Abstract
TSH receptor (TSHR) antibodies and hyperthyroidism are induced by immunizing mice with adenovirus encoding the TSHR or its A-subunit. Depleting regulatory T cells (Treg) exacerbates thyrotoxicosis in susceptible BALB/c mice and induces hyperthyroidism in normally resistant C57BL/6 mice. Vitamin D plays an important role in immunity; high dietary vitamin D intake suppresses (and low intake enhances) adaptive immune responses. Vitamin D-induced immunosuppression may enhance Treg. Therefore, we hypothesized that decreased vitamin D intake would mimic Treg depletion and enhance hyperthyroidism induced by A-subunit adenovirus immunization. BALB/c mice had a reduced ability vs. C57BL/6 mice to generate the active metabolite of vitamin D (1,25-dihydroxyvitamin D3). Vitamin D deficiency induced subtle immune changes in BALB/c (not C57BL/6) mice. Compared with mice fed regular chow, vitamin D-deprived BALB/c mice had fewer splenic B cells and decreased interferon-γ responses to mitogen and lacked memory T-cell responses to A-subunit protein. However, vitamin D deficiency did not alter TSHR antibody responses measured by ELISA, TSH binding inhibition, or cAMP generation from TSHR-expressing cells. Unexpectedly, compared with vitamin D-sufficient mice, vitamin D-deficient BALB/c mice had lower preimmunization T4 levels and developed persistent hyperthyroidism. This difference was unrelated to the immunological changes between vitamin D-deficient or -sufficient animals. Previously, we found that different chromosomes or loci confer susceptibility to TSHR antibody induction vs. thyroid function. Our present studies provide evidence that an environmental factor, vitamin D, has only minor effects on induced immunity to the TSHR but directly affects thyroid function in mice.
Vitamin D deficiency in BALB/c mice reduces baseline thyroid function and prolongs hyperthyroidism induced by thyrotropin receptor-adenovirus immunization, but surprisingly has only minor effects on immune responses.
It is now well recognized that in addition to its role in skeletal homeostasis, vitamin D plays a role in both innate and adaptive immunity (reviewed in Ref. 1). The vitamin D receptor is expressed on monocytes and activated lymphocytes (2), and the biologically active metabolite of vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], is a potent modulator of T-cell responses (3). Because of this activity, the immunological effects of vitamin D have been the subject of intense investigation in animal models of autoimmune disease. Increased vitamin D intake prevented the spontaneous development of type 1 diabetes in NOD mice (4), reduced the risk for systemic lupus erythematosus in MRL mice (5), and retarded the progression of collagen-induced arthritis in DBA/1 mice (6). Similarly, in a model of multiple sclerosis induced by immunizing SJL mice with myelin basic protein, administration of 1,25(OH)2D3 abrogated the development of myelin basic protein antibody (7) and reduced the severity of encephalomyelitis (6,7). On the other hand, in a model of thyroiditis induced by immunizing CBA mice with thyroglobulin, 1,25(OH)2D3 administration alone had little effect, and the combination of 1,25(OH)2D3 and cyclosporin A was required to reduce the severity of thyroiditis (8).
Several mechanisms may play a role in vitamin D-mediated T-cell suppression. For example, 1,25(OH)2D3 induced a T helper 1 (Th1) to Th2 cytokine shift in NOD mice immunized with the pancreatic autoantigen glutamic acid decarboxylase 65 (9). These findings are consistent with the ability of the Th2 cytokine IL-4 to inhibit the onset of diabetes in mice of this strain (10). In addition, vitamin D blocked progression to type 1 diabetes in NOD mice by enhancing the development of regulatory T cells (Treg) (11). Vitamin D also plays a role in B lymphocyte homeostasis; for example, 1,25(OH)2D3 regulates the proliferation and differentiation of activated human B cells (12).
Fewer investigations have examined the impact of vitamin D deficiency on autoimmune disease. As might be anticipated from its immunomodulatory effects, reduction of dietary vitamin D accelerated type I diabetes in NOD mice (13) and enhanced disease severity in experimental autoimmune encephalomyelitis (14). Although immune mechanisms appeared to be involved in increased disease, antigen-specific responses were not investigated in these studies. In human rheumatoid arthritis, disease activity is inversely correlated with plasma levels of the major circulating form of vitamin D, 25-hydroxyvitamin D3 [25(OH)D3], suggesting a role for reduced vitamin D intake in these individuals and/or lower exposure to sunlight (15). Genetic variability in the proteins involved in vitamin D metabolism may also contribute to autoimmune disease. In type 1 diabetes (16) and autoimmune thyroid disease (17), polymorphisms have been reported in the CYP27B1 gene that encodes the 1-α-hydroxylase enzyme responsible for converting 25(OH)D3 to active 1,25(OH)2D3. In addition, a vitamin D receptor polymorphism has been associated with Graves’ disease in some studies (18,19) although not in others (20,21).
Graves’ hyperthyroidism is a common autoimmune disease in which autoantibodies to the TSH receptor (TSHR) stimulate the thyroid gland (reviewed in Ref. 22). Thyroid-stimulating antibodies and elevated T4 levels are induced in mice by immunization with adenovirus encoding the TSHR (23) or its A-subunit (24). Some mouse strains, such as BALB/c, are susceptible to disease induction, but others (like C57BL/6) remain euthyroid despite developing TSHR antibodies (23,25,26). However, depleting Treg induces hyperthyroidism in some C57BL/6 mice and enhances thyroid hormone levels in the susceptible BALB/c strain (27,28).
As mentioned, increased vitamin D intake exerts its immunosuppressive effects in some models by enhancing Treg. Also, depleting Treg enhances Graves’-like hyperthyroidism induced by TSHR-adenovirus immunization in BALB/c and C57BL/6 mice. Therefore, we predicted that vitamin D deficiency would induce, or enhance, the severity of Graves’ disease in TSHR A-subunit adenovirus-immunized C57BL/6 and BALB/c mice, respectively. However, our expectation of increased hyperthyroidism was only partially fulfilled. Rather than modulating the immune response, our study revealed an unanticipated effect of vitamin D deficiency on the ability of the thyroid gland to maintain its hyperthyroid state.
Materials and Methods
Adenovirus preparation, mouse strains, vitamin D-deficient diet, and adenovirus immunization
Adenovirus (RGD vector) expressing the TSHR A-subunit [amino acid residues 1–289, A-sub-Ad (29) and null adenovirus (Con-Ad) (30)] have been described. Both adenoviruses were propagated in HEK293 cells (American Type Culture Collection, Manassas, VA), purified on CsCl density gradients, and viral particle concentration was determined by measuring the absorbance at 260 nm (31).
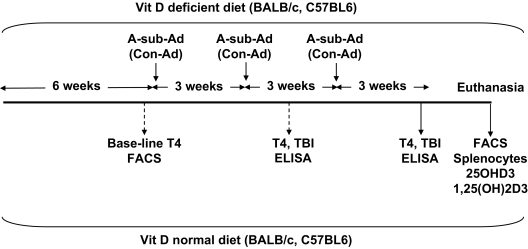
Female BALB/cJ and C57BL/6J mice (∼6 wk of age; Jackson Laboratory, Bar Harbor, ME) were divided into two groups; for each strain, one group was maintained on vitamin D-deficient chow (AIN-93M W/No Vitamin D; TestDiet, Richmond, IN), and the other group received regular mouse chow. After 6 wk, blood was drawn from mice of both strains, and 7 d later, when the animals were 13 wk old, mice were immunized three times at three weekly intervals with A-sub-Ad or Con-Ad (∼109 particles per injection). Blood was drawn 1 wk after two injections, and mice were euthanized 1 month after the third injection (Fig. 1). All animal studies were approved by the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center and performed with the highest standards of care in a pathogen-free facility.
Figure 1.
Schedule for investigating BALB/c and C57BL/6 mice maintained on vitamin D-deficient or normal diet and immunized three times with A-sub-Ad or Con-Ad. Also shown are the time intervals at which blood was drawn and mice were euthanized. Splenocytes were analyzed for lymphocyte subsets by flow cytometry (FACS) and responses to A-subunit protein and mitogens. Sera were characterized for vitamin D and its active metabolite, T4, and TSHR antibodies.
Serum T4, vitamin D, and its active metabolite
Total T4 in mouse sera was measured in undiluted serum (25 μl) by RIA using a kit (Diagnostic Products Corp., Los Angeles, CA). T4 concentrations were computed from standards provided in the kit. Sera obtained by cardiac puncture at euthanasia (and stored at −80 C) were tested for circulating levels of 25(OH)D3 and 1,25(OH)2D3 levels by Dr. Bruce Hollis (Medical University of South Carolina, Charleston, SC).
RT-PCR (real-time) analysis of mRNA for the mouse CYP27b1 gene (Cyp27b)
Mouse kidneys were removed at euthanasia, diced into pieces, and transferred to RNAlater (QIAGEN, Valencia, CA) for storage at 4 C. RNA was extracted using the RNeasy Total RNA extraction kit (QIAGEN) and eluted in ribonuclease-free elution solution, and aliquots (1.5 μg) were reverse-transcribed using Powerscript Maloney murine leukemia virus reverse transcriptase as described by the manufacturer (Applied Biosystems, Foster City, CA).
Expression of the mouse Cyp27b1 mRNA was quantified using an ABI 7700 sequence detection system (Applied Biosystems) as described previously (32). Approximately 50 ng cDNA were used per reaction. All reactions were multiplexed with the housekeeping GAPDH gene, provided as an optimized control probe labeled with VIC fluorochrome (Applied Biosystems), enabling data to be expressed in relation to an internal reference to allow for sampling differences. Data were obtained as Ct values (the cycle number at which logarithmic PCR plots cross a calculated threshold line) and used to determine ΔCt values (Ct of target gene − Ct of housekeeping GAPDH gene). PCR amplification of target gene cDNA was carried out using the TaqMan mouse gene expression assay for Cyp27b1 (Mm 01165922). Amplification of cDNA was carried out under the following conditions: 50 C for 2 min and 95 C for 10 min, followed by 40 cycles of 95 C for 15 sec and 60 C for 1 min. Reactions were performed in triplicate and expressed as mean ± sem ΔCt values.
Flow cytometric analysis
Splenocyte suspensions were prepared and tested by flow cytometry for expression of T cells (CD3), B cells (CD19), T-cell subsets (CD4, CD8), and Treg (CD4+CD25+, CD4+CD122+, and FoxP3) using the following antibodies conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE): anti-CD3-FITC, anti-CD19-PE, anti-CD4-FITC, anti-CD8-PE or -FITC, anti-CD25(clone PC61)-PE, and anti-CD122(clone TB-b1)-PE (all from BD Biosciences, San Jose CA). Flow cytometry was performed using a Becton Dickinson FACScan flow cytometer. Cells positive for FoxP3, CD25, and CD4 were enumerated using a kit (eBioscience, San Diego, CA) and CyAn ADP (DakoCytomation, Carpinteria, CA).
Splenocyte responses to TSHR protein and mitogens
Splenocytes (200-μl aliquots of ∼5 × 105 cells) were incubated in round-bottomed 96-well plates with or without purified TSHR A-subunit protein (10 μg/ml). Recombinant A-subunit protein secreted by Chinese hamster ovary (CHO) cells with an amplified transgenome (33) was purified from culture supernatants by affinity chromatography (34). Responses to the mitogens (concanavalin A, 5 μg/ml; Sigma-Aldrich, St. Louis, MO) and pokeweed mitogen (10 μg/ml; Invitrogen-Life Technologies, Inc., Carlsbad, CA) were also tested. Culture medium was RPMI 1640, 10% heat-inactivated fetal bovine serum, 2 mm glutamine, 1 mm sodium pyruvate, 50 μm β-mercaptoethanol, 50 μg/ml gentamicin, and 100 U/ml penicillin (Invitrogen). After 5–6 d (37 C, 5% CO2), the plates were centrifuged to remove cell debris and supernatants stored (−80 C). Interferon-γ (IFN-γ) was measured by ELISA (100 μl supernatant) using capture and biotinylated detection-antibodies (Biosciences, San Jose, CA).
TSHR antibodies
TSHR antibodies were studied using three assays: TSH binding inhibition (TBI), A-subunit protein ELISA, and thyroid-stimulating antibody (TSAb). TBI was measured using a kit (Kronus, Boise, ID). Serum aliquots (25 μl) were incubated with detergent-solubilized TSHR; [125I]TSH was added, and the TSHR-antibody complexes were precipitated with polyethylene glycol. TBI values were calculated from the following formula: [1 − (TSH binding in test serum − nonspecific binding)/(TSH binding in normal serum − nonspecific binding)] × 100.
ELISA for TSHR antibody was performed using wells coated with A-subunit protein (see above; 5 μg/ml) as previously described (24). After incubation with test sera (duplicate aliquots, diluted 1:100), antibody binding was detected with horseradish peroxidase-conjugated goat antimouse IgG (Sigma Chemical Co., St. Louis, MO), and the signal was developed with o-phenylenediamine and H2O2. The IgG subclass distribution of TSHR antibodies was determined using A-subunit (1 μg/ml)-coated ELISA wells and goat antimouse antibodies specific for mouse IgG1, IgG2a, and IgG2b (Caltag Laboratories, Burlingame, CA). The data for IgG class antibodies and IgG subclasses are reported as OD at 490 nm.
TSAb activity was measured using CHO cells expressing the human TSHR (35) as previously described (36). In addition, we measured cAMP generated in response to CHO cells expressing the mouse TSHR. Murine TSHR cDNA (from Dr. Peter Kopp, Northwestern University, Chicago, IL) was transferred to the vector pcDNA5/FRT (Invitrogen). Amino acid mutations in the TSHR, three reported previously (37), were converted to the wild-type sequence (GenBank U02602) using the QuikChange site-directed mutagenesis kit (Stratagene, San Diego CA). The mouse TSHR in pcDNA5/FRT was transfected into CHO cells using Fugene HD (Roche, Indianapolis, IN) and selection with hygromycin B (Invitrogen; 50 μg/ml) to generate a stable cell line. Expression was confirmed by flow cytometry as described previously (38) using mouse monoclonal antibody 2C11 (Morphosys, Raleigh, NC) and detection with fluoresceinated antimouse IgG (Caltag).
The assay for TSAb was performed as follows. TSHR-CHO cells in 96-well plates were incubated (80 min, 37 C) with test sera diluted 1:20 in Ham’s F12 supplemented with 10 mm HEPES (pH 7.4) and 1 mm isobutylmethylxanthine. After aspirating the medium, intracellular cAMP was extracted with ethanol, evaporated to dryness, and resuspended in Dulbecco’s PBS. Samples (12 μl) were assayed using the LANCE cAMP kit (PerkinElmer, Boston, MA). Human (or mouse) TSAb activity was expressed as a percentage of cAMP values attained with sera from control, unimmunized mice.
Statistical analyses
The statistical significance of differences between the magnitude of responses in multiple groups was determined by ANOVA and testing between two groups by Mann-Whitney rank sum test or, when normally distributed, by Student’s t test. Differences in proportions were tested by χ2.
Results
Vitamin D, its active metabolite, and Cyp27b1
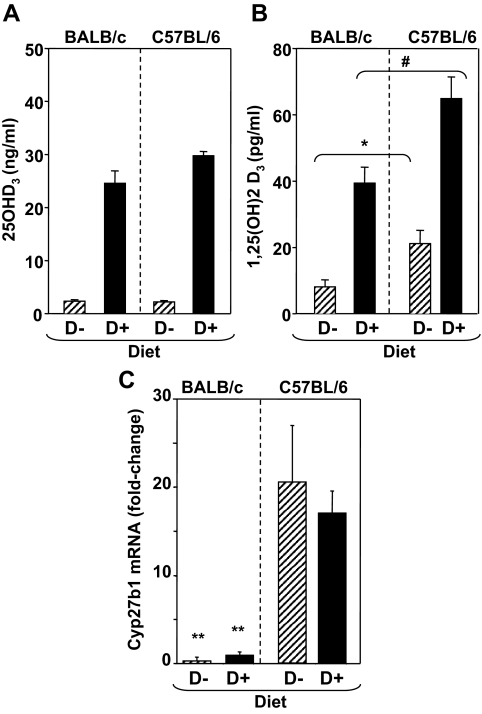
Compared with mice on regular mouse chow, BALB/c and C57BL/6 mice on the deficient (D−) diet had virtually undetectable serum levels of 25(OH)D3, the major circulating form of vitamin D (Fig. 2A). As expected, levels of the active metabolite, 1,25(OH)2D3, were also lower in mice of both strains on the deficient (D−) chow. Nevertheless, 1,25(OH)2D3 was still detectable in mice on vitamin D-deficient chow. It was also noted that 1,25(OH)2D3 levels were significantly lower in BALB/c than in C57BL/6 mice, regardless of diet (Fig. 2B). To investigate this issue further, we analyzed expression in the kidney of Cyp27b1, the gene that encodes the enzyme 1α-hydroxylase that catalyzes synthesis of 1,25(OH)2D3. Levels of Cyp27b1 mRNA were approximately 17-fold lower in BALB/c than in C57BL/6 mice, regardless of diet (Fig. 2C). These findings provide a potential explanation for the lower serum levels of 1,25(OH)2D3 in BALB/c vs. C57BL/6 mice (Fig. 2B). Overall, these differences suggested that any effects of diet were more likely to be seen in BALB/c than in C57BL/6 mice.
Figure 2.
Vitamin D [25(OH)D3], (panel A) the active metabolite [1,25(OH)2D3 (panel B)], and Cyp27B1 mRNA (panel C) in BALB/c and C57BL/6 mice maintained on vitamin D-deficient (D−) or sufficient (D+) diet. Values for 25(OH)D3 (nanograms per milliliter) and 1,25(OH)2D3 (picograms per milliliter) are shown as the mean + sem (n = 5 mice per group). Cyp27B1 mRNA values are shown as fold change in mRNA levels (+sem) relative to BALB/c mice, which were assigned an arbitrary value of 1 (n = 4 BALB/c D−, n = 5 BALB/c D+, n = 10 C57BL/6 D−, and n = 10 C57BL/6 D+). Values significantly different between BALB/c and C57BL/6 mice are indicated as follows: for 1,25(OH)2D3 on D− diet *, P = 0.001 (t test); on D+ diet #, P = 0.001 (t test); for Cyp27B1 mRNA between BALB/c and C57BL/6 (D+ or D−) **, P = 0.001 (t test).
Lymphocyte subsets and splenocyte responses to TSHR protein
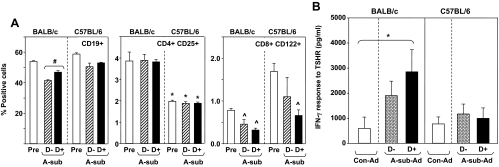
Splenocytes were isolated from mice on regular (D+) vs. deficient (D−) diet euthanized before, and a separate set after, A-sub-Ad immunization. In BALB/c (but not C57BL/6) mice, the proportion of spleen B cells (CD19+) was higher in animals on D+ vs. D− diet (Fig. 3A, left). This elevated percentage of B cells is reflected in enhanced IFN-γ generation by splenocytes stimulated with pokeweed mitogen (supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) but not with the T-cell mitogen concanavalin A (data not shown). No other lymphocyte subsets were changed by D− vs. D+ diet.
Figure 3.
A, B cells and Treg in BALB/c and C57BL/6 mice on vitamin D-deficient (D−) and sufficient (D+) diet. Cell suspensions were prepared from mice before immunization (Pre) and at euthanasia after the third A-sub-Ad immunization. Data are shown as the percentage of cells positive for CD19, CD4-CD25, and CD8-CD122; error bars are sd before immunization (n = 2 mice) and sem (n = 3, after immunization). Values significantly different are indicated as follows: left panel, CD19+ cells from BALB/c D− vs. D+ mice after immunization: #, P = 0.010 (t test); middle panel, CD4+CD25+ cells from BALB/c before immunization vs. before and after immunization from C57BL/6 D− and D+: *, P < 0.05 (ANOVA); right panel, CD122+CD8+ cells from C57BL before immunization vs. after immunization in BALB/c D+ and D− and C57BL D+: ^, P < 0.05 (ANOVA). B, Splenocyte response to A-subunit protein in BALB/c and C57BL/6 mice maintained on vitamin D-deficient (D−) or -sufficient diet (D+). Cell suspensions were prepared from mice immunized three times with A-sub-Ad immunization or Con-Ad (n = 5 mice per group). Responses were measured as IFN-γ generation. The data shown are the difference between IFN-γ generated in response to A-subunit protein vs. culture medium only. Values significantly different are indicated as follows: *, Con-Ad vs. A-sub-Ad D+, P < 0.05 (ANOVA).
Unexpectedly, these splenocyte analyses revealed striking differences between BALB/c and C57BL/6 mice: 1) reduced CD4+CD25+ cells in C57BL/6 vs. BALB/c and 2) increased CD8+CD122+ cells before immunization in C57BL/6 vs. after immunization in BALB/c (Fig. 3A middle and right, respectively). No significant strain differences were observed for the proportions of T cells; the CD4 to CD8 ratio was reduced in C57BL/6 mice compared with untreated and D+ BALB/c animals; and the small numbers of FoxP3+, CD3+, C4+ cells precluded detecting a strain difference (supplemental Fig. S2).
Turning to TSHR-specific immunity, we tested splenocyte memory responses to TSHR A-subunit protein. After A-sub-Ad immunization, splenocytes from BALB/c mice on D+ but not D− diet generated significantly more IFN-γ (P < 0.05) when challenged with A-subunit protein than splenocytes from Con-Ad immunized mice (Fig. 3B, left). In contrast, splenocytes from C57BL/6 mice had no detectable memory response to A-subunit protein, regardless of diet (Fig. 3B, right). We do not know the reason for the lack of response in this mouse strain. It should be mentioned that in our previous studies of splenocyte responses, immunization was initiated in 6-wk-old mice. In the present investigation, mice were exposed to vitamin D-sufficient or -deficient diets for 6 wk and first immunized at 12 wk of age. Therefore, another difference between BALB/c and C57BL/6 mice may be a more persistent memory response in the former than in the latter.
TSHR antibodies (TBI and ELISA)
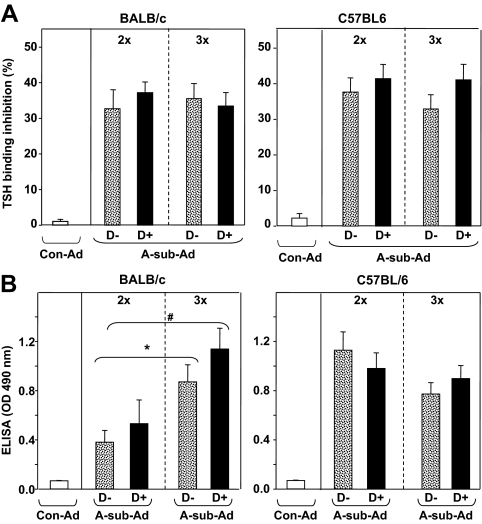
TSHR antibodies were measured by TBI, a clinical assay used in Graves’ patients. As expected, both BALB/c and C57BL/6 mice developed comparable levels of TBI activity in response to A-sub-Ad immunization. However, there were no significant differences between mice on D− vs. D+ diet after two or three immunizations (2x or 3x, Fig. 4A).
Figure 4.
TSHR antibodies, measured by TBI and ELISA induced by A-sub-Ad immunization in BALB/c and C57BL/6 mice maintained on vitamin D-deficient (D−) or -sufficient (D+) diet. Sera were tested 1 wk after two immunizations (2x) and at euthanasia after three immunizations (3x). Data are shown as the mean + sem for A-sub-Ad-injected BALB/c (n = 13 per group) and C57BL/6 (n = 17 per group) mice; Con-Ad-immunized mice, n = 4–5 mice per group. A, TBI values are shown as percent inhibition of TSH binding; B, IgG class antibody binding to TSHR A-subunit in ELISA; data are given as the OD units at 490 nm (sera diluted 1:100). Values significantly different are indicated as follows: BALB/c D− 2x vs. 3x: *, P = 0.008, t test; BALB/c D+ 2x vs. 3x: #, P = 0.010, rank sum test.
Unlike the TBI assay that detects antibody activity to conformationally intact TSHR protein, binding to TSHR A-subunit in ELISA measures antibodies to both conformational and nonconformational epitopes. By ELISA, TSHR antibody levels increased from the second to the third immunization in BALB/c mice on either D− or D+ diet (Fig. 4B, left). After two immunizations, TSHR antibody levels by ELISA were higher in C57BL/6 than BALB/c mice (OD values >1.0 vs. ∼0.5, respectively) regardless of D− and D+ diet. However, TSHR antibody levels did not increase after the third immunization in C57BL/6 mice (Fig. 4B, right). This lack of increased TSHR antibodies measured by ELISA in C57BL/6 mice may reflect the absent memory splenocyte response to A-subunit protein described above.
TSHR antibody IgG subclasses, analyzed by ELISA, were similar in mice of either strain on vitamin D-deficient or -sufficient diet. However, the two strains exhibited marked differences in the IgG subclass composition of TSHR antibodies, In BALB/c mice, TSHR antibodies were predominantly IgG2a after two immunizations and shifted after the third immunization to comparable contributions by IgG1 and IgG2a (supplemental Fig. S3, upper panels). In C57BL/6 mice, TSHR antibodies were evenly distributed between IgG1, IgG2a, and IgG2b, although the levels were lower for all three subclasses) after the third immunization (supplemental Fig. S3, lower panels).
TSAb
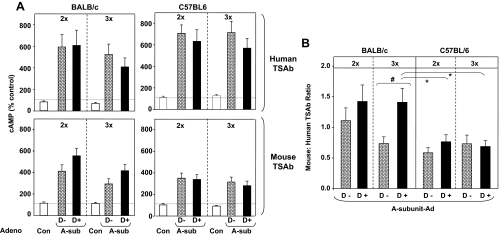
Stimulating antibodies were assessed in separate assays using CHO cells expressing the human TSHR (hTSAb) and the mouse TSHR (mTSAb). There were no statistically significant differences between D− and D+ mice of either strain or between values for mice immunized twice vs. three times. However, hTSAb levels tended to be higher for C57BL/6 than BALB/c mice, particularly after the third immunization, and for both strains, mTSAb levels were lower than hTSAb values (Fig. 5A).
Figure 5.
TSAb in BALB/c and C57BL/6 mice maintained on vitamin D-deficient (D−) or -sufficient (D+) diet. Sera were tested 1 wk after two immunizations (2x) and a month after the third immunization (3x). These sera were previously studied for TBI activity and binding to TSHR A-subunit in ELISA (Figs. 5 and 6). A, TSAb activity determined using CHO cells expressing the human TSHR (human TSAb; upper panels) and the mouse TSHR (mouse TSAb; lower panels). Data are shown as percent cAMP generated by sera from Con-Ad-immunized mice. B, Relative mouse TSAb to human TSAb activity expressed as a ratio. Values significantly different are indicated as follows: #, BALB/c D+ (3x) and D− (3x) (rank sum test, P = 0.027); *, BALB/c D+ (3x) vs. C57BL/6 D+ 2x and 3x (ANOVA, P < 0.05).
Further insight into the differences between the two mouse strains was provided by calculating the ratio of mTSAb to hTSAb. Ratios greater than 1.0, as in BALB/c mice on a vitamin D+ diet, reflect preferential recognition of the mouse TSHR. In contrast, ratios less than 1.0, as in C57BL/6 mice irrespective of diet, indicate preferential recognition of the human TSHR (Fig. 5B, right). The mTSAb to hTSAb ratios were significantly greater (P < 0.05) for BALB/c mice on D+ diet than for C57BL/6 mice on D+ or D− diet (Fig. 5B, left). In BALB/c mice, the mTSAb to hTSAb ratio was significantly higher (P = 0.027) in D+ mice immunized three times than in D− mice after the third immunization.
Development of hyperthyroidism
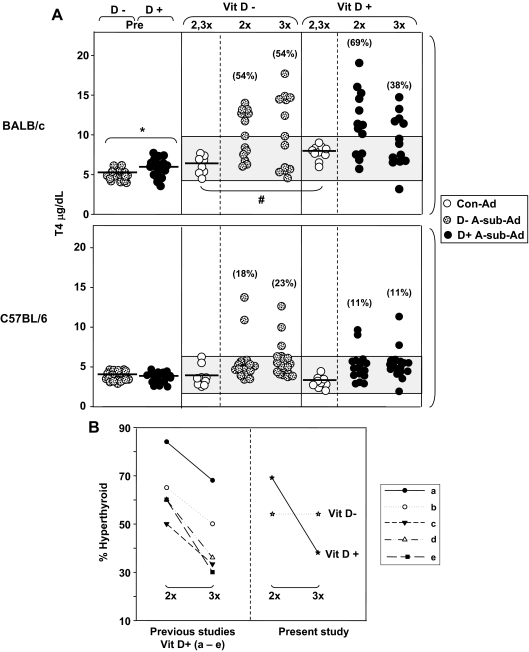
Before immunization, T4 levels in BALB/c mice were slightly but significantly lower on vitamin D− vs. D+ diet (Fig. 6A, upper left). Serum T4 was also significantly lower in Con-Ad-immunized BALB/c on D− than on D+ diet. Neither of these parameters was different for C57BL/6 mice on vitamin D-deficient vs. -sufficient diet (Fig. 6A, lower left).
Figure 6.
A, Vitamin D deficiency reduces preimmunization serum T4 and maintains hyperthyroidism in BALB/c (not C57BL/6 mice) immunized with A-sub-Ad. T4 levels (micrograms per deciliter) are shown for individual animals. Preimmunization (Pre) sera were obtained by tail bleeding after 6 wk on vitamin D-deficient (D−) or -sufficient (D+) diet (n = 18 per group). Subsequently, mice were maintained on their respective diets and immunized with A-sub-Ad (BALB/c, n = 13; C57BL/6, n = 17) or Con-Ad (n = 4–5 mice). Sera were tested 1 wk after two injections of A-sub-Ad (2x), and mice were euthanized a month after the third immunization (3x). The shaded areas represent the mean ± 2 sd values (in each strain) for D− and D+ mice after two and three (2,3x) immunizations with Con-Ad. The percentage of hyperthyroid animals for each group is in parentheses. Values significantly different are indicated as follows: preimmunization BALB/c D− vs. D+: *, P = 0.024 (t test); Con-Ad immunization BALB/c D− vs. D+: #, P = 0.006 (t test). B, Percentages of BALB/c mice that are hyperthyroid after two (2x) and three (3x) A-sub-Ad immunizations. Observations in the present study for vitamin D-sufficient (D+) and -deficient (D−) BALB/c mice are compared with data from five other studies (a–e, Refs. 24,25,26, 29, and Misharin A., B. Rapoport, and S. M. McLachlan, unpublished). Significantly fewer mice remained hyperthyroid after three immunizations (χ2 P = 0.029 for these five other studies of BALB/c mice on regular mouse chow; P = 0.013 combining previous data with those for D + mice).
Turning to A-sub-Ad-immunized animals, more BALB/c than C57BL/6 mice developed elevated T4 levels (compared with Con-Ad-immunized animals), consistent with previous studies (23,25,26). After two immunizations, over 50% of BALB/c on either D− or D+ diet developed hyperthyroidism. After the third A-sub-Ad injection, the percentage of hyperthyroid BALB/c on regular mouse chow (D+ diet) dropped from 69 to 38%. In contrast, at the same time interval, in BALB/c mice on D− diet, the proportion of hyperthyroid animals was not decreased and remained at 50% (Fig. 6A, middle and right).
These findings prompted us to examine other studies for the proportions of BALB/c mice that remain hyperthyroid after the third immunization. In five separate experiments, four previously published (24,25,26,29) and one on-going (Misharin, A., B. Rapoport, and S. M. McLachlan, unpublished data), a significantly lower number of vitamin D-sufficient mice were hyperthyroid after the third immunization compared with the second immunization (χ2; Fig. 6B, left). These findings are consistent with our present data for BALB/c mice on a vitamin D+ diet but strikingly different from the pattern in mice of the same strain maintained on a vitamin D− diet and studied in parallel (Fig 6B, right).
Discussion
Antibodies to the TSHR and hyperthyroidism are effectively induced by immunizing mice with adenovirus encoding the TSHR or its A-subunit (23,24). Depleting Treg enhances the degree of hyperthyroidism levels in the susceptible BALB/c strain and induces elevated serum T4 in some normally resistant C57BL/6 mice (27,28). Moreover, the increased T4 levels in both strains was attributed (at least in part) to higher levels of TSAb after Treg depletion (26,27). Turning to vitamin D, high dietary intake suppresses, and low intake enhances, adaptive immune responses. The immunosuppressive effects of increased vitamin D intake operates in some models by enhancing Treg (11). Consequently, in the present study, we tested the possibility that decreased vitamin D in the diet would mimic regulatory T-cell depletion and thereby increase the magnitude or incidence of hyperthyroidism in BALB/c and C57BL/6.
On a vitamin D-deficient diet, serum 25(OH)D3 levels were almost undetectable (as expected) but the active metabolite 1,25(OH)2D3 was clearly still present in both mouse strains, albeit at low concentrations. However, regardless of vitamin D-deficient or normal diet, 1,25(OH)2D3 levels were higher in C57BL/6 than BALB/c mice. Consistent with the strain differences for serum 1,25(OH)2D3, mRNA levels for Cyp27b1 [the gene encoding the enzyme 1α-hydroxylase responsible for catalyzing synthesis of 1,25(OH)2 D3] were about 17 times higher in the kidneys from C57BL/6 vs. BALB/c mice, regardless of vitamin D diet. These data for 1,25(OH)2D3 and Cyp27B1 demonstrate an important difference between BALB/c and C57BL/6 strains in handling vitamin D that has the potential to impact both systemic (endocrine) and localized responses to this hormone.
Vitamin D deficiency induced subtle changes in the response of BALB/c (but not C57BL/6) mice to immunization with A-sub-Ad. Compared with mice on regular chow, vitamin D-deprived BALB/c mice had 1) significantly fewer splenic B cells; 2) a decreased IFN-γ response to pokeweed mitogen, and 3) lack of memory T-cell responses to A-subunit protein. In contrast, the absence of dietary vitamin D did not alter TSHR antibody responses measured by ELISA (which detects binding to native or denatured TSHR protein) or assessed by TBI or thyroid stimulation (TSAb), both of which require conformationally intact TSHR. Interestingly, in BALB/c mice, the shift of TSHR antibodies from IgG2a after two immunizations toward IgG1 after the third immunization suggests a late Th2 cytokine influence. However, there is no evidence for Th1/Th2 cytokine changes associated with the absence or presence of dietary vitamin D.
Our findings of only a minor influence of vitamin D depletion on TSHR-induced immunity contrasts with that of other models in which immune responses and/or disease severity were enhanced (or reduced) by increased (or reduced) exposure to the active vitamin D metabolite. For example, we did not observe a Th2 to Th1 shift in TSHR antibody IgG subclasses as might have been expected from the reverse change (Th1 to Th2) induced by 1,25(OH)2D3 in the memory response of NOD mice to a pancreatic autoantigen (9). It should be emphasized that the mouse strains used to investigate the effect of vitamin D exposure, namely type 1 diabetes, systemic lupus erythematosus, or encephalomyelitis (NOD, MRL, and SJL) were not those used to study experimental Graves’ disease (BALB/c and C57BL/6).
Many studies to date of the interaction between vitamin D and the immune system have focused on the effects of localized production of 1,25(OH)2D3, which is profoundly influenced by circulating levels of 25(OH)D3 (39,40). However, the absence of any significant effect of 25(OH)D3 depletion in immune responses of either BALB/c or C57BL/6 mice in our study suggests an alternative model for vitamin D and thyroid autoimmunity. Specifically, the differential patterns of CD19+ cells and IFN-γ responses to the TSHR (discussed below) were consistent with the variations in 1,25(OH)2D3 between the two strains. It is therefore tempting to speculate that the effects of vitamin D on thyroid autoimmunity may be due to an endocrine rather than autocrine mechanism.
Despite the small effects of dietary vitamin D in experimentally induced Graves’ disease, we observed marked immunological differences between BALB/c and C57BL/6 mice. First, there was a difference in the proportions of Treg measured using anti-CD25 and anti-CD122. After A-subunit immunization, CD25+CD24+ T cells were higher and CD122+CD8+ cells were lower in BALB/c than in C57BL/6 mice. Second, unlike BALB/c mice on a vitamin D-sufficient diet, C57BL/6 mice lacked memory responses to A-subunit protein. Third, the increase in TSHR antibody measured by ELISA between the second and third immunizations in BALB/c mice was not observed in C57BL/6 mice. Fourth, there was a difference in TSHR antibody IgG subclasses: the IgG2a bias in BALB/c after two immunizations vs. similar contributions from IgG1, IgG2a, and IgG2b in C57BL/6 after two and three immunizations.
The final immunological difference between BALB/c and C57BL/6 mice concerned TSAb, the antibody activity most relevant to Graves’ disease. We compared TSAb measured with eukaryotic cells expressing the human TSHR vs. the same cells expressing the mouse TSHR (∼80% amino acid homology). The human TSHR A-subunit is the immunogen and would be expected to induce high levels of human-specific TSAb. On the other hand, antibodies that cross-react strongly with the mouse TSHR would theoretically be more potent stimulators of endogenous thyroid function. Indeed, BALB/c mice, that are susceptible to induced hyperthyroidism, preferentially recognized the mouse TSHR. In contrast, C57BL/6 mice, which are resistant to hyperthyroidism, preferentially recognized the human TSHR. Better antibody recognition of a related antigen than the immunogen, termed heteroclicity, has been reported for several proteins including (for example) preferential recognition of bovine or porcine insulin after immunization with human insulin (41).
Rather than affecting the immune response, the most important effect of vitamin D deficiency was on the thyroid. Before immunization, BALB/c mice deprived of dietary vitamin D had lower serum T4 levels than mice of the same strain on regular diet (D+). Similarly, T4 levels were significantly lower in D− vs. D+ mice immunized with control adenovirus. Nevertheless, these vitamin D-deprived animals developed hyperthyroidism in response to A-sub-Ad immunization. Moreover, the proportion of hyperthyroid animals (54%) after two immunizations was maintained after the third immunization. This persistent hyperthyroidism contrasts with the decline in the proportion of hyperthyroid animals on a regular vitamin D diet observed in the present study as well as in five investigations (one ongoing and four previously reported) (24,25,26,29).
The continuing hyperthyroid state in vitamin D-deprived BALB/c mice could not be attributed to any immunological difference (as described above). TSAb specific for the mouse TSHR tended to rise (rather than fall) after the third immunization in both D+ and D− BALB/c mice. Moreover, the ratio of mouse-specific TSAb: human-specific TSAb was higher in the vitamin D+-treated animals (fewer of which were hyperthyroid), not lower than in vitamin D− mice. For these reasons, it seems likely that persistent hyperthyroidism can only be attributed to a difference in the sensitivity to TSAb of the thyroid in BALB/c mice on a vitamin D-deficient diet.
Previous data suggest a direct role for vitamin D (or its active metabolite) on the thyroid. For example, 1,25(OD)2D3 dose-dependently inhibits iodide uptake and growth induced by TSH in FRTL5 cells (42,43). By up-regulating the molecule Bcl-2, the active metabolite protects human thyrocytes from programmed cell death (44). Moreover, active 1,25(OD)2D3 regulates translocation of T3, at least in the cerebellum, by increasing the binding capacity of the cytosolic T3-binding protein (45). Most recently, it was demonstrated that the vitamin D receptor antagonist Elocitol impaired cytokine responses in CD4+ T cells as well as in human thyrocytes (46). We do not know which (if any) of these mechanisms is involved in our observations concerning thyroid function in mice on a vitamin D-deficient diet.
In conclusion, we found a strain-specific difference in handling vitamin D, with BALB/c mice having reduced ability (compared with C57BL/6 mice) to generate the active metabolite. When deprived of vitamin D, BALB/c mice display important differences in the outcome of TSHR A-subunit immunization, notably persistent hyperthyroidism. None of the subtle immunological changes induced by reduced vitamin D intake were associated with the difference in thyroid function of vitamin D-deprived or -sufficient mice. Previously, in studying the genetic basis for murine Graves’ disease, we observed that different chromosomes or loci confer susceptibility to induction of TSHR antibodies vs. thyroid function (26,36). Our present studies provide evidence of a role for an environmental factor, vitamin D, on thyroid function. However, vitamin D deficiency appears to have a lesser effect than genetic factors in experimental Graves’ disease. It is nevertheless possible that, although obscured by genetic heterogeneity, vitamin D may have similar effects in humans.
Acknowledgments
We thank Dr. Bruce Hollis, Medical University of South Carolina, Charleston, SC, for analyzing vitamin D and 1,25(OH)2D3 levels in mouse sera and Dr. Peter Kopp for providing us with the mouse TSHR cDNA. We are also grateful for contributions by Dr. Boris Catz, Los Angeles.
Footnotes
This work was supported by National Institutes of Health Grants DK54684 (to S.M.M.), DK19289 (to B.R.), and AR050626 (to M.H.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 16, 2008
Abbreviations: A-sub-Ad, Adenovirus expressing the TSHR A-subunit; CHO, Chinese hamster ovary; Con-Ad, null adenovirus; FITC, fluorescein isothiocyanate; hTSAb, human TSAb; IFN-γ, interferon-γ; mTSAb, mouse TSAb; PE, phycoerythrin; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3; TBI, TSH binding inhibition; Th1, T helper 1; Treg, regulatory T cells; TSAb, thyroid-stimulating antibody; TSHR, TSH receptor.
References
- Adams JS, Hewison M 2008 Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 4:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC 1983 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science 221:1181–1183 [DOI] [PubMed] [Google Scholar]
- Lemire JM, Archer DC, Beck L, Spiegelberg HL 1995 Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr 125:1704S–1708S [DOI] [PubMed] [Google Scholar]
- Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R 1994 Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia 37:552–558 [DOI] [PubMed] [Google Scholar]
- Lemire JM, Ince A, Takashima M 1992 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity 12:143–148 [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE, DeLuca HF 1998 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr 128:68–72 [DOI] [PubMed] [Google Scholar]
- Lemire JM, Archer DC 1991 1,25-Dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest 87:1103–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier C, Gepner P, Sadouk M, Charreire J 1990 In vivo beneficial effects of cyclosporin A and 1,25-dihydroxyvitamin D3 on the induction of experimental autoimmune thyroiditis. Clin Immunol Immunopathol 54:53–63 [DOI] [PubMed] [Google Scholar]
- Overbergh L, Decallonne B, Waer M, Rutgeerts O, Valckx D, Casteels KM, Laureys J, Bouillon R, Mathieu C 2000 1α,25-Dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524–543). Diabetes 49:1301–1307 [DOI] [PubMed] [Google Scholar]
- Rapoport MJ, Jaramillo A, Zipris D, Lazarus AH, Serreze DV, Leiter EH, Cyopick P, Danska JS, Delovitch TL 1993 Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med 178:87–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L 2002 A 1α,25-dihydroxyvitamin D3 analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 51:1367–1374 [DOI] [PubMed] [Google Scholar]
- Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE 2007 Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 179:1634–1647 [DOI] [PubMed] [Google Scholar]
- Giulietti A, Gysemans C, Stoffels K, van Etten E, Decallonne B, Overbergh L, Bouillon R, Mathieu C 2004 Vitamin D deficiency in early life accelerates type 1 diabetes in non-obese diabetic mice. Diabetologia 47:451–462 [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE, DeLuca HF 1996 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA 93:7861–7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B 2007 Vitamin D in rheumatoid arthritis. Autoimmun Rev 7:59–64 [DOI] [PubMed] [Google Scholar]
- Bailey R, Cooper JD, Zeitels L, Smyth DJ, Yang JH, Walker NM, Hypponen E, Dunger DB, Ramos-Lopez E, Badenhoop K, Nejentsev S, Todd JA 2007 Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes 56:2616–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani MA, Regulla K, Segni M, Krause M, Hofmann S, Hufner M, Herwig J, Pasquino AM, Usadel KH, Badenhoop K 2002 Vitamin D 1α-hydroxylase (CYP1α) polymorphism in Graves’ disease, Hashimoto’s thyroiditis and type 1 diabetes mellitus. Eur J Endocrinol 146:777–781 [DOI] [PubMed] [Google Scholar]
- Ban Y, Ban Y, Taniyama M, Katagiri T 2000 Vitamin D receptor initiation codon polymorphism in Japanese patients with Graves’ disease. Thyroid 10:375–380 [DOI] [PubMed] [Google Scholar]
- Pani MA, Regulla K, Segni M, Hofmann S, Hufner M, Pasquino AM, Usadel KH, Badenhoop K 2002 A polymorphism within the vitamin D-binding protein gene is associated with Graves’ disease but not with Hashimoto’s thyroiditis. J Clin Endocrinol Metab 87:2564–2567 [DOI] [PubMed] [Google Scholar]
- Collins JE, Heward JM, Nithiyananthan R, Nejentsev S, Todd JA, Franklyn JA, Gough SC 2004 Lack of association of the vitamin D receptor gene with Graves’ disease in UK Caucasians. Clin Endocrinol (Oxf) 60:618–624 [DOI] [PubMed] [Google Scholar]
- Maalej A, Petit-Teixeira E, Chabchoub G, Hamad MB, Rebai A, Farid NR, Cornelis F, Ayadi H 2008 Lack of association of VDR gene polymorphisms with thyroid autoimmune disorders: familial and case/control studies. J Clin Immunol 28:21–25 [DOI] [PubMed] [Google Scholar]
- Rapoport B, McLachlan SM 2007 The thyrotropin receptor in Graves’ disease. Thyroid 17:911–922 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Kita-Furuyama M, Ando T, Nakao K, Mizuguchi H, Hayakawa T, Eguchi K, Niwa M 2002 A novel murine model of Graves’ hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol 168:2789–2794 [DOI] [PubMed] [Google Scholar]
- Chen C-R, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM 2003 The thyrotropin receptor autoantigen in Graves’ disease is the culprit as well as the victim. J Clin Invest 111:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CR, Aliesky H, Pichurin PN, Nagayama Y, McLachlan SM, Rapoport B 2004 Susceptibility rather than resistance to hyperthyroidism is dominant in a thyrotropin receptor adenovirus-induced animal model of Graves’ disease as revealed by BALB/c-C57BL/6 hybrid mice. Endocrinology 145:4927–4933 [DOI] [PubMed] [Google Scholar]
- Aliesky HA, Pichurin PN, Chen CR, Williams RW, Rapoport B, McLachlan SM 2006 Probing the genetic basis for thyrotropin receptor antibodies and hyperthyroidism in immunized CXB recombinant inbred mice. Endocrinology 147:2789–2800 [DOI] [PubMed] [Google Scholar]
- Saitoh O, Nagayama Y 2006 Regulation of Graves’ hyperthyroidism with naturally occurring CD4+CD25+ regulatory T cells in a mouse model. Endocrinology 147:2417–2422 [DOI] [PubMed] [Google Scholar]
- Saitoh O, Abiru N, Nakahara M, Nagayama Y 2007 CD8+CD122+ T cells, a newly identified regulatory T subset, negatively regulate Graves’ hyperthyroidism in a murine model. Endocrinology 148:6040–6046 [DOI] [PubMed] [Google Scholar]
- Pichurin PN, Chen C-R, Chazenbalk GD, Aliesky H, Pham N, Rapoport B, McLachlan SM 2006 Targeted expression of the human thyrotropin receptor A-subunit to the mouse thyroid: insight into overcoming the lack of response to A-subunit adenovirus immunization. J Immunol 176:668–676 [DOI] [PubMed] [Google Scholar]
- Chen CR, Aliesky HA, Guo J, Rapoport B, McLachlan SM 2006 Blockade of costimulation between T cells and antigen-presenting cells: an approach to suppress murine Graves’ disease induced using thyrotropin receptor-expressing adenovirus. Thyroid 16:427–434 [DOI] [PubMed] [Google Scholar]
- Mittereder N, March KL, Trapnell BC 1996 Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol 70:7498–7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Nguyen L, Chun RF, Lagishetty V, Ren S, Wu S, Hollis B, DeLuca HF, Adams JS, Hewison M 2008 Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology 149:4799–4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazenbalk GD, Jaume JC, McLachlan SM, Rapoport B 1997 Engineering the human thyrotropin receptor ectodomain from a non-secreted form to a secreted, highly immunoreactive glycoprotein that neutralizes autoantibodies in Graves’ patients’ sera. J Biol Chem 272:18959–18965 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Wang Y, Guo J, Hutchison JS, Segal D, Jaume JC, McLachlan SM, Rapoport B 1999 A mouse monoclonal antibody to a thyrotropin receptor ectodomain variant provides insight into the exquisite antigenic conformational requirement, epitopes and in vivo concentration of human autoantibodies. J Clin Endocrinol Metab 84:702–710 [DOI] [PubMed] [Google Scholar]
- Kakinuma A, Chazenbalk G, Filetti S, McLachlan SM, Rapoport B 1996 Both the 5′ and 3′ non-coding regions of the thyrotropin receptor messenger RNA influence the level of receptor protein expression in transfected mammalian cells. Endocrinology 137:2664–2669 [DOI] [PubMed] [Google Scholar]
- McLachlan SM, Aliesky HA, Pichurin PN, Chen C-R, Williams RW, Rapoport B 2008 Shared and unique susceptibility genes in a mouse model of Graves’ disease determined in BXH and CXB recombinant inbred mice. Endocrinology 149:2001–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu WX, Du GG, Kopp P, Rentoumis A, Albanese C, Kohn LD, Madison LD, Jameson JL 1995 The thyrotropin (TSH) receptor transmembrane domain mutation (Pro556-Leu) in the hypothyroid hyt/hyt mouse results in plasma membrane targeting but defective TSH binding. Endocrinology 136:3146–3153 [DOI] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2008 Identification of key amino acid residues in a thyrotropin receptor monoclonal antibody epitope provides insight into its inverse agonist and antagonist properties. Endocrinology 149:3427–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G 2007 Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels and extra-renal 1α-hydroxylase in the classical and nonclassical actions of 1α,25-dihydroxyvitamin D3. Semin Dial 20:316–324 [DOI] [PubMed] [Google Scholar]
- Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS 2007 Extra-renal 25-hydroxyvitamin D3–1α-hydroxylase in human health and disease. J Steroid Biochem Mol Biol 103:316–321 [DOI] [PubMed] [Google Scholar]
- Mirza IH, Wilkin TJ 1989 Antibody specificity in the immune response to insulin. Int Arch Allergy Appl Immunol 89:261–263 [DOI] [PubMed] [Google Scholar]
- Lamberg-Allardt C, Valtonen E, Polojarvi M, Stewen P 1991 Characterization of a 1,25-dihydroxy-vitamin D3 receptor in FRTL-5 cells. Evidence for an inhibitory effect of 1,25-dihydroxy-vitamin D3 on thyrotropin-induced iodide uptake. Mol Cell Endocrinol 81:25–31 [DOI] [PubMed] [Google Scholar]
- Ongphiphadhanakul B, Ebner SA, Fang SL, Lombardi A, Baran DT, Braverman LE 1992 1,25-Dihydroxycholecalciferol modulates 3H-thymidine incorporation in FRTL5 cells. J Cell Biochem 49:304–309 [DOI] [PubMed] [Google Scholar]
- Wang SH, Koenig RJ, Giordano TJ, Myc A, Thompson NW, Baker Jr JR 1999 1α,25-Dihydroxyvitamin D3 up-regulates Bcl-2 expression and protects normal human thyrocytes from programmed cell death. Endocrinology 140:1649–1656 [DOI] [PubMed] [Google Scholar]
- Hashizume K, Suzuki S, Ichikawa K, Takeda T, Kobayashi M 1991 Effect of active vitamin D3 on the levels of NADPH-dependent cytosolic 3,5,3′-triiodo-l-thyronine-binding protein. Biochem Biophys Res Commun 177:388–394 [DOI] [PubMed] [Google Scholar]
- Borgogni E, Sarchielli E, Sottili M, Santarlasci V, Cosmi L, Gelmini S, Lombardi A, Cantini G, Perigli G, Luconi M, Vannelli GB, Annunziato F, Adorini L, Serio M, Crescioli C 2008 Elocalcitol inhibits inflammatory responses in human thyroid cells and T cells. Endocrinology 149:3626–3634 [DOI] [PubMed] [Google Scholar]