Abstract
Cardiovascular complications of diabetes result from endothelial dysfunction secondary to persistent hyperglycemia. We investigated potential compensatory mechanisms in the vasculature that oppose endothelial dysfunction in diabetes. BALB/c mice were treated with streptozotocin (STZ) to induce type 1 diabetes (T1D). In mesenteric vascular beds (MVBs), isolated ex vivo from mice treated with STZ for 1 wk, dose-dependent vasorelaxation to acetylcholine (ACh) or sodium nitroprusside was comparable with that in age-matched control mice (CTRL). By contrast, MVBs from mice treated with STZ for 8 wk had severely impaired vasodilator responses to ACh consistent with endothelial dysfunction. Pretreatment of MVBs from CTRL mice with nitric oxide synthase inhibitor nearly abolished vasodilation to ACh. In MVB from 1-wk STZ-treated mice, vasodilation to ACh was only partially impaired by L-Nω-arginine methyl ester. Thus, vasculature of mice with T1D may have compensatory nitric oxide-independent mechanisms to augment vasodilation to ACh and oppose endothelial dysfunction. Indeed, pretreatment of MVBs isolated from 1-wk STZ-treated mice with NS-398 [selective cyclooxygenase (COX)-2 inhibitor] unmasked endothelial dysfunction not evident in CTRL mice pretreated without or with NS-398. Expression of COX-2 in MVBs, aortic endothelial cells, and aortic vascular smooth muscle cells from STZ-treated mice was significantly increased (vs. CTRL). Moreover, concentrations of the COX-2-dependent vasodilator 6-keto-prostaglandin F-1α was elevated in conditioned media from aorta of STZ-treated mice. We conclude that endothelial dysfunction in a mouse model of T1D is opposed by compensatory up-regulation of COX-2 expression and activity in the vasculature that may be relevant to developing novel therapeutic strategies for diabetes and its cardiovascular complications.
Endothelial dysfunction in type 1 diabetes is opposed by compensatory mechanisms involving increased expression and activity of cyclooxygenase-2 in the vasculature induced by pro-inflammatory signaling.
Endothelial dysfunction underlies cardiovascular complications of diabetes that are responsible for increased morbidity and mortality (1). In diabetes, persistent fasting and postprandial hyperglycemia induces a chronic proinflammatory state that directly contributes to endothelial dysfunction by impairing insulin signaling and altering expression of genes important for vascular homeostasis (2,3,4). Under healthy conditions, several integrated pathways regulate endothelial synthesis and release of vasodilators [e.g. nitric oxide (NO) and cyclooxygenase (COX)-2-dependent prostanoids] and opposing vasoconstrictors (e.g. endothelin-1) to maintain cardiovascular homeostasis (5,6). Endothelial dysfunction is characterized by reduced bioavailability of NO secondary to increased oxidative stress, and elevated expression of proinflammatory and prothrombotic factors that lead to abnormal vasoreactivity (7). In many disorders of cardiovascular and metabolic homeostasis, compensatory responses are often present that serve to maintain or restore physiological function. For example, when metabolic insulin resistance develops, compensatory hyperinsulinemia maintains euglycemia for as long as pancreatic β-cells are able to produce sufficiently large amounts of insulin. Likewise, compensatory mechanisms that oppose endothelial dysfunction may be present in diabetic vasculature. Endothelial dysfunction often manifests as impaired endothelium-dependent vasodilator actions secondary to decreased production and/or bioavailability of NO that contributes significantly to coronary heart disease, hypertension, and other cardiovascular disorders characterized by reciprocal relationships between endothelial dysfunction and insulin resistance (2,3). Thus, it seems likely that compensatory mechanisms opposing the endothelial dysfunction of diabetes may involve increased production of or response to vasodilators and/or decreased production of or response to vasoconstrictors.
In addition to NO, prostanoid vasodilator products of arachidonic acid metabolism generated by COX contribute to local blood flow regulation (8,9,10,11). COX-1 is constitutively expressed in most cell types, whereas COX-2 is an inducible isoform that may mediate some pathological effects of inflammation, toxic shock, and cancer (9,12,13,14,15,16). In addition, constitutive expression of COX-2 is present in the brain, vasculature, and kidney (17,18,19). COX-2 is an important regulator of cardiovascular homeostasis under healthy conditions (20,21). Indeed, in humans, COX-2 is primarily responsible for biosynthesis of the anti-atherogenic, antithrombotic, vasodilator prostacyclin in the vascular endothelium (22). Vascular expression of COX-2 protein is substantially increased in the presence of cardiovascular risk factors including elevated levels of pro-inflammatory cytokines, cholesterol, lipoproteins, and hypoxia (23,24). Expression of COX-2 in endothelial cells overlying vascular lesions in aorta or carotid and coronary arteries of diabetic animal models and humans suggests that hyperglycemia-induced overexpression of COX-2 may be a compensatory response to proatherogenic conditions (25,26,27,28). Increased expression of COX-2 with resultant enhancement in prostacyclin-mediated vasodilation is present in coronary arteries of diabetic patients (29). On the other hand, alteration in prostanoid profiles has been implicated in hyperglycemia-induced vascular abnormalities (30). Thus, the role of COX-2 in the regulation of cardiovascular physiology/pathophysiology in diabetes remains controversial. In the present study, we hypothesized that increased expression of COX-2 in the vasculature of a mouse model of type 1 diabetes may mediate a compensatory response that opposes the endothelial dysfunction of diabetes.
Materials and Methods
Drugs
Streptozotocin (STZ), noradrenaline (NA), acetylcholine (ACh), sodium nitroprusside (SNP), L-Nω-arginine methyl ester (L-NAME) were from Sigma Aldrich (St. Louis, MO). N-(2-cyclohexyloxy-4-nitrophenyl) methanesulfonamide (NS-398) was from Alexis Biochemical (San Diego, CA). Stock solutions of each drug were prepared in distilled water except for NS-398 (dissolved in dimethylsulfoxide) and STZ (dissolved in citrate buffer). Final dilutions for all drugs were prepared in modified Krebs-Henseleit solution immediately before use. None of the vehicles used induced any significant effect by themselves in vascular or animal studies (assessed by appropriate controls in preliminary studies).
Animal experiments
All experiments involving animals were performed in conformance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication 85-23, revised 1996). Male BALB/c mice from Harlan Italy (Milan, Italy) were used in all studies. Mice weighing 23–25 g were randomly assigned to treatment with STZ (240 mg/kg, ip) or vehicle control (CTRL; citrate buffer). Body weight, systolic blood pressure, blood and urine glucose, and plasma insulin were measured in mice 0, 1, and 8 wk after STZ treatment. Systolic blood pressure was measured noninvasively using a tail-cuff BP-2000 Blood Pressure Analysis System (Visitech System Inc., Apex, NC). Blood glucose was measured in samples from the tail tip using a diagnostic autoanalyzer (Accu-Chek Active; Roche Diagnostics, Indianapolis, IN). Urinary glucose was evaluated by semiquantitative determination (Diabur Test 5000; Roche Diagnostics). Serum insulin and TNF-α levels were measured by ELISA kits [Linco Research (St. Charles, MO) and R&D System Europe (Lille, France), respectively] in cardiac blood samples from mice fasted overnight and euthanized with ether.
Evaluation of vascular function ex vivo
The mesenteric vascular bed (MVB) was isolated from mice as described previously (31), mounted in a temperature-controlled moist chamber (type 834/1; Hugo Sachs Elektronik, March-Hungstetten, Germany) and perfused via the superior mesenteric artery with a modified Krebs-Henseleit solution at 37 C continuously gassed with a mixture of 95% O2-5% CO2 (pH 7.4).
A constant (total) flow rate of 2 ml/min was maintained using two distinct peristaltic pumps (101-Ismatec; Hugo Sachs Elektronik). Pump 1 was used for continuous infusion of Krebs Henseleit solution (1.9 ml/min flux), either alone or containing NA (plus or minus inhibitors including L-NAME or NS-398). Pump 2 (0.1 ml/min flux) was used to infuse either basal solution or drug-containing solutions directly into the vessel preparation. For vasoconstriction experiments, dose-response curves were obtained by adding increasing concentrations of NA (0.01–100 μm per 30 sec) in a noncumulative manner using pump 2. For vasodilation experiments, NA-containing solution was infused using pump 1, whereas ACh-containing solution was infused using pump 2. After an equilibration period (30–40 min), changes in perfusion pressure (PP) were measured with a pressure transducer system (SP 844; Capto, Horten, Norway) and recorded continuously on a polygraph (Graphtec-Watanabe type 3310) using data acquisition and analysis equipment (PowerLab system; ADInstruments, Castle Hill, Australia).
For vasorelaxation experiments (decrease in PP), MVBs were first precontracted by continuous infusion of NA to a steady-state PP of approximately 100 mm Hg. For each MVB preparation, the dose of NA was adjusted to obtain the same level of precontraction (∼80% of maximal vasoconstriction) in MVBs from either CTRL or STZ mice. Therefore, 3 μm NA were used in both 1-wk STZ and 1-wk CTRL mice, whereas 7 and 3 μm NA were used in 8-wk STZ and 8-wk CTRL mice, respectively. Dose-response curves were obtained by adding increasing concentrations of ACh (0.01–100 μm per 30 sec) or SNP (0.1–10 μm per 30 sec). In some experiments, ACh dose-response curves were performed before and after pretreatment with NS-398 (10 μm, 30 min) or L-NAME (100 μm, 30 min). Pretreatment with L-NAME significantly increased perfusion pressure to a similar extent (∼10 mm Hg) in MVBs from both CTRL- and STZ-treated mice. Data from each experiment were normalized by defining 100% as the initial steady-state perfusion pressure and 0% as the maximal reduction in perfusion pressure obtained in MVBs treated with a maximally relaxing dose of SNP (1 μm, in endothelium-denuded vessels from mice treated with vehicle control for 8 wk). Endothelial denudation was performed by bubbling air into the vessels for 30 sec. Denudation was considered successful when response to subsequent ACh administration (1 μm) was reduced by more than 90% with respect to ACh vasodilation obtained before treatment (adapted from Ref. 32).
For vasoconstriction experiments, dose-response curves were obtained by adding increasing concentrations of NA (0.01–100 μm per 30 sec) in a noncumulative manner. In separate MVB preparations, dose-response curves were repeated before and after preincubation with NS-398 (10 μm, 30 min) or L-NAME (100 μm, 30 min). Steady-state PP attained with each individual dose of NA was measured in millimeters of mercury.
Cell culture
One week after treatment of mice with STZ or CTRL, mouse aortic endothelial cells (MAECs) were obtained by collagenase digestion of isolated aortas. Briefly, the proximal end of each aorta was cannulated with a 200-μl pipette tip and the aorta was gently perfused with endothelial cell basal medium (Lonza, Basel, Switzerland). Subsequently the distal end of the aorta was clamped and the vessel was infused with 500 μl of 2 mg/ml collagenase type II solution for 75 min at 37 C. This solution was then collected and centrifuged (1000 × g, 5 min, 4 C), and cell pellets from three separate aortas were combined and cultured in collagen-coated dishes with endothelial cell growth medium (Cambrex) until cells attained confluence.
Cells were tested for purity in preliminary experiments. Endothelial phenotype was assessed by the specific ability of endothelial cells to incorporate the fluorescent probe 1,1′-dioctadecyl-1–3,3,3′,3′-tetramethyl-indocarbocianyne perchlorate (10 μg/ml, 4 h, 37 C; Biomedical Technologies, Staughton, MA). Cells were viewed with fluorescent microscopy as described (33). Aortic vascular smooth muscle cells (VSMCs) were obtained by collagenase digestion (2 mg/ml collagenase, 6 h, 37 C) of endothelium-denuded aortas isolated from mice after treatment with CTRL or STZ for 1 or 8 wk. The collagenase solution from the aortas was centrifuged (1000 × g, 5 min, 4 C) and cell pellets were resuspended in DMEM supplemented with 10% fetal bovine serum and antibiotics (100 IU/ml penicillin, 0.1 mg/ml streptomycin). Cells were cultured in gelatin-coated dishes and the phenotype of VSMCs was verified by positive staining for α-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA). More than 95% of cells maintained endothelial or VSMC phenotype up to passage 4. For subsequent experiments, MAECs and VSMCs between the second and third passages were used.
Immunoblotting
Expression of COX-2, endothelial NO synthase (eNOS), inhibitor of κB (IκB)α, phosphorylated IkBα, and Akt in cell lysates and vessel homogenates were assessed by immunoblotting with specific antibodies according to standard methods (34). For tissue samples, MVB homogenates (35 μg) were immunoblotted with α-eNOS (Transduction Laboratories, Lexington, KY), α-COX-2 (Santa Cruz Biotechnology), or α-Akt (Cell Signaling, Beverly, MA) antibodies. For MAECs, cell lysates (15 μg) were immunoblotted with α-COX-2, α-IkBα, and α-phospho-IkBα antibodies (Cell Signaling Technology). For VSMCs, cell lysates (35 μg) were immunoblotted with α-COX-2 and α-Akt antibodies. Immunoblotting results were visualized using enhanced chemiluminescence reagents (GE Healthcare Bio-Science AB, Uppsala, Sweden), and blots were quantified by scanning densitometry.
Measurement of 6-keto-prostaglandin F1α (6-keto-PGF1α) in conditioned media
Thoracic aortas were isolated from mice 1 or 8 wk after treatment with CTRL or STZ and incubated in 200 μl of modified Krebs-Henseleit solution (37 C, 20 min). For some experiments, NA was added for 20 min, followed by ACh for 2 min. In other experiments, NS-398 (10 μm) was added for 20 min alone or in combination with NA plus ACh. Conditioned media were collected for measurement of 6-keto-PGF1α (enzyme immunoassay kit; Cayman Chemicals, Ann Arbor, MI), and results were expressed as picograms per milliliter−1 per milligrams−1 of aorta (wet weight), according to a previously described protocol (34).
Detection of nuclear factor-κB (NF-κB)/p65 in intact endothelial cells
MAECs obtained from mice treated with CTRL or STZ for 1 wk were grown to 70% confluence in chamber slides (Thermo Fisher Scientific, Roskilde, Denmark), serum starved for 4 h in endothelial cell basal medium, and subsequently treated without or with TNF-α (10 ng/ml, 30 min). Cells were then fixed in 3.5% paraformaldehyde (12 min, 25 C), permeabilized with 0.5% Triton X-100, blocked with 2% BSA (1 h, 25 C), and incubated with primary antibodies against the p65 subunit of NF-κB. Alexa Fluor 568-conjugated goat antirabbit IgG was used as a secondary antibody. Cells were also stained with 4,6-diamidino-2-phenylindole (DAPI) to visualize cell nuclei. Results were visualized using an epifluorescent Axiovert TS100 microscope (Carl Zeiss Inc., Thornwood, NY) with the appropriate filters. Images captured with Axiovision Software (Zeiss).
Immunohistochemistry
Aortas were isolated from mice treated with CTRL or STZ 1 and 8 wk after treatment and then fixed in 8% paraformaldehyde. Aortic ring sections (10 μm) were blocked in 3% fat-free milk PBS buffer containing 0.5% Triton X-100, (1 h, room temperature) and subsequently immunolabeled with primary antibodies against eNOS or COX-2. Alexa Fluor 488-conjugated sheep antimouse and Alexa Fluor 568-conjugated rabbit antigoat IgG were used as secondary antibodies, respectively. In addition, sections were immunostained with either IgG-Texas Red- or IgG-fluorescein isothiocyanate-conjugated laminin antibodies. DAPI staining was used to visualize cell nuclei. Results were visualized using confocal microscopy (Nikon, Tokyo, Japan) and images were captured using appropriate cameras and software.
All immunoblotting, immunohistochemistry, 6-keto-PGF1α experiments conducted using STZ-treated mice were accompanied by experiments using age-matched vehicle-treated CTRL mice. Because no significant differences were detected when results obtained from 1-wk CTRL were compared with results from 8-wk CTRL mice, data from the 8-wk CTRL group were omitted for clarity.
Statistical analysis
A power analysis was prospectively conducted to determine the number of mice needed in this study based on coefficients of variation obtained for similar vascular tests performed in our lab in rodents (31,32,34,35,36). A total sample size of 12 (six in each group) is sufficient to detect 10% differences in dose response curves for vasodilation (α = 0.05) with a power of 0.80. Results were expressed as means ± se of n experiments, with n representing the number of mice. Two-way ANOVA for repeated measures or student t tests were used where appropriate, and values of P < 0.05 were considered to indicate statistical significance.
Results
Metabolic phenotype of STZ-treated mice
Metabolic parameters were assessed in mice 0, 1, and 8 wk after injection with vehicle control (citrate buffer) or STZ (240 mg/kg, ip) (Table 1). One week after STZ injection, mice were diabetic with significant fasting hyperglycemia, glycosuria, and hypoinsulinemia when compared with age-matched mice injected with CTRL. At this time, body weight was still similar between mice injected with either STZ or CTRL. However, 8 wk after injection, body weight was significantly lower in mice treated with STZ (vs. age matched mice treated with CTRL). This was presumably due to dehydration and protein wasting associated with diabetes. Thus, the STZ-injection protocol we used generated an experimental model of type 1 diabetes as expected (37).
Table 1.
Physiological and biochemical parameters are shown for mice 0, 1, and 8 wk after STZ treatment
| STZ treatment (wk) | Group (n) | Body weight (g) | Systolic blood pressure (mm Hg) | Blood glucose (mg/dl) | Urine glucose (mg/dl) | Plasma insulin (ng/ml) |
|---|---|---|---|---|---|---|
| 0 | CTRL (4) | 24.9 ± 1.1 | 113 ± 10 | 81 ± 6.7 | Negative | n.d. |
| 1 | CTRL (20) | 25.5 ± 0.6 | 118 ± 7 | 76 ± 6.9 | Negative | 0.47 ± 0.10 |
| STZ (20) | 23.0 ± 0.8 | 120 ± 6 | 221 ± 25a | 0–100 | 0.08 ± 0.05a | |
| 8 | CTRL (20) | 29.4 ± 0.9 | 119 ± 9 | 95 ± 2.8 | Negative | 0.51 ± 0.2 |
| STZ (20) | 23.2 ± 1.1b | 128 ± 5 | 325 ± 28b | >1000 | 0.09 ± 0.1a |
Male BaLb/c mice were treated once with STZ (240 mg/kg, ip) or vehicle control (citrate buffer, CTRL) as described in Materials and Methods. Data are mean ± sem for n independent experiments. Statistical comparisons used two-tailed unpaired Student’s t test. n.d., Not determined.
P < 0.005 vs. respective CTRL values.
P < 0.001 vs. respective CTRL values.
Endothelial dysfunction in STZ-induced diabetes is unmasked by inhibition of COX-2
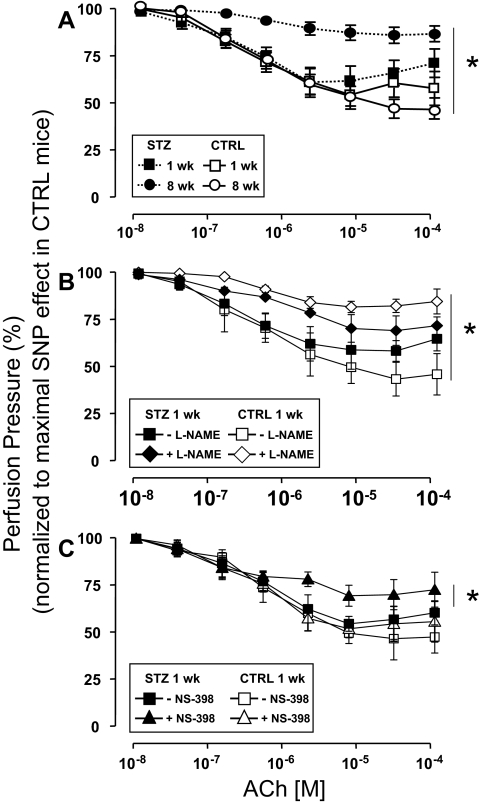
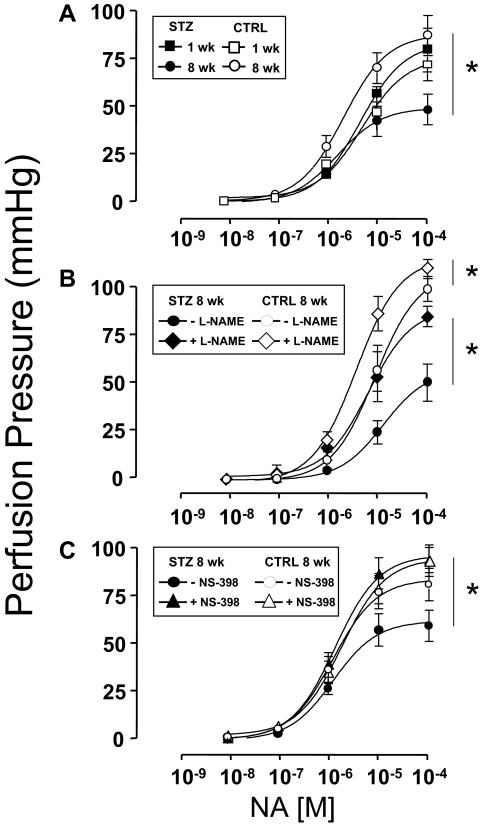
Endothelial-independent vasorelaxation in response to SNP was similar among mesenteric arteries denuded of endothelium that were isolated from mice 1 or 8 wk after injection with either STZ or CTRL (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Next, dose-dependent vasorelaxation in response to ACh was evaluated in MVBs ex vivo preconstricted with NA that were isolated from mice 1 or 8 wk after injection with either STZ or CTRL. One week after injection of mice with either STZ or CTRL, dose-dependent vasorelaxation of MVBs ex vivo in response to ACh was similar in both groups (Fig. 1A, closed vs. open squares, P > 0.12). However, 8 wk after injection with either STZ or CTRL, clear endothelial dysfunction with impaired vasorelaxation response to ACh was evident in MVBs from mice injected with STZ when compared with MVBs from mice injected with CTRL (Fig. 1A, closed vs. open circles, P < 0.001). No significant difference was observed for ACh-induced vasodilation when results from 1-wk STZ and 8-wk CTRL were compared (Fig. 1A, closed squares vs. open circles, P > 0.09). However, if a post hoc analysis with Student’s t test for grouped data are performed, differences for the last two doses between 1-wk STZ and 8-wk CTRL are observed (P < 0.05). It is important to note that the repeated-measures ANOVA comparing entire dose response curves is the primary statistical analysis for our data. Post hoc analysis revealing differences at the highest doses of ACh was evident only when comparing two groups at different ages. If anything, a statistical difference between CTRL and 1-wk STZ-treated mice in ACh-induced vasodilation present at only the highest doses reinforces our hypothesis that endothelial function is partially impaired in STZ-diabetic mice at an early stage.
Figure 1.
Endothelial dysfunction in STZ-induced diabetes is unmasked by inhibition of COX-2. MVBs were isolated from mice treated with STZ (closed symbols) or vehicle CTRL (open symbols) and prepared as described in Materials and Methods. For these experiments, MVBs were contracted to about 80% of maximal vasoconstriction. To obtain comparable levels of precontraction with a perfusion pressure of about 100 mm Hg in MVBs from CTRL and STZ mice, 3 μm NA were used in MVBs from 1-wk STZ and 1-wk CTRL mice. A, Dose-response curves for ACh-induced vasorelaxation were obtained in MVBs from mice 1 wk (squares) or 8 wk (circles) after treatment with STZ or vehicle control. Results are mean ± sem of 11 (STZ) and eight (CTRL) independent experiments. ACh-mediated vasorelaxation was significantly impaired in MVBs from mice treated with STZ for 8 wk (vs. respective CTRL, P < 0.001). No significant difference was observed for ACh-induced vasodilation when results from 1-wk STZ and 8-wk CTRL were compared (P > 0.09). B, Dose-response curves for ACh-mediated vasorelaxation were obtained in MVBs from mice 1 wk after treatment with STZ or vehicle control in the absence (squares) or presence (diamonds) of pretreatment with the NO synthase antagonist L-NAME (100 μm, 30 min). Pretreatment with L-NAME significantly increased perfusion pressure to a similar extent (∼10 mm Hg) in MVBs from both CTRL and STZ-treated mice. Results are mean ± sem of five (STZ) and four (CTRL) independent experiments. Inhibition of NO synthase significantly reduced ACh-mediated vasorelaxation in MVBs from CTRL (vs. respective basal, P < 0.001) but not 1-wk STZ-treated mice. A statistical difference was observed in ACh-induced vasodilation when results from 1-wk CTRL + L-NAME and 1-wk STZ + L-NAME were compared (P < 0.05); no significant difference was observed in ACh-induced vasodilation when results from 1-wk CTRL without L-NAME and 1-wk STZ without L-NAME were compared (P > 0.12). C, Dose-response curves for ACh-mediated vasorelaxation were obtained in MVBs from mice 1 wk after treatment with STZ or vehicle control in the absence (squares) or presence (triangles) of pretreatment with the specific COX-2 inhibitor NS-398 (10 μm, 30 min). Results are mean ± sem of seven (STZ) and four (CTRL) independent experiments. ACh-mediated vasorelaxation was significantly impaired in MVBs from mice treated with STZ for 1 wk in the presence of NS-398 pretreatment (when compared with MVBs from 1 wk STZ in the absence of NS-398, P < 0.02). No significant difference was observed in ACh-induced vasodilation when results from 1-wk CTRL without NS-398 and 1-wk STZ without NS-398 were compared (P > 0.14). Asterisks refer to significant differences found between indicated curves assessed by two-way ANOVA for repeated measures.
Because dose-dependent vasorelaxation ex vivo in response to ACh was similar among MVBs isolated from mice 1 wk after injection with either STZ or CTRL (Fig. 1A, closed vs. open squares), we wondered whether production of endothelial-derived NO in response to ACh was also similar among these two groups. In MVBs from mice 1 wk after injection with CTRL, pretreatment with the NO synthase antagonist L-NAME nearly completely abolished ACh-mediated vasorelaxation (Fig. 1B, compare open symbols, P < 0.001). By contrast, in MVBs from mice 1 wk after injection with STZ, pretreatment with L-NAME had a much smaller effect to blunt ACh-mediated vasorelaxation (Fig. 1B, compare closed symbols). A statistical difference was observed in ACh-induced vasodilation when results from 1 wk CTRL + L-NAME and 1 wk STZ + L-NAME were compared by repeated-measures ANOVA (Fig. 1B, open vs. closed rhombi, P < 0.05). Post hoc analysis using paired Student’s t test confirmed statistically significant differences (P < 0.05) for each of the last six doses of ACh when 1-wk CTRL + L-NAME and 1-wk STZ + L-NAME were compared. No significant differences were observed in ACh-induced vasodilation when results from 1-wk CTRL without L-NAME and 1-wk STZ without L-NAME were compared (Fig. 1B, open vs. closed squares, P > 0.12). Thus, the ability of L-NAME to inhibit ACh-induced vasodilation is reduced under diabetic conditions supporting our hypothesis that endothelial function is modestly impaired in mice treated with STZ for 1 wk.
This suggests that the vasculature of mice with type 1 diabetes may use NO-independent mechanisms to augment vasodilator actions of ACh and oppose endothelial dysfunction. Among potential candidates for endothelial-derived NO-independent vasodilators are prostanoids generated in response to COX-2 activation (22). Therefore, in MVBs from mice 1 wk after injection with STZ or CTRL, we repeated ACh dose-response experiments in the absence or presence of pretreatment with the specific COX-2 inhibitor NS-398. No significant difference was observed in ACh-induced vasodilation when results from 1-wk CTRL without NS-398 and 1-wk STZ without NS-398 were compared (Fig. 1C, closed vs. open squares, P > 0.14). In MVBs from mice 1 wk after injection with CTRL, pretreatment with NS-398 did not significantly alter the ACh dose-response curve (Fig. 1C, compare open symbols). By contrast, in MVBs from mice 1 wk after injection with STZ, pretreatment with NS-398 significantly and substantially blunted the vasodilator response to ACh when compared in the same preparation (Fig. 1C, compare closed symbols, P < 0.02). In MVBs from mice 1 wk after injection with STZ or CTRL, pretreatment with indomethacin (inhibitor of both COX-1 and COX-2) blunted ACh-mediated vasorelaxation to a similar extent. Indomethacin’s inhibitory effects were marginally larger in STZ-treated vs. CTRL mice, although this difference did not reach statistical significance (supplemental Fig. 2). Thus, specific inhibition of COX-2 activity unmasked endothelial dysfunction present in MVBs from mice 1 wk after STZ treatment. This suggests that COX-2 expression and/or activity may be elevated in the vasculature of STZ-treated mice as a compensatory mechanism that opposes endothelial dysfunction associated with diabetes.
Expression of COX-2 and eNOS, but not Akt, is elevated in vasculature from mice with STZ-induced diabetes
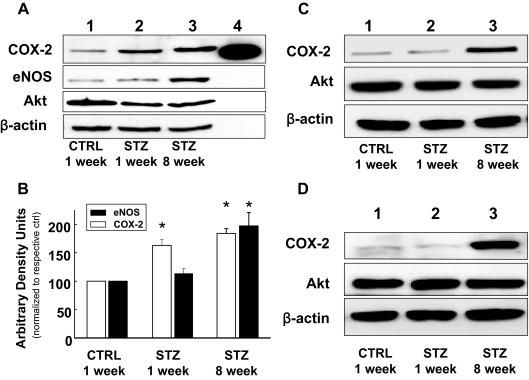
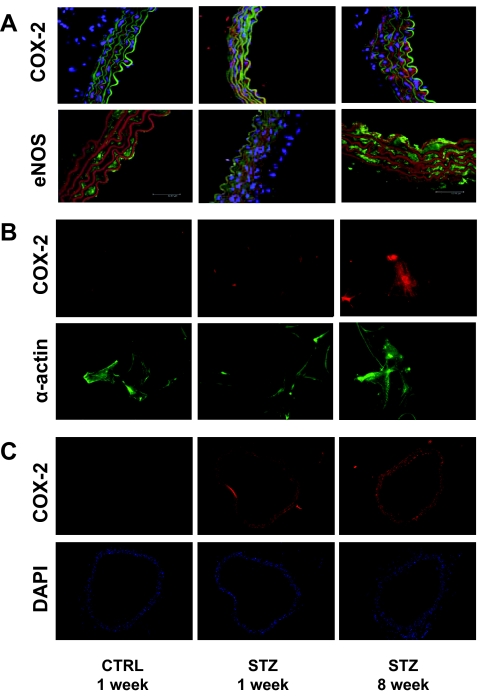
Consistent with evidence for increased COX-2 activity in MVB from mice with STZ-induced diabetes (Fig. 1C), immunoblotting of MVB homogenates demonstrated increased expression of COX-2 (but not Akt or β-actin) both 1 and 8 wk after STZ treatment (when compared with MVB from CTRL-treated mice) (Fig. 2, A and B). Expression of eNOS was also elevated in MVBs from STZ-treated mice (Fig. 2, A and B). Similar results were observed in homogenates of aorta and lysates of isolated aortic vascular smooth muscle cells (Fig. 2, C and D). Vascular expression of COX-1 was similar in all groups examined (data not shown). Using immunohistochemistry, increased expression of COX-2 and eNOS was also observed in intact aortas from STZ-treated mice (when compared with aortas from CTRL-treated mice) (Fig. 3A). Moreover, intact VSMCs isolated from aortas of mice with STZ-induced diabetes also demonstrated increased expression of COX-2 (Fig. 3B). In addition, immunofluorescent experiments in mesenteric artery demonstrated that expression of COX-2 is already increased in 1wk STZ mice when compared with CTRL mice (Fig. 3C). Thus, increased expression of COX-2 in the vasculature of mice with STZ-induced diabetes may help to explain increased COX-2-dependent vasodilator actions of ACh in MVB from STZ-treated mice.
Figure 2.
Expression of COX-2 and eNOS, but not Akt, is elevated in vasculature of mice with STZ-induced Diabetes. A, Lysates of MVBs obtained from mice treated with STZ or CTRL for the indicated times were immunoblotted with indicated antibodies. Time-dependent increase in COX-2 and eNOS expression in MVBs after STZ treatment in a representative immunoblot from three independent experiments. Lane 4, COX-2 purified protein, positive control. B, Mean ± sem of densitometric analysis for COX-2 and eNOS expression normalized for Akt expression for three independent experiments. Significant increases in protein expression were observed for COX-2 in MVBs of mice treated with STZ for 1 wk and COX-2 and eNOS in MVBs of mice treated with STZ for 8 wk (vs. CTRL, P < 0.05). Asterisks refer to significant differences found between indicated groups assessed by Student’s t test. C, Lysates from aortas of mice treated with vehicle control or STZ for the indicated times were immunoblotted with indicated antibodies. Representative immunoblots of experiments were repeated independently three times. D, Lysates of VSMCs from aortas of mice treated with vehicle control or STZ for the indicated times were immunoblotted with indicated antibodies. Representative immunoblots from experiments independently repeated three times are shown.
Figure 3.
Expression of COX-2 and eNOS is increased in aortas from mice with STZ-induced diabetes. Aortas from mice treated with CTRL or STZ for the indicated times were prepared as described in Materials and Methods. A, Aortas were fixed in 8% paraformaldehyde and ring sections (10 μm) were immunolabeled with antibodies against COX-2 (upper panel) or eNOS (lower panel). Sections were immunostained with IgG-fluorescein isothiocyanate conjugated- (upper panel) and IgG-Texas Red conjugated (lower panel)-laminin antibodies. Finally, DAPI staining was used to visualize cell nuclei. Thus, visualization by confocal microscopy shows red fluorescence for COX-2 staining and green fluorescence for laminin staining in the upper panel. In the lower panel, green fluorescence is seen for eNOS staining, whereas red fluorescence is seen for laminin staining. Cell nuclei are visualized in blue. Representative results are shown for experiments independently repeated three times. B, VSMCs were prepared from aortas of mice treated with CTRL or STZ for the indicated times as described in Materials and Methods. VSMCs were fixed in 3.5% paraformaldehyde and immunolabeled with antibodies against COX-2 (upper panel) or α-actin (lower panel). Representative results are shown for experiments that were repeated independently three times. C, Mesenteric arteries were fixed in 8% paraformaldehyde and ring sections (8 μm) were immunolabeled with COX-2 antibody (upper panel). DAPI staining was used to visualize cell nuclei (lower panel). Red fluorescence indicates COX-2 staining. Cell nuclei are visualized in blue. Representative results are shown for experiments independently repeated three times.
Production of 6-keto-PGF-1α is increased in aortas of STZ-treated mice
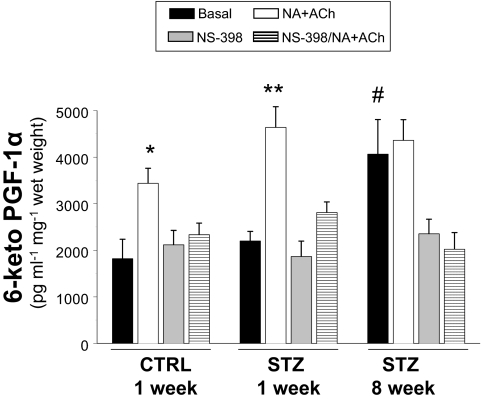
To evaluate vascular production of COX-2-dependent prostanoid vasodilators, we measured 6-keto-PGF-1α (a stable derivative of prostacyclin) in conditioned media from cultured aortas isolated from mice 1 or 8 wk after injection with STZ or CTRL (Fig. 4). Cultured aortas were treated without or with NA plus ACh in the absence or presence of pretreatment with NS-398 to mimic conditions previously tested in MVB (cf. Fig. 1). Treatment of cultured aortas from CTRL-injected mice with NA plus ACh nearly doubled the concentration of 6-keto-PGF-1α in conditioned media. This response to NA plus ACh was completely inhibited by pretreatment of aortas with NS-398. Basal levels of 6-keto-PGF-1α in conditioned media from cultured aortas isolated from mice 1 wk after STZ injection were similar to those observed in conditioned media from cultured aortas isolated from CTRL-injected mice. However, in response to NA plus ACh treatment, the increase in 6-keto-PGF-1α concentration in conditioned media from cultured aortas isolated from mice 1 wk after STZ injection was significantly greater than that observed in conditioned media from cultured aortas isolated from CTRL-injected mice. In experiments using aortas from mice 8 wk after STZ injection, significant elevations of 6-keto-PGF-1α concentrations in conditioned media were observed both before and after treatment with NA plus ACh. In all cases, pretreatment with NS-398 blocked elevations in 6-keto-PGF-1α. Differences in structure and function between aorta and mesenteric resistance vessels may partially explain limited consistency with functional data obtained from MVBs. Nevertheless, these data together suggest that there is increased formation of COX-2-dependent NO-independent prostanoid vasodilators in the vasculature of mice with STZ-induced diabetes.
Figure 4.
Production of 6-keto-PGF-1α is elevated in aortas of STZ-treated mice. Aortas from mice treated with CTRL or STZ for the indicated times were prepared as described in Materials and Methods. Aortas were incubated in Krebs-Henseleit solution (20 min, 37 C) and then treated without or with NA + ACh in the absence or presence of COX-2 inhibitor NS-398 (10 μm). Conditioned media were then collected for measurement of 6-keto-PGF-1α (ELISA). Levels of 6-keto-PGF-1α were significantly increased in response to NA + ACh stimulation in conditioned media from cultured aortas isolated from mice 1 wk after injection with CTRL or STZ (vs. respective basal). *, P < 0.005; **, P < 0.001. Significant elevations of 6-keto-PGF-1α concentrations in conditioned media were observed both before and after treatment with NA + ACh in aortas isolated from mice 8 wk after injection with STZ (vs. CTRL basal. #, P < 0.01. Results are mean ± sem (normalized to wet weight of aorta) for experiments repeated independently three times in duplicate.
Both COX-2- and NO-dependent mechanisms participate in opposing endothelial dysfunction in STZ-induced diabetes
In addition to increased production of NO-independent vasodilators such as COX-2-dependent prostanoids, other potential compensatory mechanisms to oppose endothelial dysfunction in diabetes may involve blunting of vasoconstrictor responses by NO-dependent and -independent means. To evaluate this, we examined NA-mediated vasoconstriction in MVBs from mice treated with STZ or CTRL (Fig. 5A). The NA dose-response curves were similar among MVBs isolated from mice 1 (or 8) wk after CTRL injection (maximal PP = 63.7 ± 5.3 mm Hg; ED50 = 3.8 ± 0.4 μm) and MVBs isolated from mice 1 wk after STZ injection (maximal PP = 70.6 ± 2.1 mm Hg; ED50 = 4.7 ± 0.6 μm). By contrast, substantially impaired vasoconstriction in response to NA was observed in MVBs isolated from mice 8 wk after STZ injection (maximal PP = 41.6 ± 1.1 mm Hg; ED50 = 3.8 ± 0.9 μm). Similar results were obtained using the vasoconstrictor U46619 (thromboxane A2 analog, data not shown). Different doses of NA required to achieve similar perfusion pressure in MVBs from STZ and CTRL mice indicates differential sensitivity to NA similar to that previously documented in SHR rats (35). When systolic blood pressure was measured in vivo (by tail-cuff method) in CTRL and STZ-treated mice, no statistical differences were observed. Of note, blood pressure is under tight control from multiple homeostatic mechanisms and is much less sensitive to disruption than endothelial dysfunction (38). However, a trend (that did not reach statistical significance) toward higher blood pressure was observed in STZ-treated mice 8 wk after treatment (Table 1). Taken together, our data suggest that blunted vasoconstrictor responses in the vasculature of mice with type 1 diabetes may oppose the endothelial dysfunction of diabetes.
Figure 5.
Both COX-2- and NO-dependent mechanisms participate in opposing endothelial dysfunction in STZ-induced diabetes. MVBs were isolated from mice treated with STZ (closed symbols) or vehicle control (open symbols) and prepared as described in Materials and Methods. A, Dose-response curves for NA-induced vasoconstriction were obtained in MVBs from mice 1 wk (squares) or 8 wk (circles) after treatment with STZ or vehicle control. Results are mean ± sem of 12 (STZ) and 10 (CTRL) independent experiments. NA-mediated vasoconstriction was significantly impaired in MVB from mice treated with STZ for 8 wk (vs. respective CTRL, P < 0.001). B, Dose-response curves for NA-mediated vasoconstriction were obtained in MVBs from mice 8 wk after treatment with STZ or vehicle control in the absence (circles) or presence (diamonds) of pretreatment with the NO synthase antagonist L-NAME (100 μm, 30 min). Results are mean ± sem of four (STZ) and five (CTRL) independent experiments. Inhibition of NO synthase significantly increased NA-mediated vasoconstriction in MVBs from mice treated with either STZ or vehicle control for 8 wk (vs. results without L-NAME pretreatment, P < 0.01). C, Dose-response curves for NA-mediated vasoconstriction were obtained in MVB from mice 8 wk after treatment with STZ or vehicle control in the absence (circles) or presence (triangles) of pretreatment with the specific COX-2 inhibitor NS-398 (10 μm, 30 min). Results are mean ± sem of eight (STZ) and seven (CTRL) independent experiments. Inhibition of COX-2 significantly increased NA-mediated vasoconstriction in MVB from mice treated with STZ for 8 wk (vs. results without NS-398 pretreatment, P < 0.01) but not in MVB from CTRL mice. Asterisks refer to significant differences found between indicated curves assessed by two way ANOVA for repeated measures.
To investigate potential compensatory mechanisms underlying the decrease in vasoconstrictor responses to NA in diabetes, we repeated experiments shown in Fig. 5A in the absence or presence of L-NAME or NS-398. In MVBs from mice 8 wk after STZ injection, pretreatment with L-NAME significantly increased the vasoconstrictor response to NA so that the NA dose-response curve was similar to those obtained in MVB from mice 8 wk after injection with CTRL either in the absence of L-NAME pretreatment (Fig. 5B). Likewise, in MVB from mice 8 wk after STZ injection, pretreatment with NS-398 significantly increased the vasoconstrictor response to NA so that the NA dose-response curve was similar to those obtained in MVB from mice 8 wk after injection with CTRL either in the absence or presence of NS-398 pretreatment (Fig. 5C). Biological variability may limit results from functional studies. However, our findings obtained in the same preparations before and after treatment with inhibitors imply that changes observed in vascular reactivity may be interpreted as a consequence of STZ-induced diabetes. Taken together, these results suggest that vasodilators including NO- and COX-2-dependent prostanoids may participate in opposing vasoconstrictor actions of NA that potentially contribute to endothelial dysfunction of diabetes.
Activation of proinflammatory signaling in endothelial cells from diabetic mice
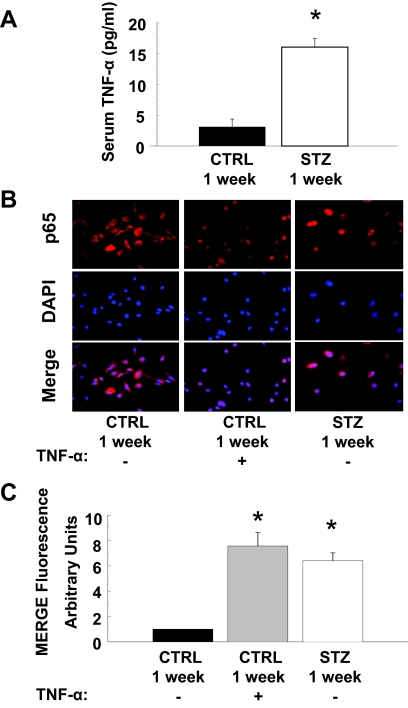
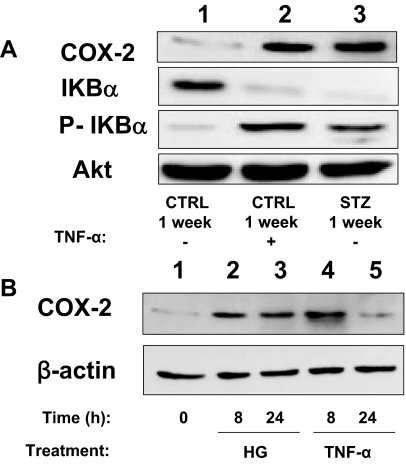
The hyperglycemia of diabetes activates proinflammatory signaling (4). Indeed, circulating levels of TNF-α were elevated 7-fold in mice 1 wk after STZ injection (when compared with levels in mice injected with CTRL) (Fig. 6A). Consistent with elevated circulating levels of TNF-α, nuclear localization of the p65 subunit of NF-κB was enhanced in quiescent MAECs isolated from mice 1 wk after STZ injection (when compared with MAECs from mice 1 wk after CTRL injection) (Fig. 6, B and C). This was similar to what was observed in MAECs from mice 1 wk after CTRL injection that were treated acutely with TNF-α for 30 min (Fig. 6B, middle column). Consistent with these findings, total IkBα expression was reduced whereas phosphorylation of IkBα and expression of COX-2 was substantially increased in MAECs isolated from mice 1 wk after STZ injection (when compared with MAECs from mice 1 wk after CTRL injection) (Fig. 7A, lanes 1 and 3). When MAECs isolated from mice 1 wk after CTRL injection were treated with TNF-α for 24 h, total IkBα expression was reduced whereas phosphorylation of IkBα and expression of COX-2 was substantially increased to levels comparable to those observed in MAECs from STZ-treated mice (Fig. 7A). Interestingly, both hyperglycemia (55 mm per 24 h) and TNF-α stimulation (10 ng/ml per 24 h) were able to increase COX-2 expression in human aortic endothelial cells (Fig. 7B). This suggests that activation of proinflammatory signaling mediates increased expression of COX-2 in the vasculature of diabetic mice.
Figure 6.
Activation of proinflammatory signaling in aortic endothelial cells leads to increased expression of COX-2. A, Serum concentrations of TNF-α (measured by ELISA) were substantially and significantly higher in mice treated with STZ for 1 wk (vs. vehicle-treated control mice, P < 0.001). Results are mean ± sem for experiments that were repeated independently three times in triplicate. B, Nuclear localization of the p65 subunit of NF-κB is enhanced in MAECs from mice treated with STZ for 1 wk. MAECs from CTRL or STZ mice were serum starved for 4 h and subsequently treated without or with TNF-α (10 ng/ml, 30 min) as indicated. Cells were then fixed in 3.5% paraformaldehyde and incubated with antibodies that detect the p65 subunit of NF-κB (upper panels). Results (red fluorescence) were visualized using an epifluorescent microscope as described in Materials and Methods. Cells were also stained with DAPI to visualize cell nuclei (middle panels, blue fluorescence). Merging of p65 and DAPI staining (lower panels) demonstrates that enhanced nuclear localization of p65 in MAECs from mice treated with STZ for 1 wk (that have elevated levels of TNF-α) is comparable with that observed in MAECs from control mice treated acutely with TNF-α. Representative images are shown from experiments that were repeated independently three times. C, Mean ± sem of densitometric analysis for merging of p65 and DAPI staining shown in B. Asterisks refer to significant differences found between indicated groups assessed by Student’s t test (vs. CTRL, P < 0.01).
Figure 7.
Activation of proinflammatory signaling in aortic endothelial cells leads to increased expression of COX-2. A, Lysates of MAECs isolated from mice treated with STZ or vehicle control for 1 wk and then stimulated without or with TNF-α (10 ng/ml, 24 h) were immunoblotted with indicated antibodies. Representative immunoblots from experiments that were repeated independently three times are shown. P-IkBα, Phosphorylated IkBα. B, Lysates of HAECs left untreated or treated with either high glucose (HG; 55 mm) or TNF-α (10 ng/ml) for the indicated time were immunoblotted with indicated antibodies. Representative immunoblots from experiments independently repeated three times are shown.
Discussion
In mice treated with STZ, we observed rapid induction of type 1 diabetes characterized by hyperglycemia, glycosuria, hypoinsulinemia, and elevated circulating levels of TNF-α. Consistent with other studies of STZ-induced diabetes (39,40,41), we observed a time-dependent worsening in metabolic homeostasis after STZ treatment. Hyperglycemia of diabetes contributes to endothelial dysfunction, in part, by altering intracellular signaling pathways, inducing changes in expression of key vascular components, shifting the cell redox balance, and by activating inflammatory macrophages (42,43,44). Proinflammatory conditions quickly stimulate up-regulation of endothelial COX-2 expression, a mediator of NO-independent vasodilation that contributes importantly to vascular homeostasis (22,45). In the present study, we discovered that increased endothelial COX-2 expression and activity represents an important compensatory response that opposes the endothelial dysfunction of diabetes.
Increased endothelial expression and activity of COX-2 opposes endothelial dysfunction in type 1 diabetes
The MVB is a prototype of small resistance vessels (46). Therefore, changes in vascular reactivity in the MVB yields useful insights for the study of vascular complications of diabetes (47). In mesenteric resistance arteries, NO (48,49), endothelial-derived hyperpolarizing factor (50), and prostacyclin (51) are among the most important mediators of ACh-dependent vasodilation. Chronic exposure to hyperglycemia and subsequent low-grade inflammation induces endothelial cells to switch from a quiescent phenotype to a more activate state that attempts to compensate for impaired production of vasoactive mediators (5). This so-called endothelial activation may represent a physiological mechanism to oppose pathophysiological forces promoting overt endothelial dysfunction. In MVBs from mice 1 wk after STZ treatment, expression of eNOS protein was unchanged despite a decrease in NO-mediated vasodilation in response to ACh. This reduced NO bioavailability in the face of normal eNOS expression may be due to effects of hyperglycemia to impair eNOS function (52,53) and/or reflects an increase in production of compounds such as reactive oxygen species that increase NO scavenging (54).
Phosphorylation of eNOS by Akt is an important mechanism regulating activation of eNOS in response to insulin, vascular endothelial growth factor, and other ligands. Although a very recent study suggests the involvement of an additional phosphorylation-dependent mechanism for ACh-induced activation of eNOS (55) in insulin-resistant states, the signaling mechanism involved with ACh-stimulated NO production is mostly calcium dependent (56). Experiments addressing the phosphorylation status of eNOS or Akt are important to explore in the context of insulin-mediated vascular actions. However, such experiments are less relevant to our present investigations related to ACh-mediated vasorelaxation under hyperglycemic conditions.
Eight weeks after STZ treatment, ACh had almost no ability to elicit vasorelaxation in MVBs isolated ex vivo from STZ-treated mice. This severe endothelial dysfunction in STZ-treated mice is consistent with the reports of others (57,58,59). Interestingly, frank endothelial dysfunction in MVBs with respect to ACh stimulation was not clearly evident 1 wk after STZ treatment, even though metabolic parameters were fully consistent with diabetes at this time. However, in MVBs from mice 1 wk after STZ treatment, L-NAME failed to completely inhibit the vasorelaxant response to ACh (unlike in MVBs from control mice). Of note, L-NAME pretreatment slightly increased perfusion pressure to a similar extent in MVB from both control and 1-wk STZ-treated mice. Because L-NAME is not a specific eNOS inhibitor and inducible NO synthase (iNOS) may be involved in early compensatory response to inflammatory conditions (58,59), the effect of L-NAME may be partially explained by inhibition of iNOS activity (60). Indeed, iNOS is mainly expressed in the VSMCs, whereas ACh-induced vasodilation is mainly mediated by eNOS. Thus, inhibition of basal NO release by L-NAME, if anything, supports our hypothesis that compensatory mechanisms are at work early in diabetes to offset the mild impairment in eNOS present in 1-wk STZ-treated mice.
Taken together, these findings raise the possibility that NO-independent vasodilator actions may be masking endothelial dysfunction caused by diminished NO bioavailability in the early stages of diabetes. That is, endothelial dysfunction secondary to metabolic derangements of STZ-induced diabetes may be obscured by a compensatory mechanism induced in response to the onset of diabetes.
Although indomethacin’s inhibitory effects were slightly larger in STZ-treated vs. CTRL mice, this difference did not reach statistical significance. Indomethacin is a general inhibitor for COX enzymes with a greater inhibitory activity toward COX-1 than COX-2 (61). Blockade of both COX-1 and COX-2 results in inhibition of both vasoconstrictor and vasodilator cyclooxygenase products (9). Thus, one possible explanation of our results is that COX-1-mediated production of vasoconstrictor prostanoids may differ between basal and diabetic conditions. That is, COX-1/COX-2 inhibition by indomethacin may blunt the individual opposing contributions of both vascular mediators. Indeed, others have previously noted reciprocal correlations between COX and NO synthase pathways under both physiological and pathological conditions (62,63,64,65). Thus, cross talk between NO synthase and COX signaling and nonspecific inhibitory effects of indomethacin may help to explain why results obtained with indomethacin are complicated to interpret. These findings reveal limitations of our experimental approach.
Nevertheless, when we pretreated MVBs from mice 1 wk after STZ treatment with the COX-2 inhibitor NS-398, we were able to unmask endothelial dysfunction with respect to ACh. Consistent with findings of increased COX-2 activity, we also observed increased expression of COX-2 protein in isolated mesenteric vessels as well as in vascular endothelial cells obtained from aortae of diabetic mice. It is important to note that endothelial cells constitute only a very small fraction of the total protein in a whole vessel. This may be one reason that immunoblotting analysis of whole aorta homogenates did not reveal differences in COX-2 protein expression when CTRL and 1-wk STZ mice were compared. Potential limitations from nonspecific effects of the COX-2 inhibitor NS-398 might be possible to address with the use of COX-2 KO mice. However, with this mouse model, cardiac fibrosis and other vascular abnormalities have been described (20) that would further complicate interpretation of results. Thus, further investigations using COX-2 knockout mice are beyond the scope of the present study.
We also observed increased production of the COX-2-dependent 6-keto-PGF1α (a stable derivative of the vasodilator prostacyclin) in response to ACh treatment of cultured aortae isolated from diabetic mice. This is consistent with our observation that aortic endothelial cells express COX-2 under basal conditions in control animals. Although results obtained in aorta may not be completely consistent with functional data from MVBs, they nevertheless suggest that increased COX-2 expression may correlate with enhanced release of vasodilator prostanoids in the vasculature of diabetic animals. Thus, similar processes are likely involved in vascular rearrangement throughout the vasculature during the early phase of diabetes in our STZ-treated mice.
Additional vasoactive mediators other than those examined may be altered under diabetic conditions. For example, endothelial-derived hyperpolarizing factor has been shown to decrease under diabetic conditions in some studies (66,67). Increased oxidative stress may profoundly alter endothelial function in response to hyperglycemia (68). Indeed, we found that superoxide production was enhanced in response to both high glucose and high TNF-α concentrations either in vivo or in vitro (see supplemental Fig. 3). A comprehensive investigation into the specific contribution of every vasoactive factor involved in endothelial dysfunction is beyond the scope of our study.
Besides increasing vasodilator responses to ACh, additional mechanisms for opposing the endothelial dysfunction of diabetes may involve factors opposing the vasoconstrictor response to NA. Indeed, the vasoconstrictor response to NA was severely blunted in MVBs isolated from mice 8 wk after STZ treatment. Pretreatment of MVBs from these mice with L-NAME or NS-398 fully restored the normal vasoconstrictor response to NA. Results from these experiments suggest that both NO- and COX-2-dependent mechanisms oppose the vasoconstrictor response to NA in diabetes and may contribute to compensatory responses that serve to oppose the endothelial dysfunction of diabetes. Indeed, both eNOS and COX-2 protein expression were increased in MVB homogenates as well as the vessel wall of aortas from 8-wk STZ-treated mice. Of note, consistent with our present findings, expression of eNOS has previously been unequivocally documented by others in human VSMCs under both basal and pathological conditions (69,70). It is important to note that in Fig. 5A, data were obtained by adding increasing concentrations of NA in a noncumulative manner (each dose was given as a 30 sec perfusion). The perfusion pressure recorded reflects the peak obtained after each single administration. By contrast, in Fig. 1, continuous infusion of NA was used to determine basal vasoconstrictor tone to perform vasodilation experiments. For these experiments, MVBs were contracted to about 80% of maximal vasoconstriction. For each MVB preparation, the dose of NA was adjusted to obtain the same level of precontraction in MVBs from either CTRL or STZ mice. Therefore, 3 μm NA were used in both 1-wk STZ and 1-wk CTRL mice, whereas 7 and 3 μm NA were used in 8-wk STZ and 8-wk CTRL mice, respectively. This helps to explain why NA doses used to obtain perfusion pressure values in curves from Fig. 5 may not precisely correspond with NA doses needed to obtain steady-state perfusion pressure of approximately 100 mm Hg in Fig 1. Clearly, multiple lines of evidence from both our functional and cellular studies strongly support a protective role for COX-2 in opposing endothelial dysfunction associated with diabetes.
Proinflammatory signaling stimulates compensatory up-regulation of COX-2 in vascular endothelium
The hyperglycemia of diabetes activates proinflammatory signaling pathways that contribute to a reciprocal relationship between insulin resistance and endothelial dysfunction (2,3). In our model of type 1 diabetes, circulating levels of the proinflammatory cytokine TNF-α were elevated 7-fold over levels measured in control mice. Moreover, intracellular activation of NF-κB in aortic endothelial cells from diabetic mice (indicated by nuclear localization of the p65 subunit of NF-κB, increased phosphorylation of IKBα, and reduction in IKBα expression) was similar to that observed in aortic endothelial cells from control mice stimulated with TNF-α for 24 h. Interestingly, treatment of endothelial cells from control mice with TNF-α also induced expression of COX-2 to levels similar to those observed in aortic endothelial cells isolated from mice 1 wk after STZ treatment. Moreover, exposure to either hyperglycemia or TNF-α increased COX-2 expression in human aortic endothelial cells. Although cultured endothelial cells obtained from aortas of 1-wk STZ-treated mice may behave differently from endothelial cells in vivo, our procedures were designed to minimize this possibility. Indeed, under these same conditions, a significant difference was observed in terms of signaling when MAECs obtained from control and STZ-treated mice were compared. Our results suggest that proinflammatory signaling in response to TNF-α is sufficient to induce increased expression of COX-2 in vascular endothelial cells. This provides at least one mechanism for the hyperglycemia of diabetes to mediate a compensatory response to oppose the endothelial dysfunction of diabetes. Additional related or independent mechanisms may also exist. Our results are consistent with previous studies implicating activation of NF-κB in increased expression of COX-2 (71,72,73).
Some rodent models of atherosclerosis and diabetes reveal associations between increased COX-2 expression and enhanced production of constrictor prostanoids (74,75,76,77). Nevertheless, other studies suggest that increased expression of COX-2 subsequently increases production of prostacyclin that limits arterial vasoconstriction and contributes to reduced platelet aggregation and thrombus formation in both human and animal models (27,78,79). Protective roles for COX-2 activity in the cardiovascular system have recently been suggested by studies demonstrating that COX-2 activity is important for protecting cardiomyocytes against oxidative stress (80) and limiting myocardial damage after ischemia/reperfusion injury (81,82,83). Moreover, activation of COX-2 in endothelium in response to shear stress increases levels of PGI2 and counteracts COX-1-dependent production of prothrombotic thromboxane A in platelets (84,85). Finally, statin-induced increases in COX-2 expression promotes production of 15 d PGJ2, a natural ligand for peroxisomal proliferator-activated receptor-γ, that may mediate some antiatherogenic actions of statins (86). It is not clear why increased expression of COX-2 seems to have beneficial cardiovascular effects in some, but not all, cases. Our results suggest that increased expression of COX-2 in endothelium is an early event and correlates with endothelial protection, whereas up-regulation of COX-2 in the VSMCs (that occurs later on during the development of diabetic vascular changes) is likely associated with impaired vascular contractility that is observed later at 8 wk. The dysregulation of vasodilator and vasoconstrictor responses seems to occur with a distinct time course. Another possibility is that COX-2 couples with several different downstream synthases which generate multiple compounds with opposing vascular actions (9). The net effect of these multiple vasoactive compounds may be determined by distinct physiological or pathophysiological contexts. For example, increased expression of COX-2 in the vasculature in response to hyperglycemia may result in either beneficial or detrimental cardiovascular consequences, depending on the timing, magnitude, tissue-specific expression, and surrounding pathophysiological settings that regulate the conversion of COX-2-dependent PGH into vasodilator or vasoconstrictor prostanoids. Indeed, several recent clinical studies demonstrating increased cardiovascular risk after administration of selective COX-2 inhibitors underscores the complexity of COX-2-mediated actions in the regulation of cardiovascular homeostasis (87,88).
Summary
We have demonstrated that increased expression and activity of COX-2 in the vasculature of an experimental mouse model of type 1 diabetes, induced in part by proinflammatory signaling, may represent a compensatory response that opposes the endothelial dysfunction of diabetes. These findings may have important implications regarding the safe use of selective COX-2 inhibitors in diabetes and may contribute to developing novel therapeutic strategies for diabetes and its cardiovascular complications.
Supplementary Material
Acknowledgments
We thank Dr. Ranganath Muniyappa for help and advice with statistical analyses.
Footnotes
This work was supported by in part by research grant awards from the Italian Ministry for University and Research and the Juvenile Diabetes Research Foundation (CDA 2-2006-32; to M.M.) and the Intramural Research Program, National Center for Complementary and Alternative Medicine, National Institutes of Health (to M.J.Q.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 9, 2008
Abbreviations: ACh, Acetylcholine; COX, cyclooxygenase; CTRL, vehicle control (citrate buffer); DAPI, 4,6-diamidino-2-phenylindole; eNOS, endothelial NO synthase; IkB, inhibitor of kappa B; iNOS, inducible NO synthase; 6-keto-PGF1α, 6-keto-prostaglandin F1α; L-NAME, L-Nω-arginine methyl ester; MAEC, mouse aortic endothelial cell; MVB, mesenteric vascular bed; NA, noradrenaline; NF-κB, nuclear factor-κB; NO, nitric oxide; NS-398, N-(2-cyclohexyloxy-4-nitrophenyl) methanesulfonamide; PP, perfusion pressure; SNP, sodium nitroprusside; STZ, streptozotocin; VSMC, vascular smooth muscle cell.
References
- Bianchi C, Miccoli R, Penno G, Del Prato S 2008 Primary prevention of cardiovascular disease in people with dysglycemia. Diabetes Care 31(Suppl 2):S208–S214 [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Montagnani M, Koh KK, Quon MJ 2007 Cardiovascular actions of insulin. Endocr Rev 28:463–491 [DOI] [PubMed] [Google Scholar]
- Kim JA, Montagnani M, Koh KK, Quon MJ 2006 Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113:1888–1904 [DOI] [PubMed] [Google Scholar]
- Sheetz MJ, King GL 2002 Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 288:2579–2588 [DOI] [PubMed] [Google Scholar]
- Deanfield JE, Halcox JP, Rabelink TJ 2007 Endothelial function and dysfunction: testing and clinical relevance. Circulation 115:1285–1295 [DOI] [PubMed] [Google Scholar]
- Marasciulo FL, Montagnani M, Potenza MA 2006 Endothelin-1: the yin and yang on vascular function. Curr Med Chem 13:1655–1665 [DOI] [PubMed] [Google Scholar]
- Lerman A, Burnett Jr JC 1992 Intact and altered endothelium in regulation of vasomotion. Circulation 86:III12–III19 [PubMed] [Google Scholar]
- Smith WL, Garavito RM, DeWitt DL 1996 Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem 271:33157–133160 [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM 1998 Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38:97–120 [DOI] [PubMed] [Google Scholar]
- Davidge ST 2001 Prostaglandin H synthase and vascular function. Circ Res 89:650–660 [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM 2000 Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69:145–182 [DOI] [PubMed] [Google Scholar]
- Wu KK 1998 Cyclooxygenase-2 induction in congestive heart failure: friend or foe? Circulation 98:95–96 [DOI] [PubMed] [Google Scholar]
- Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM 1996 Suppression of intestinal polyposis in Apc Δ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87:803–809 [DOI] [PubMed] [Google Scholar]
- Vane JR, Botting RM 1998 Anti-inflammatory drugs and their mechanism of action. Inflamm Res 47(Suppl 2):S78–S87 [DOI] [PubMed] [Google Scholar]
- Liu SF, Newton R, Evans TW, Barnes PJ 1996 Differential regulation of cyclo-oxygenase-1 and cyclo-oxygenase-2 gene expression by lipopolysaccharide treatment in vivo in the rat. Clin Sci (Lond) 90:301–306 [DOI] [PubMed] [Google Scholar]
- Liu XH, Yao S, Kirschenbaum A, Levine AC 1998 NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res 58:4245–4249 [PubMed] [Google Scholar]
- Breder CD, Dewitt D, Kraig RP 1995 Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol 355:296–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O 1995 Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 83:473–482 [DOI] [PubMed] [Google Scholar]
- Hla T, Neilson K 1992 Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA 89:7384–7388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, Gorry SA, Trzaskos JM 1995 Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378:406–409 [DOI] [PubMed] [Google Scholar]
- Syeda F, Grosjean J, Houliston RA, Keogh RJ, Carter TD, Paleolog E, Wheeler-Jones CP 2006 Cyclooxygenase-2 induction and prostacyclin release by protease-activated receptors in endothelial cells require cooperation between mitogen-activated protein kinase and NF-κB pathways. J Biol Chem 281:11792–11804 [DOI] [PubMed] [Google Scholar]
- McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA 1999 Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA 96:272–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toratani A, Sawada S, Kono Y, Higaki T, Imamura H, Tada Y, Yamasaki S, Sato T, Komatsu S, Akamatsu N, Tamagaki T, Nakagawa K, Tsuji H, Nakagawa M 1999 Interleukin-1alpha stimulated prostacyclin release by increasing gene transcription of prostaglandin H synthase and phospholipase A2 in human vascular endothelial cells. J Cardiovasc Pharmacol 33:843–851 [DOI] [PubMed] [Google Scholar]
- Pontsler AV, St Hilaire A, Marathe GK, Zimmerman GA, McIntyre TM 2002 Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor γ and oxidized alkyl phospholipids from oxidized low density lipoprotein. J Biol Chem 277:13029–13036 [DOI] [PubMed] [Google Scholar]
- Belton O, Byrne D, Kearney D, Leahy A, Fitzgerald DJ 2000 Cyclooxygenase-1 and -2-dependent prostacyclin formation in patients with atherosclerosis. Circulation 102:840–845 [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Sukhova GK, Graber P, Coulter S, Libby P 1999 Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. Am J Pathol 155:1281–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CS, Hall RJ, Evans TJ, Pomerance A, Maclouf J, Creminon C, Yacoub MH, Polak JM 1999 Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arterioscler Thromb Vasc Biol 19:646–655 [DOI] [PubMed] [Google Scholar]
- Dixon DA, Tolley ND, Bemis-Standoli K, Martinez ML, Weyrich AS, Morrow JD, Prescott SM, Zimmerman GA 2006 Expression of COX-2 in platelet-monocyte interactions occurs via combinatorial regulation involving adhesion and cytokine signaling. J Clin Invest 116:2727–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szerafin T, Erdei N, Fulop T, Pasztor ET, Edes I, Koller A, Bagi Z 2006 Increased cyclooxygenase-2 expression and prostaglandin-mediated dilation in coronary arterioles of patients with diabetes mellitus. Circ Res 99:e12–e17 [DOI] [PubMed] [Google Scholar]
- Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H, Volpe M, Luscher TF 2003 High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation 107:1017–1023 [DOI] [PubMed] [Google Scholar]
- Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M 2005 Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol 289:H813–H822 [DOI] [PubMed] [Google Scholar]
- Montagnani M, Potenza MA, Rinaldi R, Mansi G, Nacci C, Serio M, Vulpis V, Pirrelli A, Mitolo-Chieppa D 1999 Functional characterization of endothelin receptors in hypertensive resistance vessels. J Hypertens 17:45–52 [DOI] [PubMed] [Google Scholar]
- Voyta JC, Via DP, Butterfield CE, Zetter BR 1984 Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol 99:2034–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MA, Botrugno OA, De Salvia MA, Lerro G, Nacci C, Marasciulo FL, Andriantsitohaina R, Mitolo-Chieppa D 2002 Endothelial COX-1 and -2 differentially affect reactivity of MVB in portal hypertensive rats. Am J Physiol Gastrointest Liver Physiol 283:G587–G594 [DOI] [PubMed] [Google Scholar]
- Potenza MA, Marasciulo FL, Tarquinio M, Quon MJ, Montagnani M 2006 Treatment of spontaneously hypertensive rats with rosiglitazone and/or enalapril restores balance between vasodilator and vasoconstrictor actions of insulin with simultaneous improvement in hypertension and insulin resistance. Diabetes 55:3594–3603 [DOI] [PubMed] [Google Scholar]
- Cohen J 1988 Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: L. Erlbaum Associates [Google Scholar]
- Rakieten N, Rakieten ML, Nadkarni MR 1963 Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep 29:91–98 [PubMed] [Google Scholar]
- Fitzgerald SM, Kemp-Harper BK, Parkington HC, Head GA, Evans RG 2007 Endothelial dysfunction and arterial pressure regulation during early diabetes in mice: roles for nitric oxide and endothelium-derived hyperpolarizing factor. Am J Physiol Regul Integr Comp Physiol 293:R707–R713 [DOI] [PubMed] [Google Scholar]
- Bardell AL, MacLeod KM 2001 Evidence for inducible nitric-oxide synthase expression and activity in vascular smooth muscle of streptozotocin-diabetic rats. J Pharmacol Exp Ther 296:252–259 [PubMed] [Google Scholar]
- Xiong Y, Fu YF, Fu SH, Zhou HH 2003 Elevated levels of the serum endogenous inhibitor of nitric oxide synthase and metabolic control in rats with streptozotocin-induced diabetes. J Cardiovasc Pharmacol 42:191–196 [DOI] [PubMed] [Google Scholar]
- Goss JR, Goins WF, Lacomis D, Mata M, Glorioso JC, Fink DJ 2002 Herpes simplex-mediated gene transfer of nerve growth factor protects against peripheral neuropathy in streptozotocin-induced diabetes in the mouse. Diabetes 51:2227–2232 [DOI] [PubMed] [Google Scholar]
- Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, Remuzzi A, Zoja C, Remuzzi G 1998 Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-κB-dependent fashion. J Clin Invest 101:1905–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth G, Stalker TJ, Lefer AM, Scalia R 2001 Elevated ambient glucose induces acute inflammatory events in the microvasculature: effects of insulin. Am J Physiol Endocrinol Metab 280:E848–E856 [DOI] [PubMed] [Google Scholar]
- Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, Nadler JL 2006 Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology 147:2518–2525 [DOI] [PubMed] [Google Scholar]
- Inoue H, Taba Y, Miwa Y, Yokota C, Miyagi M, Sasaguri T 2002 Transcriptional and posttranscriptional regulation of cyclooxygenase-2 expression by fluid shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol 22:1415–1420 [DOI] [PubMed] [Google Scholar]
- Komidori H, Yamamoto R, Nickols GA, Takasaki K 1992 Characterization of the isolated rat mesenteric vascular-intestinal loop preparation. J Pharmacol Toxicol Methods 27:59–65 [DOI] [PubMed] [Google Scholar]
- Cooper ME, Bonnet F, Oldfield M, Jandeleit-Dahm K 2001 Mechanisms of diabetic vasculopathy: an overview. Am J Hypertens 14:475–486 [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV 1980 The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376 [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S 1987 Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524–526 [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Rubanyi GM, Miller VM, Houston DS 1986 Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol 48:307–320 [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Vanhoutte PM 1989 Endothelium-derived relaxing and contracting factors. FASEB J 3:2007–2018 [PubMed] [Google Scholar]
- Lei H, Venkatakrishnan A, Yu S, Kazlauskas A 2007 Protein kinase A-dependent translocation of Hsp90 α impairs endothelial nitric-oxide synthase activity in high glucose and diabetes. J Biol Chem 282:9364–9371 [DOI] [PubMed] [Google Scholar]
- Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA 2002 Inhibition of protein kinase Cβ prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res 90:107–111 [DOI] [PubMed] [Google Scholar]
- Salt IP, Morrow VA, Brandie FM, Connell JM, Petrie JR 2003 High glucose inhibits insulin-stimulated nitric oxide production without reducing endothelial nitric-oxide synthase Ser1177 phosphorylation in human aortic endothelial cells. J Biol Chem 278:18791–18797 [DOI] [PubMed] [Google Scholar]
- Zecchin HG, Priviero FB, Souza CT, Zecchin KG, Prada PO, Carvalheira JB, Velloso LA, Antunes E, Saad MJ 2007 Defective insulin and acetylcholine induction of endothelial cell-nitric oxide synthase through insulin receptor substrate/Akt signaling pathway in aorta of obese rats. Diabetes 56:1014–1024 [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R 1999 Signal transduction of eNOS activation. Cardiovasc Res 43:532–541 [DOI] [PubMed] [Google Scholar]
- Pieper GM 1999 Enhanced, unaltered and impaired nitric oxide-mediated endothelium-dependent relaxation in experimental diabetes mellitus: importance of disease duration. Diabetologia 42:204–213 [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Kohno F, Kawase R, Yamamoto Y, Nakayama K 2004 Contribution of nitric oxide produced by inducible nitric oxide synthase to vascular responses of mesenteric arterioles in streptozotocin-diabetic rats. Br J Pharmacol 141:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagareddy PR, Xia Z, McNeill JH, MacLeod KM 2005 Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol 289:H2144–H2152 [DOI] [PubMed] [Google Scholar]
- Cheng X, Pang CC 2004 Increased vasoconstriction to noradrenaline by 1400W, inhibitor of iNOS, in rats with streptozotocin-induced diabetes. Eur J Pharmacol 484:263–268 [DOI] [PubMed] [Google Scholar]
- Kato M, Nishida S, Kitasato H, Sakata N, Kawai S 2001 Cyclooxygenase-1 and cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs: investigation using human peripheral monocytes. J Pharm Pharmacol 53:1679–1685 [DOI] [PubMed] [Google Scholar]
- Tetsuka T, Daphna-Iken D, Srivastava SK, Baier LD, DuMaine J, Morrison AR 1994 Cross-talk between cyclooxygenase and nitric oxide pathways: prostaglandin E2 negatively modulates induction of nitric oxide synthase by interleukin 1. Proc Natl Acad Sci USA 91:12168–12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Sarthy VP, Kern TS 2004 Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol Regul Integr Comp Physiol 287:R735–R741 [DOI] [PubMed] [Google Scholar]
- Cheng HF, Zhang MZ, Harris RC 2006 Nitric oxide stimulates cyclooxygenase-2 in cultured cTAL cells through a p38-dependent pathway. Am J Physiol Renal Physiol 290:F1391–F1397 [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Salvemini D 2007 Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney Int 71:290–297 [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Van de Voorde J, Blom HJ, Vanhoutte PM, Verbeke M, Lameire NH 2000 The impaired renal vasodilator response attributed to endothelium-derived hyperpolarizing factor in streptozotocin-induced diabetic rats is restored by 5-methyltetrahydrofolate. Diabetologia 43:1116–1125 [DOI] [PubMed] [Google Scholar]
- Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A 1997 Alterations in endothelium-dependent hyperpolarization and relaxation in mesenteric arteries from streptozotocin-induced diabetic rats. Br J Pharmacol 121:1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M 2000 Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790 [DOI] [PubMed] [Google Scholar]
- Trovati M, Massucco P, Mattiello L, Costamagna C, Aldieri E, Cavalot F, Anfossi G, Bosia A, Ghigo D 1999 Human vascular smooth muscle cells express a constitutive nitric oxide synthase that insulin rapidly activates, thus increasing guanosine 3′:5′-cyclic monophosphate and adenosine 3′:5′-cyclic monophosphate concentrations. Diabetologia 42:831–839 [DOI] [PubMed] [Google Scholar]
- Liang Y, Fang M, Li J, Yew DT 2006 Immunohistochemical localization of endothelial isoform (eNOS) in human cerebral arteries and the aorta. Int J Neurosci 116:1403–1417 [DOI] [PubMed] [Google Scholar]
- Shanmugam N, Kim YS, Lanting L, Natarajan R 2003 Regulation of cyclooxygenase-2 expression in monocytes by ligation of the receptor for advanced glycation end products. J Biol Chem 278:34834–34844 [DOI] [PubMed] [Google Scholar]
- Miao F, Gonzalo IG, Lanting L, Natarajan R 2004 In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem 279:18091–18097 [DOI] [PubMed] [Google Scholar]
- Konson A, Mahajna JA, Danon A, Rimon G, Agbaria R 2006 The involvement of nuclear factor-κB in cyclooxygenase-2 overexpression in murine colon cancer cells transduced with herpes simplex virus thymidine kinase gene. Cancer Gene Ther 13:1093–1104 [DOI] [PubMed] [Google Scholar]
- Belton OA, Duffy A, Toomey S, Fitzgerald DJ 2003 Cyclooxygenase isoforms and platelet vessel wall interactions in the apolipoprotein E knockout mouse model of atherosclerosis. Circulation 108:3017–3023 [DOI] [PubMed] [Google Scholar]
- Quilley J, Chen YJ 2003 Role of COX-2 in the enhanced vasoconstrictor effect of arachidonic acid in the diabetic rat kidney. Hypertension 42:837–843 [DOI] [PubMed] [Google Scholar]
- Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, Kaley G 2005 Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol 25:1610–1616 [DOI] [PubMed] [Google Scholar]
- Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, Gong MC 2005 COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res 67:723–735 [DOI] [PubMed] [Google Scholar]
- Rudic RD, Brinster D, Cheng Y, Fries S, Song WL, Austin S, Coffman TM, FitzGerald GA 2005 COX-2-derived prostacyclin modulates vascular remodeling. Circ Res 96:1240–1247 [DOI] [PubMed] [Google Scholar]
- Bagi Z, Erdei N, Papp Z, Edes I, Koller A 2006 Up-regulation of vascular cyclooxygenase-2 in diabetes mellitus. Pharmacol Rep 58(Suppl):52–56 [PubMed] [Google Scholar]
- Adderley SR, Fitzgerald DJ 1999 Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem 274:5038–5046 [DOI] [PubMed] [Google Scholar]
- Bolli R, Shinmura K, Tang XL, Kodani E, Xuan YT, Guo Y, Dawn B 2002 Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc Res 55:506–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camitta MG, Gabel SA, Chulada P, Bradbury JA, Langenbach R, Zeldin DC, Murphy E 2001 Cyclooxygenase-1 and -2 knockout mice demonstrate increased cardiac ischemia/reperfusion injury but are protected by acute preconditioning. Circulation 104:2453–2458 [DOI] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K 2005 Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11:1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahara K, Sun B, Kambayashi J 1998 Upregulation of prostacyclin synthesis-related gene expression by shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol 18:1922–1926 [DOI] [PubMed] [Google Scholar]
- Topper JN, Cai J, Falb D, Gimbrone Jr MA 1996 Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci USA 93:10417–10422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Matsumura T, Senokuchi T, Ishii N, Murata Y, Taketa K, Motoshima H, Taguchi T, Sonoda K, Kukidome D, Takuwa Y, Kawada T, Brownlee M, Nishikawa T, Araki E 2007 Statins activate peroxisome proliferator-activated receptor γ through extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent cyclooxygenase-2 expression in macrophages. Circ Res 100:1442–1451 [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Nissen SE, Topol EJ 2001 Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 286:954–959 [DOI] [PubMed] [Google Scholar]
- Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, Shoor S, Ray WA 2005 Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet 365:475–481 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.