Abstract
Background: Studies comparing the use of basal bolus with insulin analogs vs. split-mixed regimens with human insulins in hospitalized patients with type 2 diabetes are lacking.
Research Design and Methods: In a controlled multicenter trial, we randomized 130 nonsurgical patients with blood glucose (BG) between 140 and 400 mg/dl to receive detemir once daily and aspart before meals (n = 67) or neutral protamine Hagedorn (NPH) and regular insulin twice daily (n = 63). Insulin dose was started at 0.4 U/kg · d for BG between 140 and 200 mg/dl or 0.5 U/kg · d for BG 201-400 mg/dl. Major study outcomes included differences in mean daily BG levels and frequency of hypoglycemic events between treatment groups.
Results: Glycemic control improved similarly in both groups from a mean daily BG of 228 ± 54 and 223 ± 58 mg/dl (P = 0.61) to a mean daily BG level after the first day of 160 ± 38 and 158 ± 51 mg/dl in the detemir/aspart and NPH/regular insulin groups, respectively (P = 0.80). A BG target below 140 mg/dl before meals was achieved in 45% of patients in the detemir/aspart group and 48% in the NPH/regular group (P = 0.86). During treatment, 22 patients (32.8%) in the detemir/aspart group and 16 patients (25.4%) in the NPH/regular group had at least one episode of hypoglycemia (BG <60 mg/dl) during the hospital stay (P = 0.34).
Conclusions: Treatment with basal/bolus regimen with detemir once daily and aspart before meals results in equivalent glycemic control and no differences in the frequency of hypoglycemia compared to a split-mixed regimen of NPH and regular insulin in patients with type 2 diabetes.
The investigation of in-patient hyperglycemia management shows that detemir daily with mealtime aspart offers equivalent glycemic control compared to neutral protamine Hagedorn and regular insulin in patients with type 2 diabetes.
Increasing evidence from observational studies (1,2,3,4) and a recent meta-analysis (5) indicate that hyperglycemia is associated with an increased risk of complications and mortality in hospitalized patients. Among critically ill patients, prospective randomized trials have shown that improved glycemic control reduces short- and long-term mortality, rates of multiorgan failure and systemic infections, and length of hospitalization (6,7,8). Similarly, observational studies in diabetic subjects admitted to general medical and surgical areas have also shown that poor glycemic control is a predictor of poor clinical outcome. In such patients, the presence of hyperglycemia is associated with prolonged hospital stay, infection, disability after hospital discharge, and death (1,4,9).
Hyperglycemia is commonly not well addressed in patients with diabetes admitted to general medicine wards (1,10,11,12). In the presence of altered nutrition and associated medical illness, physicians frequently discontinue their patient’s previous outpatient antidiabetic regimen and initiate sliding-scale insulin coverage with short-acting insulins, a practice associated with limited therapeutic success mainly because of the lack of basal insulin coverage (13,14,15). The use of a split-mixed insulin regimen of intermediate neutral protamine Hagedorn (NPH) and regular insulin is usually delayed due to fear of hypoglycemia (16,17). The inadequate duration of action of NPH insulin and an undesirable peak activity at 4–6 h after injection (16), as well as the high day-to-day variability in absorption (17), have been proposed as barriers to achieving appropriate targets of blood glucose (BG) control in patients with diabetes. In recent years, the basal insulin analogs glargine and detemir have been developed to overcome some of the barriers to insulin initiation, including concerns over hypoglycemia. The combination of a long-acting basal and a rapid-acting prandial insulin has been recommended as a more physiological approach to glucose control in the hospital (9,18). We recently reported that a basal/bolus insulin algorithm is a simple and more effective intervention than a sliding-scale regular insulin for glucose control in hospitalized patients with type 2 diabetes (19). The use of a basal/bolus regimen, however, has not been compared with an NPH/regular insulin regimen in the inpatient setting. Accordingly, we compared the efficacy and safety of a basal/bolus regimen of detemir once daily plus aspart insulin before meals to an NPH and regular insulin twice daily regimen in patients with type 2 diabetes mellitus in general medicine services.
Patients and Methods
In this multicenter, prospective, open-label randomized study, we enrolled 130 nonsurgical patients 18–80 yr old that were admitted to general medicine wards with BG levels between 140 and 400 mg/dl. Further inclusion criteria included a known history of type 2 diabetes for longer than 3 months, treatment with diet alone, any combination of oral antidiabetic agents and/or insulin before admission, and the absence of diabetic ketoacidosis (20). Exclusion criteria included subjects with hyperglycemia without a known history of diabetes, intensive care unit (ICU) patients, subjects expected to undergo surgery during the hospitalization course, patients with clinically relevant hepatic disease or impaired renal function (serum creatinine ≥3.0 mg/dl), pregnancy, and any mental condition rendering the subject unable to give informed consent.
This study was conducted at Grady Memorial Hospital in Atlanta, Georgia, and at Rush University Medical Center in Chicago, Illinois. The institutional review boards at Emory University and Rush University approved the study protocol. Informed consent was obtained from all subjects after explanation and understanding of the nature, purpose, and potential risks of the study. Insulin management was directed by the specific assigned protocol and was carried out daily by members of the internal medicine residency program, a research fellow, or a research nurse practitioner. The primary care teams decided on the treatment for all other medical problem(s) for which patients were admitted. No follow-up visit after discharge is included in this study. Patients were randomly assigned to receive either a basal/bolus regimen with detemir and aspart or a split-mixed regimen with NPH and regular insulin. All oral antidiabetic drugs were discontinued on admission. Subjects receiving insulin therapy before admission initially received the same total daily dose (TDD) unit for unit as their outpatient regimen. Subjects not treated with insulin before admission were started at a TDD of 0.4 U/kg · d for a BG between 140 and 200 mg/dl or 0.5 U/kg · d for a BG between 201 and 400 mg/dl based on the first BG obtained on the general medical floor after consent.
Patients treated with detemir/aspart received 50% of TDD as detemir and 50% as aspart insulin. Detemir was given (using the Flex-Pen) once daily at the same time of the day. Aspart was given (using the Flex-Pen) in three equally divided doses with each meal. To prevent hypoglycemia, if a subject was not able to eat a given meal, the dose of aspart was held. Insulin dosage was adjusted daily according to BG values. If the fasting and predinner BG were between 140 and 180 mg/dl in the absence of hypoglycemia, the dose of insulin detemir was increased by 10% every day. If the fasting and predinner BG were above 180 mg/dl in the absence of hypoglycemia, the insulin detemir dose was increased by 20% every day. If a patient developed hypoglycemia (BG <60 mg/dl), the dose of insulin detemir was decreased by 20%. Scheduled mealtime insulin aspart was supplemented for BG greater than 140 mg/dl using the supplemental insulin protocol (Table 1).
Table 1.
Insulin treatment protocols
| 1. Detemir plus aspart insulin treatment orders | |||
| A. Insulin-treated patients | |||
| Give total outpatient insulin daily dose, one half as detemir and one half as aspart insulin. | |||
| Detemir is given once daily, at the same time of the day. | |||
| Aspart is given in three equally divided doses before each meal. | |||
| To prevent hypoglycemia, if a subject is not able to eat, hold dose of aspart insulin. | |||
| B. No insulin-treated patients (diet or oral agents) | |||
| Hold oral antidiabetic drugs on admission. | |||
| Starting total daily insulin dose: | |||
| BG between 140 and 200 mg/dl: 0.4 U/kg · d | |||
| BG between 201 and 400 mg/dl: 0.5 U/kg · d | |||
| Give half of total insulin dose as detemir and half as aspart insulin. | |||
| Detemir is given once daily, at the same time of the day. | |||
| Aspart is given in three equally divided doses before each meal. | |||
| To prevent hypoglycemia, if a subject is not able to eat, hold dose of aspart insulin. | |||
| C. Insulin adjustment | |||
| If premeal BG <140 mg/dl in the absence of hypoglycemia: no change. | |||
| If premeal BG between 140 and 180 mg/dl: increase detemir insulin dose by 10%. | |||
| If premeal BG >180 mg/dl: increase detemir insulin dose by 20%. | |||
| If BG <60 mg/dl, decrease insulin detemir daily dose by 20%. | |||
| D. Supplemental insulin | |||
| Give aspart insulin after the supplemental insulin scale before each meal and at bedtime if able to eat, or every 6 h if not able to eat. | |||
| 2. NPH plus regular insulin treatment orders | |||
| A. Insulin-treated patients | |||
| Total outpatient insulin daily dose to be given two thirds in the morning and one third in the evening. | |||
| Give morning and evening dose as two thirds NPH and one third regular insulin. | |||
| To prevent hypoglycemia, if a subject is not able to eat, hold dose of regular insulin. | |||
| B. No insulin-treated patients (diet or oral agents) | |||
| Hold oral antidiabetic drugs on admission. | |||
| Starting total daily insulin dose: | |||
| BG between 140 and 200 mg/dl: 0.4 U/kg · d | |||
| BG between 201 and 400 mg/dl: 0.5 U/kg · d | |||
| Give two thirds of total insulin dose in the morning and one third in the evening. | |||
| Give morning and evening dose as two thirds NPH and one third regular insulin. | |||
| To prevent hypoglycemia, if a subject is not able to eat, hold dose of regular insulin. | |||
| C. Insulin adjustment | |||
| If premeal BG <140 mg/dl in the absence of hypoglycemia: no change. | |||
| If premeal BG between 140 and 180 mg/dl: increase NPH insulin dose by 10%. | |||
| If premeal BG >180 mg/dl: increase daily NPH insulin dose by 20%. | |||
| If BG <60 mg/dl: decrease NPH insulin dose by 20%. | |||
| D. Supplemental insulin | |||
| Give short-acting insulin after the supplemental insulin scale before each meal and at bedtime if able to eat, or every 6 h if not able to eat. | |||
| 3. Supplemental insulin protocol | |||
| Before meal: supplemental scale insulin (no. of units)—add to scheduled insulin dose. | |||
| Bedtime: give half of supplemental insulin dose. | |||
| BG (mg/dl)
|
Insulin sensitive
|
Usual
|
Insulin resistant
|
| ≥141–180 | 2 | 4 | 6 |
| 181–220 | 4 | 6 | 8 |
| 221–260 | 6 | 8 | 10 |
| 261–300 | 8 | 10 | 12 |
| 301–350 | 10 | 12 | 14 |
| 351–400 | 12 | 14 | 16 |
| >400 | 14 | 16 | 18 |
Patients treated with NPH/regular insulin received two thirds of TDD before breakfast and one third of TDD before dinner. The insulin dose was given as two thirds NPH and one third regular insulin (using vials and syringes) in the morning with breakfast, and two thirds NPH and one third regular insulin in the evening with dinner. To prevent hypoglycemia, if a subject was not able to eat a given meal, the dose of regular insulin was held. If a patient received nothing by mouth all day, the morning dose of NPH was reduced by 50%. Insulin dosage was adjusted daily according to BG values. If the fasting and predinner BG were between 140 and 180 mg/dl in the absence of hypoglycemia, the dose of NPH insulin was increased by 10% every day. If the fasting and predinner BG were above 180 mg/dl in the absence of hypoglycemia, the dose of NPH insulin was increased by 20% every day. If a patient developed hypoglycemia (BG <60 mg/dl), NPH dose was decreased by 20%. Supplemental regular insulin was given in addition to scheduled regular insulin for BG above 140 mg/dl using a supplemental insulin protocol (Table 1).
BG was measured before each meal and at bedtime (or every 6 h if a patient was not eating) using a point of care glucose meter. In addition, BG was measured at any time if a patient experienced symptoms of hypoglycemia or if requested by the treating physician. Hemoglobin A1C was measured on the first day of hospitalization. The results of BG values are presented as premeal glucose, bedtime glucose, and mean daily BG during the hospital stay after d 1.
The goal of insulin therapy was to maintain premeal and bedtime BG levels lower than 140 mg/dl while avoiding hypoglycemia. The primary outcome of the study was to determine differences in glycemic control between treatment groups as measured by mean daily BG concentration during the hospital stay. Secondary outcomes include differences between treatment groups in premeal and bedtime glucose levels, number of hypoglycemic events, length of hospital stay, and mortality rate. Hypoglycemic episodes were classified as major (BG <40 mg/dl or associated with impaired mental status or loss of consciousness), or minor (BG between 40 and 59 mg/dl) events.
Statistical analysis
Power calculation was conducted based on the data from our previous Rabbit-2 study (19), from which we hypothesized a mean daily BG difference greater than 30 mg/dl between basal bolus with insulin analogs vs. split-mixed regimens with human insulins in hospitalized patients with type 2 diabetes. Assuming a within-group sd of 40 mg/dl and α error rate of 5%, we estimated that 65 subjects per group (130 total) will be needed to achieve 80% power.
Statistical analysis was performed using the SPSS software package (SPSS Inc., Chicago, IL). Cross-sectional comparisons between groups were carried out using two-sample t-tests. Multiple comparisons across different days on therapy were adjusted conservatively by using Tukey’s adjustment.
A P value <0.05 was considered significant.
Results
A total of 130 subjects with type 2 diabetes were recruited. After randomization, 67 patients received insulin detemir once daily and insulin aspart before meals, and 63 patients received split-mixed regimen with NPH and regular insulin twice daily. The clinical and demographic characteristics of the treatment groups were similar at randomization (Table 2). The most common admitting illnesses included a variety of cardiovascular (35%), infectious (15%), pulmonary (12%), and gastrointestinal (14%) conditions. The mean hospital length of stay was 6.5 ± 7 d in patients treated with detemir plus aspart and 7.1 ± 7 d in the NPH plus regular group [P = not significant (NS)]. There was no mortality reported in this trial.
Table 2.
Characteristics of study subjects
| NPH/regular | Detemir/aspart | |
|---|---|---|
| No. of patients | 63 | 67 |
| Age (yr) | 58 ± 10 | 59 ± 10 |
| Gender (males/females) (n) | 30/33 | 30/37 |
| Race (B/W/H) | 39/12/12 | 44/14/9 |
| Body weight (kg) | 99 ± 29 | 91 ± 25 |
| Body mass index (kg/m2) | 34 ± 13 | 33 ± 8 |
| Diabetes treatment prior to admission, n (%) | ||
| Insulin | 24 (38) | 33 (49) |
| Insulin plus oral agents | 10 (16) | 6 (9) |
| Oral agent monotherapy | 14 (22) | 13 (19) |
| Multiple oral agents | 8 (13) | 10 (15) |
| No pharmacological agents | 7 (11) | 5 (8) |
| BG on admission (mg/dl) | 231 ± 145 | 230 ± 102 |
| BG prior treatment (mg/dl) | 223 ± 58 | 228 ± 54 |
| Hemoglobin A1C (%) | 8.1 ± 2 | 8.5 ± 2 |
| Creatinine (mg/dl) | 1.1 ± 0.3 | 1.2 ± 0.3 |
Data are means ± sd. To convert the values for BG from mg/dl to millimoles/liter, multiply by 0.05551. B, Black; W, White; H, Hispanic.
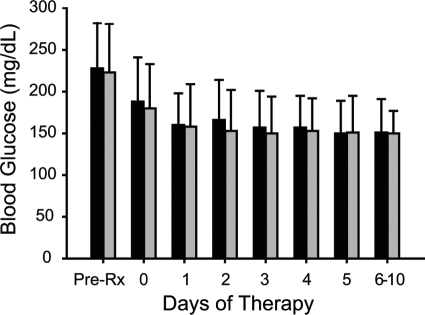
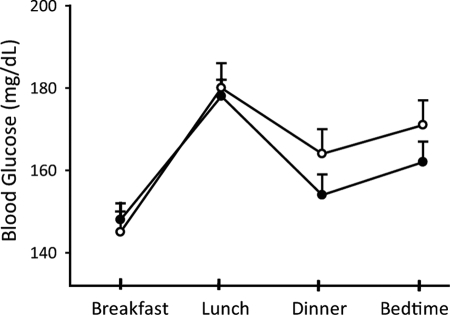
The mean admission, pretreatment, and mean daily BG levels were similar between treatment groups. The mean admission glucose levels in the detemir/aspart and NPH/regular insulin treatment groups were 230 ± 102 and 231 ± 145 mg/dl, respectively (P = NS). BG was 228 ± 54 mg/dl before starting insulin therapy in the detemir/aspart group and 223 ± 58 mg/dl in the NPH/regular group (P = NS). The mean BG level after the first day of therapy was 160 ± 38 mg/dl in the detemir/aspart group and 158 ± 51 mg/dl in the NPH/regular group (P = NS). The mean daily BG levels are shown for both groups in Fig. 1. The BG target of less than 140 mg/dl before meals was achieved in 45% of the detemir/aspart group and in 48% of the NPH/regular insulin group (P = NS). BG levels were similar at prebreakfast, predinner, and bedtime times but were higher before lunch in both treatment groups (Fig. 2). Differences in mean BG from morning to evening values in the detemir/aspart group (31 ± 80 mg/dl) were higher but not significantly different than in the NPH/regular group (15 ± 74 mg/dl) group (P = NS).
Figure 1.
Changes in mean daily BG concentration in patients treated with a basal/bolus regimen with detemir once daily and aspart before meals (open bars) and with split-mixed regimen with NPH and regular insulin twice daily (closed bars). Day 0, Randomization day. Depending on the time of admission, during the randomization day a patient could have received one or two doses of NPH/regular insulin or one dose of detemir and one to three doses of aspart insulin per day. Data are means ± sd.
Figure 2.
Mean BG concentration before breakfast, lunch, dinner, and bedtime in detemir/aspart group (open circles) and NPH/regular insulin (closed circles). Differences between treatment groups were not statistically significant. Data are means ± se.
The mean total daily insulin dose was not significantly higher in the detemir/aspart group (57 ± 45 U) compared with the NPH/regular insulin group (45 ± 32 U) (P = 0.08). The mean daily dose of detemir insulin given once daily (30 ± 28 U) was similar to the TDD of NPH insulin (27 ± 20 U) given twice daily. The TDD of aspart (27 ± 20 U) insulin given before meals was higher than the dose of regular insulin (18 ± 14 U) that was given twice daily (P < 0.05).
Nineteen patients (28.4%) in the detemir/aspart group had a BG between 40 and 59 mg/dl, and three patients (4.5%) had a BG below 40 mg/dl. Fifteen patients in the NPH/regular group (23.8%) had a BG of 40–59 mg/dl, and one patient (1.6%) had a BG below 40 mg/dl (P = 0.20 vs. detemir/aspart group). A total of 1090 glucose readings were performed in the detemir/aspart group; of them, 23 readings (2.1%) were 40–59 mg/dl, and only three readings (0.3%) were below 40 mg/dl. Of the 1021 BG readings in the NPH/regular insulin group, 19 (1.9%) were 40–59 mg/dl, and only two readings (0.2%) were below 40 mg/dl (P = 0.86 vs. detemir/aspart group). The frequency of hypoglycemic events in patients treated with insulin or with insulin plus oral agents was higher than in patients not treated with insulin before admission. Twenty-five of 73 patients (37.3%) treated with insulin before admission had at least one episode of BG below 60 mg/dl compared with 14 (24.6%) subjects treated without insulin (P = 0.32). There was no significant difference in the time of day of hypoglycemic events between groups. None of the episodes of hypoglycemia in either group were associated with loss of consciousness or seizure.
Discussion
This is the first prospective randomized multicenter trial that compared the efficacy and safety of a basal/bolus insulin regimen with detemir once daily and aspart before meals to a standard split-mixed regimen of NPH and regular insulin in non-critically ill patients with type 2 diabetes. Both treatment regimens resulted in a rapid and significant improvement in glycemic control throughout the hospital stay. Starting at a similar BG concentration of approximately 225 mg/dl, the mean BG level after the first day of hospital stay was 160 ± 38 mg/dl in the detemir/aspart group and 158 ± 51 mg/dl in NPH/regular insulin group. However, a BG target below 140 mg/dl before meals was achieved in less than half of patients in both treatment regimens, and approximately 30% of patients experienced one or more episodes of hypoglycemia during the hospital stay. These results indicate that the basal/bolus regimen with detemir once daily and aspart before meals results in equivalent glycemic control with no significant differences in the frequency of hypoglycemia compared with a twice daily split-mixed human insulin regimen with NPH and regular insulin in patients with type 2 diabetes.
Hyperglycemia in hospitalized patients is a common, serious, and costly health care problem. Evidence from observational and interventional studies indicates that hyperglycemia in critical and noncritical illness is associated with an increased risk of complications and mortality (1,9,21,22,23,24,25,26). Despite the evidence that inpatient glycemic control is important and despite guidelines recommending tighter inpatient glycemic control, clinical practice has been slow to change (27). The University Health System Consortium (UHC) benchmarking project (28), an alliance of 90 academic health centers across the United States, showed widespread gaps in processes and outcomes regarding diabetes care. In the non-ICU setting, sc insulin therapy was prescribed in 45% of patients, with a range of 12 to 77% across measured hospitals. In such patients, only 26% of patients had all BG measurements no greater than 180 mg/dl on the second measurement day, with a range of 7 to 48%. Other studies have also provided insight into gaps in diabetes care. Schnipper et al. (11) examined practices on the general medicine service of an academic medical center in Boston in 2004. Among patients with a known diagnosis of diabetes or at least one glucose reading above 200 mg/dl, they found that scheduled long-acting insulin was prescribed in only 43% of patients, and 90% of patients remained on the same sliding-scale insulin regimen despite widely varying insulin requirements. A study by Cook et al. (29) reported that among non-ICU patients, 20% of patients had persistent hyperglycemia (defined as a mean BG >200 mg/dl), and 46% of such patients did not have their insulin regimen intensified, a concept that they termed “negative therapeutic momentum.” Concern about hypoglycemia due to altered nutrition and the presence of associated medical illness is reported as the leading limiting factor in improving glycemic control in patients with diabetes (9,30). In a 3-month prospective review of consecutive medical records in 2174 hospitalized patients receiving antidiabetic agents, 206 (9.5%) experienced a total of 484 hypoglycemic episodes (31). In a noncritical care setting, factors that increase the risk of hypoglycemia include treatment with insulin and sulfonylureas, interruption of carbohydrate intake, lack of coordination between feeding and medication administration, insufficient frequency in BG monitoring, and failure to recognize changes in insulin requirements due to advanced age, renal failure, and liver disease. Minimizing the rate of hypoglycemia events is of major importance in hospitalized patients because it has been shown to be an independent risk factor of poor clinical outcome (32,33). In the present study, one fourth of the patients in both treatment groups had a BG between 40 and 59 mg/dl and 3% had a BG below 40 mg/dl during the hospital stay. The American Diabetes Association recently recommended a level below 70 mg/dl to define hypoglycemia (34). Using this definition, we observed that 24 patients (38%) in the NPH/regular insulin group and 29 patients (43%) in the detemir/aspart group had at least one episode of hypoglycemia (P = 0.67) during the hospital stay.
We recently reported the results of the Rabbit-2 trial, a prospective, randomized multicenter study comparing the efficacy and safety of a basal/bolus insulin regimen with glargine once daily and glulisine before meals and a sliding-scale regular insulin four times daily in patients with type 2 diabetes in general medicine services (19). We reported that among 130 insulin-naive patients with a mean admission BG of 229 ± 6 mg/dl and hemoglobin A1C of 8.8 ± 2%, the use of basal/bolus insulin was superior in improving BG control to a sliding-scale regimen alone. A BG target below 140 mg/dl was achieved in 66% of patients in the glargine plus glulisine group and 38% in the sliding-scale group. Only two patients (3%) in the glargine/glulisine group and in the sliding-scale regimen developed a BG below 60 mg/dl, and no patients had a BG below 40 mg/dl during the hospital stay. In this study, the frequency of hypoglycemia with detemir/aspart and NPH/regular insulin was higher than that reported with the use of glargine and glulisine in the Rabbit-2 trial. This could be explained in part by the fact that the Rabbit trial included insulin-naive patients, whereas 56% of subjects in this trial were treated with insulin before admission. In this study, about 25% of insulin-naive patients and 37% of those treated with insulin before admission developed one or more episodes of hypoglycemia during the hospital stay.
Several outpatient studies have shown that basal/bolus insulin regimens facilitate glycemic control with lower rates of hypoglycemic events than NPH/regular insulin (35,36,37,38). It is hypothesized that the use of a long-lasting basal insulin and rapid-acting mealtime insulin is more physiological and may represent a better alternative to the use of intermediate and short-acting insulin regimen in hospitalized subjects with type 2 diabetes (9,12,18,39). This study, however, failed to demonstrate differences in glycemic control between a basal/bolus regimen using detemir/aspart insulin and NPH/regular regimen in hospitalized subjects with type 2 diabetes. In this study, less than half of patients in either treatment group achieved a target BG below 140 mg/dl before meals. The relatively low rate of BG readings within target could be the result of a relatively high rate of hypoglycemic events, or in the case of detemir because the algorithm did not include an option for twice daily dosing of insulin detemir. Based on the reported pharmacodynamic profile of detemir (about 16-h duration, and a peak from 4–8 h), the use of detemir twice daily may have facilitated a more rapid insulin dose titration resulting in further improvements in glycemic control.
In summary, treatment with a basal/bolus regimen of detemir once daily and aspart before meals and a standard split-mixed regimen of NPH and regular insulin results in similar glycemic control and hypoglycemia outcomes in hospitalized patients with type 2 diabetes. Both regimens may be appropriate alternatives, and the choice depends on the physician’s preferences, hospital formulary options, cost considerations, and adequate nursing support.
Footnotes
This investigator-initiated study was supported by an unrestricted grant from Novo Nordisk Pharmaceuticals (Princeton, NJ). G.E.U. is supported by research a grant from the American Diabetes Association (7-03-CR-35), National Institutes of Health (NIH) Grant R03 DK073190-01, and General Clinical Research Center Grant M01 RR-00039. D.S. is supported by a research grant from the NIH (K12 RR-017643).
The sponsors of the study were not involved in the study design, data collection, analysis or interpretation of the results, or preparation of the manuscript.
Disclosure Statement: G.E.U. has received research support from Novo Nordisk and Sanofi-Aventis. He has received honoraria from Novo Nordisk and Sanofi-Aventis and is a member of the speakers’ bureau for Sanofi-Aventis. D.S. has received honoraria and lecture fees from Sanofi-Aventis. C.N. is a member of the speakers’ bureau and has received honoraria and lecture fees from Sanofi-Aventis. T.H. and D.B. have received grant support from Novo Nordisk. D.B. has received honoraria and lecture fees from Novo Nordisk and from Sanofi-Aventis. The other authors have nothing to disclose.
First Published Online November 18, 2008
Abbreviations: BG, Blood glucose; ICU, intensive care unit; NPH, neutral protamine Hagedorn; NS, not significant; TDD, total daily dose.
References
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE 2002 Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 87:978–982 [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Gerstein HC 2000 Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 355:773–778 [DOI] [PubMed] [Google Scholar]
- Estrada CA, Young JA, Nifong LW, Chitwood Jr WR 2003 Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting. Ann Thorac Surg 75:1392–1399 [DOI] [PubMed] [Google Scholar]
- Pomposelli JJ, Baxter 3rd JK, Babineau TJ, Pomfret EA, Driscoll DF, Forse RA, Bistrian BR 1998 Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr 22:77–81 [DOI] [PubMed] [Google Scholar]
- Kitabchi AE, Freire AX, Umpierrez GE 2008 Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients. Metabolism 57:116–120 [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R 2001 Intensive insulin therapy in the critically ill patients. N Engl J Med 345:1359–1367 [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC 2001 Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32:2426–2432 [DOI] [PubMed] [Google Scholar]
- Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, Fineberg SE, Tierney WR 2002 Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology 59:67–71 [DOI] [PubMed] [Google Scholar]
- Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, Hirsh IB 2004 Management of diabetes and hyperglycemia in hospitals. Diabetes Care 27:553–597 [DOI] [PubMed] [Google Scholar]
- Levetan CS, Magee MF 2000 Hospital management of diabetes. Endocrinol Metab Clin North Am 29:745–770 [DOI] [PubMed] [Google Scholar]
- Schnipper JL, Barsky EE, Shaykevich S, Fitzmaurice G, Pendergrass ML 2006 Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital. J Hosp Med 1:145–150 [DOI] [PubMed] [Google Scholar]
- Umpierrez GE, Maynard G 2006 Glycemic chaos (not glycemic control) still the rule for inpatient care. J Hosp Med 1:141–144 [DOI] [PubMed] [Google Scholar]
- Queale WS, Seidler AJ, Brancati FL 1997 Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med 157:545–552 [PubMed] [Google Scholar]
- Baldwin D, Villanueva G, McNutt R, Bhatnagar S 2005 Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care 28:1008–1011 [DOI] [PubMed] [Google Scholar]
- Umpierrez GE, Palacio A, Smiley D 2007 Sliding scale insulin use: myth or insanity? Am J Med 120:563–567 [DOI] [PubMed] [Google Scholar]
- Owens DR, Coates PA, Luzio SD, Tinbergen JP, Kurzhals R 2000 Pharmacokinetics of 125I-labeled insulin glargine (HOE 901) in healthy men: comparison with NPH insulin and the influence of different subcutaneous injection sites. Diabetes Care 23:813–819 [DOI] [PubMed] [Google Scholar]
- Heinemann L 2002 Variability of insulin absorption and insulin action. Diabetes Technol Ther 4:673–682 [DOI] [PubMed] [Google Scholar]
- Inzucchi SE 2006 Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med 355:1903–1911 [DOI] [PubMed] [Google Scholar]
- Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, Puig A, Mejia R 2007 Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 30:2181–2186 [DOI] [PubMed] [Google Scholar]
- Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA 2006 Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 29:2739–2748 [DOI] [PubMed] [Google Scholar]
- Finney SJ, Zekveld C, Elia A, Evans TW 2003 Glucose control and mortality in critically ill patients. JAMA 290:2041–2047 [DOI] [PubMed] [Google Scholar]
- Wahab NN, Cowden EA, Pearce NJ, Gardner MJ, Merry H, Cox JL 2002 Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol 40:1748–1754 [DOI] [PubMed] [Google Scholar]
- Norhammar AM, Ryden L, Malmberg K 1999 Admission plasma glucose. Independent risk factor for long-term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care 22:1827–1831 [DOI] [PubMed] [Google Scholar]
- Montori VM, Bistrian BR, McMahon MM 2002 Hyperglycemia in acutely ill patients. JAMA 288:2167–2169 [DOI] [PubMed] [Google Scholar]
- Stranders I, Diamant M, van Gelder RE, Spruijt HJ, Twisk JW, Heine RJ, Visser FC 2004 Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med 164:982–988 [DOI] [PubMed] [Google Scholar]
- Umpierrez GE, Kitabchi AE 2004 ICU care for patients with diabetes. Curr Opin Endocrinol 11:75–81 [Google Scholar]
- Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW 2007 Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care 30:367–369 [DOI] [PubMed] [Google Scholar]
- Baldwin D, Braithwaite S, Arnold P, Selig P, Brake H, Cuny J, Cerese J 2006 The University Health System Consortium (UHC) Benchmarking Project: evaluation of hospital glycemic control at academic medical centers (AMC). Diabetes 55(Suppl 1):104 [Google Scholar]
- Cook CB, Castro JC, Schmidt RE, Gauthier SM, Whitaker MD, Roust LR, Argueta R, Hull BP, Zimmerman RS 2007 Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum. J Hosp Med 2:203–211 [DOI] [PubMed] [Google Scholar]
- Ben-Ami H, Nagachandran P, Mendelson A, Edoute Y 1999 Drug-induced hypoglycemic coma in 102 diabetic patients. Arch Intern Med 159:281–284 [DOI] [PubMed] [Google Scholar]
- Varghese P, Gleason V, Sorokin R, Senholzi C, Jabbour S, Gottlieb JE 2007 Hypoglycemia in hospitalized patients treated with antihyperglycemic agents. J Hosp Med 2:234–240 [DOI] [PubMed] [Google Scholar]
- Krinsley JS, Grover A 2007 Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 35:2262–2267 [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P 2003 Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med 31:359–366 [DOI] [PubMed] [Google Scholar]
- 2005 Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 28:1245–1249 [DOI] [PubMed] [Google Scholar]
- Gerich JE 2004 Insulin glargine: long-acting basal insulin analog for improved metabolic control. Curr Med Res Opin 20:31–37 [DOI] [PubMed] [Google Scholar]
- Kiess W, Raile K, Galler A, Kapellen T 2004 Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes. Diabetes Care 27:2567–2568 [DOI] [PubMed] [Google Scholar]
- Hermansen K, Madsbad S, Perrild H, Kristensen A, Axelsen M 2001 Comparison of the soluble basal insulin analog insulin detemir with NPH insulin: a randomized open crossover trial in type 1 diabetic subjects on basal-bolus therapy. Diabetes Care 24:296–301 [DOI] [PubMed] [Google Scholar]
- Riddle MC, Rosenstock J, Gerich J 2003 The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 26:3080–3086 [DOI] [PubMed] [Google Scholar]
- Michota F 2007 What are the disadvantages of the sliding scale? J Hosp Medicine 2(Suppl 1):20–22 [DOI] [PubMed] [Google Scholar]