Abstract
Animal models of hematopoietic stem cell transplantation have been used to analyze the turnover of bone marrow–derived cells and to demonstrate the critical role of recipient antigen-presenting cells (APC) in graft versus host disease (GVHD). In humans, the phenotype and lineage relationships of myeloid-derived tissue APC remain incompletely understood. It has also been proposed that the risk of acute GVHD, which extends over many months, is related to the protracted survival of certain recipient APC. Human dermis contains three principal subsets of CD45+HLA-DR+ cells: CD1a+CD14− DC, CD1a−CD14+ DC, and CD1a−CD14+FXIIIa+ macrophages. In vitro, each subset has characteristic properties. After transplantation, both CD1a+ and CD14+ DC are rapidly depleted and replaced by donor cells, but recipient macrophages can be found in GVHD lesions and may persist for many months. Macrophages isolated from normal dermis secrete proinflammatory cytokines. Although they stimulate little proliferation of naive or memory CD4+ T cells, macrophages induce cytokine expression in memory CD4+ T cells and activation and proliferation of CD8+ T cells. These observations suggest that dermal macrophages and DC are from distinct lineages and that persistent recipient macrophages, although unlikely to initiate alloreactivity, may contribute to GVHD by sustaining the responses of previously activated T cells.
Most interstitial tissues contain populations of resident DC and macrophages. In conventional descriptions, there is a division of labor between DC, which are dedicated to the induction and control of adaptive immunity (1, 2), and macrophages, which are occupied with the clearance of senescent cells, extracellular debris, and maintenance of tissue homeostasis (3, 4). According to mouse models, functional specialization is mirrored by differences in rates of turnover; DC transit rapidly through the tissues (5–7), whereas macrophages are predominantly “fixed” cells (8).
Recent studies of the human dermis have emphasized the apparent phenotypic differences between resident DC and macrophages (9, 10), whereas others have argued that conversion between macrophage and DC phenotypes might occur according to conditions of quiescence or inflammation (11–13). Many investigators have focused on migratory dermal DC that are more easily obtained in an enriched form and have value as a model of maturing DC (11, 14–20). Rather less progress has been made in characterizing human dermal DC and macrophages in a freshly isolated state (9, 10, 21, 22), and the relationship between migrant and resident APC populations remains unclear.
Hematopoietic stem cell transplantation is a powerful means of defining the ontogeny of BM-derived cells and has been previously used in humans to prove the BM origin of Langerhans cells (LC) (23), alveolar macrophages (24), and Kupffer cells (25). However, the lineage relationships of macrophages and DC are unknown because it has not been possible to analyze their turnover in the same organ with sufficient resolution. Understanding the ontogeny of BM-derived APC has many clinical implications. Tandem transplant experiments in mice have shown that competent recipient APC are required for the induction of acute graft versus host disease (GVHD) (26). It has generally been assumed, without rigorous proof, that DC are the predominant APC responsible for stimulating donor T cells (for review see references 27; 28). Specific recipient DC populations such as LC are indeed sufficient to induce GVHD (29), but other experimental systems show that macrophages may also play a role (30). According to current models, macrophage involvement in GVHD has principally been described in terms of their innate immune function, such as the production of TNF-α in response to IFN-γ and LPS (31, 32), rather than through antigen-specific T cell stimulation.
The search for persistent recipient APC in humans has been further promoted by a wealth of clinical data indicating that the risk of acute GVHD after immunosuppression withdrawal or donor lymphocyte infusion (DLI) remains significant for up to 12 mo after transplantation (33–38). It is known that recipient LC persist after donor hematopoietic stem cell engraftment, especially after reduced intensity conditioning, and that donor LC engraftment is stimulated by GVHD (39, 40). However, very few recipient LC persist after 3 mo (40, 41), indicating the potential involvement of other APC in promoting GVHD. Dermal APC are indirectly implicated in the pathogenesis of GVHD by many histopathological studies (42–46). We have also previously described recipient HLA-DR+ cells in dermis for at least 1 mo after transplantation in the absence of GVHD (47, 48).
In this study, we have investigated resident dermal DC and macrophages in human skin. We characterize their phenotypes and function in vitro, determine their kinetics of turnover during transplantation, and investigate their potential to stimulate allogeneic T cells through cytokine production and antigen-specific interactions. Our observations suggest that dermal macrophages outlive all other cutaneous APC and may have the capacity to promote GVHD through both innate inflammatory properties and antigen-specific stimulation of allogeneic T cell responses.
RESULTS
Human dermal APC defined
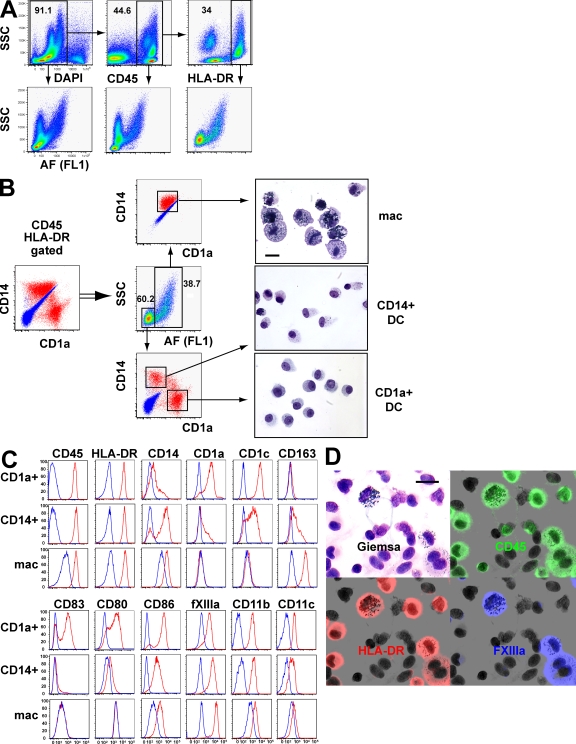
Upon 6–12-h collagenase digestion of freshly keratomed dermis, several populations of DAPI-negative cells are evident from analysis of autofluorescence (AF) and side scatter (SSC) alone (Fig. 1 A). These may be refined by successively gating on CD45+ cells, which includes DC, macrophages, mast cells, and lymphocytes, and then on HLA-DR+ cells, which excludes mast cells and lymphocytes, to produce two major fractions separable by SSC and AF: SSCloAFlo and SSChiAFhi. The SSChiAFhi fraction is especially fluorescent in channels excited by the 488-nm laser. In the experiments shown, AF was recorded in FL1.
Figure 1.
Characterization of resident dermal APC. (A) Strategy used in the characterization of resident CD45+HLA-DR+ dermal cells showing successive gating on DAPI-live cells, CD45+ cells, and HLA-DR+ cells. Progressive refinement of SSCloAFlo and SSChiAFhi fractions is depicted at each step by the corresponding panel below. CD45+ cells include macrophages, DC, mast cells, and lymphocytes. Mast cells (high SSC) are HLA-DR−, as are the majority of lymphocytes (low SSC), and are easily excluded by the final HLA-DR gate. AF was recorded in the FL1 channel (488-nm laser and 530/30 band-pass filter). Similar results were found in >50 independent preparations of skin. (B) Further analysis of SSCloAFlo and SSChiAFhi fractions of CD45+HLA-DR+ cells by expression of CD14+ and CD1a+. Blue indicates the position of isotype controls and red the expression of CD14/CD1a by each fraction. Three principal populations were sorted and show distinct morphological appearances by Giemsa staining (right). Bar, 20 µm. A representative example of three experiments is shown. (C) Surface phenotype of resident CD45+HLA-DR+ dermal cells. Gates were placed as described in A and B using CD45, HLA-DR, SSC, AF CD1a, and CD14 to define macrophages (mac), CD14+ DC, and CD1a+ DC. CD14 expression on DC cells was analyzed by gating on CD1a+ and CD1a− cells. All other markers for CD1a+ DC and CD14+ DC were determined by gating on CD14− and CD14+ populations, respectively. Staining was performed at least six times on different skin preparations with similar results. (D) Identification of large melanosome-laden macrophages by CD45, HLA-DR, and FXIIIa. Freshly isolated APC prepared on cytospin were simultaneously stained with antibodies to CD45 (green), HLA-DR (red), and FXIIIa (blue). After immunofluoresence imaging the coverslip was removed and the slide stained with Giemsa. FXIIIa staining is restricted to macrophages that are a subset of the HLA-DR+ APC in the preparation (red). A number of small agranular HLA-DR+ DC with reniform nuclei are FXIIIa negative. All HLA-DR+ cells and a number of small lymphocytes in the field are highlighted by CD45 staining. Bar, 20 µm. This finding was reproduced three times.
CD45+HLA-DR+SSChiAFhi cells comprise a striking population characterized by intracytoplasmic melanosomes (Fig. 1 B). Their appearance contrasts markedly with CD45+HLA-DR+SSCloAFlo cells, which further analysis shows to contain the classical human dermal DC populations characterized by differential CD14 and CD1a expression (11, 14–20) (Fig. 1 B). Note that plotting CD14 versus CD1a directly from gated CD45+HLA-DR+ dermal cells results in overlap of the CD14+ cell and the macrophage, which is highly autofluorescent but also expresses a low level of CD14 (Fig. 1 B).
There are consistent morphological differences between SSCloAFlo CD1a+CD14− and CD14+CD1a− subpopulations (Fig. 1 B). As shown in Figs. 1 and 2, CD14+ DC display several intermediate phenotypic and functional properties of CD1a+ DC and macrophages. We found no evidence to indicate that circulating monocytes contribute significantly to the CD14+ DC population. In particular, B cells, which are present at an ∼1:1 ratio with monocytes in blood, are equivalent to only 2–5% of the number of CD14+ cells present (unpublished data). In addition, monocytes have much higher CD52 and do not up-regulate HLA-DR to the same level after overnight exposure to medium from a dermal digest preparation (unpublished data).
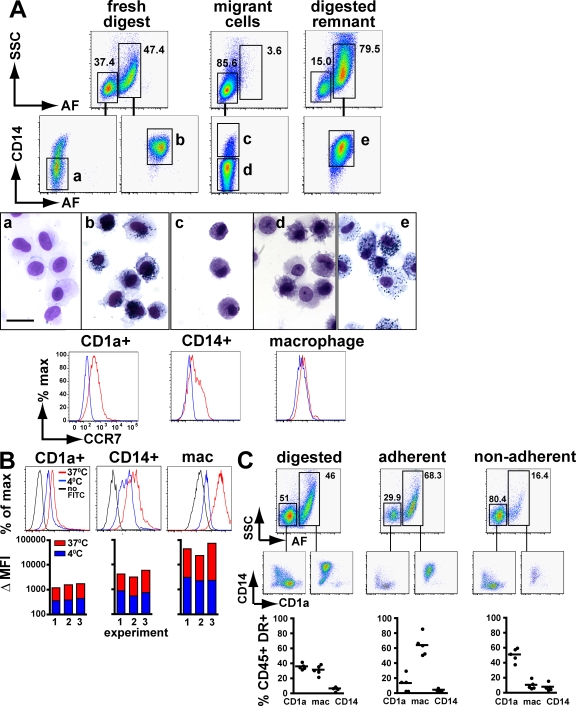
Figure 2.
Functional properties of dermal myeloid cells. (A) Differential migration of dermal myeloid cells. 1-cm square dermal sheets were freshly digested with collagenase or incubated in RPMI 10% FCS for 72 h in low adhesive culture plates, after which migrant cells were collected and the remnant dermis was digested to release nonmigrating cells. CD45+HLA-DR+ gated cells were analyzed by SSC and AF and further separated into CD14+/− fractions as described in Fig. 1. Populations a–e were sorted by flow cytometry for morphological analysis as shown. CCR7 expression was determined on freshly isolated populations. The experiment was repeated 12 times with similar findings. Bar, 20 µm. (B) Phagocytosis of FITC dextran by dermal myeloid cells. 5 × 105 collagenase-digested dermal cells were incubated with 1 mg/ml of FITC-coated dextran particles at 37 or 4°C for 1 h in RPMI containing 10% FCS. Cells were harvested and washed before immunostaining. Fluorescence histograms show a representative example. For the bar charts, all three are independent experiments. ΔMFI, change in geometric mean fluorescence compared with no dextran control. (C) Adherence of dermal myeloid cells. 5 × 105 collagenase-digested cell suspensions were cultured overnight in RPMI containing 10% FCS. Nonadherent cells and washes were collected and pooled. Adherent cells were removed by trypsinization. Flow cytometric analysis was performed using SSC, AF, CD14, and CD1a according to Fig. 1. A representative example is shown with the cumulative results of five independent experiments. Bars represent the means.
The surface phenotype of each population was defined using the strategy outlined in Fig. 1 (A and B) to identify CD1a+ DC, CD14+ DC, and macrophages (Fig. 1 C). CD1a+ DC are uniformly CD1c high but usually do not express CD163. CD14+ DC show variable CD1a and CD1c expression in addition to CD163. Macrophages show consistent CD163 and CD14 expression but lack CD1a or CD1c. Expression of CD45 and HLA-DR is lower on macrophages as determined by the change in mean fluorescence intensity or by mean fluorescence intensity ratio, but because of higher background AF the absolute brightness of HLA-DR staining always appears equivalent to the DC populations. Measurement of lectin expression was hampered by sensitivity to collagenase and is not depicted. Costimulatory molecules CD80 and CD86 are expressed predominantly by CD1a+ DC and at a lower level on CD14+ DC; macrophages usually express only a low level of CD86. CD83 expression is largely restricted to CD1a+ DC. The integrin CD11c, but not CD11b, is DC restricted as in the mouse. The intracellular antigen factor XIIIa (FXIIIa) is expressed at much higher levels by in vitro–digested macrophages than either DC (change in mean fluorescence intensity of 17,448 units above control compared with 2,181 units). On cytospin preparations, FXIIIa specifically identifies macrophages, marking a subset of large HLA-DR+ cells that contain melanosomes when the section is subsequently stained with Giemsa. Many other HLA-DR+ DC in the same field are not highlighted by FXIIIa staining (Fig. 1 D). It has previously been reported that FXIIIa identifies macrophages in situ (9). We also found that this antigen marked a subset of HLA-DR+ cells in situ that contain melanosomes when the section is subsequently lightly stained with hematoxylin and eosin (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20081633/DC1). Gene expression studies also confirm that much higher levels of FXIIIa messenger RNA are found in macrophages than in either of the DC subsets (Fig. S1 B). We therefore conclude that FXIIIa is an appropriate marker to identify macrophages for the purpose of microscopy, even though low level FXIIIa expression may be detectable in DC populations by flow cytometry.
In summary, the dermal macrophage may be defined as CD45+HLA-DR+SSChiAFhiCD14+CD1a−FXIIIa+. Two subsets of dermal DC are found in the CD45+HLA-DR+SSCloAFlo fraction that may be further divided by CD1a and CD14 expression. In the studies that follow, we use CD45, HLA-DR, SSC, AF, and CD14/CD1a for flow cytometry and HLA-DR, CD14, and FXIIIa for microscopy. As shown by others, CD163 may also substitute for FXIIIa in in situ studies of macrophages and, similar to FXIIIa, this is also detectable at low levels on CD14+ DC by flow cytometry (9, 20).
Functional properties of human dermal APC
Having defined three principal populations of dermal APC by surface phenotype, we compared the in vitro properties that conventionally separate DC from macrophages. After 72 h in culture, CD1a+ and CD14+ dermal DC migrate easily from dermal sheets, but the macrophages remain fixed (Fig. 2 A) and can only be recovered from the digested dermal “remnant.” Freshly isolated CD1a+ DC have uniform CCR7 expression but macrophages remain negative and CD14+ DC are variable (Fig. 2 A). We attempted to induce migration of macrophages using a variety of inflammatory mediators, including GM-CSF, LPS, TNF-α, and IFN-γ, but none of these stimuli induced them to leave the dermis or up-regulate expression of CCR7 (unpublished data). Differences in phagocytosis of FITC dextran (Fig. 2 B) and adherence to tissue culture plastic (Fig. 2 C) are also evident. These results are consistent with the classical view that DC are nonadherent cells with lower phagocytic activity than highly adherent macrophages. When freshly isolated, CD14+ DC show intermediate behavior.
Dermal macrophages, but not DC, survive conditioning
As a first step to defining the turnover of dermal APC during transplantation, we investigated their ability to survive conditioning therapy, the preparative regimen of cytoreductive drugs and total body irradiation which is given to patients before they receive allogeneic cells. Conditioning therapy depletes the blood of leukocytes but the effect on interstitial APC, such as dermal DC and macrophages, has not been previously studied.
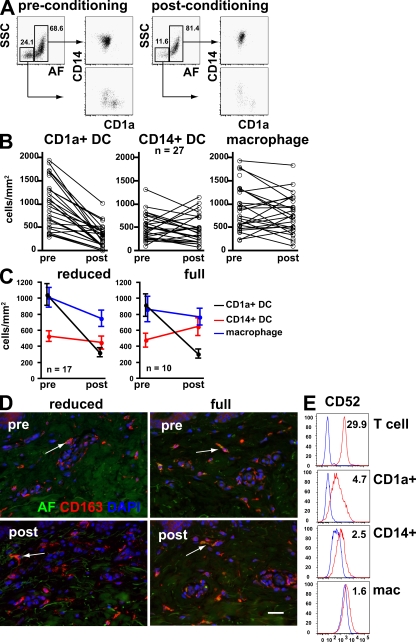
We obtained pre- and postconditioning skin shave biopsies from patients scheduled for hematopoietic stem cell transplantation. Postconditioning biopsies were taken on day 0. Dermal DC and macrophages per unit area were measured by freshly digesting a standard area of dermis and counting the total number of cells by flow cytometry. We used a similar gating strategy to that defined in Fig. 1 to count CD1a+ DC, CD14+ DC, and macrophages (Fig. 3 A). This reveals rapid and selective depletion of CD1a+ DC but preservation of macrophages and CD14+ DC (Fig. 3 B). Selective depletion of CD1a+ DC is observed in patients receiving both reduced intensity conditioning, which does not completely ablate the hematopoietic stem cell compartment, and full intensity or myeloablative conditioning (Fig. 3 C; and see Table S1 [available at http://www.jem.org/cgi/content/full/jem.20081633/DC1] for further clinical details). CD14+ DC appear to increase slightly after full intensity conditioning, possibly reflecting some recruitment caused by cytotoxic injury. The preservation of dermal macrophages is also seen directly with immunofluorescence staining, using CD163 as an alternative marker to FXIIIa (Fig. 3 D). Because alemtuzumab (humanized anti-CD52 antibody) was a component of the conditioning regimen in this cohort of patients, we also determined the level of CD52 expression by different APC populations. As shown in Fig. 3 E, the expression of CD52 is much lower on dermal APC compared with dermal T cells, which are similar to peripheral blood T cells (not depicted). Although CD1a+ DC have slightly higher CD52 levels than CD14+ DC and macrophages, it appears unlikely that direct cytotoxicity by alemtuzumab is a major cause of differential depletion of DC in this cohort of patients.
Figure 3.
Effect of conditioning therapy on recipient dermal DC and macrophages. (A) Representative flow cytometry analysis of dermal cell suspensions derived from clinical biopsies by collagenase digestion before and after conditioning. Selective depletion of SSCloAFloCD14−CD1a+ DC is evident. (B) Paired analysis of dermal cells obtained before and after conditioning from a total of 27 patients showing a decline in CD1a+ DC but preservation of CD14+ DC and macrophages. Enumeration of cells per unit area was achieved using a standard 2-mm punch of shaved dermis freshly digested into single cell suspension with collagenase. The total number of cells present in each fraction was quantified with the aid of Trucount fluorescent beads. (C) Subgroup analysis of 17 patients treated with reduced intensity and 10 patients with full intensity conditioning showing similar depletion of dermal DC in both groups. P <0.001 for before and after counts (Wilcoxon rank sum test). There is also a slight but nonsignificant increase in CD14+ DC with full intensity conditioning. Error bars indicate SD. (D) Immunofluorescence images of skin before and after conditioning showing maintenance of CD163+ perivascular macrophages. AF in the green channel can be seen in some cells (arrows). Bar, 50 µm. Two representative examples of six patients are shown. (E) Expression of CD52 antigen by dermal APC (red) compared with isotype controls (blue). Numbers indicate median fluorescence intensity ratios. Dermal T cells in the same preparation are shown for comparision. Normal skin was prepared stained and gated as described in Fig. 1.
Differential replacement of dermal DC and macrophages during transplantation
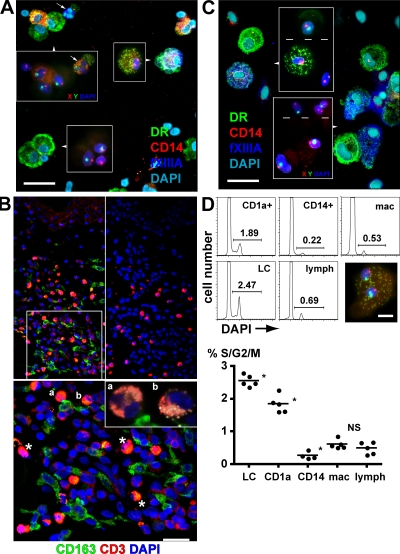
Differential rates of replacement of BM-derived cells suggest the existence of distinct lineages. In addition, the persistence of recipient APC capable of stimulating donor T cells is thought to be a critical factor in promoting GVHD. To examine these questions, we measured the repopulation of each APC compartment by donor cells. Dermal APC were prepared from shave biopsies of patients receiving hematopoietic stem cell grafts from sex-mismatched donors. Chimerism was analyzed by sequential immunofluorescence and fluorescence in situ hybridization (FISH) with X/Y probes. A combination of antibodies to HLA-DR, CD14, and FXIIIa was selected in order to define the same three populations of CD1a+ DC, CD14+ DC, and macrophages that had been previously characterized. Anti-CD3 was also added to migratory preparations to identify lymphocytes. In these experiments, CD1a+ DC were identified as HLA-DR+CD14−FXIIIa−, CD14+ DC as HLA-DR+CD14+FXIIIa−, and macrophages as HLA-DR+CD14+FXIIIa+. Although there was some low-level induction of FXIIIa on migratory CD14+ cells, the majority of strongly FXIIIa+ cells were confined to digested preparations (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20081633/DC1). These were further confirmed as macrophages either by the observation of fluorescent melanosomes during FISH or by a third sequential analysis with Giemsa staining to demonstrate intracellular melanin as described earlier in Fig. 1 D.
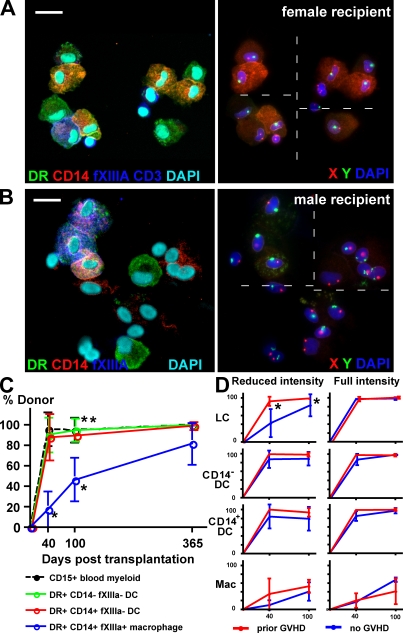
A cohort of 52 patients was biopsied at intervals of 40, 100, and 365 d after transplantation (see Table S2 [available at http://www.jem.org/cgi/content/full/jem.20081633/DC1] for further clinical details). Biopsies were either digested freshly or split into migratory and digested remnant preparations. LC from epidermal sheets of the biopsies were also prepared for comparison. Fig. 4 A shows a migratory dermal preparation containing predominantly donor-derived CD14+ and CD14− DC. A single recipient FXIIIa+ macrophage is also present. A digested remnant preparation in Fig. 4 B contains mostly recipient macrophages and some residual donor-derived CD14− (CD1a+) DC. Regardless of the mode of sample preparation, we consistently observed higher donor engraftment of both subsets of DC than macrophages (Fig. S3 and Table S3 available at http://www.jem.org/cgi/content/full/jem.20081633/DC1). The pooled data are presented in Fig. 4 C. This shows that recipient CD14+ and CD14− DC are nearly completely replaced by 40 d, which is in synchrony with peripheral blood myeloid engraftment, but that a proportion of recipient macrophages remain for at least 365 d (Fig. 4 C). The prolonged survival of recipient dermal macrophages is striking. Their median engraftment is only 17% at 40 d and 46% at 100 d, with little evidence that GVHD promotes donor engraftment (Fig. 4 D). In most samples, there is a slightly greater retention of recipient HLA-DR+CD14+ DC than HLA-DR+CD14− DC, reaching statistical significance at day 100 (Fig. 4 C). We interpret this slight recipient bias as indicating a slightly slower turnover of CD14+ cells and as evidence against their potential role as precursors of the CD14− (CD1a+) DC. Comparison of the engraftment of dermal DC and LC proves that the majority of HLA-DR+CD14− dermal DC are not derived from migrating LC but have an independent turnover from the blood. Indeed, at 40 d, patients transplanted with reduced intensity conditioning in the absence of GVHD have a median of 43.1% donor LC but 87.7% donor dermal DC (Fig. 4 D). Occasional LC of recipient origin were observed among the migrating dermal cells but they were consistently <2% of HLA-DR+ cells.
Figure 4.
Posttransplant dermal DC and macrophage chimerism. (A) Dual immunofluorescence (left) and FISH (right) of migrated dermal cells from a female recipient 40 d after transplantation. Donor-derived HLA-DR+CD14− DC and HLA-DR+CD14+ DC can be seen together with a mixture of CD3+ lymphocytes and a single recipient HLA-DR+CD14+FXIIIa+ macrophage. A small number of FXIIIa+ macrophages were seen in migratory preparations and, like the one shown, were often recipient in origin but more lightly granulated than the cells released from digested preparations. Bar, 20 µm. Dashed line indicates stitching of high-power fields. The representative example was taken from the cohort of 52 patients. (B) Dual immunofluorescence (left) and FISH (right) of remnant digested dermal cells from a male recipient 40 d after transplantation. Two donor-derived HLA-DR+CD14− dermal DC can be seen together with three recipient HLA-DR+CD14+FXIIIa+ macrophages and number of unlabeled stromal cells. Autofluorescent melanin is visible on the FISH image of the FXIIIa+ cells, confirming their identity as macrophages. Bar, 20 µm. Dashed line indicates stitching of high-power fields. The representative example was taken from the cohort of 52 patients. (C) Engraftment kinetics of dermal DC and macrophages compared with blood myeloid cells, derived from a cohort of 52 patients biopsied at 40, 100, and 365 d after transplant. Error bars show SD. *, P <0.001 compared with any other population (Mann-Whitney U test); **, P = 0.02 comparing CD14+ DC with CD14− DC (Wilcoxon rank sum test). (D) Engraftment kinetics of each cutaneous APC as a function of intensity of conditioning and prior GVHD. GVHD has a significant effect on LC engraftment both at 40 and 100 d but does not influence macrophage engraftment. Error bars show SD. *, P < 0.05 (Mann-Whitney U test).
Recipient dermal macrophages survive cutaneous GVHD
Having demonstrated that recipient dermal macrophages are replaced more slowly than either population of dermal DC or LC, we sought further evidence that they could survive the inflammatory insult of GVHD and therefore potentially contribute to alloreactivity in the skin. In patients with recent or active acute GVHD, donor DC are found together with recipient macrophages. In the example shown (Fig. 5 A), there are 92% donor CD14− DC and 90% donor CD14+ DC but 83% recipient macrophages. Eosinophils in the preparation frequently function as a cytological indicator of active GVHD. In situ staining of cutaneous GVHD with CD163, an alternative macrophage marker, reveals close association between infiltrating T cells and perivascular macrophages (Fig. 5 B, top left and magnified bottom inset). Eosinophils are also notable in these lesions (Fig. 5 B, bottom [asterisks] and inset [a and b]). These observations do not prove that recipient macrophages directly contribute to cutaneous GVHD but at least satisfy the condition that they are present in GVHD lesions.
Figure 5.
Recipient dermal macrophages survive GVHD. (A) Dual immunofluorescence and FISH (insets) of a cytospin of remnant-digested dermis from a female patient with acute GVHD 40 d after transplantation. Donor-derived male DC and lymphocytes can be seen in addition to a donor eosinophil (arrows). A recipient macrophage is clearly visible. Bar, 20 µm. (B) Immunofluorescence staining of skin affected by GVHD showing CD163+ macrophages in direct contact with infiltrating CD3+ T cells (top left and bottom magnified inset). Eosinophils appear orange because of their bright AF and nonspecific staining (asterisks, a, and b). They are also highly visible in the control with secondary antibodies only (top right) and were confirmed in the section at high magnification (bottom, inset, a and b). Bar, 20 µm. The figure shows the results of one of two patients with GVHD analyzed in the same fashion. (C) Dual immunofluorescence and FISH (insets) of a cytospin of digested dermis from a female patient who had prior acute GVHD taken at 365 d after transplantation. Recipient macrophages still persist and are highly granulated. Engrafting male donor macrophages by comparison are weakly granulated. Bar, 20 µm. Dashed lines in insets indicates stitching of high power fields. (D) DNA ploidy analysis of leukocytes from normal skin. DAPI histograms are shown from a representative experiment on the left, and the means with cumulative data from five independent experiments are shown on the right. The inset shows a binucleate dermal macrophage from a male patient. Bar, 10 µm. *, P < 0.05 by Mann-Whitney U test compared with neighbor (NS, P = 0.55).
Previous studies in mice and humans have shown that recipient LC are efficiently eliminated by GVHD (29, 40). We were surprised to discover that a substantial number of recipient macrophages remained even in patients with prior GVHD. In the example shown at 1 yr after transplantation and 10 mo after the onset of acute skin GVHD, 32% of macrophages have recipient XX chromosomes (Fig. 5 C). The ingestion of melanin appears to increase with the age of the cells because recipient cells have a much higher content than more juvenile donor cells.
To examine mechanisms that promote the survival of recipient macrophages, we measured DNA ploidy to determine their proliferative potential (Fig. 5 D). Both LC and CD1a+ dermal DC contain significant hyperdiploid fractions, 2.6 and 1.8% respectively, but CD14+ DC, macrophages, and lymphocytes are all <1%. Occasional binucleate, rather than G2/M-phase cells, probably account for 0.6% hyperdiploid macrophages because there is a virtual absence of any S-phase population and binucleate cells were occasionally observed on cytospin preparations (Fig. 5 D, inset). This observation is in keeping with their inability to migrate ex vivo and the notion that macrophages are fixed.
Dermal macrophages secrete inflammatory cytokines
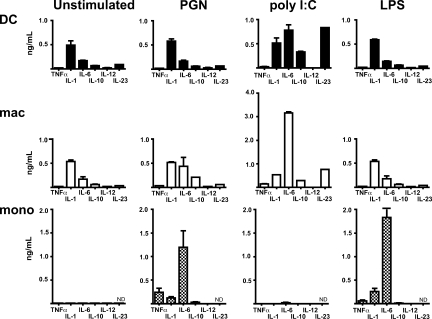
The innate immune function of resident dermal macrophages has not been previously investigated, and the conventional role of macrophages in promoting GVHD by innate mechanisms is at odds with more recent descriptions of their regulatory role in tissues such as the gut (49, 50). Spontaneous and stimulated cytokine release was measured in comparison with blood monocytes (Fig. 6). Purified CD1a+ DC and macrophages were obtained by cell sorting freshly digested dermis. Cells were gated as outlined in Fig. 1 and purity after sorting was usually at least 95% and viability at least 90% (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20081633/DC1). Both DC and macrophages exhibit proinflammatory profiles with significant production of IL-1 and IL-6. This is particularly enhanced by peptidoglycan and poly-IC stimulation, including induction of TNF-α and IL-23. Dermal macrophages make little IL-10 and do not induce Foxp3+CD25hi regulatory cells from naive T cells in the presence of TGF-β (unpublished data). In these experiments, the blood monocyte was notable for a very sensitive response to LPS.
Figure 6.
Cytokine production by dermal DC and macrophages. Cytokine analysis in the supernatant from 24-h culture of dermal DC, macrophages, and blood monocytes. 40,000 sorted cells were left unstimulated or treated with 10 ng/ml peptidoglycan (PGN), 10 µg/ml poly IC, and 0.1 µg/ml LPS. Data shown are the mean ± SEM of at least three independent experiments.
Allogeneic T cell responses to dermal macrophages
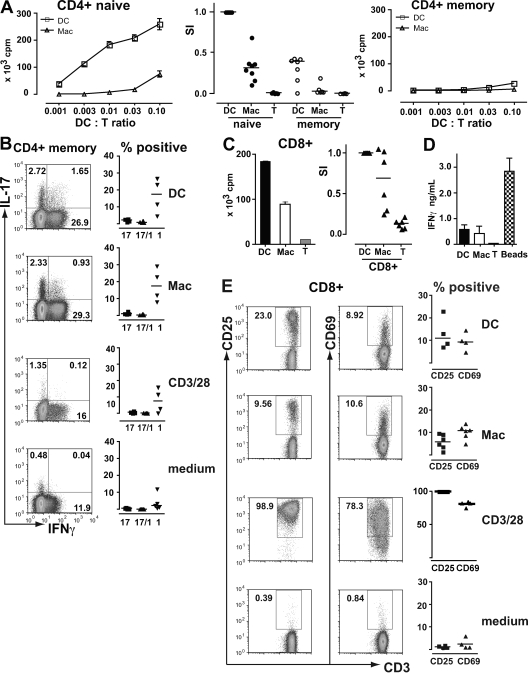
The previous results establish that dermal macrophages isolated from normal skin appear to have a proinflammatory phenotype. We sought evidence of their potential role in antigen-specific alloreactivity by purifying CD1a+ DC and macrophages from normal dermis and using them to stimulate naive CD4+ T cells, memory CD4+ T cells, and CD8+ T cells. Proliferative responses, cytokine secretion, and expression of activation antigens were measured. As expected, CD1a+ dermal DC are ∼100-fold more potent than macrophages at stimulating naive CD4+ T cells (Fig. 7 A). Surprisingly, neither DC nor macrophages are able to stimulate much proliferation in memory CD4+ T cells, compared with anti-CD3/CD28 beads, although both cells induce significant IL-17 and IFN-γ production compared with controls (Fig. 7 B). Macrophages are also able to stimulate proliferation, cytokine secretion, and expression of activation antigens by allogeneic CD8+ T cells. These responses are relatively efficient compared with dermal CD1a+ DC. Macrophages elicit ∼50% levels of proliferation and CD25 expression and similar levels of IFN-γ production and CD69 expression compared with dermal DC (Fig. 7, C–E). These results confirm the classical view of macrophages as poor stimulators of naive T cells. They also highlight a recent and unexpected finding that tissue-derived myeloid DC may be much less efficient at stimulating memory T cell proliferation than previously assumed (51). Finally, the ability of macrophages to induce cytokine responses in memory CD4+ T cells and stimulate CD8+ T cells suggests that they could potentiate the responses of alloreactive T cells during transplantation.
Figure 7.
Allostimulatory properties of dermal DC and macrophages. (A) [3H]thymidine incorporation of allogeneic CD4+ T cells stimulated by CD1a+ DC or macrophages. The left and right show representative examples of six experiments in which APC were titrated against naive and memory T cells as indicated. Stimulation with anti-CD3/28 beads + naive CD4 produced 442 × 103 cpm and with anti-CD3/28 beads + memory CD4 produced 430 × 103 cpm. Results are the mean ± SEM of triplicate wells. The middle shows the cumulative results and mean of at least seven independent experiments at an APC/T cell ratio of 1:10 compared with medium alone controls (T). The stimulation index (SI) was normalized to the proliferation of naive T cells with DC. (B) Intracellular IL-17 and IFN-γ production by memory CD4+ T cells in response to DC, macrophages, anti-CD3/28 beads, or medium alone. Representative examples of four independent experiments are shown on the left, with gating for IL-17–expressing Th17 cells and IFN-γ–expressing Th1 cells. The mean and cumulative results of four independent experiments are shown on the right, expressed as the percentage of Th17, Th17/1, or Th1 cells in each. (C) [3H]thymidine incorporation of allogeneic CD8+ T cells stimulated by dermal DC or macrophages or medium alone (T). The left shows a representative example of the data at an APC/T cell ratio of 1:10. Results are the mean ± SEM of triplicate wells. The right shows the cumulative results and mean of six independent experiments. (D) IFN-γ secretion into the medium of CD8+ T cells stimulated with DC, macrophages, medium alone (T), or anti-CD3/CD28 beads (beads). Results show the mean ± SEM of six independent experiments. (E) Activation antigen expression by CD8+ T cells stimulated by DC, macrophages, anti-CD3/28 beads, or medium alone. Representative examples of the data with gating for positive cells are shown on the left. The mean and cumulative results of four independent experiments are shown on the right, expressed as the percentage of positive cells in each.
DISCUSSION
Elucidation of the ontogeny and function of myeloid APC is critical to understanding the generation of immunity. Moreover, the initiation of graft versus host responses in hematopoietic stem cell transplantation is governed by the persistence of recipient APC of undefined origin and location. To understand this problem further, we set out to solve the complexity of human interstitial APC, characterize their survival after hematopoietic stem cell transplantation, and assess their innate and antigen-specific interactions with allogeneic T cells.
We have presented a comprehensive analysis of dermal APC that allows us to understand not only previous data using dual immunofluorescence to study DC and macrophages in situ (9, 10) but also more functional studies that have focused on the isolation of different skin migratory DC populations (11, 14–20). We find three principal subsets of myeloid cells in human dermis: HLA-DR+CD14−CD1a+ DC; HLA-DR+CD14+CD1a− DC and HLA-DR+CD14+CD1a−FXIIIa+ dermal macrophages. The CD14+ DC migrates in vitro but retains some intermediate properties of both DC and macrophages. As a migratory cell, recent evidence indicates that it has a specialized function in priming follicular helper CD4+ T cells (20). Most previous reports have not fully accounted for the existence of three distinct populations. Studies on migratory cells containing CD1a+ and CD14+ DC are unable to determine the origin of these cells in situ because they do not account for the major population of macrophages, which share a number of markers with CD14+ DC but do not migrate. In situ studies have focused on two populations, CD1a+ DC and macrophages, but do not define the place of the CD14+ DC (9, 10). Others, using freshly digested dermis, have not explicitly excluded macrophages from the CD14+ DC subset. This potentially distorts the characterization of freshly isolated CD14+ DC and makes them appear more like macrophages when they are compared with CD1a+ DC (11, 16, 18).
Human dermal macrophages can be distinguished by SSC and AF and constitute up to 40% of CD45+HLA-DR+ dermal cells. Their characteristic physical properties are a result of the ingestion of huge quantities of melanin. Tattoo pigment ingestion by dermal macrophages was recently highlighted (9), but our data suggests that melanin uptake is a significant physiological function in normal skin. Indeed the term “melanophage,” which is usually reserved for describing phagocytes seen in hyperpigmentation disorders or melanotic lesions (52–55), is entirely appropriate. Macrophages are marked by CD14, CD163, and FXIIIa as previously noted (9, 56, 57). We demonstrated the specificity of FXIIIa staining on cytospins by showing that it colocalizes with large cells containing melanosomes. A similar pattern of staining is observed in vitro. Flow cytometry reveals low level expression of FXIIIa by DC as previously reported (9, 14, 20), but the level is approximately tenfold lower than on macrophages.
Low SSC, nonautofluorescent CD45+HLA-DR+ cells of the dermis contain the two migratory APC of the dermis characterized by the expression of CD1a and CD14, respectively. Previous comparisons of skin migratory or in vitro–derived CD1a+ and CD14+ cells have concluded that the CD14+ is a form of immature or specialized DC (18, 19, 58, 59). The most recent of these analyses shows that migratory CD14+ DC have a specific function in priming follicular helper T cells (20). Others have suggested additionally that a component of these cells can differentiate into LC (16) or macrophages (11). Because the CD14+ DC has slightly delayed engraftment relative to the CD1a+ cell, it cannot be an immediate precursor of CD1a+ DC. Indeed, its phagocytic properties, lack of DNA synthesis, and morphology are reminiscent of dermal macrophages. It could be argued that the CD14+ cells, or at least a subset of them, are precursors of macrophages that have just not yet acquired a niche in the dermis. Further studies will be required to clarify that these cells actually traffic to the LN. Certainly, in vitro studies suggest a much inferior migratory capacity in response to CCR7 ligands (18). Although it is conceivable that circulating monocytes might be included among the dermal CD14+ population, we found little evidence to support this.
Having defined the myeloid cells of human dermis in detail, we examined their replacement during hematopoietic stem cell transplantation. After conditioning therapy, CD1a+ dermal DC are rapidly depleted, whereas CD14+ DC and macrophages are retained. After transplantation, both dermal DC are replaced by cells from the donor within weeks, but macrophages remain at least partly of recipient origin for >1 yr. This result supports the view that resident macrophages and immature DC are two distinct lineages at the tissue level, even though they share some markers and physiological attributes and are ultimately both BM derived. It is unlikely that resident macrophages are able to mature into migratory myeloid DC but more conceivable that they retain a distinct functional role in the dermis, even during inflammation. Their survival is consistent with our previous observation that a fraction of human HLA-DR+ dermal cells remain recipient in origin for at least 1 mo after transplantation (47). Prior reports of human alveolar macrophages and Kupffer cells have emphasized their BM origin and described complete donor engraftment within 3 mo (24, 25). Neither alveolar macrophages nor Kupffer cells may be typical of interstitial macrophages resident within epithelia such as the skin and gut. Unlike recipient LC, which turn over in response to inflammation, recipient macrophages survive GVHD and can be found in the perivascular regions of inflamed skin, interacting with T cells. They are progressively replaced by donor cells but with relatively invariant kinetics. There are also marked differences in the turnover of epidermal LC compared with dermal DC, which is most easily seen after reduced intensity conditioning. This reinforces the idea that LC have self-renewal capacity in humans.
The importance of the innate response of recipient macrophages to the induction of GVHD is well accepted (31, 32, 60). However, direct observations on human macrophages are scarce. Moreover, recent studies in the gut have assigned an antiinflammatory role to tissue macrophages through the production of IL-10 and fostering of regulatory T cell development (49, 50). Our results show, in contrast, that dermal macrophages are natural producers of IL-1 and IL-6 and synthesize TNF-α and IL-23 upon stimulation. Compared with monocytes, dermal macrophages are more polarized to respond to peptidoglycan and poly IC than to LPS, presumably reflecting the range of gram positive and viral pathogens they are likely to encounter in the skin.
We argue that in addition to providing critical inflammatory signals, recipient macrophages may also have the capacity to enhance GVHD through antigen-specific means by presenting host tissue antigens. This concept has precedence in mouse data showing that macrophages can play a primary role in the induction of antigen-specific cellular immunity (61) and allogeneic responses (62). Experiments using clodronate liposome-treated mice also suggest a role for local resident macrophages in recruiting and targeting T cells to sites of GVHD (30).
Testing the function of human APC in alloreactivity is complex because it is not immediately apparent in humans whether CD4+ or CD8+ T cells or both must be activated to induce GVHD or whether naive or memory T cell subsets contain the critical alloreactive populations. Lymphocyte recirculation must also be considered. Memory CD4+ T cells form the bulk of dermal T cells in normal skin (63, 64), and it is therefore unlikely that nonmigratory macrophages come into contact with naive CD4+ T cells, at least under quiescent conditions. However, a direct interaction with CD8+ T cells is possible because we have consistently observed CD8+ T cells in the dermis of transplant patients, even in the absence of clinical GVHD (unpublished data).
We were initially surprised to find that there was almost no proliferative response of memory CD4+ T cells to allogeneic DC or macrophages, given that memory cells are known to have a lower threshold for proliferation when stimulated via CD3/CD28 ligation (65). However, recent in vivo data show that migratory myeloid DC are very inefficient at stimulating the proliferation of CD8+ memory cells (51). The mechanism of this remains unknown and there are no previous studies in humans testing the proliferative response of memory CD4+ T cells to primary tissue DC. Recent work using in vitro–derived DC shows stimulation of memory CD4+ cell cytokine responses, which is consistent with our findings (20). An additional possibility that we cannot exclude is that the CD4+ memory T cell population in humans may not contain alloreactive cells, as has been described in mouse systems (66, 67).
Overall, it is unlikely that recipient macrophages are sufficient to induce acute GVHD, but their ability to stimulate cytokine production by memory CD4+ T cells and to activate CD8+ T cells is consistent with a role in which they may potentiate allogeneic responses locally. Clinically, this could contribute to relapses of acute GVHD mediated by antigen-experienced T cells after immunosuppression withdrawal in the manner of a secondary viral infection (68). Immunosuppression is frequently maintained until 3 mo after transplantation in humans, long after the replacement of DC and LC, but not macrophages, by donor cells. In addition, there are clear threshold effects to GVHD induction by DLI in humans (34–38), and persistent recipient macrophages could promote post-DLI GVHD by raising the frequency of alloreactive T cells above a critical level. Recipient macrophages in a number of sites might also contribute to chronic GVHD, and provide a source of recipient hematopoietic minor histocompatibility antigen, to stimulate graft versus leukemia responses.
In conclusion, this study provides a comprehensive description and functional analysis of human dermal APC, with sufficient resolution to demonstrate the differences in replacement of DC and macrophages after hematopoietic stem cell transplantation. We find evidence that dermal DC and macrophages are independent lineages and that surviving macrophages may have the potential to sustain graft versus host responses long after recipient DC have been eliminated.
MATERIALS AND METHODS
Cell isolation and culture.
Human samples were obtained after informed consent and ethical permission from the Newcastle Research Ethics Committee. Normal skin was obtained from mammoplasty surgery, and 2–4-mm shave skin biopsies were taken from the posterior iliac crest of patients undergoing transplantation. Epidermal and dermal sheets were prepared by digestion of patient shave biopsies or 300-µm skin dermatome sections of normal skin with 1 mg/ml dispase (Invitrogen) in RPMI (Invitrogen) for 60 min at 37°C. Large dermal sheets were cut into 0.5-cm squares or smaller and incubated with 0.8 mg/ml collagenase (Type IV; Worthington) in RPMI with 10% FCS for 8 h in a bacterial Petri dish to prevent adherence of leukocytes or cultured in RPMI (or X-Vivo 10; Cambrex) with 500 U/ml GM-CSF (Sargramostim; Durbin PLC) for collection of migrating cells. After 72 h of migration, dermal remnants were treated with collagenase in the same manner as fresh dermis. Viability was usually 80–90% by DAPI exclusion (Partec). Dermal macrophages and DC were isolated to >95% purity from freshly digested dermal cells suspension by fluorescence activated cell sorting using a FACS Aria Special Order System (BD) configured with 100-mW 488-nm sapphire lasers, 60-mW 350-nm UV lasers, 40-mW 640-nm red lasers, 50-mW 407-nm violet lasers, a 100-µm nozzle, and 20 psi (Fig. S4). Macrophage AF was detected using the 488-nm excitation laser with a bandpass filter of 530/30 nm.
Naive and memory CD4+ T cells were isolated to >90% purity by automated magnetic negative selection using EasySep human naive and memory CD4+ T cell isolation kits and RoboSep (StemCell Technologies Inc.) from peripheral blood mononuclear cells. CD8+ T cells were isolated to >95% purity by negative selection using RosetteSep CD8+ T cell isolation cocktail from whole blood (StemCell Technologies Inc.). Monocyte-derived DC were generated from magnetically isolated CD14+ monocytes (Miltenyi Biotec) and cultured for 6 d with 50 ng/ml GM-CSF and IL-4 (R&D Systems), followed by 24-h activation with 0.1 µg/ml LPS (Sigma-Aldrich), 10 ng/ml IL-1β (PeproTech), and 10 ng/ml TNF-α (PeproTech).
Flow cytometry.
Flow cytometry was performed with LSRII and FACSCalibur cytometers (BD) and data was analyzed with FlowJo (Tree Star, Inc.). Intracellular staining was performed after fixation and permeabilization with Cytofix/Cytoperm and Perm/Wash reagents (BD). 70,000 MW FITC coated-dextran particles were obtained from Sigma-Aldrich. For determination of DNA ploidy, cells were surfaced stained, washed, and fixed with 0.5% paraformaldehyde for 5 min. This was followed by a subsequent wash, further fixation with 50% cold ethanol for 30 min, and a final wash before resuspending in 2 µg/ml DAPI solution. The following antibodies were supplied by BD unless stated otherwise and are denoted as antigen fluorochrome (clone): CD1a FITC (NA1/34; Dako); CD1a APC (HI 149); CD1c APC (AD5-8E7; Miltenyi Biotec); CD3 PE (SK7); CD11b PE (ICRF 44); CD11c APC (B-ly6); CD14 PE and PECy7 (M5E2); CD45 APC Cy7 (2D1); CD80 PE (L307.4); CD83 APC (HB15); CD86 APC (2331:FUN-1); CD163 APC (215927; R&D Systems); HLA-DR FITC, APC, and PerCP Cy5.5 (L243); CCR7 APC (150503; R&D Systems); IL-17A Alexa Fluor 647 (eBio64DEC17; eBioscience); IFN-γ FITC (25723.11); FXIIIa (sheep polyclonal; Enzyme Research Laboratories) with APC-conjugated donkey anti–sheep (Invitrogen); CD52 PE (YTH34.5; AbD Serotec).
Microscopy.
Laser confocal microscopy was performed using a confocal microscope (TCS SP2; Leica). Fluorescence microscopy was performed using a microscope (Axioplan 2; Carl Zeiss, Inc.). The following antibodies were used to stain paraffin-embedded sections: HLA-DR (L243; BD); FXIIIa (sheep polyclonal; Enzyme Research Laboratories); CD3 (rabbit polyclonal; Abcam); CD163 (NCL-CD163; Leica). The following secondary detection reagents were supplied by Invitrogen: Alexa Fluor 555–conjugated goat anti–rabbit, Alexa Fluor 568–conjugated goat anti–mouse, Alexa Fluor 633–conjugated donkey anti–sheep, and Alexa Fluor 633–conjugated goat anti–mouse.
Enumeration of dermal DC and macrophages.
Shave biopsies were cut with a 2-mm punch and digested with dispase to separate the epidermis and obtain a 3.14-mm2 disk of dermis. This was further digested with 0.8 mg/ml of collagenase for 12 h and dispersed to obtain a single cell suspension. Cells were washed once, stained with antibodies to CD45, HLA-DR, CD14, and CD1a, washed again, and transferred to 400 µl of buffer in a Trucount tube (BD). 30,000–50,000 events were recorded. Reproducibility of biopsy procedure and cell preparation was monitored by recording the weight and the fibroblast content.
Chimerism analysis.
Dermal cells prepared from clinical biopsies were harvested by spinning onto cytospin slides at 800 rpm for 5 min using a cytocentrifuge (Shandon Cytospin 4; Thermo Fisher Scientific). Cytospin slides were air dried and stored at −20°C before sequential immunofluorescence and FISH. Slides were thawed and fixed in methanol then stained with antibodies to CD14 (rabbit polyclonal; Abcam), CD3 (SK7; BD), and FXIIIa (AC-1A1; Abcam) combined with secondary Alexa Fluor 555–conjugated goat anti–rabbit and Alexa Fluor 633–conjugated goat anti–mouse IgG1 (Invitrogen) and directly conjugated anti–HLA-DR FITC. 10–12 × 40 four-color images were acquired by confocal microscopy and assembled into montages using Photoshop CS2 (Adobe). Cytospin slides were then fixed with methanol/acetic acid 3:1 for 5 min, probed with CEP X/Y DNA probes (Vysis; Abbott Molecular), mounted with Vectastain containing DAPI (Vector Laboratories), and scored for X/Y hybridization. Migratory LC were processed in a similar manner as previously described (40). Together, a total of 18,370 interphase nuclei were examined in 149 samples taken from 52 patients.
Dermal APC stimulation with TLR ligands.
4 × 104 flow-sorted dermal macrophages or DC were cultured in 96-well flat-bottomed plates. Supernatant from unstimulated and cells stimulated with 0.1 µg/ml LPS (Sigma-Aldrich), 10 ng/ml peptidoglycan (InvivoGen), and 10 µg/ml poly I:C (InvivoGen) were collected after 24 h of culture for cytokine analysis. TNF-α, IL-1, IL-6, IL-10, and IL-12p70 were detected using cytometric bead arrays (CBA-Flex; BD) and analyzed with Array software v1.0 (BD), and IL-23 was detected by ELISA (p19/p40; eBioscience).
Proliferation and T cell cytokine production assays.
104 flow-sorted dermal macrophages or DC were cultured with 105 allogeneic naive CD4+, memory CD4+, or CD8+ T cells in round-bottomed 96-well plates. CD3/28 beads were used at 1:1 ratio. On day 5 of alloreaction, the cultures received a 16-h pulse of 0.548 Mbq/ml [3H]thymidine (TRA310; GE Healthcare). Thymidine incorporation was measured using a luminescence counter (MicroBeta TriLux; PerkinElmer). CD25 and CD69 expression on CD8+ T cells were analyzed on day 5 of alloreactions. Supernatant from CD8+ alloreactions were collected on day 5 for IFN-γ analysis using cytometric bead array (BD). On day 6 of alloreactions, memory CD4+ T cells were stimulated with 50 ng/ml PMA (Sigma-Aldrich) and 500 ng/ml ionomycin (Sigma-Aldrich) for 5 h in the presence of 10 µg/ml brefeldin A (Sigma-Aldrich) for the last 4 h before intracellular staining for IFN-γ and IL-17.
Statistical analyses.
Mann-Whitney U and Wilcoxon Rank Sum tests were performed using Prism 4.0 (GraphPad Software, Inc.). All p-values are two tailed.
Online supplemental material.
Fig. S1 A shows the expression of FXIIIa in situ by immunofluorescence and correlates this with macrophages containing intracellular melanosomes. Fig. S1 B shows the relative messenger RNA expression of FXIIIa in CD1a+ DC, CD14+ DC, and macrophages. Fig. S2 gives additional examples of cytospin preparations used to determine posttransplant engraftment of APC and Fig. S3 shows that similar results are obtained using freshly digested, migrated, or remnant digest preparations. Fig. S4 shows purity after sorting of CD1a+ DC and macrophages used in the functional studies of the paper. Table S1 describes patients analyzed before and after conditioning therapy. Table S2 shows patients analyzed for dermal APC chimerism. Table S3 presents the raw data used for chimerism analysis. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081633/DC1.
Acknowledgments
We thank the patients, Stem Cell Transplant team, and Department of Plastic and Reconstructive Surgery at the Royal Victoria Infirmary, Newcastle upon Tyne. We also gratefully acknowledge the advice of Nick Bown and Wing-Hong Kwan and helpful discussion of Gwen Randolph, Milena Bogunovic, and Julie Helft.
This work was funded by the Leukaemia Research Fund (UK), Action Medical Research, Histiocytosis Association of America, Tyneside Leukaemia Research Association, and Newcastle Healthcare Charity.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: AF, autofluorescence; DLI, donor lymphocyte infusion; FISH, fluorescence in situ hybridization; GVHD, graft versus host disease; LC, Langerhans cell; SSC, side scatter.
References
- Steinman R.M., Lustig D.S., Cohn Z.A. 1974. Identification of a novel cell type in peripheral lymphoid organs of mice. III. Functional properties in vivo.J. Exp. Med. 139:1431–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H., Klechevsky E., Morita R., Aspord C., Cao T., Matsui T., Di Pucchio T., Connolly J., Fay J.W., Pascual V., et al. 2007. Dendritic cell subsets in health and disease.Immunol. Rev. 219:118–142 [DOI] [PubMed] [Google Scholar]
- Lee S.H., Starkey P.M., Gordon S. 1985. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80.J. Exp. Med. 161:475–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Taylor P.R. 2005. Monocyte and macrophage heterogeneity.Nat. Rev. Immunol. 5:953–964 [DOI] [PubMed] [Google Scholar]
- Steinman R., Hoffman L., Pope M. 1995. Maturation and migration of cutaneous dendritic cells.J. Invest. Dermatol. 105:2S–7S [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A., Henri S., Dubois B., Laplace-Builhe C., Perrin P., Romani N., Tripp C.H., Douillard P., Leserman L., Kaiserlian D., et al. 2005. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells.Immunity. 22:643–654 [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Collin M.P., Bogunovic M., Abel M., Leboeuf M., Helft J., Ochando J., Kissenpfennig A., Malissen B., Grisotto M., et al. 2007. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state.J. Exp. Med. 204:3133–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.W., Abkowitz J.L. 1997. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model.Blood. 90:986–993 [PubMed] [Google Scholar]
- Zaba L.C., Fuentes-Duculan J., Steinman R.M., Krueger J.G., Lowes M.A. 2007. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages.J. Clin. Invest. 117:2517–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa M.T., Loncaric A., Krutzik S.R., Becker T.C., Modlin R.L. 2008. “Dermal dendritic cells” comprise two distinct populations: CD1+ dendritic cells and CD209+ macrophages.J. Invest. Dermatol. 128:2225–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gruijl T.D., Sombroek C.C., Lougheed S.M., Oosterhoff D., Buter J., van den Eertwegh A.J., Scheper R.J., Pinedo H.M. 2006. A postmigrational switch among skin-derived dendritic cells to a macrophage-like phenotype is predetermined by the intracutaneous cytokine balance.J. Immunol. 176:7232–7242 [DOI] [PubMed] [Google Scholar]
- Nestle F.O., Nickoloff B.J. 2007. Deepening our understanding of immune sentinels in the skin.J. Clin. Invest. 117:2382–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D.A. 2008. Macrophages as APC and the dendritic cell myth.J. Immunol. 181:5829–5835 [DOI] [PubMed] [Google Scholar]
- Nestle F.O., Zheng X.G., Thompson C.B., Turka L.A., Nickoloff B.J. 1993. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets.J. Immunol. 151:6535–6545 [PubMed] [Google Scholar]
- Lenz A., Heine M., Schuler G., Romani N. 1993. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization.J. Clin. Invest. 92:2587–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larregina A.T., Morelli A.E., Spencer L.A., Logar A.J., Watkins S.C., Thomson A.W., Falo L.D.J. 2001. Dermal-resident CD14+ cells differentiate into Langerhans cells.Nat. Immunol. 2:1151–1158 [DOI] [PubMed] [Google Scholar]
- Morelli A.E., Rubin J.P., Erdos G., Tkacheva O.A., Mathers A.R., Zahorchak A.F., Thomson A.W., Falo L.D.J., Larregina A.T. 2005. CD4+ T cell responses elicited by different subsets of human skin migratory dendritic cells.J. Immunol. 175:7905–7915 [DOI] [PubMed] [Google Scholar]
- Angel C.E., George E., Brooks A.E., Ostrovsky L.L., Brown T.L., Dunbar P.R. 2006. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands.J. Immunol. 176:5730–5734 [DOI] [PubMed] [Google Scholar]
- Angel C.E., Lala A., Chen C.J., Edgar S.G., Ostrovsky L.L., Dunbar P.R. 2007. CD14+ antigen-presenting cells in human dermis are less mature than their CD1a+ counterparts.Int. Immunol. 19:1271–1279 [DOI] [PubMed] [Google Scholar]
- Klechevsky E., Morita R., Liu M., Cao Y., Coquery S., Thompson-Snipes L., Briere F., Chaussabel D., Zurawski G., Palucka A.K., et al. 2008. Functional specializations of human epidermal langerhans cells and CD14+ dermal dendritic cells.Immunity. 29:497–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier L., Gonzalez-Ramos A., Cooper K.D. 1993. Heterogeneous populations of class II MHC+ cells in human dermal cell suspensions. Identification of a small subset responsible for potent dermal antigen-presenting cell activity with features analogous to Langerhans cells.J. Immunol. 151:4067–4080 [PubMed] [Google Scholar]
- McLellan A.D., Heiser A., Sorg R.V., Fearnley D.B., Hart D.N. 1998. Dermal dendritic cells associated with T lymphocytes in normal human skin display an activated phenotype.J. Invest. Dermatol. 111:841–849 [DOI] [PubMed] [Google Scholar]
- Volc-Platzer B., Stingl G., Wolff K., Hinterberg W., Schnedl W. 1984. Cytogenetic identification of allogeneic epidermal Langerhans cells in a bone-marrow-graft recipient.N. Engl. J. Med. 310:1123–1124 [DOI] [PubMed] [Google Scholar]
- Thomas E.D., Ramberg R.E., Sale G.E., Sparkes R.S., Golde D.W. 1976. Direct evidence for a bone marrow origin of the alveolar macrophage in man.Science. 192:1016–1018 [DOI] [PubMed] [Google Scholar]
- Gale R.P., Sparkes R.S., Golde D.W. 1978. Bone marrow origin of hepatic macrophages (Kupffer cells) in humans.Science. 201:937–938 [DOI] [PubMed] [Google Scholar]
- Shlomchik W.D., Couzens M.S., Tang C.B., McNiff J., Robert M.E., Liu J., Shlomchik M.J., Emerson S.G. 1999. Prevention of graft versus host disease by inactivation of host antigen-presenting cells.Science. 285:412–415 [DOI] [PubMed] [Google Scholar]
- Shlomchik W.D. 2003. Antigen presentation in graft-vs-host disease.Exp. Hematol. 31:1187–1197 [DOI] [PubMed] [Google Scholar]
- Chakraverty R., Sykes M. 2007. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia.Blood. 110:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Hoffmann P., Ranheim E., Slaymaker S., Manz M.G., Lira S.A., Charo I., Cook D.N., Weissman I.L., Strober S., Engleman E.G. 2004. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease.Nat. Med. 10:510–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Shlomchik W.D., Joe G., Louboutin J.P., Zhu J., Rivera A., Giannola D., Emerson S.G. 2002. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease.J. Immunol. 169:7111–7118 [DOI] [PubMed] [Google Scholar]
- Nestel F.P., Price K.S., Seemayer T.A., Lapp W.S. 1992. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor α during graft-versus-host disease.J. Exp. Med. 175:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill G.R., Crawford J.M., Cooke K.R., Brinson Y.S., Pan L., Ferrara J.L. 1997. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines.Blood. 90:3204–3213 [PubMed] [Google Scholar]
- Kolb H.J., Mittermuller J., Clemm C., Holler E., Ledderose G., Brehm G., Heim M., Wilmanns W. 1990. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients.Blood. 76:2462–2465 [PubMed] [Google Scholar]
- Mackinnon S., Papadopoulos E.B., Carabasi M.H., Reich L., Collins N.H., Boulad F., Castro-Malaspina H., Childs B.H., Gillio A.P., Kernan N.A., et al. 1995. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease.Blood. 86:1261–1268 [PubMed] [Google Scholar]
- Marks D.I., Lush R., Cavenagh J., Milligan D.W., Schey S., Parker A., Clark F.J., Hunt L., Yin J., Fuller S., et al. 2002. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation.Blood. 100:3108–3114 [DOI] [PubMed] [Google Scholar]
- Guglielmi C., Arcese W., Dazzi F., Brand R., Bunjes D., Verdonck L.F., Schattenberg A., Kolb H.J., Ljungman P., Devergie A., et al. 2002. Donor lymphocyte infusion for relapsed chronic myelogenous leukemia: prognostic relevance of the initial cell dose.Blood. 100:397–405 [DOI] [PubMed] [Google Scholar]
- Peggs K.S., Thomson K., Hart D.P., Geary J., Morris E.C., Yong K., Goldstone A.H., Linch D.C., Mackinnon S. 2004. Dose-escalated donor lymphocyte infusions following reduced intensity transplantation: toxicity, chimerism, and disease responses.Blood. 103:1548–1556 [DOI] [PubMed] [Google Scholar]
- Fozza C., Szydlo R.M., Abdel-Rehim M.M., Nadal E., Goldman J.M., Apperley J.F., Dazzi F. 2007. Factors for graft-versus-host disease after donor lymphocyte infusions with an escalating dose regimen: lack of association with cell dose.Br. J. Haematol. 136:833–836 [DOI] [PubMed] [Google Scholar]
- Perreault C., Pelletier M., Landry D., Gyger M. 1984. Study of Langerhans cells after allogeneic bone marrow transplantation.Blood. 63:807–811 [PubMed] [Google Scholar]
- Collin M.P., Hart D.N., Jackson G.H., Cook G., Cavet J., Mackinnon S., Middleton P.G., Dickinson A.M. 2006. The fate of human Langerhans cells in hematopoietic stem cell transplantation.J. Exp. Med. 203:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffermann-Gretzinger S., Eger L., Bornhauser M., Schakel K., Oelschlaegel U., Schaich M., Illmer T., Thiede C., Ehninger G. 2006. Fast appearance of donor dendritic cells in human skin: dynamics of skin and blood dendritic cells after allogeneic hematopoietic cell transplantation.Transplantation. 81:866–873 [DOI] [PubMed] [Google Scholar]
- Lampert I.A., Janossy G., Suitters A.J., Bofill M., Palmer S., Gordon-Smith E., Prentice H.G., Thomas J.A. 1982. Immunological analysis of the skin in graft versus host disease.Clin. Exp. Immunol. 50:123–131 [PMC free article] [PubMed] [Google Scholar]
- Hymes S.R., Farmer E.R., Lewis P.G., Tutschka P.J., Santos G.W. 1985. Cutaneous graft-versus-host reaction: prognostic features seen by light microscopy.J. Am. Acad. Dermatol. 12:468–474 [DOI] [PubMed] [Google Scholar]
- Lever R., Turbitt M., Mackie R., Hann I., Gibson B., Burnett A., Willoughby M. 1986. A prospective study of the histological changes in the skin in patients receiving bone marrow transplants.Br. J. Dermatol. 114:161–170 [DOI] [PubMed] [Google Scholar]
- Yoo Y.H., Park B.S., Whitaker-Menezes D., Korngold R., Murphy G.F. 1998. Dermal dendrocytes participate in the cellular pathology of experimental acute graft-versus-host disease.J. Cutan. Pathol. 25:426–434 [DOI] [PubMed] [Google Scholar]
- Deguchi M., Aiba S., Ohtani H., Nagura H., Tagami H. 2002. Comparison of the distribution and numbers of antigen-presenting cells among T-lymphocyte-mediated dermatoses: CD1a+, factor XIIIa+, and CD68+ cells in eczematous dermatitis, psoriasis, lichen planus and graft-versus-host disease.Arch. Dermatol. Res. 294:297–302 [DOI] [PubMed] [Google Scholar]
- Bogunovic M., Ginhoux F., Wagers A., Loubeau M., Isola L.M., Lubrano L., Najfeld V., Phelps R.G., Grosskreutz C., Scigliano E., et al. 2006. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men.J. Exp. Med. 203:2627–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M.P., Bogunovic M., Merad M. 2007. DC homeostasis in hematopoietic stem cell transplantation.Cytotherapy. 9:521–531 [DOI] [PubMed] [Google Scholar]
- Smythies L.E., Sellers M., Clements R.H., Mosteller-Barnum M., Meng G., Benjamin W.H., Orenstein J.M., Smith P.D. 2005. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity.J. Clin. Invest. 115:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning T.L., Wang Y.C., Patel S.R., Williams I.R., Pulendran B. 2007. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses.Nat. Immunol. 8:1086–1094 [DOI] [PubMed] [Google Scholar]
- Belz G.T., Bedoui S., Kupresanin F., Carbone F.R., Heath W.R. 2007. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells.Nat. Immunol. 8:1060–1066 [DOI] [PubMed] [Google Scholar]
- Cooper K.D., Neises G.R., Katz S.I. 1986. Antigen-presenting OKM5+ melanophages appear in human epidermis after ultraviolet radiation.J. Invest. Dermatol. 86:363–370 [DOI] [PubMed] [Google Scholar]
- Bolognia J.L., Lin A., Shapiro P.E. 1994. The significance of eccentric foci of hyperpigmentation (‘small dark dots’) within melanocytic nevi. Analysis of 59 cases.Arch. Dermatol. 130:1013–1017 [PubMed] [Google Scholar]
- Unver N., Freyschmidt-Paul P., Horster S., Wenck H., Stab F., Blatt T., Elsasser H.P. 2006. Alterations in the epidermal-dermal melanin axis and factor XIIIa melanophages in senile lentigo and ageing skin.Br. J. Dermatol. 155:119–128 [DOI] [PubMed] [Google Scholar]
- Handerson T., Berger A., Harigopol M., Rimm D., Nishigori C., Ueda M., Miyoshi E., Taniguchi N., Pawelek J. 2007. Melanophages reside in hypermelanotic, aberrantly glycosylated tumor areas and predict improved outcome in primary cutaneous malignant melanoma.J. Cutan. Pathol. 34:679–686 [DOI] [PubMed] [Google Scholar]
- Cupurdija K., Azzola D., Hainz U., Gratchev A., Heitger A., Takikawa O., Goerdt S., Wintersteiger R., Dohr G., Sedlmayr P. 2004. Macrophages of human first trimester decidua express markers associated to alternative activation.Am. J. Reprod. Immunol. 51:117–122 [DOI] [PubMed] [Google Scholar]
- Torocsik D., Bardos H., Nagy L., Adany R. 2005. Identification of factor XIII-A as a marker of alternative macrophage activation.Cell. Mol. Life Sci. 62:2132–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C., Vanbervliet B., Massacrier C., Dezutter-Dambuyant C., de Saint-Vis B., Jacquet C., Yoneda K., Imamura S., Schmitt D., Banchereau J. 1996. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNFα.J. Exp. Med. 184:695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C., Massacrier C., Vanbervliet B., Dubois B., Durand I., Cella M., Lanzavecchia A., Banchereau J. 1997. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis.Blood. 90:1458–1470 [PubMed] [Google Scholar]
- Hill G.R., Ferrara J.L. 2000. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation.Blood. 95:2754–2759 [PubMed] [Google Scholar]
- Jun H.S., Yoon C.S., Zbytnuik L., van Rooijen N., Yoon J.W. 1999. The role of macrophages in T cell–mediated autoimmune diabetes in nonobese diabetic mice.J. Exp. Med. 189:347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi L.A., Maciaszek J.W., Rock K.L. 2005. Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells.J. Immunol. 175:2071–2081 [DOI] [PubMed] [Google Scholar]
- Mackay C.R., Marston W.L., Dudler L. 1990. Naive and memory T cells show distinct pathways of lymphocyte recirculation.J. Exp. Med. 171:801–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.A., Chong B., Mirchandani N., Brinster N.K., Yamanaka K., Dowgiert R.K., Kupper T.S. 2006. The vast majority of CLA+ T cells are resident in normal skin.J. Immunol. 176:4431–4439 [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions.Nature. 401:708–712 [DOI] [PubMed] [Google Scholar]
- Chen B.J., Cui X., Sempowski G.D., Liu C., Chao N.J. 2004. Transfer of allogeneic CD62L- memory T cells without graft-versus-host disease.Blood. 103:1534–1541 [DOI] [PubMed] [Google Scholar]
- Anderson B.E., McNiff J., Yan J., Doyle H., Mamula M., Shlomchik M.J., Shlomchik W.D. 2003. Memory CD4+ T cells do not induce graft-versus-host disease.J. Clin. Invest. 112:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe S.R., Turner S.J., Miller S.C., Roberts A.D., Rappolo R.A., Doherty P.C., Ely K.H., Woodland D.L. 2003. Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections.J. Exp. Med. 198:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]