Abstract
Organic anion transporting polypeptide (Oatp) 1c1 is a high-affinity T4 transporter with narrow substrate specificity expressed at the blood-brain barrier. A transport model using cells overexpressing Oatp1c1 was created to identify novel Oatp1c1 substrates and inhibitors. Rat Oatp1c1 was cloned and stably expressed in human embryonic kidney 293 cells. Oatp1c1-transfected human embryonic kidney 293 cells transported 125I-labeled T4 in a time-dependent manner that was completely abolished in the presence of excess unlabeled T4. Next, various compounds, including inhibitors of thyroid hormone uptake, were screened for inhibitory effects on Oatp1c1-mediated T4 uptake. Phenytoin (64%), indocyanine green (17%), fenamic acid (68%), diclofenac (51%), and meclofenamic acid (33%) all reduced T4 uptake by Oatp1c1 when assayed at concentrations of 10 μM. Dose-response assays for the fenamic acids, iopanoic acid, indocyanine green, and phenytoin revealed IC50 values for Oatp1c1 T4 uptake below or near the blood plasma levels after therapeutic doses. Further kinetic assays and reciprocal plot analyses demonstrated that the fenamic acid diclofenac inhibited in a competitive manner. Finally, microvessels were isolated from adult rat brain and assessed for T4 uptake. Ten micromolar of fenamate concentrations inhibited T4 microvessel uptake with a similar hierarchical inhibition profile [fenamic acid (43%), diclofenac (78%), and meclofenamic acid (85%)], as observed for Oatp1c1 transfected cells. Oatp1c1 is expressed luminally and abluminally in the blood-brain barrier endothelial cell, and exhibits bidirectional transport capabilities. Together, these data suggest that Oatp1c1 transports fenamates into, and perhaps across, brain barrier cells.
The fenamate class of nonsteroidal anti-inflammatory drugs is transported by organic anion transporting polypeptides and competitively inhibits thyroxine transport in brain microvessels.
Organic anion transporting polypeptides (Oatps) are a large and multifaceted group of membrane-bound solute carriers mediating the transport of amphipathic organic substrates across cellular plasma membranes (1). Structurally, all rodent and other animal/human Oatps contain 12- transmembrane spanning domains, a superfamily signature of 13 amino acids and 11 extracellular cysteine residues (2). Substrates of these transporters include steroid conjugates, bile salts, organic dyes, thyroid hormones, and various drugs (3). Rodent and other animal/human Oatp transport mechanisms are sodium independent and likely accomplished through anion exchange (1). Currently, the driving force is unclear, but intracellular glutathione is a potential candidate (4). Many rodent and other animal/human Oatps are expressed ubiquitously, but more specialized members are expressed exclusively in a limited number of tissues (3). Oatp1c1/OATP1C1 is an Oatp/OATP superfamily member that follows the latter expression and functional pattern. Oatp1c1/OATP1C1 is a high-affinity T4 transporter [reported Michaelis-Menten constant (Km) = 180–720 nm] expressed in the blood-brain barrier (BBB), choroid plexus, human ciliary body epithelium, and Leydig cells of the testis (5,6,7). Other known substrates include rT3, cerivastatin, and estradiol-d-17 β-glucuronide (E217βG) (8). Of the Oatps expressed at the BBB, Oatp1c1 has the lowest identified Km for T4, a prohormone that plays a crucial role in the timing of multiple neurological development processes (9).
An extensive network of microvessels infiltrates the brain and is comprised of brain capillary endothelial cells that together form the BBB. Unlike other tissues in which solutes in the blood enter the parenchyma through diffusion between endothelial cells, brain capillary endothelial cells possess tight junctions that prevent paracellular diffusion. As a result, solutes must cross the luminal and abluminal membranes of the brain capillary endothelial cell to enter the brain parenchyma. Such transport is accomplished by a number of solute carriers, including the Oatps (10,11).
Oatp1c1 is the fourth most abundant transporter expressed in rat brain endothelial cells (12,13). Oatp1c1 protein has been localized to both the luminal and abluminal side of brain endothelial cells, and implicated in the transport of T4 across the BBB. T4 is an important regulator of brain development (8,12,14). In addition, Oatp1c1 is also localized to cells comprising the blood-cerebrospinal fluid barrier. Unlike other regions of the brain, choroid plexus vasculature is fenestrated, and the barrier is formed through tight junctions between epithelial cells (15). Currently, Oatp1c1 is thought to be localized to the basolateral membrane of choroid plexus epithelial cells (1). Identifying Oatp1c1 and other brain transporter substrates is important in understanding drug-drug interactions or drug-hormone interactions and for the rational design of new drugs. A comprehensive knowledge of substrate specificity will assist in the design of drugs to facilitate or inhibit uptake via brain transporters.
Compared with other Oatps, Oatp1c1 has relatively narrow substrate specificity. Our current study sought to identify novel Oatp1c1 substrates. In addition, we sought to elucidate possible functional consequences of Oatp1c1-mediated transport of these substances.
Candidates were identified from known competitive inhibitors of thyroid hormone transport (16). Because T4 is transported by Oatp1c1, we hypothesized that some of these known thyroid hormone transport inhibitors may be specific Oatp1c1 inhibitors and/or substrates. Amiodarone, bilirubin, phenytoin, iopanoic acid, indocyanine green (ICG), and members of the fenamic acid family of nonsteroidal antiinflammatory drugs (NSAIDs) were among the compounds tested for Oatp1c1-specific T4 transport inhibition using a model cell line overexpressing Oatp1c1. We found that the fenamic acid diclofenac competitively inhibited T4 uptake by Oatp1c1. In addition, the fenamates inhibited T4 uptake in isolated rat brain microvessels using an ex vivo transport assay. Molecular modeling of T4 and fenamates revealed striking structural commonalities in electrostatic surface potential and steric bulk. Together, these data suggest that Oatp1c1 may contribute to fenamate influx into, and perhaps across, brain endothelial cells.
Materials and Methods
Cloning of Oatp1c1 and plasmid construction
To clone rat Oatp1c1, cDNA was reverse transcribed from postnatal d 15 total rat brain RNA using SuperScript III Reverse Transcriptase (Invitrogen Corp., Carlsbad, CA). PCR was then performed against Oatp1c1 using forward and reverse primers designed against Oatp1c1 sequences obtained in the National Center for Biotechnology Information database (accession identification no. NM_053441). Forward primers (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCGAAGGAGATAGAACCATGGCCGACACTTCATCCAAAGAAAATGCCC-3′) and reverse primers (5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCAAGTCGGGTCTCTTGCCTG-3′) were flanked on their 5′ end by att B sites for use in Gateway cloning (Invitrogen). The stop codon was removed in the reverse primer for fusion with a C-terminal tag in the expression vector pEF-DEST 51 (Invitrogen). Oatp1c1 PCR products were gel purified using a GeneClean kit (Qbiogene, Inc., Irvine, CA) to remove att B primers and primer dimers, and then further purified using a QIAquick PCR purification kit (QIAGEN, Inc., Valencia, CA). Att B-flanked PCR products were directionally cloned into att P-containing Gateway pDONR 221 entry vectors (Invitrogen) in a 4-h BP-recombination reaction catalyzed by BP clonase according to the manufacturer’s instructions resulting in att L-containing Oatp1c1-pDONR 221 entry clones. Oatp1c1 was subcloned from pDONR 221 to the att R-containing expression vector pEF-DEST 51 in a 5-h LR-recombination reaction. pEF-DEST 51 contains a C-terminal fusion V5 epitope tag for antibody detection. The Oatp1c1 insert along with upstream elements and the V5 tag were sequence verified.
Stable transfection of Oatp1c1-pEF-DEST51 in human embryonic kidney (HEK) 293 cells
HEK293 cells were grown to 50% confluence in MEM with 10% fetal bovine serum (FBS), sodium pyruvate (1 mm), and nonessential amino acids (1×) without antibiotics. Linearized Oatp1c1-pEF-DEST 51 was introduced into the cells through lipofection using the reagent lipofectin (Invitrogen). Transfected cells were split 1:5 and cultured in the presence of blasticidin (10 μg/ml) for 2 wk. Surviving cells were pooled and cultured in MEM with 10% FBS, sodium pyruvate (1 mm), nonessential amino acids (1×), and a maintenance concentration of blasticidin (2 μg/ml).
Western blot
One confluent well of a six-well plate of Oatp1c1-pEF-DEST 51 transfected HEK293 cells was washed once with ice-cold PBS and then lysed with 150 μl radioimmunoprecipitation assay buffer [50 mm Tris-HCl (pH 8), 150 mm NaCl, 1.0% Nonidet P-40, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 50 mm NaF, 50 mm EDTA] containing Complete Mini Protease Inhibitor (Roche Diagnostics, Penzberg, Germany). Cell lysate was boiled for 5 min, and 15 μl was run on a 10% sodium dodecyl sulfate-polyacrylamide gel containing 0.36 g/ml urea. Protein was transferred to a nitrocellulose membrane at 100 V for 1 h and blocked in blotto [50 mm Tris (pH 8), 2 mm CaCl, 80 mm NaCl, 0.2% Tween 20, and 5% nonfat dry milk] for 1 h at room temperature. The membrane was then incubated with anti-V5 antibodies (Invitrogen) (1:4000 in blotto) for 1 h at room temperature. After four 10-min washes with Tris-buffered saline (pH 8), 0.1% Tween 20 at room temperature, the membrane was incubated with the secondary horseradish-peroxidase conjugated secondary antibody (1:20,000) for 1 h at room temperature. The membrane was then washed three times for 10 min in Tris-buffered saline (pH 8), 0.1% Tween 20 and developed in West Pico Chemiluminescent substrate (Pierce, Rockford, IL) for 5 min. Finally, the membrane was imaged using FluoroChem 8000 software (Alpha Innotech Corp., San Leandro, CA).
Immunocytochemistry
Transfected cells were plated in a 12-well plate containing no. 1 round glass coverslips (Dynalab Corp., Rochester, NY). Cells were then washed in PBS (pH 7.4), followed by a rinse in room temperature methanol, and fixed in −20 C methanol for 12 h. Next, cells were washed, permeabilized, and blocked during three 5-min washes in PBS, 1% BSA, and 0.1% Triton X-100. Primary anti-V5 mouse IgG antibodies (Invitrogen) were diluted 1:100 in PBS, 1% BSA, and 0.1% Triton X-100, and applied to the coverslips for 1 h in a humidity chamber placed in a 37 C incubator. After primary antibody incubation, cells received three 5-min washes in PBS. Next, goat antimouse fluorescein isothiocyanate-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were applied (1:75), and incubated and washed using the identical procedure as with the primary antibody. Cells were then mounted on slides with Prolong Antifade Reagent (Molecular Probes, Inc., Eugene, OR). Slides were examined using a Nikon TE300 inverted fluorescent microscope (Nikon Corp., Melville, NY).
Transport assay
Cell-culture transport assays were performed using a method based on a procedure published by Sugiyama et al. (8). A total of 1 × 105 cells per well was plated in four-well plates (Nunc, Rochester, NY) in standard culture medium (see Stable transfection of Oatp1c1-pEF-DEST51 in HEK293). Twenty-four hours before the assay, media were changed and supplemented with 10 mm sodium butyrate, and 10% stripped serum. Stripped serum was prepared by incubating 50 ml FBS and 0.3 μCi 125I-T4 with 2.5 g Dowex 1 × 8, 50–100 mesh, ion exchange resin (Acros Organics, Geel, Germany) three times for 2 h at room temperature. After the final incubation, the beads were pelleted with centrifugation, and the serum was filter sterilized. Thyroid hormone removal was assessed by monitoring 125I-T4 tracer elimination. To perform the assay, cells were washed and incubated with Krebs-Henseleit buffer [142 mm NaCl, 23.8 mm NaHCO3, 12.5 mm HEPES, 5 mm glucose, 4.83 mm KCl, 1.53 mm CaCl2, 1.2 mm MgSO4, and 0.96 mm KH2PO4 (pH 7.4)]. Uptake was commenced with the addition of Krebs-Henseleit containing 1 nm 125I-T4 (307 μCi/ml; PerkinElmer, Inc., Wellesley, MA) and ended at specific time points with two washes of Krebs-Henseleit without radiolabeled ligand. Cells were then lysed with 0.5% Triton X-100 (Sigma-Aldrich Corp., St. Louis, MO), and the associated radioactivity was measured with a Beckman γ counter (Beckman Coulter, Inc., Fullerton, CA). Protein concentrations were determined with a bicinchoninic acid protein assay kit (Pierce). Background radioactivity associated with the empty vector zero time point was subtracted from all samples. Zero time points were performed as follows. After equilibration in Krebs-Henseleit buffer, the buffer was aspirated from cells, and 200 μl isotopic medium was overlaid on confluent HEK293 cells and immediately removed. Cells were then rapidly washed two times with fresh Krebs-Henseleit buffer. The 125I-T4 associated counts at 0 min account for approximately 10% of uptake at 2 min for Oatp1c1-transfected cells. Uptake was calculated from the proportion of radioactivity associated with the cell lysate compared with total radioactivity associated with the isotopic Krebs-Henseleit buffer and expressed in units of pmol/min/mg protein. The uptake units were expressed as a cell to medium ratio of associated radioactivity derived from the following equality: counts in isotopic standard/volume of standard = counts in lysate sample/“volume” taken up by cells in lysate sample. The volume units were then converted to picomolar units by equating sample uptake volume to the standard volume that had a known T4 concentration. In all experiments, 125I-T4 concentrations were 1 nm and supplemented with unlabeled T4 to achieve higher total T4 concentrations when needed. All unlabeled compounds used in inhibition assays were purchased from Sigma-Aldrich.
Rat cerebral microvessel isolation
Cerebral microvessels were isolated from adult Sprague Dawley rats. Adult rats weighed approximately 250 g. Rats were anesthetized with Nembutal 50 mg/kg ip, exsanguinated, and decapitated. The brains were immediately removed and pooled. The brain matter was minced in 10 ml ice-cold buffer A (MEM supplemented with 25 mm HEPES and 1% dextran) into approximately 5-mm cubes. The minced brain matter was homogenized via 10 strokes in a glass-Teflon homogenizer (DuPont Co., Wilmington, DE) with a clearance of 0.25 mm using a motor driven drill press. The homogenate was passed over a 350-μm nylon mesh, followed by three washes with 5 ml ice-cold buffer A. The flow through was passed over a 110-μm nylon mesh and washed three times, with 5 ml ice-cold buffer A. The resulting flow through was thoroughly mixed with an equal part of ice-cold buffer A plus 40% dextran. The mixture was centrifuged in a swinging bucket rotor at 5000 × g for 15 min at 4 C. The supernatant was aspirated, and the pellet was resuspended in 5 ml ice-cold buffer A. The microvessels were collected by passing the resuspended pellet over a 25-μm nylon mesh, and the trapped microvessels were washed three times with 5 ml ice-cold buffer A. The nylon mesh containing the trapped microvessels was placed in a 12-ml centrifuge tube and washed with 5 ml ice-cold buffer A to remove the adherent microvessels. The microvessel collection procedure on the 25-μm nylon mesh was repeated for a total of six times with the resulting flow through. The microvessel preparation was assessed microscopically for purity. All animal use and protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee. Animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Rat cerebral microvessel uptake assay
The determination of T4 uptake in rat cerebral microvessels is based on the filter binding methodology of Inoue et al. (17). In brief, purified microvessels were washed with Krebs-Henseleit buffer at 37 C and resuspended to 1 μg/μl in Krebs-Henseleit buffer, and incubated at 37 C for 15 min. Protein concentration was determined using a bicinchoninic acid protein assay kit. The uptake assay was begun by the addition of 0.344 μCi 125I-T4 in 100 μl Krebs-Henseleit to 100 μg purified microvessels for a total volume of 200 μl. The reaction was incubated at 37 C for the indicated time (0, 30, 60, and 120 sec). To terminate the reaction, 800 μl ice-cold Krebs-Henseleit buffer was added to the reaction, followed by immediate filtration on nitrocellulose filter disks (Millipore HAWP02500; Millipore Corp., Billerica, MA) with suction. The filter disk was rapidly washed three times with approximately 10 ml PBS. The filters were placed in vials, and 10-min γ counts were performed. Controls were included for nonspecific binding of 125I-T4 to the filter, which was typically 1.2% of total activity.
Determination of kinetic parameters
Dose response and uptake curves were fitted using nonlinear regression with GraphPad Prism Version 4 (GraphPad Software Inc., San Diego, CA). Dose-response curves followed the “four-parametric logistic equation” of the form: response = bottom + (top − bottom)/[1 + 10(LogIC50-[drug])]. Uptake curves followed the Michaelis-Menten equation: v = maximum velocity × [S]/[S] + Km.
Substrate-inhibitor comparison
Structures of the T4 and Oatp1c1 inhibitors were retrieved from PubChem in Simplified Molecular Input Line Entry System notation and converted into their three-dimensional structures using Sybyl 7.2 (Tripos, Inc., St. Louis, MO). The structures were minimized by the Powell method in Sybyl 7.2 using the Tripos force field, atomic charges assigned by the Gasteiger-Marsili method. To optimize the structural alignment, simulated annealing was used to sample conformational space. There were 10 cycles of heating to 700 K for 1000 fsec and cooling to 200 K for 1000 fsec using an exponential temperature vs. time decay used. The energetics was calculated as described previously. The simulation was followed by another minimization and the process repeated until structural minima were obtained having a high degree of structural overlap. The structural alignments were done using the Multifit algorithm in Sybyl 7.2. MOLecular Computer Aided Design (Tripos) was used to compare the electrostatic potential surface of T4 and meclofenamic acid.
Results
Oatp1c1 cloning and expression in HEK293 cells
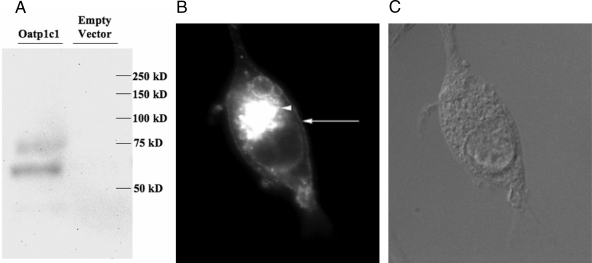
The coding sequence of Oatp1c1 was PCR amplified from postnatal d-15 rat brain total RNA, inserted into the Gateway expression vector pEF-DEST 51, and sequence verified. Western blot and immunocytochemistry were performed to verify full-length expression and proper subcellular localization of Oatp1c1-pEF-DEST51 transfected HEK293 cells. Oatp1c1 was expressed with an apparent molecular mass of approximately 75 kDa, consistent with predicted size, along with a lower mass band likely resulting from a degradation product or incomplete denaturing of the protein (Fig. 1A). Immunocytochemical analysis revealed expected Oatp1c1 plasma membrane localization along with deposition in the endoplasmic reticulum (ER) and/or Golgi apparatus in transfected HEK293 cells (Fig. 1, B and C).
Figure 1.
Oatp1c1 expression in transfected HEK293 cells. A, Full-length Oatp1c1 is expressed in transfected HEK293 cells. Lysate from HEK293 cells transfected with Oatp1c1-pEF-DEST 51 (lane 1) and empty vector (lane 2) was transferred to a nitrocellulose membrane and probed with anti-V5 antibodies. B and C, Oatp1c1 is expressed at the plasma membrane (arrow) and perinuclear regions (arrowhead) in transfected HEK293 cells. Methanol-fixed HEK293 cells expressing Oatp1c1 were probed with anti-V5 antibodies to reveal V5 tagged Oatp1c1 localization. Green stain corresponds to Oatp1c1 localization.
Establishing a cell-based model for assessing Oatp1c1-mediated transport
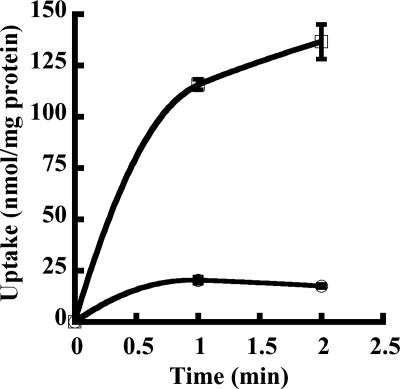
125I-T4 was used as a model high-affinity Oatp1c1 substrate for all assays. Oatp1c1 transported 125I-T4 in a time-dependent manner into transfected HEK293 cells (Fig. 2). 125I-T4 uptake was almost 9-fold higher in Oatp1c1 transfected cells compared with empty vector transfected cells. The addition of excess unlabeled T4 quenched labeled T4 transport.
Figure 2.
T4 is transported in a time-dependent manner by Oatp1c1-pEF-DEST 51 transfected HEK293 cells. Uptake of 125I-T4 was examined at different time points in cells transfected with Oatp1c1-pEF-DEST 51 (open square) or empty vector (open circle) at 37 C. The zero time point was subtracted from each time point to account for background. Each point represents the mean uptake ± se (n = 3).
Inhibition of Oatp1c1-mediated T4 transport
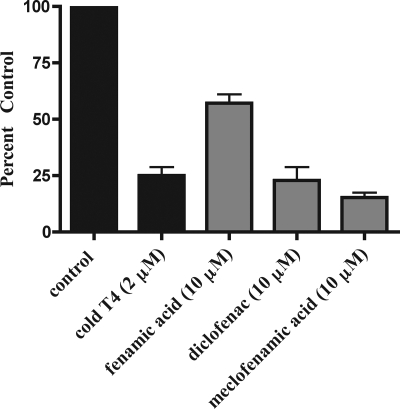
Next, putative inhibitors of Oatp1c1-mediated T4 uptake were screened at 10-μm concentrations. We first tested the known thyroid hormone transport inhibitors (amiodarone, bilirubin, ICG, iopanoic acid, phenytoin, and fenamic acid) to assess Oatp1c1-specific transport inhibition. Additional NSAIDs were tested, including the fenamates diclofenac and meclofenamic acid. Aspirin, flurbiprofen, ibuprofen, and indomethacin had no effect on T4 uptake (Fig. 3). Amiodarone, bilirubin, phenytoin, fenamic acid, diclofenac, meclofenamic acid, and ICG all reduced T4 uptake significantly. ICG was the most potent inhibitor (82.5 ± 1.5%), followed by members of the fenamic acid family (diclofenac 48.5 ± 4.6%; meclofenamic acid 66.8 ± 2.9%) and iopanoic acid (58.8 ± 1.0%).
Figure 3.
Fenamic acids, ICG, iopanoic acid, and phenytoin inhibit Oatp1c1-mediated T4 transport. The effect of various unlabeled compounds (10 μm) on Oatp1c1-mediated 125I-T4 (1 nm) transport was examined at 2-min time points at 37 C. Each graph represents the mean uptake ± se (n = 3). **, P ≤ 0.01; ***, P ≤ 0.001.
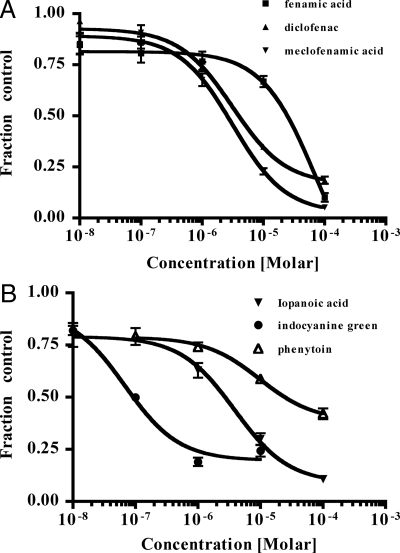
Dose-response analyses were performed for the fenamates, phenytoin, ICG, and iopanoic acids (Table 1). IC50 values followed the same pattern as the initial inhibition screen, with ICG displaying the lowest value, followed by meclofenamic acid, iopanoic acid, diclofenac, phenytoin, and fenamic acid (Fig. 4). T4 is light sensitive and known to undergo photodegradation in the presence of colored compounds. Therefore, we examined whether the measured inhibition by the fenamates, ICG, phenytoin, and iopanoic acid was due to interactions with Oatp1c1 or photodegradation of the 125I-T4. To test this we assessed the ability of these six compounds to inhibit Oatp1c1-mediated transport when coincubated under ambient light with 125I-T4 immediately and 1 h before uptake was measured. We found that the time of light exposure had no effect on the degree of inhibition, suggesting that photodegradation did not occur in our assays (data not shown).
Table 1.
IC50 values for inhibitors of Oatp1c1-mediated transport
| Compound | IC50 (μm) |
|---|---|
| Fenamic acid | 25 |
| Diclofenac | 4 |
| Meclofenamic acid | 3 |
| Iopanoic acid | 3 |
| ICG | 0.1 |
| Phenytoin | 26 |
Figure 4.
Fenamate, iopanoic acid, ICG, and phenytoin dose responses for Oatp1c1-mediated T4 transport. Uptake of 125I-T4 was examined in the presence of different concentrations of fenamic acid, diclofenac, meclofenamic acid (A), and iopanoic acid, ICG, and phenytoin (B). Dose-response curves were fitted, and IC50 values were calculated using nonlinear regression analysis (GraphPad Prism). Each point represents the mean uptake ± se (n = 3).
Identification of competitive inhibitors of Oatp1c1-mediated T4 transport
We determined initial velocities at 2-min time points of Oatp1c1 T4 uptake at varying concentrations in the presence of the fenamic acid diclofenac. As seen in Fig. 5, increasing concentrations of T4 attenuated, then abolished diclofenac inhibition, suggesting competitive inhibition by this fenamic acid.
Figure 5.
Characterization of Oatp1c1-mediated T4 uptake inhibition. Uptake of increasing concentrations of 125I-T4 supplemented with cold T4 (total concentration 1–100 nm) was monitored at 2 min at 37 C with and without the inhibitor diclofenac. Each point represents the mean velocity ± se (n = 3).
Inhibition of T4 uptake into microvessels
Because Oatp1c1 is expressed nearly exclusively at the BBB, the physiological relevance of NSAID inhibition of T4 uptake was examined using an ex vivo transport system in isolated brain microvessels. T4 uptake inhibition into microvessels was most strongly inhibited by meclofenamic acid (14.82 ± 3.11%), followed by diclofenac (19.87 ± 6.56%) and fenamic acid (28.54 ± 4.73%), duplicating the hierarchy of inhibition assumed in the in vitro cell culture model (Fig. 6).
Figure 6.
Effect of fenamic acid, diclofenac, and meclofenamic acid (10 and 100 μm) on the uptake of 125I-T4 in adult rat cerebral microvessels over 5 min at 37 C. Values represent mean uptake ± se (n = 3–5).
Discussion
Chalmers et al. (18) previously observed inhibition of T3 uptake in cultured hepatocytes in the presence of mono and disubstituted fenamic acids. We now report that members of the fenamic acid family of NSAIDs inhibit Oatp1c1-mediated T4 transport in a competitive manner, suggesting that Oatp1c1 transports this family of drugs across the plasma membrane. Interestingly, meclofenamic acid is the strongest T4 transport competitor, followed by diclofenac and fenamic acid. Similar to our data (Fig. 3), when a library of 26 fenamic acid derivatives was screened for l-T3 uptake inhibition in H4 rat hepatoma cells, meclofenamic acid was the most potent inhibitor (18). This hierarchical pattern of Oatp1c1-mediated T4 transport inhibition mirrors the degree of cyclooxygenase (COX)-1 inhibition in whole blood assays by these NSAIDs (19). Thus, the relatively stronger COX inhibitory effect of meclofenamic acid may reflect enhanced intracellular delivery.
COX-1 and COX-2 are ER-localized enzymes responsible for prostaglandin synthesis. NSAIDs inhibit COX activity through binding to the active site in the ER lumen. Interestingly, our immunocytochemistry data suggest that Oatp1c1 protein is localized both to plasma and perinuclear membranes (Fig. 1B). Oatp expression in perinuclear membranes such as the ER may have functional significance. For example, ER-localized Oatps may facilitate NSAID delivery into the ER lumen, where the drug is now free to bind to the COX active site. In support of a role for Oatps in ER transport, Battaglia and Gollan (20) have demonstrated saturable transport of the Oatp substrate E217βG across ER-derived microsomal membranes. Interestingly, this transport process was inhibited by model organic anions, including bromosulfophthalein.
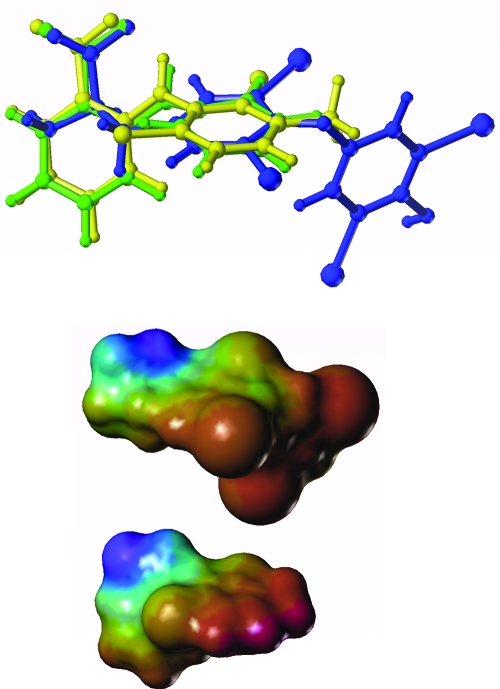
A structural comparison suggests why the fenamates are able to inhibit T4 transport and presumably can themselves be transported by Oatp1c1 (Fig. 7). A structural overlay (Fig. 7, upper panel) was optimized by searching conformational space using simulated annealing for alternative low-energy conformations until the resulting structures could be superimposed. The superposition shows alignment of the carboxylate (Fig. 7, upper left) and one benzylic ring, as well as a number of atoms between them. The electrostatic surface potential in Fig. 7 (lower panel) further highlights these commonalities. The volume occupied by meclofenamic acid also correlates very highly with T4. These features compare well with the determinants of binding affinity identified in a recent Oatp1a5 QSAR paper (21). Also in that paper, it was shown that additional steric bulk near the negative charge can have a positive effect on binding. Although Oatp1c1 substrates may differ in detail, this may play a role in the fenamates’ binding affinity, having the steric bulk of a six-membered ring near the carboxylate. In addition, the higher affinity of meclofenamic acid for Oatp1c1 could be due to the added steric bulk of the methyl group on its benzylic ring, also shown to be important in the previous study (21). Not obvious from that work is the importance of the iodine or chlorine substituents on the benzene ring. The increase in affinity of diclofenac with respect to fenamic acid to Oatp1c1 suggests that this substitution is important. Furthermore, it improves the volume overlap with the very high-affinity T4.
Figure 7.
Structural and electrostatic comparison of T4 and fenamate inhibitors of Oatp1c1. Upper panel, Overlay of T4 (blue), fenamic acid (green), and meclofenamic acid (yellow) using Multifit in Sybyl 7.2. There were 12 atoms in each molecule used in the alignment. Simulated annealing was used to sample viable structural conformations. Lower panel, Electrostatic surface potential of T4 (top) and meclofenamic acid (bottom). The electrostatic potential was mapped onto the Connolly surface using MOLecular Computer Aided Design.
Tight junctions prevent paracellular diffusion of molecules into brain interstitial spaces. Compounds of smaller molecular masses with high lipophilicity and low polarizability and hydrogen bonding potential are more likely to diffuse passively across the BBB (22). Most NSAIDs are lipophilic with ClogP values (logP octanol/water) approximately in the four to six range for fenamic acid analogs (18). Despite the inherent lipophilic nature of these compounds, over 99% of NSAIDs circulate as organic anions due to their acidic character (pKa<6) (23). This severely limits availability of the diffusible nonionized fraction. As a result, NSAIDs must cross the BBB through carrier-mediated transport at the plasma membrane (24). In our study all fenamates tested significantly inhibited T4 uptake in isolated rat brain microvessels, likely due in part through inhibition of Oatp1c1 (Fig. 6). In addition to our present studies, other work implicates Oatps in NSAID transport at the BBB. For example, Oatp1a4 transport, another Oatp expressed at the BBB, is inhibited by ketoprofen and indomethacin at high concentrations, and in addition to the fenamates, Oatp1c1 transports ketoprofen with low affinity (8,25).
In addition to transporting NSAIDs across the BBB, Oatp-mediated NSAID uptake into the endothelial cell may have a direct functional consequence because these cells express both COX-1 and COX-2 isoforms (26,27,28,29). Endothelium-derived vasodilatory prostanoids are critical mediators of cerebral blood flow under both basal and stimulated conditions. Oatp1c1 transported fenamic acids, active on both COX-1 and COX-2, could potentially inhibit endothelial prostanoid synthesis. Under certain conditions this event could have potentially severe consequences in the regulation of cerebral circulation and result in serious neurological complications, including hypoxic injuries, especially in the developing neonate (28).
In addition to the fenamates, iopanoic acid, phenytoin, and ICG also inhibited Oatp1c1-mediated T4 uptake. Iopanoic acid inhibits T4 uptake in cultured hepatocytes (30). This radioopaque, cholecystographic agent is a known inhibitor of type II deiodinase, the enzyme that catalyzes the outer ring deiodination of T4 to produce the active thyroid hormone T3 (31). This drug is used to treat T4 overdoses and hyperthyroidism (32). The reasons behind the efficacious nature of iopanoic acid treatment are likely 2-fold. In addition to type II deiodinase activity, iopanoic acid may also reduce T4 transport (via Oatp1c1 in some tissues), leading to decreased intracellular T4 availability for T3 conversion. The anticonvulsant phenytoin has multiple effects on the thyroid hormone system, including inhibiting anterior pituitary cell T3 uptake (33,34). This drug acted as a partial antagonist of Oatp1c1 T4 uptake because complete inhibition was not achieved. Indeed, T4 uptake was quenched nearly maximally at phenytoin concentrations near the IC50. ICG, a dye used in measuring cardiac output, was the most potent inhibitor of Oatp1c1-mediated T4 uptake. This model organic anion also inhibits transport by other Oatps, but the inhibitory effect is not global among family members. For example, E217βG uptake is inhibited by ICG in Oatp1a4, but not Oatp1b3 transfected HEK293 cells (35). It is conceivable that T4 binding site similarities in Oatp1c1 and Oatp1a4 account for the ICG inhibition observed in cells transfected with these two transporters.
The fenamic acids, phenytoin, ICG, and iopanoic acids are all clinically used. Our T4 dose-response assays indicated that ICG, phenytoin, iopanoic acid, and all fenamic acid analogs have IC50 values below or near the blood plasma levels after therapeutic dose administration. Thus, antagonistic drug-hormone interactions may influence the uptake of Oatp1c1 substrates into the brain. In our in vitro transport system, inhibition assays were performed with Krebs-Henseleit buffer in the absence of serum. Therefore, our reported IC50 measurements (Table 1) correspond to the capacity of free drug concentrations to inhibit uptake of 1 nm 125I-T4 uptake. Reported blood plasma levels after therapeutic dosing refer to total drug concentrations, of which, a significant portion is protein bound. For example, 99% of diclofenac circulates bound to plasma albumin (36). The blood plasma concentration of diclofenac after standard dosage is 7.1 μm (37). Therefore, the pharmacologically active free concentration is 71 nm. In contrast, free T4 concentrations in adult humans are approximately 12 pm. Thus, diclofenac blood plasma levels achieved after standard dosing maintain the potential to influence T4 uptake via Oatp1c1. However, full inhibition of T4 transport will not likely occur because free drug levels are well below the measured Oatp1c1 IC50. This pattern repeats for the other inhibitors tested. Meclofenamic acid, ICG, iopanoic acid, and phenytoin all have IC50 values (Table 1) near the blood plasma levels after standard dosing (16.2, 3.6, 8, and 6.7 μm, respectively) but are highly bound in plasma (38,39,40). Thus, the unbound fraction of these drugs is well below the IC50 for Oatp1c1 T4 transport. Nonetheless, the rate of T4 transport will likely be reduced. Given the time-dependent developmental regulation of many thyroid hormone-responsive brain genes, this delay may exert a deleterious biological effect (41). In addition, drug-drug interactions between these tested compounds are possible, if administered simultaneously.
In conclusion, we have identified multiple inhibitors of Oatp1c1 T4 transport. These compounds, including the fenamic acids, iopanoic acid, ICG, and phenytoin, demonstrated IC50 values in the low micromolar to high nanomolar range. The competitive nature of fenamate inhibition of T4 transport along with structural alignments suggests that Oatp1c1 transports this family of NSAIDs. Oatp1c1 expression in brain endothelial cells suggests an important role for Oatps in brain NSAID transport. Future animal experiments will further elucidate the in vivo scope and relevance of Oatp1c1-T4-fenamate interactions.
Acknowledgments
We thank David R. Salo for his insight and technical contributions to this work.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant R01 DK054060 and a fellowship from the University of Minnesota Graduate School (to G.W.A.), Research Corporation Grant CC6681 (to J.N.R.), NIH/National Institute of Neurological Disorders and Stroke Grant R01-NS37764, American Heart Association Grant 0255935Z, the University of Minnesota Academic Health Center (to L.R.D.), and a Melendy Summer Research Scholarship (to D.D.S.).
Disclosure Statement: The authors have nothing to declare.
First Published Online October 9, 2008
Abbreviations: BBB, Blood-brain barrier; COX, cyclooxygenase; ER, endoplasmic reticulum; E217βG, estradiol-d-17 β-glucuronide; FBS, fetal bovine serum; HEK, human embryonic kidney; ICG, indocyanine green; NSAID, nonsteroidal antiinflammatory drug; Oatp, organic anion transporting polypeptide.
References
- Hagenbuch B, Meier PJ 2003 The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta 1609:1–18 [DOI] [PubMed] [Google Scholar]
- Meier-Abt F, Mokrab Y, Mizuguchi K 2005 Organic anion transporting polypeptides of the OATP/SLCO superfamily: identification of new members in nonmammalian species, comparative modeling and a potential transport mode. J Membr Biol 208:213–227 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ 2004 Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 447:653–665 [DOI] [PubMed] [Google Scholar]
- Li L, Lee TK, Meier PJ, Ballatori N 1998 Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. J Biol Chem 273:16184–16191 [DOI] [PubMed] [Google Scholar]
- Gao B, Huber RD, Wenzel A, Vavricka SR, Ismair MG, Reme C, Meier PJ 2005 Localization of organic anion transporting polypeptides in the rat and human ciliary body epithelium. Exp Eye Res 80:61–72 [DOI] [PubMed] [Google Scholar]
- Pizzagalli F, Hagenbuch B, Stieger B, Klenk U, Folkers G, Meier PJ 2002 Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol Endocrinol 16:2283–2296 [DOI] [PubMed] [Google Scholar]
- Chu C, Li JY, Boado RJ, Pardridge WM 2008 Blood-brain barrier genomics and cloning of a novel organic anion transporter. J Cereb Blood Flow Metab 28:291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, Sugiyama Y 2003 Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem 278:43489–43495 [DOI] [PubMed] [Google Scholar]
- Jones SA, Thoemke KR, Anderson GW 2005 The role of thyroid hormone in fetal and neonatal brain development. Curr Opin Endocrinol Diabetes Obes 12:10–16 [Google Scholar]
- Kusuhara H, Sugiyama Y 2005 Active efflux across the blood-brain barrier: role of the solute carrier family. NeuroRx 2:73–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westholm DE, Rumbley JN, Salo DR, Rich TP, Anderson GW 2008 Organic anion-transporting polypeptides at the blood-brain and blood-cerebrospinal fluid barriers. Curr Top Dev Biol 80:135–170 [DOI] [PubMed] [Google Scholar]
- Tohyama K, Kusuhara H, Sugiyama Y 2004 Involvement of multispecific organic anion transporter, Oatp14 (Slc21a14), in the transport of thyroxine across the blood-brain barrier. Endocrinology 145:4384–4391 [DOI] [PubMed] [Google Scholar]
- Enerson BE, Drewes LR 2006 The rat blood-brain barrier transcriptome. J Cereb Blood Flow Metab 26:959–973 [DOI] [PubMed] [Google Scholar]
- Anderson GW 2001 Thyroid hormones and the brain. Front Neuroendocrinol 22:1–17 [DOI] [PubMed] [Google Scholar]
- Segal MB 2000 The choroid plexuses and the barriers between the blood and the cerebrospinal fluid. Cell Mol Neurobiol 20:183–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennemann G, Docter R, Friesema EC, de Jong M, Krenning EP, Visser TJ 2001 Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev 22:451–476 [DOI] [PubMed] [Google Scholar]
- Inoue M, Kinne R, Tran T, Arias IM 1984 Taurocholate transport by rat liver canalicular membrane vesicles. Evidence for the presence of an Na+-independent transport system. J Clin Invest 73:659–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DK, Scholz GH, Topliss DJ, Kolliniatis E, Munro SL, Craik DJ, Iskander MN, Stockigt JR 1993 Thyroid hormone uptake by hepatocytes: structure-activity relationships of phenylanthranilic acids with inhibitory activity. J Med Chem 36:1272–1277 [DOI] [PubMed] [Google Scholar]
- Vane SJ 2000 Aspirin and other anti-inflammatory drugs. Thorax 55(Suppl 2):S3–S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia E, Gollan J 2001 A unique multifunctional transporter translocates estradiol-17β-glucuronide in rat liver microsomal vesicles. J Biol Chem 276:23492–23498 [DOI] [PubMed] [Google Scholar]
- Yarim M, Moro S, Huber R, Meier PJ, Kaseda C, Kashima T, Hagenbuch B, Folkers G 2005 Application of QSAR analysis to organic anion transporting polypeptide 1a5 (Oatp1a5) substrates. Bioorg Med Chem 13:463–471 [DOI] [PubMed] [Google Scholar]
- Habgood MD, Begley DJ, Abbott NJ 2000 Determinants of passive drug entry into the central nervous system. Cell Mol Neurobiol 20:231–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parepally JM, Mandula H, Smith QR 2006 Brain uptake of nonsteroidal anti-inflammatory drugs: ibuprofen, flurbiprofen, and indomethacin. Pharm Res 23:873–881 [DOI] [PubMed] [Google Scholar]
- Chen Y, Dalwadi G, Benson HA 2004 Drug delivery across the blood-brain barrier. Curr Drug Deliv 1:361–376 [DOI] [PubMed] [Google Scholar]
- Shitara Y, Sugiyama D, Kusuhara H, Kato Y, Abe T, Meier PJ, Itoh T, Sugiyama Y 2002 Comparative inhibitory effects of different compounds on rat oatpl (slc21a1)- and Oatp2 (Slc21a5)-mediated transport. Pharm Res 19:147–153 [DOI] [PubMed] [Google Scholar]
- Parfenova H, Eidson TH, Leffler CW 1997 Upregulation of COX-2 in cerebral microvascular endothelial cells by smooth muscle cell signals. Am J Physiol 273(1 Pt 1):C277–C288 [DOI] [PubMed] [Google Scholar]
- Parfenova H, Parfenov VN, Shlopov BV, Levine V, Falkos S, Pourcyrous M, Leffler CW 2001 Dynamics of nuclear localization sites for COX-2 in vascular endothelial cells. Am J Physiol Cell Physiol 281:C166–C178 [DOI] [PubMed] [Google Scholar]
- Parfenova H, Levine V, Gunther WM, Pourcyrous M, Leffler CW 2002 COX-1 and COX-2 contributions to basal and IL-1 β-stimulated prostanoid synthesis in human neonatal cerebral microvascular endothelial cells. Pediatr Res 52:342–348 [DOI] [PubMed] [Google Scholar]
- Glasgow JF, Middleton B 2001 Reye syndrome—insights on causation and prognosis. Arch Dis Child 85:351–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topliss DJ, Kolliniatis E, Barlow JW, Lim CF, Stockigt JR 1989 Uptake of 3,5,3′-triiodothyronine by cultured rat hepatoma cells is inhibitable by nonbile acid cholephils, diphenylhydantoin, and nonsteroidal antiinflammatory drugs. Endocrinology 124:980–986 [DOI] [PubMed] [Google Scholar]
- Coppola A, Hughes J, Esposito E, Schiavo L, Meli R, Diano S 2005 Suppression of hypothalamic deiodinase type II activity blunts TRH mRNA decline during fasting. FEBS Lett 579:4654–4658 [DOI] [PubMed] [Google Scholar]
- Bal CS, Kumar A, Chandra P 2005 Effect of iopanoic acid on radioiodine therapy of hyperthyroidism: long-term outcome of a randomized controlled trial. J Clin Endocrinol Metab 90:6536–6540 [DOI] [PubMed] [Google Scholar]
- Smith PJ, Surks MI 1984 Multiple effects of 5,5′-diphenylhydantoin on the thyroid hormone system. Endocr Rev 5:514–524 [DOI] [PubMed] [Google Scholar]
- Lim CF, Loidl NM, Kennedy JA, Topliss DJ, Stockigt JR 1996 Drug effects on triiodothyronine uptake by rat anterior pituitary cells in vitro. Exp Clin Endocrinol Diabetes 104:151–157 [DOI] [PubMed] [Google Scholar]
- Cui Y, Konig J, Leier I, Buchholz U, Keppler D 2001 Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem 276:9626–9630 [DOI] [PubMed] [Google Scholar]
- Davies NM, Skjodt NM 2000 Choosing the right nonsteroidal anti-inflammatory drug for the right patient: a pharmacokinetic approach. Clin Pharmacokinet 38:377–392 [DOI] [PubMed] [Google Scholar]
- Product information 2003 Voltaren. East Hanover, NJ: Norvartis Pharmaceuticals Corp. [Google Scholar]
- Meyer MC, Straughn AB, Mhatre RM, Shah VP, Chen ML, Williams RL, Lesko LJ 2001 Variability in the bioavailability of phenytoin capsules in males and females. Pharm Res 18:394–397 [DOI] [PubMed] [Google Scholar]
- Kennedy JM, van Rij AM 2006 Drug absorption from the small intestine in immediate postoperative patients. Br J Anaesth 97:171–180 [DOI] [PubMed] [Google Scholar]
- Moss AA, Lin SK, Margules ER, Motson RW, Riegelman S 1979 Pharmacokinetics of iopanoic acid in the rhesus monkey: biliary excretion, plasma protein binding and biotransformation. Invest Radiol 14:171–176 [DOI] [PubMed] [Google Scholar]
- Anderson GW, Schoonover CM, Jones SA 2003 Control of thyroid hormone action in the developing rat brain. Thyroid 13:1039–1056 [DOI] [PubMed] [Google Scholar]