Abstract
Extrathymic induction of regulatory T (T reg) cells is essential to the regulation of effector T cell responses in the periphery. In addition to Foxp3, T reg cell expression of suppressive cytokines, such as IL-10, is essential for peripheral tolerance, particularly in the intestines. TGF-β has been shown to induce expression of Foxp3 as well as IL10 and the vitamin A metabolite; all-trans retinoic acid (RA [at-RA]) has been found to enhance the former. We report that in contrast to its enhancement of TGF-β–mediated Foxp3 induction, at-RA potently inhibits the TGF-β–mediated induction of Il10 in naive CD4 T cells. Thus, mucosal DC subsets that are active producers of at-RA inhibit induction of Il10 in naive CD4 T cells while promoting induction of Foxp3. Accordingly, mice with vitamin A deficiency have increased numbers of IL-10–competent T reg cells. Activation of DCs by certain Toll-like receptors (TLRs), particularly TLR9, suppresses T cell induction of Foxp3 and enables induction of Il10. Collectively, our data indicate that at-RA has reciprocal effects on the induction of Foxp3 and Il10 in developing CD4+ T reg cells and suggest that TLR9-dependent inhibition of at-RA production by antigen-presenting cells might represent one mechanism to promote the development of IL-10–expressing T cells.
An important mechanism used by the adaptive immune system to limit effector responses is active suppression of immune cells by subsets of CD4 T cells, which are generally classified as regulatory T (T reg) cells (1). Among the molecules commonly associated with T reg cell suppressive activity are the transcription factor Foxp3 and the immunosuppressive cytokine IL-10. Expression of Foxp3 is induced in a subset of CD4 T cells generated during thymic differentiation, which are commonly referred to as natural T reg cells but can also be induced extrathymically to produce so-called adaptive or induced T reg cells (2). It is now clear that, at a minimum, Foxp3 is essential for amplifying and sustaining a transcriptional program that is essential for T reg cell function (3).
IL-10 is expressed by numerous hematopoietic cells, including CD4 T cells, and is typically linked to immunosuppression (4). Elevated expression of IL-10 has been used as a defining feature of a subset of T reg cells referred to as T reg type 1 (Tr1), which do not express Foxp3 (5, 6). However, on a population level, Il10 is one of the genes expressed by Foxp3+ T reg cells and is thus part of the T reg cell transcriptional signature (7). Yet in the steady-state in mice, only in intestinal tissues are the majority of Foxp3-expressing cells competent to coexpress IL-10 (8). Moreover, the intestines, as well as all peripheral lymphoid tissues, harbor CD4+Foxp3− cells that are competent for IL-10 expression and do not express effector cytokines, which is consistent with a Tr1 phenotype (8, 9). Additionally, IL-10–expressing cells develop in association with every effector CD4 T cell subset yet described, apparently as an intrinsic Foxp3-independent mechanism of effector T cell regulation (10–13). Thus, although CD4 T cells with regulatory function can coexpress Foxp3 and IL-10, the expression of these two molecules is not always linked, and IL-10 represents a cytokine whose expression appears to be universal among subsets of both regulatory and effector CD4 T cell lineages (4, 14).
TGF-β is important for the peripheral induction of both Foxp3 and Il10 (15, 16). Recently, the vitamin A metabolite all-trans retinoic acid (RA [at-RA]) has been shown to be a cofactor that enhances TGF-β–mediated induction of Foxp3 (17–20). However, a possible role for RA in the TGF-β–mediated induction of IL-10 has not been explored. Based on the elevated production of at-RA by mucosal DCs (21), it is reasonable that at-RA might also participate in the differentiation of IL-10–producing T reg cells that are enriched in the intestines. Indeed, the Il10 locus is known to harbor at least one RA-responsive element (22). However, it has also been reported that the absence of dietary vitamin A enhances the development of IL-10–producing Th2 cells while reducing Th1 development (23), raising the possibility that at-RA might, in fact, down-regulate transcription of Il10. Furthermore, induction of IL-10 has been linked to the development of Th17 cells (13, 24) which is antagonized by RA (17). Because RA receptor (RAR) agonists are under consideration as promising therapeutic agents in oral tolerance induction, it is critical to understand the effects of this signaling pathway on regulation of IL-10, a nonredundant regulator of gut immunity.
In addition to IL-10, the role of Toll-like receptor (TLR) pathways in limiting deleterious responses to the commensal flora has been established. Mice with a targeted deletion of the Il10 gene develop spontaneous colitis, but mice that are doubly deficient for Il10 and the gene encoding MyD88, which is essential for most TLR signaling, do not (25). Additionally, mice in which IL-10 deficiency is restricted to CD4 T cells also develop colitis resembling that seen in complete IL-10 deficiency (26). Thus, T cell–derived IL-10 is essential to quell unwanted TLR-dependent responses to commensal antigens. We and others have shown that TGF-β can mediate the induction of IL-10 in CD4 T cells both in vitro and in vivo (8, 16), but it is unknown whether TLR-dependent activation of the innate immune system can contribute to this inductive pathway.
In a study originally designed to examine whether conditioning of APCs by TLR activation enhanced their ability to promote expression of Il10 in naive CD4 T cells, we observed that in the presence of TGF-β, CpG prestimulation rendered DCs potent inducers of Il10. Our efforts to elucidate the mechanism whereby this occurs revealed that in contrast to its ability to enhance TGF-β–mediated induction of Foxp3, at-RA potently inhibits the induction of Il10 transcription in CD4 T cells while having no detectable effects on posttranscriptional regulation of Il10. Thus, splenic, but not mesenteric LN (MLN) and intestinal lamina propria DC, supported the TGF-β–mediated induction of Il10 in CD4 T cells. However, prestimulation of MLN DC with CpG rendered them capable of inducing Il10 while limiting their induction of Foxp3 through combined induction of IL-6 and inhibition of synthesis and/or secretion of at-RA. Interestingly, the CD103− subset of MLN DC, which has been shown to be less permissive to Foxp3 induction (19), also inhibited Il10 induction in an at-RA–dependent manner. Collectively, our data demonstrate differential effects of at-RA on the induction of Foxp3 and Il10 in naive CD4 T cells, and they indicate that TLR ligands can regulate the reciprocal expression of these genes both by promoting proinflammatory cytokine production and possibly inhibiting production of ligands that activate the RAR.
RESULTS
CpG-activated DCs promote the differentiation of Il10-expressing T cells
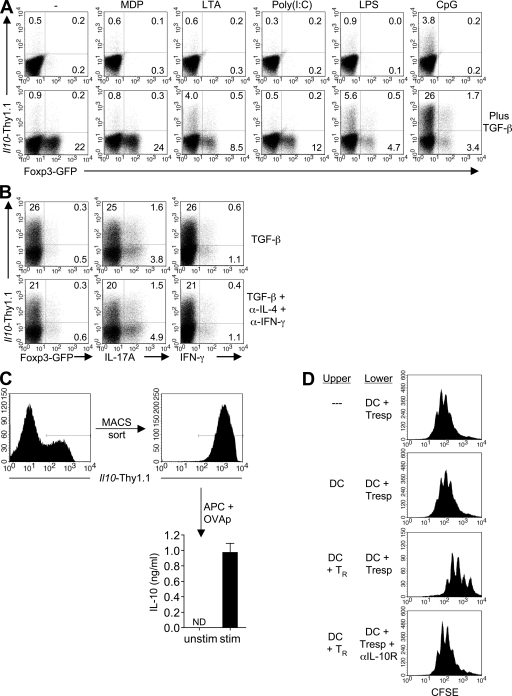
The ability of DCs activated by distinct pathogen-associated molecular patterns to induce expression of Foxp3 and/or Il10 in antigen-activated naive T cells was examined. BM-derived DCs (BMDCs) were activated overnight with the TLR ligands lipoteichoic acid (LTA), poly I:C, LPS or CpG (CpG oligodeoxynucleotide), or the nucleotide oligomerization domain (NOD) 2 agonist muramyl dipeptide (MDP) before use as APCs in cocultures with CD4 T cells (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20080950/DC1). CD4 T cells were isolated from OT-II TCR transgenic mice that had been crossed with the previously described 10BiT (Il10.Thy1.1 BAC-In Transgenic) (8) and Foxp3gfp (27) dual-reporter mice (10BiT.Foxp3gfp.OT-II; [reference 8]). These mice harbor two reporter transgenes: one in which Thy1.1 (CD90.1) is under the control of the mouse Il10 promoter, and the other in which the gene encoding GFP has been knocked into the Foxp3 gene and is expressed as a fusion protein. Notably, the Thy1.1 reporter controlled by the 10BiT transgene rapidly identifies all cells that have activated Il10 gene transcription with high fidelity and sensitivity (8), including cells with active IL-10 production from endogenous Il10 alleles as well as those poised for IL-10 production that is restrained by a posttranscriptional block on translation of Il10 transcripts (unpublished data) (8). The presence of both reporter transgenes has no adverse effects on the expression of either transgene when compared with singly transgenic mice, nor does either transgene perturb normal levels of IL-10 or Foxp3 (unpublished data) (8, 27). As such, these mice have been used previously to isolate polyclonal T reg cell subsets defined by coordinate or differential expression of Il10 and Foxp3 that develop in vivo in the steady state (8). In the present study, TCR transgenic cells from dual-reporter mice are used to track antigen-specific induction of both IL10 and Foxp3 by detection of Thy1.1 and GFP, respectively. For all experiments, isolated CD4 T cells were sorted to enrich the naive CD44loGFP−Thy1.1− fraction before stimulation (Fig. S1 B).
There was no appreciable induction of GFP (Foxp3) in T cells activated by any of the BMDC populations and only a modest, but reproducible, induction of Thy1.1 (Il10) in CD4 T cells stimulated with CpG-activated BMDC (Fig. 1 A, top). However, in the presence of exogenous TGF-β, unactivated, MDP-activated, and Poly I:C-activated BMDCs induced expression of GFP by a substantial fraction of T cells (22, 24, and 12%, respectively) without appreciable induction of Thy1.1 (Fig. 1 A, bottom). In contrast, LTA- and LPS-activated BMDCs induced a more limited fraction of GFP (Foxp3)+ cells (8.5 and 4.7%, respectively) but also induced an appreciable fraction of Thy1.1 (Il10)+ cells (4.0 and 5.6%, respectively). Interestingly, CpG-activated BMDCs promoted expression of Thy1.1 by a significantly higher fraction of CD4 T cells (>25%) with limited induction of GFP (<4%). Notably, GFP and Thy1.1 tended toward differential expression by separate fractions of T cells, although a small fraction of cells that expressed both was observed under some conditions. Collectively, these results indicate that activation of DCs by distinct TLR/NOD ligands promotes differential expression of Foxp3 and IL-10 by naive T cells stimulated in the presence of TGF-β, with extremes of expression induced by unactivated DCs or DCs activated via TLR9. Thus, CpG-activated DCs promote preferential induction of IL-10 relative to Foxp3.
Figure 1.
The combination of TGF-β– and CpG-activated BMDC induces IL-10–expressing CD4 T cells. (A) 105 D10 BMDC that were either left unactivated or preactivated with the indicated agonists were used in combination with 1 µg/ml OVA peptide to stimulate 5 × 105 naive CD4 T cells from 10BiT.Foxp3gfp.OT-II mice, in the absence (top) or presence (bottom) of 5 ng/ml of recombinant human TGF-β1. On day 3, CD4 T cells were examined for expression of Thy1.1 and GFP. Numbers represent the percentage of CD4 T cells in the respective quadrants. (B) Naive CD4 T cells were stimulated as described in A with CpG-BMDC in the presence of TGF-β only or TGF-β plus anti–IFN-γ and anti–IL-4 (each at a final concentration of 10 µg/ml). On day 5, cells were restimulated for 5 h with PMA/ionomycin in the presence of brefeldin A, and viable CD4 T cells were analyzed for expression of Thy1.1, Foxp3, IL-17A, and IFN-γ. (C) Day-5 Thy1.1+ T cells (induced in the presence of CpG-BMDC and TGF-β) were MACS sorted and cultured for 24 h with Il10−/− splenic DC in the absence (−) or presence (+) of 1 µg/ml OVA peptide. IL-10 protein in culture supernatants was quantitated by cytometric bead array. Mean cytokine concentrations ±SEM are shown. (ND, not detected). (D) 5 × 105 CFSE-labeled OT-II CD4 T cells were stimulated with 1.0 µg/ml OVA peptide and 105 IL-10–deficient splenic DC in the lower chamber of a transwell plate. In some cases, an equal number of DC or DC plus 5 × 105 day-5 Thy1.1+ T cells (differentiated as in C) were similarly activated in the upper chamber. Where indicated, anti–IL-10R antibody was added to the lower chamber at a final concentration of 10 µg/ml. After 4 d, cells were harvested from the bottom chamber and the CFSE profile of the CD4 T cells was analyzed by FACS. Numbers in A and B indicate the percentages of CD4 T cells in the given quadrants. All data are representative of at least two experiments.
Considering the established role of TGF-β in promoting Th17 development, particularly in the presence of certain proinflammatory cytokines (28–30), we examined the Thy1.1-expressing T cells for the expression of IL-17. Importantly, we saw very little expression of IL-17 by CD4 T cells activated with CpG-BMDC in the presence of TGF-β, even when blocking antibodies against IFN-γ and IL-4 were included (Fig. 1 B). In fact, the majority of IL-17+ cells were negative for the Thy1.1 reporter molecule. Moreover, we observed little or no production of IFN-γ or IL-4, suggesting that the IL-10–expressing cells were distinct from defined lineages of effector CD4 T cells.
Although we observed relatively modest expression of intracellular IL-10 after 5 h of restimulation with PMA and ionomycin (unpublished data), messenger RNA (mRNA) analysis indicated that Il10 transcripts were comparably induced in CpG-BMDC–activated T cells isolated from both WT and reporter transgenic mice, and, as reflected in enhanced Thy1.1 reporter expression, Il10 mRNA levels were substantially increased by the addition of TGF-β (Fig. 1 A and Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20080950/DC1). Accordingly, antigenic restimulation of TGF-β plus CpG-BMDC–induced Thy1.1+ T cells for 24 h resulted in the accumulation of IL-10 protein in culture supernatants (Fig. 1 C). This is consistent with previous reports that have demonstrated discordance of kinetics of induction of Il10 transcript and protein after activation as a result of posttranscriptional regulation of Il10 expression (31–33). As previously reported (8), sorted Thy1.1− cells failed to produce IL-10. To test the functional activity of Thy1.1+ T cells induced in the presence of CpG-BMDCs and TGF-β, induced Thy1.1+ T cells were stimulated with Il10-deficient splenic DCs plus antigen in the upper chamber of a transwell system, and CFSE-labeled naive responder T cells were similarly activated in the lower chamber. After 4 d, each group of responder T cells had undergone cell division. However, when Thy1.1+ T cells were placed in the upper chamber, the proliferation of responder cells was significantly reduced, and this suppression was reversed in the presence of an IL-10 receptor-blocking antibody (Fig. 1 D). These data, coupled with the lack of significant expression of Foxp3 (Fig. 1 A) or effector cytokines (Fig. 1 B), suggest that the combination of CpG-BMDC and TGF-β induce a T cell population with characteristics of Tr1 cells.
Induction of Il10 by CpG-activated BMDCs is dependent on MyD88 signaling but independent of inducible costimulator (ICOS)–ICOS ligand (ICOSL) interactions
Activation of TLR9 induces a signaling cascade that requires the adaptor molecule MyD88. In addition, it has been reported that maturation of plasmacytoid DC with the TLR9 agonist induces stable expression of the ligand for the ICOS (ICOSL), which can then promote the differentiation of IL-10–expressing Tr1-like cells (34). In the presence of BMDCs deficient for MyD88, CpG-mediated induction of Thy1.1 was inhibited, whereas GFP expression was increased, most likely as a result of diminished production of cytokines such as IL-6 which inhibit the induction of Foxp3 (30) (Fig. 2 A). Therefore, in this system, the IL-10–inducing effect of the CpG stimulation is a result of MyD88-dependent TLR signaling in BMDCs and, importantly, not a result of stimulation of the CD4 T cells by residual CpG. However, in the presence of BMDCs deficient in ICOSL, the frequency of Thy1.1, as well as GFP, resembled that observed with WT CpG-activated BMDC in the presence of TGF-β only (Fig. 2 A), indicating that ICOS signaling is dispensable for the induction of Il10 under these conditions.
Figure 2.
CpG-BMDC–mediated induction of IL-10 in CD4 T cells is Myd88 and ICOSL independent but IL-6 and IL-21 dependent. (A) Naive T cells were activated on CpG-BMDC from WT, Myd88−/−, or Icosl−/− mice in the presence of TGF-β and analyzed on day 3 for expression of Thy1.1 and GFP. (B) CD4 T cells were cultured with WT CpG-BMDC plus TGF-β in the presence of the additives indicated and analyzed on day 3 for expression of Thy1.1 and GFP. (C) D10 BMDC (left) were left unstimulated or stimulated for 24 h with the indicated TLR/NOD agonists. MACS purified CD4+Thy1.1+ cells (right), induced in the presence of CpG-BMDC plus TGF-β, were cultured for 24 h with Il10−/− splenic DC in the absence or presence of OVA peptide. IL-6 was quantified by cytometric bead array and IL-21 by ELISA. Mean cytokine concentrations ±SEM are shown (ND, not detected; *, P < 0.05; ***, P < 0.001). All data are representative of at least three experiments.
Induction of Il10 by CpG-activated BMDCs is IL-6 and IL-21 dependent but IL-27 independent
Activation of DCs via TLR9 culminates in the expression of numerous cytokines, including type 1 IFNs. In addition, studies have shown that IL-12 can induce expression of IL-10 in developing Th1 cells and, more recently, that IL-27 can induce expression of IL-10 in effector T cells (13, 35, 36). It was also reported that TGF-β and IL-6 inhibit, whereas IL-23 enhances, the pathogenic potential of Th17 cells and that the reduced pathogenicity can be reversed by IL-10 receptor blockade (24). Accordingly, we interrogated the possible roles of type 1 IFNs, IL-12, and IL-27 in the CpG-mediated induction of Il10 as well as the ability of IL-23 to inhibit this process. The addition of blocking antibodies to the IFN-α/β receptor (IFN-α/βR1) or to the p40 subunit of IL-12, IL-27, or recombinant IL-23 did not significantly diminish expression of Thy1.1 (Fig. 2 B). Thus, the CpG-mediated induction of Il10 is independent of type 1 IFNs, IL-12, and IL-27 and is not blocked by the addition of IL-23.
In the presence of TGF-β, IL-6 promotes the expression of IL-17 in T cells (13, 24). We therefore examined the possible contribution of IL-6 to the CpG-mediated induction of Thy1.1 by stimulating naive T cells with CpG-activated BMDC in the presence of TGF-β and an IL-6–neutralizing antibody (Fig. 2 B, bottom). Neutralization of IL-6 inhibited the induction of Thy1.1 and, as expected, promoted increased expression of GFP. There was a similar inhibition of Thy1.1 induction by IL-21 neutralization, suggesting that, similar to TGF-β–mediated induction of IL-17, CpG-BMDC and TGF-β cooperatively induce Il10 via a mechanism that is dependent on IL-6 and IL-21.
Given the critical roles for IL-6 and IL-21 in the CpG-mediated induction of IL-10, we examined whether expression of these two cytokines was a distinguishing feature of CpG-activated BMDCs compared with BMDCs activated with other TLR/NOD ligands. BMDCs were stimulated with the previous panel of pathogen-associated molecular patterns, and the production of IL-6 and IL-21 was quantitated. As expected, CpG-activated BMDCs produced a substantial quantity of IL-6 (17.5 ± 0.4 ng/ml) compared with unactivated D10 BMDC (Fig. 2 C, left). However, LPS-activated BMDCs produced more than twice as much IL-6 as their CpG-stimulated counterparts (38.2 ± 1.3 ng/ml). BMDCs activated with LTA and poly I:C also produced readily detectable quantities of IL-6 (4.1 ± 0.2 and 2.1 ± 0.1 ng/ml, respectively), albeit substantially less than those of CpG- and LPS-BMDCs. Interestingly, sorted CpG-induced Thy1.1+ cells produced limited amounts of IL-6 upon restimulation (0.6 ± 0.0 ng/ml). All BMDC populations were negative for IL-21 (unpublished data). However, upon antigenic restimulation, sorted Thy1.1+ cells induced in the presence of CpG-BMDC produced a significant amount of IL-21 (1.3 ± 0.1 ng/ml), suggesting that T cell–derived IL-21 functions in an autocrine manner to induce T cell expression of Il10 (Fig. 2 C, right). Collectively, these data establish a parallel between induction of Il10 and Il17 in CD4 T cells, where IL-6 induces early expression of IL-21, which then functions in an autocrine manner to promote Il10 gene expression. However, the elevated levels of IL-6 produced by other activated BMDCs, particularly LPS-activated BMDCs, suggested that IL-6, and/or the subsequent induction of IL-21, could not solely account for the differential induction of Il10 by CpG-activated BMDCs in comparison to unactivated BMDCs or BMDCs activated with other TLR/NOD agonists.
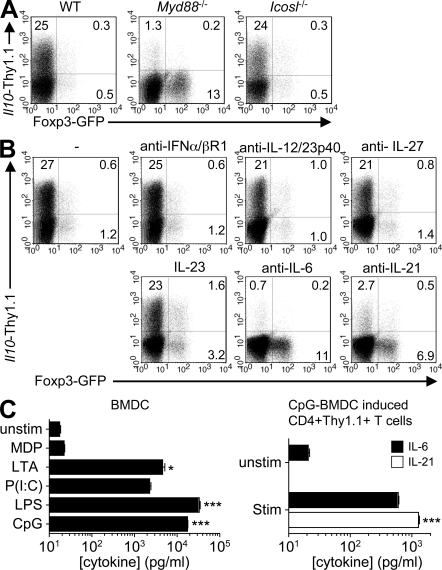
RA inhibits the induction of Il10 in CD4 T cells
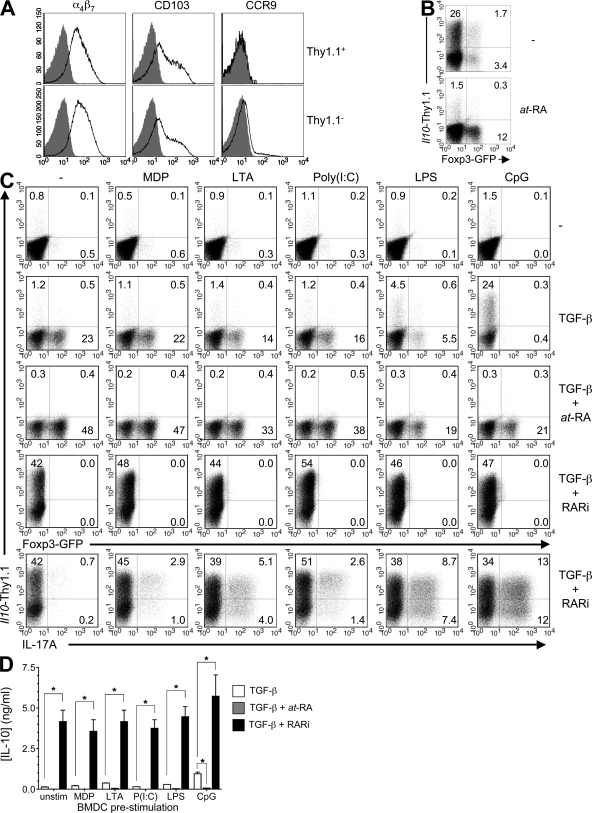
Our group and others have previously demonstrated substantial enrichment of IL-10–expressing T cells in intestinal tissues (8, 9, 37). Examination of the CpG-BMDC–induced Thy1.1-expressing cells revealed elevated expression of the gut-homing receptors α4β7 and CD103 but, importantly, not CCR9 (Fig. 3 A). Expression of CCR9 can be induced by at-RA (21), which also enhances TGF-β–mediated induction of Foxp3-expressing T reg cells from naive precursors. Considering that a significant fraction of intestinal T cells express Foxp3 and/or IL-10, we reasoned that perhaps at-RA also contributed to the observed induction of Il10 but that (CpG-induced) IL-6 actively inhibited the induction of Foxp3 under these conditions. Therefore, we examined a possible role for at-RA in the TGF-β–mediated induction of IL-10 in the presence of CpG-BMDC. Surprisingly, the addition of at-RA in the presence of TGF-β, although resulting in increased expression of GFP and CCR9, completely suppressed the induction of Thy1.1 (Fig. 3 B and not depicted).
Figure 3.
at-RA inhibits TGF-β–mediated induction of IL-10 in CD4 T cells. (A) Analysis of gut-homing receptor expression on CD4 T cells activated on CpG-BMDC in the presence of TGF-β. Shaded histograms, isotype control; open histograms, antibody to indicated marker. (B) Naive CD4 T cells were stimulated with CpG-BMDC and TGF-β only or TGF-β plus 100 nM at-RA for 3 d before being analyzed for expression of GFP and Thy1.1. (C) Naive CD4 T cells were stimulated on unactivated or preactivated BMDC alone or in the presence of TGF-β only, TGF-β plus 100 nM at-RA, or TGF-β plus 1 µM LE540. On day 5, cells were restimulated for 5 h with D10 BMDC and 1 µg/ml OVA peptide in the presence of brefeldin A and analyzed for expression of Thy1.1, GFP, and IL-17A. All FACS plots are gated on CD4 T cells and numbers represent the percentages of cells in the respective quadrants. (D) The polarized T cell populations were restimulated on day 5 with anti-CD3 and anti-CD28 antibodies for 24 h before the IL-10 content of culture supernatants was determined by cytometric bead array. Mean cytokine concentrations ± SEM are shown. *, P < 0.05. All data are representative of at least three experiments.
In view of this, we next examined whether the presence of at-RA could account for the inability of BMDC stimulated with other agonists to induce expression of IL-10. Naive T cells were stimulated with unactivated BMDCs or BMDCs activated with the various agonists in the absence or presence of TGF-β plus at-RA or the RAR inhibitor (RARi) LE540, which inhibits RAR-mediated transcriptional activation. In accordance with previous reports (17–20), addition of at-RA increased the frequency of GFP+ cells, whereas inhibition of at-RA completely inhibited induction of GFP under all conditions examined (Fig. 3 C, rows 3 and 4, respectively), suggesting that at-RA does not just enhance but is absolutely required for TGF-β–mediated induction of Foxp3. Moreover, because the precursor population in all experiments was FACS-sorted CD44lo naive T cells, the effect of RA was not a result of suppression of the activity of CD44hi memory cells as was recently suggested (38). In contrast, inhibition of RA signaling by LE540 resulted in potent induction of Thy1.1 expression in 40–60% of all T cells, irrespective of the activation state of the BMDC or the agonist used for preactivation (Fig. 3 C, fourth row). In addition, consistent with previous findings (17), inhibition of RAR in the presence of TGF-β resulted in enhanced expression of IL-17A (Fig. 3 C, bottom), which correlated with the levels of IL-6 produced by the respective BMDC subsets. Importantly, in the presence of unactivated BMDCs whose IL-6 output was just at the limit of detection (20 pg/ml; Fig. 2 C, left), there was minimal induction of IL-17 (Fig. 3 C, bottom), demonstrating that induction of Il10 in the absence of RAR signaling is not merely a byproduct of Th17 differentiation. Accordingly, in an APC-free system with the RAR antagonist and a low dose of IL-6 (100 pg/ml), we observed induction of Il10 but minimal expression of IL-17 despite increasing doses of TGF-β (Fig. S3, top, available at http://www.jem.org/cgi/content/full/jem.20080950/DC1). However, at a 100-fold higher dose of IL-6, induction of both Il10 and IL-17 was observed, both of which increased with increasing doses of TGF-β (Fig. S3, bottom). Thus, in the absence of at-RA signaling, TGF-β favors induction of Il10 in the presence of low doses of IL-6, whereas IL-17 is induced by higher doses of IL-6.
We then quantified the IL-10 protein output of CD4 T cells stimulated with the various BMDC subsets in the presence of TGF-β, TGF-β plus at-RA, and TGF-β plus RARi (Fig. 3 D). Consistent with the elevated expression of the Thy1.1 reporter molecule, T cells activated in the presence of CpG-BMDC and TGF-β produced significantly higher amounts of IL-10 relative to other conditions. Furthermore, the addition of at-RA in the presence of TGF-β suppressed the production of IL-10 to near baseline levels. In contrast, RAR blockade in the presence of TGF-β resulted in a significant increase in IL-10 production by T cells activated by all BMDC populations in the presence of TGF-β. Collectively, these data establish that at-RA potently blocks induction of Il10 and suggest that an important effect of CpG in promoting T cell induction of Il10, in addition to its stimulation of IL-6 production, is likely a result of its inhibition of production of RAR ligands such as at-RA by DCs.
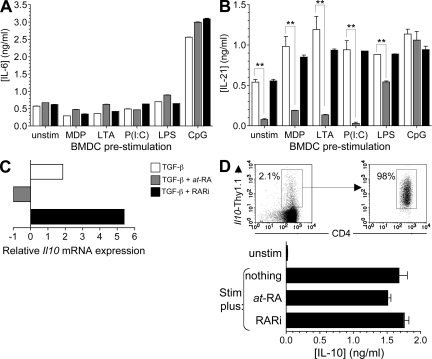
RA principally inhibits transcription but not translation of Il10
Having established the critical roles of IL-6 and IL-21 in the induction of Il10 in response to TGF-β and CpG-BMDC stimulation, we next examined whether the ability of at-RA to suppress Il10 induction involved repression of these two cytokines. The production of IL-6 and IL-21 by CD4 T cells stimulated with the various BMDC populations in the presence of TGF-β, TGF-β plus at-RA, and TGF-β plus RARi was determined. We found that IL-6 was induced under all conditions examined and that the production was not significantly affected by the addition of at-RA or blockade of RAR signaling (Fig. 4 A), indicating that the mechanism of at-RA suppression of Il10 did not involve suppression of IL-6. Notably, CD4 T cells stimulated with CpG-BMDC and TGF-β, which contained the largest fraction of Thy1.1+ cells, produced significantly higher levels of IL-6, which is suggestive of a role for autocrine IL-6 in amplifying the induction of Il10 in CD4 T cells. In contrast, IL-21, which was also produced by all polarized T cell subsets, was significantly down-regulated by the addition of at-RA and rebounded in the presence of RARi, except in the CpG-BMDC–induced T cells (Fig. 4 B). Collectively, these data indicate that despite the demonstrated involvement of IL-6 and IL-21 in the CpG-mediated induction of IL-10, the ability of at-RA to inhibit this induction is independent of these two cytokines.
Figure 4.
RA inhibits transcription but not translation of IL-10. (A and B) CD4 T cells differentiated as described in Fig. 3 C were reactivated for 24 h with anti-CD3 and anti-CD28 antibodies, and IL-10 (A) and IL-21 (B) production was quantified by cytometric bead array. (C) On day 5 after activation, CD4 T cells differentiated in the presence of CpG-activated BMDC plus the additives indicated were harvested for analysis of Il10 mRNA by real-time PCR. Data are normalized to cells activated with CpG-activated BMDC and antigen only and are from one of two experiments with similar results. (D) CD4+Thy1.1+ cells were isolated from naive 10BiT mice as shown (top left) and left unstimulated or stimulated for 24 h with anti-CD3 and anti-CD28 antibodies only or with the addition of 100 nM at-RA or 1 µM LE540 before IL-10 protein in supernatants was quantified by cytometric bead array. Mean cytokine concentrations ±SEM are shown in A, B, and D, and all data are representative of three independent experiments. **, P < 0.005.
We next examined the specific effect of at-RA on Il10 mRNA induction in the presence of CpG-BMDC and TGF-β (Fig. 4 C). Naive T cells were stimulated using CpG-BMDC in the presence of TGF-β only, TGF-β plus at-RA, or TGF-β plus RARi, and Il10 transcript levels were measured relative to cells activated by the same BMDC population in the absence of TGF-β. In accord with previous data examining Thy1.1 reporter expression, stimulation in the presence of TGF-β induced up-regulation of Il10 mRNA transcripts, whereas the addition of at-RA resulted in reduced Il10 transcript levels. Importantly, RAR blockade allowed for increased Il10 mRNA expression above that induced by addition of TGF-β alone, suggesting that although CpG treatment of DCs likely suppresses production of RAR ligands to promote Il10 induction, this is incomplete.
Because the Thy1.1 reporter transcript generated by activation of the Il10 gene in the 10BiT transgene utilizes an exogenous 3′ untranslated region that lacks the degradation signals present in the native Il10 3′ untranslated region (8), the observed profound blockade of both transgenic reporter and endogenous Il10 transcripts by at-RA suggested inhibition primarily at the level of conversion of the Il10 gene to a transcriptionally poised state and not mRNA stability (Fig. 3 C, Fig. 4 C, and not depicted). Given the substantial posttranscriptional regulation of Il10, however, we further examined whether at-RA inhibited production of IL-10 protein by cells already activated to express Il10. To this end, we sorted CD4+Thy1.1+ cells from 10BiT mice (Fig. 4 D, top) and restimulated with anti-CD3 and anti-CD28 antibodies for 24 h before quantifying the IL-10 protein content of the culture supernatants. Il10-competent CD4 T cells stimulated ex vivo produced a substantial amount of IL-10 protein (1.7 ± 0.1 ng/ml) (Fig. 4 D). Although stimulation in the presence of at-RA showed modest reduction of IL-10 production, this did not achieve statistical significance. Accordingly, restimulation in the presence of the RARi did not affect production relative to cells restimulated in the absence of any additives (1.8 ± 0.1 ng/ml). Therefore, although at-RA inhibits induction of transcription of Il10 by naive CD4 T cells stimulated in the presence of TGF-β, it does not impede the production of IL-10 by cells already committed to expression of Il10, indicating principal effects at the level of transcription and not translation.
RA produced by mucosal DCs inhibits TGF-β–mediated induction of Il10
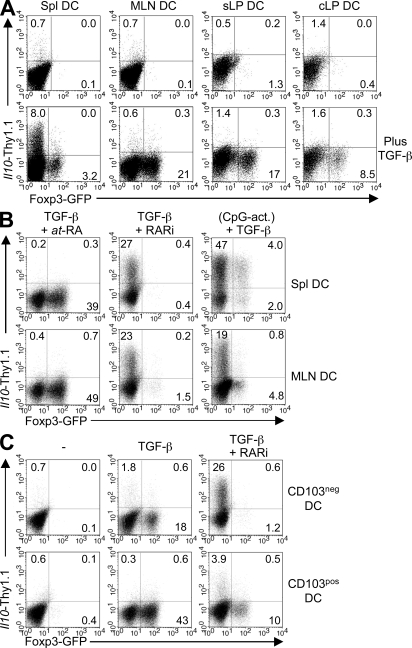
Mucosal but not splenic DCs are known to possess the enzymatic machinery required to convert vitamin A to at-RA and have thus been shown to promote expression of Foxp3 and gut-homing receptors in T cells (21, 39). Therefore, we purified total CD11c+ DCs from the spleen, MLN, small intestine lamina propria (sLP), and colon lamina propria (cLP) and compared their abilities to induce IL-10 and/or Foxp3 reporter expression in naive CD4 T cells. In response to antigenic stimulation only, none of the DC subsets induced significant levels of the Il10 reporter (Fig. 5 A, top). However, in the presence of TGF-β, splenic DCs induced low-level expression of GFP and readily detectable levels of Thy1.1 (3.2 and 8% of CD4 T cells, respectively). In contrast, MLN, sLP, and cLP DC failed to induce Thy1.1 expression but, as expected, induced a significant population of GFP-expressing cells (21, 17, and 8.5% of CD4 T cells, respectively; Fig. 5 A, bottom). Similar to what was observed with the CpG-BMDC–stimulated T cells, the addition of at-RA resulted in an increase in GFP+ cells in the presence of both splenic and MLN DCs (39 and 49%, respectively) but completely inhibited the induction of Thy1.1 in the presence of splenic DCs and TGF-β (Fig. 5 B). Interestingly, in the presence of TGF-β and the RA inhibitor, both splenic and MLN DCs induced a significant fraction of Thy1.1-expressing cells (27 and 23%, respectively) and a reduced percentage of GFP-expressing cells (0.8 and 1.7%, respectively). In addition, prestimulation of splenic and MLN DCs with CpG also resulted in increased Thy1.1 (47 and 19%, respectively) and reduced GFP expression (6.0 and 5.6%, respectively). Collectively, these data indicate that, similar to BMDCs, MLN DC-derived at-RA inhibits the induction of Thy1.1 (hence IL-10) while favoring Foxp3 induction.
Figure 5.
Mucosal DC-derived RA inhibits the induction of IL-10 in CD4 T cells. (A) 5 × 105 naive 10BiT.Foxp3gfp.OT-II CD4 T cells were stimulated with 1 µg/ml OVA peptide and 105 CD11c+ DC from the spleen, MLN, sLP, or cLP in the absence (top) or presence (bottom) of 5 ng/ml TGF-β1. (B) CD4 T cells were stimulated with splenic or MLN DC as described in A in the presence of 100 µM TGF-β1 and RA or 1 µM LE540. CpG-act are DC that were preactivated for 16 h with CpG and then washed and cocultured with CD4 T cells in the presence of OVA peptide and TGF-β1. (C) CD11c+ MLN DC were FACS sorted into CD103− and CD103+ fractions before being used in cocultures with T cells as in A. Data are representative of three to four independent experiments.
Elevated expression of at-RA and the consequent ability to induce expression of Foxp3 has been associated with the CD103+ subset of mucosal DCs (20, 40). Induction of Foxp3 and the corresponding inhibition of IL-10 induction appear to correlate with an enrichment of CD103+ DCs within the total DC population isolated from MLN as well as sLP and cLP (21, 37, and 31%, respectively). In contrast, splenic DCs, which are essentially devoid of this subset, were permissive to induction of IL-10 (Fig. S4 A, available at http://www.jem.org/cgi/content/full/jem.20080950/DC1). To address specific inductive roles of DCs divided on the basis of CD103, we sorted MLN DCs into CD103− and CD103+ fractions (Fig. S4 B) and examined their respective abilities to induce Il10 expression. Consistent with published reports, both CD103− and CD103+ MLN DC induced expression of GFP (17 and 53% of CD4 T cells, respectively) when stimulated in the presence of TGF-β. However, under the same conditions, both DC subsets induced minimal levels of Thy1.1 (2.4 and 0.9% of CD4 T cells, respectively; Fig. 5 C). The induction of GFP and the minimal induction of Thy1.1 suggest that the at-RA production by CD103− DC is sufficient to inhibit induction of IL-10 and demonstrate that expression of CD103 does not absolutely correlate with a differential capacity of mucosal DCs to mediate induction of Foxp3 or Il10. Accordingly, inclusion of an RARi in the cultures of CD103− MLN DCs almost completely inhibited induction of GFP (1.2% of CD4 T cells) and allowed for the robust induction of Thy1.1 (26% of CD4 T cells; Fig. 5 C). Although the RARi also greatly blocked GFP induction by CD103+ DCs (>75% inhibition), GFP expression was incompletely extinguished, apparently as a result of incomplete inhibition of RAR by RARi at the high levels of at-RA generated by this DC population (Fig. 5 C and not depicted). Similarly, addition of the RARi promoted a more modest induction of Thy1.1 by CD103+ DCs. Collectively, these data demonstrate that mucosal DCs promote induction of Foxp3 and inhibit induction of Il10 via an RA–dependent mechanism.
Reciprocal effects on expression of Il10 and Foxp3 by dietary modulation of vitamin A
Given the potency of mucosal DC-derived at-RA at suppressing induction of Il10 in vitro, we examined the effects of modulation of the dietary RA precursor vitamin A on development of T reg cell subsets in vivo. 10BiT.Foxp3gfp mice were placed on diets with normal (vitamin A control [VAC]), deficient (vitamin A deficient [VAD]), or high (vitamin A high [VAH]) levels of vitamin A and examined for the development of Il10- and Foxp3-expressing T cell subsets (Fig. 6). By introducing the dietary changes to mothers of preterm fetuses, significant alterations in vitamin A levels generally begin to manifest at 6–8 wk of age (41). At 6 wk, both dietary deficient (VAD) and high (VAH) mice had reduced body weight compared with control (VAC) mice. In addition, although VAH mice grew at approximately the same rate as VAC mice, VAD mice began to lose weight at 12 wk of age (Fig. S5 A, available at http://www.jem.org/cgi/content/full/jem.20080950/DC1). All cohorts were killed at 14 wk of age and evaluated. There was a significant reduction in serum retinol levels in mice fed the VAD diet compared with those on the VAC diet (Fig. S5 B). No significant difference was found in serum retinol levels of VAH mice relative to VAC mice, as circulating levels of serum retinol typically plateau despite high dietary intake and increased tissue stores (41). Notably, no evidence of intestinal inflammation was found in any of the cohorts at the time of termination (unpublished data).
Figure 6.
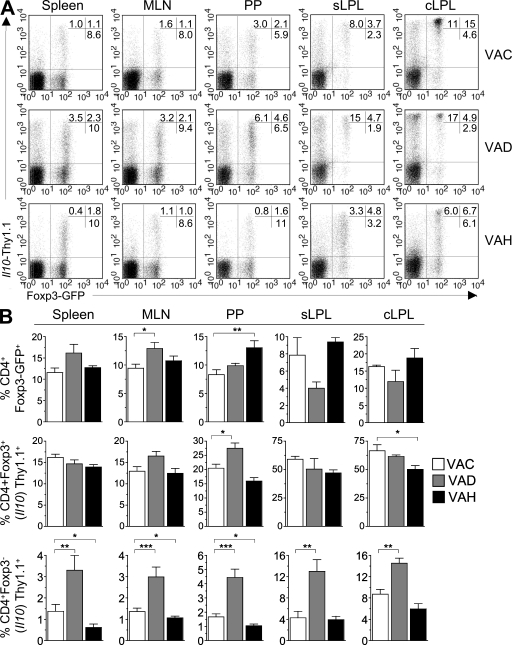
Reduced dietary vitamin A enhances and increased dietary vitamin A inhibits the induction of Il10 in vivo in the steady state. (A) Male 10BiT.Foxp3gfp mice that were exposed to vitamin A–modified diets as described were killed at 12–14 wk of age. Single cell suspensions were prepared from the indicated tissues, and expression of GFP and Thy1.1 by CD4 T cells was determined by FACS. Plots are gated on CD4 T cells and numbers represent the percentage of CD4 T cells in quadrants 1, 2, and 4. (B) Graphical summary of data represented in A to denote the frequency of Il10-Thy1.1–expressing cells among Foxp3− (bottom) and Foxp3+ (middle) cells in the respective tissue sites among the three diet groups. Graphs show mean frequencies ±SEM and all data are pooled from 8–12 mice per diet group. *, P < 0.05; **, P < 0.005; ***, P < 0.001. Data are representative of three independent experiments.
CD4 T cells isolated from spleen, MLN, Peyer's patches, sLP, and cLP were examined for expression of GFP and Thy1.1 (Fig. 6). Increased frequencies of Thy1.1+Foxp3+ cells were observed in the gut-associated lymphoid tissue (GALT) of VAD mice. Conversely, this subset was reduced in both Peyer's patches and the lamina propriae of VAH mice (Fig. 6, A [middle] and B). Notably, the number of CD4 T cells recovered from the intestinal lamina propriae of VAH mice was comparable to that of VAC mice (unpublished data), suggesting that the reduced frequencies of IL-10–competent cells in these tissues is a result of inhibition of Il10 induction in the face of elevated vitamin A and its metabolite at-RA.
The effect of dietary manipulations of vitamin A on the induction of Il10 in Foxp3− cells was quite pronounced. In every tissue examined, a significant increase in the frequency of Foxp3−IL-10+ Tr1-like T cells was found in VAD mice compared with VAC mice (Fig. 6, A and B [bottom]). A reciprocal decrease in this population was evident in VAH mice relative to VAC mice. Notably, the intensity of Thy1.1 expression mirrored the effects of vitamin A manipulation on frequencies of Thy1.1-expressing T cells. Thus, compared with mice on the control diet, the mean fluorescence intensities of Thy1.1 expression by flow cytometric analyses were relatively increased or reduced in VAD and VAH mice, respectively (Fig. 6 A and not depicted). Collectively, these data indicate that reduced dietary vitamin A, which results in reduced synthesis of at-RA, promotes elevated induction of Il10 in CD4 T cells. Conversely elevated dietary vitamin A, which leads to increased precursor for at-RA, contributes to diminished induction of IL-10–competent T reg cells in the steady state.
DISCUSSION
In this study, we identify reciprocal effects of the vitamin A metabolite at-RA on expression by CD4 T cells of two key molecules associated with immune regulation and mucosal homeostasis: IL-10 and Foxp3. In contrast to its established effects on the induction of Foxp3 and, thus, promotion of iT reg cell development (17–20), we find that at-RA potently suppresses Il10 gene expression, primarily at the level of transcription. In support of this, VAD mice developed increased frequencies of IL-10–competent cells in all lymphoid tissues and in effector sites of the intestinal tissues. In a survey of TLR and NOD agonist effects on DCs, it was found that activation of the TLR9 pathway most potently promoted TGF-β–mediated induction of the Il10 gene in naive CD4 T cells, in part as a result of its induction of IL-6 and, seemingly, its suppression of the synthesis and/or secretion of at-RA compared with other TLR and NOD ligands. Accordingly, CpG activation of MLN DCs, which normally synthesize at-RA and strongly induce T reg cell development, resulted in suppressed Foxp3 induction in favor of enhanced Il10 induction. Collectively, these results extend prior studies on differential induction of iT reg and Th17 lineages by RA and indicate that the balance of at-RA, TGF-β, and the proinflammatory mediator IL-6 also differentially regulate development of distinct subsets of Foxp3+ and Foxp3− T reg cells from naive CD4 precursors. These findings add further complexity to our emerging understanding of the diverse pathways to IL-10 expression by T cells (4, 14) but indicate that, at least initially, induction of Foxp3 and Il10 in naive CD4 T cells are largely divergent and can be controlled by differential actions of TLR/NOD ligands on APCs.
Although at-RA has been shown to limit development of Th17 cells in response to TGF-β and IL-6 (17), the findings herein indicate that although there is significant overlap in the programming of IL-17– and IL-10–competent cells (e.g., requirement for IL-21 by both), the at-RA–mediated inhibition of IL-10 is more sensitive and can be dissociated from Th17 development. Thus, although not inconsistent with previous studies that have demonstrated overlap between the TGF-β–mediated inductive pathways of IL-10 and IL-17 (13, 24), we find that inhibition of at-RA signaling allows substantial induction of Il10 even under conditions that are suboptimal for Th17 development. Activation of naive T cells in the presence of TGF-β promoted induction of Il10 irrespective of the activation state of the APC and the presence of specific determinants of effector cell differentiation, contingent upon limited or absent RAR signaling. Accordingly, DCs that were not activated by TLR or NOD signals and did not promote Th17 development potently induced IL-10 reporter expression. Under conditions where RAR activity was inhibited, Il10 expression was induced even by low levels of IL-6 that were insufficient to promote IL-17 expression. Naive CD4 T cells can, therefore, develop along a Tr1-type program that diverges from Th17 effector cell development contingent upon the relative levels of RA, TGF-β, and IL-6. The finding that vitamin A deficiency promotes increased development of Il10-expressing T cells in vivo supports the role of at-RA in suppressing development of IL-10–competent T cells, particularly the Foxp3− IL-10–competent Tr1-type subset. The relative frequencies of Foxp3+ and Foxp3− T reg cells are therefore regulated at least in part by dietary intake of vitamin A.
Other variables are also likely to affect the relationship of developing IL-10–competent T cells as a subset of effector lineages (such as Th17) or as an independent pathway. Prior studies of Tr1 cell development showed effects of IL-10 itself, corticosteroids, and vitamin D3 as determinants promoting IL-10 expression (4, 5, 14). Furthermore, it is not unlikely that developing intestinal Th17 cells might extinguish expression of the Il17a/Il17f cytokine locus late in the inflammatory response and give rise to IL-10–competent cells that lack Th17 effector cytokines, which is analogous to infectious settings wherein chronic antigenic persistence promotes the emergence of IL-10 expression from effector T cell responses. Accordingly, we have found modest increases in the frequencies of Th17 effectors in the GALT of VAD mice, as well as a fraction of cells that coexpress IL-17A and high levels of the Thy1.1 reporter (unpublished data). These findings highlight the overlapping development of IL-10 competency under conditions of diminished RA availability, whether through Th17-associated or -independent pathways.
In accordance with recent studies (17, 19, 20), the CD103+ subset of mucosal DCs was found to be particularly efficient at inducing Foxp3+ iT reg cells. However, although at-RA–producing DCs were enriched in the CD103+ subset, the levels of at-RA produced by CD103− DCs were sufficient to potently inhibit induction of Il10 despite more limited effects on induction of Foxp3, suggesting exquisite sensitivity of the Il10 gene to repression by RAR signaling. Further work will be necessary to better characterize both the at-RA–producing and –nonproducing subsets and to compare their respective roles in mucosal tolerance. However, the finding that RA blockade nearly completely abolished induction of Foxp3 expression suggests that RA does not just enhance but is a critical cofactor for TGF-β–mediated Foxp3 iT reg cell development.
Given that splenic DCs are deficient in the enzymatic pathway for conversion of vitamin A to RA, the observed inhibition of Foxp3 induction by splenic DCs plus TGF-β in the presence of RAR blockade (Fig. 5 B) raised the possibility that in our and some previous studies, an unappreciated amount of at-RA activity might have been derived from the culture medium. Indeed, we have found sufficient at-RA present in many lots of fetal bovine serum that is adequate to induce high expression of Foxp3 under APC-free conditions of activation of naive CD4 T cells by addition of TGF-β alone, as indicated by nearly complete inhibition of Foxp3 expression by addition of RARi (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20080950/DC1), as well as direct quantitation of at-RA (42) (unpublished data). Notably, in contrast to cultures with BM- or tissue-derived DCs supplemented with TGF-β, at-RA blockade under APC-free conditions did not result in expression of Il10, which is consistent with the requirement for at least low levels of IL-6 for induction of Il10 under conditions of RAR suppression (Fig. S6).
The finding that TLR9 had the greatest efficacy in its reciprocal effects on expression of the Foxp3gfp and 10BiT reporters is consistent with a recent study demonstrating that Foxp3+ cells are increased in small intestinal tissues of Tlr9−/− mice at steady state. Hall et al. (43) found corresponding decreases in IL-17– and IFN-γ–expressing effector T cells, as well as more modest decreases in IL-10–expressing cells, at steady-state in Tlr9−/− mice. Furthermore, CpG or DNA of the commensal bacteria markedly inhibited Foxp3+ iT reg cell development induced by DCs isolated from the intestinal lamina propria. Those data and data herein are consistent with in vivo modulation of at-RA levels in the GALT by TLR9 agonists derived from the intestinal microbiota as a major mechanism by which the balance of T reg cell subsets is achieved. Ivanov et al. (44) found reciprocal induction of Th17 cells and Foxp3+ T reg cells in the GALT by specific commensal bacteria, identifying a role for some but not all bacterial species in regulating the balance of T reg cells and Th17 cells. Because expression of IL-10 was not examined in that study, it is unknown whether similar bacterial species-specific effects regulate the balance of Foxp3 and IL-10 competent T reg cell subsets and whether there is overlap or segregation of bacterial species that might induce IL-17– and IL-10–expressing cells in the GALT.
Vitamin A or at-RA has been reported to inhibit development of IFN-γ– and IL-17–producing CD4 T cells (17, 23, 45), and RA has been reported to enhance induction of IL-4 production by Th2 cells (45), possibly as a result of RA-mediated suppression of IL-10. Conversely, factors that promote Th1, Th2, and Th17 development have been shown to limit Foxp3+ T reg cell development (30, 46). In contrast, it is now clear that IL-10 can be induced in association with all CD4 T cells subsets, regulatory and effector (14). This likely represents a strategic adaptation to allow T cell–mediated immune regulation at effector sites where the proinflammatory millieu would be antagonistic to Foxp3 expression. In this context, it is notable that the characteristics of the 10BiT reporter model, which sensitively identifies cells in which the Il10 gene has become transcriptionally active, has identified a remarkable degree of posttranscriptional regulation of IL-10 protein production, such that many reporter-positive cells induced by TGF-β and IL-6 under RA-deficient conditions require additional signals to produce high amounts of IL-10, despite high levels of Il10 transcripts both in Th17-associated and Tr1-type cells (unpublished data). This implies a mechanism akin to that recently reported for NK cells (33), whereby derepression of blocks on translation of Il10 mRNA transcripts represents a principal strategy by which expression of IL-10 is primed but not fully executed until late in CD4 T cell development, whether in association with effector or regulatory lineages.
Considering the nonredundant role for T cell–derived IL-10 in maintaining intestinal immune homeostasis and the marked predominance of IL-10–competent T cells in the intestinal tissues, it is not immediately clear why at-RA, which is prevalent in the intestines and associated lymphoid tissues, would be inhibitory to Il10 expression by naive CD4 T cells. This is particularly intriguing given the fact that the majority of Foxp3+ CD4 T cells in the intestines are competent for IL-10 expression, especially in the colon (8). Notably, however, the intestines are also enriched for IL-10–competent T cells that lack Foxp3 (8), a population which is increased by VAD, which is consistent with their development via an RA-independent pathway. Although a fraction of Foxp3− IL-10–competent cells coexpress effector cytokines, particularly IFN-γ and IL-17, the large majority do not, suggesting that development of Tr1-like cells in the intestines arise independently of effector lineage development, extinguish effector cytokines caused by chronic reactivity to the commensal microbiota, or both (8, 14).
Concerning T cells that coexpress Foxp3 and Il10, although rapid induction of this phenotype was observed in a minor fraction of naive CD4 T cells activated with TGF-β and IL-6 under conditions of limited at-RA in vitro, we have previously shown that Foxp3+IL-10+ T reg cells arise in the periphery from Foxp3+IL-10− precursors, especially in intestinal tissues (8). Thus, mechanisms yet to be defined must exist for induction of Il10 transcription in committed Foxp3+ T reg cells, i.e., late in the T reg cell development. Given the potent inhibitory effects of at-RA on Il10 induction in naive CD4 T cells, this suggests that mature T reg cells are resistant to inhibitory effects of at-RA on Il10 induction, respond to at-RA differently from naive T cells, and/or interact preferentially with a subset of APCs that lack the RA biosynthetic pathway.
Further studies defining the inductive pathways to Il10 gene expression will be needed to understand how RAR signaling might impact Il10 suppression in early versus late CD4 lineage development. Notably, however, in unpublished studies of 10BiT.Foxp3gfp mice, we have found that both Foxp3+ and Foxp3− subsets of Thy1.1 reporter-positive (IL-10 competent) T cells are highly enriched for expression of the gene Prdm1, which encodes the transcription factor B lymphocyte–induced maturation protein-1 (Blimp-1). Martins et al. (47) reported that CD4 T cells with targeted ablation of Blimp-1 showed reduced expression of IL-10 and developed spontaneous colitis, implicating this factor as a critical inducer of IL-10 in T cells. Importantly, expression of Blimp-1 in B cells is induced by STAT3-dependent signaling, particularly that activated by IL-6, IL-21, and IL-10 (48). Although similar induction of Blimp-1 by these same factors in CD4 T cells has not yet been reported, we speculate that the induction of Il10 transcription and, by extension, repression of Il10 transcription by at-RA results from interplay of these same signaling pathways, which converge on the expression of Blimp-1 to control Il10 transcription. Implementation of 10BiT. Foxp3gfp mice to directly test these and other pathways to coordinate and differential expression of Il10 and Foxp3 should be informative.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6), C57BL/6.Ly5.1 (CD45.1), and C57BL/6.Icosl−/− mice were purchased from The Jackson Laboratory. C57BL/6.Myd88−/− mice were provided by D. Chaplin and S. Michalek (University of Alabama at Birmingham, Birmingham AL). 10BiT.Foxp3gfp mice have been described previously (8). All mice were bred and maintained and all animal experimentation was approved in accordance with guidelines and approval of the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Antibodies and reagents.
PE-conjugated anti-CD44 (IM7), anti-α4β7 (LPAM), anti-CD90.1 (OX-7), anti-CD103 (M290), anti-IL-17 (TC11-18H10), PerCP (peridinin chlorophyll-a protein)-conjugated anti-CD90.1 (OX-7), and functional grade anti-IL-10R (1b1.3a) were purchased from BD; allophycocyanin-conjugated anti-CD4 (GK1.5), anti-IL-10 (JES5-16S3), anti–IFN-γ (XMG1.2), PE-conjugated and anti–IL-10 (JES5-16S3), PE-Cy7–conjugated anti-CD4 (GK1.5), affinity-purified anti-CCR9 (CW-1.2), and functional grade anti–IL-6 (MP5-20F3) were purchased from eBioscience. The following reagents were purchased from R&D Systems: recombinant human TGF-β1, IL-21 R/Fc chimera, WSSX-1 R/Fc chimera, and anti–IFN-α/βR1. at-RA was purchased from Sigma-Aldrich and the RA inhibitor LE540 was purchased from Wako Chemicals USA, Inc. MDP, LTA, poly (I:C), LPS, and CpG ODN were purchased from InvivoGen.
Differentiation and activation of BMDCs.
BMDC were prepared according the protocol described by Lutz et al. (49) with slight modifications. In brief, BM cells were plated in 6-well plates at a density of 5 × 105/ml in RPMI 1640 medium containing 10% FCS, 100 IU/ml penicillin, 100 µg/ml streptomycin, nonessential amino acids, 1 µM sodium pyruvate, 2.5 µM β-mercaptoethanol, and 2 µM l-glutamine (R-10 medium) with 2% GM-CSF supernatant for 10 d. On day 10, BMDCs were left unstimulated or were activated with 1 µg/ml MDP, 1 µg/ml LTA, 2.5 µg/ml Poly (I:C), 0.1 µg/ml LPS, or 2.5 µg/ml CpG ODN for 24 h. BMDCs were then washed twice with serum-free media then resuspended in R-10 in preparation for culture with naive T cells.
Ex vivo DC isolation.
Spleens and MLN were digested with collagenase VIII and DNase 1 (both obtained from Sigma-Aldrich) for 30 min at 37°C and then incubated for 5 min in PBS plus EDTA. The intestines were removed and Peyer's patches were dissected from the small intestines. After removal of the epithelial layer by sequential incubation with in HBSS containing 154 µg/liter l-dithioerythreitol followed by 2 µM EDTA, the remaining gut tissue was completely digested with 100 U/ml collagenase VIII and 20 µg/ml DNase (Sigma-Aldrich) in R-10 for 45 min at 37°C with gentle magnetic stirring to isolate lamina propria cells. Total lamina propria cells were purified on a 40/75% Percoll gradient by room temperature centrifugation for 20 min at 2,000 rpm with no brake. CD11c+ DCs from all tissues were enriched using CD11c microbeads according to the manufacturer's protocol (Miltenyi Biotec). Where indicated, MLN DCs were labeled with APC-conjugated anti-CD11c and PE-conjugated anti-CD103 and then sorted into CD103− and CD103+ fractions using a FACS Aria (BD).
CD4 T cell isolation and activation.
Spleens and LN were collected from 10BiT.Foxp3gfp.OT-II mice and single-cell suspensions were prepared by mechanical disruption. CD4+ T cells were isolated using Dynabeads mouse CD4 beads followed by DETACHaBEAD mouse CD4 according to the manufacturer's directions (Invitrogen). Cells were then stained with PE-conjugated anti-CD44, PerCP-conjugated CD90.1 (Thy1.1), and APC-conjugated CD4, and the CD44loGFP−Thy1.1− fraction was sorted by FACS. 5 × 105 sorted naive T cells were stimulated with 1.0 µg/ml OVA peptide and 105 DC and supplemented or not with recombinant cytokines or blocking antibodies as indicated. In all cases, CD4+ T cells were examined by FACS on day 3 or 4 after activation for expression of GFP and Thy1.1 and the remaining cells were split and supplemented with fresh media. On day 5, cells were restimulated with PMA and ionomycin before being stained intracellularly with antibodies specific for IFN-γ, IL-10, and IL-17.
Generation of vitamin A–modified mice.
Semipurified casein-based diets were purchased from Harlan with vitamin A content modified as follows: VAD diet, 0.2 IU/g (TD 88407); VAC diet, 4 IU/g (TD 96007); and VAH diet, 250 IU/g (TD 96008). Timed pregnant females were fed the respective diets starting early in the second week of pregnancy and maintained on the same diets throughout the pregnancy and postnatally through weaning. Progeny were weaned and maintained on the same diet that the dams had been fed until they were killed for analysis.
Statistical analyses.
Statistical significance was calculated by unpaired Student's t test, Mann-Whitney U test, or analysis of variance as appropriate. All p-values ≤0.05 are considered significant unless specifically indicated in the text.
Online supplemental material.
Fig. S1 includes a schematic representation of the experimental design for Figs. 1–4 as well as purification of naive T cells used in in vitro experiments. Fig. S2 shows that Il10 transcript is expressed in both WT and 10BiT.Foxp3gfp CD4 T cells stimulated with CpG-BMDC and TGF-β. Fig. S3 shows a dose titration of TGF-β in the presence an RA inhibitor and a high or low dose of IL-6. Fig. S4 shows CD103 expression by tissue DC, including FACS-purified CD103− and CD103+ MLN DC. Fig. S5 shows the weight chart and serum retinol levels of mice fed control or modified vitamin A diets. Fig. S6 shows that TGF-β–dependent induction of Foxp3 is aided by RA present in the culture media. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20080950/DC1.
Acknowledgments
The authors are grateful to members of the Weaver laboratory for their helpful comments and suggestions. We are also grateful to Maureen Kane and Joe Napoli (University of California, Berkeley) for helpful discussions and assistance in quantifying vitamin A metabolites. We thank Chunyan Song, Michael Blake, and Alina Wettstein for expert technical assistance and Gloria Gaskins for editorial assistance.
This work was supported by grants from the National Institutes of Health (AI057956, DK071167, and DK064400 to C.T. Weaver) and the Crohn's and Colitis Foundation of America (to C.T. Weaver).
The authors have no conflicting financial interests to declare.
Footnotes
Abbreviations used: at-RA, all-trans RA; Blimp-1, B lymphocyte–induced maturation protein-1; BMDC, BM-derived DC; cLP, colon lamina propria; GALT, gut-associated lymphoid tissue; ICOS, inducible costimulator; ICOSL, ICOS ligand; LTA, lipoteichoic acid; MDP, muramyl dipeptide; MLN, mesenteric LN; mRNA, messenger RNA; NOD, nucleotide oligomerization domain; RA, retinoic acid; RAR, RA receptor; RARi, RAR inhibitor; sLP, small intestine lamina propria; TLR, Toll-like receptor; TR1, T reg type 1; T reg cell, regulatory T cell; VAC, vitamin A control; VAD, vitamin A deficient; VAH, vitamin A high.
References
- Shevach E.M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers.Nat. Rev. Immunol. 2:389–400 [DOI] [PubMed] [Google Scholar]
- Bluestone J.A., Abbas A.K. 2003. Natural versus adaptive regulatory T cells.Nat. Rev. Immunol. 3:253–257 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Rudensky A.Y. 2007. Foxp3 in control of the regulatory T cell lineage.Nat. Immunol. 8:457–462 [DOI] [PubMed] [Google Scholar]
- O'Garra A., Vieira P. 2007. T(H)1 cells control themselves by producing interleukin-10.Nat. Rev. Immunol. 7:425–428 [DOI] [PubMed] [Google Scholar]
- Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis.Nature. 389:737–742 [DOI] [PubMed] [Google Scholar]
- Vieira P.L., Christensen J.R., Minaee S., O'Neill E.J., Barrat F.J., Boonstra A., Barthlott T., Stockinger B., Wraith D.C., O'Garra A. 2004. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells.J. Immunol. 172:5986–5993 [DOI] [PubMed] [Google Scholar]
- Gavin M.A., Rasmussen J.P., Fontenot J.D., Vasta V., Manganiello V.C., Beavo J.A., Rudensky A.Y. 2007. Foxp3-dependent programme of regulatory T-cell differentiation.Nature. 445:771–775 [DOI] [PubMed] [Google Scholar]
- Maynard C.L., Harrington L.E., Janowski K.M., Oliver J.R., Zindl C.L., Rudensky A.Y., Weaver C.T. 2007. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10.Nat. Immunol. 8:931–941 [DOI] [PubMed] [Google Scholar]
- Kamanaka M., Kim S.T., Wan Y.Y., Sutterwala F.S., Lara-Tejero M., Galan J.E., Harhaj E., Flavell R.A. 2006. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse.Immunity. 25:941–952 [DOI] [PubMed] [Google Scholar]
- Fiorentino D.F., Bond M.W., Mosmann T.R. 1989. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones.J. Exp. Med. 170:2081–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C.F., Oukka M., Kuchroo V.J., Sacks D. 2007. CD4+CD25−Foxp3− Th1 cells are the source of IL-10–mediated immune suppression in chronic cutaneous leishmaniasis.J. Exp. Med. 204:285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Feng C.G., Goldszmid R.S., Collazo C.M., Wilson M., Wynn T.A., Kamanaka M., Flavell R.A., Sher A. 2007. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection.J. Exp. Med. 204:273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer J.S., Silver J.S., Laurence A., Porrett P.M., Harris T.H., Turka L.A., Ernst M., Saris C.J., O'Shea J.J., Hunter C.A. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10.Nat. Immunol. 8:1363–1371 [DOI] [PubMed] [Google Scholar]
- Maynard C.L., Weaver C.T. 2008. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation.Immunol. Rev. 226:219–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N., McGrady G., Wahl S.M. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3.J. Exp. Med. 198:1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani A., Fuss I., Nakamura K., Kumaki F., Usui T., Strober W. 2003. Transforming growth factor (TGF)-β1–producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-β1–mediated fibrosis.J. Exp. Med. 198:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid.Science. 317:256–260 [DOI] [PubMed] [Google Scholar]
- Benson M.J., Pino-Lagos K., Rosemblatt M., Noelle R.J. 2007. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation.J. Exp. Med. 204:1765–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Carcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism.J. Exp. Med. 204:1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid.J. Exp. Med. 204:1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M., Hirakiyama A., Eshima Y., Kagechika H., Kato C., Song S.Y. 2004. Retinoic acid imprints gut-homing specificity on T cells.Immunity. 21:527–538 [DOI] [PubMed] [Google Scholar]
- Tavera-Mendoza L., Wang T.T., Lallemant B., Zhang R., Nagai Y., Bourdeau V., Ramirez-Calderon M., Desbarats J., Mader S., White J.H. 2006. Convergence of vitamin D and retinoic acid signalling at a common hormone response element.EMBO Rep. 7:180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephensen C.B., Jiang X., Freytag T. 2004. Vitamin A deficiency increases the in vivo development of IL-10-positive Th2 cells and decreases development of Th1 cells in mice.J. Nutr. 134:2660–2666 [DOI] [PubMed] [Google Scholar]
- McGeachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., McClanahan T., Cua D.J. 2007. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology.Nat. Immunol. 8:1390–1397 [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Hao L., Medzhitov R. 2006. Role of toll-like receptors in spontaneous commensal-dependent colitis.Immunity. 25:319–329 [DOI] [PubMed] [Google Scholar]
- Roers A., Siewe L., Strittmatter E., Deckert M., Schluter D., Stenzel W., Gruber A.D., Krieg T., Rajewsky K., Muller W. 2004. T cell–specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation.J. Exp. Med. 200:1289–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3.Immunity. 22:329–341 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells.Immunity. 24:179–189 [DOI] [PubMed] [Google Scholar]
- Mangan P.R., Harrington L.E., O'Quinn D.B., Helms W.S., Bullard D.C., Elson C.O., Hatton R.D., Wahl S.M., Schoeb T.R., Weaver C.T. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage.Nature. 441:231–234 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells.Nature. 441:235–238 [DOI] [PubMed] [Google Scholar]
- Powell M.J., Thompson S.A., Tone Y., Waldmann H., Tone M. 2000. Posttranscriptional regulation of IL-10 gene expression through sequences in the 3′-untranslated region.J. Immunol. 165:292–296 [DOI] [PubMed] [Google Scholar]
- Tone M., Powell M.J., Tone Y., Thompson S.A., Waldmann H. 2000. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3.J. Immunol. 165:286–291 [DOI] [PubMed] [Google Scholar]
- Maroof A., Beattie L., Zubairi S., Svensson M., Stager S., Kaye P.M. 2008. Posttranscriptional regulation of Il10 gene expression allows natural killer cells to express immunoregulatory function.Immunity. 29:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Yang M., Wang Y.H., Lande R., Gregorio J., Perng O.A., Qin X.F., Liu Y.J., Gilliet M. 2007. Plasmacytoid dendritic cells prime IL-10–producing T regulatory cells by inducible costimulator ligand.J. Exp. Med. 204:105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A., Carrier Y., Peron J.P., Bettelli E., Kamanaka M., Flavell R.A., Kuchroo V.K., Oukka M., Weiner H.L. 2007. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells.Nat. Immunol. 8:1380–1389 [DOI] [PubMed] [Google Scholar]
- Fitzgerald D.C., Zhang G.X., El-Behi M., Fonseca-Kelly Z., Li H., Yu S., Saris C.J., Gran B., Ciric B., Rostami A. 2007. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells.Nat. Immunol. 8:1372–1379 [DOI] [PubMed] [Google Scholar]
- Uhlig H.H., Coombes J., Mottet C., Izcue A., Thompson C., Fanger A., Tannapfel A., Fontenot J.D., Ramsdell F., Powrie F. 2006. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis.J. Immunol. 177:5852–5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J.A., Hall J.A., Sun C.M., Cai Q., Ghyselinck N., Chambon P., Belkaid Y., Mathis D., Benoist C. 2008. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells.Immunity. 29:758–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora J.R., Iwata M., Eksteen B., Song S.Y., Junt T., Senman B., Otipoby K.L., Yokota A., Takeuchi H., Ricciardi-Castagnoli P., et al. 2006. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells.Science. 314:1157–1160 [DOI] [PubMed] [Google Scholar]
- Davidson T.S., DiPaolo R.J., Andersson J., Shevach E.M. 2007. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells.J. Immunol. 178:4022–4026 [DOI] [PubMed] [Google Scholar]
- Schuster G.U., Kenyon N.J., Stephensen C.B. 2008. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma.J. Immunol. 180:1834–1842 [DOI] [PubMed] [Google Scholar]
- Kane M.A., Folias A.E., Wang C., Napoli J.L. 2008. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry.Anal. Chem. 80:1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.A., Bouladoux N., Sun C.M., Wohlfert E.A., Blank R.B., Zhu Q., Grigg M.E., Berzofsky J.A., Belkaid Y. 2008. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses.Immunity. 29:637–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., Frutos Rde L., Manel N., Yoshinaga K., Rifkin D.B., Sartor R.B., Finlay B.B., Littman D.R. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine.Cell Host Microbe. 4:337–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M., Eshima Y., Kagechika H. 2003. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors.Int. Immunol. 15:1017–1025 [DOI] [PubMed] [Google Scholar]
- Wei J., Duramad O., Perng O.A., Reiner S.L., Liu Y.J., Qin F.X. 2007. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells.Proc. Natl. Acad. Sci. USA. 104:18169–18174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins G.A., Cimmino L., Shapiro-Shelef M., Szabolcs M., Herron A., Magnusdottir E., Calame K. 2006. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function.Nat. Immunol. 7:457–465 [DOI] [PubMed] [Google Scholar]
- Martins G., Calame K. 2008. Regulation and functions of Blimp-1 in T and B lymphocytes.Annu. Rev. Immunol. 26:133–169 [DOI] [PubMed] [Google Scholar]
- Lutz M.B., Kukutsch N., Ogilvie A.L., Rossner S., Koch F., Romani N., Schuler G. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow.J. Immunol. Methods. 223:77–92 [DOI] [PubMed] [Google Scholar]