Abstract
Calcitriol actions are mediated by the vitamin D receptor (VDR), a nuclear transcription factor of the steroid-retinoid-thyroid nuclear receptor gene superfamily. Calcitriol inhibits the growth of many cells including cancer cells by inducing cell cycle arrest. In some cancer cell lines, calcitriol also induces apoptosis. In the LNCaP prostate cancer cell line, induction of apoptosis and caspase-3/7 activities by staurosporine (STS) abolished [3H]1,25-dihydroxy vitamin D3 binding and VDR protein, suggesting that the VDR may be targeted for inactivation by caspases during apoptosis. A potential caspase-3 site (D195MMD198S) was identified in the human VDR ligand-binding domain. Mutations D195A, D198A, and S199A were generated in the putative capase-3 cleavage site. In transfected COS-7 cells, STS treatment resulted in the cleavage of the wild-type (WT) VDR and S199A mutant VDR but not the D195A or D198A mutants. In in vitro assays, the WT VDR and S199A mutant VDR were cleaved by caspase-3, although the D195A and D198A mutants were resistant to caspase-3. In vitro, the WT VDR was also cleaved by caspase-6 and caspase-7 and in extracts of STS-treated LNCaP cells. In STS-treated LNCaP cells and human skin fibroblasts, the proteasome inhibitor MG-132 protected the VDR caspase cleavage fragment from further degradation by the 26S proteasome. The rat VDR that does not contain the caspase-3 cleavage site was not cleaved in STS-treated COS-7 cells. In conclusion, our results demonstrate that the human VDR is a target of caspase-3 and suggest that activation of caspase-3 may limit VDR activity.
The vitamin D receptor contains a caspase-3 cleavage site in the ligand-binding domain that can be cleaved by caspase-3 in vitro and in intact cells.
The physiological actions of 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3 or calcitriol], the active form of vitamin D, are mediated by the vitamin D receptor (VDR), a member of the steroid-thyroid-retinoid receptor superfamily of nuclear transcription factors (1). In addition to the classical actions on calcium homeostasis, calcitriol regulates other cellular processes including cell proliferation, apoptosis, and differentiation (2,3,4,5,6). Calcitriol inhibits the growth of many cells, including cancer cells such as breast, colon, ovarian, pancreatic, and prostate cancer cells (2,3,4,5,6). In breast and prostate cancer cells, calcitriol inhibits cell proliferation by inducing G1 arrest (2,3,4,5,6). In some cancer cells, calcitriol also induces apoptosis (2,3,4,5,6,7,8). In some breast cancer cells, calcitriol induces apoptosis by a caspase-independent mechanism (9), whereas in prostate cancer cells and other breast cancer cells, calcitriol induces apoptosis by a caspase-dependent mechanism (2). It is these properties that make calcitriol an attractive therapeutic agent for treating numerous disease processes including cancer (2,3,4,5,6,7,8).
Caspases are a family of 14 cysteine proteases that specifically cleave proteins following aspartic acid residues (10,11). The main executioner caspases activated during apoptosis are caspase-3, -6, and -7 (12,13). Caspase-3 and -7 share a common cleavage site at aspartic acid followed by any two amino acids followed by aspartic acid, a so-called DxxD motif (14). These caspases are activated during programmed cell death and lead to apoptosis (12,13). Under nonapoptotic conditions, caspases are also important regulators of cell survival, proliferation, differentiation, and inflammation (15). Because many proteins including p53 and retinoblastoma that regulate cell growth are cleaved by caspases during apoptosis (16,17), we were interested in determining whether the VDR was also a target of caspases. In this study, we have demonstrated that the VDR is cleaved and inactivated by caspases during induction of apoptosis.
Materials and Methods
Cell culture
COS-7 monkey kidney cells and LNCaP prostate cancer cells were obtained from the American Type Culture Collection (Manassas, VA). LNCaP and PC-3 cells were cultured in RPMI medium supplemented with 5% fetal calf serum. COS-7 cells were cultured in DMEM with 10% bovine growth serum (Hyclone Laboratories, Logan, UT).
Reagents and assays
The anti-VDR monoclonal antibody D-6 and polyclonal antibody C-20 that recognize the VDR COOH-terminal domain and polyclonal antibody N-20 that recognizes the VDR NH2-terminal domain were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The poly-(ADP-ribose) polymerase (PARP) monoclonal antibody was purchased from Cell Signaling Technology (Danvers, MA). Staurosporine (STS) was purchased from Sigma Aldrich (St. Louis, MO). MG-132 was purchased from EMD Biosciences (La Jolla, CA). Protein concentrations were determined by the Bradford method (18).
Induction of apoptosis in LNCaP prostate cancer cells
LNCaP cells were induced to undergo apoptosis by the addition of 1 μm STS (19,20). Samples were collected over time and the VDR analyzed by ligand binding and immunoblotting. DEVDase activity was determined using a caspase-3 colorimetric assay kit (Biovision, Mountain View, CA). In some assays the proteasome inhibitor MG-132 was added to a final concentration of 10 μm.
Ligand binding
Ligand binding assays using [3H]1,25-(OH)2D3 (specific activity 109 Ci/mmol; Amersham Biosciences, Piscataway, NJ) were performed as previously described (21).
Immunoblotting
For immunoblot analysis, cell extracts were mixed with lithium dodecyl sulfate-sample buffer (Invitrogen, Carlsbad, CA), incubated for 10 min at 70 C, and electrophoresed on 10% NuPAGE gels in MOPS-SDS running buffer (Invitrogen). Proteins were transferred to 0.2-μm nitrocellulose membranes in NuPAGE transfer buffer (Invitrogen) containing 10% methanol. The membranes were incubated for 1 h at ambient temperature in 5% BLOT-QuickBlocker (Geno Technology, Inc., St. Louis, MO) in Tris-buffered saline containing 0.1% Tween 20 (TBST) and then incubated with a 1:250 dilution of the rabbit anti-VDR N-20 polyclonal antibody in 5% BLOT-QuickBlotter in TBST or a 1:250 dilution of the mouse anti-VDR D-6 monoclonal antibody in 5% BSA in TBST. After overnight incubation at 4 C, the membranes were washed in TBST and incubated for 1 h at ambient temperature with a 1:4000 dilution of goat antirabbit IgG-horseradish peroxidase or antimouse IgG-horseradish peroxidase (Santa Cruz Biotechnology) in 5% QuickBlocker in TBST. The blots were washed and then developed using ECL Plus Western blotting reagent (Amersham Biosciences).
VDR cDNA mutagenesis
Site-directed mutagenesis of the wild-type (WT) VDR cDNA in pSG5 (Stratagene, La Jolla, CA) was performed using the Gene Editor system (Promega Corp., Madison, WI) as previously described (22).
Studies in transfected COS-7 cells
COS-7 cells were transfected with human WT and mutant VDR cDNA expression vectors for 24 h using Polyfect transfection reagent (QIAGEN, Valencia, CA). In some experiments, the rat VDR expression vector was also transfected. The transfected cells were then treated with 1 μm STS and samples taken over time and the VDR analyzed by immunoblotting.
Preparation of naive and apoptotic extracts
LNCaP cells were treated with vehicle or 1 μm STS for 3 h. The cells were washed with ice-cold PBS, pelleted by centrifugation, and then resuspended in cell lysis buffer (Biovision). After 30 min at 4 C, the lysed cells were centrifuged at maximum speed in a microfuge for 20 min at 4 C. Supernatants (naive and apoptotic extracts) were divided into small aliquots and stored at −80 C.
In vitro cleavage with cell extracts
Human WT and mutant VDRs expressed in COS-7 cells were incubated with 20 μg LNCaP cell naive or apoptotic extract in caspase assay buffer (Biovision) at 37 C for 1 h. The samples were then electrophoresed on 10% NuPAGE gels and immunoblotted with anti-VDR antibody D-6 as described above.
In vitro cleavage with caspases
Human WT and mutant VDRs expressed in COS-7 cells were incubated with 1 U purified caspase-3, -6, -7, or -8 in caspase assay buffer (Biovision) at 37 C for 1 h and then separated on a 10% SDS gel and immunoblotted with anti-VDR antibody D-6 as described above.
Transactivation
COS-7 cells seeded in 12-well tissue culture plates were transfected in triplicate using Polyfect transfection reagent (5 μl/well; QIAGEN). Cells were transfected with the CYP24A1 promoter-luciferase reporter plasmid (125 ng/well) and WT and mutant VDR expression vectors (62.5 ng/well). A pRLnull reporter (1 ng/well; Promega) was included to control for transfection efficiency (21). Cells were transfected overnight and then treated with graded concentrations of 1,25-(OH)2D3 for 24 h. Luciferase activity was determined using the dual luciferase assay (Promega) and a Turner luminometer. Protein expression was analyzed by immunoblotting.
Results
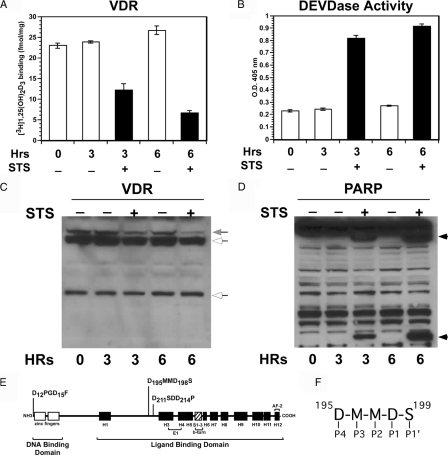
During apoptosis and other nonapoptotic circumstances, many proteins that regulate the cell cycle are inactivated by caspases (23). In this study, we were interested in determining whether the VDR, a regulator of cell growth, is inactivated during apoptosis or conditions in which caspases are activated. For these initial studies, we used the LNCaP human prostate cancer cells because these cells express the VDR and readily undergo apoptosis when treated with STS. As shown in Fig. 1A, when LNCaP prostate cancer cells were treated with 1 μm STS, a concentration that induces caspase-3 activity and activates apoptosis in these cells (24), a decrease in [3H]1,25-(OH)2D3 binding was observed over time. After 3 h incubation, the [3H]1,25-(OH)2D3 binding had decreased by about 50% and by 6 h had decreased by about 80% compared with the vehicle control. DEVDase, a measure of caspase-3 and -7 activities, on the other hand, was stimulated by STS treatment at both time points (Fig. 1B). In addition, immunoblot analysis using anti-VDR N-20 antibody that recognizes the VDR N terminus showed a decrease in the approximately 50-kDa VDR protein after 3 and 6 h treatment with STS (Fig. 1C). PARP, a target of caspase-3 and -7 and marker of apoptosis, was also cleaved when LNCaP cells were treated with STS (Fig. 1D). Based on these results, we hypothesized that caspases inactivated the VDR during early stimulation of apoptosis.
Figure 1.
Decreased [3H]1,25-(OH)2D3 binding and VDR protein in LNCaP prostate cancer cells during apoptosis. LNCaP prostate cancer cells were treated with 1 μm STS to induce apoptosis (19). Samples were taken over time and examined for [3H]1,25-(OH)2D3 binding (A) and DEVDase activity (B), a measure of caspase-3 and caspase-7 activities. Results shown represent the average of triplicate experiments ± sd. C, Immunoblot of VDR protein. Intact VDR migrates as approximately 50 kDa (gray arrow). White arrows indicate nonspecific bands. D, Immunoblot of PARP, a target of caspase-3 and -7 and marker of apoptosis. Arrowhead indicates PARP-cleaved fragments. E, Potential caspase-3 cleavage sites in the VDR. The motif recognized by caspase-3 is DxxD, where D is aspartic acid and x is any amino acid. Three potential caspase-3 sites with the DxxD motif in the VDR are shown. F, Caspases recognize a four-amino-acid motif in potential substrates named P4-P3-P2-P1 that are shown for the site at amino acids 195-198 in the VDR LBD. Cleavage occurs after the P1 residue that is usually aspartic acid. The site at 195-198 has a serine in the P1′ position, the most favored residue for caspase-3 in this position. The site at amino acids 211–214 have a proline in the P1′ site that is the least favorable residue by caspase-3 in this position.
Because caspase-3 is the main executioner caspase, we examined the VDR amino acid sequence for caspase-3 cleavage sites. Caspase-3 exhibits a preference for motifs containing DxxD (where D is aspartic acid and x is any amino acid). As shown in Fig. 1E, three potential caspase-3 cleavage sites are present in the VDR (D12PDG15F, D195MMD198S and D211SDD214P). Cleavage of either of the two sites located in the ligand-binding domain (LBD), D195MMD198S and D211SDD214P, would inactivate the VDR, whereas cleavage at the N-terminal site D12PDG15F would likely not inactivate the VDR. Because serine is the most favored residue in the P1′ position (Fig. 1F) by caspase-3 immediately following the DxxD motif and proline is the least favorable residue (10,11), we focused on the DMMDS sequence at amino acids 195-199 (Fig. 1E). We created three mutations in the putative caspase-3 cleavage site D195A at position P4, D198A at position P1, and S199A at position P1′ (Fig. 1F) and then determined their affects on cleavage of the VDR by caspases.
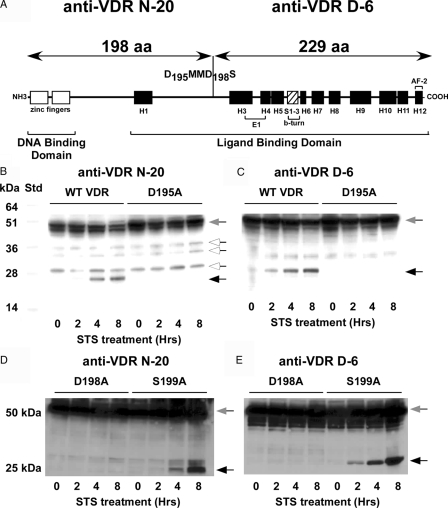
To determine whether the WT VDR or the VDR mutants were cleaved during apoptosis in intact cells, we used the COS-7 monkey kidney cell line that previously had been shown to respond to STS treatment both with an increase in caspase-3 activity and induction of apoptosis (25). COS-7 cells were transfected with the WT VDR and VDR mutant expression vectors and treated with 1 μm STS to activate caspase-3 and induce apoptosis. Cleavage of the VDR at the D198 site would generate two fragments of 198 and 229 amino acids in length that could be detected with VDR antibodies N-20 and D-6, respectively (Fig. 2A). When we examined the VDR by Western blot using the N-20 antibody to the N-terminal region of the VDR, we observed a time-dependent decrease in the approximately 50-kDa WT VDR protein along with the appearance of novel approximately 22-kDa immunoreactive band (Fig. 2B). An immunoreactive band of about 25 kDa was also observed using the D-6 antibody to the C terminus of the VDR (Fig. 2C). In contrast, the approximately 22- and 25-kDa bands were not produced during STS treatment in cells expressing the D195A (Fig. 2, B and C) or D198A (Fig. 2, D and E) mutant VDRs. The S199A mutant VDR exhibited a cleavage pattern similar to the WT VDR during the STS treatment (Fig. 2, D and E). These results also demonstrate that the D211SDD214P site was not cleaved during induction of apoptosis because no cleavage products were generated from the D195A or D198A mutant VDRs. It could not be determined from the immunoblots whether the D12PDG15F was cleaved because the amino acids 1-15 fragment would not be detected by the N-20 antibody and that the larger fragment amino acids 16-427 would be hard to distinguish from the amino acids 1-427 band and from other bands originating from use of downstream ATG start sites that were detected by the D-6 antibody.
Figure 2.
STS induced cleavage of the VDR in COS-7 cells. A, A putative caspase cleavage site with the DxxD motif was located at amino acids 195-198 in the LBD in the loop structure between helix H2n and helix H3. The size of the predicted cleavage fragments is depicted above. VDR antibodies N-20 and D-6 recognize the 198 and 229 amino acid fragments, respectively. Residues D195, D198, and S199 were mutated to alanine. B–E, COS-7 cells expressing the WT VDR or the D195A, D198A, or S199A mutants were treated with 1 μm STS and samples taken at the indicated times. Protein extracts were subjected to SDS-PAGE and immunoblotted with VDR antibodies that recognize either the N-terminal (antibody N-20) (B and D) or the C-terminal (antibody D-6) (C and E) cleavage fragments. Intact VDR migrates as approximately 50 kDa (gray arrow). Black arrows indicate the VDR cleavage fragments. White arrows indicate nonspecific bands.
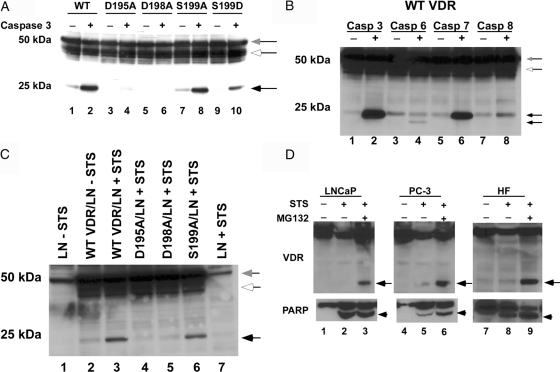
To determine whether caspase-3 actually cleaved the VDR at the 195-198 site, we incubated COS-7 cell extracts expressing the different VDR mutants with purified caspase-3 and examined them by immunoblot using the D-6 antibody (Fig. 3A). The blot shows that the WT VDR and the S199A mutant were cleaved by caspase-3, whereas the D195A and D198A mutants were resistant to cleavage by caspase-3. These results demonstrated that the VDR can be cleaved by caspase-3 and that the two aspartic acid residues at amino acids 195 and 198 define the cleavage site.
Figure 3.
Cleavage of the VDR at the caspase-3 site in vitro and in intact cells. A, COS-7 cell extracts expressing the WT VDR and mutant VDRs were incubated with 1 U caspase-3 for 1 h at 37 C. Cleavage products were then detected by immunoblot using anti-VDR D-6 antibody. B, Differential sensitivity of VDR to caspases in vitro. Recombinant human caspase-3, -6, -7, and -8 (1 U each) were incubated with COS-7-expressed WT VDR for 1 h at 37 C and then analyzed by immunoblot with anti-VDR D-6 antibody. C, Extracts from naive and STS-treated LNCaP cells were incubated with COS-7 cell extracts expressing the WT VDR and mutant VDRs for 1 h at 37 C. Cleavage products were then detected by immunoblot using anti-VDR D-6 antibody. LN, LNCaP cell extracts. D, LNCaP and PC-3 prostate cancer cells and human skin fibroblasts were treated with 1 μm STS for 3 h in the absence and presence of 10 μm MG-132. VDR cleavage products were then detected by immunoblot using anti-VDR D-6 and PARP cleavage detected using anti-PARP antibodies. Gray arrows show intact VDR. Black arrows show the VDR cleavage fragment. White arrows indicate nonspecific bands. Arrowhead indicates PARP cleaved fragment. HF, Human fibroblasts.
Because phosphorylation of serine residues at the P1′ position inhibit cleavage of some proteins by caspase-3 (26), we created an S199D mutation in the VDR to mimic phosphorylation of this serine residue adjacent to the caspase-3 cleavage site. Incubation of the VDR S199D mutant with caspase-3 resulted in a weaker production of the approximately 25-kDa fragment, suggesting that phosphorylation of S199 may inhibit cleavage of the VDR by caspase-3 at this site (Fig. 3A, lane 10). Whether this site is actually phosphorylated in the VDR is currently unknown.
We next determined whether the VDR was susceptible to cleavage by other caspases in vitro. As shown in the immunoblot with the D-6 antibody in Fig. 3B, incubation of the WT VDR with caspase-3 or caspase-7 resulted in the generation of the approximately 25-kDa cleavage fragment. Incubation with caspase-6 also generated a unique approximately 15-kDa cleavage fragment, whereas caspase-8 exhibited some minor overlapping cleavage of the VDR at the caspase-3 site.
In the LNCaP cells, it was difficult to visualize the approximately 22- or 25-kDa caspase-3 cleavage fragments of the VDR in the cell extracts after STS treatment (Fig. 3C, lane 7). To determine whether the VDR was likely cleaved at the caspase-3 site in LNCaP cells undergoing apoptosis, we incubated COS-7 expressed WT VDR and mutant VDRs with naive and STS-treated extracts from LNCaP cells. In Fig. 3C, we demonstrated that the WT VDR and the S199A mutant were cleaved at the caspase-3 site when incubated with STS-treated LNCaP cell extracts. No cleavage fragments were detected when the WT VDR was incubated with naive LNCaP cell extracts or when the D195A or D198A VDRs were incubated with STS-treated LNCaP cell extracts (Fig. 3C). Although there was a decrease in VDR in the LNCaP cells treated with STS compared with the VDR in the untreated naive LNCaP cells after 3 h (Fig. 3C, compare lane 1 and lane 7), no caspase cleavage fragments were detected on the blot under these conditions.
Caspase cleavage fragments generated from a number of other proteins have been shown to undergo further degradation by the 26S proteasome (27). To examine whether the VDR caspase cleavage fragments were subjected to further degradation by the proteasome, we treated LNCaP and PC-3 prostate cancer cells and human fibroblasts with 1 μm STS to induce apoptosis in the absence and presence of the proteasome inhibitor MG-132. As shown in Fig. 3D, no caspase cleavage fragments were detected in the samples treated with STS alone. However, in the presence of MG-132, the caspase cleavage fragments were detected, indicating that in LNCaP and PC-3 cells and human skin fibroblasts, the VDR is cleaved at the caspase-3 site and that the cleavage fragments are further degraded by the 26S proteasome.
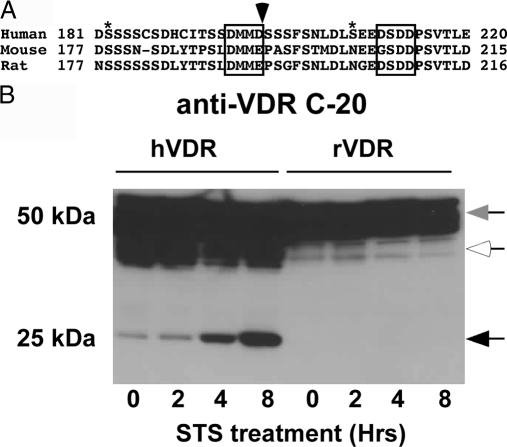
Figure 4A shows the alignment of the mouse and rat WT VDR sequences surrounding the caspase-3 site compared with the human VDR sequence. In the mouse and rat VDRs, the critical aspartic acid (D) in the P1 position in the caspase cleavage site is replaced by glutamic acid (E). In addition, proline (P) is present in the P1′ position in the mouse and rat sequence (proline has been shown to be the least favorable residue in the P1′ position following the caspase-3 site). To examine whether these amino acid differences in the rat and mouse VDRs affect cleavage by caspase-3, we transfected a rat VDR cDNA expression vector in COS-7 cells and then treated the cells with STS. We then analyzed the cleavage products using an antibody that recognizes the C terminus of the rat and human VDRs. As shown in Fig. 4B, the rat VDR was resistant to caspase-3 cleavage, whereas the human VDR was cleaved during STS treatment, indicating that the D to E amino acid transition at position 194 in the rat VDR prevents cleavage by caspase-3.
Figure 4.
The aspartic acid at the critical P1 position in the human VDR is not conserved in the mouse and rat VDRs. A, Alignment of human, mouse, and rat VDR sequences surrounding caspase-3 site. Asterisks indicate phosphorylation sites (S182 and S208), arrow indicates caspase-3 cleavage site, and boxes indicate DxxD motifs. B, COS-7 cells expressing the human or rat WT VDRs were treated with 1 μm STS and samples taken at the indicated times. Protein extracts were subjected to SDS-PAGE and immunoblotted with a VDR antibody (anti-VDR C-20) that recognizes the C terminus of human and rat VDRs. Intact VDR migrates as approximately 50 kDa (gray arrow). Black arrows indicate the VDR cleavage fragments. White arrows indicate nonspecific bands.
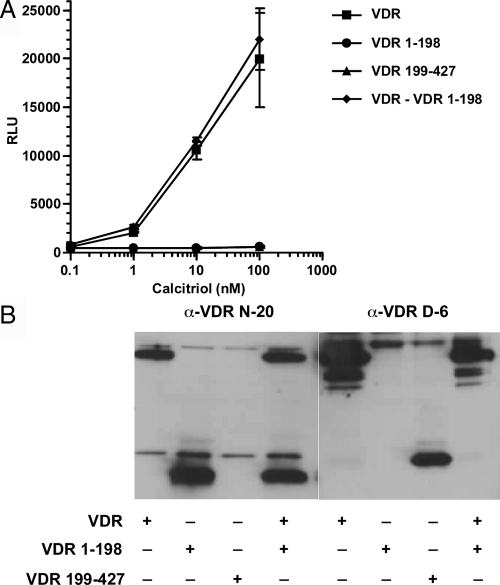
As shown in Fig. 5A, the individual caspase fragments (VDR 1-198 and VDR 199-427) exhibited no calcitriol-induced transactivation, demonstrating that caspase cleavage inactivates the VDR. It was, however, possible that the VDR N-terminal caspase cleavage fragment that contains the DNA-binding domain (DBD) might interfere with the function of the intact VDR by competing for DNA binding sites. Coexpression of the VDR N-terminal 1–198 fragment that contains the DBD with the intact WT VDR failed to inhibit transactivation by the intact WT VDR (Fig. 5A). Immunoblotting demonstrated that both caspase fragments were expressed (Fig. 5B).
Figure 5.
The caspase fragments are transcriptionally inactive, and the N-terminal fragment does not compete with the WT VDR. A, COS-7 cells were singly transfected with WT VDR, VDR 1-198, and VDR 199-427 mutants or the combination of the WT VDR and the VDR 1-198 mutant and the CYP24A1 promoter luciferase reporter. The cells were treated with graded concentrations of calcitriol. After 24 h, luciferase activity was determined. Results shown represent the average of triplicate transfection ± sd. B, VDR and VDR fragments were detected by immunoblot using anti-VDR N-20 or D-6 antibodies.
Discussion
We have demonstrated that the VDR is inactivated by caspase-3 during induction of apoptosis and/or stimulation of caspase activity. The VDR contains three potential caspase-3 cleavage sites (Fig. 1E). We found that cleavage of the VDR occurs at amino acid D198 in the motif D195MMD198S in the LBD and generates two fragments of approximately 22 and 25 kDa. Substitution of alanine for either of the two aspartic acid (D) residues inhibits cleavage by caspase-3. Similarly, substitution of glutamic acid (E) for aspartic acid at position 194, as present in rat VDR (Fig. 4A), prevents caspase-3 cleavage. A second potential caspase-3 cleavage site, D211SDD214P, in the LBD was not cleaved most likely because a proline residue occupies the P1′ position that is unfavorable for caspase-3 cleavage (11). We could not determine from our immunoblots whether a third potential caspase-3 site at the N terminus (D12PGD15F) was cleaved or not. Although we showed that the individual cleavage fragments lack transactivation activity and that the N-terminal cleavage fragment that contains the DBD does not compete with WT VDR transactivation (Fig. 5), the caspase-3 cleavage fragments may exhibit functions that are independent of and different from the intact VDR.
We demonstrated in LNCaP prostate cancer cells that treatment with STS for 6 h reduced the [3H]1,25-(OH)2D3 binding by more than 80% and stimulated caspase-3/7 activities. Although we could not detect any specific cleavage fragments, we showed that the VDR protein levels were significantly reduced during induction of apoptosis by STS. However, addition of the proteasome inhibitor MG-132 stabilized the caspase cleavage fragment generated during STS treatment in LNCaP and PC-3 prostate cancer cells and human skin fibroblasts, indicating that the cleavage fragment was subjected to further degradation by the 26S proteasome. STS treatment also resulted in the cleavage of PARP, a caspase-3/7 substrate and marker of apoptosis. In addition, we demonstrated that the WT VDR and the S199A mutant VDR were cleaved at the casapse-3 site when incubated with apoptotic extracts from LNCaP cells.
In vitro, the VDR was cleaved by caspase-6, -7, and -8. Cleavage by caspase-7 occurred at the D198 site because mutations D195A and D198A prevented cleavage by caspase-7 (data not shown). Cleavage by caspase-6 generated a unique fragment; however, the cleavage was not as robust as cleavage by caspase-3, indicating that the caspase-6 site is not an optimal site or is not as accessible as the caspase-3 site. Cleavage by caspase-8 also occurred at the caspase-3 site, indicating that there may be some overlapping activity. Alternatively, caspase-8 may have activated endogenous caspase-3 that resulted in the cleavage of the VDR.
As shown in Fig. 4, a number of serine residues surround the caspase-3 cleavage site in the human VDR. Phosphorylation of serine residues in the P1′ position have been shown to inhibit cleavage by caspase-3 (26). When we mutated S199 at the P1′ position to aspartic acid to mimic phosphorylation of this residue, the cleavage by caspase-3 was partially inhibited (Fig. 3A). Phosphorylation of S199 or other nearby serine residues may serve as a mechanism to protect the VDR from cleavage by caspase-3 under nonapoptotic conditions. Whether the VDR is inactivated by caspases in the absence of apoptotic signals remains to be determined. It is of interest that in studies of the crystal structure of the VDR LBD, to obtain good crystals, amino acids 165-215 of the VDR that include the sites of phosphorylation and the caspase cleavage site were deleted (28,29).
The caspase-3 site is located in a region of the human VDR that exhibits the greatest divergence among the VDRs from other species. Aspartic acid in the P1 position is thought to be critical for caspase recognition. In mouse and rat VDRs, however, glutamic acid is in the P1 position, making it unlikely that the mouse and rat VDRs are cleaved by caspase-3. Indeed, we have shown that the rat VDR is not cleaved by caspases during STS treatment in COS-7 cells (Fig. 4). The caspase-3 site is conserved only among the primates including orangutan, monkey, and tamarin but not in bovine, chicken, dog, horse, or pig VDRs. The importance of this pattern of evolution is not clear at present.
Caspase-3 is found in the nucleus as well as the cytoplasm and is the major executioner caspase (30). But caspase-3 also regulates cell proliferation, differentiation, survival, and inflammation under nonapoptotic conditions (15,23). Caspase cleavage can lead to protein inactivation (e.g. p53, retinoblastoma) or to protein activation (e.g. Mst1 kinase) (15). Thus, we hypothesize that caspase cleavage of VDR may serve as a mechanism to limit liganded and unliganded actions of the VDR. Examples of such situations might involve effects on cell growth. For example, because calcitriol is a major inhibitor of cell growth, inactivation of the VDR by caspases may be a mechanism to suppress the actions of calcitriol during critical stages of cell growth. Another setting might involve degradation of the VDR during the catagen phase of the hair cycle during which time the cells are undergoing apoptosis. The restriction of unliganded VDR actions here may be an important aspect of the hair cycle in humans. We are currently investigating this hypothesis.
In breast cancer, calcitriol has been shown to induce apoptosis by a caspase-independent pathway involving translocation of the proapoptotic protein Bax to the outer mitochondrial membrane and mitochondrial disruption (9). Calcitriol-induced apoptosis can be inhibited by the antiapoptotic protein Bcl-2 (31). In prostate cancer cells, calcitriol induces caspase-dependent and caspase-independent apoptotic pathways. Calcitriol induced cleavage of both procaspase-3 and procaspase-9 to their active forms (32). This proapoptotic activity of calcitriol was inhibited by the pan-caspase inhibitor z-VAD-fmk (32). Calcitriol also induced cytochrome c release from mitochondria, and this activity was not inhibited by z-VAD-fmk, indicating it was caspase independent (32). It has been well documented that the extent of apoptosis induced by calcitriol is greater in some target cells than in others (6,8,33). We suggest that this differential effect may be dependent, in part, on the extent of caspase induction that would limit the scope of calcitriol-induced apoptosis.
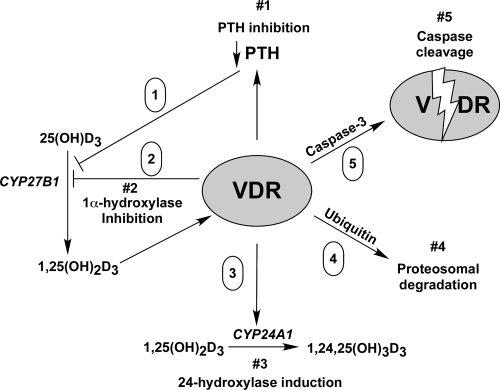
Several mechanisms appear to be operative to prevent the overstimulation by calcitriol in target cells. In our model shown in Fig. 6, the negative autoregulation of vitamin D action occurs at both the ligand and receptor levels. At the ligand level, calcitriol synthesis is impeded by 1) inhibitory actions of the liganded VDR on PTH, an inducer of the 1α-hydroxylase gene CYP27B1 (34); 2) by a direct suppression of CYP27B1 gene expression (35); and 3) by induction of the 24-hydroxylase (CYP24A1) gene that leads to inactivation of both 25(OH)D or calcitriol (36), and calcitriol induces fibroblast growth factor-23 expression, which then acts directly on the kidney to suppress CYP27B1 expression (37) (not shown in the figure). At the receptor level, calcitriol induces ubiquitination of the VDR leading to its degradation by the ubiquitin-proteasomal pathway (38,39), and as we have shown in this paper, cleavage of the VDR by caspases such as caspase-3 results in VDR inactivation. Caspase cleavage may function as an autoregulation pathway in settings where calcitriol induces apoptosis but may also be induced by other proapoptotic stimuli as well as nonapoptotic caspase activation.
Figure 6.
Negative regulation of vitamin D action at the ligand and receptor levels. The model shows five pathways that negatively regulate vitamin D action: 1) down-regulation of PTH, a stimulator of CYP27B1 (1α-hydroxylase gene); 2, down-regulation of CYP27B1; 3) degradation of ligand by CYP24A1 (24-hydroxylase gene); 4) regulation of VDR activity by ubiquitin-mediated proteasomal degradation; and 5) regulation of VDR activity by caspase-3 cleavage.
In summary, we have shown that the VDR is a caspase-3 target and is inactivated during apoptosis. Because caspases also exhibit nonapoptotic functions, it will be important to determine whether the VDR is cleaved under nonapoptotic conditions as a mechanism to regulate its activity.
Acknowledgments
We thank Drs. Hector DeLuca for the rat VDR expression vector and Milan Uskokovic for the calcitriol.
Footnotes
This work was supported by a grant from the National Institutes of Health (DK42482) to D.F.
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 1, 2008
Abbreviations: DBD, DNA-binding domain; LBD, ligand-binding domain; 1,25-(OH)2D3, 1,25-dihydroxyvitamin D3; PARP, poly-(ADP-ribose) polymerase; TBST, Tris-buffered saline containing 0.1% Tween 20; STS, staurosporine; VDR, vitamin D receptor; WT, wild type.
References
- Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR 2001 Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord 2:203–216 [DOI] [PubMed] [Google Scholar]
- Stewart LV, Weigel NL 2004 Vitamin D and prostate cancer. Exp Biol Med (Maywood) 229:277–284 [DOI] [PubMed] [Google Scholar]
- Nagpal S, Na S, Rathnachalam R 2005 Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 26:662–687 [DOI] [PubMed] [Google Scholar]
- Feldman D, Malloy PJ, Krishnan AV, Balint E 2007 Vitamin D: biology, action, and clinical implications. In: Marcus R, Feldman D, Nelson DA, Rosen CJ, eds. Osteoporosis. 3rd ed. San Diego: Academic Press; 317–382 [Google Scholar]
- Deeb KK, Trump DL, Johnson CS 2007 Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 7:684–700 [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Moreno J, Nonn L, Malloy P, Swami S, Peng L, Peehl DM, Feldman D 2007 Novel pathways that contribute to the anti-proliferative and chemopreventive activities of calcitriol in prostate cancer. J Steroid Biochem Mol Biol 103:694–702 [DOI] [PubMed] [Google Scholar]
- Byrne B, Welsh J 2007 Identification of novel mediators of vitamin D signaling and 1,25(OH)2D3 resistance in mammary cells. J Steroid Biochem Mol Biol 103:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valrance ME, Brunet AH, Acosta A, Welsh J 2007 Dissociation of growth arrest and CYP24 induction by VDR ligands in mammary tumor cells. J Cell Biochem 101:1505–1519 [DOI] [PubMed] [Google Scholar]
- Narvaez CJ, Welsh J 2001 Role of mitochondria and caspases in vitamin D-mediated apoptosis of MCF-7 breast cancer cells. J Biol Chem 276:9101–9107 [DOI] [PubMed] [Google Scholar]
- Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D, Ghayur T, Brady KD, Wong WW 1997 Substrate specificities of caspase family proteases. J Biol Chem 272:9677–9682 [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Renatus M, Meldal M, Salvesen GS 2000 Internally quenched fluorescent peptide substrates disclose the subsite preferences of human caspases 1, 3, 6, 7 and 8. Biochem J 350(Pt 2):563–568 [PMC free article] [PubMed] [Google Scholar]
- Stennicke HR, Ryan CA, Salvesen GS 2002 Reprieval from execution: the molecular basis of caspase inhibition. Trends Biochem Sci 27:94–101 [DOI] [PubMed] [Google Scholar]
- Degterev A, Boyce M, Yuan J 2003 A decade of caspases. Oncogene 22:8543–8567 [DOI] [PubMed] [Google Scholar]
- Cohen GM 1997 Caspases: the executioners of apoptosis. Biochem J 326(Pt 1):1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P 2007 Caspases in cell survival, proliferation and differentiation. Cell Death Differ 14:44–55 [DOI] [PubMed] [Google Scholar]
- Chau BN, Borges HL, Chen TT, Masselli A, Hunton IC, Wang JY 2002 Signal-dependent protection from apoptosis in mice expressing caspase-resistant Rb. Nat Cell Biol 4:757–765 [DOI] [PubMed] [Google Scholar]
- Sayan BS, Sayan AE, Knight RA, Melino G, Cohen GM 2006 p53 is cleaved by caspases generating fragments localizing to mitochondria. J Biol Chem 281:13566–13573 [DOI] [PubMed] [Google Scholar]
- Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Marcelli M, Marani M, Li X, Sturgis L, Haidacher SJ, Trial JA, Mannucci R, Nicoletti I, Denner L 2000 Heterogeneous apoptotic responses of prostate cancer cell lines identify an association between sensitivity to staurosporine-induced apoptosis, expression of Bcl-2 family members, and caspase activation. Prostate 42:260–273 [DOI] [PubMed] [Google Scholar]
- Li X, Marani M, Mannucci R, Kinsey B, Andriani F, Nicoletti I, Denner L, Marcelli M 2001 Overexpression of BCL-XL underlies the molecular basis for resistance to staurosporine-induced apoptosis in PC-3 cells. Cancer Res 61:1699–1706 [PubMed] [Google Scholar]
- Malloy PJ, Xu R, Peng L, Clark PA, Feldman D 2002 A novel mutation in helix 12 of the vitamin D receptor impairs coactivator interaction and causes hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia. Mol Endocrinol 16:2538–2546 [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Eccleshall TR, Gross C, Van Maldergem L, Bouillon R, Feldman D 1997 Hereditary vitamin D resistant rickets caused by a novel mutation in the vitamin D receptor that results in decreased affinity for hormone and cellular hyporesponsiveness. J Clin Invest 99:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranaga E, Miura M 2007 Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol 17:135–144 [DOI] [PubMed] [Google Scholar]
- Marcelli M, Cunningham GR, Walkup M, He Z, Sturgis L, Kagan C, Mannucci R, Nicoletti I, Teng B, Denner L 1999 Signaling pathway activated during apoptosis of the prostate cancer cell line LNCaP: overexpression of caspase-7 as a new gene therapy strategy for prostate cancer. Cancer Res 59:382–390 [PubMed] [Google Scholar]
- Brophy VA, Tavare JM, Rivett AJ 2002 Treatment of COS-7 cells with proteasome inhibitors or γ-interferon reduces the increase in caspase 3 activity associated with staurosporine-induced apoptosis. Arch Biochem Biophys 397:199–205 [DOI] [PubMed] [Google Scholar]
- Tozser J, Bagossi P, Zahuczky G, Specht SI, Majerova E, Copeland TD 2003 Effect of caspase cleavage-site phosphorylation on proteolysis. Biochem J 372:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belizario JE, Alves J, Garay-Malpartida M, Occhiucci JM 2008 Coupling caspase cleavage and proteasomal degradation of proteins carrying PEST motif. Curr Protein Pept Sci 9:210–220 [DOI] [PubMed] [Google Scholar]
- Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D 2000 The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell 5:173–179 [DOI] [PubMed] [Google Scholar]
- Rochel N, Tocchini-Valentini G, Egea PF, Juntunen K, Garnier JM, Vihko P, Moras D 2001 Functional and structural characterization of the insertion region in the ligand binding domain of the vitamin D nuclear receptor. Eur J Biochem 268:971–979 [DOI] [PubMed] [Google Scholar]
- An S, Park MJ, Park IC, Hong SI, Knox K 2003 Procaspase-3 and its active large subunit localized in both cytoplasm and nucleus are activated following application of apoptotic stimulus in Ramos-Burkitt lymphoma B cells. Int J Mol Med 12:311–317 [PubMed] [Google Scholar]
- Mathiasen IS, Lademann U, Jaattela M 1999 Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res 59:4848–4856 [PubMed] [Google Scholar]
- Guzey M, Kitada S, Reed JC 2002 Apoptosis induction by 1α,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther 1:667–677 [PubMed] [Google Scholar]
- Polek TC, Stewart LV, Ryu EJ, Cohen MB, Allegretto EA, Weigel NL 2003 p53 is required for 1,25-dihydroxyvitamin D3-induced G0 arrest but is not required for G1 accumulation or apoptosis of LNCaP prostate cancer cells. Endocrinology 144:50–60 [DOI] [PubMed] [Google Scholar]
- Kim MS, Fujiki R, Murayama A, Kitagawa H, Yamaoka K, Yamamoto Y, Mihara M, Takeyama K, Kato S 2007 1α,25(OH)2D3-induced transrepression by vitamin D receptor through E-box-type elements in the human parathyroid hormone gene promoter. Mol Endocrinol 21:334–342 [DOI] [PubMed] [Google Scholar]
- Kim MS, Fujiki R, Kitagawa H, Kato S 2007 1α,25(OH)2D3-induced DNA methylation suppresses the human CYP27B1 gene. Mol Cell Endocrinol 265–266:168–173 [DOI] [PubMed] [Google Scholar]
- Ly LH, Zhao XY, Holloway L, Feldman D 1999 Liarozole acts synergistically with 1α,25-dihydroxyvitamin D3 to inhibit growth of DU 145 human prostate cancer cells by blocking 24-hydroxylase activity. Endocrinology 140:2071–2076 [DOI] [PubMed] [Google Scholar]
- Perwad F, Zhang MY, Tenenhouse HS, Portale AA 2007 Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1α-hydroxylase expression in vitro. Am J Physiol Renal Physiol 293:1577–1583 [DOI] [PubMed] [Google Scholar]
- Masuyama H, MacDonald PN 1998 Proteasome-mediated degradation of the vitamin D receptor (VDR) and a putative role for SUG1 interaction with the AF-2 domain of VDR. J Cell Biochem 71:429–440 [PubMed] [Google Scholar]
- Li XY, Boudjelal M, Xiao JH, Peng ZH, Asuru A, Kang S, Fisher GJ, Voorhees JJ 1999 1,25-Dihydroxyvitamin D3 increases nuclear vitamin D3 receptors by blocking ubiquitin/proteasome-mediated degradation in human skin. Mol Endocrinol 13:1686–1694 [DOI] [PubMed] [Google Scholar]