Abstract
Implantation of mouse embryos is dependent on the proliferation and differentiation of uterine stromal cells in a process called decidualization. Decidualization both supports and limits the invasion of the implanting embryo and is regulated in part by the expression of matrix metalloproteinases (MMPs) and their inhibitors, the tissue inhibitors of metalloproteinases (TIMPs). Molecules that alter the balance between MMP and TIMP expression could prevent implantation of the embryo. The membrane glycoprotein basigin (CD147/EMMPRIN), a known inducer of MMPs, is necessary for normal implantation in the mouse. The purpose of this study was to investigate the potential roles of basigin during implantation in the mouse. Using an in vitro stromal cell culture system, we found that recombinant human basigin protein (rBSG) increases MMP-3 and MMP-9 expression without altering TIMP-3 expression. Our results also showed rBSG induces expression of cytokines IL-1α/β and leukocyte chemoattractants, CCL3, CCL20, CXCL2, and CXCL5. More importantly, rBSG significantly suppressed stromal cell decidualization as shown by the inhibition of alkaline phosphatase-2 expression and activity by rBSG. However, rBSG did not affect stromal cell proliferation. Taken together, our data indicate that basigin mediates gene expression changes in mouse uterine stromal cells and suggests that temporal and spatial regulation of basigin expression may be involved in the recruitment of leukocytes to the mouse uterus during early pregnancy.
The role of basigin during embryo implantation in mice is examined. Basigin regulates matrix metalloproteinase, IL-1, and leukocyte chemoattractant production by uterine stromal cells.
Implantation of the mouse embryo into the uterus is a highly invasive process that is accompanied by the remodeling of the uterine tissue by locally produced factors as well as by factors produced by the embryo and infiltrating immune cells. After apposition and attachment of the blastocyst to the uterine luminal epithelia, the uterine stromal fibroblasts near the site of embryo attachment proliferate and differentiate to form decidua (1). During the process of decidualization, the interstitial-type extracellular matrix (ECM; containing collagen type I, III, V, VI, and fibronectin) surrounding stromal cells is remodeled into a basement membrane-type matrix ECM (containing collagen type IV, laminin, enactin) (2,3). Matrix metalloproteinases (MMPs), a family of zinc-dependent proteases, play a significant role in the ECM remodeling and degradation during decidualization (4). Several MMPs including gelatinase A (MMP-2), stromelysin-1 (MMP-3), gelatinase B (MMP-9), and stromelysin-3 (MMP-11) are expressed within the undifferentiated stromal fibroblasts during implantation (4,5,6). The implanting embryo expresses MMP-9, and the immune cells that infiltrate the uterus during pregnancy express a number of proteases including MMP-2 and MMP-9 (7,8). The importance of MMP activity for decidualization was demonstrated by treating pregnant mice with the peptide hydroxamic acid metalloproteinase inhibitor (4). Inhibition of total MMP activity with metalloproteinase inhibitor significantly reduced both the length and size of the decidua (4).
MMP activity is regulated by a family of proteins called tissue inhibitors of metalloproteinases (TIMPs), which function by reversibly binding to the catalytic domain of MMPs (9). TIMP-3 is expressed by decidualized cells surrounding the invading embryo and can effectively inhibit the activity of MMP-9 secreted by the embryonic cytotrophoblasts (4,10,11). Therefore, the temporal and spatial balance of MMP and TIMP activity in the mouse uterus is critical for promoting trophoblast invasion whereas also limiting the extent of invasion. Consequently, molecules that alter the balance between MMP and TIMP expression are potentially important regulators of implantation. Basigin is one potential candidate for such a molecule. Basigin is a glycosylated membrane protein belonging to the immunoglobulin superfamily (12). As a broadly distributed protein, basigin exerts multiple functions in both physiological and pathological processes including fertilization (13), inflammation (14,15), and tumor progression (16,17,18). More importantly, most embryos lacking basigin expression fail to implant in the uterus (19), and the transfer of wild-type embryos to pseudopregnant basigin null females results in a significant reduction of live births (13). These data suggest that there are important embryonic and maternal functions for basigin during implantation and early development. One well-characterized function for basigin is its ability to induce the expression of MMPs in fibroblasts and cancer cells. Human basigin protein shed from the surface of cells as a component of membrane microvesicles (20,21) can stimulate the expression of MMP-1, -2, -3, -9, and -14 in fibroblasts and tumor cells without altering the expression level of TIMPs (22,23,24,25). Therefore, basigin may function as a regulator of MMP activity by altering the balance of MMP and TIMP expression levels within tissues. However, it is unknown whether basigin can mediate similar gene expression changes in uterine stromal cells during implantation and decidualization.
The purpose of the current study was to characterize the expression pattern of basigin in the mouse uterus and to investigate the potential roles of basigin to mediate gene expression changes in uterine stromal cells during implantation. Immunohistochemical analysis of mouse uterine tissue revealed that basigin expression is restricted to luminal and glandular epithelial cells before embryo attachment. After embryo attachment at d 4 of pregnancy, basigin is expressed within the uterine stroma, and as the cells surrounding the embryo decidualize, basigin protein is rapidly lost from these cells. This pattern of basigin expression correlates with the expression of MMP-2 and MMP-9 (6), suggesting that basigin may influence gene expression changes in uterine stromal cells during early pregnancy. To explore this possibility, we employed an in vitro uterine stromal cell culture model for decidualization (26). Characterization of the in vitro system demonstrated that the uterine stromal cells express basigin and MMPs in a temporal pattern similar to that of intact tissues before and after decidualization. Addition of exogenous recombinant human basigin protein (rBSG) to nondecidualized cells in culture significantly increased mRNA and protein levels for MMP-3 and -9 without affecting MMP-2 or TIMP-3 mRNA levels. Inflammatory cytokines are also potent inducers of MMP and chemokine expression in stromal fibroblasts (27,28), and recent work by Rossi et al. (29) identified numerous MMPs, cytokines, and chemokines that are up-regulated in response to treatment of human endometrial stromal cells with Il-1β. We hypothesized that basigin might also regulate MMP activity indirectly within the uterine stroma by inducing expression of inflammatory cytokines and chemokines. To explore this possibility, we determined the expression levels for the cytokines IL-1α/β as well as the chemoattractant cytokines specific for mononuclear cells [C-C motif chemokines (CCL)-3, CCL20], and neutrophils [C-X-C chemokines (CXCL)-2 and CXCL5]. The results revealed that basigin is sufficient to induce MMP, cytokine, and chemokine gene expression changes in mouse uterine stromal cells and suggests that the temporal and spatial regulation of basigin expression may be involved in the recruitment of leukocytes to the mouse uterus during early pregnancy.
Materials and Methods
Animals and reagents
Animals used in this research were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee at the University of Illinois-Urbana. Six to16-wk-old CD-1 female and 12- to 36-wk-old BDF-1 vasectomized male mice were obtained from Harlan Sprague Dawley (Indianapolis, IN). To induce pseudopregnancy, females were caged individually with vasectomized males and were checked for vaginal plugs on the following morning. The day on which the vaginal plug was noted was considered as d 1 of pseudopregnancy. For pregnant females, the female mice were caged individually with an intact male and checked for the presence of a vaginal plug each morning. Day 1 of gestation was the day on which the vaginal plug was observed. Uterine tissues were collected at noon on d 1–8 of gestation.
Phenol red-free DMEM and Ham’s F-12 nutrient mixture (1:1), and the Hanks’ balanced salt solution (HBSS) were from Cambrex (Walkersville, MD). Fetal bovine serum (FBS) was from Atlanta Biologicals (Norcross, GA). Streptomycin, penicillin, and dispase were purchased from Life Technologies, Inc. (Grand island, NY). Pancreatin, 17β-estradiol, progesterone, and gelatin were from Sigma (St. Louis, MO). Collagenase III was from Worthington (Lakewood, NJ), TRIzol was from Invitrogen (Carlsbad, CA), and the RETROscript first-strand cDNA synthesis kit was from Ambion (Austin, TX). The universal PCR master mix No AmpErase UNG and the gene expression assays were purchased from Applied Biosystems (Atlanta, GA). Amicon Ultra-4 10-kDa centrifugal filters were from Millipore (Billercia, MA), the antihuman MMP-2 antibody from Oncogene (Cambridge, MA), the rabbit antimouse IgG-horseradish peroxidase secondary antibody from Cell Signaling Technology (Danvers, MA), and the SuperSignal West Pico substrate kit was from Pierce (Rockford, IL). The goat antimouse basigin polyclonal antibody, MMP-2, and MMP-3 ELISA kits were from R&D Systems (Minneapolis, MN). The biotinylated rabbit antigoat IgG and ABC kit were from Vector Laboratories (Burlingame, CA).
Immunohistochemistry
Dissected mouse uterine horns were cut into 0.5-cm segments, fixed in 10% neutral-buffered formalin for 24 h and embedded in paraffin. Five-micrometer sections were mounted on poly-l-lysine-coated slides and subjected to antigen retrieval in boiling 10 mm citrate buffer (pH 6.0) for 10 min. Endogenous peroxidase activity was blocked in methanol containing 0.3% hydrogen peroxide for 15 min. Basigin immunoreactivity was detected according to the ABC method. Briefly, slides were incubated with 5% normal rabbit serum in PBS for 20 min and then incubated with the goat antimouse basigin polyclonal antibody (2 μg/ml) overnight at 4 C. Nonspecific goat IgG was used as a negative control. After washing in PBS, sections were incubated with biotinylated rabbit antigoat IgG diluted 1:100 in PBS for 60 min at room temperature. Sections were incubated in ABC solution for 45 min and then in diaminobenzidine solution for 3 min and counterstained with hemotoxylin.
Cell culture and recombinant basigin
Mouse uterine stromal cells were isolated from d 4 pseudopregnant CD-1 mice as described previously (26). Uteri were trimmed of fat, split longitudinally, and cut into 3- to 4-mm pieces. The tissue was washed in HBSS without calcium and magnesium containing 100 μg/ml streptomycin and 100 U/ml penicillin. The tissue was digested with 6 mg/ml dispase and 2.5 mg/ml pancreatin for 1 h at 4 C, followed by 1 h at room temperature and 10 min at 37 C. Digestion was stopped by adding HBSS containing 10% FBS. Uterine tissues were pipetted vigorously to remove epithelial cell sheets. The remaining uterine tissue was digested with 0.5 mg/ml collagenase III for 30 min at 37 C, pipetted thoroughly to release cells, and filtered through a 200-mesh screen to remove remaining epithelial cell clumps. Stromal cells were seeded onto 6-well plates at 4 × 105 cells/well in phenol red-free DMEM and Ham’s F-12 nutrient mixture (1:1) containing 10% FBS for 90 min. Unbound cells were removed by thorough washing with HBSS. Adherent cells, consisting mostly of stromal fibroblast cells, were cultured in fresh DMEM/F12 media supplemented with 2% charcoal stripped FBS, 10 nm 17β-estradiol, and 1 μm progesterone. Cells were harvested at 0, 2, 6, 24, 48, and 72 h after initial attachment for RNA isolation.
rBSG protein was expressed in the pASK-IBA44 bacterial expression vector (IBA GmBH, Gottingen, Germany) and purified by immobilized metal affinity chromatography as described previously (30). We have shown that this recombinant basigin protein is biologically active and able to stimulate MMP production by both mouse and human endometrial stromal cells (30,31). Endotoxin levels in the rBSG protein preparations and purified lipopolysaccharide (LPS; Roche, Indianapolis, IN) were measured using limulus amoebocyte lysate testing at Cambrex Bio Sciences Walkersville, Inc. (Walkersville, MD). The rBSG used for this study contained 0.55 endotoxin units per microgram of protein. In comparison, the only commercially available preparation of recombinant basigin (R&D Systems) contains less than 1.0 endotoxin units per microgram protein. Whereas this protein is expressed in a mouse myeloma cell line, it is not entirely free of endotoxins either. Equivalent amounts of endotoxin in the form of LPS were added to all control experiments to differentiate the effects of endotoxin from rBSG. Isolated stromal cells were cultured in DMEM/F12 containing 10% FBS until the cells reached 70–80% confluence, the cells were serum starved for 24 h and then treated with rBSG protein at 0, 1, 10, or 100 ng/ml for 24 h. RNA was isolated for quantitative RT-PCR and the conditioned media harvested for immunoblotting, zymography, and ELISA analysis.
RNA isolation and quantitative RT-PCR
Total RNA was extracted from cells using TRIzol (Invitrogen) according to the manufacturer’s instructions. Two micrograms of total RNA were reverse transcribed using the RETROscript first-strand cDNA synthesis kit and quantitative RT-PCR analysis was performed using TaqMan universal PCR master mix No AmpErase UNG (Applied Biosystems, Atlanta, GA). All genes except basigin were amplified using the 20 × Assays-on-Demand gene expression assays (Applied Biosystems; sequences listed in Table 1). Primer and probe set for basigin were designed using Primer Express software (PerkinElmer, Boston, MA). The 200 nm forward primer (5′-TGGACCGTGTTCACATCCAT-3′), 200 nm reverse primer (5′-TGGCACCTACGTGTGTAATGC-3′), and 200 nm probe (5′-TGGACCGTGTTCACATCCAT-3′) were used for basigin cDNA amplification. Gene amplification and detection were performed in MicroAmp optical 384-well reaction plates using the ABI 7900 sequence detection system. Relative mRNA levels were calculated using the standard curve method and normalized to ribosomal 18S.
Table 1.
Reference sequences used for quantitative RT-PCR
| Gene | NCBI gene reference | Assay ID | Reference sequence |
|---|---|---|---|
| IL-1α | NM_010554 | Mm00439620_m1 | ACCTGCAACAGGAAGTAAAATTTGA |
| IL-1β | NM_008361 | Mm00434228_m1 | GACCCCAAAAGATGAAGGGCTGCTT |
| CCL3 | NM_011337.1 | Mm00441258_m1 | CTTCTCAGCGCCATATGGAGCTGAC |
| CCL20 | NM_016960.1 | Mm00444228_m1 | AGCCAGGCAGAAGCAGCAAGCAACT |
| CXCL2 | NM_009140.1 | Mm00436450_m1 | CCAAAAGATACTGAACAAAGGCAAG |
| CXCL5 | NM_009141.1 | Mm00436451_g1 | TAGCTGAAGCTGCCCCTTCCTCAGT |
| MMP-2 | NM_008610 | Mm00439508_m1 | GGACCTGCAGGGCGGTGGTCATAGC |
| MMP-3 | NM_010809.1 | Mm00440295_m1 | GGATGTCACTGGTACCAACCTATTC |
| MMP-9 | NM_013599.2 | Mm00442991_m1 | CATCTTCCAGTACCAAGACAAAGCC |
| TIMP-3 | NM_011595.1 | Mm00441826_m1 | CGACATCGTGATCCGGGCCAAAGTG |
| 18S | X03205.1 | Hs99999901_s1 | GGAGGGCAAGTCTGGTGCCAGCAG |
NCBI, National Center for Biotechnology Information (Bethesda, MD).
Immunoblotting
Cell conditioned media were concentrated 50-fold with Amicon Ultra-4 10-kDa centrifugal filters (Millipore), and equal volumes of concentrated media were resolved by 10% SDS-PAGE. The gels were transferred to nitrocellulose membranes, blocked in 5% nonfat dry milk, and probed with the antihuman MMP-2 antibody at a 1:500 dilution for 1 h. The membranes were washed and incubated with the antimouse IgG-horseradish peroxidase at a 1:20,000 dilution for 1 h at room temperature. The bound secondary antibody was detected using SuperSignal West Pico substrate kit (Pierce).
Gelatin and casein zymography
The presence of MMP-2 and MMP-9 bioactivity in the media was detected by gelatin zymography. Three microliters of 50-fold concentrated conditioned media were resolved by 10% PAGE gel containing 1 mg/ml gelatin under nonreducing conditions. After electrophoresis the gels were washed in 2.5% Triton X-100 and 50 mm Tris-HCl (pH 7.5) for 1 h at room temperature and incubated in 150 mm NaCl, 5 mm CaCl2, and 50 mm Tris-HCl (pH 7.6) overnight at 37 C. The gels were then stained with 0.1% (wt/vol) Coomassie Brilliant blue R-250 in 30% (vol/vol) isopropyl alcohol and 10% (vol/vol) glacial acetic acid for 60 min and destained in 10% (vol/vol) methanol and 5% (vol/vol) glacial acetic acid for 2 h.
ELISA
The secreted MMP-2 and MMP-3 proteins in the medium from stromal cells were quantified by commercial ELISA kits.
Measurement of alkaline phosphatase activity
Cultured stromal cells were lysed with 250 μl of 0.25% deoxycholate and kept at −80 C until alkaline phosphatase (ALP) measurement. After spinning down cellular debris, protein concentrations in the lysate were determined using the BCA protein assay kit (Pierce). Fifty microgram of cell lysate were mixed with 50 μl p-nitrophenyl phosphate substrate (Sigma) in a 96-well plate, and incubated at 37 C for 30 min. The optical absorbance that represented the ALP activity was read at 410 nm. The OD values were normalized to the amount of protein we used.
ALP staining
Cultured stromal cells were rinsed with PBS and fixed with fresh 4% paraformaldehyde (Sigma) in PBS for 2 min at room temperature. After three gentle washings with PBS, cells were incubated in the staining solution containing 0.1 m Tris-HCl (pH 8.5), 0.1 mg/ml naphthol AS-MX phosphate (Sigma), 0.5% N,N-dimethyl formamide (Fisher, Fair Lawn, NY), 2 mm magnesium chloride, and 0.6 mg/ml fast blue BB salt (Sigma) for 30 min at room temperature. For uterine sections, uterine horns were collected and snap frozen in liquid nitrogen and kept at −80 C until later use. Frozen sections (10 μm thick) were fixed in 4% paraformaldehyde for 30 min followed by washing with PBS twice. The sections were then incubated with the above staining solution for 30 min. The development of blue color indicated alkaline phosphatase expression.
Cell proliferation
Uterine stromal cells isolated from d 4 pseudopregnant mice were seeded at a density of 4 × 105 cells/well in 6-well plates and cultured in the DMEM/F12 medium containing 1% FBS. Cells were treated with 100 ng/ml basigin or 3 pg/ml LPS (control) for 24 h. Six fields per each well were randomly selected and photographed. The number of cells in each field was counted. For tritiated thymidine incorporation arrays, uterine stromal cells were cultured in 6-well plates in the DMEM/F12 medium containing 1% FBS and treated with 100 ng/ml rBSG, 100 ng/ml epidermal growth factor (positive control), or 3 pg/ml LPS (negative control). Cells received 2 μCi [3H]thymidine per well (PerkinElmer) after the initial attachment. After 24 h, cells were harvested to measure the rate of incorporated [3H]thymidine.
Statistics
Results are presented as the average ± se of at least three separate experiments. Statistical analysis was performed by ANOVA. Values of P < 0.05 were considered significant.
Results
Immunohistochemical localization of basigin in the mouse uterus during early pregnancy
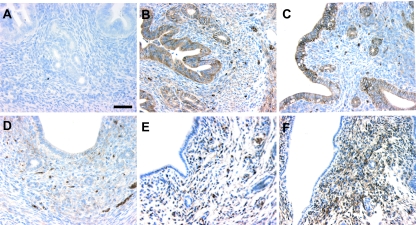
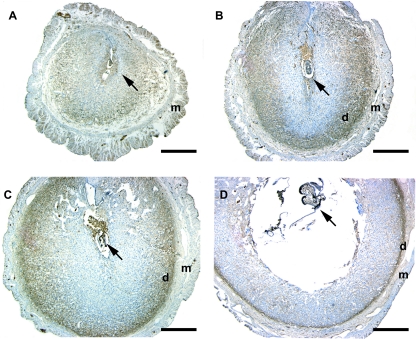
In a previous study by Xiao et al. (32), it was demonstrated that basigin mRNA transcripts are present within the luminal and glandular epithelial cells on d 1 of pregnancy and are gradually lost from these cells on d 2–4 of pregnancy. These authors also demonstrated that basigin transcripts appeared within the uterine stroma in the periimplantation period beginning on d 3. However, the results of the immunohistochemical staining for basigin protein in the study by Xiao et al. did not correlate with their in situ data. Therefore, we sought to clarify the issue of basigin protein localization within the uterus during early pregnancy. Staining of uterine tissues with basigin antibodies show that basigin protein is expressed within the luminal and glandular epithelial cells at d 1 and 2 of pregnancy (Fig. 1, B and C) and is subsequently lost over the following 2 d (Fig. 1, D and E). Within the uterine stroma, basigin expression is detectable at d 4. On d 5, when the uterine stromal cells surrounding the invading embryo began to undergo decidualization, intense basigin staining was evident in these stromal cells (Fig. 2A). On d 6 when these stromal cells had transformed into mature decidual cells and formed the secondary decidualization zone, basigin expression disappeared in the secondary decidualization zone, whereas strong basigin immunoreactivity was still detected in the predecidual cells and the deep, undifferentiated stromal cells adjacent to the myometrium (Fig. 2B). On d 7 and 8, as the pregnancy progressed and the number of decidual cells increased, the area of basigin-positive cells was reduced (Fig. 2, C and D), suggesting that basigin expression is down-regulated during decidualization.
Figure 1.
Basigin expression in the mouse uterus during early pregnancy. Photos show d 1 (B), d 2 (C), d 3 (D), d 4 (E), and d 5 (F). Mouse uterine sections were immunostained with antimouse basigin antibody (B–F) or nonspecific IgG (A). The brown color represents basigin-positive cells. Bar, 50 μm.
Figure 2.
Basigin expression in implantation sites of the mouse uterus on d 5 (A), d 6 (B), d 7 (C), and d 8 (D) of pregnancy. Sections in implantation sites were immunostained with antimouse basigin antibody. Basigin is expressed in deep undifferentiated cells close to myometrium, and its expression is lost in decidual cells. d, Deep undifferentiated cell; m, myometrium. Arrow, Embryo. Bar, 500 μm.
Expression profiles for MMP-2, MMP-3, MMP-9, TIMP-3, and basigin mRNAs during in vitro decidualization
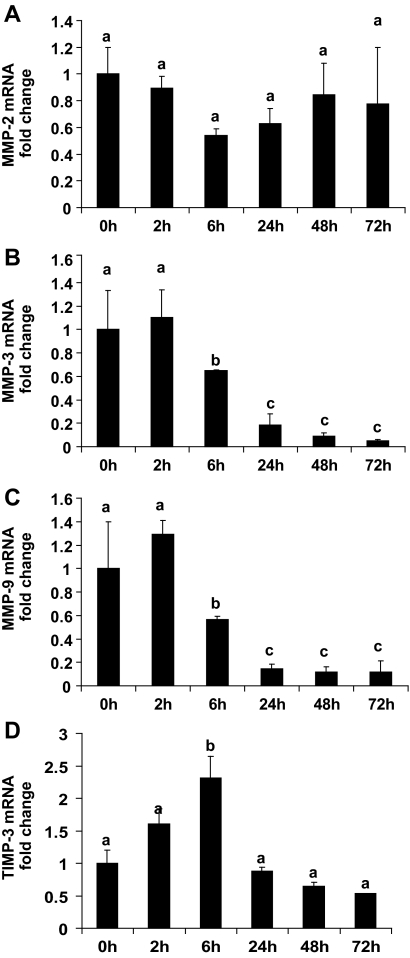
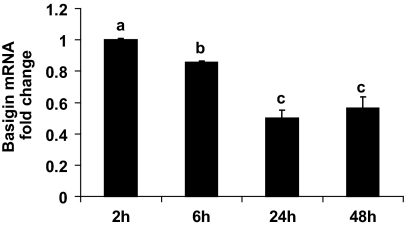
Several MMPs are expressed within the undifferentiated uterine stromal cells during early pregnancy, and their expression is subsequently lost as stromal cells decidualize (4,5,6). This expression pattern of MMPs is similar to that of basigin. To examine the potential role for basigin in regulating MMP gene expression in stromal cells, an in vitro uterine stromal cell culture system was characterized. Mouse uterine stromal cells isolated from d 4 pseudopregnant mice were cultured in the presence of estrogen and progesterone to induce decidualization, and the presence of MMP-2, MMP-3, MMP-9, TIMP-3, and basigin transcripts was determined by quantitative RT-PCR. After 6 h of culture, we observed a 10-fold increase in the mRNA level for ALP (data not shown), which is a well-known marker of decidualization (33). After 48 h of culture, most cells displayed the morphological characteristics of decidualizing stromal fibroblasts as they appeared larger, binucleate, and their cytoplasm was filled with lipid droplets (data not shown). More importantly, the mRNA levels for MMP-3 and MMP-9 in these cells were markedly decreased within 6 h of culture (Fig. 3). MMP-3 mRNA transcripts were reduced approximately 35% within 6 h, 82% within 24 h, and remained low over the following 48 h (Fig. 3B). A similar down-regulation of MMP-9 mRNA levels was observed (Fig. 3C), whereas the mRNA levels for MMP-2 remained constant during in vitro decidualization (Fig. 3A). Interestingly, there was a rapid and transient increase in TIMP-3 mRNA levels within 6 h of culture (Fig. 3D) with TIMP-3 mRNA levels elevated approximately 2.4-fold before decreasing to basal levels over the remaining time course. Furthermore, basigin mRNA levels decreased as a result of in vitro decidualization. Within 6 h the basigin mRNA levels were significantly reduced, and by 24 h the basigin transcript level dropped to approximately 50% of that present at 2 h (Fig. 4).
Figure 3.
Expression profiles of MMP-2, MMP-3, MMP-9, and TIMP-3 mRNA during in vitro decidualization. Stromal cells were isolated from d 4 pseudopregnant mouse uteri, cultured in the presence of estrogen and progesterone to induce decidualization and analyzed by quantitative RT-PCR for MMP-2 (A), MMP-3 (B), MMP-9 (C), and TIMP-3 (D). RNA was isolated from stromal cells at different time points during the 72 h after establishment of the primary cell culture. RNA levels were normalized to endogenous 18S and calibrated to the transcript levels at 0 h. The MMP-3 and MMP-9 mRNA levels are significantly reduced as stromal cells decidualize (B and C), whereas MMP-2 mRNA levels do not significantly change (A). The mRNA levels for TIMP-3 transiently increase. Data are means ± se from three separate experiments. Bars with different letters are significantly different from each other (P < 0.05).
Figure 4.
Expression profile of basigin mRNA during in vitro decidualization. The mRNA levels for basigin decrease during the 48 h after establishment of the culture. Data are means ± se from three separate experiments. Bars with different letters are significantly different from each other (P < 0.05).
Basigin treatment of stromal cells increases MMP-3 and MMP-9 expression
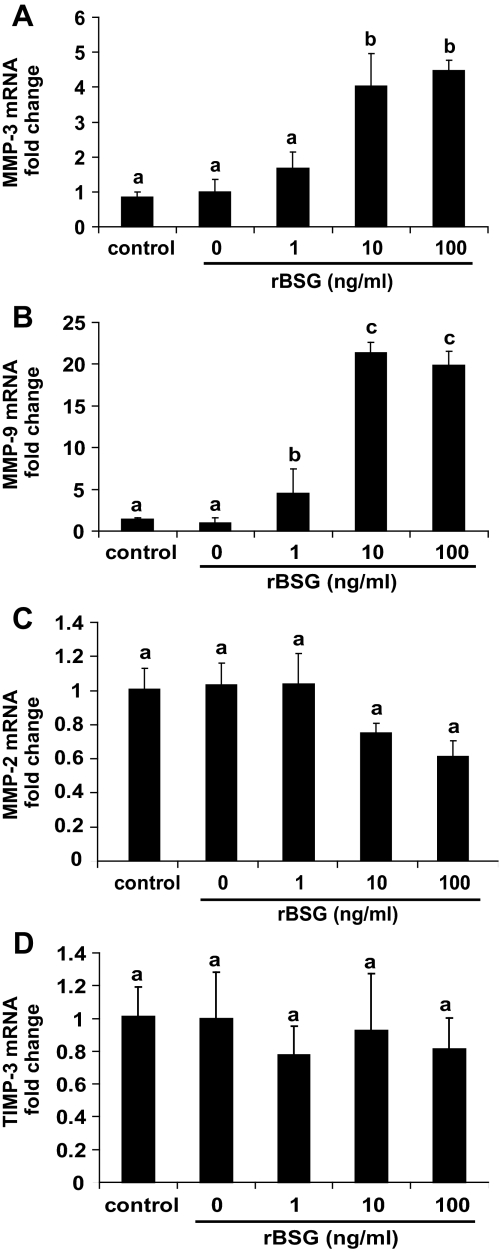
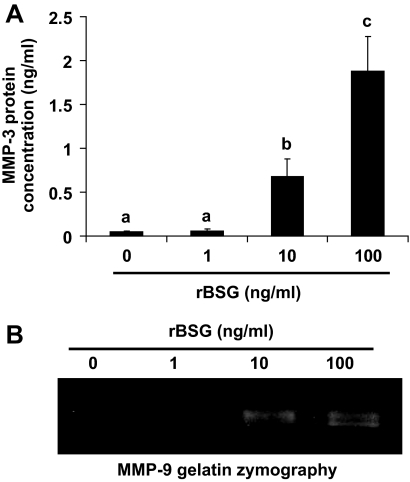
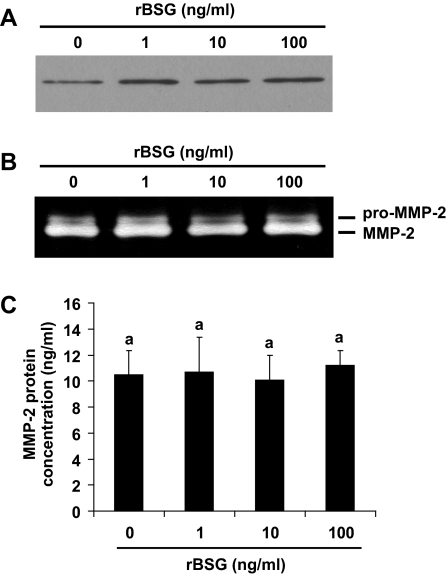
To test the possibility that the presence of basigin is sufficient to induce MMP expression by uterine stromal cells, cultures were treated with purified rBSG protein (30,31). Cells were grown in medium lacking progesterone and estradiol to prevent decidualization and treated with rBSG (0–100 ng/ml) for 24 h, and the expression of MMP-2, MMP-3, and MMP-9 was measured using quantitative RT-PCR. The rBSG used for the study was purified from a bacterial expression system and tested for the presence of endotoxin using the limulus amoebocyte lysate assay. To control for the effect of endotoxin on gene expression, all control samples were treated with LPS (containing endotoxin) equivalent to the amount of endotoxin present in the highest dose of rBSG protein. The results demonstrated that treatment with LPS does not have a measurable effect on MMP expression (Fig. 5, control). However, treatment of stromal cells with rBSG increased MMP-3 mRNA levels at least 4-fold (Fig. 5A), and MMP-9 mRNA levels 20-fold (Fig. 5B). In contrast, rBSG treatment did not affect MMP-2 mRNA levels (Fig. 5C) or TIMP-3 mRNA levels (Fig. 5D). To measure changes in MMPs secreted from stromal cells, conditioned medium was collected 24 h after treatment with rBSG and subjected to ELISA for MMP-3 (Fig. 6A) or gelatin zymography for MMP-9 (Fig. 6B). The results indicated that rBSG could stimulate significant increases in MMP-3 and MMP-9 secretion and activity but had no effect on the expression, secretion, or activity of MMP-2 (Fig. 7).
Figure 5.
Treatment of uterine stromal cells with rBSG increases MMP-3 and MMP-9 mRNA levels without affecting MMP-2 or TIMP-3 expression. Stromal cells were grown in the absence of estradiol and progesterone to prevent decidualization and treated with rBSG at 0 (untreated), 1, 10, or 100 ng/ml for 24 h. To measure the effects of LPS contamination on gene expression, the stromal cells were treated with 3 pg/ml LPS for 24 h (control). This is the amount of LPS present within the highest dose of rBSG being used. MMP-3 (A) and MMP-9 (B) mRNA levels were significantly increased by treatment with 10 ng/ml rBSG. MMP-2 (C) and TIMP-3 (D) mRNA levels were unaffected by the rBSG treatment. Fold changes in mRNA levels were analyzed by quantitative RT-PCR, normalized to 18S, and calibrated to control levels. Data are means ± se from four separate experiments. Bars with the different letter are significantly different from each other (P < 0.05).
Figure 6.
rBSG stimulates MMP-3 and -9 protein secretion from stromal cells. Stromal cells were treated with rBSG at 0, 1, 10, and 100 ng/ml for 24 h as described, and the conditioned medium from the cultures was collected. Concentrated samples were analyzed for MMP-3 (A) using ELISA or for MMP-9 (B) using gelatin zymography. Data are means ± se from three separate experiments. Bars with the different letter are significantly different from each other (P < 0.05).
Figure 7.
rBSG does not alter MMP-2 protein secretion. Stromal cells were treated with rBSG at 0 (control), 1, 10, and 100 ng/ml for 24 h as described, and the secretion of MMP-2 from stromal cells measured by immunoblotting for MMP-2 (A), gelatin zymography (B), and ELISA for MMP-2 (C).
Proinflammatory gene expression changes in response to rBSG
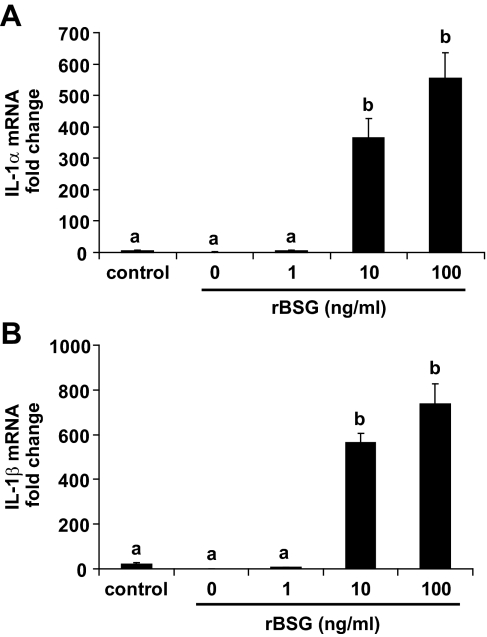
The proinflammatory cytokines IL-1α/β are potent inducers of MMP gene expression in uterine fibroblasts and play a critical role in uterine tissue remodeling (27). Because IL-1α/β are expressed in a number of cell types, including monocytes, macrophages, and fibroblasts (34), it is possible that basigin may indirectly stimulate MMP expression by inducing uterine stromal cells to produce IL-1α/β. To explore this possibility, quantitative RT-PCR analyses for IL-1α and IL-1β were performed using RNA from rBSG-treated stromal cells. The results demonstrated that rBSG treatment stimulated a profound increase (>400-fold) in both IL-1α and IL-1β using 10 ng/ml of rBSG (Fig. 8). This response was not a consequence of LPS contamination of the rBSG because treatment of the cells with 10-fold more LPS than that found in the rBSG treatment did not significantly increase expression of either cytokine.
Figure 8.
rBSG increases IL-1α (A) and IL-1β (B) mRNA levels in mouse uterine stromal cells. Stromal cells were treated with rBSG at 1, 10, and 100 ng/ml or 3 pg/ml LPS (control) for 24 h. RNA was isolated and subjected to quantitative RT-PCR for IL-1α and IL-1β. The data are presented as fold changes relative to untreated cells and represent the means from four independent experiments (means ± se) Bars with different letters are significantly different from each other (P < 0.05).
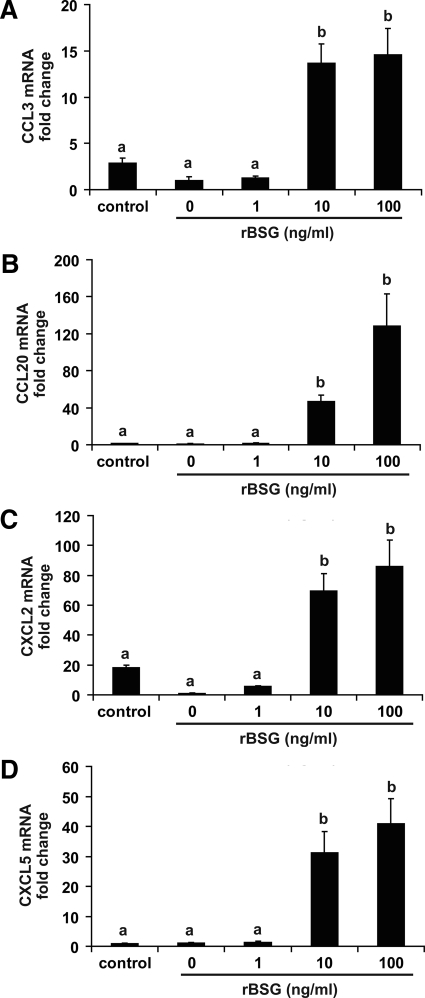
A number of chemokines are also expressed by stromal fibroblasts during implantation and are proposed to act as chemoattractants for a variety of leukocytes, including uterine natural killer cells, monocytes/macrophages, neutrophils, dendritic cells, and memory T cells in both the mouse (35,36,37,38) and human (39,40,41,42,43,44). Because IL-1 is a potent inducer of chemokine expression in fibroblasts, the evidence that rBSG stimulates IL-1 expression in stromal cells suggested the possibility that basigin might induce expression of chemokines needed to promote the recruitment of leukocytes thought to be important during early pregnancy. Two different classes of chemokines were examined based on their ability to attract different immune cells. The C-C motif chemokines, CCL3 [macrophage inflammatory protein (MIP)-1α] and CCL20 (MIP-3α), which are macrophage and dendritic cell/memory T-cell chemoattractants, as well as the C-X-C motif chemokines, CXCL2 (Groβ) and CXCL5 (ENA-78), which are monocyte and neutrophil chemoattractants, were measured in rBSG-treated cells. The results showed that all four chemokines were significantly up-regulated in response to stimulation with 10–100 ng/ml rBSG (Fig. 9).
Figure 9.
The mRNA levels for the cytokines CCL3, CCL20, CXCL2, and CXCL5 are significantly increased by treatment of mouse uterine stromal cells with rBSG. Cells were treated with rBSG at 1, 10, or 100 ng/ml or 3 pg/ml LPS (control) for 24 h as described. RNA were isolated and subjected to quantitative RT-PCR for CCL3 (A), CCL20 (B), CXCL2 (C), and CXCL5 (D). The data are presented as fold changes relative to untreated cells and represent the means from four independent experiments (means ± se). Bars with different letters are significantly different from each other (P < 0.05).
Basigin inhibits uterine stromal cell decidualization in vitro
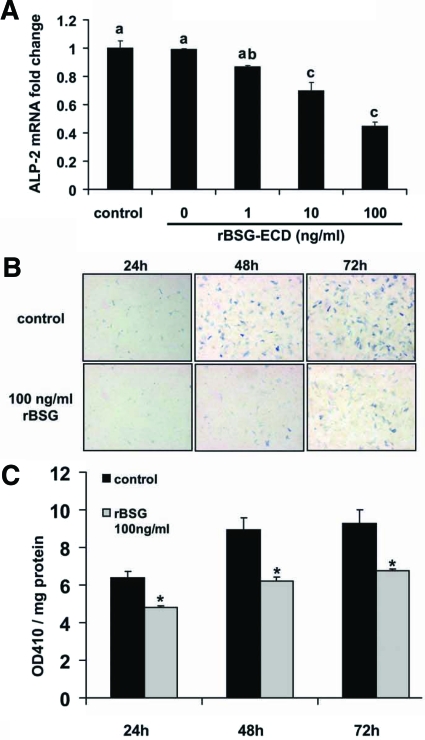
The expression of basigin in the deep undifferentiated stromal cells and down-regulation of basigin expression during decidualization suggests that basigin may inhibit uterine stromal cell decidualization. To test the effect of basigin on decidualization, we treated stromal cells with rBSG at 1, 10, and 100 ng/ml and measured ALP-2 mRNA levels and protein activities. Quantitative RT-PCR results indicated that the treatment of stromal cells with rBSG protein for 24 h significantly suppressed ALP-2 mRNA in a dose-dependent manner. Ten and 100 ng/ml rBSG protein suppressed ALP-2 mRNA levels by 30% (P < 0.05) and 50% (P < 0.05), respectively (Fig. 10A). We next compared the ALP histochemical staining of rBSG-treated (100 ng/ml) stromal cells with that of control (3 pg/ml LPS treated) cells. Consistent with the quantitative RT-PCR data, ALP histochemical staining was greatly reduced in the rBSG-treated cells compared with control cells at 24, 48, and 72 h, confirming that basigin had an inhibitory effect on stromal cell decidualization (Fig. 10B). We also measured ALP enzymatic activity in stromal cell lysate. This method is based on the ability of ALP protein to convert p-nitrophenyl phosphate to p-nitrophenol that presents as a yellow color in alkaline solution and can be read at OD 410 nm. A significant reduction in ALP activity was detected in 100 ng/ml rBSG-treated cells at 24, 48, and 72 h compared with the control (3 pg/ml LPS treated) cells (Fig. 10C).
Figure 10.
rBSG inhibits the expression and activity of ALP-2 in mouse uterine stromal cells. Mouse uterine stromal cells were treated with rBSG-ECD at 1, 10, and 100 or 3 pg/ml LPS (control) for 24 h. RNA was isolated and subjected to quantitative RT-PCR for ALP-2 (A). Fold changes in ALP-2 mRNA levels were quantitated and calibrated to control levels. Data are means ± se from three separate experiments. Bars with different letters are significantly different from each other (P < 0.05). Stromal cells were treated with 100 ng/ml rBSG or 3 pg/ml LPS (control) for 24, 48, or 72 h and then fixed and stained for ALP (B), or protein lysate was harvested for ALP enzyme activity measurement (C) (n = 6, means ± se). Blue color represents ALP protein. *, Significant difference between control and rBSG-treated cells (P < 0.05).
Basigin does not affect uterine stromal proliferation
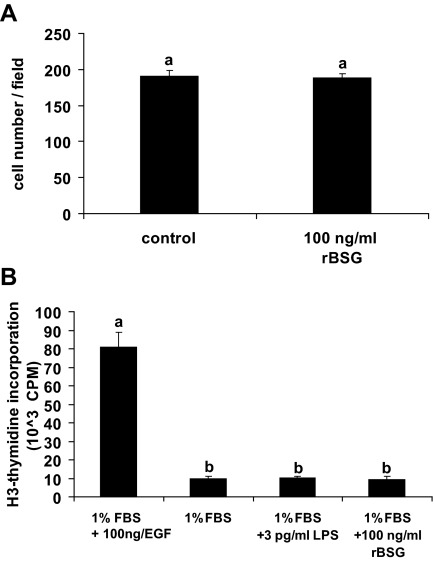
Stromal cell proliferation is the first step of decidualization. To examine the effect of basigin on stromal cell proliferation, we cultured stromal cells in the absence (treated with 3 pg/ml LPS) or presence of 100 ng/ml rBSG for 24 h. Wells were photographed and the number of cells in each field was counted. No difference was observed between control and rBSG-treated groups (Fig. 11A), suggesting that rBSG did not significantly affect stromal cell proliferation in vitro. Tritiated thymidine incorporation assays showed similar results as treatment of uterine stromal cells with 100 ng/ml rBSG for 24 h did not promote thymidine incorporation (Fig. 11B).
Figure 11.
rBSG does not affect stromal cell proliferation. A, Stromal cells isolated from d 4 pseudopregnant mouse uteri were treated with 100 ng/ml rBSG or 3 pg/ml LPS (control) for 24 h. Six fields per each well were randomly selected and photographed. The number of cells in each field was counted. B, Stromal cells were cultured in medium with 1% FBS and treated with 100 ng/ml rBSG-ECD, 100 ng/ml EGF (positive control), or 3 pg/ml LPS (negative control) for 24 h. Incorporation of [3H]thymidine was measured. Each bar represents the mean ± se of six wells. Bars with the same letter are not significantly different from one another. CPM, Counts per minute.
Discussion
Implantation in the mouse is a highly invasive process that requires the activity of MMPs expressed by the embryo, stromal fibroblasts, and the leukocytes that infiltrate the uterus during pregnancy. The differentiation of stromal fibroblasts surrounding each implanting embryo into decidual cells prevents the uncontrolled invasion of embryonic cytotrophoblast cells into the uterus. In the absence of decidualization, mouse blastocysts can invade deep into surrounding tissue as demonstrated in experiments using blastocysts transplanted under the kidney capsule (45). Therefore, the localized control of MMP expression within the uterus is critical for successful implantation. In this study, we present evidence supporting a role for basigin as an inducer of MMP-3 and MMP-9 expression by uterine stromal fibroblasts. Furthermore, the data presented indicate that basigin can induce the local expression of proinflammatory cytokines IL-1α/β and chemokines CCL3, CCL20, CXCL2, and CXCL5 by stromal cells. Our data suggest that the transient expression of basigin in the stroma before decidualization may contribute to the recruitment of leukocytes to the sites of implantation during early pregnancy in which they could participate in the process of stromal cell decidualization. In addition, our results showed that basigin expression declines as stromal cells decidualize and treatment with exogenous basigin inhibits or delays decidualization.
Previous studies examining the expression of MMPs by mouse uterine stromal cells during implantation demonstrated that undifferentiated uterine stromal fibroblasts express MMPs (4,5,6). The study by Bany et al. (6) showed that stromal cells express MMP-9 on d 4 of pregnancy. By d 6, when cytotrophoblast invasion into the stroma is established, MMP-9 was not expressed within the decidua, even though its expression persisted in the undifferentiated stromal cells surrounding the decidual zone. By d 8, MMP-9 expression was limited to the thin layer of undifferentiated stromal cells adjacent to the myometrium. Alexander et al. (4) demonstrated a similar expression pattern for MMP-3 during implantation.
The results shown in Figs. 1 and 2 demonstrate that the expression pattern for basigin within the pregnant mouse uterus correlates closely with those previously reported for MMP-3 and MMP-9. Basigin expression first appears within the uterine stroma before the differentiation of stromal fibroblasts into decidual cells. Subsequently, as these cells undergo decidualization, basigin protein is cleared from the decidual zone. This tight correlation between basigin and MMP-3 and MMP-9 expression in vivo led us to hypothesize that basigin might regulate MMP expression in mouse uterine fibroblasts. To test this hypothesis, we used a uterine stromal cell culture system in which uterine stromal cells can be induced to undergo in vitro decidualization in the presence of estradiol and progesterone (26). Uterine stromal cells isolated from d 4 pseudopregnant mice were characterized for changes in basigin, MMP-2, MMP-3, MMP-9, and TIMP-3 expression during the process of decidualization. After 6 h, basigin and MMP-3 and MMP-9 mRNA levels were significantly decreased. In contrast, TIMP-3 mRNA levels were significantly and transiently elevated before returning to baseline levels within 24 h. This transient increase in TIMP-3 mRNA is consistent with the observation that TIMP-3 mRNA expression in vivo is high on d 7 of pregnancy, decreases at d 8, and ceases by d 9 (4). The fact that MMP-2 mRNA levels remained relatively constant throughout the in vitro decidualization process is consistent with previous studies demonstrating that MMP-2 mRNA tends to be constitutively expressed in a variety of cells (46,47,48). These results further demonstrate that the in vitro decidualization procedure accurately recapitulates the temporal gene expression changes occurring in vivo for basigin, MMP-3, MMP-9, and TIMP-3 and supports the hypothesis that basigin may regulate MMP-3 and MMP-9 expression within the uterine stroma.
To directly test whether basigin is sufficient to induce gene expression changes in the undifferentiated stroma, cultures were treated with a recombinant form of human basigin. The rBSG protein used in this study is a biologically active, nonglycosylated form of human basigin consisting of the extracellular domain of human basigin (30,31). The results demonstrated that basigin markedly increases MMP-3 and MMP-9 mRNA and protein levels in a dose- and time-dependent manner without affecting the expression of MMP-2 or TIMP-3. The physiological significance of MMP-3 and MMP-9 up-regulation by basigin in stromal fibroblasts before implantation is not entirely clear. MMP-3 and MMP-9 digest many ECM components including gelatin, laminin, fibronectin, vitronectin, collagen IV, and proteoglycans (49). MMPs are involved in many biological processes including growth, development, wound healing, arthritis, and cancer (50), and they may contribute to these processes by degrading the ECM or releasing and activating growth factors embedded within the ECM (51,52). For example, cleavage of proteoglycans by MMP-3 and MMP-9 leads to release of fibroblast growth factor and vascular endothelial growth factor, which facilitates tumor progression (52,53). In addition, MMP-3 can digest IGF-binding proteins to release free IGFs, leading to increases in cellular proliferation (54). Because decidualization of uterine stromal cells is a process involving first cell proliferation followed by cell differentiation, the expression of basigin in the undifferentiated uterine stromal fibroblasts might promote stromal cell proliferation and/or inhibit cell decidualization. Using ALP-2 as a marker for decidual cells, we found that rBSG suppressed transformation of stromal cells into decidual cells. However, rBSG did not directly stimulate uterine stromal cell proliferation. These results suggest that expression of basigin by uterine stromal cells somehow regulates the timing of decidualization and the loss of basigin expression allows the stromal cells to undergo full differentiation to become decidual cells.
MMP expression in human uterine stromal cells can also be regulated by the cytokine IL-1 (27). IL-1 is expressed by a number of cell types, including fibroblasts, and it is a potent stimulator of MMP expression in monocytes, macrophages and neutrophils (34). Previous work from our laboratory demonstrated that both IL-1β and basigin (EMMPRIN) induce expression of MMPs in cultured human uterine fibroblasts (55). However, the possibility that basigin might stimulate IL-1β expression in stromal cells was not explored. In the current study, we demonstrate for the first time that basigin can induce the expression of IL-1α and IL-1β in mouse uterine fibroblasts. These novel findings suggest that basigin may play a central role in the process of decidualization by transiently inducing MMP expression directly as well as indirectly through its ability to promote IL-1 expression. Furthermore, human uterine stromal cells lose their responsiveness to IL-1 as they undergo decidualization (56), suggesting that the down-regulation of basigin in the decidual cells in vivo provides a possible mechanism to limit MMP and Il-1 expression during implantation.
Embryo implantation can be considered an inflammatory process due to the numerous leukocytes that infiltrate the uterus and the increased expression of inflammatory factors. Increased levels of cytokines, including Il-1β, can induce the expression of chemoattractant cytokines (chemokines) by uterine stromal cells, and it has been proposed that such chemokines function to induce leukocyte infiltration to the endometrium in the mouse (56) and human (41,42,44). Two types of chemokines involved in the recruitment of leukocytes to the uterus during early pregnancy include the C-C and C-X-C ligands. In this study, we examined the possibility that the C-C chemokines, CCL3 (MIP-1α) and CCL20 (MIP-3α), which are macrophage and dendritic cell/memory T cell chemoattractants (40,57,58), and the C-X-C motif chemokines CXCL2 (Groβ) and CXCL5 (ENA-78), which are monocyte and neutrophil chemoattractants (40,44), might be expressed in response to basigin treatment. Mouse and human uterine stromal fibroblasts express these four chemokines during implantation, and the expression of two C-X-C chemokines (CXCL2 and CXCL5) is directly regulated by IL-1β. The novel finding that basigin can increase the transcript levels of these chemokines in cultured uterine stromal cells supports the hypothesis that basigin can regulate gene expression in stromal fibroblasts and leukocytes during implantation. The mechanism(s) by which basigin mediates changes in gene expression has not yet been explored, and there are at least two possible explanations for these changes. It is possible that basigin might induce the localized expression of these leukocyte chemoattractants by first increasing IL-1 expression, or basigin may directly induce expression of chemokines by uterine stromal cells. In either case, the evidence presented herein demonstrating a temporal-spatial regulation of basigin expression within the uterus during early pregnancy is consistent with basigin playing a role in leukocyte recruitment to the uterus. To further clarify the mechanism of basigin action during implantation in vivo, future studies will require the development and use of a conditional basigin knockout mouse model.
Footnotes
This work was supported by National Institutes of Health/National Institute of Child Health and Human Development Grant U54 HD40093 (to R.A.N.) and a Lalor Foundation Postdoctoral Fellowship (to R.J.B.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 1, 2008
Abbreviations: ALP, Alkaline phosphatase; CCL, C-C motif chemokine; CXCL, C-X-C chemokine; ECM, extracellular matrix; FBS, fetal bovine serum; HBSS, Hanks’ balanced salt solution; LPS, lipopolysaccharide; MIP, macrophage inflammatory protein; MMP, matrix metalloproteinase; rBSG, recombinant human basigin; TIMP, tissue inhibitors of metalloproteinase.
References
- Abrahamsohn PA, Zorn TM 1993 Implantation and decidualization in rodents. J Exp Zool 266:603–628 [DOI] [PubMed] [Google Scholar]
- Glasser SR, Lampelo S, Munir MI, Julian J 1987 Expression of desmin, laminin and fibronectin during in situ differentiation (decidualization) of rat uterine stromal cells. Differentiation 35:132–142 [DOI] [PubMed] [Google Scholar]
- Wewer UM, Damjanov A, Weiss J, Liotta LA, Damjanov I 1986 Mouse endometrial stromal cells produce basement-membrane components. Differentiation 32:49–58 [DOI] [PubMed] [Google Scholar]
- Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, Werb Z 1996 Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development 122:1723–1736 [DOI] [PubMed] [Google Scholar]
- Das SK, Yano S, Wang J, Edwards DR, Nagase H, Dey SK 1997 Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse uterus during the peri-implantation period. Dev Genet 21:44–54 [DOI] [PubMed] [Google Scholar]
- Bany BM, Harvey MB, Schultz GA 2000 Expression of matrix metalloproteinases 2 and 9 in the mouse uterus during implantation and oil-induced decidualization. J Reprod Fertil 120:125–134 [DOI] [PubMed] [Google Scholar]
- Daimon E, Wada Y 2005 Role of neutrophils in matrix metalloproteinase activity in the preimplantation mouse uterus. Biol Reprod 73:163–171 [DOI] [PubMed] [Google Scholar]
- Salamonsen LA, Zhang J, Brasted M 2002 Leukocyte networks and human endometrial remodelling. J Reprod Immunol 57:95–108 [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z 2001 How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside EJ, Jackson MM, Herington AC, Edwards DR, Harvey MB 2001 Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 are key regulators of extracellular matrix degradation by mouse embryos. Biol Reprod 64:1331–1337 [DOI] [PubMed] [Google Scholar]
- Leco KJ, Edwards DR, Schultz GA 1996 Tissue inhibitor of metalloproteinases-3 is the major metalloproteinase inhibitor in the decidualizing murine uterus. Mol Reprod Dev 45:458–465 [DOI] [PubMed] [Google Scholar]
- Miyauchi T, Kanekura T, Yamaoka A, Ozawa M, Miyazawa S, Muramatsu T 1990 Basigin, a new, broadly distributed member of the immunoglobulin superfamily, has strong homology with both the immunoglobulin V domain and the β-chain of major histocompatibility complex class II antigen. J Biochem (Tokyo) 107:316–323 [DOI] [PubMed] [Google Scholar]
- Kuno N, Kadomatsu K, Fan QW, Hagihara M, Senda T, Mizutani S, Muramatsu T 1998 Female sterility in mice lacking the basigin gene, which encodes a transmembrane glycoprotein belonging to the immunoglobulin superfamily. FEBS Lett 425:191–194 [DOI] [PubMed] [Google Scholar]
- Arora K, Gwinn WM, Bower MA, Watson A, Okwumabua I, MacDonald HR, Bukrinsky MI, Constant SL 2005 Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol 175:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenko V, Zybarth G, O'Connor M, Dai WW, Franchin G, Hao T, Guo H, Hung HC, Toole B, Gallay P, Sherry B, Bukrinsky M 2002 Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem 277:22959–22965 [DOI] [PubMed] [Google Scholar]
- Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K 1995 The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res 55:434–439 [PubMed] [Google Scholar]
- Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Bugelski P, Yan L 2005 Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res 65:3193–3199 [DOI] [PubMed] [Google Scholar]
- Foda HD, Rollo EE, Drews M, Conner C, Appelt K, Shalinsky DR, Zucker S 2001 Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340). Am J Respir Cell Mol Biol 25:717–724 [DOI] [PubMed] [Google Scholar]
- Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan QW, Miyauchi T, Toyama Y, Kuno N, Yuasa S, Takahashi M, Senda T, Taguchi O, Yamamura K, Arimura K, Muramatsu T 1998 A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol 194:152–165 [DOI] [PubMed] [Google Scholar]
- Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C 2004 The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene 23:956–963 [DOI] [PubMed] [Google Scholar]
- Taylor PM, Woodfield RJ, Hodgkin MN, Pettitt TR, Martin A, Kerr DJ, Wakelam MJ 2002 Breast cancer cell-derived EMMPRIN stimulates fibroblast MMP2 release through a phospholipase A(2) and 5-lipoxygenase catalyzed pathway. Oncogene 21:5765–5772 [DOI] [PubMed] [Google Scholar]
- Kataoka H, DeCastro R, Zucker S, Biswas C 1993 Tumor cell-derived collagenase-stimulatory factor increases expression of interstitial collagenase, stromelysin, and 72-kDa gelatinase. Cancer Res 53:3154–3158 [PubMed] [Google Scholar]
- Guo H, Zucker S, Gordon MK, Toole BP, Biswas C 1997 Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem 272:24–27 [PubMed] [Google Scholar]
- Sameshima T, Nabeshima K, Toole BP, Yokogami K, Okada Y, Goya T, Koono M, Wakisaka S 2000 Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett 157:177–184 [DOI] [PubMed] [Google Scholar]
- Yang JM, Xu Z, Wu H, Zhu H, Wu X, Hait WN 2003 Overexpression of extracellular matrix metalloproteinase inducer in multidrug resistant cancer cells. Mol Cancer Res 1:420–427 [PubMed] [Google Scholar]
- Daikoku T, Tranguch S, Friedman DB, Das SK, Smith DF, Dey SK 2005 Proteomic analysis identifies immunophilin FK506 binding protein 4 (FKBP52) as a downstream target of Hoxa10 in the periimplantation mouse uterus. Mol Endocrinol 19:683–697 [DOI] [PubMed] [Google Scholar]
- Singer CF, Marbaix E, Lemoine P, Courtoy PJ, Eeckhout Y 1999 Local cytokines induce differential expression of matrix metalloproteinases but not their tissue inhibitors in human endometrial fibroblasts. Eur J Biochem 259:40–45 [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, White CA, Jones RL, Salamonsen LA 2005 Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update 11:613–630 [DOI] [PubMed] [Google Scholar]
- Rossi M, Sharkey AM, Vigano P, Fiore G, Furlong R, Florio P, Ambrosini G, Smith SK, Petraglia F 2005 Identification of genes regulated by interleukin-1β in human endometrial stromal cells. Reproduction 130:721–729 [DOI] [PubMed] [Google Scholar]
- Chen L, Nakai M, Belton Jr RJ, Nowak RA 2007 Expression of extracellular matrix metalloproteinase inducer and matrix metalloproteinases during mouse embryonic development. Reproduction 133:405–414 [DOI] [PubMed] [Google Scholar]
- Belton Jr RJ, Chen L, Mesquita FS, Nowak RA 2008 Basigin-2 is a cell surface receptor for soluble basigin ligand. J Biol Chem 283:17805–17814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao LJ, Chang H, Ding NZ, Ni H, Kadomatsu K, Yang ZM 2002 Basigin expression and hormonal regulation in mouse uterus during the peri-implantation period. Mol Reprod Dev 63:47–54 [DOI] [PubMed] [Google Scholar]
- Finn CA, McLaren A 1967 A study of the early stages of implantation in mice. J Reprod Fertil 13:259–267 [DOI] [PubMed] [Google Scholar]
- Chung KF 2001 Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl 34:50s–59s [PubMed] [Google Scholar]
- Wood GW, Hausmann E, Choudhuri R 1997 Relative role of CSF-1, MCP-1/JE, and RANTES in macrophage recruitment during successful pregnancy. Mol Reprod Dev 46:62–69; discussion 69–70 [DOI] [PubMed] [Google Scholar]
- Pollard JW, Lin EY, Zhu L 1998 Complexity in uterine macrophage responses to cytokines in mice. Biol Reprod 58:1469–1475 [DOI] [PubMed] [Google Scholar]
- Kyaw Y, Hasegawa G, Takatsuka H, Shimada-Hiratsuka M, Umezu H, Arakawa M, Naito M 1998 Expression of macrophage colony-stimulating factor, scavenger receptors, and macrophage proliferation in the pregnant mouse uterus. Arch Histol Cytol 61:383–393 [DOI] [PubMed] [Google Scholar]
- Chantakru S, Kuziel WA, Maeda N, Croy BA 2001 A study on the density and distribution of uterine natural killer cells at mid pregnancy in mice genetically ablated for CCR2, CCR 5 and the CCR5 receptor ligand, MIP-1α. J Reprod Immunol 49:33–47 [DOI] [PubMed] [Google Scholar]
- Arima K, Nasu K, Narahara H, Fujisawa K, Matsui N, Miyakawa I 2000 Effects of lipopolysaccharide and cytokines on production of RANTES by cultured human endometrial stromal cells. Mol Hum Reprod 6:246–251 [DOI] [PubMed] [Google Scholar]
- Nasu K, Arima K, Kai K, Fujisawa K, Nishida M, Miyakawa I 2001 Expression of epithelial neutrophil-activating peptide 78 in cultured human endometrial stromal cells. Mol Hum Reprod 7:453–458 [DOI] [PubMed] [Google Scholar]
- Sun B, Nasu K, Fukuda J, Mine S, Nishida M, Miyakawa I 2002 Expression of macrophage inflammatory protein-3α in an endometrial epithelial cell line, HHUA, and cultured human endometrial stromal cells. Mol Hum Reprod 8:930–933 [DOI] [PubMed] [Google Scholar]
- Fukuda J, Nasu K, Sun B, Mine S, Kawano Y, Miyakawa I 2003 Expression of growth-regulated oncogene beta in an endometrial epithelial cell line, HHUA, and cultured human endometrial cells. J Reprod Immunol 59:61–70 [DOI] [PubMed] [Google Scholar]
- Nishida M, Nasu K, Fukuda J, Kawano Y, Narahara H, Miyakawa I 2004 Down-regulation of interleukin-1 receptor type 1 expression causes the dysregulated expression of CXC chemokines in endometriotic stromal cells: a possible mechanism for the altered immunological functions in endometriosis. J Clin Endocrinol Metab 89:5094–5100 [DOI] [PubMed] [Google Scholar]
- Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, Tang C, Abrahams VM, Krikun G, Lockwood CJ 2006 Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol 72:60–73 [DOI] [PubMed] [Google Scholar]
- Kirby DR 1960 Development of mouse eggs beneath the kidney capsule. Nature 187:707–708 [DOI] [PubMed] [Google Scholar]
- Xu P, Wang Y, Piao Y, Bai S, Xiao Z, Jia Y, Luo S, Zhuang L 2001 Effects of matrix proteins on the expression of matrix metalloproteinase-2, -9, and -14 and tissue inhibitors of metalloproteinases in human cytotrophoblast cells during the first trimester. Biol Reprod 65:240–246 [DOI] [PubMed] [Google Scholar]
- Crawford HC, Matrisian LM 1996 Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein 49:20–37 [DOI] [PubMed] [Google Scholar]
- Curry Jr TE, Osteen KG 2003 The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 24:428–465 [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM 2001 Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol 13:534–540 [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z 2002 New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174 [DOI] [PubMed] [Google Scholar]
- Mott JD, Werb Z 2004 Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 16:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D 2000 Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2:737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA 1996 The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem 271:10079–10086 [DOI] [PubMed] [Google Scholar]
- Fowlkes JL, Serra DM, Bunn RC, Thrailkill KM, Enghild JJ, Nagase H 2004 Regulation of insulin-like growth factor (IGF)-I action by matrix metalloproteinase-3 involves selective disruption of IGF-I/IGF-binding protein-3 complexes. Endocrinology 145:620–626 [DOI] [PubMed] [Google Scholar]
- Braundmeier AG, Fazleabas AT, Lessey BA, Guo H, Toole BP, Nowak RA 2006 Extracellular matrix metalloproteinase inducer regulates metalloproteinases in human uterine endometrium. J Clin Endocrinol Metab 91:2358–2365 [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Hirota Y, Koga K, Hirata T, Yano T, Ayabe T, Tsutsumi O, Taketani Y 2003 Endometrial stromal cells undergoing decidualization down-regulate their properties to produce proinflammatory cytokines in response to interleukin-1β via reduced p38 mitogen-activated protein kinase phosphorylation. J Clin Endocrinol Metab 88:2236–2241 [DOI] [PubMed] [Google Scholar]
- Dudley DJ, Spencer S, Edwin S, Mitchell MD 1995 Regulation of human decidual cell macrophage inflammatory protein-1α (MIP-1α) production by inflammatory cytokines. Am J Reprod Immunol 34:231–235 [DOI] [PubMed] [Google Scholar]
- Nasu K, Narahara H, Matsui N, Kawano Y, Tanaka Y, Miyakawa I 1999 Platelet-activating factor stimulates cytokine production by human endometrial stromal cells. Mol Hum Reprod 5:548–553 [DOI] [PubMed] [Google Scholar]