Abstract
Adiponectin plays important roles in the control of energy homeostasis and autonomic function through peripheral and central nervous system actions. The paraventricular nucleus (PVN) of the hypothalamus is a primary site of neuroendocrine (NE) and autonomic integration, and, thus, a potential target for adiponectin actions. Here, we investigate actions of adiponectin on parvocellular PVN neurons. Adiponectin influenced the majority (65%) of parvocellular PVN neurons, depolarizing 47%, whereas hyperpolarizing 18% of neurons tested. Post hoc identification (single-cell RT-PCR) after recordings revealed that adiponectin depolarizes NE-CRH neurons, whereas intracerebroventricular injections of adiponectin in vivo caused increased plasma ACTH concentrations. Adiponectin also depolarized the majority of TRH neurons, however, NE-TRH neurons were unaffected, in accordance with in vivo experiments showing that intracerebroventricular adiponectin was without effect on plasma TSH. In addition, bath administration of adiponectin also depolarized both preautonomic TRH and oxytocin neurons. These results show that adiponectin acts in the central nervous system to coordinate NE and autonomic function through actions on specific functional groups of PVN neurons.
Adiponectin, through actions at its two receptors AdipoR1 and AdipoR2, in addition to a potentially third uniqe mechanism, controls the excitability of specific paraventricular nucleus neurons, including neuroendocrine CRH and pre-autonomic TRH and oxytocin parvocellular neurons.
Energy homeostasis is coordinated in large part through peripherally produced hormones that interact with the central nervous system (CNS) (1). Adipose tissues produce a number of these signaling molecules that function in the CNS to dictate feeding patterns and responses to stress, and maintain dynamic equilibrium. Adiponectin is one such protein that, like leptin and resistin, is produced by adipocytes, circulates in the plasma, and controls network excitability in important autonomic nuclei (2,3,4,5,6). However, unlike leptin and resistin, adiponectin circulates at extremely high concentrations that vary inversely with adiposity (7,8). Adiponectin knockout mice show impaired responses to glucose challenges, and have compromised insulin and metabolic function among other pathologies (9). When injected into the brain, adiponectin has a marked effect on autonomic function. Mice show a substantial decrease in body weight, increased thermogenesis, increased oxygen consumption, and increased CRH mRNA production in the paraventricular nucleus (PVN) (10). This evidence suggests that adiponectin plays a critical role in controlling energy homeostasis.
Adiponectin signals through two known receptors: adiponectin receptors (AdipoRs) 1 and 2 (11). AdipoRs are located throughout the CNS, notably in regions of the hypothalamus and brainstem, important in controlling autonomic function and feeding behavior, including the area postrema (12), arcuate nucleus (13), and the PVN (3).
The PVN contains neurons that play important roles in controlling autonomic state (14,15). Electrophysiologically, the PVN can be divided into three populations of neurons (16,17): magnocellular (MNC), preautonomic (PA) parvocellular, and neuroendocrine (NE) parvocellular. Distinct groups of PA and NE neurons contain CRH, TRH, and oxytocin (OT) that participate as neuromodulators (PA) or hormones (NE) translating output information from the PVN to its targets (15).
We recently reported that adiponectin controls the excitability of PVN MNC neurons; hyperpolarizing OT neurons, having varying effects on vasopressin neurons and having no effect on neurons that expressed both peptides (3). Here, we examine the effects of adiponectin on the parvocellular neurons in the PVN (PA and NE), specifically investigating three chemically distinct types of neurons within this group: CRH-, TRH- and OT-expressing cells, while also correlating electrophysiological effects with integrated NE function in conscious freely moving animals.
Materials and Methods
All procedures involving the use of animals conformed to the standards of the Canadian Counsel on Animal Care and were approved by the Queen’s University Animal Care Committee. All chemicals used in making solutions were obtained from Sigma Pharmaceuticals (Oakville, Ontario, Canada). Tetrodotoxin (TTX) citrate was obtained from Alomone Laboratories (Jerusalem, Israel), made into working aliquots, and stored at −80 C. Globular adiponectin was obtained from Phoenix Pharmaceuticals (Belmont, CA), made into working aliquots, and stored at −20 C until experimentation.
Electrophysiology
Coronal hypothalamic slices (300 μm) containing the PVN were taken daily from male Sprague Dawley rats (Charles River, Quebec, Canada) between postnatal d 19 and 26 that were maintained on a 12-h light, 12-h dark cycle, and provided with food and water ad libitum. Rats were decapitated, and brains were removed and placed in ice-cold slicing solution consisting of (in mm) 87 NaCl, 2.5 KCl, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 1.25 NaH2PO4, 25 glucose, and 75 sucrose bubbled with 95% O2/5% CO2 for 3–5 min. Brains were then trimmed to size, mounted, immersed with slicing solutions, and 300 μm hypothalamic slices containing PVN (typically three) were cut using a vibratome (Leica, Nussloch, Germany). Slices were then bisected and incubated at 32.5 C in artificial cerebral spinal fluid (aCSF) containing (in mm) 126 NaCl, 2.5 KCl, 26 NaHCO3, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, and 10 glucose saturated with 95% O2/5% CO2 for at least 1 h before recording. aCSF was used as external recording solution for all electrophysiological experiments unless otherwise indicated.
Parvocellular PVN neurons were visualized using a ×40 water-immersion objective mounted to an upright Nikon E600FN microscope fitted with differential interference contrast/infrared optics (Nikon Corp., Tokyo, Japan). Borosilicate glass (World Precision Instruments, Sarasota, FL) was pulled by a flaming micropipette puller (Sutter Instruments Co., Novato, CA), and when filled with internal recording solution containing (in mm) 130 K+-gluconate, 10 KCl, 2 MgCl2, 0.1 CaCl2, 5.5 EGTA, 10 HEPES, 2 NaATP, adjusted to pH 7.2 with KOH, had resistances of between 2 and 5 mΩ. Whole cell configuration was obtained following the formation of a GΩ seal by applying negative pressure to the pipette and monitoring resistance changes on an oscilloscope.
Current clamp recordings from parvocellular PVN neurons were obtained with a Multiclamp 700B (Molecular Devices, Palo Alto, CA) patch clamp amplifier. Previous reports have shown that PVN parvocellular neurons can be divided into two main groups based on anatomical projection patterns and electrophysiological profile (16,18). Parvocellular PVN neurons were classified as either PA (type 2) or NE (type 3) neurons using a current pulse protocol designed to identify the presence of a low-threshold spike (LTS), or nonaccommodating spike firing, identifying electrophysiological features unique to PA and NE neurons in the PVN. The pulse protocol consisted of an initial 250-msec step of −60 pA from baseline membrane potential followed by sweeps of 10 pA (from −60 to 40 pA) steps for 250 msec until action potential threshold was reached. Neurons that displayed an LTS were classified as PA (see example in Fig. 4A, inset), whereas parvocellular neurons that showed no discernable LTS and no accommodation of spike frequency during depolarization were classified as NE (example Fig. 3A, inset). Neurons that had an ambiguous electrophysiological fingerprint were removed from the study. Experiments were recorded in real-time using acquisition software Spike 2 (Cambridge Electronic Devices, Cambridge, UK).Capacitance transients and series resistance error was minimized at the start of all recordings.
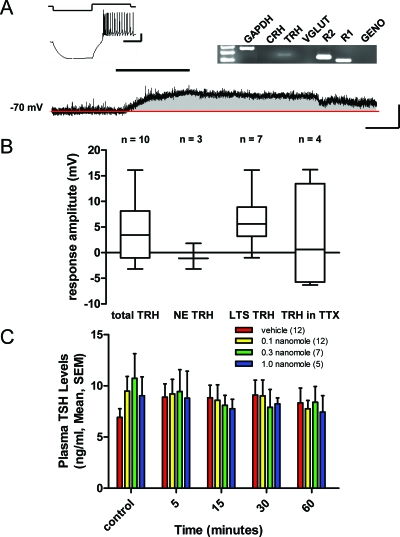
Figure 4.
Adiponectin selectively depolarizes type 2 PA TRH neurons. A, Current clamp recording of an identified type 2 PA neuron (inset) that expresses mRNA for TRH (gel inset) and depolarizes to bath application of 10 nm adiponectin (black bar). Scale bars, 20 mV and 100 msec (inset), 10 mV and 100 sec. B, Box and whisker plots showing different subgroups of PVN cells that express TRH as measured by single-cell RT-PCR and their response to adiponectin. C, Plasma TSH concentrations after icv injections of vehicle vs. three increasing doses of adiponectin measured at increasing time points up to 60 min after injection. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; R1, receptor 1; R2, receptor 2; VGLUT, vesicular glutamate transporter; GENO, genome.
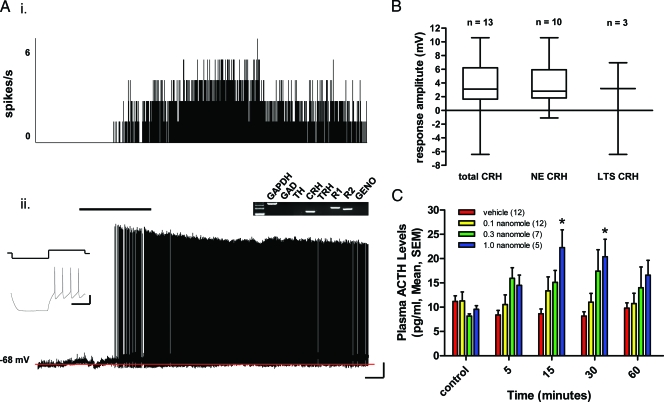
Figure 3.
Adiponectin activates the hypothalamic-pituitary stress axis through excitatory actions on NE CRH neurons in the PVN. A, The electrophysiological trace on the bottom (ii) is a current clamp recording from an electrophysiologically identified NE neuron (inset) whose post hoc single-cell RT-PCR profile (gel inset) identifies it as expressing CRH, depolarizing after bath application of 10 nm adiponectin (black bar). The red line in this and following figures indicates the control period membrane potential of the neuron. This neuron had a robust increase in action potential frequency as illustrated in a rate meter recording above the trace (i) showing a change in spike frequency before and after adiponectin administration. Scale bars, 30 mV and 100 msec (inset), 10 mV and 50 sec. B, A series of box and whisker plots showing the response of cells that expressed CRH as detected by single-cell RT-PCR to adiponectin administration. All but one NE CRH cell favored depolarization, whereas PA CRH neurons were mixed in their response to adiponectin. C, Plasma ACTH levels after icv injection of vehicle or adiponectin at three different doses, measured at different time points up to 1 h after injection (*, P < 0.05, between groups ANOVA, Scheffé multiple comparisons). GAD, γ-Amino butyric acid; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; R1, receptor 1; R2, receptor 2; GENO, genomic.
After establishing a stable control membrane potential for at least 100 sec, adiponectin, dissolved in aCSF to a concentration of 10 nm [previous studies indicate that 1 nm is the minimum effective dose (3)] was bath applied to the slice at a rate of 1–2 ml/min. Responsiveness of neurons to adiponectin was assessed by averaging all membrane potential points in 100-sec units starting from the time of application of peptide measuring 10 U. The peak mean voltage change in a 100-sec unit was compared with the mean membrane potential of points in the control unit. Neurons were categorized as responsive if the peak mean within the 10 U exceeded two times the sd value of the control membrane potential. Membrane potential was recorded until values returned to control levels, and only one recording was obtained from each hypothalamic slice. Neurons were eliminated from the data set if they did not show at least partial recovery to baseline.
Single-cell RT-PCR
After experimentation a post hoc single-cell RT-PCR was performed to detect mRNA for prominent neuropeptides expressed in parvocellular neurons (Table 1). Gentle suction was applied to the recording pipette to aspirate cytoplasm. Membrane resistance was continuously monitored as an outside-out patch was pulled, sealing the cytoplasmic contents within the pipette. Cells that failed to form outside-out patches were eliminated from sense-specific RT-PCR analysis but were included in the analysis of changes in membrane potential. After pipette withdrawal from the bath, the electrode tip was broken, and its contents were expelled into a 0.5-ml PCR tube. The following were added to the tube to set up the deoxyribonuclease (DNase) reaction: (approximate concentrations) 10 U DNase I and 10 mm 10× reaction buffer with MgCl2 (Fermentas Life Sciences, Burlington, Ontario, Canada) and incubated at 37 C for 30 min. After incubation, 2.5 mM EDTA was added and incubated at 65 C for 10 min to stop the DNase reaction. Reverse transcriptase reaction proceeded immediately after by adding dithiothreitol (26 mm), deoxynucleotide triphosphates (3 mm), random hexamers (3 μm), MgCl2 (4 mm), ribonuclease inhibitor (20 U), and SuperScript II (100 U) (Invitrogen Canada, Burlington, Ontario, Canada), and allowed to incubate overnight at 37 C to allow the reaction to proceed. A negative control reaction in which SuperScript II was omitted from the reaction mixture was also performed. Samples were stored at −80 C until the multiplex reaction.
Table 1.
Primer sets used in the detection of mRNA from single cells
| Gene | Primer | Position | Sequence | Product size (bp) | Accession no. |
|---|---|---|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | F (outside) | gatggtgaaggtcggtgtg | 469 | NM_017008 |
| R | gggctaagcagttggtggt | ||||
| F (nested) | taccaggctgccttctct | 360 | |||
| R | ctcgtggttcacacccatc | ||||
| γ-Amino butyric acid | GAD67 | F (outside) | cacaaactcagcggcataga | 550 | NM_017007 |
| R | gagatgaccatccggaagaa | ||||
| F (nested) | cacaaactcagcggcataga | 149 | |||
| R | ctggaagaggtagcctgcac | ||||
| Tyrosine hydroxylase | TH | F (outside) | cacctggagtattttgtgcg | 1138 | NM_012740 |
| R | cctgtgggtggtaccctatg | ||||
| F (nested) | tcgacccagtatatccgcca | 376 | |||
| F. | tcggacacaaagtacacagg | ||||
| Vasopressin | VP | F (outside) | cctcacctctgcctgctactt | 237 | NM_016992 |
| R | agccagctgtaccagcctaa | ||||
| F (nested) | acctctgcctgctacttcca | 216 | |||
| R | agccagctgtaccagcctaa | ||||
| Oxytocin | OT | F (outside) | ctgccccagtctcgcttg | 281 | NM_012996 |
| R | cctccgcttccgcaaggcttctggc | ||||
| F (nested) | ctgccccagtctcgcttg | 244 | |||
| F | gcgagggcaggtagttctcc | ||||
| Corticotropin-releasing hormone | CRH | F (outside) | ggggaaaggcaaagaaaagg | 184 | X03036 |
| R | gacagagccaccagcagcat | ||||
| F (nested) | ggagaagagaaaggagaagaggaa | 140 | |||
| R | ggacaccagcagccgcag | ||||
| Thyrotropin-releasing hormone | TRH | F (outside) | agaggggagacttgggagaa | 44? | M23632 |
| R | ctttgcttcaccagggtctc | ||||
| F (nested) | attcatgggcagatgaggag | 245 | |||
| R | ggcgtttctcaggcattaag | ||||
| Vesicular glutamate transporter-2 | VGLUT2 | F (outside) | aggttggctaccacctcct | 583 | AF271235 |
| R | tgagagtagccaacaaccagaagca | ||||
| F (nested) | cccgcaaagcatccaacca | 164 | |||
| R | cctgcagaagtttgcaacaa | ||||
| Adiponectin receptor 1 | AdipoRl | F (outside) | gtcccctggctctattctcct | 509 | BC061839 |
| R | agcacttggctgtgatgt | ||||
| F (nested) | tcttcctcatggctgtgatgt | 223 | |||
| R | ggctcagagaagggagtcatc | ||||
| Adiponectin receptor 2 | AdipoR2 | F (outside) | ggagccattctctgcctttc | 464 | NM_001037979 |
| R | ccagatgtcacatttgcca | ||||
| F (nested) | actgtaacccacaaccttgcttc | 191 | |||
| R | tcaggaacccttctgagatgac |
F, Forward; R, reverse.
A multiplex PCR strategy was used to amplify the cDNA produced in the reverse transcriptase reaction. This protocol consisted of two different amplification steps: outside and nested. First, primer sets (0.2 μm) specific for all genes of interest (outside) were added to the cDNA from the single cell, which then underwent an amplification protocol. Reactions were performed in 100-μl volumes with reagents provided in the QIAGEN Multiplex kit (QIAGEN, Mississauga, Ontario, Canada). The reaction mixture was denatured at 95 C for 15 min, then cycled 20 times through a temperature protocol of 94 C for 30 sec, 60 C for 90 sec, and 72 C for 90 sec. The second step consisted of individual reactions for each gene of interest using “nested” primer sets. Reactions were performed in 50-μl volumes consisting of QIAGEN Multiplex reaction reagents, 0.2 μm primers and 2 μl solution from the multiplex reaction to act as template. The reaction mixture was subject to 35 cycles of the same amplification protocol. Two control reactions were performed during the multiplex protocol: 1) 1 reaction was run with PCR H2O instead of cDNA to detect for genomic contamination, and 2) a second reaction was run with cDNA from whole PVN to assess primer integrity and eliminate false negatives. PCR products from the nested reaction were run on a 2% (wt/vol) agarose gel containing ethidium bromide and sequenced to confirm their identity (Robarts Research Institute, London, Ontario, Canada). PCRs that failed to pass any of the control tests were eliminated from the study.
In vivo experiments
lp;&-2qUnder ketamine (Ketaset; Fort Dodge Animal Health, Fort Dodge, IA)/xylazine (TranquiVed; Vedco Inc., St. Joseph, MO) anesthesia (60 mg/8 mg mixture/ml, 0.1 ml/100 g body weight, ip injection), rats were placed in a stereotaxic device, and a 23-gauge, stainless steel cannula (17 mm) was implanted into the right lateral cerebroventricle as previously described (19). Minimally, 5 d later, after the animals had returned to preimplantation body weights, an indwelling jugular vein cannula was implanted as previously described (20) under isoflurane-induced anesthesia (3% in O2 for induction, 2% in O2 for maintenance of anesthesia; IsoSol; Vedco). The jugular cannula was exteriorized at the back of the neck and sealed with heparinized saline (200 U/ml 0.9% NaCl). On the following day, an extension tubing (PE-50) was attached to the jugular cannula to facilitate blood sampling, and rats were left undisturbed for minimally 120 min. An initial blood sample was then withdrawn from the jugular vein without disturbing the animal. All blood samples (0.3 ml) were removed from conscious, unrestrained rats into heparinized syringes and replaced with an equal volume of 0.9% NaCl (37 C). Sampling was conducted between 0900 and 1100 h (lights on 0600 h). Blood samples were stored on ice before plasma was separated (10,000 × g, 5 min) and stored at −20 C until hormone assays were conducted. Immediately after the removal of the initial (zero time) blood sample, a 2-μl injection of isotonic saline vehicle alone or vehicle containing 0.1, 0.3, or 1.0 nmol adiponectin was conducted via the indwelling cerebroventricular cannula. Subsequent blood samples were removed 5, 15, 30, and 60 min after intracerebroventricular (icv) injections.
Plasma TSH levels were measured (20 μl aliquots) using the materials obtained from the National Peptide and Pituitary Program (National Institutes of Health (rTSH-RP-2 standard, minimum detectable level: 0.5 ng/ml; interassay and intraassay coefficients of variability were less than 8%). ACTH concentrations in plasma (33 μl aliquots) were determined by RIA using the unextracted plasma protocol described by the supplier (Peninsula Laboratories, Inc., San Carlos, CA). Minimum detectable ACTH levels were 1 pg/ml, and interassay and intraassay coefficients of variability were less than 8%.
Data from the RIAs were analyzed by one-way ANOVA both within treatment groups across time and between treatment groups at any sampling time point, followed by Scheffé’s multiple comparison testing. Homogeneity of variance was established using the S test. Significance was assigned to results that occurred with less than 5% probability.
Results
Adiponectin influences the excitability of parvocellular neurons in the PVN
Current clamp recordings were obtained from 122 identified (58 PA and 64 NE) parvocellular PVN neurons, and the effects of bath administration of 10 nm adiponectin on membrane potential were examined.
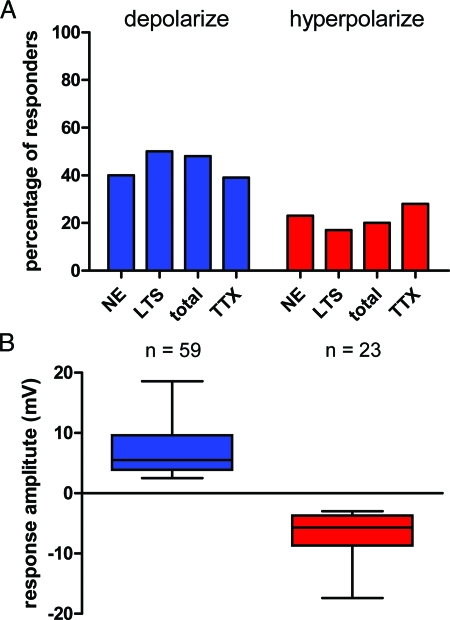
Adiponectin influenced the membrane potential of the majority of both NE and PA neurons with depolarizing and hyperpolarizing effects observed in different cells in each group (Fig. 1). The majority of cells influenced by adiponectin in each group depolarized (7.0 ± 0.5 mV, n = 59) (PA 50%, NE 40%, total 48%), whereas a smaller proportion of cells hyperpolarized (−6.7 ± 0.7 mV, n = 23) (PA 17%, NE 23%, total 20%) (Fig. 2). A third population of parvocellular neurons did not respond to 10 nm adiponectin. Both depolarizing (8.4 ± 1.3 mV, n = 15) and hyperpolarizing (−5.9 ± 0.9 mV, n = 11) effects of adiponectin were maintained in the presence of 1 μm TTX (n = 39), and were observed in similar proportions of neurons (Fig. 2A), suggesting that mechanistically, adiponectin acts directly at the cell membrane of parvocellular neurons to modulate their electrical excitability.
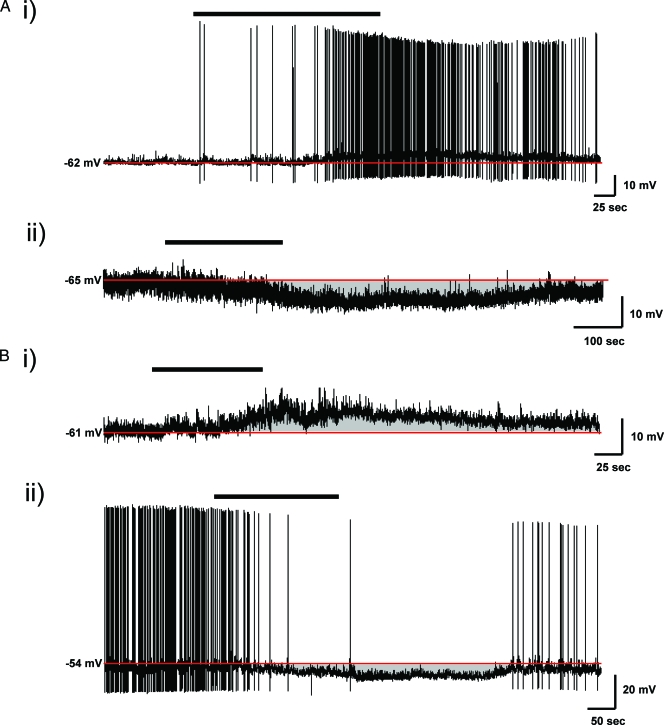
Figure 1.
Adiponectin effects on the membrane potential of NE and PA neurons in the PVN. A, Bath administration of 10 nm adiponectin (black bar) induces both depolarizing (i) and hyperpolarizing (ii) effects on membrane potential in different populations of type 3 NE neurons. Membrane potential routinely recovered to near control membrane potential (red line) after removal of adiponectin from the bath. B, Similar effects of adiponectin on membrane potential were seen in type 2 PA neurons, depolarizing (i) a population of cells while hyperpolarizing (ii) a separate group of neurons.
Figure 2.
The effects of adiponectin are direct and proportionally similar across parvocellular PVN neurons. A, Histogram showing percentage of each cell type; NE, PA, total combined and when action potential induced neurotransmitter release was inhibited through the application of 1 μm TTX of neurons depolarizing (blue) or hyperpolarizing (red) in the presence of 10 nm adiponectin. B, Summary box and whisker plot indicating for both depolarization and hyperpolarization the smallest observation, the lowest quartile, median, upper quartile, and the largest observation in each data set. The median depolarization was 5.5 mV, whereas the median hyperpolarization was −5.7 mV.
Adiponectin depolarizes NE CRH neurons
To assess adiponectin’s effects on specific groups of neurons in the PVN after membrane potential recordings, we performed post hoc single-cell RT-PCR analysis for markers of interest (Table 1). Of the 122 neurons recorded from, 13 were positively identified as CRH mRNA expressing neurons. Of 13 CRH neurons, 10 were NE neurons, and 70% of those neurons showed a depolarizing shift in membrane potential in response to 10 nm adiponectin (5.2 ± 1.1 mV) (Fig. 3, A and B). In addition, three PA CRH neurons responded to 10 nm adiponectin (two depolarized, one hyperpolarized).
AdipoR profile in CRH NE neurons was also assessed by single-cell RT-PCR, and all seven of the responding CRH neurons expressed mRNA for at least one AdipoR (R1, n = 3; R2, n = 1; and R1/R2, n = 3). NE CRH neurons that did not express either receptor did not respond to adiponectin (0.7 ± 0.9 mV, n = 3).
We next examined the effect of adiponectin on the hypothalamic-pituitary-adrenal axis by measuring the effects of adiponectin injected into the lateral cerebroventricle (icv) on plasma ACTH concentrations. Injections (icv) of adiponectin caused a dose-related increase in plasma ACTH concentrations 15 and 30 min after injections when compared with saline-injected controls (Fig. 3C). The injection of 1.0 nmole adiponectin showed the largest increase in plasma ACTH concentration compared with vehicle at 15 min [22.3 ± 3.7 pg/ml (1.0 nmole adiponectin) vs. 8.6 ± 1.0 pg/ml (vehicle); P = 0.014), (ANOVA, Scheffé multiple comparisons)]. Plasma ACTH concentrations had returned to baseline at the 60-min time point, suggesting that the actions of adiponectin are transient and reversible. These results indicate that adiponectin acts on CRH neurons in the PVN to control CRH release from the median eminence, and subsequent ACTH release from the anterior pituitary gland.
Adiponectin depolarizes PA TRH neurons
We also obtained current clamp recordings from a total of 10 neurons that were identified post hoc as TRH mRNA expressing neurons in which we were able to assess the effects of bath administration of 10 nm adiponectin. The majority of TRH neurons (six of 10) depolarized in response to 10 nm adiponectin (7.6 ± 1.9 mV) (Fig. 4A). More interestingly, classification of TRH neurons as NE or PA revealed that a majority of the TRH neurons depolarized by adiponectin were PA cells (six of seven), whereas zero of three NE TRH neurons were affected by adiponectin (Fig. 4B). Analysis of the expression patterns of AdipoRs in TRH neurons showed that one of six responsive PA TRH neurons expressed receptors (R1, n = 0; R2, n = 0; and R1/R2, n = 1), whereas a significant number that responded to adiponectin (4.0 ± 2.9 mV, n = 5) did not. We hypothesized that this may be due to the effect of adiponectin on a presynaptic interneuron and, therefore, performed additional current clamp recordings from a total of four identified PA TRH neurons that did not express either AdipoR in the presence of 1 μm TTX. We observed clear effects of adiponectin in all of these neurons (depolarization in two and hyperpolarization in two), as shown in Fig. 4B, suggesting that adiponectin may influence PA TRH neurons, at least in part, through an indirect sodium channel-independent mechanism. The mechanisms underlying such indirect effects are not known at the current time.
In addition to measuring plasma ACTH concentrations after lateral ventricle icv injections of adiponectin, plasma TSH concentrations were measured. TSH concentrations showed no significant change compared with vehicle control at any concentration or time point (15 min, vehicle: 8.8 ± 1.2 ng/ml vs. 1.0 nmole: 7.8 ± 0.9 ng/ml, ANOVA, Scheffé multiple comparisons) after adiponectin administration. These data suggest that adiponectin selectively regulates ACTH secretion in the hypothalamic-pituitary axis but has no effect on TRH/TSH secretion.
Adiponectin depolarizes PA OT neurons
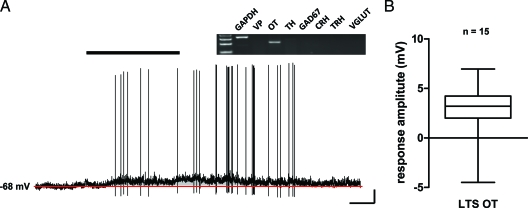
A significant proportion of PA neurons that express OT send axonal projections to the nucleus of the solitary tract (NTS), and have been suggested to modify feeding by sensitizing NTS neurons to satiety signals such as cholecystokinin (21). We recorded from 15 PA neurons that were positively identified as OT expressing neurons. The majority of these PA OT neurons (11/15) depolarized in response to 10 nm adiponectin (4.1 ± 0.4 mV) (Fig. 5), whereas one of the remaining four cells tested was hyperpolarized by peptide administration. The majority of adiponectin responsive PA OT neurons expressed AdipoRs [R1, n = 2; R2, n = 2; and R1/R2, n = 3 (seven of 11)], whereas in accordance with our recordings from PA TRH neurons, a proportion of these adiponectin responsive OT PA neurons (four of 11) did not express mRNA for either of these receptors. These data suggest that adiponectin may interact with OT PA neurons, and importantly highlight opposite actions of this adipokine of functionally distinct subpopulations of OT expressing neurons in the PVN.
Figure 5.
The majority of type 2 PA OT neurons are depolarized by adiponectin. A, Current clamp recording of a type 2 PA OT neuron in response to bath administration of 10 nm adiponectin. A majority of PA OT neurons showed a depolarizing shift in membrane potential followed by a return to baseline upon removal of adiponectin from the bath. Scale bars, 10 mV and 50 sec. B, Box and whisker summary plot of type 2 PA OT neurons and their response to adiponectin. GAD67, γ-Amino butyric acid 67; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; R1, receptor 1; R2, receptor 2; VGLUT, vesicular glutamate transporter; VP, vasopressin.
Discussion
These data show both endocrine and autonomic effects of adiponectin acting upon specific groups of neurons in the PVN, confirming and identifying new and potentially critical roles played by this peptide in regulating energy and autonomic homeostasis. We have shown that adiponectin is primarily an excitatory peptide in the parvocellular regions of the PVN, depolarizing a majority of identified parvocellular neurons, while hyperpolarizing only a minority of neurons in the same groups. We have also shown that adiponectin directly excites NE CRH neurons that express AdipoRs, leading potentially to an increase in CRH release into pituitary portal circulation. Indeed, we have measured an increase in plasma ACTH concentrations after lateral cerebroventricular injections of adiponectin.
In addition, we have identified two chemically distinct groups of PA neurons: TRH expressing and OT expressing cells whose electrical excitability is controlled by adiponectin, suggesting that the peptide may play alternate regulatory roles in addition to hypothalamic-pituitary axis induction through modulation of caudally projecting, PA cells.
CRH is a potent anorexigenic peptide that when released from parvocellular neurons into the median eminence, regulates the secretion of ACTH from anterior pituitary cells, ultimately controlling plasma glucocorticoid levels (22). Our observations that adiponectin depolarizes the majority of NE CRH neurons while also increasing plasma concentrations of ACTH suggests mechanisms through which this peptide may control activation of the stress axis. Previous reports indicate that when injected intracerebroventricularly, adiponectin increases respiration, energy metabolism, and body temperature in mice (10), all indicators of elevated sympathetic tone. Intriguingly, iv injections of adiponectin increase feeding through actions at the arcuate nucleus (13), which has recently been shown to express AdipoR1 and AdipoR2 (23), suggesting a site specificity for autonomic function. Our results clarify the mechanism responsible for this, and suggest that adiponectin’s actions at CRH neurons involved in the hypothalamic-pituitary axis partially dictate the metabolic and autonomic state of the animal.
PVN TRH neurons also control feeding behavior, participate in the stress response, and control metabolic function (24). Increases in TRH secretion at the median eminence are associated with elevated thyroid function ultimately increasing the drive to promote negative energy expenditure. Given that icv injections of adiponectin increase energy expenditure (10), we were surprised to find that plasma concentrations of TSH after central adiponectin injections were unchanged, an observation that ultimately correlates well with our electrophysiological data showing that the TRH NE cells tested were also unaffected by adiponectin.
In contrast to neurosecretory TRH neurons, PA TRH neurons were selectively depolarized by adiponectin. These neurons have been suggested to project to autonomic preganglionic neurons in the dorsal vagal complex (18) and spinal cord, and send information about core body temperature to peripheral targets (25). Thus, adipocyte derived adiponectin acting through PA TRH neurons may represent a vital connection coupling adiposity stores to central thermoregulation. Although an attractive hypothesis, further experiments will be needed to investigate this possibility.
Activation of PA OT neurons that project to the NTS has exerted profound effects on feeding patterns (21). The excitation of these PVN neurons leading to the release of OT into the hindbrain promotes the sensitization of NTS neurons to satiety cues such as cholecystokinin, advancing meal termination (26). The depolarizing effect of adiponectin on PA OT neurons would suggest that adiponectin may act centrally to control meal size. These effects would have profound consequences on feeding behavior and may contribute to the effects seen on weight loss when adiponectin is injected intracerebroventricularly (10). The effect of adiponectin on PA OT neurons may not be limited to alterations in feeding behavior but may be heterogeneous in nature. Caudally projecting PA neurons have functionally diverse roles in the autonomic nervous system (27,28), and further experiments will be needed to examine other systems.
Intriguingly, the depolarizing effects of adiponectin on type 2 PA OT neurons are in contrast to adiponectin’s hyperpolarizing effects on PVN OT MNC neurons (3). Although these are two functionally and anatomically independent systems, they share the same signaling molecule OT and apparently are differentially modulated by adiponectin. Together, these data would indicate that the function of adiponectin in the PVN may not be to control the release of a specific peptide but to coordinate diverse networks of neurons that may use the same signaling molecule but have unique targets and, thus, different effects in vivo. The effective icv dose of adiponectin was 1 nmole, a gram per body weight value similar to that used in previous in vivo studies (10). Assuming a 1-ml volume of cerebral spinal fluid (CSF), the calculated CSF concentration of adiponectin is 1 μm. Although this concentration is larger than the 10 nm used in the in vitro current clamp studies, enzymatic degradation and diffusion to the presumed active site in PVN will both result in further reductions in the active concentration reaching the PVN. These considerations suggest that our icv injections likely achieve concentrations in PVN that are closer to the 10-nm concentration used in vitro.
Adiponectin is unlikely to cross the blood-brain barrier (29,30), however, concentrations of the peptide have been detected in the CSF of humans and rats (31,32), and CSF levels have increased upon iv injections of the peptide (10,23). These data prompt speculation as to the source of the adiponectin for actions in the CNS. Recent evidence suggests that adiponectin mRNA is readily expressed in chicken and mouse brain (33,34). Although we cannot exclude that peripherally derived adiponectin controls the excitability of CNS structures, the case for brain-derived adiponectin as a modulator of these systems is an attractive alternative hypothesis and may play an important role in vivo.
Our data correlating AdipoR expression with cell responsiveness confirm previous reports from our laboratory (3,12) showing that neurons expressing AdipoR1/R2, AdipoR1 alone, or AdipoR2 alone all respond to adiponectin. Similarly, all responding CRH neurons expressed at least one AdipoR. These observations suggest that the response of the neuron to adiponectin may not be dictated by receptor expression but, rather, by the ion channel(s) targets expressed by the neuron and modulated by activation of receptor(s). Elucidation of these target ion channels will provide more information on the intracellular signaling mechanisms responsible for adiponectin induced neuronal excitability.
Several PA neurons that expressed TRH or OT and responded to adiponectin did not express mRNA for either AdipoR. However, the response to adiponectin in these neurons was maintained in TTX, suggesting an effect, independent of AdipoR activation at the cell surface of these recorded neurons. Several possibilities could explain these observations. Leptin and ghrelin have been shown to confer their central effects on feeding through activation of the PVN endocannabinoid system, leptin specifically reducing excitatory synaptic drive to PVN neurons through an endocannabinoid-dependent fast inhibitory feedback mechanism (35,36). Whether adiponectin also signals through the endocannabinoid system is unknown at the current time but could represent a receptor-independent mechanism of signaling in these neurons. A second possibility of how adiponectin could influence these neurons is the modulation of TTX insensitive dendritic release of neuromodulatory peptides. This system has been shown to act as an autocrine or paracrine messaging system and can modulate neuronal output within the PVN (37). A third explanation for the unique effect of adiponectin is the interaction with another receptor. The cell surface receptor T cadherin has been proposed to be a third receptor for circulating adiponectin; however, it is unlikely that activation of this receptor is responsible for the effects seen here because T cadherin preferentially binds only high-molecular or hexameric forms of adiponectin (38). Finally, although our serial dilution controls have shown that both receptors can be detected in dilutions of whole PVN cDNA, reflecting far less volume than a single cell (R1, 1/10,000,000; R2, 1/10,000,000), we cannot totally exclude the possibility that AdipoRs are expressed in these cells that our single-cell RT-PCR technology cannot detect.
In conclusion, we have shown that adiponectin, through actions at its two receptors AdipoR1 and AdipoR2, in addition to potentially a third unique mechanism, controls the excitability of specific groups of neurons in the parvocellular regions of the PVN, depolarizing NE CRH, PA TRH, and PA OT neurons. Furthermore, through in vivo studies, we have shown that when injected centrally, adiponectin selectively controls the release of ACTH into the plasma while having no effect on TSH release. This study highlights the diverse central roles played by adiponectin in controlling energy homeostasis and autonomic function, and may provide insight into the pathophysiology of disrupted adiponectin signaling in diabetes and obesity.
Acknowledgments
We thank Ms. Christie Hopf for her technical assistance with the single-cell RT-PCR experiments.
Footnotes
This work was supported by grants from the Heart and Stroke Foundation (to A.V.F.), National Institutes of Health (to W.K.S.), and a Canadian Institute of Health Research Doctoral Research Award (to T.D.H.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 23, 2008
For editorial see page 559
Abbreviations: aCSF, Artificial cerebral spinal fluid; AdipoR, adiponectin receptor; CNS, central nervous system; CSF, cerebral spinal fluid; DNase, deoxyribonuclease; icv, intracerebroventricular; LTS, low-threshold spike; MNC, magnocellular; NE, neuroendocrine; NTS, nucleus of the solitary tract; OT, oxytocin; PA, preautonomic; PVN, paraventricular nucleus; TTX, tetrodotoxin.
References
- Murphy KG, Bloom SR 2006 Gut hormones and the regulation of energy homeostasis. Nature 444:854–859 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Lazar MA 2008 Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol 22:1023–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyda TD, Fry M, Ahima RS, Ferguson AV 2007 Adiponectin selectively inhibits oxytocin neurons of the paraventricular nucleus of the hypothalamus. J Physiol 585(Pt 3):805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal NS, Lazar MA, Ahima RS 2007 Central resistin induces hepatic insulin resistance via neuropeptide Y. J Neurosci 27:12924–12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis JE, Bains JS, Ferguson AV 1998 Leptin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol 274(5 Pt 2):R14682–R1472 [DOI] [PubMed] [Google Scholar]
- Spanswick R, Smith MA, Groppi VE, Logan SD, Ashford MLJ 1997 Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390:521–525 [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM 1996 AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271:10697–10703 [DOI] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF 1995 A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270:26746–26749 [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T 2002 Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277:25863–25866 [DOI] [PubMed] [Google Scholar]
- Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS 2004 Adiponectin acts in the brain to decrease body weight. Nat Med 10:524–529 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T 2003 Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769 [DOI] [PubMed] [Google Scholar]
- Fry M, Smith PM, Hoyda TD, Duncan M, Ahima RS, Sharkey KA, Ferguson AV 2006 Area postrema neurons are modulated by the adipocyte hormone adiponectin. J Neurosci 26:9695–9702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T 2007 Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6:55–68 [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Washburn DLS 1998 Angiotensin II: a peptidergic neurotransmitter in central autonomic pathways. Prog Neurobiol 54:169–192 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE 1980 Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31:410–417 [DOI] [PubMed] [Google Scholar]
- Luther JA, Daftary SS, Boudaba C, Gould GC, Halmos KC, Tasker JG 2002 Neurosecretory and non-neurosecretory parvocellular neurones of the hypothalamic paraventricular nucleus express distinct electrophysiological properties. J Neuroendocrinol 14:929–932 [DOI] [PubMed] [Google Scholar]
- Tasker JG, Dudek FE 1991 Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol (Lond) 434:271–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE 2001 Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol 537(Pt 1):161–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes-Rodrigues J, McCann SM, Rogers LC, Samson WK 1985 Atrial natriuretic factor inhibits dehydration- and angiotensin II-induced water intake in the conscious, unrestrained rat. Proc Natl Acad Sci USA 82:8720–8723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms PG, Ojeda SR 1974 A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol 36:391–392 [DOI] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG 2004 Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287:R87–R96 [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G 2005 Endocrinology of the stress response. Annu Rev Physiol 67:259–284 [DOI] [PubMed] [Google Scholar]
- Guillod-Maximin E, Roy AF, Vacher CM, Aubourg A, Bailleux V, Lorsignol A, Penicaud L, Parquet M, Taouis M 2009 Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J Endocrinol 200:93–105 [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C 2006 The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res 153:209–235 [DOI] [PubMed] [Google Scholar]
- Arancibia S, Rage F, Astier H, Tapia-Arancibia L 1996 Neuroendocrine and autonomous mechanisms underlying thermoregulation in cold environment. Neuroendocrinology 64:257–267 [DOI] [PubMed] [Google Scholar]
- Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG 2003 Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res 993:30–41 [DOI] [PubMed] [Google Scholar]
- Rinaman L 1998 Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol 399:101–109 [DOI] [PubMed] [Google Scholar]
- Toth ZE, Gallatz K, Fodor M, Palkovits M 1999 Decussations of the descending paraventricular pathways to the brainstem and spinal cord autonomic centers. J Comp Neurol 414:255–266 [PubMed] [Google Scholar]
- Spranger J, Verma S, Gohring I, Bobbert T, Seifert J, Sindler AL, Pfeiffer A, Hileman SM, Tschop M, Banks WA 2006 Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes 55:141–147 [PubMed] [Google Scholar]
- Pan W, Tu H, Kastin AJ 2006 Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides 27:911–916 [DOI] [PubMed] [Google Scholar]
- Kusminski CM, McTernan PG, Schraw T, Kos K, O'Hare JP, Ahima R, Kumar S, Scherer PE 2007 Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia 50:634–642 [DOI] [PubMed] [Google Scholar]
- Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, Snead DR, Hoggart B, O'Hare JP, McTernan PG, Kumar S 2007 Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab 92:1129–1136 [DOI] [PubMed] [Google Scholar]
- Maddineni S, Metzger S, Ocon O, Hendricks III G, Ramachandran R 2005 Adiponectin gene is expressed in multiple tissues in the chicken: food deprivation influences adiponectin messenger ribonucleic acid expression. Endocrinology 146:4250–4256 [DOI] [PubMed] [Google Scholar]
- Wilkinson M, Brown R, Imran SA, Ur E 2007 Adipokine gene expression in brain and pituitary gland. Neuroendocrinology 86:191–209 [DOI] [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG 2006 Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci 26:6643–6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci SA, Rogers EK, Korbonits M, Kirkham TC 2004 The cannabinoid CB1 receptor antagonist SR141716 blocks the orexigenic effects of intrahypothalamic ghrelin. Br J Pharmacol 143:520–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G 2006 Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7:126–136 [DOI] [PubMed] [Google Scholar]
- Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF 2004 T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 101:10308–10313 [DOI] [PMC free article] [PubMed] [Google Scholar]