Abstract
Our laboratory has developed a paradigm of psychosocial stress (sequential layering of isolation, blindfold, and predator cues) that robustly elevates cortisol secretion and decreases LH pulse amplitude in ovariectomized ewes. This decrease in LH pulse amplitude is due, at least in part, to a reduction in pituitary responsiveness to GnRH, caused by cortisol acting via the type II glucocorticoid receptor (GR). The first experiment of the current study aimed to determine whether this layered psychosocial stress also inhibits pulsatile GnRH release into pituitary portal blood. The stress paradigm significantly reduced GnRH pulse amplitude compared with nonstressed ovariectomized ewes. The second experiment tested if this stress-induced decrease in GnRH pulse amplitude is mediated by cortisol action on the type II GR. Ovariectomized ewes were allocated to three groups: nonstress control, stress, and stress plus the type II GR antagonist RU486. The layered psychosocial stress paradigm decreased GnRH and LH pulse amplitude compared with nonstress controls. Importantly, the stress also lowered GnRH pulse amplitude to a comparable extent in ewes in which cortisol action via the type II GR was antagonized. Therefore, we conclude that psychosocial stress reduces the amplitude of GnRH pulses independent of cortisol action on the type II GR. The present findings, combined with our recent observations, suggest that the mechanisms by which psychosocial stress inhibits reproductive neuroendocrine activity at the hypothalamic and pituitary levels are fundamentally different.
A paradigm of psychosocial stress inhibits GnRH pulse amplitude. This effect is not reversed by treatment with RU486, a type II glucocorticoid receptor antagonist.
Various types of stress potently stimulate the hypothalamic-pituitary-adrenal axis and simultaneously suppress reproductive neuroendocrine activity. For example, psychosocial stress increases circulating levels of glucocorticoids and inhibits pulsatile LH secretion (1,2,3,4). Recent studies in ovariectomized sheep have demonstrated that a stress-like elevation of plasma cortisol decreases pulsatile LH secretion in the absence of stress and that this occurs via suppression of pituitary responsiveness to GnRH (5,6,7). This effect of cortisol, which reflects a direct action on the pituitary and mediation by the type II glucocorticoid receptor (GR), occurs without concurrent inhibition of pulsatile GnRH secretion (6,8,9).
Our laboratory has recently described a paradigm of psychosocial stress that consists of sequential layering of isolation, restraint, blindfold, and predator cues (barking dog sound and odor) (10). This model of psychosocial stress (“layered stress paradigm”) causes a robust elevation in circulating cortisol along with a profound decrease in LH pulse amplitude and steroid-induced sexual behavior in ovariectomized ewes (10,11). The decrease in LH pulse amplitude was observed not only in ovariectomized ewes in which LH pulses were driven by endogenous GnRH pulses, it was also evident in a pituitary-clamp model in which endogenous GnRH pulses were blocked and LH pulses were driven by fixed hourly boluses of exogenous GnRH (10). Thus, the lowering of LH pulse amplitude during the layered stress is due, at least in part, to a reduction in pituitary responsiveness to GnRH. Importantly, the stress-induced suppression of pituitary responsiveness to GnRH in the pituitary-clamp model was blocked by RU486, an antagonist of the type II GR, implying that the elevation in plasma cortisol mediates the stress-induced suppression of pituitary responsiveness via action on this receptor (10). However, RU486 failed to reverse the suppressive effect of the layered stress on LH pulse amplitude in ovariectomized ewes in which pulsatile LH secretion was driven by endogenous GnRH pulses.
Collectively, these findings suggest that psychosocial stress reduces LH pulse amplitude by two mechanisms: one involving cortisol acting via the type II GR to inhibit pituitary responsiveness to GnRH, and the second not involving cortisol action via this receptor. Increasing evidence suggests that stress inhibits reproductive neuroendocrine function by hypothalamic mechanisms that reduce pulsatile GnRH release (12,13,14,15). However, the only definitive approach to assess this is by monitoring GnRH directly, and this has proven to be technically difficult. Thus, characteristics of pulsatile GnRH secretion are typically inferred from the LH secretory pattern. This indirect approach can be problematic, especially for assessing amplitude, because LH pulse amplitude reflects not only the amount of GnRH released during a pulse but also responsiveness of the pituitary to GnRH, both of which might be influenced by stress (5,10,16,17,18).
The present study used a powerful model to monitor GnRH pulsatility directly in serial samples of hypophyseal portal blood of sheep to test two hypotheses: 1) psychosocial stress reduces the amplitude of GnRH pulses; and 2) this effect is not mediated by cortisol acting upon the type II GR.
Materials and Methods
Experiments were conducted during two consecutive anestrous seasons (March-July) of 2006 (experiment 1) and 2007 (experiment 2) on mature Suffolk ewes maintained under standard husbandry conditions at the Sheep Research Facility near Ann Arbor, MI. Animals were fed hay and alfalfa pellets, and had free access to water and mineral licks. All ewes were ovariectomized using aseptic conditions and general anesthesia at least 6 months before the study. Two weeks before sampling, the animals were surgically fitted with a pituitary portal blood collection device, as described by Caraty et al. (19). All procedures were approved by the Committee for the Use and Care of Animals at the University of Michigan.
Psychosocial stress model
Our recently developed model of psychosocial stress consists of sequential hourly application of: isolation (removal from flock mates), restraint (confinement in a pen), blindfold (removal of visual cues), and the sound of a barking dog (predatory cues). This layered stress paradigm consistently and robustly increases circulating levels of cortisol and suppresses pulsatile LH secretion in ovariectomized ewes by reducing LH pulse amplitude (10,11). The layered stress paradigm was modified in the present study to exclude the acute restraint component because animals must necessarily be confined in individual pens (0.5 × 1.2 m) to sample pituitary portal blood. The modification was as follows: isolation (80 min), blindfold (80 min), and barking dog (80 min). To lessen the potential for restraint stress due to penning the animals for portal blood collection, the ewes were acclimated to their pens for at least 2 d (experiment 1) or 1 wk (experiment 2) before sampling.
Experiment 1: Does psychosocial stress inhibit pulsatile GnRH secretion?
Sixteen ovariectomized ewes surgically fitted with a pituitary portal blood collection device and allocated to two groups: nonstress control (n = 8), or stress (n = 8). After a 2-wk recovery from surgery and 1 d before sampling, animals were equipped with two jugular catheters, one for peripheral blood collection and the other for heparin-saline infusion (200 U/min). Pituitary portal and jugular blood were withdrawn continuously via an automated collection system (19) that permits remote sampling with the investigator absent from the animal room. To minimize GnRH degradation, portal blood was collected in tubes containing ice-cold bacitracin and extracted with methanol within 1.5 h of collection. Simultaneous samples of pituitary portal and jugular blood were obtained at 10-min intervals for 8 h. Ewes in both groups were maintained under calm basal conditions for the first 4 h of sampling (period 1). During the final 4 h (period 2), ewes in the experimental group were exposed to the layered stress paradigm, beginning with relocation to a new pen in a separate isolation room; controls remained under basal conditions throughout. To minimize disturbance during the basal period, animals were sampled as pairs in adjacent pens (both of the same treatment group). In addition, to minimize the influence of the transient increase in plasma cortisol that we observe in response to feeding, sampling was begun 5 h after daily feeding. After sampling, animals were killed with a barbiturate overdose (Fatal Plus; Vortech Pharmaceuticals, Dearborn, MI), and the pituitary was removed to determine proper lesion placement relative to pituitary portal vessels.
Experiment 2: Does antagonism of the type II GR prevent stress-induced suppression of pulsatile GnRH secretion?
The goal of this experiment was to confirm the inhibitory effect of the layered stress paradigm on pulsatile GnRH secretion and determine whether this suppression is mediated by cortisol acting via the type II GR. Thirty ovariectomized ewes were surgically fitted with a pituitary portal blood collection device and randomly allocated to three groups: nonstress control (n = 10), stress (n = 9), or stress plus RU486 (n = 11). RU486 (Sigma-Aldrich Corp., St. Louis, MO) is a nonspecific antagonist of the type II GR, which blocks suppressive effects of both cortisol and progesterone on pulsatile LH and GnRH secretion in sheep (8,20). The antagonist was suspended in vehicle (equal parts sesame oil and 75% ethanol) and injected (10 mg/kg, im) at 24 and 12 h before stress initiation. We previously determined that this dose of RU486 reverses the suppressive effects of both cortisol (8) and the layered stress (10) on pituitary responsiveness to GnRH in ovariectomized ewes. A vehicle control group was not included in this experiment because our recent work determined that the vehicle did not affect pulsatile LH secretion in ovariectomized ewes (8,10). The lack of effect of vehicle (with RU486) was confirmed in this study in that GnRH and LH pulse parameters before stress in ewes treated with RU486 were virtually identical to those in nonstressed animals that did not receive RU486 (see Results). Experiment 2 was conducted in the same manner as experiment 1 with the exception that animals were acclimated to their individual pens for 1 wk before sampling because the results from experiment 1 suggested that a longer preconditioning period might be optimal for minimizing the potential for penning stress on the day of sampling.
Hormone assays
GnRH was assayed in duplicate reconstituted methanol extracts of portal plasma (∼300 μl plasma) using a previously described RIA (21,22). Intraassay and interassay coefficients of variation were 5.8 and 10.5%, respectively, and assay sensitivity averaged 0.07 pg/tube (25 assays).
LH was measured in duplicate aliquots of plasma (25–100 μl) using a modification (23) of a previously described RIA (24,25) and is expressed in terms of NIH-LH-S12. Intraassay and interassay coefficients of variation were 6.8 and 10.5%, respectively, and assay sensitivity averaged 0.6 ng/ml (12 assays).
Plasma cortisol was determined in duplicate aliquots (50 μl) using the Coat-a-Count cortisol kit (Siemens Healthcare Diagnostics Inc., Los Angeles, CA) validated for use in sheep (16). Intraassay and interassay coefficients of variation were 2.2 and 8.5%, respectively. Assay sensitivity averaged 0.5 ng/ml (15 assays).
Data analysis
GnRH is reported as the rate of collection (pg/min) rather than concentration in portal blood. This minimizes error due to changes in the rate of portal blood withdrawal caused by changes in head position or contamination of portal blood with cerebrospinal fluid or peripheral blood. The latter was judged to be minimal based on little or no blood aspirated into the sampling lines during the hour before lesion of the portal vessels; cerebrospinal fluid contamination was negligible based on hourly hematocrit measures during sampling. GnRH and LH pulses were identified by the Cluster pulse-detection algorithm (26). As in our previous studies (5), peak and nadir cluster sizes were set at one and one for GnRH, and one and two for LH, and the t statistic used to determine significant increases or decreases in hormone concentration was 3.8 for GnRH and 2.6 for LH. Because the sampling procedure measures GnRH only in its initial pass down the portal system (GnRH recirculating from periphery is undetectable) and because many GnRH pulses were split between two consecutive 10-min samples [GnRH pulses last 5–6 min (27)], the amplitude of a given GnRH pulse was calculated by adding all values greater than baseline and subtracting the value of the preceding nadir. LH pulse amplitude was defined as the difference between the peak and preceding nadir. Frequency was calculated as interpulse interval.
For statistical analyses, hormone concentrations were log transformed to normalize variation across a broad range of values. A mean GnRH and LH pulse amplitude, interpulse interval, and plasma cortisol concentration was calculated for each ewe during the first and last 4 h sampling (period 1 and period 2, respectively). To test for treatment effects, a repeated measures ANOVA was conducted on these means to identify significant interactions (treatment × time) between experimental groups. The between-subjects factor was treatment (i.e. nonstress, stress, and in experiment 2, stress plus RU486) and the within-subjects factor was time (i.e. period 1 and period 2). In experiment 2 a repeated measures ANOVA analysis of the three treatment groups resulted in a significant treatment × time interaction, which identified a treatment effect but did not determine the group(s) that differed significantly from the other(s). To test for significance between specific treatment groups, separate repeated measures ANOVAs were performed in which one treatment group was removed, and analyses were conducted on the remaining two groups. Statistical significance was defined as P < 0.05.
Criteria for animal inclusion
Accurate assessment of the effects of the psychosocial stress paradigm required that we monitor GnRH secretion in animals that were not compromised by the unintended stress of the surgical preparation and manipulation associated with sampling (e.g. penning and lesion of portal vessels). Although most animals behaved normally and remained calm during sampling, some appeared stressed (agitation, vocalization) before the application of our experimental stress paradigm. Therefore, we established three criteria to ensure that statistical analyses included only animals in which pituitary portal blood had been collected successfully from animals in which reproductive neuroendocrine activity had not already been compromised by the stress of the surgical preparation and sampling.
Ewes must have a mean plasma cortisol level less than 20 ng/ml during the first 4 h sampling (period 1); our experience indicates higher values are indicative of stress (10) (E.R.W. and F.J.K., unpublished observations).
Stressed ewes must have a greater mean plasma cortisol level in the stress period (period 2) than in the prestress period (period 1).
Autopsy must reveal a clear lesion of the portal vessels, and GnRH must be detectable and unambiguously pulsatile during the prestress period.
Animals that did not satisfy these criteria were excluded from the statistical analysis and are reported separately at the end of Results.
Results
Experiment 1: Does psychosocial stress inhibit pulsatile GnRH secretion?
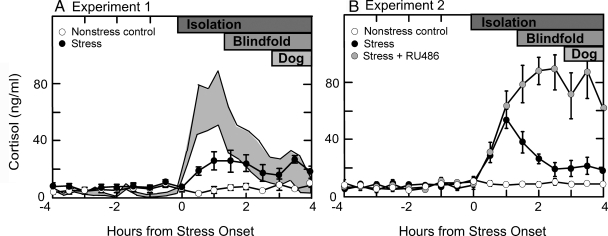
Using the criteria for inclusion of animals as described in Materials and Methods, data from seven stressed ewes and four nonstressed controls were included in the statistical analyses. Figure 1A depicts mean plasma cortisol values during the 8 h sampling. In control ewes, values remained low throughout the sampling period (mean ± sem, 6.3 ± 1.3 ng/ml) and did not differ from the first to second 4 h sampling. Cortisol increased during the layered stress (7.6 ± 1.5 vs. 21.7 ± 4.1 ng/ml, period 1 vs. period 2; P < 0.01; F = 8.1; df = 1,9). Average individual peak values (37.5 ± 4.8 ng/ml) averaged 5-fold over basal levels. Both the mean and peak cortisol values appeared to be less than observed previously in the laboratory with the layered stress paradigm (Fig. 1A).
Figure 1.
Mean ± sem plasma cortisol concentrations in control ewes (open circles) and ewes subjected to the layered stress paradigm (black circles) in experiment 1 (A, left panel), and in control ewes (open circles), ewes subjected to the layered stress paradigm (black circles), and those pretreated with the GR antagonist, RU486, before stress (gray circles) in experiment 2 (B, right panel). The se about the mean plasma cortisol responses to the layered stress achieved in previous work (10) is illustrated by the shaded area in A. The layered stress paradigm is depicted at the top of the panels, and consisted of isolation, blindfold, and predator cues (dog).
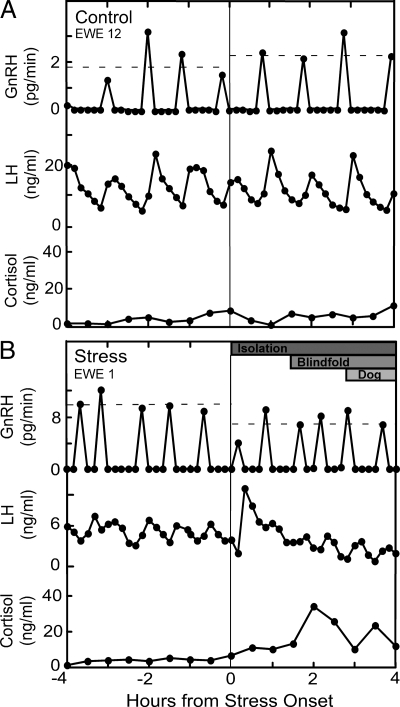
Representative GnRH and LH profiles from each treatment group are shown together with cortisol in Fig. 2; composite results and summary of statistical analyses are depicted in Table 1. GnRH and LH pulsatility remained stable throughout sampling in all four control ewes; repeated measures ANOVA revealed no change in pulse amplitude or interpulse interval between the first and second 4 h sampling. In contrast, GnRH pulse amplitude decreased during the layered stress, as revealed by a significant treatment × time interaction (28% suppression, period 1 vs. period 2; P < 0.05). In addition, there was 24% suppression in LH pulse amplitude during stress, but this did not reach statistical significance (period 1 vs. period 2; P = 0.0968). The layered stress did not significantly affect either GnRH or LH interpulse interval.
Figure 2.
GnRH, LH, and cortisol profiles for one representative ewe kept under nonstress conditions (control; top panel) (A) or exposed to the layered stress paradigm (stress; bottom panel) (B) in experiment 1. Vertical line depicts time of stress onset in stressed ewes (and a comparable time in nonstress controls), and the horizontal dashed lines represent the mean GnRH pulse amplitude in each period (period 1 vs. period 2). The layered stress paradigm is depicted at the top of the panel for the stressed ewe, and consisted of isolation, blindfold, and predator cues (dog).
Table 1.
Effects of psychosocial stress on GnRH and LH pulse parameters in experiment 1
| Pulse amplitudea
|
Interpulse intervala
|
|||
|---|---|---|---|---|
| Period 1 | Period 2 | Period 1 | Period 2 | |
| GnRH | ||||
| Control | 1.6 ± 0.5 | 1.9 ± 0.7 | 48.3 ± 2.8 | 48.5 ± 4.3 |
| Stress | 3.2 ± 1.2 | 2.3 ± 0.8b | 55.5 ± 6.1 | 49.9 ± 4.0 |
| LH | ||||
| Control | 7.9 ± 2.3 | 8.3 ± 2.7 | 49.4 ± 3.6 | 51.5 ± 3.6 |
| Stress | 8.3 ± 2.4 | 6.3 ± 1.5 | 55.5 ± 6.6 | 52.4 ± 3.6 |
All values are mean ± sem across periods 1 and 2.
LH is shown as ng/ml, GnRH as pg/min, and interpulse interval as minutes.
Significant treatment × time interaction in repeated measures ANOVA (P < 0.05 F = 11.6; df = 1,9).
Experiment 2: Does antagonism of the type II GR prevent stress-induced suppression of pulsatile GnRH secretion?
The statistical analyses included data from nine control ewes, six stressed ewes, and six stressed ewes treated with RU486. As illustrated in Fig. 1B, plasma cortisol remained low in nonstressed controls (mean ± sem, 8.9 ± 0.5 ng/ml) and was elevated during stress (28.8 ± 3.0 ng/ml; P < 0.001; F = 60.8; df = 1,13). This cortisol response was more typical of that seen in response to the layered stress (Fig. 1). RU486 enhanced mean plasma cortisol concentrations during stress by approximately 2.5-fold (72.0 ± 11.7 ng/ml; P < 0.01; F = 48.7; df = 1,13) and prolonged the peak response compared with stress only, documenting its efficacy in blocking cortisol negative feedback via the type II GR (28,29,30). Maximal cortisol levels during stress were also higher in the presence of RU486 compared with stress alone (92.8 ± 10.3 vs. 56.8 ± 6.3 ng/ml; P < 0.01).
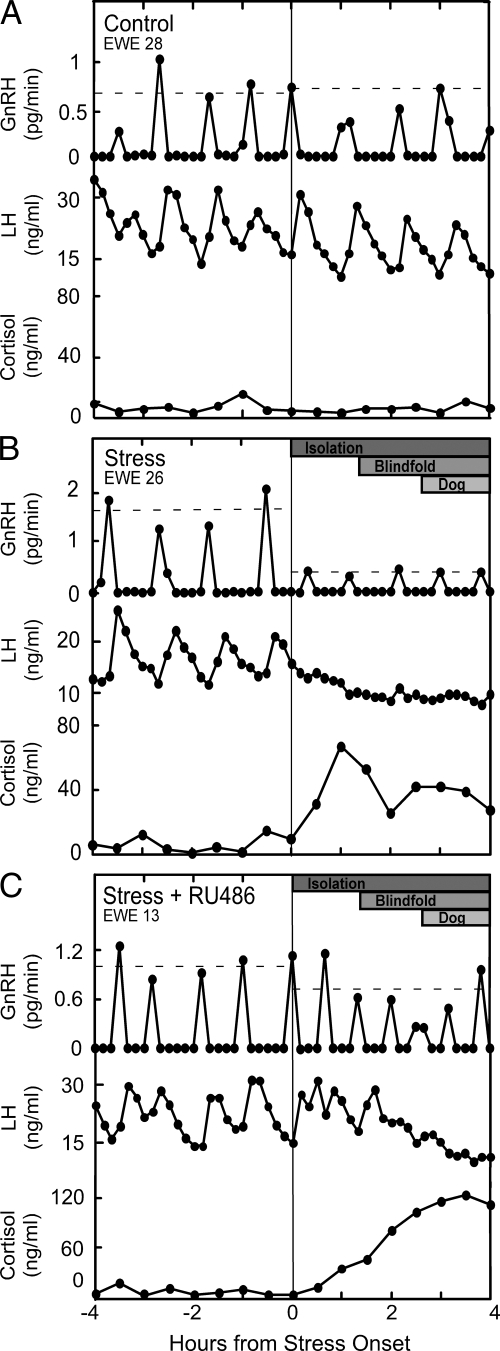
Representative GnRH and LH profiles for each treatment are shown along with cortisol values in Fig. 3; composite results and summary of statistical analyses are depicted in Table 2. Repeated measures ANOVA indicated no change in GnRH or LH pulse amplitude or interpulse interval in control ewes between the first and second 4 h sampling. Again, the layered stress reduced GnRH pulse amplitude, as determined by a significant treatment × time interaction (43% suppression; P < 0.05), and, in this experiment, the decrease in LH pulse amplitude achieved significance (52% suppression; P < 0.05; Table 2). Importantly, the layered stress also lowered GnRH and LH pulse amplitude in the RU486 group (P < 0.05), and the magnitude of the reduction in GnRH did not differ in the presence or absence of RU486 (30 vs. 43% suppression respectively; P > 0.1). Unexpectedly, the layered stress decreased GnRH interpulse interval in both the presence and absence of RU486; this was not accompanied by a similar increase in LH pulse frequency. Finally, GnRH and LH pulse parameters before stress in ewes treated with RU486 were comparable to those in nonstressed controls, which did not receive RU486 (Table 2).
Figure 3.
GnRH, LH, and cortisol profiles for one representative ewe kept under nonstress conditions (control; top panel) (A), exposed to the layered stress paradigm (stress; middle panel) (B), or treated with RU486 before stress (stress + RU486; bottom panel) (C) in experiment 2. Vertical line depicts time of stress onset in stressed ewes (and a comparable time in nonstress controls), and the horizontal dashed lines represent the mean GnRH pulse amplitude in each period (period 1 vs. period 2). The layered stress paradigm is depicted at the top of the panel for the stressed ewes, and consisted of isolation, blindfold, and predator cues (dog).
Table 2.
Effects of psychosocial stress on GnRH and LH pulse parameters in experiment 2
| Pulse amplitudea
|
Interpulse intervala
|
|||
|---|---|---|---|---|
| Period 1 | Period 2 | Period 1 | Period 2 | |
| GnRH | ||||
| Control | 0.9 ± 0.2 | 0.9 ± 0.2 | 48.8 ± 4.1 | 50.0 ± 4.7 |
| Stress | 1.4 ± 0.3 | 0.8 ± 0.2b | 56.4 ± 6.1 | 46.7 ± 4.7b |
| Stress + RU486 | 1.0 ± 0.1 | 0.7 ± 0.2c | 51.9 ± 3.1 | 42.3 ± 3.7c |
| LH | ||||
| Control | 10.4 ± 1.3 | 8.8 ± 1.2 | 54.8 ± 4.7 | 52.4 ± 4.7 |
| Stress | 11.2 ± 2.7 | 5.4 ± 1.2b | 66.1 ± 7.4 | 71.6 ± 12.0 |
| Stress + RU486 | 9.3 ± 1.1 | 5.3 ± 1.3c | 52.9 ± 1.5 | 50.5 ± 4.5 |
All values are mean ± sem across periods 1 and 2.
LH is shown as ng/ml, GnRH as pg/min, and interpulse interval as minutes.
Significant treatment × time interaction. Control vs. stress (P < 0.05). For GnRH pulse amplitude: F = 7.2; df = 1,13. For LH pulse amplitude: F = 14.8; df = 1,13. For GnRH interpulse interval: F = 10.9; df = 1,13.
Significant treatment × time interaction. Control vs. stress plus RU486 (P < 0.05). For GnRH pulse amplitude: F = 5.3; df = 1,13. For LH pulse amplitude: F = 9.0; df = 1,13. For GnRH interpulse interval: F = 14.4; df = 1,13.
Animals excluded from data analysis
In experiment 1, three controls and one stressed ewe were excluded based on elevated mean plasma cortisol concentrations (>20 ng/ml) during the first 4 h sampling, suggesting that these animals were stressed during period 1 (see criteria for including animals described in Materials and Methods). Another control was omitted because clear-cut GnRH pulses were not evident, and autopsy failed to reveal an unambiguous lesion in the portal vasculature, indicating that portal blood was not successfully sampled. In experiment 2, one control and one stress ewe were excluded because clear-cut GnRH pulses were not evident in period 1; another stressed ewe was omitted due to elevated plasma cortisol during the first 4 h sampling. Five of the 11 RU486 ewes were excluded due to the lack of GnRH pulses (one ewe), or elevated plasma cortisol before stress and/or lack of a stress-induced cortisol rise (four ewes). The larger number of RU486 ewes exhibiting elevated cortisol before stress likely reflects antagonism of negative feedback action of cortisol (28,29,30) rather than the stress of portal sampling. One additional stress animal was judged to be an outlier. Notes during sampling indicated little or no behavioral responses to stress (agitation, vocalization), and subsequent hormone analyses failed to reveal clear-cut neuroendocrine responses to stress: mean plasma cortisol level was 11 ng/ml, and the ratio of stress to prestress GnRH and LH pulse amplitudes were outside the 95% confidence limit of the other ewes in this group.
Discussion
Recent studies demonstrate that a paradigm of layered psychosocial stress reduces LH pulse amplitude in ovariectomized ewes, and this is due, at least in part, to inhibition of pituitary responsiveness to GnRH (10). Furthermore, this effect on the pituitary is blocked by antagonism of the type II GR, suggesting that cortisol action via this receptor mediates the response (10). The present work used a procedure for directly monitoring the GnRH secretory profile to tease apart hypothalamic from pituitary effects. Our findings permit two conclusions. First, a similar psychosocial stress also has a central effect resulting in decreased GnRH pulse amplitude. Second, antagonism of the type II GR does not reverse this response, suggesting that cortisol acting via the type II GR does not mediate this hypothalamic effect. Collectively, these findings imply that psychosocial stress disrupts reproductive neuroendocrine activity at both the hypothalamic and pituitary levels. Furthermore, the mechanisms and mediators of the disruption at these two sites differ in that cortisol mediates the inhibitory response only at the pituitary level. This interpretation is in full accord with our recent finding that the suppressive effect of the layered stress on LH pulse amplitude could not be accounted for entirely by inhibition of pituitary responsiveness to GnRH (10).
Results from both experiments support our primary conclusion: psychosocial stress has a hypothalamic effect to lower GnRH pulse amplitude. However, the cortisol and LH responses in experiments 1 and 2 differed in a quantitative sense. These differences are fully explicable and predictable based on our prior observations and the procedural details of the present studies, and they reinforce our understanding of these neuroendocrine responses to stress. For example, the cortisol response was attenuated in experiment 1 relative to that in experiment 2 and that seen previously with the layered stress paradigm (10). The reduced response compared with previous studies is likely due to the removal of one of the stressors, acute restraint, typically used in this paradigm. This was necessary because the animals had to be confined to pens in a sampling room to collect pituitary portal blood. Furthermore, the period of acclimation to the sampling pens before the experimental day was relatively brief in experiment 1 (2–4 d). Penning in a novel environment itself is a form of stress and, given the brief acclimation period, could have attenuated the cortisol response due to habituation. Therefore, the acclimation period was lengthened to 7 d in experiment 2 to allow animals to become more accustomed to their new environment. The cortisol response in experiment 2 was more typical of that seen in our earlier studies, although maximal values still only reached the lower portion of the range seen previously (Fig. 1), most likely due to the omission of acute restraint from the layered stress paradigm.
With respect to the differing LH responses in the two experiments, LH pulse amplitude was significantly reduced by the layered stress in experiment 2, whereas it was not in experiment 1, although it is worth noting that mean amplitude during stress in experiment 1 was 24% lower than the prestress value (P = 0.0968). The attenuated LH response in experiment 1 is predictable based on the decreased cortisol increase. Cortisol is necessary for the suppression of pituitary responsiveness to GnRH that occurs during exposure to the layered stress (10). Of interest, GnRH pulse amplitude was reduced in experiment 1, despite the attenuated cortisol response. This fits with the finding that cortisol is neither necessary (this study) nor sufficient (6) for stress-induced inhibition of GnRH pulse amplitude. Collectively, these considerations reinforce the concept that stress inhibits LH pulse amplitude via separate hypothalamic and pituitary effects. The pituitary effect, reduced responsiveness to GnRH, requires increased cortisol secretion, whereas the hypothalamic effect, reduced GnRH pulse amplitude, does not.
There is a potential caveat to the conclusion that cortisol does not mediate the stress-induced suppression of pulsatile GnRH secretion. How can we be sure that RU486 had the desired effect: block neuroendocrine responses to cortisol mediated by the type II GR? There are two reasons why we are confident that RU486 was effective in this regard. First, we used the same dose and delivery method in two of our previous studies, which demonstrated that RU486 prevented both cortisol- and stress-induced suppression of pituitary responsiveness to GnRH (8,10). Second, and more importantly, RU486 markedly amplified and prolonged the stress-induced increase in plasma cortisol in all animals in experiment 2. Because the type II GR mediates the negative feedback actions of stress-like levels of cortisol (28,29,30), this intensified cortisol response in the presence of RU486 indicates a blockade of glucocorticoid negative feedback and confirms efficacy of the antagonist in blocking neuroendocrine actions of cortisol mediated via the type II GR. It is also worth noting that the antagonist clearly gains access to central sites of suppression of GnRH pulsatility because peripheral injection of RU486 blocks the inhibitory action of progesterone on GnRH pulse frequency in ewes (20). Collectively, these considerations document antagonist efficacy and reinforce the conclusion that cortisol action via the type II GR does not mediate the reduction in GnRH pulse amplitude in response to psychosocial stress.
This conclusion does not exclude the possibility that cortisol acts via a different receptor, e.g. the type I mineralocorticoid receptor (30), to mediate the suppressive effect of psychosocial stress on pulsatile GnRH release. Nevertheless, two prior observations would argue against this possibility. Antagonism of the type I mineralocorticoid receptor does not block the suppressive effect of cortisol on pulsatile LH secretion in the ovariectomized ewe (8). More notably, an increment of plasma cortisol similar to the values induced by the layered stress does not inhibit pulsatile GnRH release in the nonstressed ovariectomized ewe (6). These observations complement the present findings and strengthen our conclusion that suppression of pulsatile GnRH secretion in ovariectomized ewes during psychosocial stress is not mediated by cortisol.
If not cortisol, then what does cause the decrease in GnRH pulse amplitude in response to the layered stress? One possibility arises from the results of experiment 2 is that the decrease in GnRH pulse amplitude might be secondary to an increase in pulse frequency, which would be expected to lower releasable GnRH stores between pulsatile discharges. However, this possibility can be discounted by findings in experiment 1 that stress lowered GnRH pulse amplitude without increasing the frequency. A more likely mechanism by which psychosocial stress decreases GnRH pulse amplitude is via induction of inhibitory molecules and neurotransmitters. In this regard, neuropeptides of the hypothalamo-pituitary-adrenal axis (CRH, arginine vasopressin), endogenous opioid peptides (β-endorphin, dynorphin), cytokines (IL-1, IL-6, TNFα), and prostaglandins have all been suggested to mediate stress-induced suppression of pulsatile GnRH secretion (12,13,15,17,31,32,33). However, few studies have directly linked effects of these molecules to suppression of GnRH pulse amplitude. Two interesting studies that have provided this link determined that treatment with naloxone, an endogenous opioid peptide antagonist, increased the amplitude of GnRH pulses in ovariectomized ewes (34) and castrated rams (21). This is noteworthy because there is evidence that endogenous opioid peptides play an inhibitory role in GnRH pulsatile regulation and are increased in response to stress (21,35,36,37). Clearly, further work is needed to determine whether endogenous opioid peptides, or other inhibitory molecules, mediate the suppression of GnRH pulse amplitude in response to psychosocial stress.
Finally, we are puzzled why psychosocial stress enhanced GnRH pulse frequency in experiment 2 but not in experiment 1. Although we have no explanation for this, it is noteworthy that another stress type, immune-inflammatory stress (endotoxin), has caused an increase in GnRH pulse frequency in sheep, but this effect, too, was inconsistent across studies (5,16,17). Possibly related to this, endotoxin stimulates CRH secretion in sheep (38,39), and central delivery of CRH in sheep increased LH pulse frequency in some studies, but not in others (40,41,42). Although further work is necessary to explain the variable effect of the layered stress paradigm on GnRH pulse frequency, it is probably unrelated to cortisol action via the type II GR because the effect was evident both in the presence and absence of RU486.
In conclusion, the present study directly demonstrates that psychosocial stress decreases GnRH pulse amplitude and, thus, adds further insight into how stress can act to reduce the amplitude of LH pulses secreted by the pituitary. As demonstrated previously, one such mechanism involves an elevated level of plasma cortisol acting upon the pituitary to inhibit responsiveness to GnRH (9,10). The results reported here indicate that stress can also act centrally to inhibit GnRH pulse amplitude. Thus, hypothalamic as well as pituitary mechanisms likely contribute to the reduction of LH pulse amplitude in response to psychosocial stress. Importantly, an elevated level of cortisol mediates stress-induced suppression of pituitary responsiveness by acting via the type II GR (10), but, as shown here, this is not true for the hypothalamic effect of stress to decrease GnRH pulse amplitude. These findings encourage further studies to identify the factor(s) responsible for the central effect of psychosocial stress on GnRH pulsatility as well as to determine the importance of a decrease in GnRH pulse amplitude on overall reproductive function.
Acknowledgments
We thank Doug Doop for his expert animal care, and Alison Cooper, Christopher McCrum, Sarah Musil, Leslie Phillips, and Drs. Bree Pierce and Anne Turner for their assistance in the planning, conducting, and interpreting of the experiments. We also thank Drs. Alan Caraty, Gordon D. Niswender, and Leo E. Richert, Jr., for supplying RIA reagents.
Footnotes
This work was supported by National Science Foundation Grant IOB0520597, and National Institutes of Health Grants HD30773 and HD051360.
Present address for K.M.B.: Department of Reproductive Medicine, University of California, San Diego, Leichtag Biomedical Research Building, Room 349, La Jolla, California 92093-0674.
Present address for A.E.O.: Department of Physiology and Biophysics, University of Washington, Health Sciences Building, Room BB604, Box 356460, 1959 NE Pacific Street, Seattle, WA 98195-6460.
Preliminary reports have appeared in the Society for Neuroscience Abstract Viewer/Itinerary Planner, 2007 (Abstract 732.23) and the Program for the Endocrine Society’s 90th Annual Meeting, 2008 (Abstract P1-688).
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 1, 2008
Abbreviation: GR, Glucocorticoid receptor.
References
- Tilbrook AJ, Canny BJ, Serapiglia MD, Ambrose TJ, Clarke IJ 1999 Suppression of the secretion of luteinizing hormone due to isolation/restraint stress in gonadectomised rams and ewes is influenced by sex steroids. J Endocrinol 160:469–481 [DOI] [PubMed] [Google Scholar]
- Dobson H, Tebble JE, Phogat JB, Smith RF 1999 Effect of transport on pulsatile and surge secretion of LH in ewes in the breeding season. J Reprod Fertil 116:1–8 [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Ferin M 2002 Inadequate luteal function is the initial clinical cyclic defect in a 12-day stress model that includes a psychogenic component in the rhesus monkey. J Clin Endocrinol Metab 87:2232–2237 [DOI] [PubMed] [Google Scholar]
- Li XF, Edward J, Mitchell JC, Shao B, Bowes JE, Coen CW, Lightman SL, O'Byrne KT 2004 Differential effects of repeated restraint stress on pulsatile luteinizing hormone secretion in female Fischer, Lewis and Wistar rats. J Neuroendocrinol 16:620–627 [DOI] [PubMed] [Google Scholar]
- Debus N, Breen KM, Barrell GK, Billings HJ, Brown M, Young EA, Karsch FJ 2002 Does cortisol mediate endotoxin-induced inhibition of pulsatile luteinizing hormone and gonadotropin-releasing hormone secretion? Endocrinology 143:3748–3758 [DOI] [PubMed] [Google Scholar]
- Breen KM, Karsch FJ 2004 Does cortisol inhibit pulsatile hormone secretion at the hypothalamic or pituitary level? Endocrinology 145:692–698 [DOI] [PubMed] [Google Scholar]
- Stackpole CA, Clarke IJ, Breen KM, Turner AI, Karsch FJ, Tilbrook AJ 2006 Sex difference in the suppressive effect of cortisol on pulsatile secretion of luteinizing hormone in the sheep. Endocrinology 147:5921–5931 [DOI] [PubMed] [Google Scholar]
- Breen KM, Stackpole CA, Clarke IJ, Pytiak AV, Tilbrook AJ, Wagenmaker ER, Young EA, Karsch FJ 2004 Does the type II glucocorticoid receptor mediate cortisol-induced suppression in pituitary responsiveness to gonadotropin-releasing hormone? Endocrinology 145:2739–2746 [DOI] [PubMed] [Google Scholar]
- Breen KM, Davis TL, Doro LC, Nett TM, Oakley AE, Padmanabhan V, Rispoli LA, Wagenmaker EW, Karsch FJ 2008 Insight into the neuroendocrine site and cellular mechanism by which cortisol suppresses pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology 149:767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen KM, Oakley AE, Pytiak AV, Tilbook AJ, Wagenmaker ER, Karsch FJ 2007 Does cortisol acting via the type II glucocorticoid receptor mediate suppression of pulsatile luteinizing hormone secretion in response to psychosocial stress? Endocrinology 148:1882–1890 [DOI] [PubMed] [Google Scholar]
- Pierce BN, Hemsworth PH, Rivalland ETA, Wagenmaker ER, Morrissey AD, Papagiris MM, Clarke IJ, Karsch FJ, Turner AI, Tilbrook AJ 2008 Psychosocial stress suppresses attractivity, proceptivity and pulsatile LH secretion in the ewe. Horm Behav 54:424–434 [DOI] [PubMed] [Google Scholar]
- Ferin M 1999 Clinical review 105: stress and the reproductive cycle. J Clin Endocrinol Metab 84:1768–1774 [DOI] [PubMed] [Google Scholar]
- Rivier C, Rivest S 1991 Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod 45:523–532 [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Clarke IJ 2006 Neuroendocrine mechanisms of innate states of attenuated responsiveness of the hypothalamo-pituitary adrenal axis to stress. Front Neuroendocrinol 27:285–307 [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Battaglia DF, Breen KM, Debus N, Harris H 2002 Mechanisms for ovarian cycle disruption by immune/inflammatory stress. Stress 5:101–112 [DOI] [PubMed] [Google Scholar]
- Battaglia DF, Bowen JM, Krasa HB, Thrun LA, Viguie C, Karsch FJ 1997 Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: a simultaneous view from hypophyseal portal and peripheral blood. Endocrinology 138:4273–4281 [DOI] [PubMed] [Google Scholar]
- Harris TG, Battaglia DF, Brown ME, Brown MB, Carlson NE, Viguie C, Williams CY, Karsch FJ 2000 Prostaglandins mediate the endotoxin-induced suppression of pulsatile gonadotropin-releasing hormone and luteinizing hormone secretion in the ewe. Endocrinology 141:1050–1058 [DOI] [PubMed] [Google Scholar]
- Williams CY, Harris TG, Battaglia DF, Viguie C, Karsch FJ 2001 Endotoxin inhibits pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology 142:1915–1922 [DOI] [PubMed] [Google Scholar]
- Caraty A, Locatelli A, Moenter SM, Karsch FJ 1994 Sampling of hypophyseal portal blood of the conscious sheep for direct monitoring of hypothalamic neurosecretory substances. Methods Neurosci 20:162–183 [Google Scholar]
- Skinner DC, Evans NP, Delaleu B, Goodman RL, Bouchard P, Caraty A 1998 The negative feedback actions of progesterone on gonadotropin-releasing hormone secretion are transduced by the classical progesterone receptor. Proc Natl Acad Sci USA 95:10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraty A, Locatelli A, Schanbacher B 1987 [Augmentation, by naloxone, of the frequency and amplitude of LH-RH pulses in hypothalamo-hypophyseal portal blood in the castrated ram]. C R Acad Sci III 305:369–374 (French) [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Karsch FJ 1990 The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 127:1375–1384 [DOI] [PubMed] [Google Scholar]
- Hauger RL, Karsch FJ, Foster DL 1977 A new concept for control of the estrous cycle of the ewe based on the temporal relationships between luteinizing hormone, estradiol and progesterone in peripheral serum and evidence that progesterone inhibits tonic LH secretion. Endocrinology 101:807–817 [DOI] [PubMed] [Google Scholar]
- Niswender GD, Midgley Jr AR, Reichert Jr LE 1968 Radioimmunologic studies with murine, ovine and porcine luteinizing hormone. In: Rosenborg E, ed. Gonadotropins. Los Altos, CA: Geron-X; 299–306 [Google Scholar]
- Niswender GD, Reichert Jr LE, Midgley Jr AR, Nalbandov AV 1969 Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology 84:1166–1173 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML 1986 Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol 250(4 Pt 1):E486–E493 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Brand RM, Midgley AR, Karsch FJ 1992 Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology 130:503–510 [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER 1985 Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117: 2505–2511 [DOI] [PubMed] [Google Scholar]
- Spencer RL, Miller AH, Moday H, Stein M, McEwen BS 1993 Diurnal differences in basal and acute stress levels of type I and type II adrenal steroid receptor activation in neural and immune tissues. Endocrinology 133:1941–1950 [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M 1998 Brain corticosteroid receptor balance in health and disease. Endocr Rev 19:269–301 [DOI] [PubMed] [Google Scholar]
- Feleder C, Wuttke W, Moguilevsky JA 1998 Hypothalamic relationships between interleukin-6 and LHRH release affected by bacterial endotoxin in adult male rats. Involvement of the inhibitory amino acid system. Biol Signals Recept 7:7–14 [DOI] [PubMed] [Google Scholar]
- Yoo MJ, Nishihara M, Takahashi M 1997 Tumor necrosis factor-α mediates endotoxin induced suppression of gonadotropin-releasing hormone pulse generator activity in the rat. Endocr J 44:141–148 [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Turner AI, Clarke IJ 2002 Stress and reproduction: central mechanisms and sex differences in non-rodent species. Stress 5:83–100 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Parfitt DB, Evans NP, Dahl GE, Karsch FJ 1995 Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology 136:2414–2420 [DOI] [PubMed] [Google Scholar]
- Fabbri A, Jannini EA, Gnessi L, Ulisse S, Moretti C, Isidori A 1989 Neuroendocrine control of male reproductive function. The opioid system as a model of control at multiple sites. J Steroid Biochem 32:145–150 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Gibson M, Skinner DC, Lehman MN 2002 Neuroendocrine control of pulsatile GnRH secretion during the ovarian cycle: evidence from the ewe. Reprod Suppl 59:41–56 [PubMed] [Google Scholar]
- Tomaszewska D, Mateusiak K, Przekop F 1999 Changes in extracellular LHRH and β-endorphin-like immunoreactivity in the nucleus infundibularis-median eminence of anestrous ewes under stress condition. J Neural Transm 106:265–274 [DOI] [PubMed] [Google Scholar]
- Battaglia DF, Brown ME, Krasa HB, Thrun LA, Viguie C, Karsch FJ 1998 Systemic challenge with endotoxin stimulates corticotropin-releasing hormone and arginine vasopressin secretion into hypophyseal portal blood: coincidence with gonadotropin-releasing hormone suppression. Endocrinology 139:4175–4181 [DOI] [PubMed] [Google Scholar]
- Dadoun F, Guillaume V, Sauze N, Farisse J, Velut JG, Orsoni JC, Gaillard R, Oliver C 1998 Effect of endotoxin on the hypothalamic-pituitary-adrenal axis in sheep. Eur J Endocrinol 138:193–197 [DOI] [PubMed] [Google Scholar]
- Naylor AM, Porter DWF, Lincoln DW 1990 Central administration of corticotrophin-releasing factor in the sheep: effects on secretion of gonadotrophins, prolactin and cortisol. J Endocrinol 124:117–125 [DOI] [PubMed] [Google Scholar]
- Caraty A, Miller DW, Delaleu B, Martin GB 1997 Stimulation of LH secretion in sheep by central administration of corticotrophin-releasing hormone. J Reprod Fertil 111:249–257 [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Canny BJ, Stewart BJ, Serapiglia MD, Clarke IJ 1999 Central administration of corticotrophin releasing hormone but not arginine vasopressin stimulates the secretion of luteinizing hormone in rams in the presence and absence of testosterone. J Endocrinol 162:301–311 [DOI] [PubMed] [Google Scholar]