Abstract
Context: Products of at least five specific steroidogenic genes, including steroidogenic acute regulatory protein (StAR), which facilitates the entry of cytosolic cholesterol into the mitochondrion, side chain cleavage P450 enzyme, 3β-hydroxysteroid-dehydrogenase-2, 17-hydroxylase/17-20-lyase, and aromatase, which catalyzes the final step, are necessary for the conversion of cholesterol to estrogen. Expression and biological activity of StAR and aromatase were previously demonstrated in endometriosis but not in normal endometrium. Prostaglandin E2 (PGE2) induces aromatase expression via the transcriptional factor steroidogenic factor-1 (SF1) in endometriosis, which is opposed by chicken-ovalbumin upstream-transcription factor (COUP-TF) and Wilms’ tumor-1 (WT1) in endometrium.
Objective: The aim of the study was to demonstrate a complete steroidogenic pathway leading to estrogen biosynthesis in endometriotic cells and the transcriptional mechanisms that regulate basal and PGE2-stimulated estrogen production in endometriotic cells and endometrium.
Results: Compared with normal endometrial tissues, mRNA levels of StAR, side chain cleavage P450, 3β-hydroxysteroid-dehydrogenase-2, 17-hydroxylase/17-20-lyase, aromatase, and SF1 were significantly higher in endometriotic tissues. PGE2 induced the expression of all steroidogenic genes; production of progesterone, estrone, and estradiol; and StAR promoter activity in endometriotic cells. Overexpression of SF1 induced, whereas COUP-TFII or WT1 suppressed, StAR promoter activity. PGE2 induced coordinate binding of SF1 to StAR and aromatase promoters but decreased COUP-TFII binding in endometriotic cells. COUP-TFII or WT1 binding to both promoters was significantly higher in endometrial compared with endometriotic cells.
Conclusion: Endometriotic cells contain the full complement of steroidogenic genes for de novo synthesis of estradiol from cholesterol, which is stimulated by PGE2 via enhanced binding of SF1 to promoters of StAR and aromatase genes in a synchronous fashion.
In stromal cells of endometriosis, steroidogenic factor-1 coordinately orchestrates prostaglandin-E2 induction of the key genes responsible for the biosynthesis of estradiol from cholesterol.
Endometriosis is a common, chronic, and estrogen-dependent gynecological disorder associated with pelvic pain and infertility. The prevalence of pelvic endometriosis approaches 6–10% among women; in women with pelvic pain, infertility, or both, its frequency is 35–50% (1,2). Retrograde menstruation has been suggested as the crucial element in the development of endometriosis, yet other factors that allow the implantation and propagation of endometriotic lesions are largely unknown (3). In addition to, or perhaps as a consequence of, immune, environmental, and genetic factors, endometriotic lesions show high estradiol biosynthesis and low estradiol inactivation compared with normal endometrium (4,5).
In a woman with endometriosis, estrogen in the circulation arises from two sources (4). Estradiol secreted by the ovary reaches endometriotic tissue by circulation. In the preovulatory ovarian follicle, two cell types, namely, theca and granulosa, collaborate to produce estradiol. Additionally, aromatase (P450arom) that resides in peripheral adipose and skin tissue catalyzes the conversion of circulating androstenedione to estrone that may reach endometriosis via circulation and be converted locally to estradiol. In contrast to circulating estrogen, steroidogenic proteins present within endometriotic tissue may give rise to local production of estrogen (4). In particular, high levels of steroidogenic acute regulatory protein (StAR) and P450arom have been demonstrated in endometriotic stromal cells (4). A molecular link between inflammation and estrogen production in endometriotic tissue was recently uncovered (4). This is mediated by a positive feedback cycle that favors overexpression of key steroidogenic genes, most notably aromatase, overexpression of COX2, and continuous local production of estradiol and prostaglandin E2 (PGE2) in endometriotic tissue (4).
In the ovarian follicle, expression of steroidogenic gene products is compartmentalized into two cells types that cooperate to make estradiol. Theca cells express StAR, side chain cleavage P450 (P450scc), 3β-hydroxysteroid-dehydrogenase type 2 (HSD3B2), and 17-hydroxylase/17-20-lyase (P450c17) to convert cholesterol to androstenedione, which diffuses into the neighboring granulosa cell where it is converted by P450arom and 17β-hydroxysteroid type 1 (HSD17B1) to estrone and estradiol (4,6,7). StAR facilitates the first step of steroidogenesis, the entry of cholesterol into the mitochondrion, where cholesterol is converted by the mitochondrial enzyme P450scc to pregnenolone, which is then converted to progesterone via HSD3B2 (8,9). It was proposed that StAR activity accounts for the majority of progesterone production in ovarian cells (8,9). P450c17 catalyzes the conversion of progesterone to androstenedione, which is the primary substrate for P450arom in ovarian granulosa cells (6,7). P450arom is responsible for the conversion of androstenedione to estrone, which is further converted to the biologically active estradiol by the enzyme HSD17B1 (4).
The products of some of these steroidogenic genes that catalyze the formation of estradiol from cholesterol, namely StAR, P450arom (CYP19A1), and HSD17B1 were demonstrated in stromal cells of endometriotic tissue (10,11,12,13). P450arom expression in endometriosis and peripheral tissues was shown to be critical because its inhibitors have been used successfully to treat postmenopausal endometriosis (14). The question remains then whether estradiol is produced de novo from cholesterol in endometriosis, or whether endometriotic tissue P450arom is dependent on circulating androstenedione and testosterone as substrates for estrogen formation. The overall aim of this study is to demonstrate whether StAR, P450arom, and other essential steroidogenic genes are coordinately induced by a common signal, e.g. PGE2, leading to synthesis of estradiol de novo from cholesterol in endometriotic stromal cells.
PGE2 is the most potent known stimulator of P450arom in endometriotic stromal cells (10,13,15). The transcription factor steroidogenic factor-1 (SF1), which is elevated in endometriotic tissue, mediates the stimulatory action of PGE2 via binding to the P450arom promoter. Conversely, the transcription factors chicken ovalbumin upstream promoter-transcription factor (COUP-TF) and Wilms’ tumor-1 (WT1) bind to and inhibit the P450arom promoter in endometrial stromal cells (16,17). PGE2 also stimulates StAR expression in endometriotic stromal cells (10,18). Although the transcriptional factors that mediate LH-, ACTH-, or cAMP-dependent regulation of StAR in gonadal or adrenal cells have been well characterized (19,20,21,22), the transcriptional mechanisms responsible for the PGE2 induction of StAR in endometriotic cells are unknown. Here, we characterize the PGE2-cAMP-dependent coactivation of the promoters of multiple steroidogenic genes, most notably StAR and P450arom, in endometriotic cells. A better understanding of the common mechanism responsible for regulation of estrogen biosynthesis within endometriotic tissue may lead to the discovery of new diagnostic and therapeutic targets for endometriosis.
Subjects and Methods
Tissue acquisition and processing
Cyst walls of ovarian endometriomas (n = 17) and eutopic endometrial tissues (n = 16) were processed to collect and culture stromal cells using a protocol previously described by Ryan et al. (23) with minor modifications (13). Briefly, endometriotic or endometrial tissues were digested with collagenase B (1 mg/ml) and deoxyribonuclease I (0.1 mg/ml). Epithelial cells were removed by filtration of stromal cells through a 75-μm sieve. Stromal cells were then cultured in DMEM/F-12 containing 10% fetal bovine serum to confluency. We routinely used passage 1 cells for all experiments. Eutopic endometrial samples were obtained from endometriosis-free women at the time of hysterectomy for cervical dysplasia. A portion of these tissues were snap-frozen before being processed for cell culture. Extraovarian endometriotic tissue samples were obtained from a different group of women (n = 13) undergoing laparoscopy for endometriosis during either the proliferative (n = 5) or luteal phase (n = 8), as confirmed histologically. The age range of the subjects was 23–45 yr. Tissues were collected after obtaining written informed consent for the study protocol approved by the Institutional Review Board of Northwestern University.
Real-time RT-PCR
The RNeasy Mini Kit was used to extract total RNA (QIAGEN, Crawley, UK) following the manufacturer’s protocol. Two micrograms of total RNA were subjected to reverse transcription using the ABI High Capacity cDNA Archieve Kit (Applied Biosystems, Warrington, UK). For real-time PCR, we used ABI SYBR Green master mix and the ABI 7000 apparatus, as previously described (24,25). The PCR primers (50 nm final concentration) are listed in Table 1. Absolute quantities of transcripts were determined from a standard curve. Equivalent use of RNA was ensured by amplifying 18S RNA using diluted (1:1000) samples.
Table 1.
The primers used for real-time PCR
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Exons | Size (bp) |
|---|---|---|---|---|
| CYP11A1 (P450scc) | TCCAGAAGTATGGCCCGATT | CATCTTCAGGGTCGATGACATAAA | 1 and 3 | 75 |
| LRH1 | TTACCGACAAGTGGTACATGGAA | CGGCTTGTGATGCTATTATGGA | 6 and 7 | 89 |
| CYP17 (P450c17) | TCTCTGGGCGGCCTCAA | AGGCGATACCCTTACGGTTGT | 1 and 2 | 63 |
| 3BHSD1 | CGGCTAACGGGTGGAATCTG | CCCCATAGATATACATGGGTCGTAAG | 4 | 75 |
| 3BHSD2 | GCGGCTAATGGGTGGAATCTA | CATTGTTGTTCAGGGCCTCAT | 4 | 127 |
| CYP19A1 (P450arom) | TCACTGGCCTTTTTCTCTTGGT | GGGTCCAATTCCCATGCA | 3 and 4 | 83 |
| SF1 | GGAGTTTGTCTGCCTCAAGTTCA | CGTCTTTCACCAGGATGTGGTT | 6 and 7 | 80 |
| STAR | CCACCCCTAGCACGTGGA | TCCTGGTCACTGTAGAGAGTCTCTTC | 2 and 3 | 88 |
Progesterone and estradiol assays
Steroid levels in the media were measured after treatment of cells. Briefly, cells were treated with vehicle or PGE2 and supplements of exogenous low-density lipoprotein (LDL) or 22 (R)-hydroxycholesterol for 48 h. Culture medium was collected from the cells, and the steroids were extracted by phenol from the medium. Progesterone, estrone, and estradiol concentrations were determined using ELISA kits (Cayman Chemical, Ann Arbor, MI) as per the manufacturer’s protocol. The lower limits of these assays were 10 pg/ml for progesterone and estradiol and 5 pg/ml for estrone.
Immunoblot assays
Total protein was extracted from whole cells using M-PER Mammalian Protein Extraction Reagent (Pierce Chemical Co., Rockford, IL) following the protocol suggested by the manufacturer. Immunoblot assays were performed as previously described (26,27).
Plasmid constructs
The human (h) −885 bp StAR promoter 5′-flanking sequence in the pGL3-basic luciferase vector (J. Strauss, Richmond, VA), and the full-length cDNAs encoding hSF1 (K. L. Parker, Dallas, TX), hC/EBPα (G. D. Darlington, Houston, TX), rat C/EBPβ (G. S. Hotamisligil, Boston, MA), mouse (m) LRH1 (D. J. Mangelsdorf, Dallas, TX), mCOUP-TFII (M. J. Tsai, Houston, TX), and mWT1 (D. A. Haber, Boston, MA) were generous gifts. The cDNAs were cut and ligated into the pcDNA3.1 expression plasmid (Invitrogen, Carlsbad, CA). The fidelity of each cDNA was confirmed by sequencing.
Transient transfections and luciferase assays
Primary endometrial and endometriotic stromal cells were transfected using Lipofectamine Plus (Life Technologies, Inc.-BRL, Grand Island, NY) with the following plasmids: 1) 0.5 μg −885 bp StAR promoter luciferase reporter plasmid; 2) 0.5 μg pcDNA3.1 expression plasmid encoding SF1, liver receptor homolog-1 (LRH1), WT1, C/EBPα, C/EBPβ or COUP-TFII; and 3) 5 ng Renilla luciferase reporter plasmid (Promega Corp., Madison, WI) as an internal control for transfection efficiency. Empty pcDNA3 vector was added as necessary to ensure transfection of equimolar quantities of DNA per well. The day before transfection, primary stromal cells were seeded into 35-mm dishes at 2 × 105 cells per dish. At the time of transfection, stromal cells were 80% confluent. The transfection solution was comprised of 200 μl Opti-MEM reduced-serum medium containing PLUS reagent (6 μl), precomplexed DNA, and 4 μl of Lipofectamine reagent. After transfection for 3 h in transfection solution at 37 C in 5% CO2, the medium was changed to antibiotic-free DMEM/F-12 containing 10% fetal bovine serum for overnight recovery. Transfected cells were washed twice in PBS and lysed in 250 μl luciferase lysis buffer [0.1 m potassium phosphate (pH 7.8), 1% Triton X-100, 1 mm dithiothreitol, 2 mm EDTA]. Luciferase and Renilla luciferase readings were obtained using a dual-luciferase reporter assay system (Promega Corp.) and LUMAT LB9507 luminometer (Berthold Technologies GmbH & Co. KG, Bad Wildbad, Germany).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using a ChIP assay kit (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer’s instructions. Briefly, cells were cross-linked with 1% formaldehyde at 37 C for 10 min, rinsed three times with ice-cold PBS, collected into PBS, and centrifuged for 5 min at 1000 rpm. Crude nuclei were isolated using the protocol described for coimmunoprecipitation assays. Nuclei were prepared in the sodium dodecyl sulfate lysis buffer provided in the kit. Lysates were sonicated at 30% power, 5 × 10 sec using a Sonic Dismembrator model 300 (Fisher Scientific, Pittsburgh, PA) to shear genomic DNA. The following antibodies were used to perform immunoprecipitation: polyclonal SF1, polyclonal WT1, polyclonal COUP-TF, and polyclonal rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). PCR amplification of the StAR promoter region was performed using the following primers: 5′-TCCTGGTCACTGTAGAG-3′ and 5′-CCACCCCTAGCACGTGG-3′. Five microliters of extracted DNA and 35 rounds of amplification were used. PCR amplification of the P450arom promoter I.3/II region was performed as previously described (28).
Statistical analyses
Data were expressed as mean ± sem for cells cultured from six different subjects for experiments represented in Figs. 2 and 3. For experiments represented in Figs. 4 and 5, mean ± sem was calculated from cells cultured in triplicate replicates. These data were reproduced using cells from three different subjects. Statistical analyses for comparison of treatment groups were performed by one-way ANOVA followed by Tukey multiple comparison test. For data illustrated in Fig. 1, sample sizes for tissues were indicated, and the Student’s t test was used to assess the differences between mRNA levels. Significance was defined as α:0.05 and β:0.20.
Figure 2.
mRNA levels of steroidogenic genes in stromal cells isolated from normal eutopic endometrium vs. endometriosis incubated in the absence or presence of PGE2 (10−7 m). PGE2 significantly stimulated mRNA levels of each steroidogenic gene except for HSD3B1 in endometriotic stromal cells (E-OSIS; n = 6). The mRNA levels of these genes in normal endometrium (E-IUM; n = 6) were very low regardless of the absence or presence of PGE2.
Figure 3.
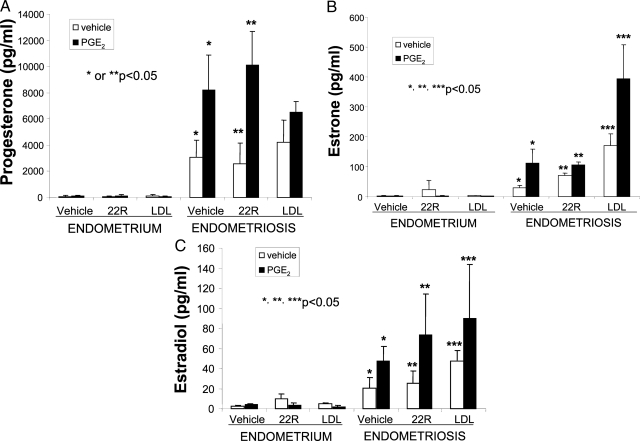
Hormone concentrations in media conditioned by stromal cells isolated from endometrium (n = 3) or endometriosis (n = 3) incubated in the absence or presence of PGE2 (10−7 m), 22(R)-OH-cholesterol (22R; 10 μm), or LDL (50 μg/ml). 22(R)-OH-cholesterol can diffuse into the mitochondria without a requirement for StAR. LDL was added in an attempt to increase the availability of cholesterol. PGE2 stimulated the levels progesterone (A), estrone (B), and estradiol (C) in media from endometriosis-derived stromal cells. The addition of 22R-OH-cholesterol or LDL did not modify this effect of PGE2 significantly, except for the enhancing effects of LDL on estrone levels.
Figure 4.
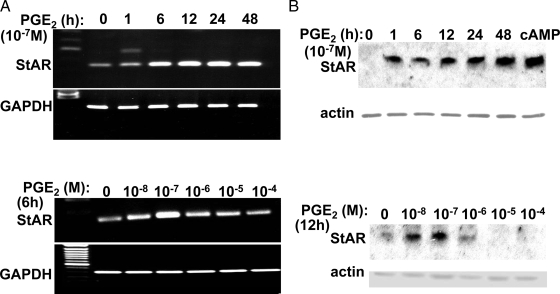
Time and dose-dependent induction of StAR mRNA and protein by PGE2 in endometriotic stromal cells. PGE2 stimulated StAR mRNA (A) and protein levels (B) in a time- and dose-dependent manner in endometriotic stromal cells. StAR protein in the last lane in the upper panel B was detected in endometriotic stromal cells treated for 48 h with dibutyryl cAMP. mRNA or protein levels of the house-keeping genes, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and actin, were used as loading controls.
Figure 5.
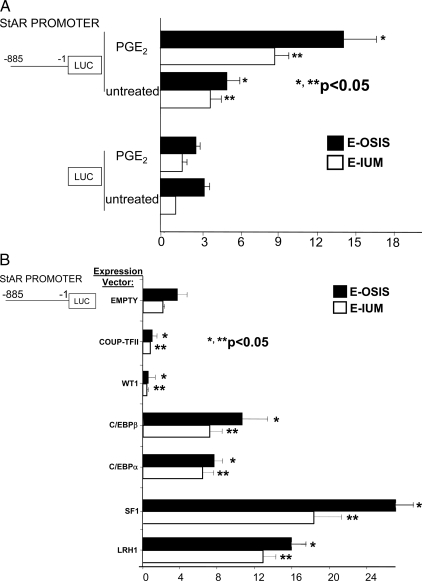
Regulation of StAR promoter activity in endometriotic and endometrial cells. A, PGE2 stimulated the activity of the −885 bp StAR promoter fused to the luciferase (LUC) reporter gene in both endometriotic and endometrial cells, whereas it did not stimulate luciferase activity of the empty vector. *, P value for endometriotic cells; **, P value for endometrial cells. B, A vector overexpressing a transcription factor was cotransfected together with the −885 bp StAR-luciferase (LUC) into endometriotic or endometrial stromal cells. C/EBPβ, C/EBPα, SF1, or LRH1 induced StAR promoter activity in both cell types. On the other hand, WT1 or COUP-TFII inhibited StAR promoter activity. *, P value for endometriotic cells comparing an expression vector with the empty vector; **, P value for endometrial cells.
Figure 1.
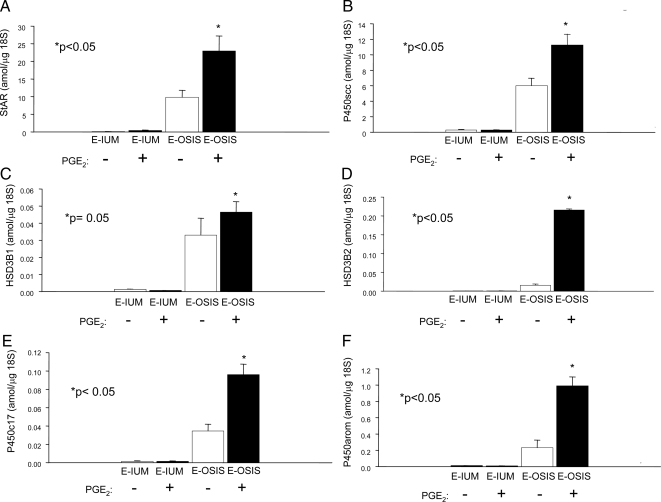
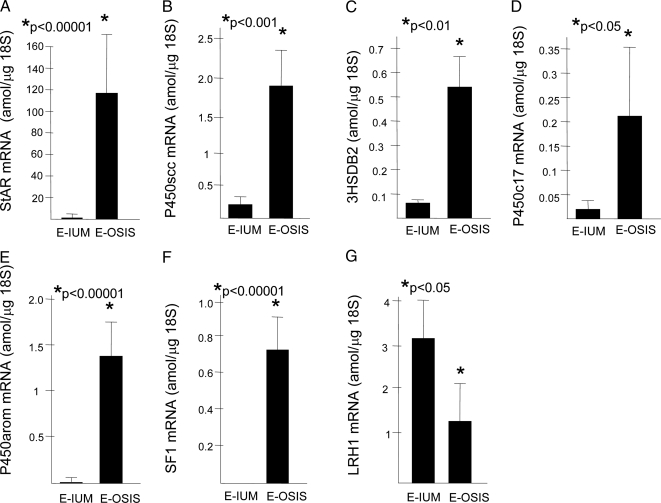
In vivo (tissue) mRNA levels of steroidogenic genes (real-time PCR). mRNA levels of the steroidogenic genes including the mitochondrial cholesterol transporter, StAR (STAR); the enzymes P450scc (CYP11A1), HSD3B2, P450c17 (CYP17A1), and P450arom (CYP19A1); and the transcription factor SF1 were found to be strikingly higher in tissues of endometriosis (n = 13) compared with endometrium (n = 13). On the other hand, mRNA levels of the SF1 homolog gene, LRH1, were higher in endometrial tissue. E-IUM, Endometrium; E-OSIS, endometriosis.
Results
Expression of steroidogenic genes in eutopic and ectopic endometrium
We demonstrated by real-time RT-PCR the differential expression of steroidogenic genes in extraovarian (peritoneal) endometriosis tissues (n = 13 patients) and eutopic endometrial tissues from age- and cycle-matched disease-free women (n = 13) (Fig. 1, A–D). We found that StAR, P450scc, P450c17, and P450arom mRNA levels were significantly higher in endometriotic tissue than in normal endometrium. The most striking differences, however, were observed for StAR and P450arom mRNA levels (Fig. 1, A and E). The differences in P450scc, HSD3B2, and P450c17 mRNA levels were apparent but less significant (Fig. 1, B–D). The transcription factors SF1 and LRH1 have been shown to play key roles in the induction of steroidogenic gene expression in various cell types (29,30). Interestingly, LRH1 mRNA levels were slightly but significantly lower in endometriotic cells vs. endometrial cells (Fig. 1G). On the other hand, SF1 mRNA was barely detectable in endometrium and expressed at strikingly high levels in endometriotic tissue (0.7 attomoles/μg 18S RNA; Fig. 1F). These in vivo findings strongly suggest that SF1 may be the key transcriptional regulator of steroidogenic gene expression in endometriosis. The mRNA levels of each of the tested genes were not significantly different between endometriotic or endometrial tissues collected during the proliferative vs. luteal phase of the menstrual cycle.
We employed real-time PCR to quantify the mRNA levels of steroidogenic genes in eutopic endometrial stromal cells (n = 6) vs. endometriotic stromal cells (n = 6) incubated in the presence or absence of PGE2 (10−7 m) for 24 h based on our previous data (13). In endometriotic stromal cells, PGE2 induced robustly the mRNA levels of StAR (2.3-fold) (P < 0.05) and P450arom (4.3-fold) (P < 0.05), whereas inductions of P450scc, P450c17, and HSD3B2 mRNA were modest but significant (1.4- to 1.9-fold; P < 0.05; Fig. 2). Eutopic endometrial stromal cells from disease-free women contained extremely low levels of steroidogenic genes, which could not be stimulated by PGE2 (except for a 3-fold increase in StAR; Fig. 2). These findings are consistent with the data shown in Fig. 1 and indicate that StAR and P450arom appear to be the key PGE2-induced genes in endometriotic tissue. Furthermore, when compared with normal endometrial tissue, all steroidogenic genes involved in estrogen production in endometriotic tissue are responsive to PGE2. Induction of HSD3B1 mRNA did not reach statistical significance (P = 0.05).
Progesterone and estradiol production by stromal cells
Levels of progesterone, estrone, and estradiol were significantly higher in vehicle or PGE2-treated endometriotic cells compared with endometrial cells (n = 3; P < 0.05; Fig. 3, A–C). Treatment of endometriotic stromal cells with PGE2 significantly increased the levels of progesterone, estrone, and estradiol in culture media (n = 3; P < 0.05; Fig. 3, A–C). The addition of 22(R)–OH-cholesterol (10 μm), which can pass directly through the mitochondrial membrane (independent of StAR) (31), did not stimulate the production of these steroids to the same extent as PGE2 treatment, suggesting that StAR up-regulation by PGE2 is necessary for steroid production in endometriotic cells (Fig. 3). We did not observe a stimulatory effect on progesterone or estradiol production with the addition of LDLs to the media (LDL, 50 μg/ml) (Fig. 3, A and C). Addition of LDL increases the formation of estrone by endometriotic cells (Fig. 3B).
These findings indicate that PGE2 stimulates the expression of StAR, P450scc, and HDS3B2 to catalyze the conversion of cholesterol to progesterone in endometrial stromal cells. PGE2 also stimulated estrone and estradiol formation through additional up-regulation of P450c17 and P450arom. Taken together, our data suggest that PGE2 can stimulate the full conversion of cholesterol to estradiol within the endometriotic stromal cell.
Regulation of StAR mRNA and protein levels by PGE2 in endometriotic stromal cells
We determined the time course and dose-dependent regulation of StAR expression by PGE2 in cultured endometriotic stromal cells (Fig. 4). Time-dependent PGE2 induction of both StAR mRNA and protein started as early as 1 h after treatment. Peak levels were observed at 24 h for mRNA and 48 h for protein. Administration of physiological quantities of PGE2 (10−7 or 10−8 m) significantly stimulated StAR mRNA expression, whereas this stimulatory effect was not observed at higher concentrations (Fig. 4A). The dose-dependent regulation of StAR mRNA by PGE2 was accompanied by similar changes in protein levels (Fig. 4B).
Transcriptional regulation of StAR in endometriotic cells
We transfected the −885 bp StAR promoter-luciferase reporter gene into endometriotic and endometrial stromal cells. PGE2 stimulated StAR promoter activity 2.5-fold in endometriotic stromal cells (P < 0.05) (Fig. 5A). A 1.9-fold induction (P < 0.05) was observed in endometrial stromal cells.
The regulation of StAR promoter by various transcription factors was very similar to that described for the P450arom promoter (16). Previously, we demonstrated that in vitro overexpression studies reflected the in vivo effects of these factors on differential P450arom expression (16,17,22). Upon cotransfection of expression plasmids of various transcription factors, the −885 StAR-luciferase reporter was up-regulated significantly by overexpression of SF1 (Fig. 5B; P < 0.05). Modest inductions were also observed with overexpression of LRH1 (an SF1 homolog), C/EBPα, and C/EBPβ. Conversely, overexpression of WT1 or COUP-TFII effectively suppressed SF1-dependent StAR promoter activation (Fig. 5B). We used the minus KTS splice isoform of WT1, which is a more potent transcriptional regulator compared with the plus KTS isoform (17). Similar results were observed upon cotransfection of endometrial stromal cells with the StAR promoter-luciferase vector and these expression plasmids.
Binding of key regulatory transcription factors to StAR and P450arom promoters in a synchronized manner
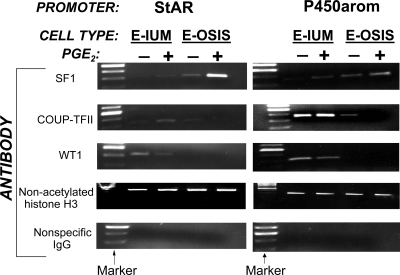
Using ChIP, we demonstrated differential binding of key transcription factors and coregulators (SF1, COUP-TFII, and WT1) to the StAR and P450arom promoters in intact endometrial and endometriotic stromal cells in primary culture. The PGE2-dependent binding pattern of these three factors was remarkably similar with respect to both the StAR and P450arom promoters, but distinct in endometriotic and endometrial stromal cells. Under basal conditions, SF1 bound to both promoters in endometriotic but not in endometrial cells (Fig. 6), and PGE2 significantly enhanced SF1 binding in both cell types. SF1 binding activity was strikingly higher in PGE2-treated endometriotic cells. On the other hand, binding activity of the inhibitory transcription factor COUP-TFII was much higher in endometrial cells compared with endometriotic cells, where PGE2 treatment eliminated its binding to either promoter. Similarly, WT1 binding activity was either minimal or absent in endometriotic cells. PGE2 suppressed the binding activity of WT1, which functions as a corepressor of SF1, to either promoter in endometrial cells (Fig. 6). Taken in the context of previous findings that SF1 expression is higher and WT1 expression is lower in endometriotic tissues than in endometrium (17) (see also Fig. 1), these ChIP data suggest that, compared with normal endometrium, the transcriptional environment within endometriotic cells is permissive for estradiol synthesis, which is further increased in the presence of PGE2.
Figure 6.
ChIP assay demonstrating coordinate binding activity of transcription factors to StAR and P450arom promoters. In endometriotic (E-OSIS) stromal cells, PGE2 induced binding of SF1 to both StAR and P450arom promoters, whereas very low SF1 binding was observed in endometrial (E-IUM) stromal cells. On the other hand, the inhibitory transcription factors COUP-TFII and WT1 were associated with StAR and P450arom promoters more avidly in endometrial cells compared with endometriotic cells. Note that PGE2 reduced binding of COUP-TFII or WT1 in endometriotic or endometrial cells. The association of the control protein, nonacetylated histone H3, to chromatin did not vary with cell type or treatment. No binding activity was observed after chromatin was immunoprecipitated with nonspecific IgG.
Discussion
We made a number of conclusions from the data presented. First, expression of all components of the complete steroidogenic cascade for estrogen biosynthesis was present in endometriotic stromal cells, and PGE2 stimulated these cells to produce progesterone, estrone, and estradiol. Because we previously demonstrated constitutive expression of HSD17B1, which catalyzes the conversion of weakly estrogenic estrone to biologically potent estradiol in both endometrial and endometriotic tissues, this experiment was not repeated here (11). Thus, the full enzymatic cascade for production of estradiol from cholesterol is present in the endometriotic stromal cell.
Second, we demonstrated the synchronized regulation of the key steroidogenic genes StAR and P450arom via binding of identical transactivating factors to their promoters in response to PGE2. These two findings complement each other and support the notion that the PGE2-cAMP-SF1 pathway as the key regulator of steroidogenesis, particularly estradiol production, in endometriotic stromal cells. We also found that the same transcriptional mechanism is responsible for silencing the two genes, leading to suppression of estrogen production in normal endometrial stromal cells.
Third, endometriotic cells do not only have the ability to produce estrogen, but they can also produce a baseline level of estrogen even in the absence of PGE2 that is higher than in normal endometrial cells (Fig. 3). The endometriotic cells express more endogenous SF1, StAR, P450arom, and other steroidogenic enzymes compared with normal cells, and SF1 is bound to the StAR and P450arom promoters, whereas the corepressors are not recruited. This is the exact opposite of the normal endometrial cells, in which corepressors are bound to the target promoters to limit estrogen synthesis.
Our current results regarding PGE2 induction of StAR expression and progesterone production are in general consistent with the previously published data by Tsai et al. (10) regarding StAR expression. Our data, however, differ significantly with respect to expression of P450scc, HSD3B2, and P450c17, all of which were strikingly higher in endometriotic vs. endometrial tissues in our hands (Fig. 1). Moreover, we found that PGE2 significantly induced the expression of each gene in endometriotic stromal cells (Fig. 2). In contrast, Tsai et al. (10) did not find induction of these genes by PGE2 and attributed progesterone production solely to StAR activity as evidenced by the substitution of PGE2 induction of progesterone production by the 22(R)-OH-cholesterol that can pass directly through the mitochondrial membrane (independent of StAR). In our hands, addition of 22(R)-OH-cholesterol to endometriotic cells did not fully substitute PGE2 induction of progesterone production (Fig. 3A). There are several potential explanations for this discrepancy. First, potentially diverse mechanisms in the patient populations in two studies (Taiwan vs. U.S.) may account for this. Second, real time RT-PCR used in our study may be more sensitive for detecting differential gene expression, in contrast to semiquantitative PCR used by Tsai et al. Finally, possible differences between the two studies with respect to surgical methods for excision of endometriotic tissue specimens may account, in part, for different results.
The cis-regulatory elements in the StAR and aromatase promoters are very similar. Additionally, similar transcription factors (SF1, C/EBPs, and CREB) mediate PGE2-dependent regulation of both promoters (19,20,21,22). These promoters remain quiescent under the influence of inhibitory transcription factors (e.g. COUP-TF) and corepressors (e.g. WT1) in normal endometrial stromal cells (4). Binding of SF1 to the promoter region of the StAR may be the key event that brings the entire transcriptional complex to initiate StAR expression in endometriosis. As in the case of the P450arom gene, SF1 possibly serves as a dominant activator for StAR by competing with COUP-TF for binding to its promoter in endometriotic stromal cells (16). In addition to COUP-TF, there may be additional fail-safe mechanisms to silence both StAR and P450arom in normal endometrium. Significant binding of the SF1 corepressor WT1 to both StAR and P450arom promoters in endometrial but not endometriotic stromal cells (Fig. 6) likely represents one of these redundant mechanisms for the inhibition of estrogen synthesis in eutopic endometrium. Interestingly, minimal levels of StAR and steroidogenic enzymes are expressed in endometrium despite practically absent SF1 in this tissue. It is possible that LRH1 present in endometrial stromal cells may act as a surrogate of SF1 and be responsible for low levels of steroidogenic gene expression in these cells (see Figs. 1 and 5B).
In vivo and in vitro demonstration of synchronous regulation of StAR and a complete set of steroidogenic enzymes including P450arom by the same complement of transcription factors in endometriotic cells shows that estrogen production in endometriosis is up-regulated by a distinct mechanism. Moreover, steroid measurements and parallel gene expression data support the notion that estrogen is synthesized de novo from cholesterol and that endometriotic P450arom is not solely dependent for substrate on circulating androstenedione secreted by the adrenal or ovary. These new findings may revise our view of the pathogenesis and hormonal treatment strategies for endometriosis (14,15).
Acknowledgments
We thank Dr. Zongjuan Fang for her technical contributions.
Footnotes
This work was supported by grants from the National Institutes of Health (HD38691 and HD40093) and Friends of Prentice.
Disclosure Statement: E.A., H.T., G.I., M.B.Y., D.R., M.P., B.G., R.A., N.Y., and D.B.H. have nothing to declare. S.E.B. serves as a consultant for Meditrina Pharmaceuticals, Inc., GlaxoSmithKline, and Novartis.
First Published Online November 11, 2008
Abbreviations: ChIP, Chromatin immunoprecipitation; COUP-TF, chicken ovalbumin upstream promoter-transcription factor; h, human; HSD17B1, 17β-hydroxysteroid dehydrogenase type 1; HSD3B2, 3β-hydroxysteroid-dehydrogenase type 2; LDL, low-density lipoprotein; LRH1, liver receptor homolog-1; m, mouse; PGE2, prostaglandin E2; P450arom, aromatase; P450c17, 17-hydroxylase/17-20-lyase; P450scc, side chain cleavage P450; SF1, steroidogenic factor-1; StAR, steroidogenic acute regulatory protein; WT1, Wilms’ tumor-1.
References
- Giudice LC, Kao LC 2004 Endometriosis. Lancet 364:1789–1799 [DOI] [PubMed] [Google Scholar]
- Ryan IP, Taylor RN 1997 Endometriosis and infertility: new concepts. Obstet Gynecol Surv 52:365–371 [DOI] [PubMed] [Google Scholar]
- Sampson JA 1927 Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 14:422–469 [Google Scholar]
- Tremblay Y, Ringler GE, Morel Y, Mohandas TK, Labrie F, Strauss 3rd JF, Miller WL 1989 Regulation of the gene for estrogenic 17-ketosteroid reductase lying on chromosome 17cen–q25. J Biol Chem 264:20458–20462 [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J 2006 Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol 248:94–103 [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Lee TC, Miller WL 1998 Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M 1995 Modulation of the activity of human 17 α-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. Biochem J 308:901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM 2001 StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol 63:193–213 [DOI] [PubMed] [Google Scholar]
- Miller WL, Strauss 3rd JF 1999 Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J Steroid Biochem Mol Biol 69:131–141 [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wu MH, Lin CC, Sun HS, Chan HM 2001 Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J Clin Endocrinol Metab 86:5765–5773 [DOI] [PubMed] [Google Scholar]
- Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, Johns A, Meng L, Putman M, Carr B, Bulun SE 1998 Deficient 17β-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17β-estradiol. J Clin Endocrinol Metab 83:4474–4480 [DOI] [PubMed] [Google Scholar]
- Noble LS, Simpson ER, Johns A, Bulun SE 1996 Aromatase expression in endometriosis. J Clin Endocrinol Metab 81:174–179 [DOI] [PubMed] [Google Scholar]
- Noble LS, Takayama K, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE 1997 Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab 82:600–606 [DOI] [PubMed] [Google Scholar]
- Attar E, Bulun SE 2006 Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril 85:1307–1318 [DOI] [PubMed] [Google Scholar]
- Attar E, Bulun SE 2006 Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Update 12:49–56 [DOI] [PubMed] [Google Scholar]
- Zeitoun K, Takayama K, Michael MD, Bulun SE 1999 Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of SF-1 and COUP-TF to the same cis-acting element. Mol Endocrinol 13:239–253 [DOI] [PubMed] [Google Scholar]
- Gurates B, Sebastian S, Yang S, Zhou J, Tamura M, Fang Z, Suzuki T, Sasano H, Bulun SE 2002 WT1 and DAX-1 inhibit aromatase P450 expression in human endometrial and endometriotic stromal cells. J Clin Endocrinol Metab 87:4369–4377 [DOI] [PubMed] [Google Scholar]
- Sun HS, Hsiao KY, Hsu CC, Wu MH, Tsai SJ 2003 Transactivation of steroidogenic acute regulatory protein in human endometriotic stromal cells is mediated by the prostaglandin EP2 receptor. Endocrinology 144:3934–3942 [DOI] [PubMed] [Google Scholar]
- Manna P, Dyson M, Eubank D, Clark B, Lalli E, Sassone-Corsi P, Zeleznik A, Stocco D 2002 Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol Endocrinol 16:184–199 [DOI] [PubMed] [Google Scholar]
- Manna P, Eubank D, Lalli E, Sassone-Corsi P, Stocco D 2003 Transcriptional regulation of the mouse steroidogenic acute regulatory protein gene by the cAMP response-element binding protein and steroidogenic factor 1. J Mol Endocrinol 30:381–397 [DOI] [PubMed] [Google Scholar]
- Christenson L, Strauss JI 2001 Steroidogenic acute regulatory protein: an update on its regulation and mechanism of action. Arch Med Res 32:576–586 [DOI] [PubMed] [Google Scholar]
- Yang S, Fang Z, Takashi S, Hironobu S, Jianfeng Z, Gurates B, Tamura M, Ferrer K, Bulun SE 2002 Regulation of aromatase P450 expression in endometriotic and endometrial stromal cells by CCAT/enhancer binding proteins: decreased C/EBPβ in endometriosis is associated with overexpression of aromatase. J Clin Endocrinol Metab 87:2336–2345 [DOI] [PubMed] [Google Scholar]
- Ryan I, Schriock ED, Taylor R 1994 Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab 78:642–649 [DOI] [PubMed] [Google Scholar]
- Lin Z, Reierstad S, Huang CC, Bulun SE 2007 Novel estrogen receptor-α binding sites and estradiol target genes identified by chromatin immunoprecipitation cloning in breast cancer. Cancer Res 67:5017–5024 [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Cheng YH, Lin Z, Reierstad S, Yin P, Attar E, Xue Q, Imir G, Thung S, Trukhacheva E, Suzuki T, Sasano H, Kim JJ, Yaegashi N, Bulun SE 2008 Upstream stimulatory factor-2 regulates steroidogenic factor-1 expression in endometriosis. Mol Endocrinol 22:904–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Reierstad S, Lin Z, Lu M, Brooks C, Li N, Innes J, Bulun SE 2007 Prostaglandin E(2) induces breast cancer related aromatase promoters via activation of p38 and c-Jun NH(2)-terminal kinase in adipose fibroblasts. Cancer Res 67:8914–8922 [DOI] [PubMed] [Google Scholar]
- Ronen-Fuhrmann T, Timberg R, King SR, Hales KH, Hales DB, Stocco DM, Orly J 1998 Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology 139:303–315 [DOI] [PubMed] [Google Scholar]
- Deb S, Jianfeng Z, Amin SA, Gonca IA, Bertan YM, Zihong L, Bulun SE 2006 A novel role of sodium butyrate in the regulation of cancer-associated aromatase promoters I. 3 and II by disrupting a transcriptional complex in breast adipose fibroblasts. J Biol Chem 281:2585–2597 [DOI] [PubMed] [Google Scholar]
- Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA 1998 Wilms’ tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93:445–454 [DOI] [PubMed] [Google Scholar]
- Hanley N, Rainey W, Wilson D, Ball S, Parker K 2001 Expression profiles of SF-1, DAX1, and CYP17 in the human fetal adrenal gland: potential interactions in gene regulation. Mol Endocrinol 15:57–68 [DOI] [PubMed] [Google Scholar]
- Toaff ME, Schleyer H, Strauss 3rd JF 1982 Metabolism of 25-hydroxycholesterol by rat luteal mitochondria and dispersed cells. Endocrinology 111:1785–1790 [DOI] [PubMed] [Google Scholar]