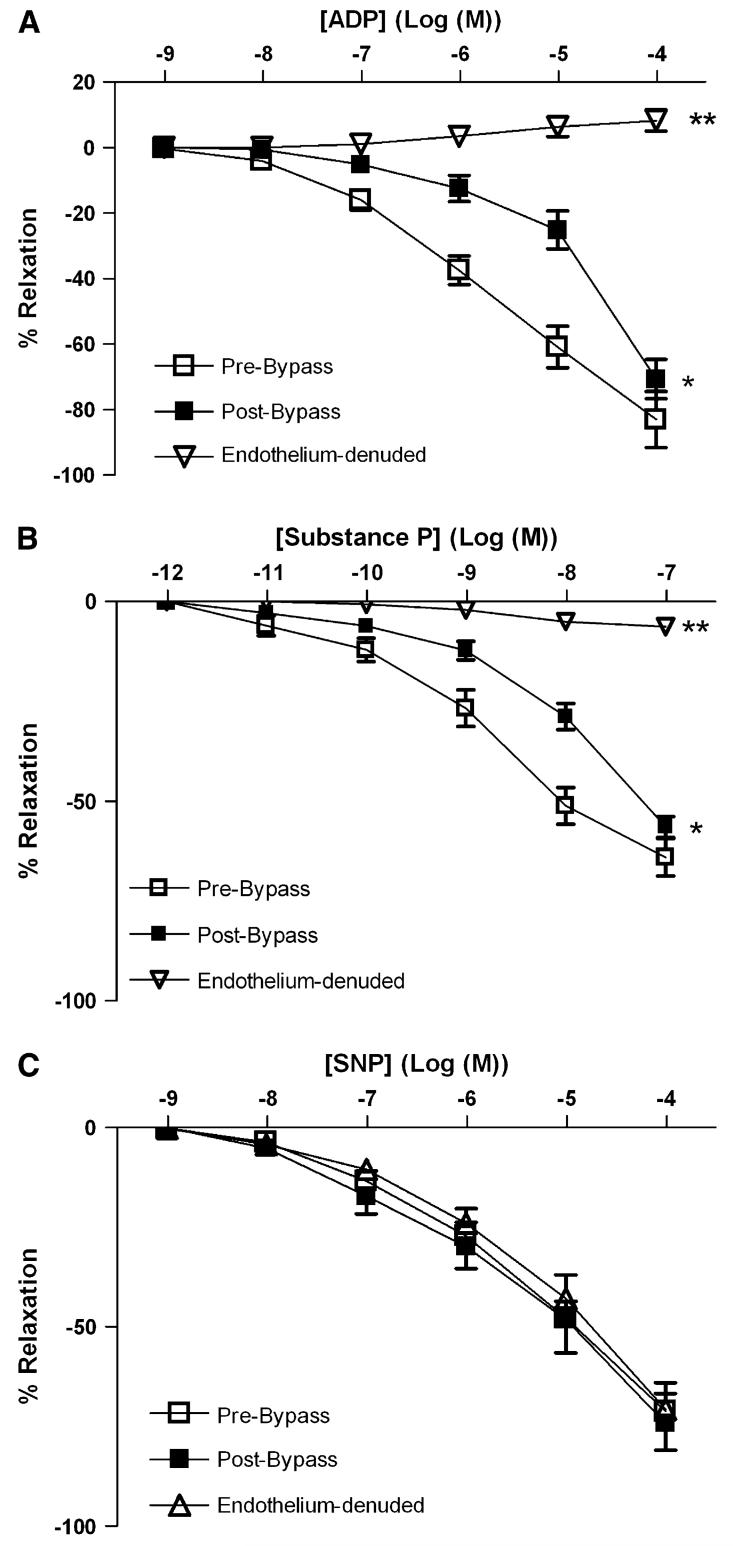
Responses to ADP, substance P, and SNP
ADP, substance P, and SNP induced a dose-dependent relaxation of coronary arterioles (Fig 3, A-C). CPB markedly decreased the relaxation responses both to ADP and to substance P compared with pre-CPB responses (P < .05; Fig 3, A, B). In contrast, the responses to SNP were unchanged pre- and post-CPB (Fig 3, C). Endothelium denudation abolished responses to both ADP and substance P (P < .001;Fig 3, A, B), but left intact SNP-induced relaxation.
Fig 3.
In vitro response of preconstricted human skeletal muscle arterioles to endogenous vasodilators. Arterioles were compared pre- and post-CPB (n = 8 per group). Pre-CPB arterioles were compared with intact endothelium and after endothelial denudation (n =6 per group) as indicated. A, Response to the endothelium-dependent vasodilator, ADP. *P < .05 versus pre-CPB; **P < .001 versus intact pre-CPB. B, Response to substance P. *P < .05 versus pre-CP-Rep; *P <.001 versus pre-CP-Rep. C, Response to the endothelium-independent vasodilator, SNP.
Effect of CPB on levels of KCa polypeptides
Pre-CPB skeletal muscle artery levels of the KCa channel polypeptides BKCa-β1, IKCa, and SK3Ca were unchanged in the post-CPB period as detected by immunoblot (Fig 4).
Fig 4.
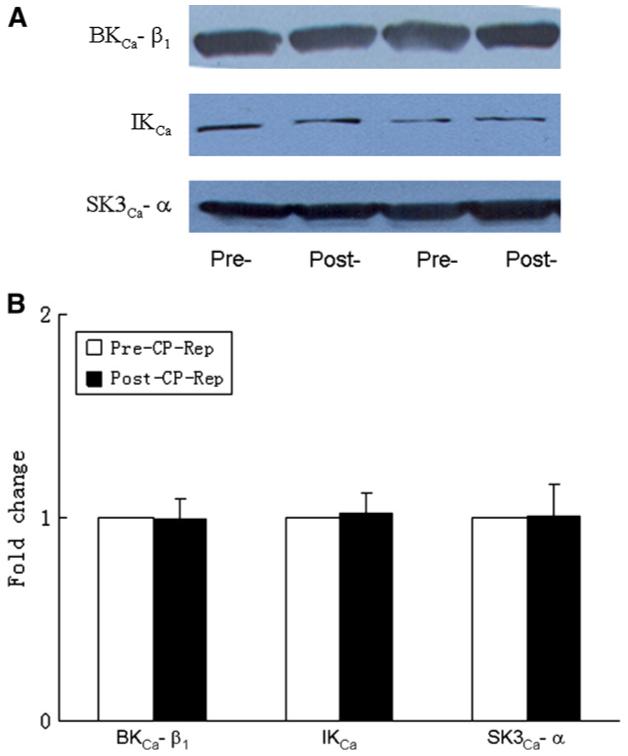
A, Representative immunoblots of human skeletal muscle microvessels (40 μg loaded protein) developed with antibodies to BK-β1,IKCa, and SK3Ca polypeptides. B, Densitometric evaluation of immunoblot band intensity normalized to pre-CPB values shows unaltered levels of KCa polypeptides after CPB (n = 6 per group).
DISCUSSION
We have shown previously that relaxation responses of porcine skeletal muscle arterioles to the endothelium-dependent vasodilator acetylcholine are decreased after hypothermic CPB.2,3 The present study, indicating that relaxation responses of human skeletal muscle arterioles to the endothelium-dependent vasodilators ADP and substance P are impaired post-CPB, suggests that CPB is associated with endothelial dysfunction of human peripheral microvasculature, and is consistent with the earlier study in porcine arterioles. The vascular endothelium is important not only for regulation of permeability, but also as the site of interaction with circulating leukocytes in the inflammatory response associated with CPB. Thus, CPB-induced activation of complement and neutrophils, release of proinflammatory cytokines and free radicals, and excessive adrenergic stimulation may all contribute to increased vascular permeability and endothelial swelling, resulting in peripheral microvascular endothelial dysfunction.
There are 3 types of vascular KCa channels—BK Ca, IK Ca, and SK Ca.7,8 In the present study, relaxation responses to NS309 were blocked completely in the presence of the IKCa blocker TRAM 34 and the SKCa inhibitor apamin, consistent with the proposed specificity of NS309 as a IKCa/SKCa activator. In contrast, relaxation responses to NS1619 were abolished in the presence of the BKCa blocker iberiotoxin, indicating specificity of NS1619 as a specific BKCa activator. Endothelium denudation significantly diminished NS309-induced relaxation, but failed to affect the response to NS1619, suggesting that relaxation induced by the IKCa/SKCa opener NS309 is endothelium dependent. Remarkably, CPB impaired the dose-dependent vasodilatation induced by the IKCa/SKCa channel activator NS309 but not that induced by the BKCa opener NS1619, suggesting that IKCa/SKCa inactivation may contribute to endothelial dysfunction of human skeletal muscle microvasculature. The molecular mechanism of CPB-related IKCa/SKCa dysfunction is unclear. CPB may modulate activity of serine and/or tyrosine kinases, leading to possible downregulation of IKCa/SKCa channels.1,4,9 Alternatively or additionally, release of free radicals and/or cytokines during CPB might inhibit IKCa/SKCa channel activity of endothelium.1,10
To further test the presence in human skeletal muscle microvasculature of IKCa/SKCa and BKCa polypeptides, we performed immunoblot analyses to detect these channel proteins. BKCa, IKCa, and SKCa polypeptides were highly expressed in human skeletal muscle microvessels. CPB did not, however, alter total microvessel polypeptide abundance of any of these channels, suggesting that brief hypothermic ischemia and reperfusion modifies channel activity without change in channel polypeptide abundance. Recently, we also found that the responses to NS309 were significantly impaired in the coronary microvasculature after cardioplegic arrest and reperfusion, suggesting that SK and IK may be responsible for coronary microvascular endothelial dysfunction after cardiac operations.11
In conclusion, this study demonstrated that KCa channels are expressed in human skeletal muscle microvasculature. Inactivation of IK/SK channels may contribute to CPB-related microvascular endothelial dysfunction of human peripheral tissue.
Acknowledgments
Supported in part by NIH-R01 grants (HL-69024 and HL-46716 [F.W.S]; HL-077765 [SLA]) and a NIH-T32 research training grant (HL076130-02).
Footnotes
Presented at the Third Annual Academic Surgical Congress, Huntington Beach, California, February 13-15, 2008.
REFERENCES
- 1.Ruel M, Khan TA, Voisine P, Bianchi C, Sellke FW. Vasomotor dysfunction after cardiac surgery. Eur J Cardiothorac Surg. 2004;26:1002–14. doi: 10.1016/j.ejcts.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 2.Wang SY, Stamler A, Li J, Johnson RG, Sellke FW. Decreased myogenic reactivity in skeletal muscle arterioles after hypothermic cardiopulmonary bypass. J Surg Res. 1997;69:40–4. doi: 10.1006/jsre.1997.5020. [DOI] [PubMed] [Google Scholar]
- 3.Stamler A, Wang SY, Aguirre DE, Johnson RG, Sellke FW. Cardiopulmonary bypass alters vasomotor regulation of the skeletal muscle microcirculation. Ann Thorac Surg. 1997;64:460–5. doi: 10.1016/S0003-4975(97)00539-0. [DOI] [PubMed] [Google Scholar]
- 4.Khan TA, Bianchi C, Araujo EG, Ruel M, Voisine P, Li J, et al. Cardiopulmonary bypass reduces peripheral microvascular contractile function by inhibition of mitogen-activated protein kinase activity. Surgery. 2003;134:247–54. doi: 10.1067/msy.2003.229. [DOI] [PubMed] [Google Scholar]
- 5.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology. 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 6.Feng J, Sellke ME, Ramlawi B, Boodhwani M, Clements R, Li JY, et al. Bradykinin induces microvascular preconditioning via opening of calcium-activated potassium channels. Surgery. 2006;140:192–7. doi: 10.1016/j.surg.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. No-menclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;5:463–72. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 8.Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, et al. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–7. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- 9.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–12. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Terata K, Chai Q, Li H, Kleinman LH, Gutterman DD. Peroxynitrite inhibits Ca2+-activated K+ channel activity in smooth muscle of human coronary arterioles. Circ Res. 2002;9:1070–6. doi: 10.1161/01.res.0000046003.14031.98. [DOI] [PubMed] [Google Scholar]
- 11.Liu YH, Feng J, Sodha NR, Khabbaz KR, Senthilnathan V, Alper SL, et al. Calcium activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegia arrest. Circulation. 2007;116(Suppl II):II–324. doi: 10.1161/CIRCULATIONAHA.107.755827. [DOI] [PMC free article] [PubMed] [Google Scholar]