Abstract
Direct mineralocorticoid receptor (MR) activation with deoxycorticosterone acetate has deleterious effects on the cerebral vasculature. Inhibition of 11β-hydroxysteroid dehydrogenase type II (11βHSD2) mimics the detrimental effects of elevated mineralocorticoids in the heart, but the effect of enzyme inactivation on the cerebral vasculature is unknown. Therefore, we hypothesized that systemic 11βHSD2 inhibition with carbenoxolone (CBX) would cause remodeling of the middle cerebral artery (MCA) and increase the damage caused by cerebral ischemia. Six-week-old male Sprague Dawley rats were divided into control and CBX (2.5 mg/d) + 0.9% NaCl treated. After 4 wk treatment, rats were used to assess either structure and reactivity of the MCA or the response to cerebral ischemia using the MCA occlusion technique. Cerebral damage was assessed by 2,3,5-triphenyltetrazolium chloride staining and expressed as a percentage of the hemisphere infarcted. CBX treatment increased systolic blood pressure (153.2 ± 7.3 vs. 122.1 ± 4.4 mm Hg; P < 0.05) compared with control rats. MCAs from CBX treated rats were smaller and stiffer than control MCAs over a range of intralumenal pressures, indicating inward remodeling of the vessel. CBX treatment significantly increased ischemic cerebral infarct size compared with control rats (27.1 ± 5.4% vs. 14.8 ± 4.2%; P < 0.05). These data indicate that inhibition of 11βHSD2, and, thus, disproportionate glucocorticoid activation of the MR, results in remodeling of the MCA and worsens the outcome of cerebral ischemia, further underscoring the importance of understanding the mechanism by which MR activation leads to cerebrovascular disease.
11β-Hydroxysteroid dehydrogenase inhibition increases blood pressure, altering cerebral vessel structure in a manner that increases the damaged caused by focal cerebral ischemia in rats.
Several studies have highlighted the beneficial effects of mineralocorticoid receptor (MR) antagonism in the cardiovascular system, even when aldosterone levels are not elevated (1,2,3). Cortisol and aldosterone have the same affinity for the MR (4,5,6), and cortisol circulates at much higher levels than aldosterone. In aldosterone-sensitive tissues, the enzyme 11β-hydroxysteroid dehydrogenase type II (11βHSD2) preserves MR specificity for aldosterone by converting the glucocorticoid cortisol to its inactive metabolite cortisone (corticosterone to 11-dehydrocorticosterone in rodents) (6,7). Meanwhile, 11βHSD2 has no effect on aldosterone. Conditions in which the activity of this enzyme is disrupted, such as the congenital syndrome of apparent mineralocorticoid excess (AME) or exogenous inhibition by the liquorice ingredient glycyrrhetinic acid, result in excess glucocorticoid activation of the MR despite normal aldosterone levels (7,8,9,10). Patients with AME present with symptoms of aldosterone excess such as sodium retention, increased potassium excretion, and hypertension (11,12). Importantly, complications of AME are fatal in more than 10% of these patients, with the majority of deaths resulting from stroke or cerebral hemorrhage (13).
Although cortisol gains access to the MR when 11βHSD2 activity is impaired, binding of the receptor may not be sufficient for activation. It has been suggested that the redox status of the aldosterone target cell is important for cortisol activation of the MR because 11βHSD2 requires nicotinamide adenine dinucleotide-+ for its activity (14,15); this concept has been reviewed in detail (16). Ward et al. (17) demonstrated that eplerenone inhibits constrictive remodeling of coronary arteries after angioplasty induced damage. Eplerenone increased lumen area of coronary arteries, but infusion of aldosterone did not have an effect on lumen area. This interesting finding raises the possibility that eplerenone is inhibiting the actions of cortisol rather than aldosterone during angioplasty induced injury. If cortisol becomes an MR agonist when vascular damage is present, it seems possible that some cardiovascular pathologies may have a component of disproportionate MR activation by cortisol, further underscoring the importance of understanding the cardiovascular effects of this “alternative” MR activation.
Inhibition of 11βHSD2 with the glycyrrhetinic acid derivative carbenoxolone (CBX) has mimicked the effects of excess MR activation in the heart (18). In this study, CBX treatment and direct MR activation with the mineralocorticoid deoxycorticosterone resulted in similar levels of cardiac fibrosis and increased infiltration of inflammatory markers such as osteopontin and ED1+ macrophages, and the effects of both treatments were inhibited by the specific MR antagonist eplerenone.
The outcome of cerebral ischemia and the damage that occurs are dependent on the ability of the cerebral vasculature to perfuse adequately the tissue. Dilation of collateral blood vessels near the obstruction of flow is another important factor (19,20). As hemodynamics change, as in the case of hypertension, vessel structure can change. For example, vessels can become stiffer, wall thickness may increase, whereas lumen diameter may decrease (21,22). Although the effects of 11βHSD2 inhibition in the cerebral vasculature have not been studied, there is a clear link between MR activation and cerebrovascular complications. Our laboratory has shown previously that direct activation of the MR with deoxycorticosterone acetate (DOCA) reduces lumen diameter of the middle cerebral artery (MCA) and results in a larger infarct size after induction of cerebral ischemia (23). We have also demonstrated improvement in cerebral vascular remodeling and infarct size in stroke-prone spontaneously hypertensive rats (SHRSPs) after administration of the MR antagonist spironolactone (3,24). Based on the evidence for involvement for MR activation in stroke and cerebrovascular remodeling and the effects of CBX treatment in the heart, we hypothesized that inhibition of 11βHSD with CBX would cause remodeling of the MCA and worsen the outcome of cerebral ischemia.
Materials and Methods
Animals
Six-week-old male Sprague Dawley rats were purchased from Harlan (Indianapolis, IN), and were maintained on a 12-h light, 12-h dark cycle with access to food and water ad libitum. Rats were housed in an American Association for Accreditation of Laboratory Animal Care accredited facility, and all protocols were approved by the Institutional Animal Care and Use Committee. Control rats received regular tap water, whereas a second group received the nonselective 11βHSD inhibitor CBX in the drinking water (2.5 mg/d) + 0.9% NaCl. Rats were subsequently randomly divided into separate groups for either cerebral ischemia or vessel structure and reactivity studies. Rats were treated for 4 wk with blood pressure measurements being made by tail-cuff plesythmography (Kent Scientific Corp., Torrington, CT) throughout the treatment period. After euthanization with a lethal dose of sodium pentobarbital, hearts and kidneys from each rat were weighed. An additional group of rats was treated for 48 h with CBX to assess acute effects of 11βHSD inhibition on cerebral infarct size.
MCA occlusion
The intralumenal suture model developed by Longa et al. and previously used in our laboratory (24,25,26) was used to induce cerebral ischemia. Rats were initially anesthetized with isoflurane in an induction chamber, and anesthesia was maintained with 2% isoflurane in oxygen; body temperature was maintained at 37 C. An incision was made in the top of the head to expose the skull for attachment of a laser Doppler flow probe to measure blood flow to the region supplied by the MCA. A midline incision was made to expose the carotid artery. The lingual and thyroid arteries were cauterized, and the external carotid and pterygopalatine arteries were tied off with suture. A 3-0 nylon monofilament with a rounded end was inserted into the common carotid artery. This monofilament was then advanced through the internal carotid artery to block blood flow to the MCA where it branches from the circle of Willis. MCA occlusion was verified by a decrease in flow as measured by laser Doppler. After 24 h ischemia, rats were anesthetized and decapitated, and the brain was removed, sliced into 2-mm sections, and stained with 2% 2,3,5-triphenyltetrazolium chloride to assess ischemic damage. Brains were fixed in 2% paraformaldehyde, and digital images of brain slices were taken. The percentage of infarction was determined by the following equation:
 |
where VL is the volume of the lesioned hemisphere, and VC is the volume of the control hemisphere (27).
MCA reactivity and structure assessment
MCAs were dissected from the brain after decapitation and placed in HEPES buffered physiological salt solution (PSS) (contents in mm: 119 NaCl, 4.7 KCl, 3.5 MgSO4, 10.0 HEPES, 1.18 KH2PO4, 0.40 EDTA, 5.0 glucose, and 3.7 CaCl2) on ice. Vessels were mounted on glass micropipettes in a pressure myograph (Living Systems Instrumentation, Burlington, VT) and secured using nylon suture. Only vessels that held pressure at 80 mm Hg were used for experimentation. The arteriograph was connected to a pressure servomechanism controller by a pressure transducer, allowing for pressure to be manipulated under zero-flow conditions. A video dimension analyzer connected to a monitor permitted measurement of lumen and outer diameters. After equilibration at 80 mm Hg in PSS heated to 37 C and gassed with 95% O2/5%CO2, dose-response curves to 5-hydroxytryptamine (5-HT) (10−8 to 10−5 m) and ADP (10−9 to 10−5 m) were conducted. In addition, the myogenic response was determined by measuring lumen diameter as pressure was increased by 20 mm Hg increments over a range of intralumenal pressures (0–180 mm Hg). The same measurements were made in the absence of calcium to determine passive vessel structure. From the passive data, wall thickness, circumferential wall stress, and circumferential wall strain could be extrapolated using the following equations:
 |
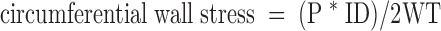 |
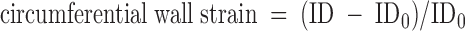 |
where ID is inner diameter, OD is outer diameter, P is intralumenal pressure, WT is wall thickness, and ID0 is inner diameter at 0 mm Hg. Individual stress-strain curves were fit with an exponential regression equation to determine the slope coefficient, or β-coefficient, an indicator of vessel stiffness.
Comparing the lumen diameters under active and passive conditions, the percentage of myogenic tone was calculated:
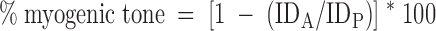 |
where IDA is inner diameter under active conditions, and IDP is inner diameter under passive conditions.
Aorta reactivity
Thoracic aortae were dissected from rats after euthanization with sodium pentobarbital and placed in ice-cold PSS (contents in mm: 130 NaCl, 4.7 KCl, 1.17 MgSO4, 1.18 KH2PO4, 0.03 EDTA, 5.5 glucose, and 1.6 CaCl2). Aortae were cleaned of adipose and connective tissues, and cut into approximately 3-mm rings. Each ring was mounted on stainless steel pins in a modified myograph system (DanishMyo Technologies, Aarhus, Sweden), and passive tension was adjusted to 36–37 mN. Vessels were allowed to equilibrate in PSS warmed to 37 C and gassed with 95% O2/5%CO2, after which a contractile response to phenylephrine (PE) (10−7 m) and dilation of acetylcholine (Ach) (10−7 m) was confirmed to ensure vessel viability and an intact endothelium. Dose-response curves to PE (10−10 to 10−5 m), 5-HT (10−10 to 10−5 m), ACh, (10−10 to 10−5 m), and sodium nitroprusside (10−10 to 10−5 m) were conducted.
Statistics
Physiological parameters, cerebral infarct size, and β-coefficients were compared using a Student’s t test. A two-way ANOVA was used to compare lumen diameter, outer diameter, wall to lumen ratio, myogenic response, myogenic tone, and the response to 5-HT between control and CBX treated rats. A P value less than 0.05 was considered statistically significant. Values are presented as mean ± sem.
Results
Physiological parameters
Summary data for several physiological parameters are shown in Table 1. Rats treated with the 11βHSD inhibitor CBX had significantly increased body weight. CBX treated rats also showed evidence of cardiac and renal hypertrophy, as indicated by increased heart-body weight and kidney-body weight. In addition, systolic blood pressure was significantly increased at the end of 4 wk CBX treatment.
Table 1.
Physiological parameters of control (n = 7–10) and CBX (n = 6–20) treated rats at the end of 4 wk treatment
| Parameter | Control | CBX |
|---|---|---|
| Body weight (g) | 304.3 ± 3.4 | 336.5 ± 8.2a |
| Heart-body weight (mg/g) | 3.71 ± 0.06 | 4.07 ± 0.15a |
| Kidney-body weight (mg/g) | 3.49 ± 0.11 | 3.89 ± 0.12a |
| Systolic blood pressure (mm Hg) | 122.1 ± 4.4 | 153.2 ± 7.3a |
Values are mean ± sem.
P < 0.05.
Cerebral infarct size
To determine the effect of 11βHSD inhibition on cerebral infarct size, control and CBX treated rats were exposed to 24 h cerebral ischemia using an intralumenal suture technique that blocks blood flow to the MCA. Damage due to cerebral ischemia was greater in CBX treated rats than control (27.1 ± 5.4% vs. 14.8 ± 4.2% CBX vs. control; P < 0.05), as indicated by the percentage of the hemisphere infarcted in Fig. 1. Chronic administration of the drug was required for this increase in infarct size. The percentage of the hemisphere infarcted in rats treated with CBX for only 48 h was not different from control rats (14.8 ± 4.6%). Importantly, the degree of occlusion of the MCA was the same in each rat, as confirmed by laser Doppler flowmetry.
Figure 1.

A, Representative brain slices after 2,3,5-triphenyltetrazolium chloride staining. Pink area indicates viable tissue, and white area indicates damaged tissue. B, Percentage of the hemisphere infarcted after 24 h cerebral ischemia in control (n = 6) and CBX (n = 12) treated rats. Values are mean ± sem. *, P < 0.05 vs. control.
Cerebral vessel structure and compliance
Passive lumen (Fig. 2A) and outer (Fig. 2B) diameters of MCAs measured under calcium-free conditions were significantly decreased in CBX treated rats (ANOVA, P < 0.05) over a range of intralumenal pressures. Specifically, lumen diameter was smaller from 40–180 mm Hg, and outer diameter was decreased from 60–180 mm Hg in CBX treated rats. In addition, the wall to lumen ratio was increased (ANOVA, P < 0.05) in the CBX group (Fig. 2C). Stress-strain curves were produced to assess compliance of the MCA in the two groups. CBX treatment resulted in a leftward shift in the stress-strain curve, indicating decreased compliance of the MCA (Fig. 3A). In addition, these rats had a significant increase in the stiffness of the MCA, as indicated by a larger β-coefficient after 11βHSD inhibition (Fig. 3B).
Figure 2.
Lumen diameter (A), outer diameter (B), and wall to lumen ratio (C) of MCAs from control (n = 7) and CBX (n = 8) treated rats. Measurements were made in calcium-free conditions over the range of intralumenal pressures. Values are mean ± sem. *, P < 0.05 vs. control.
Figure 3.
Stress-strain curves (A) and β-coefficients (B) of control (n = 6) and CBX (n = 7) treated rats. Values are mean ± sem. *, P < 0.05 vs. control.
MCA myogenic response and tone
CBX treatment altered the myogenic response of the MCA (Fig. 4A). CBX treated rats maintained a smaller lumen diameter as intralumenal pressure was increased over the range of pressures compared with control rats (ANOVA, P < 0.05). Interestingly, the force-mediated dilation appears to occur around the same pressure in both groups of rats, implying that CBX treatment does not affect the autoregulatory range in the MCA. 11βHSD inhibition also resulted in an increase in the percentage of myogenic tone in the MCA at 80 and 120 mm Hg (Fig. 4B).
Figure 4.

A, Myogenic response of MCAs from control (n = 7) and CBX (n = 6) treated rats. Lumen diameter was measured over the range of intralumenal pressures under active conditions. B, Percentage of myogenic tone of MCAs from control (n = 7) and CBX (n = 5) treated rats at 80 and 120 mm Hg. Values are mean ± sem. *, P < 0.05.
Constriction to 5-HT
The constrictor activity of the MCA in response to 5-HT was assessed by conducting a dose-response curve to 5-HT at a constant pressure of 80 mm Hg. As shown in Fig. 5, there was a significant increase in the percentage of constriction in CBX treated rats at the higher doses of 5-HT. Relaxation to ADP was also assessed in these vessels, but no differences were observed between control and CBX treated rats. Vasoreactivity to several pharmacological agents was also investigated in the aorta to determine whether 11βHSD inhibition altered the reactivity of larger vessels. Interestingly, the percentage of constriction to 5-HT compared with baseline values was also noticeably increased in aortae from CBX treated rats (59.8 ± 3.0% vs. 46.4 ± 3.3% CBX vs. control at 10−5 m 5-HT; P < 0.05) compared with the control. However, responses to PE, ACh, and sodium nitroprusside were not different between groups.
Figure 5.

Percentage of constriction to 5-HT of MCAs at 80 mm Hg from control (n = 10) and CBX (n = 7) treated rats. Values are mean ± sem. *, P < 0.05.
Discussion
A key finding of this study is that inhibition of 11βHSD2 results in increased infarct size after 24 h permanent cerebral ischemia, much like direct MR activation via DOCA (23). Also, like direct MR activation, 11βHSD2 inhibition results in inward hypertrophic remodeling and increased stiffness of the MCA. In addition, increased corticosterone access to the MR alters the myogenic and constrictor profile of the MCA to produce a vessel with heightened tone and reactivity to the vasoconstrictor 5-HT. CBX treatment resulted in cardiac and renal hypertrophy, as well as increased body weight. Although we have not explored the mechanism behind the increase in body weight, it is possible that it is partially due to fluid retention as a result in increased MR activation. The increase in body weight is too great to be explained by fluid retention alone. Another possibility is a difference in food consumption between control and CBX treated rats. Food consumption was not directly measured in this study but could be assessed in future studies. However, in a previous study using a higher dose of CBX without NaCl supplementation, there was no difference in food consumption according to preliminary measurements. Our results are consistent with the findings of Young et al. (18) because we have shown that altering pre-receptor regulation of the MR mimics direct MR activation, leading to a pathological condition in the cardiovascular system.
Human studies indicated a link between increased levels of plasma aldosterone and stroke (28,29). The role for MR activation in the pathology of stroke is further underscored by studies from our laboratory indicating that MR antagonism with spironolactone improves infarct size after cerebral ischemia (24). In our model of alternative MR activation, the cerebrovascular remodeling and heightened constriction may provide an explanation for the observed increase in infarct size. An inward, hypertrophic remodeling, increased stiffness, and increased tone can all impair the ability of a vessel to respond to ischemia. The hypertrophic remodeling likely involves an increase in vascular smooth muscle cell (VSMC) proliferation, and increased collagen deposition likely explains the increased vessel stiffness. Both of these pathways have been suggested to be downstream of MR activation.
The observed increase in infarct size cannot simply be explained by a difference in the effectiveness of the occlusion of the MCA. Laser Doppler flowmetry was used to ensure that the degree of occlusion was comparable in each rat, indicating that the worse outcome after cerebral ischemia in CBX treated rats is due to an alteration in the response to ischemia rather than variability in the technique used to induce ischemia. Of interest though, the magnitude of the increase in infarct size and decrease in MCA diameter in our model was less than what was reported in the DOCA model. Therefore, it is possible that the extent of the increase in MR activation due to CBX treatment is much less than DOCA. Importantly, the duration of CBX treatment was 2 wk less than that of DOCA. Although 4 wk CBX treatment is sufficient to induce cerebrovascular changes, a longer treatment would likely result in a more robust response. Another alternative is a difference between strains of rats because these studies have been conducted in Sprague Dawley rats, whereas the DOCA studies were done in Wistars. Interestingly, the blood pressure of the control rats in the two studies, as well as the CBX and DOCA rats, was comparable despite strain differences.
The increased 5-HT-induced constriction of the MCA in the CBX treated group may be important upon consideration of the nature of an ischemic stroke. The majority of ischemic strokes are caused by clots, and platelets are a source of 5-HT, which causes constriction of the MCA. Therefore, the heightened constriction to 5-HT could be involved in the impaired response to ischemia in CBX treated rats. In addition, our findings indicate that constriction to 5-HT is also increased in the aorta of CBX treated rats, indicating that 11βHSD inhibition alters the response to 5-HT in both conduit and resistance vessels. This alteration in vasoconstriction seems to be specific to 5-HT because constriction to PE was not different in the aorta of control and CBX treated rats. Previous studies have examined the effects of DOCA-salt hypertension on 5-HT vascular reactivity, but ours is the first to investigate whether alternative MR activation alters constriction to 5-HT. Watts et al. (30) demonstrated an increase in the constriction to 5-HT in isolated mesenteric arteries of DOCA-salt treated rats and found that this constriction was mediated by the 5-HT2B receptor. A subsequent study indicated that DOCA-salt treatment up-regulates 5-HT1B and 5-HT2B receptors in the aorta (31). Clearly, further studies are required to investigate this important constrictor pathway in the cerebral vasculature.
There has been much debate over the expression of 11βHSD2 in blood vessels. Some groups have reported expression of the type II isoform of the enzyme in both endothelial and VSMCs (32), others have reported that only 11βHSD1 is expressed in smooth muscle cells with 11βHSD2 restricted to the endothelium (33). Although it is likely that 11βHSD2 is differentially expressed in various vascular beds and vessel sizes, our results may lend support to VSMC expression of the enzyme because 11βHSD2 inhibition appears to alter the phenotype of VSMCs in our model.
It is important to note that CBX is not specific for the type II isoform of 11βHSD. This may appear to be a confounding factor when interpreting our results, however, the effect of 11βHSD1 inhibition must be considered. The role of this enzyme is to convert inactive glucocorticoids to their active counterparts. 11βHSD1 inhibition has been shown to be beneficial in models of obesity and metabolic syndrome, and is a potential therapeutic intervention for these health problems. However, it is unclear to what extent this enzyme may have been inhibited in our studies or to what extent inhibition may have affected vascular structure. However, studies using a specific 11βHSD1 inhibitor suggest that its blockade would be beneficial for the cerebral vasculature rather than deleterious.
A moderate increase in blood pressure was observed in the CBX treated rats, consistent with other studies using the use of systemic administration of CBX (18). Gomez-Sanchez and Gomez-Sanchez (34) provided evidence that both oral and intracerebroventricular (ICV) infusion of CBX increase blood pressure, with ICV infusion rapidly increasing pressure but to a lesser extent than what we report. Importantly, the increase in pressure by oral or ICV CBX was blocked by central infusion of the MR antagonist RU28318 at a dose that does not affect pressure when given sc. These results imply a role for mineralocorticoids in the central control of blood pressure. Although there are several possibilities for mechanisms involved in the CBX induced increased in blood pressure, including direct actions in the kidney to increase sodium reabsorption as well as increased activation of the sympathetic nervous system and direct effects on the vasculature, the exact mechanism is not clearly defined. However, interpretation of the increase in blood pressure in this study is further complicated by a study by Leshchenko et al. (35), in which HPLC was used to demonstrate that CBX does not cross the blood-brain barrier. This would imply that systemic administration of CBX vs. ICV administration may result in differential MR activation and, thus, differential outcomes on parameters such as blood pressure. It is also important to note that blood pressure was measured by tail-cuff plethysmography in our study. Although it is possible that our measurements are not as accurate as telemetry measurements, a previous study from our laboratory provided evidence that the Kent Scientific tail-cuff system used in our laboratory provides readings that are comparable to telemetry measurements (3).
It is possible that the observed effects are mediated strictly by the increase in blood pressure. However, in a recent study from our laboratory, MR antagonism prevented the inward remodeling in male SHRSPs and improved the outcome of cerebral ischemia without affecting blood pressure (3). In addition, in the study investigating the cardiac effects of CBX treatment, MR antagonism with eplerenone prevented the deleterious effects of CBX treatment without affecting blood pressure (18). Although the aims of this study were different from ours, these results support the blood pressure independence of our findings. Importantly, the increased risk of stroke in patients with primary hyperaldosteronism is blood pressure independent (36).
The Randomized ALdactone Evaluation Study and EPlerenone neuroHormonal Efficacy and SUrvival Study provided evidence that MR antagonism can have beneficial effects on the morbidity and mortality associated with cardiac failure (1,2). Interestingly, the patients in these trials did not have elevated aldosterone levels, yet benefited from MR antagonism. Previous studies have shown the protective effects of MR antagonism on ischemic cerebral infarct size and vessel structure in the SHRSP model (3,24). Aldosterone levels are not increased in the SHRSPs, suggesting the possibility that a steroid other than aldosterone is binding to and activating the MR, perhaps because of the increased oxidant load in these rats. Together with our findings, these studies suggest that patients who are at risk for disproportionate MR activation by glucocorticoids due to disruption of 11βHSD2 activity may have an improvement in overall cardiovascular health upon treatment with an MR antagonist, including a reduction in the risk of or damage caused by ischemic stroke. With stroke ranking as the third leading cause of death in the United States, these studies are clinically relevant and underscore the potential therapeutic effect of MR antagonists in patients who are at risk for stroke.
Footnotes
This work was supported by National Heart Lung and Blood Institute Grant HL077385 and American Heart Association Grant 0840122N (to A.M.D.).
Disclosure Summary: J.M.O. has nothing to declare. A.M.D. has received grant funding from the National Institutes of Health (2004–2008), American Diabetes Association (2007–2010), and American Heart Association (2008–2012).
First Published Online October 9, 2008
Abbreviations: Ach, Acetylcholine; AME, apparent mineralocorticoid excess; CBX, carbenoxolone; DOCA, deoxycorticosterone acetate; 11βHSD2, 11β-hydroxysteroid dehydrogenase type II; 5-HT, 5-hydroxytryptamine; ICV, intracerebroventricular; MCA, middle cerebral artery; MR, mineralocorticoid receptor; PE, phenylephrine; PSS, physiological salt solution; SHRSP, stroke-prone spontaneously hypertensive rat; VSMC, vascular smooth muscle cell.
References
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M 2003 Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348:1309–1321 [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J 1999 The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341:709–717 [DOI] [PubMed] [Google Scholar]
- Rigsby CS, Pollock DM, Dorrance AM 2007 Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res 73:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM 1987 Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237:268–275 [DOI] [PubMed] [Google Scholar]
- Krozowski ZS, Funder JW 1983 Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc Natl Acad Sci USA 80:6056–6060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder JW, Pearce PT, Smith R, Smith AI 1988 Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science 242:583–585 [DOI] [PubMed] [Google Scholar]
- Stewart PM, Wallace AM, Valentino R, Burt D, Shackleton CH, Edwards CR 1987 Mineralocorticoid activity of liquorice: 11-β-hydroxysteroid dehydrogenase deficiency comes of age. Lancet 2:821–824 [DOI] [PubMed] [Google Scholar]
- Kuhnle U, Lewicka S, Fuller PJ 2004 Endocrine disorders of sodium regulation. Role of adrenal steroids in genetic defects causing sodium loss or sodium retention. Horm Res 61:68–83 [DOI] [PubMed] [Google Scholar]
- Lifton RP, Gharavi AG, Geller DS 2001 Molecular mechanisms of human hypertension. Cell 104:545–556 [DOI] [PubMed] [Google Scholar]
- Edwards CR, Stewart PM, Burt D, Brett L, McIntyre MA, Sutanto WS, de Kloet ER, Monder C 1988 Localisation of 11 β-hydroxysteroid dehydrogenase—tissue specific protector of the mineralocorticoid receptor. Lancet 2:986–989 [DOI] [PubMed] [Google Scholar]
- New MI, Levine LS, Biglieri EG, Pareira J, Ulick S 1977 Evidence for an unidentified steroid in a child with apparent mineralocorticoid hypertension. J Clin Endocrinol Metab 44:924–933 [DOI] [PubMed] [Google Scholar]
- Stewart PM, Corrie JE, Shackleton CH, Edwards CR 1988 Syndrome of apparent mineralocorticoid excess. A defect in the cortisol-cortisone shuttle. J Clin Invest 82:340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantero F, Palermo M, Petrelli MD, Tedde R, Stewart PM, Shackleton CH 1996 Apparent mineralocorticoid excess: type I and type II. Steroids 61:193–196 [DOI] [PubMed] [Google Scholar]
- Albiston AL, Obeyesekere VR, Smith RE, Krozowski ZS 1994 Cloning and tissue distribution of the human 11 β-hydroxysteroid dehydrogenase type 2 enzyme. Mol Cell Endocrinol 105:R11–R17 [DOI] [PubMed] [Google Scholar]
- Rusvai E, Naray-Fejes-Toth A 1993 A new isoform of 11 β-hydroxysteroid dehydrogenase in aldosterone target cells. J Biol Chem 268:10717–10720 [PubMed] [Google Scholar]
- Funder JW 2005 RALES, EPHESUS and redox. J Steroid Biochem Mol Biol 93:121–125 [DOI] [PubMed] [Google Scholar]
- Ward MR, Kanellakis P, Ramsey D, Funder J, Bobik A 2001 Eplerenone suppresses constrictive remodeling and collagen accumulation after angioplasty in porcine coronary arteries. Circulation 104:467–472 [DOI] [PubMed] [Google Scholar]
- Young MJ, Moussa L, Dilley R, Funder JW 2003 Early inflammatory responses in experimental cardiac hypertrophy and fibrosis: effects of 11 β-hydroxysteroid dehydrogenase inactivation. Endocrinology 144:1121–1125 [DOI] [PubMed] [Google Scholar]
- Coyle P 1989 Altered cerebral collaterals and protection from infarction. In: Hartmann A, Kuschinsky W, eds. Cerebral ischemia and calcium. Heidelberg, Germany: Springer-Verlag; 69–78 [Google Scholar]
- Coyle P, Heistad DD 1991 Development of collaterals in the cerebral circulation. Blood Vessels 28:183–189 [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Heistad DD 1989 Remodeling of cerebral arterioles in chronic hypertension. Hypertension 13:968–972 [DOI] [PubMed] [Google Scholar]
- Heistad DD, Mayhan WG, Coyle P, Baumbach GL 1990 Impaired dilatation of cerebral arterioles in chronic hypertension. Blood Vessels 27:258–262 [DOI] [PubMed] [Google Scholar]
- Dorrance AM, Rupp NC, Nogueira EF 2006 Mineralocorticoid receptor activation causes cerebral vessel remodeling and exacerbates the damage caused by cerebral ischemia. Hypertension 47:590–595 [DOI] [PubMed] [Google Scholar]
- Dorrance AM, Osborn HL, Grekin R, Webb RC 2001 Spironolactone reduces cerebral infarct size and EGF-receptor mRNA in stroke-prone rats. Am J Physiol Regul Integr Comp Physiol 281:R944–R950 [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R 1989 Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91 [DOI] [PubMed] [Google Scholar]
- Dorrance AM, Pollock DM, Romanko OP, Stepp DW 2007 A high-potassium diet reduces infarct size and improves vascular structure in hypertensive rats. Am J Physiol Regul Integr Comp Physiol 292:R415–R422 [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR 1990 A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10:290–293 [DOI] [PubMed] [Google Scholar]
- Litchfield WR, Anderson BF, Weiss RJ, Lifton RP, Dluhy RG 1998 Intracranial aneurysm and hemorrhagic stroke in glucocorticoid-remediable aldosteronism. Hypertension 31:445–450 [DOI] [PubMed] [Google Scholar]
- McMahon GT, Dluhy RG 2004 Glucocorticoid-remediable aldosteronism. Cardiol Rev 12:44–48 [DOI] [PubMed] [Google Scholar]
- Watts SW, Gilbert L, Webb RC 1995 5-Hydroxytryptamine2B receptor mediates contraction in the mesenteric artery of mineralocorticoid hypertensive rats. Hypertension 26(6 Pt 2):1056–1059 [DOI] [PubMed] [Google Scholar]
- Banes AK, Watts SW 2002 Upregulation of arterial serotonin 1B and 2B receptors in deoxycorticosterone acetate-salt hypertension. Hypertension 39(2 Pt 2):394–398 [DOI] [PubMed] [Google Scholar]
- Alzamora R, Michea L, Marusic ET 2000 Role of 11β-hydroxysteroid dehydrogenase in nongenomic aldosterone effects in human arteries. Hypertension 35:1099–1104 [DOI] [PubMed] [Google Scholar]
- Christy C, Hadoke PW, Paterson JM, Mullins JJ, Seckl JR, Walker BR 2003 11β-hydroxysteroid dehydrogenase type 2 in mouse aorta: localization and influence on response to glucocorticoids. Hypertension 42:580–587 [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Gomez-Sanchez CE 1992 Central hypertensinogenic effects of glycyrrhizic acid and carbenoxolone. Am J Physiol 263(6 Pt 1):E1125–E1130 [DOI] [PubMed] [Google Scholar]
- Leshchenko Y, Likhodii S, Yue W, Burnham WM, Perez Velazquez JL 2006 Carbenoxolone does not cross the blood brain barrier: an HPLC study. BMC Neurosci 7:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda R, Matsubara T, Miyamori I, Hatakeyama H, Morise T 1995 Vascular complications in patients with aldosterone producing adenoma in Japan: comparative study with essential hypertension. The Research Committee of Disorders of Adrenal Hormones in Japan. J Endocrinol Invest 18:370–373 [DOI] [PubMed] [Google Scholar]




