Abstract
It is thought that differentiation of β-cell precursors into mature cells is largely autonomous, but under certain conditions differentiation can be modified by external factors. The factors that modify β-cell differentiation have not been identified. In this study, we tested whether adult islet cells can affect the differentiation process in mouse and human pancreatic anlage cells. We assessed β-cell proliferation and differentiation in mouse and human pancreatic anlage cells cocultured with adult islet cells or βTC3 cells using cellular, molecular, and immunohistochemical methods. Differentiation of murine anlage cells into β-cells was induced by mature islet cells. It was specific for β-cells and not a general feature of endodermal derived cells. β-Cell differentiation required cell-cell contact. The induced cells acquired features of mature β-cells including increased expression of β-cell transcription factors and surface expression of receptor for stromal cell-derived factor 1 and glucose transporter-2 (GLUT-2). They secreted insulin in response to glucose and could correct hyperglycemia in vivo when cotransplanted with vascular cells. Human pancreatic anlage cells responded in a similar manner and showed increased expression of pancreatic duodenal homeobox 1 and v-maf musculoaponeurotic fibrosarcoma oncogene homolog A and increased production of proinsulin when cocultured with adult islets. We conclude that mature β-cells can modify the differentiation of precursor cells and suggest a mechanism whereby changes in differentiation of β-cells can be affected by other β-cells.
Mature β cells affect differentiation of pancreatic anlage cells into functional β cells. The differentiated cells respond to glucose and ameliorate diabetes.
β-Cell mass is a key determinant for maintaining glucose homeostasis, and β-cell deficiency is the hallmark of type 1 and type 2 diabetes. Correction of β-cell deficiency is considered the ultimate cure of type 1 diabetes when autoimmunity is blocked by immune regulatory therapies and is also likely to improve treatment of type 2 diabetes. Cell replacement with either β-cells generated ex vivo or regeneration of endogenous β-cells are two approaches that have been studied intensively during the last decade. Although considerable progress has been made recently in exploring islet stem cells and new sources of β-cells, defining developmental regulatory factors, and manipulating embryonic stem cells into insulin-producing cells, numerous hurdles to successfully achieve the goal of β-cell replacement remain (1,2,3). Understanding of the developmental mechanisms is crucial for attempts to employ regenerative therapies to restore lost β-cells in diabetes.
In adults, β-cell mass is determined by the number of embryonic progenitor cells and dynamic balance of proliferation, neogenesis, and apoptosis (4,5,6). The number of pancreatic precursor cells is established early in embryonic life [by embryonic day (E) 10.5 in the mouse] and under normal growth conditions, other cells do not compensate if these cells are lost (4). This suggests that fulfillment of final β-cell mass is an instructive process that is ultimately dependent on the absolute number of precursor cells. However, extrinsic factors may also affect growth and differentiation of β-cells during pregnancy, growth, and with obesity, both insulin content of existing β-cells and the number of new cells are postulated to increase. Moreover, after pancreatectomy or ablation of β-cells in rodents, there is an increase in β-cell mass (7,8). In the presence of insulitis, we and others have previously shown that there is an increase in new β-cell formation in rodents (9,10). These observations suggest that compensatory increases in β-cell mass can be induced by extrinsic factors. Some investigators have postulated that glucose and/or insulin may stimulate β-cell growth and differentiation (11,12,13,14). Two recent studies have suggested that external factors, such as those derived from fetal pancreatic tissues can also affect growth and differentiation of islet cells (15,16). It is also possible that under certain conditions such as pregnancy or obesity, or in rodents after pancreatectomy, factors derived from adult islets can also affect the differentiation of β-cells (17,18,19). Here we studied differentiation of β-cell precursors in pancreatic anlage, which are composed of the precursors of mature β-cells (20). We hypothesized that islet cells themselves may provide instructive signals to precursor cells that affect differentiation into mature β-cells.
Materials and Methods
Animals
BALB/c and BALB/c/enhanced green fluorescent protein (GFP) transgenic mouse strains were used (21). These latter mice express a human histone protein with GFP for ubiquitous fluorescent labeling of nucleosomes. Cells from these mice were used to identify the source of cells in studies that used flow cytometry or immunohistochemistry. The use of mice was reviewed and approved by Animal Care and Use Committees at Yale University and Columbia University Medical Center. All protocols with human tissues were reviewed and approved by the Yale University Human Investigation Committee.
Cell lines
The murine pancreatic endocrine cell lines (βTC3 and αTC6) and pancreatic ductal cell line T31 were kind gifts from Dr. Domenico Accili (Columbia University, New York, NY) and were maintained in culture medium reported previously (22).
Islet and tissue isolation
Islets and hepatocytes were isolated from 8- to 12-wk-old mice after digestion with collagenase (Liberase; Roche, Indianapolis, IN) as described previously (23). Islets were hand picked with an inverted microscope. For the coculture studies, islets were used intact or dissociated into single cells using enzyme-free dissociation buffer (Invitrogen, Carlsbad, CA). The isolated islets or islet cells were used immediately or permeabilized and fixed in Cyto/Perm (BD Biosciences, San Jose, CA) and washed with PBS with 1% BSA. Thymocytes were prepared in PBS by mechanical dispersion of the thymus, and exocrine pancreatic cells were harvested from pancreatic digests, on the basis of morphology, and fixed.
In vitro culture and analysis of differentiation of mouse pancreatic anlage
The dorsal and ventral murine pancreatic anlage were harvested from E13.5–14.5 embryos by microscopic dissection. Certain studies used anlage harvested from E12.5 embryos. Anlage were identified by their budding appearance and removed by microscopic dissection. They were placed directly into culture wells (three to five pieces per well) or dissociated into single cells with 0.05% trypsin/EDTA and placed (5–10 × 104 cell/well) in culture with or without adult islets, islet, or other cells on organotypic membrane inserts (Millipore, Billerica, MA). The cells were maintained in culture media [DMEM/F-12 (Invitrogen) with B-27 supplement (Invitrogen), penicillin, streptomycin, HEPES, NaHCO3, glutamine, and heparin] at 37 C with 5% CO2 and the medium was replaced every 2 d. The insulin content of the media was 12–14 μg/ml. To monitor cell proliferation, anlage were pulsed with 5-bromo-2′-deoxyuridine (BrdU; 10 μg/ml; Sigma, St. Louis, MO) for 24–48 h at the indicated times. In certain studies human insulin (Sigma), exendin-4 (Bachem, San Carols, CA), or human TGF-β (R&D Systems, Minneapolis, MN) were added to cultures of the pancreatic anlage at the indicated concentrations. In some experiments, the anlage were separated from islets by placing the islets below the insert.
For immunofluororescence and confocal microscopy, anlage cells were grown on chamber slides, then fixed with 4% parafomaldehyde, and permeablized with 0.1% Triton X-100. They were stained with primary antibodies: guinea pig antiinsulin serum (1:2000; Linco Research, St. Charles, MO), rabbit antiglucagon (1:2000; Linco), rabbit antiglucose transporter-2 (1:1000; Calbiochem, San Diego, CA), or rabbit antipancreatic duodenal homeobox (Pdx)-1 antibody (1:5000, a generous gift of Dr. C. V. E. Wright, Vanderbilt University, Nashville, TN). Secondary antibodies were Texas red or Cy3-conjugated donkey antiguinea pig IgG and fluorescein isothiocyanate-conjugated antirabbit IgG (Jackson ImmunoResearch, West Grove, PA). 4′,6′-Diamino-2-phenylindole (Molecular Probes, Eugene, OR) was used for nuclear staining. Fluorescent images were captured with an Olympus BX51 fluorescence microscope (Center Valley, PA) and TSC SP5 confocal microscope (Leica, Bannockburn, IL). The frequency of +-stained cells was determined in 10 fields.
In vitro culture and differentiation of human fetal pancreatic cells
Human fetal pancreas tissues (gestational age 13–16 wk) were obtained from the human fetal tissue repository of Albert Einstein College of Medicine (New York, NY). The fetal pancreatic tissue was cut into 1-mm3 pieces and incubated with 0.05% trypsin-EDTA to generate small cell clusters. The clusters were placed in tissue culture flasks with supplemented DMEM as described above for 10–14 d for cell expansion. Human islets (>90% viability) were obtained from Emory University (Atlanta, GA) or the Islet Cell Resource Center Basic Science (ICR Islet Distribution Program) from adult cadaveric donors. The islets were isolated by collagenase digestion and purified by Ficoll gradients (24). The islets were dispersed into single cells using enzyme-free cell dissociation buffer (Invitrogen). After brief fixation with 0.05% paraformaldehyde, the islet cells were stored in human serum+10% dimethylsulfoxide at −80 C. Before adding the islets, the anlage cells were labeled with fluorescent PKH-67 dye (Sigma).
Flow cytometry analysis
The cultured pancreatic anlage or anlage cells were washed with PBS and dissociated into single cell suspensions with trypsin/EDTA. They were fixed and permeablized with Cytofix/Cytoperm solution kit (BD Biosciences). Monoclonal antibodies were obtained from BD Biosciences [antireceptor for stromal cell derived factor 1 (CXCR4), anti-BrdU] or R&D Systems [antirat IgG, antiproinsulin (clone 253627), and anti-Pdx-1]. The data were acquired on a FACSCalibur instrument (BD Biosciences) and analyzed by using FlowJo software (Tree Star, San Carlos, CA). Electronic gates were placed around the viable population based on scatter and on the GFP+ murine or the PKH-67+ human cells to exclude the nonanlage cells that had been added to the cultures. The threshold for positive staining was based on staining with isotype controls in which less than 1% of cells were above the threshold.
The murine proinsulin monoclonal antibody (mAb), 253627, is specific for murine proinsulin and does not recognize murine insulin. To verify that the mAb does not recognize human insulin that was present in the culture media, we coated Luminex beads with human insulin (Sigma) and measured binding of the mAb by indirect immunofluorescence. The staining of the mAb 253627 to the insulin-coated beads resulted in binding of eight fluorescent units, whereas the equivalent concentration of a control antiinsulin antibody showed binding of 5632 fluorescent units.
Insulin secretion assay
Cultured anlage cells were placed in CRML-1066 medium containing 10 mm glucose for 2 d before assay. The cells were washed twice and incubated with Krebs-Ringer bicarbonate (KRB; 3 mm glucose) for 4 h at 37 C as basal level. The anlage cells were stimulated with KRB containing 25 mm glucose for 2 h. Isolated adult mouse islets were stimulated in parallel as a positive control. The supernatants from each culture period were collected and the insulin concentration was measured with a mouse insulin ELISA kit (ALPCO, Salem, NH). The lower limit of detection of insulin in this assay was 0.019 ng/ml.
Cell implantation into diabetic nonobese diabetic/scid (NOD/scid) mice
The function of the differentiated anlage cells was evaluated in NOD/scid mice with hyperglycemia induced with five daily doses of streptozotocin (50 mg/kg·d × 5, ip) and confirmed (>300 mg/dl × 2) before the mice were used. The mice received a single slow-release insulin pellet (bovine insulin; Linshin Canada Inc., Ontario, Canada) implanted sc. Human vascular endothelial cells (HUVECs) transfected with Bcl-2 (gift of Dr. Jordan Pober, Yale University, New Haven, CT) were isolated by from human umbilical veins and infected with a retroviral vector containing caspase-resistant form of Bcl-2 as described (25).
Pancreatic anlage cells (2 × 106), which were cultured with or without fixed βTC3 cells, were collected after 7 d in culture and were mixed with or without Bcl-2-transduced HUVECs (0.5 × 106) in ice-cold Matrigel (Invitrogen) and were injected sc into the dorsal neck. The insulin pellet was removed after 20 d. Blood glucose concentrations were monitored in blood samples from tail veins.
Gene expression analysis by quantitative RT-PCR
Total RNA of the cultured cells was isolated using the RNeasy kit (QIAGEN, Valencia, CA). RNA samples were prepared for real-time PCR by random-primed reverse transcriptase using oligo(dT) primer (SuperScript first-strand; Invitrogen). The following forward and reverse gene primers were used for mouse: insulin-1, 5′-GGAGACCTTAATGGGCCAAACAG, 5′-GCCCACCAGCTTTATAGTCCAAA; PDX-1, 5′-GCTGGAGCTGGAGAAGGAATT, 5′-CTCTCGGTCAAGTTCAACATCACT; v-maf musculoaponeurotic fibrosarcoma oncogene homolog A (MafA), 5′-GAGGTCATCCGACTGAAACAGAAG, 5′-ACTTCTCGCTCTCCAGAATGTGC; v-maf musculoaponeurotic fibrosarcoma oncogene homolog B (MafB), 5′-AGACAGGCTTTGCGTCTAA, 5′-CGTTAGTTGCCAATGTGTGG; Ngn3, 5′-CCCATAGATGATGTTCGTCGTCTT, 5′-GCAGAATGGAGAAGCTCAGAAAC; Nkx6.1, 5′-CTGGCTGTGGGATGTTAGCT, 5′-GGGCTGCTGAGCAGGAA; Nkx2.2, 5′-TAGGACCACAATGCCAAAGAAAGA, 5′-AAGGCAGACGAAAGAAAAAGGTTG; actin-B, 5′-GCAAGTGCTTCTAGGCGGAC, 5′-AAGAAAGGGTGTAAAACGCAGC.
The following forward and reverse gene primers were used for human: insulin, 5′-CCAGCATCTGCTCCCTCTAC, 5′-TGCTGGTTCAAGGGCTTTAT; PDX-1, 5′-CATTGGAAGGCTCCCTAACA, 5′-TTCCACTGGCATCAATTTCA; MafA, 5′-CTTCAGCAAGGAGGAGGTCAT, 5′-ACTTCTCGCTCTCCAGAATGTG; MafB, 5′-GATCCTCCCCTCTGCTTTTTAT, 5′-GGGTTTCTGGTACATTCTGAGC; actin-B, 5′-AGATTGGCATGGCTTTATTTGT, 5′-ACCTTCACCGTTCCAGTTTTTA.
Quantitative real-time PCR was performed on a MX3000P real-time PCR system (Stratagene, La Jolla, CA) using the iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). The amplification was performed as follows: 10 min at 95 C and then 40 cycles at 95 C for 15 sec and 60.5 C for 70 sec. Reactions were performed in triplicate. The threshold cycle numbers were averaged for each gene, normalizing expression levels with β-actin. In preliminary studies, we were unable to isolate RNA from fixed islet or βTC3 cells or generate a signal using real-time PCR.
Statistical analyses
The group data are presented as the mean ± sem. The group means were compared by Student’s t test. Fisher’s exact test was used to compare the frequencies of two experimental groups. Glucose levels after transplantation of anlage cells into diabetic mice were compared by repeated-measures ANOVA. P = 0.05 was considered to be statistically significant.
Results
Adult islets enhance β-cell differentiation in mouse pancreatic anlage
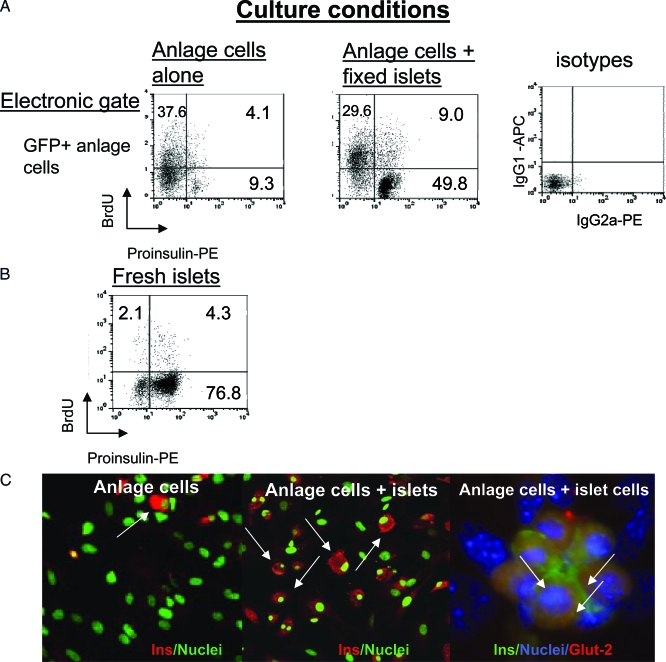
To examine effects of adult islets on β-cell differentiation, we established a primary culture system to grow pancreatic anlage and anlage cells. Intact pancreatic anlage from E13.5–14.5 GFP+ mice, were cultured for 7 d with or without coculture of freshly isolated murine adult islets. β-Cell differentiation was studied by fluorescence-activated cell sorting (FACS) analysis of enzymatically dissociated cells after pulsing with BrdU and staining for BrdU and proinsulin. The anlage-derived cells could be identified by staining with GFP because the histone fluorescent fusion protein labeled the nuclei of the cells and all daughter cells. This allowed us to exclude the adult islet cells from the analysis because the islet cells were GFP−. The proportion of anlage cells that stained with BrdU was high, generally greater than 45%, and the total number of cells in the wells increased about 3-fold during the 7-d culture. In the anlage cells cultured alone, less than 10% of the total cells stained for proinsulin, and about one third of the proinsulin+ cells stained for BrdU and proinsulin (Table 1). When freshly isolated islets were added to the cultures, there was an approximately 2-fold increase in total proportion of proinsulin+ cells (9.7 ± 2.7 to 19.7 ± 5.0%, P < 0.01) and a higher percentage of the proinsulin+ cells were BrdU− (Table 1). These studies indicate that coculture of fresh islets with anlage cells induces the expression of proinsulin in the anlage cells.
Table 1.
FACS analysis of cell proliferation and differentiation in mouse pancreatic anlage cell culture
| Pancreatic anlage | Pancreatic anlage + fresh islets | |
|---|---|---|
| Proinsulin+/BrdU− cells (%) | 6.8 ± 1.1 | 14.2 ± 2.6a |
| Proinsulin+/BrdU+ cells (%) | 3.5 ± 1.3 | 5.5 ± 1.1a |
| Total proinsulin+ cells (%) | 9.7 ± 2.7 | 19.7 ± 5.0b |
| Pancreatic anlage cells | Pancreatic anlage cells+ fixed islets | |
|---|---|---|
| Proinsulin+/BrdU− cells (%) | 7.3 ± 1.6 | 41.0 ± 8.4b |
| Proinsulin+/BrdU+ cells (%) | 10.6 ± 2.0 | 21.0 ± 3.7a |
| Total proinsulin+ cells (%) | 15.9 ± 1.8 | 58.5 ± 8.7b |
Anlage cells were cultured with or without fresh or fixed adult islets. After 7 d of culture, dissociated pancreatic H2b: enhanced GFP anlage cells were intracellularly stained with fluorescently labeled mAbs to proinsulin and BrdU and were analyzed by FACS. Representative staining is shown in Fig. 1. The data shown represent the proportion of total anlage cells that stained (by flow cytometry) for proinsulin alone, proinsulin and BrdU, and the total proportion of cells that stain for proinsulin (the sum of the two previous groups). Data represent mean ± sem, (n = 3 and 6 experiments, respectively).
P < 0.05.
P < 0.01.
When the anlage were dissociated into single cells, the induction of proinsulin+ cells was even greater. Therefore, dispersed anlage cells were used in subsequent experiments.
Surface-bound factor(s) are responsible for enhanced differentiation of β-cells in pancreatic anlage cell culture
The enhanced differentiation of anlage cells may have been due to a soluble factor and/or cell contact between the anlage cells and islets. To test the requirement for cell contact, we separated islets from anlage cells by placing them on opposite sides of a culture well membrane insert and found that the enhanced differentiation no longer occurred suggesting that soluble factors were not involved (9.6 ± 4.5 vs. 10.3 ± 4.9% of proinsulin+ cells, P > 0.1). Moreover, we did not find any effect of adding insulin (10 or 100 μg/ml), exendin-4 (10 or100 ng/ml), or TGF-β (10 ng/ml) at the start of cultures on the differentiation of the anlage cells after 7 d in culture (not shown) (26,27,28,29).
We then tested whether fixed islets would also affect anlage differentiation. The islets were prepared as described and stored until they were used for coculture. There was a greater than 4-fold increase of proinsulin+ cells when pancreatic anlage cells were cultured with fixed islets compared with culture without islets (58.5 ± 8.7 vs. 15.8 ± 1.82%, P < 0.01, Table 1, Fig. 1). The staining for proinsulin that was seen in the anlage cells cocultured with fixed islets was not due to staining of the fixed islet cells because when an electronic gate was placed around the GFP− cells, the total proportion of proinsulin+ cells was 2% (not shown). The proinsulin content of the anlage-derived cells cocultured with islets was comparable with the insulin content of freshly isolated mature islet β-cells on a per-cell basis: the mean fluorescence intensity of proinsulin staining was 32.9 ± 6.2 in anlage cells cultured with islets vs. 36.6 ± 4.8 in mature, freshly isolated islets (n = 4, P > 0.05) (Fig. 1B). In addition, when the fixed islets were placed into the cultures of dissociated anlage cells, the anlage cells formed clusters at a greater frequency than when the cells were cultured alone (10.5 ± 1.8 vs. 3.5 ± 0.3 per chamber, n = 4 separate experiments, P < 0.01) (not shown).
Figure 1.
Induction of proinsulin in anlage cells cultured with fixed adult islets: A, Proinsulin expression and BrdU labeling was analyzed by flow cytometry. Cells from 7 d cultures of pancreatic anlage from GFP+ mice were intracellularly stained with fluorescently labeled mAbs to proinsulin and BrdU. Dot plots of the FACS staining are shown. Gating was placed on GFP+ anlage cells. The percentage of cells in quadrants are shown. The staining with control isotype antibodies is shown at the far right. B, Comparative staining of freshly isolated islets. C, Representative immunohistochemical staining of insulin in cells from cultures of anlage alone (left) or anlage with adult islets (middle). Proinsulin+GFP+ cells are identified with arrows. The panel on the far right is from another experiment in which anlage cells from nontransgenic mice were cocultured with islet cells and stained for insulin and Glut-2 (arrows). The cells from the mature islets that had been permeabilized, fixed, and stored did not stain with antibody to insulin. PE, Phycoerythrin.
Our finding of increased proinsulin expression after coculture with fixed islets suggested that the staining of proinsulin in the anlage cells was not due to the uptake of insulin or proinsulin from the media because the fixed cells did not release insulin. Moreover, the staining did not reflect the uptake of insulin from the media itself because the mAb used for these studies (mAb 253627) did not recognize human insulin that was the constituent of the media (see Materials and Methods).
Immunocytochemical characterization of endocrine cell differentiation.
To characterize the differentiation process in vitro, we also studied cells in the cultures by immunohistochemistry and determined whether the effect on differentiation was limited to β-cells or whether there was a general increase in maturation of endocrine cells (Fig. 1C). To identify cells that stained for insulin and glucagon separately and together, we used anlage from non-GFP+ transgenic mice. The anlage were dispersed into single cells and cultured alone or with fixed islet cells for 7 d. They were then harvested, fixed, and stained with antibodies to insulin and glucagon. The number of single- and dual-positive cells was counted in 20 fields from four separate cultures per group. In cultures with pancreatic anlage cells alone, only a small percentage of the total cells (n = 195) were insulin+ (8.9%) or glucagon+ (0.5%), and 23% were dual hormone+. After culture with fixed islets, a higher proportion of the cells were only insulin+ (45%) and lower proportion were dual hormone+ (20%) (n = 224 cells) (P < 0.0001 by Fischer’s exact test).
The expression of a chemokine receptor, CXCR-4, a ligand for stromal cell-derived factor-1, has been described to increase during differentiation of β-cells (30). We analyzed the expression of this marker on the anlage cells. GFP+ anlage cells were placed into culture with or without fixed islets. After 7 d in culture, the cells were harvested and stained with mAbs to proinsulin and CXCR4 and analyzed by flow cytometry. Electronic gates were placed around the GFP+ anlagen cells and then around the GFP+, proinsulin+ cells. CXCR4 expression was then measured on these dual+ cells. Among the proinsulin-positive cells cultured without fixed islets, 28.3 ± 3.6% were CXCR4+. In contrast, after coculture with fixed islets, 80.4 ± 7.5% of the proinsulin+ cells expressed CXCR4 (P < 0.001, n = 2 separate experiments). Thus, coincident with the increased expression of insulin, the cocultured cells expressed cellular markers of differentiated β-cells.
Cell specificity on promotion of β-cell differentiation
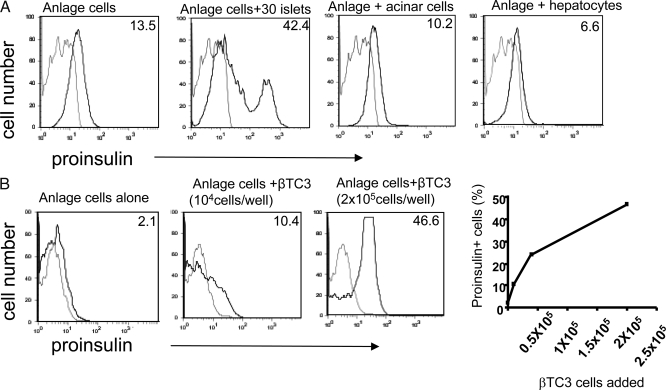
To establish the specificity of islets in promoting differentiation of the proinsulin+ cells, we tested other tissues and cell lines in our coculture system (Fig. 2A). Neither fixed murine hepatocytes nor fixed pancreatic acinar cells promoted β-cell differentiation when cocultured with anlage cells. We also tested a β- (βTC3) and other islet cell lines and found that fixed βTC3 cells induced differentiation of anlage cells in a dose-dependent manner (Fig. 2B), but neither αTC6, the ductal cell line T31, nor thymocytes affected differentiation of proinsulin+ cells (data not shown). In other studies, adult islets failed to induce lung anlage cells to become proinsulin+ when lung anlage from E14.5 embryos were cocultured in the same manner as pancreatic anlage with adult islets (data not shown).
Figure 2.
Effects of other adult cell types and a β-cell line on β-cell differentiation from anlage cells. A, Histograms of the percentage of proinsulin-expressing mouse pancreatic anlage cells cultured with the indicated tissue fixed with paraformaldehyde. Data are representative of two separate experiments. The numbers in the top right hand corner refer to the percentage of cells that stain with the antiproinsulin antibody (black line) above staining with the isotype control antibody (gray line). B, The histograms show the percentages of proinsulin-expressing cells in cultures with fixed βTC3 cells (0.5 × 105 and 2 × 105 cells/well). Staining with the isotype control antibody is shown in gray. The relationship between the number of βTC3 cells added to cultures of pancreatic anlage cells and the proportion of proinsulin+ anlage cells is shown in the graph. The data are from a single experiment representative of three experiments.
Gene expression analysis of β-cell developmental pathways
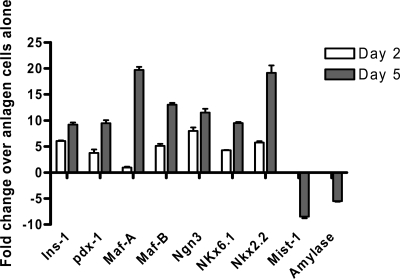
To discriminate an effect of the cocultured islets on precommitted β-cells or induction of uncommitted precursors into a β-cell pathway, we established cultures with E12.5 anlage cells, the earliest stage at which we could identify and isolate the pancreatic bud, and studied the expression of transcription factors after culture (Fig. 3). The transcription factor MafA is unique in being exclusively expressed at the secondary transition and principal phase of insulin-expressing cell production and is the only transcriptional activator present exclusively in islet β-cells (31,32,33). Pdx-1 is expressed in adult β-cells (34). In contrast, MafB is expressed in α- and β-cells during organogenesis but exclusively in α-cells in mature islets (35). We studied the expression of these genes after 2 and 5 d in culture to establish whether differentiation of cells was induced or, alternatively, represented stabilization of cell-specific factors that may have been expressed soon after anlage cell isolation. There was an increase in Ins-1, Pdx-1, and Maf-A over time. The expression of the following genes were increased (fold increase above anlage cells cultured without islets): Ins-1 (6.4 times), Pdx1 (3.7 times), Nkx6.1 (4.3 times) (all P < 0.0005, Fig. 3) as well as Ngn3 (8.7 times), Nkx2.2 (5.7 times) and MafB (5.1 times). Mist1 and amylase, markers of exocrine lineage, were in contrast decreased in anlage cells cocultured with islets compared with cells cultured alone. Collectively, these findings suggest that coculture with islet cells directs anlage cells into the β-cell lineage of differentiation.
Figure 3.
mRNA levels of genes involved in the β-cell differentiation pathway in pancreatic anlage cells. Pancreatic anlage cells from E12.5 embryos were used. RNA was prepared from the cells after 2 (open bars) or 5 d (filled bars) in culture with or without fixed islets. The change in the indicated gene expression at these two time points in anlage cells cultured with fixed islets is shown relative to the levels of RNA in the anlage cells cultured alone for the same period of time (all P values of data with islets are <0.005 except for MafA at d 2, which is ns) The data are from a single experiment representative of two independent experiments.
Differentiated β-cells from pancreatic anlage respond to glucose
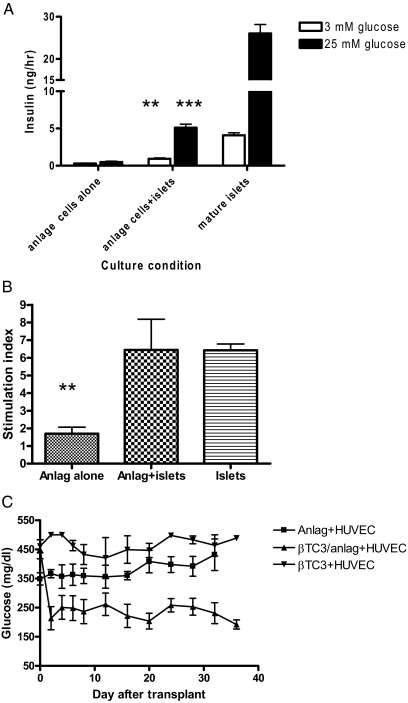
To test whether cells from pancreatic anlage cocultures had acquired functional responses after 7 d in culture, we stimulated them with glucose for 2 h and measured the insulin released into the supernatants (Fig. 4A). There was a poor response to glucose by anlage cells that had been cultured alone (0.4920 ± 0.118 vs. 0.297 ± 0.038 ng/h, P > 0.05). The release of insulin was significantly greater under both low and high glucose concentrations from cells cultured with fixed islets (0.923 ± 0.118 ng/h at 3 mm glucose, P < 0.001; 5.105 ± 0.478 vs. 0.492 ± 0.118 ng/h at 25 mm glucose, P < 0.0001). The stimulation index of the cocultured cells (6.45 ± 1.74) was similar to mature islets (6.43 ± 0.36), and both were significantly greater than the stimulation index of pancreatic anlage cells cultured alone (1.70 ± 0.37, P = 0.02) (Fig. 4B).
Figure 4.
Anlage cells cocultured with mature islets acquire insulin secretory responses to glucose in vitro and in vivo. A, Anlage cells that were cultured alone or with fixed mature islets were placed into culture with KRB solution containing 3 or 25 mm glucose. The concentration of insulin in the supernatants was measured by ELISA. **, P < 0.005 in comparison with insulin release in anlage cells plus islets vs. anlage cells alone at 3 mm glucose level. ***, P < 0.0001 in comparison with insulin release in anlage plus islets between 3 mm glucose and 25 mm glucose levels. The data are from six replicate cultures. B, Stimulation index (insulin concentration at 25 mm glucose/insulin concentration at 3 mm glucose calculated from the data in A (P = 0.02 vs. anlage cells cocultured with islets). C, NOD/scid mice with streptozotocin-induced diabetes received anlage cells placed into matrigel with HUVECs (▪-▪), anlage cells cocultured with βTC3 cells, and placed into matrigel with HUVECs (▴-▴; n = 5 mice/group) or fixed βTC3 cells with HUVECs alone (▾-▾; n = 4 mice) (P = 0.01 comparison of glucose levels of mice receiving anlage cells cultured with βTC3 and implanted in Matrigel with HUVECs vs. anlage cells implanted in Matrigel with HUVECs).
We also transplanted the cultured anlage cells with HUVECs in Matrigel into NOD/scid mice with streptozotocin-induced diabetes. In preliminary studies, we found that the viability of the anlage cells transplanted sc or under the kidney capsule was poor, and therefore, to provide a matrix and vascular supply, we mixed the anlage cells with HUVECs in Matrigel (Fig. 4C). The mice that received Matrigel loaded with HUVECs and anlage cells cultured without islets (n = 5) did not show significant reduction of glucose concentrations after transplantation, and none of the recipients showed a glucose concentration less than 250 mg/dl, even after the insulin pellet was removed on d 20. The glucose concentrations were also not reduced in mice that received Matrigel loaded with the fixed βTC3 cells and HUVECs. The glucose concentrations were significantly lower after the insulin pellet was removed (392 ± 41 vs. 224 ± 34 mg/dl, P = 0.01 by repeated measures ANOVA) in the mice transplanted with anlage cells cultured with fixed βTC3 cells (n = 5 mice/group). Four of five mice had glucose levels less than 250 mg/dl 30 d after transplantation.
Human islets promote differentiation of β-cells in human pancreatic anlage
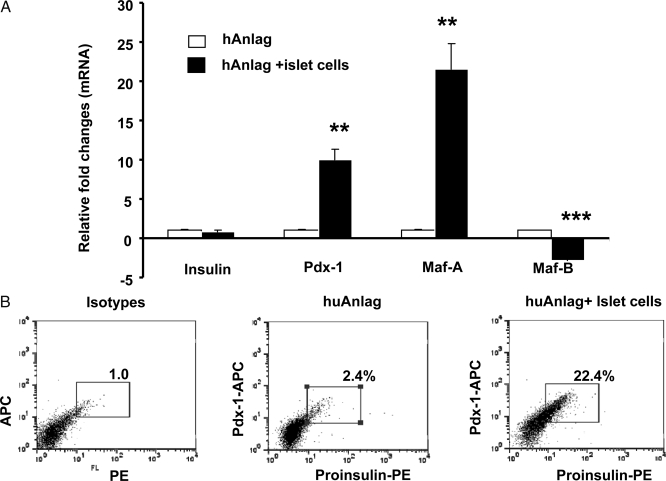
To test whether our findings with murine cells also were seen with human cells, we isolated human pancreatic anlage at wk 16 of gestation and placed the dissociated tissue in culture with or without fixed adult human islet cells. The anlage cells were labeled with PKH-67 dye so that insulin expression in the anlage cells could be differentiated from the mature islets by FACS and gene expression was studied by RT-PCR. After 6–8 d in culture with fixed islet cells, the expression of Pdx-1 and MafA were increased in human pancreatic anlage cells compared with the anlage cells cultured alone (9- and 22-fold increase, respectively Fig. 5A, P = 0.01 and 0.003), whereas in the same samples, there was a decline in MafB gene expression (P = 0.0001). Insulin gene expression was not noticeably different in human pancreatic anlage cells cultured with or without fixed adult islet cells.
Figure 5.
Effects of human adult islets on differentiation of fetal pancreatic cells. A, Real-time RT-PCR analysis of gene expression during culture of human anlage with and without fixed human islets. Bars show relative fold changes of mRNA levels of indicated gene expression in cells cocultured with fixed islets over those cultured without islets. Data are from a single experiment representative of two independent experiments. B, Flow cytometry analysis of proinsulin and Pdx-1 expression in anlage cells. Electronic gates were placed on anlage cells. The numbers refer to the percentage of proinsulin/Pdx-1+ cells. The data, shown as dot plots, are from a single experiment representative of three independent experiments. The isotype controls are on the left. **, P < 0.01; ***, P < 0.001. PE, Phycoerythrin.
Although insulin gene expression was unchanged in pancreatic anlage cells, it was possible that posttranscriptional regulation of the insulin gene could be affected during late stages of β-cell maturation (36). To test this, we examined insulin and Pdx-1 expression by flow cytometry. As shown in Fig. 5B, very few cells (2.0 ± 0.6%) among the pancreatic anlage cells cultured alone stained for both proinsulin and Pdx-1 at the end of 6 d of culture, but when fixed adult islet cells were added the culture, there was a 10-fold increase of proinsulin+/Pdx-1+ cells (19.4 ± 4.8%, P < 0.001). These results suggest that differentiation of human β-cells is promoted by coculture with islets. There is an increase in the expression of proinsulin in the differentiated cells, and this expression appears to be regulated at the posttranscriptional level.
Discussion
We have shown that adult islets promote the differentiation of pancreatic precursor cells into insulin-producing β-cells with a mature phenotype. The induction of β-cells involves a surface molecule because fixed islets were able to induce β-cell differentiation of the anlage cells. This effect of islet β-cells is cell type specific because other endoderm derived cells or non-β-endocrine cell lines did not induce differentiation of insulin+ cells. The differentiated cells secreted insulin in response to glucose and ameliorated hyperglycemia in diabetic mice when the cells were cotransplanted with vascular endothelial cells. These functional responses represent an important final stage in development of β-cells that was achieved in a previous study of differentiated human embryonic stem cells only by transplantation of cells in vivo (37,38). A similar effect of mature islet cells is seen with human pancreatic anlage cells from gestational age 13–16 wk, corresponding to E13–E16 of mouse fetal pancreas.
Previous studies have described the intrinsic transcriptional control of β-cell development (20,39). Our new observations suggest that this instructional control of β-cell development can be altered, reflected by the changes in the expression of transcriptional markers of islet cell differentiation. There was enhanced expression of Pdx-1 and MafA in murine and human anlage cells cocultured with islets that increased with time in culture. These findings suggest, but do not prove, that the effect of the coculture was to redirect the differentiation of pluripotential cells into the β-cell lineage because β-cell transcription factors were increased over time: a more formal proof of this notion would require tracing of lineage-specific markers. With human anlage cells, similar effects of islets were seen. However, in human cells we found an increase in Pdx-1 but not insulin gene expression in the cocultured anlage cells. It is possible that with longer cultures we may have found increased insulin gene transcription or alternatively, the maturation of β-cells may have enhanced proinsulin expression through a nontranscriptional mechanism supported by our FACS data.
We analyzed cell differentiation in an artificial environment, but the effects of mature islets in vivo on differentiation of precursor cells may be different. Under normal developmental conditions, the anlage spontaneously differentiates into endocrine and exocrine pancreas in a complex physical niche. The signals that are delivered by the cells in close proximity may be important in determining the fate of the developing organ. Previous investigations have emphasized the importance of the overlying mesenchyme and the vascular basement membrane matrix for inducing differentiation of the primordial pancreas (1,40). Our studies suggest that, in addition, β-cells are an important component of the physical niche.
In other studies, unidentified soluble factors released from islet cells have been thought to affect differentiation of pancreatic and embryonic stem cells. For example, Vaca et al. (15) found that factors released by developing pancreas (conditioned media from E16.5 pancreatic buds) stimulated endocrine pancreatic differentiation from embryonic stem cells. Our studies suggest that cell-cell contact is required rather than soluble products because enhanced differentiation was not seen when the mature islets and anlage cells were separated by a transwell, and fixed islets or β-cells could also induce differentiation. The pathways and molecules responsible for this effect are not clear: molecules in the islet basement membrane could be involved, but the basement membrane components alone are not sufficient. TGFβ and epidermal growth factor are important in lineage specification of β-cells and differentiation of islets from precursors, whereas the Fgf, Notch, Wnt, TGFβ, and epidermal growth factor pathways are involved in early pancreatic growth (41). Members of the TGFβ signaling pathway have been detected in mature islets. However, further studies will be needed to establish the role of these or other mediators in causing β-cell differentiation.
A role for direct cell contact for inducing differentiation of precursor cells has been described with developing neural cells (42). Previous studies suggested that cell matrix components may be important in differentiation of β-cells from precursors. Jiang et al. (28,43) described that signals from extracellular matrix, like laminin-1 and bone marrow proteins, promote β-cell development in mouse fetal pancreatic tissue. We believe, however, that the basement membrane matrix alone is not likely to account for the effects of the islets on differentiation. In other experiments, we cocultured anlage cells with the geltrex, which contains laminin, collagen IV, entactin, and heparin sulfate proteoglycan, components of the extracellular matrix. Compared with culture without geltrex, there were modest increases in the expression of Ins-1 (3.1-fold), Pdx-1 (3.5-fold), Maf-A (1.0-fold), Maf-B (5.8-fold), Ngn3 (1.1-fold), Nkx (6.1- to 1.3-fold), and Nkx (2.2- to 2.2-fold) (data not shown) and not of the magnitude that we found when the anlage cells were cultured with islets.
In summary, we found that adult β-cells can promote differentiation of fetal murine and human pancreatic anlage cells in vitro into functional β-cells. Our findings suggest that a cell surface molecule is involved in inducing differentiation. Our studies may have relevance to the control of β-cell formation and growth during physiological conditions such as growth and normal pregnancy or even factors that control β-cell differentiation under pathological conditions such as obesity or insulitis. Moreover, these studies suggest an approach that may be useful to promote differentiation of β-cells from their precursors under pathological conditions in which differentiation of mature β-cells is limited or possibly the acquisition of a mature functional phenotype from embryonic stem cells.
Footnotes
This work was supported by National Institutes of Health Grants R01DK068661 (to V.E.P.), R01DK068678 (to K.C.H.); Juvenile Diabetes Research Foundation Grant 2007-234 (to K.C.H.); and a gift from the Russel Berrie Foundation. Human islets were obtained from the ICR Basic Science Islet Distribution Program.
Disclosure Statement: W.C., S.B., V.E.P., and K.C.H. are coinventors on a patent application. L.O.-A.J.G., T.F.G., and A.L.M.B. have nothing to declare.
First Published Online October 9, 2008
Abbreviations: BrdU, 5-Bromo-2′-deoxyuridine; CXCR4, receptor for stromal cell-derived factor 1; E, embryonic day; GFP, green fluorescent protein; FACS, fluorescence-activated cell sorting; H2, histone protein type 2; HUVEC, human vascular endothelial cell; KRB, Krebs-Ringer bicarbonate; mAb, monoclonal antibody; MafA, v-maf musculoaponeurotic fibrosarcoma oncogene homolog A; MafB, v-maf musculoaponeurotic fibrosarcoma oncogene homolog B; NOD/scid, nonobese diabetic/scid; PDX, pancreatic duodenal homeobox factor.
References
- Murtaugh LC, Melton DA 2003 Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol 19:71–89 [DOI] [PubMed] [Google Scholar]
- Hao E, Tyrberg B, Itkin-Ansari P, Lakey J, Geron I, Monosov E, Barcova M, Mercola M, Levine F 2006 β-Cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med 12:310–316 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Weir G 2005 New sources of pancreatic β-cells. Nat Biotechnol 23:857–861 [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Tanaka A, Melton D 2007 Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 445:886–891 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S 2000 Islet growth and development in the adult. J Mol Endocrinol 24:297–302 [DOI] [PubMed] [Google Scholar]
- Nir T, Melton DA, Dor Y 2007 Recovery from diabetes in mice by β cell regeneration. J Clin Invest 117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, DeLeon D, Kaestner KH, Stoffers DA 2006 Regeneration of pancreatic islets after partial pancreatectomy in mice does not involve the reactivation of neurogenin-3. Diabetes 55:269–272 [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA 2004 Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41–46 [DOI] [PubMed] [Google Scholar]
- Sreenan S, Pick AJ, Levisetti M, Baldwin AC, Pugh W, Polonsky KS 1999 Increased β-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes 48:989–996 [DOI] [PubMed] [Google Scholar]
- Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC 2006 Effects of autoimmunity and immune therapy on β-cell turnover in type 1 diabetes. Diabetes 55:3238–3245 [DOI] [PubMed] [Google Scholar]
- Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O'Donnell CP, Garcia-Ocana A 2007 Glucose infusion in mice: a new model to induce β-cell replication. Diabetes 56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S 2007 A dominant role for glucose in β cell compensation of insulin resistance. J Clin Invest 117:81–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF 1998 Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900–904 [DOI] [PubMed] [Google Scholar]
- White MF 2002 IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 283:E413–E422 [DOI] [PubMed] [Google Scholar]
- Vaca P, Martin F, Vegara-Meseguer J, Rovira J, Berna G, Soria B 2006 Induction of differentiation of embryonic stem cells into insulin secreting cells by fetal soluble factors. Stem Cells:258–265 [DOI] [PubMed] [Google Scholar]
- Brolen GK, Heins N, Edsbagge J, Semb H 2005 Signals from the embryonic mouse pancreas induce differentiation of human embryonic stem cells into insulin-producing β-cell-like cells. Diabetes 54:2867–2874 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S 2000 Perspective: postnatal pancreatic β cell growth. Endocrinology 141:1926–1929 [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Desai BM, DeLeon DD, Simmons RA 2003 Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes 52:734–740 [DOI] [PubMed] [Google Scholar]
- Bertin E, Gangnerau MN, Bellon G, Bailbe D, Arbelot De Vacqueur A, Portha B 2002 Development of β-cell mass in fetuses of rats deprived of protein and/or energy in last trimester of pregnancy. Am J Physiol Regul Integr Comp Physiol 283:R623–R630 [DOI] [PubMed] [Google Scholar]
- Edlund H 2001 Developmental biology of the pancreas. Diabetes 50(Suppl 1):S5–S9 [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Papaioannou VE 2004 Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice. BMC Biotechnol 4:33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitout V, Stout LE, Armstrong MB, Walseth TF, Sorenson RL, Robertson RP 1995 Morphological and functional characterization of βTC-6 cells—an insulin-secreting cell line derived from transgenic mice. Diabetes 44:306–313 [DOI] [PubMed] [Google Scholar]
- Salvalaggio PR, Deng S, Ariyan CE, Millet I, Zawalich WS, Basadonna GP, Rothstein DM 2002 Islet filtration: a simple and rapid new purification procedure that avoids Ficoll and improves islet mass and function. Transplantation 74:877–879 [DOI] [PubMed] [Google Scholar]
- Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW 1988 Automated method for isolation of human pancreatic islets. Diabetes 37:413–420 [DOI] [PubMed] [Google Scholar]
- Schechner JS, Nath AK, Zheng L, Kluger MS, Hughes CC, Sierra-Honigmann MR, Lorber MI, Tellides G, Kashgarian M, Bothwell AL, Pober JS 2000 In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci USA 97:9191–9196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Yang H, Kim IS, Saint-Hilaire F, Thomas DA, De BP, Ozkaynak E, Muthukumar T, Hancock WW, Crystal RG, Suthanthiran M 2005 Systemic transforming growth factor-β1 gene therapy induces Foxp3+ regulatory cells, restores self-tolerance, and facilitates regeneration of β cell function in overtly diabetic nonobese diabetic mice. Transplantation 79:1091–1096 [DOI] [PubMed] [Google Scholar]
- Bai L, Meredith G, Tuch BE 2005 Glucagon-like peptide-1 enhances production of insulin in insulin-producing cells derived from mouse embryonic stem cells. J Endocrinol 186:343–352 [DOI] [PubMed] [Google Scholar]
- Jiang FX, Naselli G, Harrison LC 2002 Distinct distribution of laminin and its integrin receptors in the pancreas. J Histochem Cytochem 50:1625–1632 [DOI] [PubMed] [Google Scholar]
- Crisera CA, Rose MI, Connelly PR, Li M, Colen KL, Longaker MT, Gittes GK 1999 The ontogeny of TGF-β1, -β2, -β3, and TGF-β receptor-II expression in the pancreas: implications for regulation of growth and differentiation. J Pediatr Surg 34:689–693; discussion 693–684 [DOI] [PubMed] [Google Scholar]
- Koblas T, Zacharovova K, Berkova Z, Mindlova M, Girman P, Dovolilova E, Karasova L, Saudek F 2007 Isolation and characterization of human CXCR4-positive pancreatic cells. Folia Biol (Praha) 53:13–22 [DOI] [PubMed] [Google Scholar]
- Zhao L, Guo M, Matsuoka TA, Hagman DK, Parazzoli SD, Poitout V, Stein R 2005 The islet β cell-enriched MafA activator is a key regulator of insulin gene transcription. J Biol Chem 280:11887–11894 [DOI] [PubMed] [Google Scholar]
- Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S 2005 MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol 25:4969–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H, Miyatsuka T, Kawamori D, Yamamoto K, Kato K, Shiraiwa T, Katakami N, Yamasaki Y, Matsuhisa M, Matsuoka TA 2008 PDX-1 and MafA play a crucial role in pancreatic β-cell differentiation and maintenance of mature β-cell function. Endocr J 55:235–252 [DOI] [PubMed] [Google Scholar]
- Movassat J, Beattie GM, Lopez AD, Hayek A 2002 Exendin 4 up-regulates expression of PDX 1 and hastens differentiation and maturation of human fetal pancreatic cells. J Clin Endocrinol Metab 87:4775–4781 [DOI] [PubMed] [Google Scholar]
- Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R 2007 MafB is required for islet β cell maturation. Proc Natl Acad Sci USA 104:3853–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Scherberg N, Gilmore R, Steiner DF 1986 Translational control of insulin biosynthesis. Evidence for regulation of elongation, initiation and signal-recognition-particle-mediated translational arrest by glucose. Biochem J 235:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE 2008 Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26:443–452 [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE 2006 Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24:1392–1401 [DOI] [PubMed] [Google Scholar]
- Gu G, Brown JR, Melton DA 2003 Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev 120:35–43 [DOI] [PubMed] [Google Scholar]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E 2006 The vascular basement membrane: a niche for insulin gene expression and β cell proliferation. Dev Cell 10:397–405 [DOI] [PubMed] [Google Scholar]
- Oliver-Krasinski JM, Stoffers DA 2008 On the origin of the β cell. Genes Dev 22:1998–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R, Gomes FC 2005 Proliferation of cerebellar neurons induced by astrocytes treated with thyroid hormone is mediated by a cooperation between cell contact and soluble factors and involves the epidermal growth factor-protein kinase a pathway. J Neurosci Res 80:341–349 [DOI] [PubMed] [Google Scholar]
- Jiang FX, Stanley EG, Gonez LJ, Harrison LC 2002 Bone morphogenetic proteins promote development of fetal pancreas epithelial colonies containing insulin-positive cells. J Cell Sci 115:753–760 [DOI] [PubMed] [Google Scholar]