Abstract
Context: Polycystic ovary syndrome (PCOS) is characterized by insulin resistance, compensatory hyperinsulinemia, increased prevalence of impaired glucose tolerance, and increased ovarian androgen biosynthesis.
Objective: The aim of the study was to evaluate effects of pioglitazone on whole body insulin action and ovarian androgen biosynthesis in PCOS.
Design: We performed a randomized placebo-controlled trial.
Setting: The study was conducted at the Special Diagnostic and Treatment Unit of the Veterans Affairs Medical Center, San Diego, and the University of California, San Diego, General Clinical Research Center.
Patients or Other Participants: A total of 23 subjects with PCOS were evaluated at baseline and end of treatment. Six age- and body mass index-matched women without PCOS were normal controls for baseline evaluation.
Intervention: Subjects with PCOS were randomized to oral placebo or pioglitazone 45 mg daily for 6 months.
Main Outcome Measure(s): The primary outcome measures were whole body insulin action as measured by hyperinsulinemic euglycemic clamp and ovarian androgen biosynthesis as measured by leuprolide-stimulated production of 17-hydroxyprogesterone (17-OHP).
Results: Compared with placebo, pioglitazone treatment significantly improved multiple measures of insulin action, including glucose disposal rate (P < 0.01), 2-h glucose during 75-g oral glucose tolerance test (P < 0.01), area under the curve glucose during oral glucose tolerance test (P < 0.01), serum adiponectin (P < 0.01), and fasting hyperinsulinemia (P < 0.01). Compared to placebo, pioglitazone treatment reduced the increment of leuprolide-stimulated 17-OHP (P < 0.02). Improvements in glucose disposal rate correlated with reductions in 17-OHP stimulation (P < 0.02).
Conclusions: Compared to placebo, pioglitazone treatment in PCOS was associated with improvements in insulin action and glucose homeostasis and ameliorated the hyperandrogenic ovarian response.
Improvements in insulin sensitivity in overweight/obese women with polycystic ovary syndrome following pioglitazone treatment are associated with reduced ovarian androgen responsiveness to gonadotropin stimulation.
Polycystic ovary syndrome (PCOS) is a common endocrine disorder affecting 6–10% of women of reproductive age (1,2). PCOS is characterized by chronic anovulation and hyperandrogenism. PCOS is often associated with insulin resistance, hyperinsulinemia, and abnormal glucose tolerance, leading to significantly increased risk of type 2 diabetes (3).
Insulin action is impaired in PCOS and is made worse by obesity. Insulin resistance in PCOS results in impaired insulin-stimulated glucose disposal in skeletal muscle, increased hepatic glucose production, and increased lipolysis (4,5). Adiponectin levels are also reported to be reduced in PCOS, which may be related to level of glucose tolerance and insulin resistance (6,7,8).
Insulin resistance and hyperinsulinemia may contribute to excess ovarian androgen production in PCOS. Specifically, it has been shown that women with PCOS exhibit increased expression of cytochrome P450c17 (CYP17), which results in increased activity of 17α-hydroxylase and 17,20-lyase in ovarian theca cells. This dysregulation of CYP17 promotes enhanced production of 17-hydroxyprogesterone (17-OHP), androstenedione (A4), and testosterone. Accordingly, women with PCOS display elevated mean circulating levels of 17-OHP and have exaggerated serum 17-OHP responses to gonadotropin stimulation compared with responses observed in normal women (9,10). In vitro studies have demonstrated that cultured theca cells from normal and PCOS women produce significantly more androgen in response to LH stimulation after coincubation with insulin (11). Such data support an underlying connection between hyperinsulinemia and insulin resistance and ovarian hyperandrogenism seen in PCOS.
In this study, we hypothesized that pioglitazone would not only improve insulin action in women with PCOS, but may also impact the hyperandrogenic response of the ovary.
Subjects and Methods
Subjects
Twenty-three women with PCOS were recruited for study. The diagnosis was based on criteria established at the 1990 National Institutes of Health Conference on PCOS: 1) evidence of chronic anovulation or oligoamenorrhea by clinical history; 2) clinical [Ferriman-Gallwey (FG) score ≥8] and/or biochemical evidence of hyperandrogenism; and 3) the exclusion of other conditions (12). Pregnancy was ruled out by urine pregnancy test. Normal thyroid and prolactin function were established by hormonal evaluation. Late-onset nonclassic congenital hyperplasia was excluded by basal 17-OHP values of less than 3 ng/ml (9.1 nmol/liter). Exclusionary medications included glucocorticoids, antiandrogens, ovulation induction agents, metformin, and antiobesity medications within the 60 d before screening. None of the participants had a history of thiazolidinedione use. Subjects who were previously on oral contraceptive pills had to discontinue these for a minimum of 1 month before screening for eligibility for the study (13). For comparative purposes, six age- and weight-matched women with regular menstrual cycles were also evaluated as baseline control subjects.
None of the subjects were affected by other significant unstable medical illness. None of the subjects had a history of type 2 diabetes. The procedures were in accordance with the guidelines of the Helsinki Declaration on Human Experimentation. The study was conducted in the Special Diagnostic and Treatment Unit (SDTU) of the Veterans Affairs Medical Center (San Diego, CA) and the General Clinical Research Center (GCRC), University of California, San Diego. The experimental protocol was approved by the appropriate institutional review boards. The purpose of the protocol was explained to the research subjects, and written informed consent was obtained from all subjects before beginning the study. Subjects who were confirmed to have PCOS on the screening visit were admitted to the SDTU on another date and had the following procedures.
Procedure
Each subject was admitted to the SDTU the evening before evaluation. Subjects who had predictable periods were studied during the midfollicular phase at baseline and at the end of the 6-month treatment period. All subjects underwent an overnight fast. An oral glucose tolerance test (OGTT) was performed as follows: blood samples were obtained through an iv catheter before and at 30, 60, 90, and 120 min after ingestion of a 75-g glucose load.
At the end of the OGTT, two additional baseline blood samples were obtained 20 min apart for determination of A4, total testosterone, 17-OHP, dehydroepiandrosterone (DHEA), DHEA sulfate (DHEA-S), and SHBG. Values were averaged to provide the baseline value. After baseline sampling, each subject received a sc injection of a GnRH agonist, leuprolide acetate (10 μg/kg). A subsequent blood sample was obtained 24 h later for A4, total testosterone, 17-OHP, and DHEA. All blood samples were immediately centrifuged, and the serum was stored at −70 C until analysis.
After an overnight fast, each subject underwent determination of in vivo insulin action as assessed by a 3-h hyperinsulinemic (300 mU/m2 · min) euglycemic (5 mm) clamp as described by DeFronzo et al. (14). A hyperinsulinemic clamp was used to ensure suppression of hepatic glucose production and to evaluate peripheral insulin action as we have previously shown (15). During the clamp, plasma insulin concentration was raised with a prime-continuous infusion of insulin, and plasma glucose concentrations were assessed every 5 min and held constant at basal levels by a variable glucose infusion. The glucose infusion rate (milliliters per minute) was calculated at the end of the 3-h clamp over the final two 20-min periods to ensure steady state. Because steady-state euglycemia was achieved, the calculated glucose infusion rate equals the glucose uptake by whole body, or glucose disposal rate (GDR), a reflection of tissue insulin sensitivity (14,16). Preclamp steady-state free fatty acid levels were calculated by averaging values obtained at time −30, −20, −10, and 0 minutes. A single value at the end of the clamp was used in the calculation of free fatty acid suppression.
Subjects also had a dual energy x-ray absorptiometry (DEXA) for measurement of total body fat. A urine pregnancy test was done before DEXA to rule out pregnancy.
After all pretreatment procedures, subjects with PCOS were randomly assigned to receive either oral placebo or pioglitazone (45 mg) daily for 6 months. Subjects were instructed to continue their usual diet and activity behaviors. At the end of treatment, each subject underwent the same procedures as described above.
Assays
Plasma glucose levels were determined immediately by the glucose oxidase method with a YSI Glucose Analyzer (YSI, Yellow Springs, OH). Serum insulin levels were performed by RIA (Linco Research Inc., St. Louis, MO) with intraassay coefficient of variation (CV) less than 7%. Serum free fatty acid levels were measured using enzymatic colorimetric endpoint (Wako Chemical, Neuss, Germany) with intraassay CV less than 7%. Circulating serum adiponectin was measured using a commercially available RIA kit (Linco). The lower limit of detection with this assay was 2 μg/ml; the inter- and intraassay CVs were 7 and 11%, respectively.
Serum concentrations of testosterone, 17-OHP, DHEA, and A4 were measured by well-established internal RIA with intraassay and interassay CVs less than 7%. DHEA-S was measured by chemiluminescent immunoassay (ARUP Laboratories, Salt Lake City, UT), with inter- and intraassay CVs less than 2 and 5%, respectively. SHBG was measured by chemiluminescent immunoassay (ARUP Laboratories) with inter- and intraassay CVs less than 2%.
Data analysis
Within-group treatment effects were evaluated using paired Student’s t test analysis. Comparisons between groups (PCOS vs. control, placebo vs. pioglitazone) were made using t tests for independent samples. Where variables were not normally distributed, significance was confirmed by a Mann-Whitney U test. Where groups were not equivalent at baseline, significance was evaluated using analysis of covariance, with the baseline variable as the covariate. Data are presented as mean ± sem with two-tailed P < 0.05 considered statistically significant. Correlation analyses report that Spearman’s coefficient and linear regression was performed on correlation analyses. All analyses were run using Prism4 statistical software (GraphPad Inc., San Diego, CA) or SPSS (SPSS Inc., Chicago, IL).
Results
Subjects
Six normal control (NC) subjects were evaluated as baseline controls. Twenty-eight subjects with PCOS were recruited and randomized to placebo or pioglitazone 45 mg daily. In the placebo group, one subject was withdrawn due to unanticipated pregnancy, and three subjects were lost to follow-up. In the pioglitazone group, one subject was lost to follow-up. Baseline characteristics of these subjects who were lost to follow-up or withdrawn include: mean age, 27.8 ± 2.75 yr; mean fasting glucose, 5.40 ± 0.39 mmol/liter; mean body mass index (BMI), 35.47 ± 1.79 kg/m2. One subject in the placebo group developed mild nonpitting edema, and three subjects in the pioglitazone group developed lower extremity edema. Data are reported on six NC subjects and the 23 subjects with PCOS who completed all procedures. Tables 1 and 2 summarize the results.
Table 1.
Clinical, metabolic, and hormonal characteristics of subjects with PCOS and NC subjects
| All PCOS (n = 23) | NC (n = 6) | P value | |
|---|---|---|---|
| Age (yr) | 27.87 ± 0.87 | 31.83 ± 1.92 | ns |
| BMI (kg/m2) | 36.29 ± 1.34 | 35.93 ± 2.84 | ns |
| Weight (kg) | 99.67 ± 3.63 | 98.10 ± 10.17 | ns |
| Waist circumference (cm) | 106.2 ± 3.29 | 102.3 ± 6.61 | ns |
| Waist-hip ratio | 0.89 ± 0.02 | 0.87 ± 0.06 | ns |
| % Body fat | 42.27 ± 1.01 (n = 21) | 39.90 ± 3.79 (n = 4) | ns |
| Fasting glucose (mg/dl) | 93.77 ± 1.67 | 97.70 ± 2.43 | ns |
| 2-h Glucose (mg/dl) | 150.9 ± 8.10 | 123.5 ± 4.40 | ns (P = 0.10) |
| AUCg | 18,970 ± 714.7 | 17,520 ± 695.2 | ns |
| Hemoglobin A1c (%) | 5.29 ± 0.09 (n = 22) | 5.32 ± 0.15 | ns |
| Adiponectin (μg/ml) | 9.43 ± 0.88 | 16.51 ± 1.85 | ≤0.01 |
| Fasting insulin (μIU/ml) | 28.78 ± 2.96 | 17.54 ± 1.93 | 0.03 |
| GDR (mg/kg · min) | 6.26 ± 0.48 | 8.55 ± 0.69 | <0.03 |
| Free fatty acid suppression during clamp (% change) | −74.09 ± 2.46% | −90.79 ± 3.31% | <0.01 |
| FG score | 13.57 ± 1.72 | 3.29 ± 0.89 | <0.01 |
| Testosterone (ng/ml) | 0.41 ± 0.04 | 0.19 ± 0.03 | <0.02 |
| Free testosterone index | 10.38 ± 1.34 | 3.42 ± 0.53 | <0.02 |
| DHEA-S (ng/ml) | 2,433 ± 191.9 | 1,215 ± 139.2 | <0.01 |
| SHBG (nmol/liter) | 16.65 ± 1.64 | 22.13 ± 5.04 | 0.19 |
| Basal 17-OHP (ng/ml) | 1.99 ± 0.18 | 1.20 ± 0.18 | <0.05 |
| Stimulated 17-OHP (ng/ml) | 4.21 ± 0.44 | 2.14 ± 0.17 | <0.05 |
| Change in 17-OHP with leuprolide stimulation (ng/ml) | 2.22 ± 0.50 | 0.94 ± 0.10 | 0.20 |
| Basal DHEA (ng/ml) | 5.82 ± 0.76 | 2.35 ± 0.30 | <0.02 |
| Stimulated DHEA (ng/ml) | 6.65 ± 0.90 | 2.93 ± 0.62 | <0.04 |
| Change in DHEA with leuprolide stimulation (ng/ml) | 0.83 ± 0.76 | 0.58 ± 0.66 | ns |
| Basal A4 (ng/dl) | 153 ± 12.89 | 67.91 ± 6.02 | <0.01 |
| Stimulated A4 (ng/dl) | 223.78 ± 17.77 | 89.97 ± 8.02 | <0.01 |
| Change in A4 with leuprolide stimulation (ng/dl) | 70.77 ± 10.89 | 22.06 ± 6.30 | <0.04 |
To convert to SI units, multiply by the following conversion factors: 0.05551 for glucose, 7.175 for insulin, 3.47 for testosterone, 0.0027 for DHEA-S, 3.03 for 17-OHP, 3.47 for DHEA, 0.0349 for A4. Free testosterone index is calculated as (total testosterone/SHBG)*100. Data are expressed as mean ± sem. ns, Not significant.
Table 2.
Treatment effects of placebo or pioglitazone (45 mg/d) on anthropometric, metabolic, and hormonal parameters
| Placebo (n = 10)
|
Pioglitazone (n = 13)
|
|||
|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | |
| Age (yr) | 27 ± 1 | 28 ± 1 | ||
| BMI (kg/m2) | 35.63 ± 2.10 | 35.22 ± 2.14 | 36.80 ± 1.80 | 38.55 ± 2.04a,b |
| Weight (kg) | 97.41 ± 6.27 | 96.57 ± 6.71 | 101.4 ± 4.41 | 106.0 ± 5.04a,b |
| Waist circumference (cm) | 106.7 ± 4.62 | 104.1 ± 4.52 | 106.9 ± 5.54 (n = 11) | 110.7 ± 4.39 (n = 11) |
| Waist-hip ratio | 0.89 ± 0.02 | 0.89 ± 0.02 | 0.89 ± 0.04 (n = 11) | 0.87 ± 0.03 (n = 11) |
| % Body fat | 44.44 ± 1.70 (n = 8) | 43.18 ± 2.06 (n = 8) | 40.62 ± 1.19 (n = 12) | 42.17 ± 1.28 (n = 12)a |
| Fasting glucose (mg/dl) | 91.38 ± 1.26 | 91.01 ± 2.16 | 95.62 ± 2.74 | 88.78 ± 1.92a |
| 2-h Glucose (mg/dl) | 129.4 ± 10.53 | 151.8 ± 15.75a | 167.4 ± 9.84c | 134.5 ± 8.98a,b |
| Hemoglobin A1c (%) | 5.28 ± 0.13 (n = 9) | 5.11 ± 0.14 (n = 9) | 5.23 ± 0.13 (n = 11) | 5.13 ± 0.08 (n = 11) |
| AUCg | 17,019 ± 964 | 18,486 ± 829 | 20,466 ± 831c | 17,863 ± 586a,b |
| Adiponectin (μg/ml) | 9.10 ± 1.37 (n = 9) | 11.33 ± 1.68 (n = 9) | 9.31 ± 1.24 | 20.08 ± 2.12a,b |
| Free fatty acid suppression during clamp (% change) | −74.30 ± 3.54 | −74.23 ± 4.57 | −73.70 ± 3.81 (n = 12) | −78.59 ± 4.41(n = 12) |
| Fasting insulin (μIU/ml) | 23.23 ± 2.62 | 26.64 ± 2.96 | 33.05 ± 4.58 | 21.30 ± 3.64a,b |
| GDR (mg/kg · min) | 7.04 ± 0.90 | 6.89 ± 0.75 | 5.66 ± 0.48 | 7.26 ± 0.62a,b |
| FG score | 11 ± 3 | 9 ± 2 | 16 ± 2 | 11 ± 2a,b |
| Total testosterone (ng/ml) | 0.45 ± 0.07 | 0.41 ± 0.04 | 0.38 ± 0.05 | 0.45 ± 0.06 |
| Free testosterone index | 10.06 ± 2.14 | 8.01 ± 0.97 | 10.63 ± 1.89 | 10.07 ± 1.75 |
| DHEA-S (ng/ml) | 2,299 ± 367.0 | 2,211 ± 333.9 | 2,493 ± 389.1 (n = 11) | 2,340 ± 306.0 (n = 11) |
| SHBG (nmol/liter) | 20.03 ± 3.20 | 20.21 ± 2.63 | 14.05 ± 1.21 | 17.34 ± 1.56a |
| Basal 17-OHP (ng/ml) | 2.41 ± 0.23 | 1.68 ± 0.05 | 1.67 ± 0.23c | 1.51 ± 0.19 |
| Stimulated 17-OHP (ng/ml) | 3.97 ± 0.46 | 4.38 ± 0.54 | 4.39 ± 0.70 | 3.23 ± 0.33 |
| Change in 17-OHP with leuprolide stimulation (ng/ml) | 1.56 ± 0.63 | 2.70 ± 0.51 | 2.73 ± 0.72 | 1.73 ± 0.31b |
| Basal DHEA (ng/ml) | 5.11 ± 1.07 (n = 8) | 4.69 ± 0.58 (n = 8) | 6.39 ± 1.07 (n = 10) | 5.18 ± 0.89 (n = 10) |
| Stimulated DHEA (ng/ml) | 6.07 ± 1.04 (n = 8) | 4.95 ± 0.63 (n = 8) | 7.13 ± 1.44 (n = 10) | 5.81 ± 0.88 (n = 10) |
| Change in DHEA with leuprolide stimulation (ng/ml) | 0.96 ± 0.71 (n = 8) | 0.26 ± 0.28 (n = 8) | 0.73 ± 1.28 (n = 10) | 0.63 ± 1.03 (n = 10) |
| Basal A4 (ng/dl) | 163.04 ± 16.62 | 154.73 ± 10.60 | 145.56 ± 19.48 | 139.83 ± 22.35 |
| Stimulated A4 (ng/dl) | 227.22 ± 28.08 | 194.84 ± 18.34 | 221.20 ± 23.78 | 183.38 ± 27.51 |
| Change in A4 with leuprolide stimulation (ng/dl) | 63.89 ± 20.06 | 40.11 ± 12.89 | 75.64 ± 11.75 | 43.84 ± 8.88a |
To convert to SI units, multiply by the following conversion factors: 0.05551 for glucose, 7.175 for insulin, 3.47 for testosterone, 0.0027 for DHEA-S, 3.03 for 17-OHP, 3.47 for DHEA, and 0.0349 for A4. Free testosterone index is calculated as (total testosterone/SHBG)*100. Data are expressed as mean ± sem.
P < 0.05 compared with baseline.
P < 0.05 treatment effect of pioglitazone vs. placebo.
P < 0.05 compared with placebo baseline.
Anthropometric measures
Anthropometric measures were similar in the PCOS subjects compared with NC subjects (Table 1). PCOS subjects that comprised the two treatment groups did not differ in age, obesity, and baseline anthropometric measurements. Pioglitazone treatment was associated with a significant increase in body weight, BMI, and percentage body fat as measured by DEXA. Similar changes were not observed in the group of women receiving placebo therapy. Compared with placebo, pioglitazone increased body weight and BMI. There were no significant changes in waist circumference or waist-hip ratio (Table 2).
Glucose tolerance
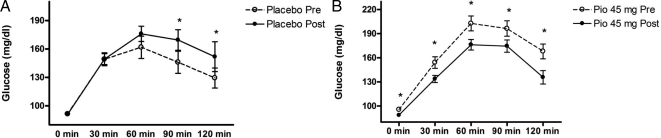
Baseline metabolic parameters, including fasting glucose, 2-h glucose, area under the curve for glucose (AUCg), and hemogloblin A1c were similar between PCOS and NC subjects (Table 1). Pretreatment fasting glucose and hemoglobin A1c were similar between the two PCOS treatment groups, although baseline 2-h glucose and AUCg were higher in the pioglitazone treatment group. Numerous metabolic parameters improved in the pioglitazone-treated PCOS subjects compared with a lack of response in the placebo-treated subjects. Responses of glucose to the 75-g oral glucose challenge are illustrated in Fig. 1. Although placebo-treated subjects had no change in fasting glucose, they displayed a deterioration in glucose tolerance manifested as a significant increase in 90 and 120 min glucose and a trend toward increase in AUCg (P = 0.05). In contrast, pioglitazone-treated subjects had a lowering of glucose at each time point during the 2-h OGTT and a reduced AUCg. Compared with placebo, pioglitazone improved AUCg by an average of 22% (P < 0.0001) (Table 2).
Figure 1.
Glucose tolerance curves after 75-g oral glucose challenge in subjects with PCOS before and after 6 months of placebo (n = 10) (A) and subjects with PCOS before and after 6 months of pioglitazone 45 mg/d (n = 13) (B). To convert glucose to SI units, multiply by the conversion factor 0.05551. Data are depicted as mean ± sem. *, P < 0.05.
Notably, after 6 months of placebo treatment, 29% of the normal glucose-tolerant subjects developed impaired glucose responses during the OGTT, whereas none of the impaired glucose-tolerant individuals in this group reverted to normal glucose tolerance. In contrast, 40% of the subjects with impaired glucose responses at baseline within the pioglitazone group reverted to normal glucose tolerance. None of the normal glucose-tolerant subjects in the pioglitazone group progressed to impaired glucose tolerance.
Insulin action
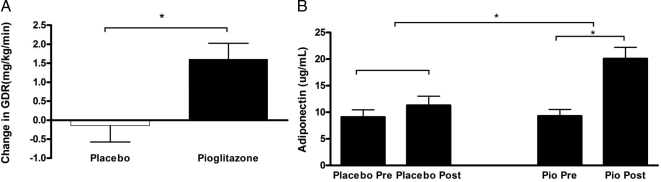
Baseline GDR and circulating adiponectin levels were lower in PCOS subjects compared with control subjects (Table 1). Baseline insulin-stimulated GDRs, as determined by the hyperinsulinemic (300 mU/m2 · min) euglycemic (5 mm) clamp technique, and adiponectin levels did not differ between subjects randomized to placebo or pioglitazone. Pioglitazone treatment significantly improved GDR (from 5.66 ± 0.48 to 7.26 ± 0.62 mg/kg · min; P < 0.01), representing a 31 ± 7% increase over the paired baseline and a significant treatment effect compared with placebo. Likewise, pioglitazone more than doubled circulating adiponectin levels (9.31 ± 1.24 to 20.08 ± 2.12 μg/ml; P < 0.0001), whereas placebo treatment had no impact on either GDR or circulating adiponectin levels (Table 2 and Fig. 2).
Figure 2.
Change in insulin sensitivity with pioglitazone treatment. A, Change in GDR during hyperinsulinemic euglycemic clamp after 6 months of treatment with placebo or pioglitazone. B, Change in circulating serum adiponectin levels before and after 6 months of placebo or pioglitazone (Pio) treatment. Data are depicted as mean ± sem. *, P < 0.05.
Free fatty acid suppression during the clamp was significantly less in PCOS subjects compared with NCs (Table 1). The extent of free fatty acid suppression was unaffected by treatment with either placebo or pioglitazone (Table 2).
Reproductive/hormonal measures
Women with PCOS exhibited significantly greater circulating concentrations of A4, testosterone, 17-OHP, DHEA, and DHEA-S compared with corresponding values in NC women (Table 1). Pioglitazone treatment did not change basal testosterone, DHEA, or DHEA-S levels (Table 2).
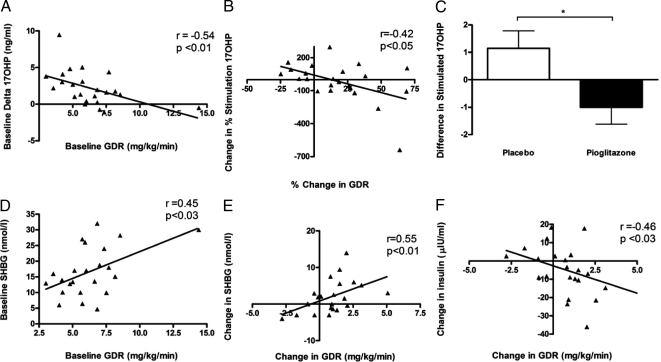
At baseline, there was a trend toward greater increase in 17-OHP response to leuprolide stimulation in the PCOS subjects compared with NC (P = 0.12), as is already known with PCOS (Table 1). Compared with placebo treatment, pioglitazone treatment blunted this increased response (P < 0.02) (Table 2 and Fig. 3). In addition, baseline GDR significantly correlated with 17-OHP response to leuprolide (P < 0.01; r = −0.54). Improvement in GDR also correlated with reductions in the 17-OHP response to leuprolide (Fig. 3). Baseline insulin and 17-OHP responses were also positively correlated (P < 0.01; r = 0.63). There was a trend toward a correlation between reductions in the 17-OHP response to leuprolide and percentage change in basal insulin levels (P = 0.05; r = 0.41) (data not illustrated).
Figure 3.
A, Correlation between baseline GDR during hyperinsulinemic euglycemic clamp and increment of leuprolide-stimulated 17OH progesterone. B, Correlation of improvement in GDR during hyperinsulinemic euglycemic clamp with attenuation of leuprolide-stimulated 17-OHP responses. C, Change in increment of leuprolide-stimulated 17-OHP with 6 months of treatment with placebo or pioglitazone. D, Correlation of baseline GDR and SHBG. E, Correlation of improvement in GDR with improvement in SHBG. F, Correlation of change in GDR with change in fasting insulin. To convert 17-OHP to SI units, multiply by the conversion factor 3.03. *, P < 0.05.
In women with PCOS, serum SHBG levels were lower than those of NCs, although the difference failed to achieve statistical significance (Table 1). SHBG concentration increased significantly within the pioglitazone treatment group (P < 0.05), compared with no change in the placebo group (Table 2). Moreover, baseline SHBG positively correlated with baseline GDR (r = 0.45; P < 0.03). Improvements in GDR correlated with increases in SHBG (r = 0.55; P < 0.01) and decreases in fasting serum insulin (r = −0.46; P < 0.03).
As expected, FG scores were higher in women with PCOS than scores determined for NC subjects (Table 1). In both groups of PCOS women undergoing treatment, FG scores before therapy were equivalent. FG scores were significantly reduced after 6 months of pioglitazone treatment (16 ± 2 to 11 ± 2; P = 0.0015), whereas in the group treated with placebo no change was detected (Table 2). Thirty-seven percent of placebo subjects had more than three regular menstrual cycles during the 6 months of treatment, and 54% of subjects treated with pioglitazone had more than three regular menstrual cycles during the 6 months of treatment.
Discussion
Two distinct but likely related features of PCOS are insulin resistance with compensatory hyperinsulinemia and ovarian hyperandrogenemia. In this study, we explored whether intervention with a potent insulin sensitizer, pioglitazone, would improve the insulin resistance as well as basal and gonadotropin-stimulated androgen secretion characteristic of PCOS. We demonstrated that pioglitazone improved whole body insulin action, as evidenced by increased glucose uptake during the hyperinsulinemic euglycemic clamp, improved glucose tolerance, increased circulating adiponectin levels, and reduced hyperinsulinemia. Pioglitazone may improve glucose tolerance in PCOS by directly improving insulin sensitivity. Indeed, both fasting glucose and 2-h glucose improved with pioglitazone treatment, suggesting an effect on both hepatic insulin resistance and skeletal muscle insulin resistance. There was no change in free fatty acid suppression, suggesting less of an effect on insulin action in adipose tissue.
Adiponectin levels more than doubled in pioglitazone-treated subjects with PCOS over the 6-month study period, an increase difficult to achieve with other treatment modalities (17). Another thiazolidinedione, rosiglitazone, has also been shown to similarly increase adiponectin levels in PCOS with 4 months of treatment (18). Whether adiponectin is a marker or a mediator of insulin sensitivity in PCOS remains undetermined. Our data support that hypoadiponectinemia is a significant metabolic problem in PCOS and is responsive to treatment. Hypoadiponectinemia is associated with an increased risk of type 2 diabetes and hypertension and may be associated with increased cardiovascular risk (17,19,20,21). Thus, our finding of a doubling of circulating adiponectin and improvement in whole body insulin action in a placebo-controlled fashion suggests that pioglitazone may positively impact the natural history of development of diabetes in individuals with PCOS.
Measures of insulin action improved despite an increase in total body weight. Although we did not assess for fat depot differences in this study, such an improvement in insulin action despite increase in body weight may reflect a redistribution of fat depots, as has been reported for thiazolidinedione treatment in other subject populations (22).
Beyond these metabolic improvements, pioglitazone also ameliorated the hyperandrogenic response of the ovary compared with placebo. This was evident in the improvement in leuprolide-stimulated 17-OHP responses in the pioglitazone group compared with placebo. The level of improvement in the 17-OHP response directly correlated with improvements in GDR (Fig. 3). The stimulated A4 response also significantly improved within the pioglitazone group. All of these findings support the hypothesis that insulin sensitization with pioglitazone improves the up-regulation of P450c17 characteristic of PCOS.
Pioglitazone may exert its reproductive hormonal effects in PCOS either directly on the ovary or indirectly by reducing circulating insulin levels. There is evidence to support both possibilities. To support a direct effect on the ovary, the thiazolidinediones have been shown to inhibit the steroidogenic enzymes p450c17 and 3β-hydroxysteroid dehydrogenase in “humanized yeast” (23,24). In addition, in human adrenal NCI-H295R cells, pioglitazone has been shown to inhibit the expression of the CYP17 and HSD3B2 genes responsible for encoding P450c17 and 3β-hydroxysteroid dehydrogenase II enzymes (25). However, in these studies the amount of pioglitazone required to achieve an equivalent inhibition constant (IC50) was much greater than that resulting from the therapeutic dose employed in the current study, indicating limited potential for steroid suppression (23).
Insulin, IGFs, and inhibin have been shown to augment the ovarian androgenic response to LH and ACTH (26). Insulin augmentation of the 17α-hydroxylase activity of P450c17 appears in part to be mediated by phosphatidylinositol-3-kinase activity (27). Insulin sensitizers have previously been demonstrated to reduce circulating androgen levels. In addition, metformin has been shown to attenuate the ovarian androgen response to gonadotropin stimulation, presumably by reducing hyperinsulinemia (28,29,30,31). In this study, pioglitazone improved insulin sensitivity. There appeared to be a relationship between the level of improvement in circulating insulin levels and amelioration of the 17-OHP response to leuprolide (P = 0.05). These observations suggest that pioglitazone may impact ovarian androgen biosynthesis by reducing circulating insulin levels, which could reduce stimulation of phosphatidylinositol-3-kinase in the ovary.
An alternative pathway by which pioglitazone may impact ovarian androgen biosynthesis is by regulation of ovulation and menstrual cycles. Regulation of ovulation may restore normal feedback effects of luteal steroids, which would in effect help normalize serum LH levels, causing improvements in ovarian responses to leuprolide. Current published data, however, are mixed on the effect of insulin sensitization on mean LH levels in women with PCOS (32,33,34).
Although basal circulating levels of androgens did not change, the subjects treated with pioglitazone had an increase in SHBG. This improvement in SHBG appeared to reflect the improvement in insulin sensitivity (Fig. 3) and may potentially have consequences on the available metabolically active free circulating testosterone. Accordingly, although total testosterone levels and free testosterone index did not change, FG scores did improve.
This is the only currently published placebo-controlled trial of 6-month duration in women with PCOS evaluating the effect of a maximal dose of pioglitazone (45 mg) on whole body insulin action by the gold standard euglycemic hyperinsulinemic clamp technique and its effects on ovarian androgen biosynthesis via the P450c17 pathway. Despite significant metabolic improvements that were seen, subjects did gain weight. In this study, subjects were not given specific diet or lifestyle instructions for weight loss. Given the potential long-term consequences of weight gain, future studies with thiazolidinediones in PCOS may also need to explore caloric intake, expenditure, and measures to prevent weight gain while retaining metabolic benefit.
In summary, this study has demonstrated that improvements in insulin sensitivity in overweight/obese women with PCOS after pioglitazone treatment are associated with reduced ovarian androgen responsiveness to gonadotropin stimulation. The beneficial effects of thiazolidinedione on insulin resistance and excess ovarian androgen production were realized despite weight gain during treatment. Further investigation of the long-term effects of insulin sensitization combined with behavioral modification appears warranted in overweight/obese women with PCOS.
Acknowledgments
We are grateful to Leslie Carter, for her assistance with the adiponectin assays; Jeff Wong and David Herold, M.D., for assistance with the reproductive hormone assays; Gina Reggiardo, M.D., for database assistance; and Sunita Baxi, M.D., for assistance with subject recruitment.
Footnotes
This work was supported by grants from the American Diabetes Association (to T.P.C. and R.R.H.); the Medical Research Service, Department of Veterans Affairs, VA San Diego Healthcare System (to R.R.H.); Takeda Pharmaceuticals North America (to R.R.H.); and Grant M01 RR-00827 in support of the General Clinical Research Center from the General Clinical Research Branch, Division of Research Resources, National Institutes of Health (NIH). This research was also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through cooperative agreement U54 HD012303-25 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Disclosure Statement: V.R.A., T.P.C., P.B., P.C., S.P., R.J.C. have nothing to declare. S.M. has received lecture fees from Takeda Pharmaceuticals, North America. R.R.H. has received lecture fees, consulting fees, and grant support from Takeda Pharmaceuticals, North America.
First Published Online November 4, 2008
Abbreviations: A4, Androstenedione; AUCg, area under the curve for glucose; CV, coefficient of variation; CYP17, cytochrome P450c17; DEXA, dual energy x-ray absorptiometry; DHEA, dehydroepiandrosterone; DHEA-S, DHEA sulfate; FG, Ferriman-Gallwey; GDR, glucose disposal rate; OGTT, oral glucose tolerance test; 17-OHP, 17-hydroxyprogesterone; NC, normal control; PCOS, polycystic ovary syndrome.
References
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO 2004 The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749 [DOI] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R 1998 Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 83:3078–3082 [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dodson WC, Dunaif A 1999 Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169 [DOI] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A 1989 Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 38:1165–1174 [DOI] [PubMed] [Google Scholar]
- Ek I, Arner P, Bergqvist A, Carlström K, Wahrenberg H 1997 Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab 82:1147–1153 [DOI] [PubMed] [Google Scholar]
- Sepilian V, Nagamani M 2005 Adiponectin levels in women with polycystic ovary syndrome and severe insulin resistance. J Soc Gynecol Investig 12:129–134 [DOI] [PubMed] [Google Scholar]
- Sieminska L, Marek B, Kos-Kudla B, Niedziolka D, Kajdaniuk D, Nowak M, Glogowska-Szelag J 2004 Serum adiponectin in women with polycystic ovarian syndrome and its relation to clinical, metabolic and endocrine parameters. J Endocrinol Invest 27:528–534 [DOI] [PubMed] [Google Scholar]
- Aroda V, Ciaraldi TP, Chang SA, Dahan MH, Chang RJ, Henry RR 2008 Circulating and cellular adiponectin in polycystic ovary syndrome: relationship to glucose tolerance and insulin action. Fertil Steril 89:1200–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield RL, Barnes RB, Ehrmann DA 1994 Studies of the nature of 17-hydroxyprogesterone hyperresponsiveness to gonadotropin-releasing hormone agonist challenge in functional ovarian hyperandrogenism. J Clin Endocrinol Metab 79:1686–1692 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Rosenfield RL, Barnes RB, Brigell DF, Sheikh Z 1992 Detection of functional ovarian hyperandrogenism in women with androgen excess. N Engl J Med 327:157–162 [DOI] [PubMed] [Google Scholar]
- Nahum R, Thong KJ, Hillier SG 1995 Metabolic regulation of androgen production by human thecal cells in vitro. Hum Reprod 10:75–81 [DOI] [PubMed] [Google Scholar]
- Zawadzki JK, Dunaif A 1992 Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, eds. Polycystic ovary syndrome. Oxford, UK: Blackwell; 59–69 [Google Scholar]
- Fauser BC, Van Heusden AM 1997 Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocr Rev 18:71–106 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Mudaliar S, Mohideen P, Deutsch R, Ciaraldi TP, Armstrong D, Kim B, Sha X, Henry RR 2002 Intravenous glargine and regular insulin have similar effects on endogenous glucose output and peripheral activation/deactivation kinetic profiles. Diabetes Care 25:1597–1602 [DOI] [PubMed] [Google Scholar]
- Thorburn AW, Gumbiner B, Bulacan F, Wallace P, Henry RR 1990 Intracellular glucose oxidation and glycogen synthase activity are reduced in non-insulin-dependent (type II) diabetes independent of impaired glucose uptake. J Clin Invest 85:522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather KJ, Funahashi T, Matsuzawa Y, Edelstein S, Bray GA, Kahn SE, Crandall J, Marcovina S, Goldstein B, Goldberg R2008 Diabetes prevention program adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes 57:980–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majuri A, Santaniemi M, Rautio K, Kunnari A, Vartiainen J, Ruokonen A, Kesäniemi YA, Tapanainen JS, Ukkola O, Morin-Papunen L 2007 Rosiglitazone treatment increases plasma levels of adiponectin and decreases levels of resistin in overweight women with PCOS: a randomized placebo-controlled study. Eur J Endocrinol 156:263–269 [DOI] [PubMed] [Google Scholar]
- Imatoh T, Miyazaki M, Momose Y, Tanihara S, Une H 2008 Adiponectin levels associated with the development of hypertension: a prospective study. Hypertens Res 31:229–233 [DOI] [PubMed] [Google Scholar]
- Chow WS, Cheung BM, Tso AW, Xu A, Wat NM, Fong CH, Ong LH, Tam S, Tan KC, Janus ED, Lam TH, Lam KS 2007 Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. Hypertension 49:1455–1461 [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, May S, Langenberg C 2007 Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol 165:164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadid S, Stehouwer CD, Jensen MD 2006 Diet/exercise versus pioglitazone: effects of insulin sensitization with decreasing or increasing fat mass on adipokines and inflammatory markers. J Clin Endocrinol Metab 91:3418–3425 [DOI] [PubMed] [Google Scholar]
- Arlt W, Auchus RJ, Miller WL 2001 Thiazolidinediones but not metformin directly inhibit the steroidogenic enzymes p450c17 and 3b-hydroxysteroid dehydrogenase. J Biol Chem 276:16767–16771 [DOI] [PubMed] [Google Scholar]
- Gasic S, Nagamani M, Green A, Urban RJ 2001 Troglitazone is a competitive inhibitor of 3β-hydroxysteroid dehydrogenase enzyme in the ovary. Am J Obstet Gynecol 184:575–579 [DOI] [PubMed] [Google Scholar]
- Kempná P, Hofer G, Mullis PE, Flück CE 2006 Pioglitazone inhibits androgen production in NCI-H295R cells by regulating gene expression of CYP17 and HSD3B2. Mol Pharmacol 71:787–798 [DOI] [PubMed] [Google Scholar]
- Qin KN, Rosenfield RL 1998 Role of cytochrome P450c17 in polycystic ovary syndrome. Mol Cell Endocrinol 145:111–121 [DOI] [PubMed] [Google Scholar]
- Munir I, Yen HW, Geller DH, Torbati D, Bierden RM, Weitsman SR, Agarwal SK, Magoffin DA 2004 Insulin augmentation of 17α-hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/2 in human ovarian theca cells. Endocrinology 145:175–183 [DOI] [PubMed] [Google Scholar]
- Nestler JE, Jakubowicz DJ 1996 Decreases in ovarian cytochrome p450c17α activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med 335:617–623 [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Biri A, Karkoc A, Törüner F, Bingöl B, Cakir N, Tiras B, Ayvaz G, Arslan M 2005 The effects of rosiglitazone and metformin on insulin resistance and serum androgen levels in obese and lean patients with polycystic ovary syndrome. J Endocrinol Invest 28:1003–1008 [DOI] [PubMed] [Google Scholar]
- Stout DL, Fugate SE 2005 Thiazolidinediones for treatment of polycystic ovary syndrome. Pharmacotherapy 25:244–252 [DOI] [PubMed] [Google Scholar]
- la Marca A, Egbe TO, Morgante G, Paglia T, Cianci A, De Leo V 2000 Metformin treatment reduces ovarian cytochrome P-450c17α response to human chorionic gonadotrophin in women with insulin resistance-related polycystic ovary syndrome. Hum Reprod 15:21–23 [DOI] [PubMed] [Google Scholar]
- Glintborg D, Hermann AP, Andersen M, Hagen C, Beck-Nielsen H, Veldhuis JD, Henriksen JE 2006 Effect of pioglitazone on glucose metabolism and luteinizing hormone secretion in women with polycystic ovary syndrome. Fertil Steril 86:385–397 [DOI] [PubMed] [Google Scholar]
- Mehta RV, Patel KS, Coffler MS, Dahan MH, Yoo RY, Archer JS, Malcom PJ, Chang RJ 2005 Luteinizing hormone secretion is not influenced by insulin infusion in women with polycystic ovary syndrome despite improved insulin sensitivity during pioglitazone treatment. J Clin Endocrinol Metab 90:2136–2141 [DOI] [PubMed] [Google Scholar]
- Lawson MA, Jain S, Sun S, Patel K, Malcolm PJ, Chang RJ 2008 Evidence for insulin suppression of baseline luteinizing hormone in women with polycystic ovarian syndrome and normal women. J Clin Endocrinol Metab 93:2089–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]