Abstract
Context: Estradiol and its nuclear receptors, estrogen receptor (ER) α and ERβ, play critical roles in endometrium and endometriosis. Levels of ERβ, due to pathological hypomethylation of its promoter, are significantly higher in endometriotic vs. endometrial tissue and stromal cells, whereas ERα levels are lower in endometriosis. Estradiol regulates ERα gene expression via its alternatively used promoters A, B, and C.
Objective: The aim of the study was to determine whether high levels of ERβ in endometriotic stromal cells from ovarian endometriomas regulate ERα gene expression.
Results: ERβ knockdown significantly increased ERα mRNA and protein levels in endometriotic stromal cells. Conversely, ERβ overexpression in endometrial stromal cells decreased ERα mRNA and protein levels. ERβ knockdown significantly decreased proliferation of endometriotic stromal cells. Chromatin immunoprecipitation assays demonstrated that estradiol enhanced ERβ binding to nonclassical activator protein 1 and specificity protein 1 motifs in the ERα gene promoters A and C and a classic estrogen response element in promoter B in endometriotic stromal cells.
Conclusions: High levels of ERβ suppress ERα expression and response to estradiol in endometrial and endometriotic stromal cells via binding to classic and nonclassic DNA motifs in alternatively used ERα promoters. ERβ also regulates cell cycle progression and might contribute to proliferation of endometriotic stromal cells. We speculate that a significantly increased ratio of ERβ:ERα in endometriotic tissues may also suppress progesterone receptor expression and contribute to progesterone resistance. Thus, ERβ may serve as a significant therapeutic target for endometriosis.
Estrogen receptor β (ERβ) acts as a suppressor of ERα in endometrial and endometriotic stromal cells via binding to classical and non-classical DNA motifs in specific ERα promoters.
Endometriosis is an estrogen-dependent disease that affects 6–10% of women of reproductive age. It is characterized by the presence of endometrium-like tissue outside the uterine cavity, primarily on the ovaries and pelvic peritoneum, and represents one of the most common causes of chronic pelvic pain, dysmenorrhea, and infertility (1). Similar to other chronic diseases, such as asthma and diabetes mellitus, endometriosis is inherited in a polygenic manner and has a complex and multifactorial etiology (2). Although the exact mechanism for the development of endometriosis remains unclear, there is a large body of research data and circumstantial evidence that suggests a crucial role of estrogen in the establishment and maintenance of this disease (2,3).
Despite its sensitivity to estrogen, endometriosis appears to contain a unique but severely altered complement of steroid hormone receptors compared with that of its normal tissue counterpart, eutopic endometrium. The levels of both isoforms of progesterone receptor (PR), particularly PR B, are significantly lower in endometriosis compared with eutopic endometrium (4,5). Moreover, a number of investigators have reported markedly elevated levels of estrogen receptor (ER) β and lower levels of ERα in human endometriotic tissues and primary stromal cells when compared with eutopic endometrial tissues and cells (6,7). The classical human ERα was cloned in 1986, and a second ER, ERβ, was cloned from rat prostate and human testis in 1996 (8,9,10). Both ERs act as transcription factors and are believed to play key roles in endometrium and endometriosis for regulation of growth differentiation and a number of other biological functions.
The human ERα gene is regulated via multiple promoters; the three major promoters are A, B, and C and are alternatively used in various tissues (11,12,13). Promoters A and B are located within the 2-kb region proximal to the translation start site, whereas promoter C lies some 101 kb upstream of this site (11,14). In vivo observations strongly suggest that estradiol (E2) regulates ERα expression in endometrium (15). On the other hand, strikingly high quantities of E2 produced via local aromatase activity in addition to high ERβ levels in stromal cells of endometriosis may perturb the regulation of ERα expression (7,16). However, the mechanisms involving promoter-specific regulation of the ERα gene expression by E2 or the role of ERβ in this regulation are not known.
Currently, the biological roles of ERβ in endometrium and endometriosis are not well understood. We chose to investigate ERβ-dependent regulation of ERα expression and response to E2 via specific promoters in endometrial and endometriotic stromal cells for several reasons. First, one of the most striking differences between endometriosis and endometrium was observed with respect to ERβ levels compared with other steroid receptors, where ERβ mRNA levels were found to be more than 36-fold higher in endometriosis compared with normal endometrium (7). Second, an ERβ-selective analog was shown to be therapeutic in a rodent endometriosis model. Third, in eutopic endometrium, ERα stimulates PR production. If ERβ acts as a suppressor of ERα in endometriosis, then decreased ERα levels might lead to decreased PR levels and contribute to the state of progesterone resistance observed in endometriosis. Moreover, ERβ was shown to interact with cell cycle spindle assembly checkpoint protein, MAD2, which may indicate a distinct role of this nuclear receptor in cell cycle regulation and proliferation of endometriotic cells (17).
Subjects and Methods
Subjects and primary cell culture
Eutopic endometrium samples from disease-free subjects (n = 8) and the walls of cystic endometriosis lesions of the ovaries (n = 10) were obtained immediately after surgery. Written informed consent was obtained before surgeries, including a consent form and protocol approved by the Institutional Review Boards at Northwestern University. The average age of subjects was 40.11 ± 6.07 yr (endometrium) and 36.09 ± 3.11 yr (endometriosis), and there were no statistically significant differences between the two groups with respect to age. None of the patients had received any preoperative hormonal therapy. All samples were histologically confirmed. Eutopic endometrial samples were obtained during the proliferative stage of the menstrual cycle from premenopausal women undergoing hysterectomy for cervical dysplasia, uterine leiomyoma, or pelvic prolapse. The phase of the menstrual cycle was determined by preoperative history and histological examination. Stromal cells were isolated from these two tissue types using a protocol previously described (18,19) with minor modifications and suspended in DMEM/F12 1:1 (GIBCO/BRL, Grand Island, NY) containing 10% fetal bovine serum.
Hormonal treatments
After serum starvation, endometrial and endometriotic stromal cells were incubated in serum-free DMEM/F12 medium (GIBCO/BRL) containing 10−7 m E2 (Sigma, St. Louis, MO) for various periods of time.
RNA extraction and quantitative analysis by real-time PCR
Total RNA was isolated from stromal cells with TRIzol (Sigma) according to the manufacturer’s protocol. RNA samples were treated with DNase I to avoid genomic DNA contamination using the Turbo-DNA free kit (Ambion, Austin, TX) according to the manufacturer’s protocol. Two micrograms of total RNA were used to generate cDNA with the Superscript III first-strand synthesis system (Invitrogen, Carlsbad, CA). Real-time quantitative PCR was performed using the ABI 7900 Sequence Detection system and the ABI Power Syber Green gene expression system (Applied Biosystems, Foster City, CA) to quantify total ERα mRNA, ERα mRNA species containing promoter A-, B-, and C-specific exons, ERβ mRNA, and human 18S RNA. 18S values were used for normalization. Relative quantification for all transcripts was analyzed by the comparative threshold cycles method described previously (20). Expression of promoter A, B, or C-specific ERα mRNA was determined in all analyzed samples (n = 18) with semiquantitative RT-PCR with the products analyzed on the 1% agarose gel. Isolated endometrial and endometriotic stromal cells in primary culture were treated with E2 (10−7 m) or vehicle for 15 or 30 min or 1, 3, 6, 12, or 24 h. Real-time PCR employing exon-specific primers was used to quantify total and promoter A, B, or C-specific ERα mRNA levels.
The following primers were used for the ERα coding region: forward, 5′-CACCAACCAGTGCACCATTG-3′; reverse, 5′-AAGGTTGGCAGCTCTCATGTC-3′; for ERα promoter A: forward, 5′-CTGGGAGCTGCACTTGCT-3′; reverse, 5′-GTGGAGGGTCATGA-3′; for ERα promoter B: forward, 5′- ACACTGAGCCACTCGCACAT-3′; reverse, 5′-GGTCATGGTCATGAAGGCTCAG-3′; for ERα promoter C: forward, 5′-GGACTGCGGTACCAAATATCAGC-3′; reverse, 5′-GGTCATGGTCATGAAGGCTCAG-3′; for ERβ: forward, 5′-CCATGATCCTGCTCAATTCC-3′; reverse, 5′-CTCTTGGCAATCACCCAAAC-3′; and for human 18S, forward: 5′-AGGAATTCCCAGTAAGTGCG-3′; reverse, 5′-GCCTCACTAAACCATCCAA-3′.
Small interfering RNA (siRNA) knockdown
Endometrial and endometriotic stromal cells were cultured in the growth medium as described above to achieve approximately 50–60% confluence at the time of transfection. Transfections were performed using a nontargeting negative control siRNA (Dharmacon, Chicago, IL) or siRNAs against human ERβ (Dharmacon) at a final concentration of 100 nmol/liter using Lipofectamine RNAiMAX (Invitrogen). Thirty-six hours after transfection, the cells were serum starved for 12 h, treated with E2 or vehicle for 24 h, and processed for real-time PCR, immunoblotting, or apoptosis/cell cycle analysis.
ERβ overexpression using plasmid DNA
Transfections were performed using a plasmid pRST7ER (Addgene Inc., Cambridge, MA) containing the human ERβ gene that was described previously (21) or control plasmid vector. Both control and pRST7ER plasmids were confirmed by DNA sequencing before transfection. Endometrial and endometriotic stromal cells were cultured and transfected at approximately 50–60% confluence. DNA was introduced into the cells using FuGene HD (Roche Applied Science, Indianapolis, IN) according to manufacturer’s instructions. Briefly, transfections were performed in 100-mm dishes using 0.7 μg total plasmid DNA, with a ratio of plasmid DNA to transfection reagent of 1:4. Thirty-six hours after transfection, cells were serum starved for 12 h, treated with E2 or vehicle for 24 h, and processed for real-time PCR, immunoblotting, or apoptosis/cell cycle analysis.
Chromatin immunoprecipitation (ChIP) assay
Endometrial and endometriotic stromal cells were incubated with 10−7 m E2 or vehicle (ethanol) for 24 h. ChIP assays were performed as described previously using a kit from Upstate Biotechnology (Lake Placid, NY) (22). Cells were washed twice with cold PBS and cross-linked with 1% formaldehyde at room temperature for 10 min. The cross-linked chromatin was lysed and sonicated to shear the DNA into 0.6–1.0 kb fragments. The soluble chromatin fraction was immunoprecipitated with an equal amount of either rabbit IgG or rabbit polyclonal antihuman ERβ antibody (Upstate) overnight and washed three times with low-salt buffer and four times with high-salt buffer. The immunoprecipitated chromatin was purified and analyzed by PCR. ERα promoter A, B, and C sequences were analyzed using Dragon ERE Finder version 2 and Transcription Element Search System software (University of Pennsylvania, Philadelphia, PA). The only classic estrogen response element (ERE) region was identified with ERα promoter B. Multiple activator protein 1 (AP1) and specificity protein 1 (Sp1) sites were identified in ERα promoters A and C. The primer sequences used for PCR were: for ERα promoter B ERE site (−839/−709 bp), forward, 5′-GACAGAGCTGGGTCATGTCA-3′; reverse, 5′-TGTCCTAACGGCCTCCTAAG-3′; for ERα promoter A AP1 site (−347/−98 bp), forward, 5′-GCACACCCCATTCTATCTGC-3′; reverse, 5′-GCACGAGGATCTGCTAAAGG-3′; for ERα promoter C Sp1 site (+298/+591 bp), forward, 5′-GCACTCTACATGCGTTGCTT-3′; reverse, 5′-CAACTGGCAAGAGGAGAAGG-3′; and for ERα promoter C AP1 site (−237/−19 bp), forward, 5′-CCAATGGCATCAGTGGTAAC-3′; reverse, 5′-GAGAAGGAAAGGGAGATGTGG-3′.
Immunoblot analysis
Cells were washed with ice-cold PBS and suspended in protein extraction reagent (Pierce, Rockford, IL). Lysates were cleared by centrifugation at 14,000 × g for 10 min. Equal amounts of protein (10 μg) were resolved on 10% Ready Gel Precast Gels (Bio-Rad Laboratories, Hercules, CA) for 90 min at 50 mAmp and transferred to nitrocellulose membranes at 150 mAmp for 2 h. The gels were run in Tris/glycine/sodium dodecyl sulfate buffer (Bio-Rad); for transfers, Tris/glycine buffer (Bio-Rad) was used. The nitrocellulose membranes were later incubated with that rabbit polyclonal antihuman ERβ antibodies at a dilution of 1:2000 (Upstate) or antihuman ERα antibodies at a dilution of 1:100 (Calbiochem, an affiliate of Merck KGaA, Darmstadt, Germany). Anti-β-actin antibody was used as a loading control. Detection was performed using a supersignal west femto maximum sensitivity substrate system (Pierce). Immunoblots were quantified using Image J program (National Institutes of Health).
Apoptosis and cell cycle analysis
To determine apoptosis and cell cycle specific changes in endometrial and endometriotic stromal cells, the APO-DIRECT assay kit (BD, Franklin Lakes, NJ) was used to label single step DNA breaks with fluorescein isothiocyanate 2′-deoxyuridine-5′-triphosphate. After DNA labeling, flow cytometric analysis for detection of apoptotic or DNA-replicating cells was performed according to the technique described previously (23).
Statistical analysis
Statistical analysis for comparisons between different treatments or over time was performed by ANOVA followed by the Tukey multiple comparisons procedure. A P value of <0.05 was considered significant. All values are given as the mean ± sem.
Results
Transcriptional regulation of ERα gene by E2 via specific promoters
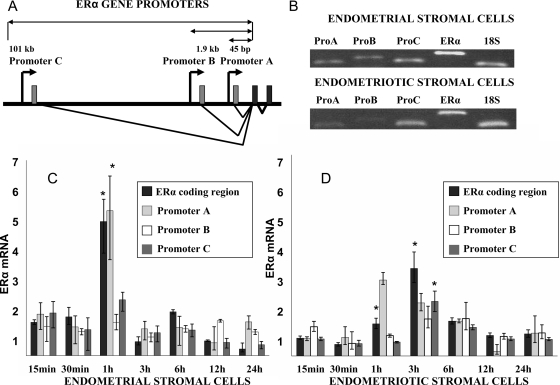
The ERα gene is regulated via three alternatively used promoters (A, B, and C) in endometrial and endometriotic stromal cells (Fig. 1, A and B). As depicted in Fig. 1B, we detected various ERα mRNA species (coding region and untranslated promoter-specific first exons A, B, or C) by exon-specific PCR or simply by amplifying the coding region. Next, using real-time PCR, we characterized PCR promoter-specific ERα mRNA species in endometrial and endometriotic stromal cells treated with E2 (10−7 m). Treatment with E2 regulated total ERα mRNA levels in a time-dependent manner in both endometrial and endometriotic stromal cells primarily via the proximally located promoter A and distal promoter C (Fig. 1, C and D, respectively). At 1- and 3-h time points, E2 significantly induced ERα promoter A or C-specific mRNA species in endometrial or endometriotic stromal cells. Of note, ERα expression was relatively lower in endometriotic vs. endometrial stromal cells.
Figure 1.
Transcriptional regulation of ERα gene by E2 via specific promoters in endometrial stromal cells and endometriotic stromal cells (from ovarian endometriomas). A, ERα promoters A and B are located proximally to the coding region, and distal promoter C is located at 101 kb upstream of the transcription start site. B, ERα total and promoter A, B, or C-specific mRNA expression in endometrial and endometriotic stromal cells was determined by semiquantitative RT-PCR. Products were analyzed on 1% agarose gel. Total ERα mRNA levels were determined by PCR and primers targeting only the coding region. C, In endometrial stromal cells, total and promoter-specific ERα mRNA up-regulation was noted after 1 h of E2 treatment (*, P < 0.001), with promoter A-specific mRNA being most significantly up-regulated (*, P < 0.05). P values were derived after comparing E2-treatment with vehicle treatment expressed as fold-change. D, In endometriotic stromal cells, ERα mRNA up-regulation was increased significantly 3 h after E2 treatment (*, P < 0.001), with promoter C-specific mRNA being up-regulated (*, P < 0.05). Promoter A-specific mRNA was significantly up-regulated after 1 h of E2 treatment (*, P < 0.05). P values were derived after comparing E2-treatment with vehicle treatment expressed as fold change. Experiments were repeated in both endometrial and endometriotic stromal cells from four different subjects. All graphs are derived from experiments performed in triplicate in one representative subject. ANOVA followed by the Tukey multiple comparisons procedure was used for statistical analysis.
ERβ knockdown with siRNA resulted in significant ERα up-regulation in endometriotic stromal cells
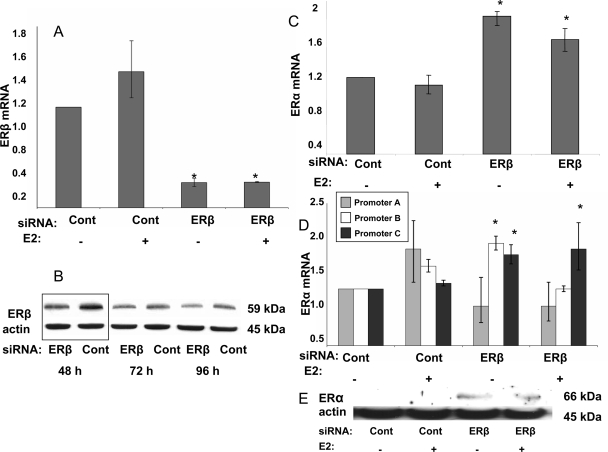
ERβ siRNA knockdown was verified at the mRNA level by real-time PCR and immunoblot (Fig. 2, A and B). ERβ protein knockdown was recognizable at 48 h after siRNA transfection (Fig. 2B). siRNA transfection for 48 h was carried out in the following experiments. ERβ mRNA knockdown above 70% was confirmed in all analyzed samples.
Figure 2.
ERβ knockdown with siRNA resulted in significant ERα up-regulation in endometriotic stromal cells (from ovarian endometriomas). A, ERβ mRNA levels significantly decreased in the presence of ERβ-specific siRNAs (*, P < 0.0001). B, Maximum ERβ protein knockdown occurred 48 h after siRNA transfections. The ratio of ER-β protein in ER-β specific siRNA vs. control siRNA-treated cells was the lowest at 48 h after transfections. C, ERβ knockdown resulted in significantly increased total ERα mRNA levels in endometriotic stromal cells (*, P < 0.0001). D, ERα promoter A-, B-, and C-specific mRNA levels in endometriotic stromal cells with ERβ knockdown. Promoter B- and promoter C-specific mRNA levels were significantly up-regulated (*, P < 0.0001; and *, P = 0.0026, respectively). E, Immunoblotting confirmed increased ERα protein levels when ERβ is knocked down. Experiments were repeated in endometriotic stromal cells from five different subjects. All graphs are derived from experiments performed in triplicate in one representative subject. ANOVA followed by the Tukey multiple comparisons procedure was used for statistical analysis.
In response to ERβ knockdown, ERα mRNA levels in endometriotic stromal cells significantly increased (Fig. 2C). ERβ knockdown resulted in significant elevation of promoter B- and C-specific ERα mRNA levels in endometriotic stromal cells (Fig. 2D). ERβ ablation also increased ERα protein levels (Fig. 2E).
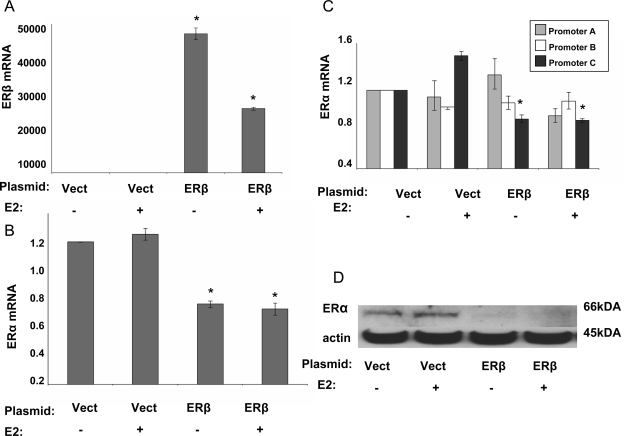
ERβ overexpression resulted in significant ERα down-regulation in endometrial stromal cells
First, increased ERβ levels in endometrial stromal cells with ERβ overexpression were confirmed at the mRNA levels using real-time PCR (Fig. 3A). ERβ overexpression reduced ERα mRNA levels significantly (Fig. 3B). In particular, ERα promoter C-specific mRNA levels were significantly down-regulated in the presence or absence of E2 (Fig. 3C). Immunoblot confirmed decreased ERα protein levels in the presence of ERβ overexpression (Fig. 3D).
Figure 3.
ERβ overexpression resulted in significant ERα down-regulation in endometrial stromal cells. A, Increased ERβ mRNA levels in endometrial stromal cells upon overexpression with and without E2 treatment were confirmed with real-time PCR (*, P < 0.0001). E2 treatment had a suppressive effect on the ERβ levels (*, P < 0.0001). B, In endometrial stromal cells, ERβ overexpression resulted in a significant decrease in ERα mRNA levels (*, P < 0.001). C, ERα promoter C-specific mRNA level was significantly down-regulated in the presence or absence of E2 (*, P < 0.001). D, Immunoblotting confirmed decreased ERα protein levels in the presence of ERβ overexpression. Experiments were repeated in endometrial stromal cells from five different subjects with reproducible results. Data are presented from one representative subject. ANOVA followed by the Tukey multiple comparisons procedure used for statistical analysis. Vect, Vector.
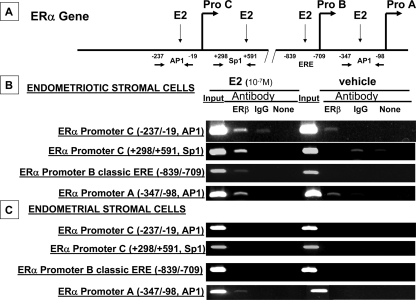
ChIP assay revealed ERβ binding to specific ERα promoter regions in endometriotic and endometrial stromal cells
First, we analyzed the 5′ and 3′ flanking sequences of ERα promoters A, B, and C for cis-regulatory elements. The only classic ERE was identified within ERα promoter B. However, multiple AP1 and Sp1 sites, which may mediate nonclassical ERβ action, were identified in ERα promoters A and C (Fig. 4A). In endometriotic stromal cells, ERβ binding activity to four sequences in three ERα promoter regions was determined. ERβ recruitment to the far distal ERα promoter C at the Sp1 site (+298/+591) and the AP1 site (−237/−19) was demonstrated and found to be enhanced by E2 treatment (Fig. 4B). Recruitment of ERβ to the ERα promoter B ERE (−839/−709) was also shown in endometriotic stromal cells incubated with E2 (Fig. 4B). ERβ binding to the AP1 site (−347/−98) in promoter A was found to be increased by E2 treatment in endometriotic stromal cells (Fig. 4B). Because promoters A and C did not contain a classic ERE, we also screened other potential nonclassical ERβ binding sites; however, primer pairs covering the +445/+675 or +1181/+1379 sites in promoter A and the +36/+183, +300/+589, or −239/−38 sites at promoter C did not show ERβ binding in the presence or absence of E2 (data not shown). Thus, we concluded that ERβ binding to distinct sequences in Fig. 4B was specific. In endometrial stromal cells (Fig. 4C), ERβ binding to the ERα promoter A region AP1 site (−347/−98 bp) was also found to be increased by E2 treatment (Fig. 4C). No binding of ERβ to other ERα promoter regions described above was observed in endometrial stromal cells (Fig. 4C).
Figure 4.
ChIP assay characterized ERβ binding to specific ERα promoter regions in stromal cells from ovarian endometriomas and endometrial stromal cells. A, ERα gene structure with selected potential cis-acting elements within each promoter region characterized in the ChIP assay. B, In the presence or absence of E2, ERβ binds via Sp1 and AP1 sites in ERα promoter C and the AP1 site in promoter A. ERβ binds to a classical ERE within ERα promoter B only in the presence of E2. C, In an E2-dependent manner, ERβ interacts only with the ERα promoter A via an AP1 site. All experiments were repeated in at least three subjects. Data are presented from one representative subject.
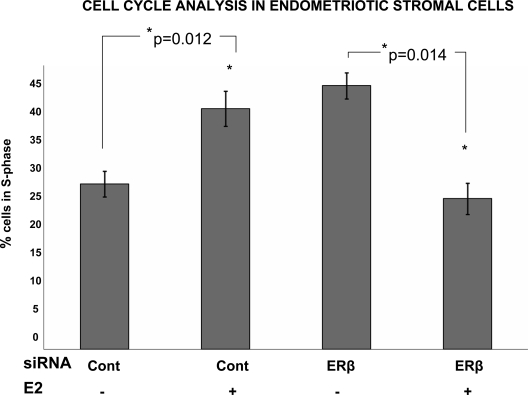
E2 modulates cell cycle via ERβ in endometriotic stromal cells
Treatment with E2 induced proliferation of endometriotic stromal cells, causing a significantly increased percentage of cells to shift into the S-phase (Fig. 5). Although ablation of ERβ abolished E2-induced proliferation of endometriotic stromal cells, ERβ ablation did not alter proliferation of endometrial stromal cells, which were used as controls (data not shown). ERβ knockdown did not alter apoptosis as determined by flow cytometry in endometrial or endometriotic stromal cells (data not shown). ERβ overexpression did not affect cell cycle progression or apoptosis in endometrial or endometriotic stromal cells, which were used as controls (data not shown). This collectively suggests that ERβ is necessary, but not sufficient to increase proliferation of endometriotic stromal cells.
Figure 5.
Cell cycle analysis in stromal cells from ovarian endometriomas. Treatment with E2 induced proliferation of endometriotic stromal cells as indicated by a significant shift of cells into the S-phase (*P = 0.012). Ablation of ERβ eliminated E2-induced proliferation in endometriotic stromal cells (*, P = 0.014). All experiments were repeated in at least three subjects. Data are derived from experiments performed in triplicate in one representative subject. ANOVA followed by the Tukey multiple comparisons procedure was used for statistical analysis. Cont, Control.
Discussion
Although circumstantial and laboratory evidence point to a major role for E2 in regulating the levels of its target receptor ERα in the endometrium, the underlying transcriptional mechanism remained elusive. Here, we reveal for the first time a significant role for ERβ in regulation of ERα gene expression in endometrial and endometriotic stromal cells. These findings are particularly relevant to the pathology of endometriosis because multiple investigators have reported significantly elevated ERβ levels and decreased ERα levels in stromal cells and endometriotic tissue when compared with eutopic endometrium (7,24). Indeed, ERβ levels in endometriotic tissue and stromal cells are about 36 times higher than those in normal endometrium or its stromal cells (7), suggesting that the biological influence of estrogen on endometriosis is mediated at least partially through ERβ. We hypothesized that a primary consequence of strikingly high levels of ERβ in endometriotic stromal cells is suppression of transcription of the ERα gene via its alternatively used promoters.
Recently reported biological functions of ERβ in the regulation of inflammatory processes in autoimmune diseases and in endometriosis further support a key role for severely increased ERβ levels in endometriotic tissue (25,26,27). An ERβ-selective analog, ERB-041, has been shown to reduce the size and number of endometriotic implants in a rodent model (27). This analog has been shown to be inactive on classic estrogenic targets, such as the uterus, mammary gland, and bone, but demonstrated potent antiinflammatory activity in two in vivo models: the HLA-B27 transgenic rat, and Lewis rat adjuvant-induced arthritis.
Differences in the ERα:ERβ ratio in endometriotic and endometrial stromal cells could have important functional implications because these ERs have different ligand-binding characteristics (28,29). It also has been proposed that heterodimers of ERα and ERβ can associate with estrogen-responsive elements in vitro (30). Therefore, it is conceivable that the regulation of estrogen target genes varies significantly in endometriotic vs. endometrial stromal cells.
An interesting finding was that ERβ interacted with regulatory sequences in the ERα promoters primarily in a nonclassic fashion, because only one ERβ binding site out of four motifs identified contained a classic ERE. Our findings are supported by studies showing that ERβ is recruited to AP1 and Sp1 sites at other promoters (31,32). Moreover, recent publications have shown that far distant ER-binding sites, even in other chromosomes, may regulate the expression of certain genes (33,34,35).
Another interesting finding is that ERα levels were regulated by E2 and ERβ primarily via promoter A in endometrial cells and via both promoters A and C in endometriotic cells (Figs. 1 and 4). The mechanism for this partial switch in promoter use may be due to extremely high ERβ levels in endometriosis. This is the first demonstration of promoter switch for ERα gene regulation in a normal tissue compared with its disease state. We do not know whether this molecular abnormality also exists in endometrial tissue of women with endometriosis because this tissue was shown to have significant molecular abnormalities compared with endometrium of disease-free women (2).
We observed a time-dependent effect of E2 on the ERα gene expression in nontransfected endometrial and endometriotic stromal cells (Fig. 1, C and D). During the knockdown and overexpression experiments, however, we have not observed a significant E2 effect on ER-α regulation. Most likely, the addition of the siRNAs, overexpression plasmids, and transfection reagents needed to knockdown or overexpress ERβ altered the cell biology and its ability to respond to E2 in culture.
Although ERβ regulation of ERα gene expression has not been extensively studied, our data are consistent with several previous reports. Hall and McDonnell (21) determined that ERβ suppresses ERα activity and that coexpression of ERβ results in suppression of both the efficacy and the potency of E2-stimulated responses in several human cell lines. Relative expression levels of the two ER subtypes constitute an important determinant of the target genes regulated by estrogens and antiestrogens (21). Additionally, ERβ has been reported to affect the gene network regulated by ERα in breast cancer cells, and ERα down-regulation was noted in the presence of high ERβ levels in MCF-7 cells (36).
In mice with selective disruption of ERα, the effect of estrogen on the uterus is ablated (37). In contrast, ERβ knockout mice did not display an apparent uterine phenotype (37,38). In humans, however, the roles of ERα and ERβ on proliferation and apoptosis in endometrium and endometriosis are not well known. For example, the relationship between ER levels and proliferation in endometriosis remains poorly understood. Beliard et al. (39) reported a positive correlation between ER levels and proliferation in eutopic endometrium. However, the authors did not differentiate between ERα and ERβ. Because the levels of ERβ are barely detectable in eutopic endometrium, this finding probably describes ERα regulation of cell cycle in eutopic endometrium. The same study reported no correlation between the ER levels and proliferation in endometriosis; however, this finding needs further clarification because ERα and ERβ are both present in endometriosis and might differentially affect cell cycle and apoptosis. Another study showed that adenovirus-delivery of dominant negative ER genes inhibits cell proliferation and induces apoptosis in endometriotic stromal cells (40). Recently, ERβ was shown to interact with cell cycle spindle assembly checkpoint protein, MAD2 (17). This suggests potential importance of ERβ in cell cycle regulation and supports a function of ERβ distinct from the established role of ERs as transcription factors. We have demonstrated that E2 treatment in the presence of ERβ knockdown results in decreased proliferation. Further studies are needed in this area, but our findings suggest that ERβ present in high levels in endometriotic stromal cells might stimulate progression through the cycle and increase the rate of proliferation and growth of this pathological tissue in vivo.
In summary, this is the first report to demonstrate that ERβ acts as a suppressor of ERα in both endometrial and endometriotic stromal cells via binding to classical and nonclassical cis-regulatory elements in specific promoters of the ERα gene. We presented evidence that ERα expression and response to E2 is regulated through its three major promoters—A, B, and C. ERβ not only regulates ERα in endometrial and endometriotic stromal cells but also affects the cell cycle in endometriotic stromal cells and might contribute to proliferation in this pathological condition in vivo.
These findings may have several clinical applications. In eutopic endometrium, ERα stimulates PR production. Thus, ERβ, acting as ERα suppressor, might contribute to decreased PR levels and to progesterone resistance that is observed in endometriosis. New drugs that regulate ERβ action to preserve the normal ERα:ERβ ratio may prevent progesterone resistance and may be useful as potential therapeutic agents for endometriosis.
Footnotes
This work was supported by National Institutes of Health Grants HD38691 and HD40093 and by the Friends of Prentice (to S.E.B.).
Disclosure Statement: All other authors have nothing to disclose.
First Published Online November 11, 2008
Abbreviations: AP1, Activator protein 1; ChIP assay, chromatin immunoprecipitation-PCR assay; E2, estradiol; ER, estrogen receptor; ERE, estrogen response element; PR, progesterone receptor; siRNA, small interfering RNA; Sp1, specificity protein 1.
References
- Eskenazi B, Warner ML 1997 Epidemiology of endometriosis. Obstet Gynecol Clin North Am 24:235–258 [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC 2004 Endometriosis. Lancet 364:1789–1799 [DOI] [PubMed] [Google Scholar]
- Thomas EJ 1995 Endometriosis, 1995–confusion or sense? Int J Gynaecol Obstet 48:149–155 [DOI] [PubMed] [Google Scholar]
- Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE 2000 Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab 85:2897–2902 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J 2006 Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol 248:94–103 [DOI] [PubMed] [Google Scholar]
- Brandenberger AW, Lebovic DI, Tee MK, Ryan IP, Tseng JF, Jaffe RB, Taylor RN 1999 Oestrogen receptor (ER)-α and ER-β isoforms in normal endometrial and endometriosis-derived stromal cells. Mol Hum Reprod 5:651–655 [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE 2007 Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod 77:681–687 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA 1996 Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R 1996 ER β: identification and characterization of a novel human estrogen receptor. FEBS Lett 392:49–53 [DOI] [PubMed] [Google Scholar]
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P 1986 Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 320:134–139 [DOI] [PubMed] [Google Scholar]
- Grandien K 1996 Determination of transcription start sites in the human estrogen receptor gene and identification of a novel, tissue-specific, estrogen receptor-mRNA isoform. Mol Cell Endocrinol 116:207–212 [DOI] [PubMed] [Google Scholar]
- Donaghue C, Westley BR, May FE 1999 Selective promoter usage of the human estrogen receptor-α gene and its regulation by estrogen. Mol Endocrinol 13:1934–1950 [DOI] [PubMed] [Google Scholar]
- Grandien K, Backdahl M, Ljunggren O, Gustafsson JA, Berkenstam A 1995 Estrogen target tissue determines alternative promoter utilization of the human estrogen receptor gene in osteoblasts and tumor cell lines. Endocrinology 136:2223–2229 [DOI] [PubMed] [Google Scholar]
- Grandien KF, Berkenstam A, Nilsson S, Gustafsson JA 1993 Localization of DNase I hypersensitive sites in the human oestrogen receptor gene correlates with the transcriptional activity of two differentially used promoters. J Mol Endocrinol 10:269–277 [DOI] [PubMed] [Google Scholar]
- Bergman MD, Schachter BS, Karelus K, Combatsiaris EP, Garcia T, Nelson JF 1992 Up-regulation of the uterine estrogen receptor and its messenger ribonucleic acid during the mouse estrous cycle: the role of estradiol. Endocrinology 130:1923–1930 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S 2005 Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev 57:359–383 [DOI] [PubMed] [Google Scholar]
- Poelzl G, Kasai Y, Mochizuki N, Shaul PW, Brown M, Mendelsohn ME 2000 Specific association of estrogen receptor β with the cell cycle spindle assembly checkpoint protein, MAD2. Proc Natl Acad Sci USA 97:2836–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE 1997 Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab 82:600–606 [DOI] [PubMed] [Google Scholar]
- Ryan IP, Schriock ED, Taylor RN 1994 Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab 78:642–649 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP 1999 The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 [DOI] [PubMed] [Google Scholar]
- Deb S, Zhou J, Amin SA, Imir AG, Yilmaz MB, Lin Z, Bulun SE 2006 A novel role of sodium butyrate in the regulation of cancer-associated aromatase promoters I. 3 and II by disrupting a transcriptional complex in breast adipose fibroblasts. J Biol Chem 281:2585–2597 [DOI] [PubMed] [Google Scholar]
- Li X, Darzynkiewicz Z 1995 Labelling DNA strand breaks with BrdUTP. Detection of apoptosis and cell proliferation. Cell Prolif 28:571–579 [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Chai D, Langoi D, Bulun SE 2003 Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil Steril 80(Suppl 2):820–827 [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA 2007 Estrogen receptor-β: recent lessons from in vivo studies. Mol Endocrinol 21:1–13 [DOI] [PubMed] [Google Scholar]
- Harris HA, Bruner-Tran KL, Zhang X, Osteen KG, Lyttle CR 2005 A selective estrogen receptor-β agonist causes lesion regression in an experimentally induced model of endometriosis. Hum Reprod 20:936–941 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA 1997 Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138:863–870 [DOI] [PubMed] [Google Scholar]
- Tong W, Perkins R, Xing L, Welsh WJ, Sheehan DM 1997 QSAR models for binding of estrogenic compounds to estrogen receptor α and β subtypes. Endocrinology 138:4022–4025 [DOI] [PubMed] [Google Scholar]
- Cowley SM, Hoare S, Mosselman S, Parker MG 1997 Estrogen receptors α and β form heterodimers on DNA. J Biol Chem 272:19858–19862 [DOI] [PubMed] [Google Scholar]
- Pearce ST, Jordan VC 2004 The biological role of estrogen receptors α and β in cancer. Crit Rev Oncol Hematol 50:3–22 [DOI] [PubMed] [Google Scholar]
- Gustafsson JA 2000 An update on estrogen receptors. Semin Perinatol 24:66–69 [DOI] [PubMed] [Google Scholar]
- Nunez E, Kwon YS, Hutt KR, Hu Q, Cardamone MD, Ohgi KA, Garcia-Bassets I, Rose DW, Glass CK, Rosenfeld MG, Fu XD 2008 Nuclear receptor-enhanced transcription requires motor- and LSD1-dependent gene networking in interchromatin granules. Cell 132:996–1010 [DOI] [PubMed] [Google Scholar]
- Lin Z, Reierstad S, Huang CC, Bulun SE 2007 Novel estrogen receptor-α binding sites and estradiol target genes identified by chromatin immunoprecipitation cloning in breast cancer. Cancer Res 67:5017–5024 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M 2006 Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- Chang EC, Frasor J, Komm B, Katzenellenbogen BS 2006 Impact of estrogen receptor β on gene networks regulated by estrogen receptor α in breast cancer cells. Endocrinology 147:4831–4842 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada-Hiraike O, Hiraike H, Okinaga H, Imamov O, Barros RP, Morani A, Omoto Y, Warner M, Gustafsson JA 2006 Role of estrogen receptor β in uterine stroma and epithelium: insights from estrogen receptor β−/− mice. Proc Natl Acad Sci USA 103:18350–18355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliard A, Noel A, Foidart JM 2004 Reduction of apoptosis and proliferation in endometriosis. Fertil Steril 82:80–85 [DOI] [PubMed] [Google Scholar]
- Othman EE, Salama S, Ismail N, Al-Hendy A 2007 Toward gene therapy of endometriosis: adenovirus-mediated delivery of dominant negative estrogen receptor genes inhibits cell proliferation, reduces cytokine production, and induces apoptosis of endometriotic cells. Fertil Steril 88:462–471 [DOI] [PubMed] [Google Scholar]