Abstract
Context: Fibroblast growth factor (FGF)-23 is produced in bone, and circulating levels are markedly elevated in patients with end-stage kidney disease, but the relationship between plasma levels of FGF-23 and bone histology in dialysis patients with secondary hyperparathyroidism is unknown.
Objective: The aim of the study was to evaluate the correlation between plasma levels of FGF-23 and bone histology in pediatric patients with end-stage kidney disease who display biochemical evidence of secondary hyperparathyroidism.
Design: We performed a cross-sectional analysis of the relationship between plasma FGF-23 levels and bone histomorphometry.
Setting: The study was conducted in a referral center.
Study Participants: Participants consisted of forty-nine pediatric patients who were treated with maintenance peritoneal dialysis and who had serum PTH levels (1st generation Nichols assay) greater than 400 pg/ml.
Intervention: There were no interventions.
Main Outcome Measure: Plasma FGF-23 levels and bone histomorphometry were measured.
Results: No correlation existed between values of PTH and FGF-23. Bone formation rates correlated with PTH (r = 0.44; P < 0.01), but not with FGF-23. Higher FGF-23 concentrations were associated with decreased osteoid thickness (r = −0.49; P < 0.01) and shorter osteoid maturation time (r = −0.48; P < 0.01).
Conclusions: High levels of FGF-23 are associated with improved indices of skeletal mineralization in dialyzed pediatric patients with high turnover renal osteodystrophy. Together with other biomarkers, FGF-23 measurements may indicate skeletal mineralization status in this patient population.
High levels of fibroblast growth factor-23 are associated with improved indices of skeletal mineralization in dialyzed pediatric patients with high turnover renal osteodystrophy.
The skeletal lesions of chronic kidney disease (CKD), termed renal osteodystrophy, have been traditionally classified according to alterations in bone turnover. PTH stimulates osteoblastic activity, and serum levels of this peptide, which correlate with rates of bone formation, are widely used to diagnose lesions ranging from high turnover (osteitis fibrosa) to low bone turnover (adynamic bone) (1). However, abnormalities in skeletal mineralization are also present in many patients with CKD (2,3), and these may contribute to increased fracture rates, prevalence of skeletal deformities, and growth failure, which persist despite improvements in the control of secondary hyperparathyroidism (4). The pathophysiology of excessive osteoid accumulation in CKD is, however, incompletely understood, and little information exists as to how circulating biomarkers relate to changes in skeletal mineralization in patients with end-stage kidney disease (ESKD). The Kidney Disease Improving Global Outcomes (KDIGO) working group recently underscored these issues by emphasizing the necessity of evaluating three areas of bone histology, namely turnover, mineralization, and volume (TMV), in all patients with CKD and in developing noninvasive biomarkers for their assessment (3).
Fibroblast growth factor (FGF)-23 is a phosphaturic hormone that also reduces the formation of the biologically active 1,25-dihydroxyvitamin D [1,25(OH)2 vitamin D] (5,6). Mineralized bone is the primary source of FGF-23 (7), and FGF-23 overexpression is associated with osteomalacia in individuals with normal kidney function (8). FGF-23 levels rise as glomerular filtration declines (9), and values are markedly elevated in patients treated with dialysis (9). However, the relationship between circulating FGF-23 levels and skeletal histology in patients with CKD remains unknown. Thus, the current study was designed to examine the relationship between plasma levels of FGF-23 and skeletal turnover, mineralization, and/or volume in pediatric dialysis patients with secondary hyperparathyroidism.
Patients and Methods
A cross-sectional evaluation of the relationship between FGF-23 and bone histomorphometry was performed in 49 patients (24 males/25 females) aged 13 ± 6 yr, who were treated with maintenance continuous cycling peritoneal dialysis and who had serum PTH levels greater than 400 pg/ml [first-generation immunometric PTH assay (1st PTH-IMA); Nichols Institute Diagnostics, San Juan Capistrano, CA]. None of these patients were part of any previous clinical trials. The average duration of dialysis before the current analysis was 14 ± 12 months, and underlying renal pathology was equally divided between obstructive/dysplastic uropathy, glomerular disease, and unknown etiology. Thirty-three subjects had a residual glomerular filtration rate of 5 ± 2 ml/min/1.73 m2. All patients were dialyzed with Dianeal (Baxter, Deerfield, IL) containing 2.5 mEq/liter of calcium and a glucose concentration determined according to ultrafiltration need. All subjects were treated with oral calcium carbonate as a phosphate binder. Before the study, the majority of patients had been treated with daily oral calcitriol. Some had received no vitamin D sterols at all, and vitamin D therapy was discontinued in all patients for at least 4 wk before the bone biopsy. At the time of biopsy, serum levels of calcium, phosphorus, alkaline phosphatase, PTH, 25-hydroxyvitamin D [25(OH) vitamin D], and FGF-23 were obtained.
Biochemical determinations for calcium, albumin, phosphorus, and alkaline phosphatase were obtained using an Olympus AU5400 (Olympus America Incorporated, Center Valley, PA). Serum calcium levels were adjusted based on serum albumin level by the formula: serum Ca = measured calcium + [0.8 × (4 − serum albumin)]. PTH levels were measured by the 1st generation Nichols assay, which detects full-length PTH as well as large aminoterminally truncated fragments (normal range, 10–65 pg/ml). 25(OH) Vitamin D levels were measured by RIA (10). FGF-23 levels were determined in plasma using two immunoassays, termed “intact” and “C-terminal” assays (Immutopics, San Clemente, CA). The intact assay is a two-site ELISA using antibodies directed against the carboxyl- and amino-terminal portions of the molecule, measuring exclusively full-length FGF-23 in plasma (normal range, 13.3 ± 19.0 pg/ml) (11). The C-terminal assay, also an ELISA, uses two antibodies directed against the carboxyl-terminal portion of the molecule and detects both total intact and C-terminal FGF-23 (normal range, 72.9 ± 38.2 RU/ml) (11).
Patients were admitted to the UCLA General Clinical Research Center, and full thickness bone biopsies were obtained from the anterior iliac crest (2 cm below the anterior superior iliac spine) using a modified Bordier trephine needle under general anesthesia. Double tetracycline-labeling (10 mg/kg/d for 2 d, followed by a 10-d tetracycline free period, a subsequent 2 d of relabeling, and a final 2- to 4-d tetracycline free period) was performed in 48 patients before bone biopsy. Biopsy specimens were 0.5 cm in diameter by 1–2 cm in length. Specimens were dehydrated in alcohol, cleared with xylene, and embedded in methylmethacrylate. Static histomorphometric parameters were evaluated in undecalcified 5-μm sections treated with Goldner stain; tetracycline labeling was assessed in unstained 10-μm sections.
Primary bone histomorphometric parameters were assessed in trabecular bone under 200× magnification using the OsteoMetrics system (OsteoMetrics, Decatur, GA). Mineralized bone was defined by green-staining areas; red-staining seams at least 1.5 μm in width were included in measurements of unmineralized osteoid. Derived indices were calculated by formulae displayed in Table 1. The TMV classification for renal osteodystrophy (3) was used to characterize bone pathology.
Table 1.
Formulae used to calculate derived bone histomorphometric indices from primary measurements
| Abbreviation | Parameter | Unit | Formula |
|---|---|---|---|
| Structural | |||
| BV/TV | Bone volume/tissue volume | % | Bone area/tissue area ∗ 100 |
| BS/TV | Bone surface/tissue volume | mm2/mm3 | Bone perimeter/tissue area ∗ 1.2 |
| Static formation | |||
| OV/BV | Osteoid volume/bone volume | % | Osteoid area/bone area ∗ 100 |
| OS/BS | Osteoid surface/bone surface | % | Osteoid perimeter/bone perimeter ∗ 100 |
| O.Th | Osteoid thickness | μm | (Osteoid area/osteoid perimeter) ∗ 2/1.2 |
| Fb/TV | Fibrosis volume/tissue volume | % | Fibrosis area/tissue area ∗ 100 |
| Dynamic formation | |||
| MS/BS | Mineralizing surface/bone surface | % | (Double label perimeter + 1/2 single label perimeter)/bone perimeter ∗ 100 |
| MAR | Mineral apposition rate | μm/d | (Distance between labels/interlabel period)/1.2 |
| AjAr | Adjusted apposition rate | μm/d | [(MAR ∗ MS/BS)/OS/BS] ∗ 0.01 |
| MLT | Mineralization lag time | d | O.Th/AjAr |
| OMT | Osteoid maturation time | d | O.Th/MAR |
| BFR/TV | Bone formation rate/tissue volume | %/yr | MAR ∗ MS/BS ∗ BS/TV ∗ 3.65 |
Normal values for all histomorphometric parameters were previously obtained from double-tetracycline-labeled iliac crest bone biopsy specimens from 31 pediatric patients with normal kidney function who were undergoing elective orthopedic surgery (12). Bone specimen acquisition, fixation, staining, and measurement for these normal volunteers were performed as described above for study subjects.
This study was approved by the UCLA Human Subjects Protection Committee, and consent forms were obtained from all patients and/or parents/guardians.
Statistical analysis
Measurements for bone and biochemical variables are reported as mean ± sd. For variables with skewed distributions (PTH, alkaline phosphatase, intact FGF-23, C-terminal FGF-23, mineralization lag time, and bone formation rate/tissue volume), the median (95% confidence intervals) are also presented. The relationship between individual biochemical and bone parameters was assessed using Spearman correlation coefficients. To improve visual representation, log transformation was applied to all nonnormally distributed variables before graphic depiction.
To determine the predictive significance of FGF-23 on bone histological variables while adjusting for other biochemical parameters, multiple linear regression was performed. To avoid overfitting, values of alkaline phosphatase, PTH, and FGF-23 were included in the model (13). FGF-23 was chosen as the parameter of interest for this study; PTH and alkaline phosphatase were included due to their previously reported relationship with bone skeletal histology (1,14). Log-transformation was applied to all nonnormally distributed variables before analysis. Robust regression method with methods of moment-estimation (15) was used to limit the influence of outliers and leverage points.
Lower plasma FGF-23 levels were observed in patients with residual renal function compared with completely anuric subjects, but this difference did not alter the relationship between FGF-23 levels and bone. Thus, results from the entire cohort are presented together.
All statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC); all tests were two-sided. Ordinary P values without correction for multiple testing are reported.
Results
Biochemical determinations
Biochemical parameters are provided in Table 2. Serum calcium levels were within the normal range, whereas serum phosphorus and the calcium × phosphorus product were elevated. Alkaline phosphatase activity and PTH levels were increased, and serum 25(OH) vitamin D levels were low (16). The median value for intact plasma FGF-23 was 74 pg/ml (95% confidence interval, 57–115 pg/ml), whereas the median value for C-terminal FGF-23 was 514 RU/ml (95% confidence interval, 373–893 RU/ml). Serum levels of FGF-23 differed between anuric patients and those with residual renal function. Irrespective of residual renal function, plasma C-terminal FGF-23 levels correlated with phosphorus (r = 0.52; P < 0.01) and with the calcium × phosphorus product (r = 0.70; P < 0.01). There was an inverse relationship between FGF-23 levels and alkaline phosphatase (r = −0.39; P < 0.01), but values of PTH did not correlate with FGF-23 levels. Despite a high degree of correlation between FGF-23 as assessed by the two assays (r = 0.86; P < 0.01), a consistently higher degree of correlation with all parameters was found with C-terminal FGF-23 values.
Table 2.
Biochemical parameters
| Calcium (mg/dl) | 9.2 ± 0.8 |
| Phosphorus (mg/dl) | 6.4 ± 1.5 |
| Calcium × phosphorus (mg2/dl2) | 56 ± 15 |
| Alkaline phosphatase (IU/liter) | 416 ± 287 |
| 361 (231–445) | |
| 1st PTH-IMA (Nichols) (pg/ml) | 951 ± 444 |
| 822 (736–1,063) | |
| 25(OH) vitamin D (ng/ml) | 15.8 ± 7.8 |
| Intact FGF-23 (pg/ml) | 330 ± 752 |
| 74 (57–115) | |
| RRF (no) | 563 ± 921 |
| 240 (93–638)a | |
| RRF (yes) | 213 ± 636 |
| 58 (43–77)a | |
| C-terminal FGF-23 (RU/ml) | 4,797 ± 11,351 |
| 514 (373–893) | |
| RRF (no) | 8,587 ± 12,477 |
| 1,612 (605–13,590)a | |
| RRF (yes) | 2,959 ± 10,470 |
| 383 (199–561)a |
Data represent mean ± sd and median (95% confidence interval). RRF, Residual renal function.
The difference between anuric patients and those with residual renal function (P < 0.05).
Relationship between bone histomorphometric variables and biochemical parameters
Bone histomorphometry was performed on 49 specimens; double-tetracycline labeling was present in 48. Consistent with secondary hyperparathyroidism, the majority of patients displayed increased bone turnover (Tables 3 and 4). Impaired mineralization, defined by an abnormality in static indices (osteoid volume/bone volume) as well as impaired rates of osteoid mineralization (osteoid maturation time and mineralization lag time), was also highly prevalent (Tables 3 and 4). All patients had increased osteoid thickness whereas 48 of the 49 (98%) had increased osteoid volume/bone volume. Forty-three of 48 (90%) showed increases in osteoid maturation time, and 11 of 48 (23%) displayed prolonged mineralization lag times. As defined as an osteoid volume greater than 12% in combination with bone marrow fibrosis and increased bone turnover (1), the mixed lesion of renal osteodystrophy was present in 31 of 49 (63%) subjects. Bone volume/tissue volume was normal in 14 subjects (29%) and increased in the remainder (n = 35 or 71%).
Table 3.
Bone histomorphometry by TMV classification (3)
| Bone variable | Valuea | Normal range (median) (n = 28) |
|---|---|---|
| Turnover | ||
| BFR/TV (%/yr) | 48.9 ± 35.8 [36.7 (38.7–52.2)] | 3.4–22.4 (8) |
| Mineralization | ||
| OV/BV (%) | 15.8 ± 6.9 | 0.2–5.8 (1.4) |
| OS/BS (%) | 53.8 ± 1.8 | 4.3–31.7 (16.4) |
| O.Th (μm) | 22.4 ± 6.4 | 2.0–13.2 (5.5) |
| OMT (d) | 20.3 ± 7.3 | 1.2–11.5 (4.9) |
| MLT (d) | 51.9 ± 6.9 [42.7 (12.5–278.3)] | 2.3–63.8 (11.0) |
| Volume | ||
| BV/TV (%) | 30.9 ± 7.0 | 8.9–34.4 (17.2) |
| Fibrosis | ||
| Fb/TV (%) | 0.94 ± 0.40 | 0 |
Abbreviations are defined in Table 1.
Values represent mean ± sd [median (95% confidence interval)].
Table 4.
Patients with bone histomorphometric abnormalities by TMV classification
| Parameter | High | Normal | Low | Missinga |
|---|---|---|---|---|
| Turnover | ||||
| BFR/TV | 39 | 8 | 1 | 1 |
| Mineralization | ||||
| OV/BV | 48 | 1 | ||
| OS/BS | 42 | 7 | ||
| O.Th | 49 | |||
| OMT | 43 | 5 | 1 | |
| MLT | 11 | 37 | 1 | |
| Volume | ||||
| BV/TV | 35 | 14 |
Abbreviations are defined in Table 1.
Double-tetracycline labeling was not assessed in one patient. This subject displayed increased numbers of cells, osteoid, and fibrosis consistent with a diagnosis of high turnover bone disease.
Serum alkaline phosphatase and PTH levels correlated with bone formation rate/tissue volume (Table 5 and Fig. 1). Additionally, alkaline phosphatase and PTH correlated to a similar degree with static parameters of unmineralized bone (osteoid volume/bone volume, osteoid surface/bone surface, and osteoid thickness) (Table 5). Serum 25(OH) vitamin D levels did not correlate with bone formation rate/tissue volume or with any of the mineralization parameters. In contrast to PTH, FGF-23 concentrations did not correlate with bone formation rate (Fig. 2) but were inversely related to osteoid thickness (Table 5 and Fig. 3), to osteoid maturation time (Table 5 and Fig. 4), and to mineralization lag time (Table 5). The relationship between FGF-23 and osteoid thickness, osteoid maturation time, and mineralization lag time persisted in multivariate analysis when serum PTH, alkaline phosphatase, and FGF-23 levels were considered simultaneously (Table 6). FGF-23 levels were also inversely related to bone volume/tissue volume but did not correlate with tissue fibrosis [fibrosis volume (Fb)/tissue volume] (Table 5).
Table 5.
Coefficients of correlation between biochemical and bone parameters
| Bone parameter | 1st PTH-IMA (Nichols) | Alkaline phosphatase | Intact FGF-23 | C-terminal FGF-23 |
|---|---|---|---|---|
| Turnover | ||||
| BFR/TV | r = 0.44, P < 0.01 | r = 0.50, P < 0.01 | r = 0.25, P = NS | r = 0.20, P = NS |
| Mineralization | ||||
| OV/BV | r = 0.44, P < 0.01 | r = 0.60, P < 0.01 | r = 0.05, P = NS | r = −0.17, P = NS |
| OS/BS | r = 0.47, P < 0.01 | r = 0.57, P < 0.01 | r = −0.01, P = NS | r = −0.11, P = NS |
| O.Th | r = 0.37, P < 0.01 | r = 0.60, P < 0.01 | r = −0.38, P < 0.01 | r = −0.49, P < 0.01 |
| OMT | r = 0.19, P = NS | r = 0.26, P = NS | r = −0.37, P < 0.01 | r = −0.48, P < 0.01 |
| MLT | r = −0.13, P = NS | r = −0.10, P = NS | r = −0.35, P < 0.05 | r = −0.35, P < 0.05 |
| Volume | ||||
| BV/TV | r = −0.18, P = NS | r = 0.29, P < 0.05 | r = −0.38, P < 0.01 | r = −0.37, P < 0.01 |
| Fibrosis | ||||
| Fb/TV | r = 0.51, P < 0.01 | r = 0.36, P < 0.05 | r = 0.11, P = NS | r = 0.09, P = NS |
NS, Not significant. Other abbreviations are defined in Table 1.
Figure 1.
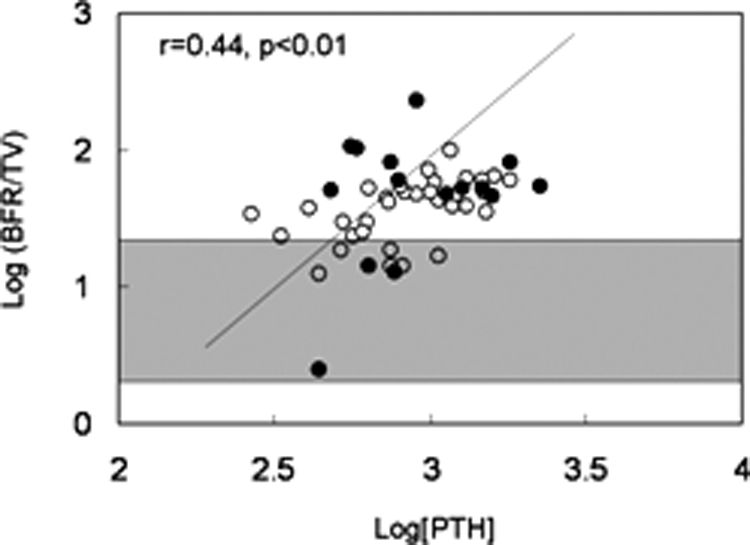
Relationship between log[PTH] and log[bone formation rate/tissue volume]. Open circles depict patients with residual renal function; closed circles depict anuric patients. The shaded area represents the normal range for bone formation rate/tissue volume in children with normal kidney function.
Figure 2.
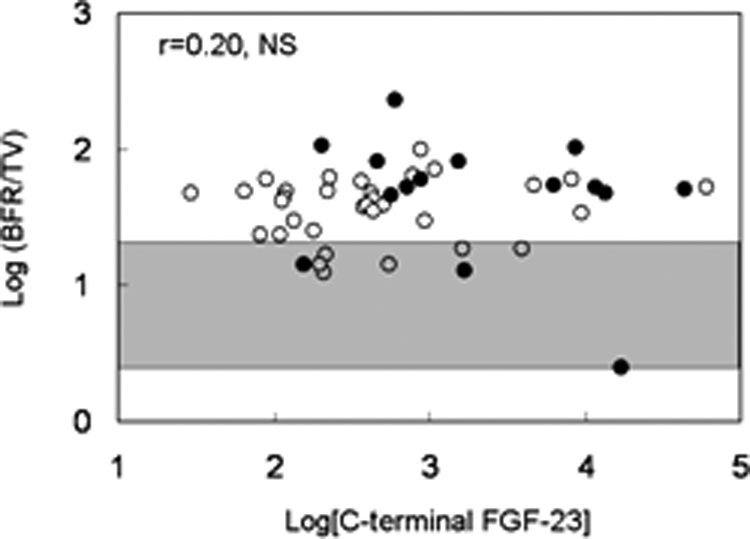
Relationship between log[C-terminal FGF-23] and log[bone formation rate/tissue volume]. Open circles depict patients with residual renal function; closed circles depict anuric patients. The shaded area represents the normal range for bone formation rate/tissue volume in children with normal kidney function.
Figure 3.
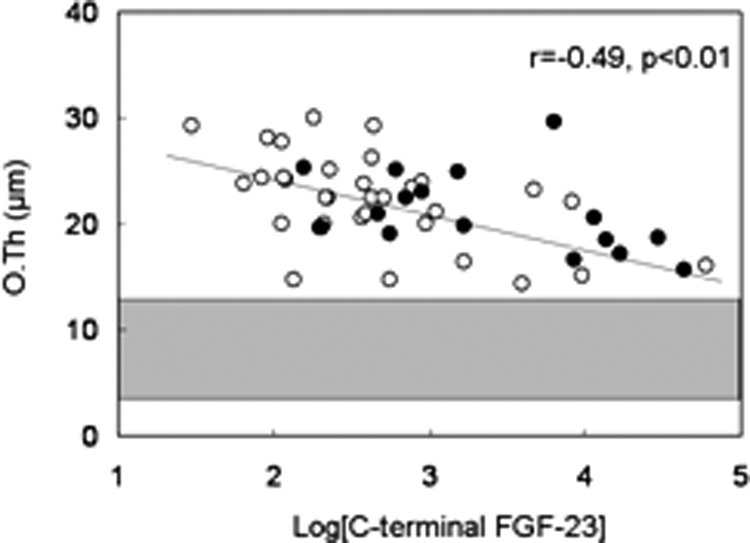
Relationship between log[C-terminal FGF-23] and osteoid thickness (O.Th). Open circles depict patients with residual renal function; closed circles depict anuric patients. The shaded area represents the normal range for osteoid thickness in children with normal kidney function.
Figure 4.
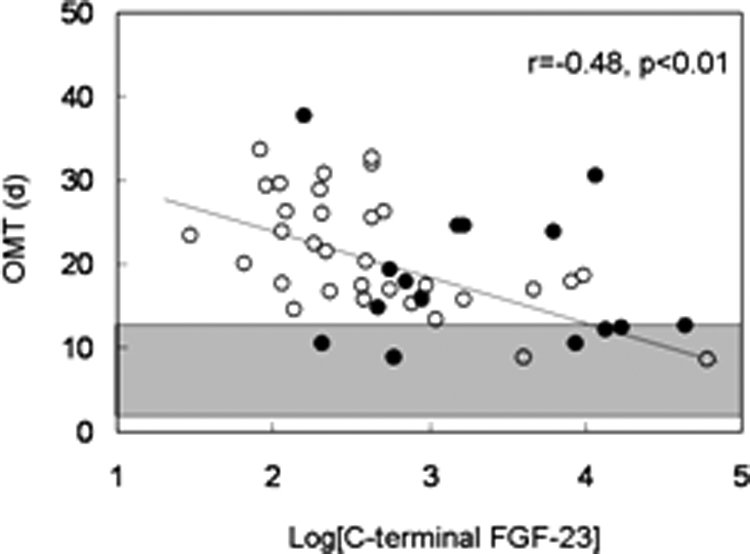
Relationship between log[C-terminal FGF-23] and osteoid maturation time (OMT). Open circles depict patients with residual renal function; closed circles depict anuric patients. The shaded area represents the normal range for osteoid maturation time in children with normal kidney function.
Table 6.
Parameter estimates and statistical significance of FGF-23, alkaline phosphatase, and PTH as predictors of skeletal mineralization in multivariate analysis
| Skeletal mineralization parameter | Parameter estimate, P value
|
||
|---|---|---|---|
| FGF-23 (C-terminal)a | Alkaline phosphatasea | PTHa | |
| OV/BV | 0.42 ± 0.40, P = 0.2896 | 5.52 ± 1.28, P < 0.0001 | 1.24 ± 1.72, P = 0.4701 |
| OS/BS | 0.84 ± 0.72, P = 0.2470 | 12.9 ± 2.27, P < 0.0001 | 3.01 ± 3.06, P = 0.3246 |
| O.Th | −0.90 ± 0.29, P = 0.0019 | 1.84 ± 0.88, P = 0.0370 | 1.06 ± 1.26, P = 0.4006 |
| OMT | −1.88 ± 0.66, P = 0.0043 | 0.49 ± 1.89, P = 0.7965 | 0.39 ± 2.65, P = 0.8822 |
| MLTa | −0.18 ± 0.05, P = 0.0003 | −0.21 ± 0.14, P = 0.1431 | 0.02 ± 0.20, P = 0.9375 |
Abbreviations are defined in Table 1.
Log transformation performed before analysis.
Discussion
This study confirmed previous findings that serum PTH, alkaline phosphatase (14,16,17,18,19,20,21), and plasma FGF-23 levels (22,23,24) are markedly elevated in ESKD patients with secondary hyperparathyroidism. A high degree of correlation existed between the results obtained with both FGF-23 assays, but consistently stronger coefficients of correlation were found between the C-terminal assay and biochemical or bone parameters. Anuric subjects displayed higher levels of FGF-23 than patients with residual urine output, suggesting that this phosphaturic hormone is cleared to some extent by the kidney. However, the relationship between FGF-23 and biochemical or bone variables was not altered by the presence of residual renal function. FGF-23 levels directly correlated with serum phosphorus and the calcium × phosphorus product, which is consistent with previous observations and confirms that elevation in serum phosphate level is a major stimulus for the release of FGF-23 from bone (23,24,25). There was no relationship between FGF-23 and PTH levels. As has been previously demonstrated (1,14,26), serum PTH levels were predictors of bone turnover; values of PTH were also directly correlated to osteoid thickness, which is likely a reflection of PTH-stimulated collagen synthesis by osteoblasts. In contrast to PTH, plasma FGF-23 levels were inversely correlated with both static (osteoid thickness) and dynamic (osteoid maturation time and mineralization lag time) indices of osteoid mineralization. Plasma FGF-23 levels were also inversely correlated with bone volume.
Serum PTH levels have been traditionally used as a surrogate marker of bone turnover in the diagnosis and treatment of renal osteodystrophy. However, defects in mineralization are also prevalent in patients with CKD, yet bone biopsy, an invasive technique that is not readily available to the majority of practicing clinicians, is currently the only method for assessing these changes. Although serum PTH levels were directly related to the amount of unmineralized osteoid, PTH values were not related to the rate of mineral deposition. In the current study, plasma levels of FGF-23 inversely correlated with osteoid thickness in patients with end-stage kidney disease. FGF-23 levels were also inversely related to the time between the deposition of unmineralized osteoid and the completion of mineralization, whether evaluated at each bone forming site (osteoid maturation time) or normalized across the entire osteoid seam (mineralization lag time). In multivariate analysis, when PTH, alkaline phosphatase, and FGF-23 were considered simultaneously, the relationship between FGF-23 and osteoid thickness, osteoid maturation time, and mineralization lag time persisted.
The current cohort of patients was neither hypocalcemic nor hypophosphatemic—indeed, all were treated with calcium-based phosphate binders; thus, sufficient substrate was available for skeletal mineralization yet defective mineralization was prevalent. Low levels of 25(OH) vitamin D could have contributed to the prevalence of mineralization defects in this cohort. However, the degree of vitamin D deficiency was associated neither with the quantity of unmineralized osteoid nor with the rate of mineral deposition. Although the prevalence of very low levels of 25(OH) vitamin D in this population potentially limited our ability to detect differences in mineralization over a range of 25(OH) vitamin D values, the findings in this study are consistent with previous cross-sectional data suggesting no relationship between circulating 25(OH) vitamin D and bone mineralization (27).
An inverse relationship between FGF-23 and osteoid thickness, osteoid maturation time, and mineralization lag time was not anticipated, because elevated levels of FGF-23 in individuals with normal kidney function are associated with osteomalacia. Although surprising, these findings are not completely unexpected because the ablation of FGF-23 results in abnormal mineralization of osteoid (5,6). The mechanisms leading to impaired mineralization in FGF-23-null animals, which have severe hyperphosphatemia, normal or elevated calcium levels, and high levels of 1,25(OH)2 vitamin D, remains uncertain but could also be related to a direct role for FGF-23 in skeletal mineral deposition (5,6), either through a direct effect on skeletal mineralization or through actions involving other mineralization factors (28,29). In contrast to these undefined mechanisms, hypophosphatemia and thus the resulting insufficient mineral substrate is likely to be responsible for the rickets and osteomalacia observed in humans and in rodents with increased FGF-23 secretion and normal renal function (30). FGF-23 secretion appears to be regulated by members of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family of proteins. Some of these proteins, including matrix extracellular phosphoglycoprotein (MEPE) and dentrin matrix protein (DMP1) are exclusively expressed in osteoblasts, osteocytes, and odontoblasts (31); are essential to mineralization in vitro (32); and appear to regulate FGF-23 secretion. Increases in MEPE (28) and loss of DMP1 expression (33) are both linked to defects in skeletal mineralization along with increased levels of FGF-23. Whether alterations in FGF-23 expression themselves, in turn, regulate these mineralization proteins remains to be determined but could potentially be part of a feedback loop, suppressing MEPE or increasing DMP1 expression at the level of bone.
On the other hand, serum FGF-23 levels may reflect the actions of other hormones on skeletal mineralization. 1,25(OH)2 Vitamin D, the active form of vitamin D, improves skeletal mineralization (34) and has been shown to increase serum FGF-23 levels in animal studies (5,6,35,36). Although no patients in this study had received vitamin D sterols for at least 4 wk before bone biopsy, the duration of action of 1,25(OH)2 vitamin D on osteocytic FGF-23 production is unknown. Furthermore, serum levels of 1,25(OH)2 vitamin D were not measured in this cohort of patients, and it is possible that these levels contributed to circulating values of FGF-23. However, we have previously demonstrated that 1,25(OH)2 vitamin D levels in this patient population are universally low (an average of 12 ± 7 pg/ml) (37), suggesting that it is unlikely that increased circulating 1,25(OH)2 vitamin D is the sole factor affecting FGF-23 levels and improved mineralization.
The findings in this study are not without their limitations. Indeed, although the data suggested a direct correlation between FGF-23 and both mineral apposition rate (MAR) and mineralizing surface/bone surface (MS/BS), there was no correlation between FGF-23 and bone formation rate/tissue volume in this study. This may have been due to the modest strength of the relationships between MAR and bone formation rate/tissue volume and between MS/BS and FGF-23, in combination with the limited sample size. When bone formation rate/tissue volume is calculated as a product of MAR and MS/BS, variation in the two parameters is combined and may have obscured any relationship between FGF-23 and bone formation rate/tissue volume. Furthermore, despite an inverse correlation with osteoid thickness, FGF-23 levels were not related to osteoid volume/bone volume or osteoid surface/bone surface. It is possible that the relatively small sample size and inclusion of only patients with secondary hyperparathyroidism contributes to these discrepancies and that evaluation of patients across the spectrum of renal osteodystrophy might be needed to assess these relationships more completely. However, it is also possible that osteocytes within bone might use local FGF-23 production to communicate with surface osteoblasts. Indeed, FGF-23 is expressed at high levels in osteocytes (38), and animals with a complete lack of FGF-23 expression demonstrate focal areas of defective skeletal mineralization, suggesting that FGF-23 may control skeletal mineralization at a local level (39).
Despite its inability to prove causation, this cross-sectional study establishes an association between FGF-23 and parameters of skeletal mineralization in pediatric ESKD patients with secondary hyperparathyroidism. The findings in this study may not be applicable to the adult population. However, comparable elevations in serum PTH levels are present in adults and children, and these are consistently associated with skeletal lesions of secondary hyperparathyroidism (1,14). Other biomarkers, including FGF-23, are similarly elevated in adults and children with ESKD. The variation in FGF-23 levels among dialysis patients was very high, as has been previously shown (9). This finding could be related, at least in part, to circulating factors such as ferritin that appear to affect its synthesis or secretion (40). Furthermore, the biological activity of FGF-23 may not be accurately reflected by the current immunological assays for the detection of FGF-23. The results obtained with the two FGF-23 assays used in this study demonstrated a high degree of correlation, suggesting that circulating FGF-23 does not undergo significant degradation after secretion. Despite the high degree of correlation between the two assays, FGF-23 levels obtained from the C-terminal assay yielded consistently stronger correlations with different biochemical and bone parameters. These findings suggest that the C-terminal assay provides a better assessment of biologically active FGF-23 in circulation than the intact assay used in this study. However, the predictive value of another intact FGF-23 assay (40) for the assessment of skeletal mineralization remains to be evaluated.
In conclusion, FGF-23 levels are elevated in patients with secondary hyperparathyroidism who are treated with maintenance dialysis. In this patient population, FGF-23 levels were inversely correlated with osteoid thickness and accelerated rates of osteoid mineralization. Although the mechanism by which FGF-23 is associated with skeletal mineralization remains incompletely defined, our findings suggest that FGF-23 may, in combination with other markers, offer noninvasive information for the diagnosis of skeletal mineralization defects in pediatric ESKD patients with secondary hyperparathyroidism.
Footnotes
This work was supported by grants and fellowships supporting the writing of the paper: U.S. Public Health Service Grants DK-35423, DK-67563, and MO1-RR00865, as well as funds from the Casey Lee Ball Foundation. K.W.-P. is a recipient of a National Kidney Foundation Award.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 2, 2008
Abbreviations: CKD, chronic kidney disease; DMP1, dentrin matrix protein; ESKD, end-stage kidney disease; Fb, fibrosis volume; FGF-23, fibroblast growth factor-23; MAR, mineral apposition rate; MEPE, matrix extracellular phosphoglycoprotein; MS/BS, mineralizing surface/bone surface; 25(OH) vitamin D, 25-hydroxyvitamin D; 1,25(OH)2 vitamin D, 1,25-dihydroxyvitamin D; 1st PTH-IMA, first-generation immunometric PTH assay; TMV, turnover, mineralization, and volume.
References
- Salusky IB, Ramirez JA, Oppenheim W, Gales B, Segre GV, Goodman WG 1994 Biochemical markers of renal osteodystrophy in pediatric patients undergoing CAPD/CCPD. Kidney Int 45:253–258 [DOI] [PubMed] [Google Scholar]
- Malluche H, Faugere MC 1990 Renal bone disease 1990: an unmet challenge for the nephrologist. Kidney Int 38:193–211 [DOI] [PubMed] [Google Scholar]
- Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G 2006 Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69:1945–1953 [DOI] [PubMed] [Google Scholar]
- Groothoff JW, Offringa M, Van Eck-Smit BL, Gruppen MP, Van De Kar NJ, Wolff ED, Lilien MR, Davin JC, Heymans HS, Dekker FW 2003 Severe bone disease and low bone mineral density after juvenile renal failure. Kidney Int 63:266–275 [DOI] [PubMed] [Google Scholar]
- Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T 2004 Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Jüppner H, Lanske B 2004 Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol 23:421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiko Y, Wang H, Minamizaki T, Ijuin C, Yamamoto R, Suemune S, Kozai K, Tanne K, Aubin JE, Maeda N 2007 Mineralized tissue cells are a principal source of FGF23. Bone 40:1565–1573 [DOI] [PubMed] [Google Scholar]
- 2000 Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348 [DOI] [PubMed] [Google Scholar]
- Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB 2003 Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64:2272–2279 [DOI] [PubMed] [Google Scholar]
- Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL 1993 Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem 39:529–533 [PubMed] [Google Scholar]
- Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D, Kassem M, Rackoff P, Zimering M, Dalkin A, Drobny E, Colussi G, Shaker JL, Hoogendoorn EH, Hui SL, Econs MJ 2006 Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab 91:2055–2061 [DOI] [PubMed] [Google Scholar]
- Sanchez CP, Salusky IB, Kuizon BD, Ramirez JA, Gales B, Ettenger RB, Goodman WG 1998 Bone disease in children and adolescents undergoing successful renal transplantation. Kidney Int 53:1358–1364 [DOI] [PubMed] [Google Scholar]
- Harrell FE 2001 Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer-Verlag [Google Scholar]
- Sherrard DJ, Hercz G, Pei Y, Maloney NA, Greenwood C, Manuel A, Saiphoo C, Fenton SS, Segre GV 1993 The spectrum of bone disease in end-stage renal failure—an evolving disorder. Kidney Int 43:436–442 [DOI] [PubMed] [Google Scholar]
- 2008 The Robustreg procedure. SAS/STAT 9.1 User’s Guide. Chap 62. Cary, NC: SAS Institute Inc. [Google Scholar]
- Salusky IB, Goodman WG, Sahney S, Gales B, Perilloux A, Wang HJ, Elashoff RM, Jüppner H 2005 Sevelamer controls parathyroid hormone-induced bone disease as efficiently as calcium carbonate without increasing serum calcium levels during therapy with active vitamin D sterols. J Am Soc Nephrol 16:2501–2508 [DOI] [PubMed] [Google Scholar]
- Wang M, Hercz G, Sherrard DJ, Maloney NA, Segre GV, Pei Y 1995 Relationship between intact 1-84 parathyroid hormone and bone histomorphometric parameters in dialysis patients without aluminum toxicity. Am J Kidney Dis 26:836–844 [DOI] [PubMed] [Google Scholar]
- Monier-Faugere MC, Geng Z, Mawad H, Friedler RM, Gao P, Cantor TL, Malluche HH 2001 Improved assessment of bone turnover by the PTH-(1-84)/large C-PTH fragments ratio in ESRD patients. Kidney Int 60:1460–1468 [DOI] [PubMed] [Google Scholar]
- Lehmann G, Stein G, Huller M, Schemer R, Ramakrishnan K, Goodman WG 2005 Specific measurement of PTH (1–84) in various forms of renal osteodystrophy (ROD) as assessed by bone histomorphometry. Kidney Int 68:1206–1214 [DOI] [PubMed] [Google Scholar]
- Salusky IB, Goodman WG, Kuizon BD, Lavigne JR, Zahranik RJ, Gales B, Wang HJ, Elashoff RM, Jüppner H 2003 Similar predictive value of bone turnover using first- and second-generation immunometric PTH assays in pediatric patients treated with peritoneal dialysis. Kidney Int 63:1801–1808 [DOI] [PubMed] [Google Scholar]
- Coen G, Bonucci E, Ballanti P, Balducci A, Calabria S, Nicolai GA, Fischer MS, Lifrieri F, Manni M, Morosetti M, Moscaritolo E, Sardella D 2002 PTH 1-84 and PTH “7-84” in the noninvasive diagnosis of renal bone disease. Am J Kidney Dis 40:348–354 [DOI] [PubMed] [Google Scholar]
- Nishi H, Nii-Kono T, Nakanishi S, Yamazaki Y, Yamashita T, Fukumoto S, Ikeda K, Fujimori A, Fukagawa M 2005 Intravenous calcitriol therapy increases serum concentrations of fibroblast growth factor-23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin Pract 101:c94–c99 [DOI] [PubMed] [Google Scholar]
- Kazama JJ, Sato F, Omori K, Hama H, Yamamoto S, Maruyama H, Narita I, Gejyo F, Yamashita T, Fukumoto S, Fukagawa M 2005 Pretreatment serum FGF-23 levels predict the efficacy of calcitriol therapy in dialysis patients. Kidney Int 67:1120–1125 [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Kazama JJ, Nii-Kono T, Omori K, Yamashita T, Fukumoto S, Gejyo F, Shigematsu T, Fukagawa M 2005 Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int 67:1171–1178 [DOI] [PubMed] [Google Scholar]
- Urena TP, Friedlander G, de Vernejoul MC, Silve C, Prie D 2008 Bone mass does not correlate with the serum fibroblast growth factor 23 in hemodialysis patients. Kidney Int 73:102–107 [DOI] [PubMed] [Google Scholar]
- Qi Q, Monier-Faugere MC, Geng Z, Malluche HH 1995 Predictive value of serum parathyroid hormone levels for bone turnover in patients on chronic maintenance dialysis. Am J Kidney Dis 26:622–631 [DOI] [PubMed] [Google Scholar]
- Coen G, Mantella D, Manni M, Balducci A, Nofroni I, Sardella D, Ballanti P, Bonucci E 2005 25-Hydroxyvitamin D levels and bone histomorphometry in hemodialysis renal osteodystrophy. Kidney Int 68:1840–1848 [DOI] [PubMed] [Google Scholar]
- Liu S, Rowe PS, Vierthaler L, Zhou J, Quarles LD 2007 Phosphorylated acidic serine-aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J Endocrinol 192:261–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe PS, Matsumoto N, Jo OD, Shih RN, Oconnor J, Roudier MP, Bain S, Liu S, Harrison J, Yanagawa N 2006 Correction of the mineralization defect in hyp mice treated with protease inhibitors CA074 and pepstatin. Bone 39:773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H 2003 Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348:1656–1663 [DOI] [PubMed] [Google Scholar]
- Petersen DN, Tkalcevic GT, Mansolf AL, Rivera-Gonzalez R, Brown TA 2000 Identification of osteoblast/osteocyte factor 45 (OF45), a bone-specific cDNA encoding an RGD-containing protein that is highly expressed in osteoblasts and osteocytes. J Biol Chem 275:36172–36180 [DOI] [PubMed] [Google Scholar]
- Rowe PS, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, Cundy J, Navvab S, Chen D, Drezner MK, Quarles LD, Mundy GR 2004 MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone 34:303–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE 2006 Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie PJ, Glorieux FH 1981 Stimulation of cortical bone mineralization and remodeling by phosphate and 1,25-dihydroxyvitamin D in vitamin D-resistant rickets. Metab Bone Dis Relat Res 3:159–164 [DOI] [PubMed] [Google Scholar]
- Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T 2001 Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98:6500–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T 2004 FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435 [DOI] [PubMed] [Google Scholar]
- Salusky IB, Kuizon BD, Belin TR, Ramirez JA, Gales B, Segre GV, Goodman WG 1998 Intermittent calcitriol therapy in secondary hyperparathyroidism: a comparison between oral and intraperitoneal administration. Kidney Int 54:907–914 [DOI] [PubMed] [Google Scholar]
- Liu S, Tang W, Zhou J, Vierthaler L, Quarles LD 2007 Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice. Am J Physiol Endocrinol Metab 293:E1636–E1644 [DOI] [PubMed] [Google Scholar]
- Liu S, Zhou J, Tang W, Menard R, Feng JQ, Quarles LD 2008 Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab 295:E254–E261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham BH, Joseph F, Bailey LM, Fraser WD 2007 The association of circulating ferritin with serum concentrations of fibroblast growth factor-23 measured by three commercial assays. Ann Clin Biochem 44:463–466 [DOI] [PubMed] [Google Scholar]


