Abstract
The commensal fungus Candida albicans causes oropharyngeal candidiasis (OPC; thrush) in settings of immunodeficiency. Although disseminated, vaginal, and oral candidiasis are all caused by C. albicans species, host defense against C. albicans varies by anatomical location. T helper 1 (Th1) cells have long been implicated in defense against candidiasis, whereas the role of Th17 cells remains controversial. IL-17 mediates inflammatory pathology in a gastric model of mucosal candidiasis, but is host protective in disseminated disease. Here, we directly compared Th1 and Th17 function in a model of OPC. Th17-deficient (IL-23p19−/−) and IL-17R–deficient (IL-17RA−/−) mice experienced severe OPC, whereas Th1-deficient (IL-12p35−/−) mice showed low fungal burdens and no overt disease. Neutrophil recruitment was impaired in IL-23p19−/− and IL-17RA−/−, but not IL-12−/−, mice, and TCR-αβ cells were more important than TCR-γδ cells. Surprisingly, mice deficient in the Th17 cytokine IL-22 were only mildly susceptible to OPC, indicating that IL-17 rather than IL-22 is vital in defense against oral candidiasis. Gene profiling of oral mucosal tissue showed strong induction of Th17 signature genes, including CXC chemokines and β defensin-3. Saliva from Th17-deficient, but not Th1-deficient, mice exhibited reduced candidacidal activity. Thus, the Th17 lineage, acting largely through IL-17, confers the dominant response to oral candidiasis through neutrophils and antimicrobial factors.
Oropharyngeal candidiasis (OPC; “thrush”) is an AIDS-defining illness caused mainly by the commensal fungus Candida albicans and related species (1). C. albicans also causes vaginal candidiasis, onychomycosis, and disseminated candidiasis. Disseminated candidiasis typically occurs as a nosocomial infection in neutropenic patients and has a 40% mortality rate (2). Interestingly, host defense mechanisms against C. albicans vary by anatomical site, as HIV-infected patients are primarily susceptible to oropharyngeal, but not vaginal or disseminated, candidiasis (1, 3, 4). Thus, CD4+ T cells play a more central role in OPC compared with other forms of candidiasis, but the mechanisms by which this occurs remain poorly understood.
Recently, a new CD4+ T effector cell was discovered that was distinct in lineage and cytokine profile from classical Th1 and Th2 populations. Termed “Th17,” these cells secrete IL-17 (IL-17A) and IL-17F, IL-21, and IL-22 (5). Whereas Th1 cells develop in response to IL-12 (a heterodimeric cytokine composed of IL-12p35 and IL-12p40 subunits), Th17 cells are induced to differentiate by a combination of TGF-β, IL-6, IL-1, and IL-21. The IL-12 family cytokine IL-23, comprised of IL-12p40 and the unique partner IL-23p19, is essential for Th17 cell expansion and function. Despite a common subunit, IL-12 and IL-23 have dichotomous functions in vivo (5, 6).
Recent studies have revealed an essential role for IL-17 and other Th17 cytokines in host defense at mucosal surfaces such as lung and gut, mediated by induction of prototypical innate immune genes including neutrophil-activating factors (CXC chemokines, G-CSF, and ICAM-1), antimicrobial peptides (defensins, mucins, and S100 proteins), and acute phase proteins (IL-6, lipocalin 2, CRP, and SAA) (7–10). Consistent with a mucosal function, Th17 cells express the CCR6 and CCR4 receptors, which facilitate targeting to mucosal areas (11–13). The oral cavity has many features that distinguish it from other mucosal regions, such as saliva and unique microbiota (14). We recently showed that IL-17 plays a host-protective role in preventing periodontal bone loss after infection with a human periodontal pathogen through activation of neutrophils (15, 16). However, the role of IL-17 and Th17 cells in coordinating host defense in the oral mucosa is largely undefined.
Accumulating evidence implicates IL-17 and Th17 cells in host defense against fungi, but no prior studies have examined these cells in OPC. IL-17 receptor–deficient (IL-17RAKO) mice are susceptible to disseminated C. albicans through regulation of neutrophils, particularly via CXC chemokines (17). The pattern recognition receptor dectin-1 binds to β-glucan components of yeast cell walls and was reported to drive a protective Th17 response in models of disseminated candidiasis (18, 19). In humans, resident memory T cells specific for C. albicans are primarily of the Th17 subset (11, 20). IL-17 also contributes to host defense against other fungi, including Pneumocystis carinii (21). Conversely, in mouse models of fungal gastric candidiasis or aspergillosis, IL-17 and IL-23 have been reported to play a counterproductive role by amplifying inflammatory pathology (22).
It has long been believed that immunity to OPC is mediated by both neutrophils and Th1 cells (2). Although neutrophils are classically considered part of the innate immune response, IL-17 is a critical regulator of neutrophils through CXC chemokines and granulopoietic cytokines (23). IL-12p40KO mice are deficient in Th1 cells and are susceptible to OPC (24); however, IFN-γKO mice also have an impaired Th1 response and are resistant to oral candidiasis (24). Because IL-12p40 is a common component of IL-12 and IL-23, we hypothesized that a Th17-mediated response rather than a defective Th1 response is responsible for the increased susceptibility of the IL-12p40KO mice to OPC.
To dissect host responses to OPC systematically, we used a model of OPC (25) using mice deficient in Th1 cells (IL-12p35KO), Th17 cells (IL-23p19KO), IL-17RA (the receptor for IL-17 and IL-17F), or IL-22. Here, we show that IL-17RAKO and IL-23KO mice are profoundly susceptible to oral candidiasis, whereas IL-12KO mice show only a slight increase in fungal burden and no overt fungal lesions. Analyses of the oral mucosa revealed induction of IL-17 target genes encoding neutrophil-activating factors, CXC-chemokines, and antimicrobial proteins in WT mice after oral C. albicans infection. The majority of these genes were suppressed in IL-17RAKO mice after infection, particularly β defensin 3 (BD3). Saliva from IL-17RAKO and IL-23KO mice showed a reduced capacity to kill C. albicans in vitro, which correlated with reduced levels of BD3 in the oral mucosa. Thus, immunity to OPC appears to be dominantly regulated by IL-17 signaling rather than Th1 cells.
RESULTS
Th17-deficient and IL-17 receptor–deficient mice are susceptible to oral candidiasis
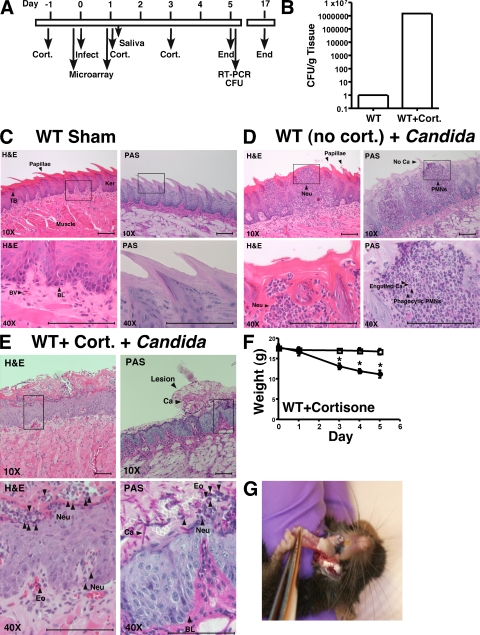
To dissect T cell responses during OPC, we used a quantitative mouse model of OPC (Fig. 1 A) in which mice were infected sublingually with 2 × 107 C. albicans blastospores/ml. The extent of candidiasis was evaluated 5 d later by tissue fungal burden (Fig. 1 B) and histology (Fig. 1, C–E). None of the mice exhibited detectable carriage of oral C. albicans based on an oral swab taken before the first infection (unpublished data). Similar to immunocompetent humans, WT mice infected with C. albicans are almost completely resistant to infection, with an extremely low fungal burden 5 d after inoculation (Fig. 1, B–D). Inoculation of C. albicans into immunocompetent WT mice was associated with a strong neutrophilic infiltration into the oral cavity, in the epithelial, basal, and muscular layers of the tongue (Fig. 1 D). When WT mice were immunosuppressed with cortisone acetate, a mean of 1.5 × 106 CFU/g (geometric mean of 6.4 × 105) was recovered from tongue (n = 16; Fig. 1 B; see also Fig. 2 A), which is consistent with prior findings (25). These mice lost considerable body weight (Fig. 1 F) and exhibited fungal patches covering the dorsal layer of the tongue, palate, and buccal mucosa (Fig. 1 G). Microscopic analysis indicated that yeast invaded the superficial epithelial layer of the mucosa, destroying the papillae and the overall architecture of the keratinized superficial epithelium. There was extensive proliferation of the basal germinal layer, which is indicative of ongoing tissue repair. Although some neutrophils and eosinophils were found at infected sites, this was substantially reduced compared with WT mice (Fig. 1 D vs. Fig. 1 E). Both hyphal and blastoconidial forms of C. albicans were present (Fig. 1 E). Thus, this model faithfully reproduces key clinical features of human OPC (25).
Figure 1.
Model of murine OPC. (A) Timeline of infection model. (B) WT mice are resistant to OPC unless they are immunosuppressed. WT mice (n = 8) or WT mice treated with cortisone acetate (n = 16) were infected sublingually with C. albicans, and after 5 d CFU/g of tongue tissue was assessed in triplicate (log scale). These data are from at six independent experiments. (C–E) Histological evaluation of C. albicans infection. At day 5, sections of tongue from the indicated mice were stained with PAS to visualize C. albicans (Ca) or hematoxylin and eosin (H&E; which does not stain fungi) and viewed at 10X or 40X magnification. Fungal lesions, papillae, and keratinized layer are indicated. (C) WT mice subjected to sham infection. (D) WT mice without cortisone were infected orally with C. albicans. (E) WT mice treated with cortisone acetate were infected orally with C. albicans. TB, taste bud; BV, blood vessel; BL, basal layer; ker, keratinized layer; Neu, neutrophil; Eo, eosinophils; Ca, C. albicans. Data are representative of 2–3 mice per strain. Bars, 100 μm. (F) OPC induces weight loss in cortisone-treated WT mice. All mice were weighed daily, and weight (gram) on each day of infection is indicated. *, P < 0.05. Circles, Sham-infected mice; squares, C. albicans–infected mice. (G) OPC on day 5 after inoculation with C. albicans. Fungal lesions on tongue of WT mice treated with cortisone acetate.
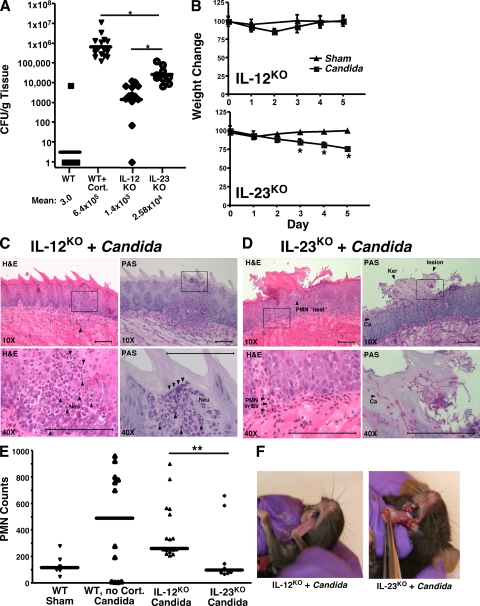
Figure 2.
Th17-deficient mice are more susceptible to OPC than Th1-deficient mice. (A) Quantitation of C. albicans infection in Th1-deficient (IL-12KO) and Th17-deficient (IL-23KO) mouse strains. IL-12KO mice (n = 13) and IL-23KO mice (n = 9) were infected orally with C. albicans and CFU/gram tongue tissue was assessed. Each dot represents an individual mouse, and horizontal bar indicates the geometric mean, which is also indicated below. *, P < 0.05 by Mann-Whitney t test. Note, the WT mouse data are the same as that shown in Fig. 1 B. IL-12KO mice were evaluated in three independent experiments, and IL-23KO mice were evaluated in two independent experiments. (B) OPC-induced weight loss in Th1-deficient or Th17-deficient strains. Mice were weighed daily and percentage of weight change compared with day 0 is shown for each strain. *, P < 0.05. Triangles, Sham-infected mice; squares, C. albicans–infected mice. (C and D) Histological evaluation of C. albicans infection in IL-12KO versus IL-23KO mice. At day 5, sections of tongue from the indicated mice were stained with PAS or hematoxylin and eosin (H&E) and viewed at 10–40X magnification. Data are representative of two mice per strain. Bars, 100 μm. (E) Quantitation of histological evaluation of PMNs. Six sections per mouse from two mice per group were evaluated for the number of PMNs per microscopic field, either in the vicinity of fungal lesions or parallel to areas of sham-infected tongue. Evaluators were blinded to the identity of the samples. (F) OPC on day 5 after inoculation with C. albicans. Fungal lesions on tongues of representative IL-12KO or IL-23KO mice are shown.
To compare Th1 and Th17 cells in OPC directly, IL-12p35– and IL-23p19–deficient mice (hereafter, IL-12KO and IL-23KO) were infected with C. albicans. IL-23KO mice showed a fungal burden of 2.6 × 104 CFU/g, which was less than cortisone-treated WT mice, but nonetheless indicated considerable susceptibility to OPC (Fig. 2 A). In contrast, IL-12KO mice (1.4 × 103 CFU/g) were ∼450-fold less susceptible than cortisone-treated WT mice and ∼20-fold less susceptible than IL-23KO mice, indicating that IL-23 is far more important for mediating host defense against OPC than IL-12. Weight loss values were consistent with recovery of C. albicans cells from tongue, as IL-23KO mice showed continuous weight loss, whereas IL-12KO mice showed transient weight loss at day 2, but recovered by day 5 (Fig. 2 B). Although characteristic C. albicans lesions were readily visible on the palates and tongues of IL-23KO mice, no fungal lesions were found in the IL-12KO mice (Fig. 2 F). Hyphal formation and invasion of the superficial epithelial layer were visible in IL-23KO tongue sections, confirming invasive candidiasis (Fig. 2 D). Disruption of the superficial epithelium and some infiltration of the infected area with PMNs was observed. However, the degree of neutrophilic infiltrate was significantly reduced compared with WT or IL-12KO mice, and PMNs appeared to be clustered in “nests” rather than crossing into the infected areas (Fig. 2, D and E). Fungal invasion in the IL-23KO mice was less profound than in cortisone-treated mice (Fig. 1 E vs. Fig. 2 D). In contrast to IL-23KO or cortisone-treated mice, there was little damage to the epithelial layer in the IL-12KO mice, and C. albicans cells were almost never found on the surface or invading epithelial tissue (Fig. 2 C and not depicted). A prominent neutrophilic infiltrate occurred both in the muscular area and the subepithelial layer, indicative of an ongoing immune response (Fig. 2, C and E). Thus, IL-23, but not IL-12, is required to prevent invasive oral candidiasis and permit a robust PMN response.
IL-23 drives expansion of the Th17 lineage, and IL-17 and IL-17F are hallmark Th17 cytokines that bind to a common receptor, IL-17RA. The oral fungal burden (2.64 × 104 CFU/g; Fig. 3 A) and weight loss (Fig. 3 B) in IL-17RAKO mice were not statistically different from the IL-23KO mice and were higher than the IL-12KO mice (compare Fig. 3 to Fig. 2; statistical comparisons not depicted). Fungal lesions were visible on the tongue and palate at levels similar to the IL-23KO mice (not depicted), and invading hyphae were frequently found in IL-17RAKO tongue sections (Fig. 3 D). Destruction of the epithelial and basal layers was observed in IL-17RAKO sections, similar to the IL-23KO mice. Some neutrophilic infiltrates were present, similar to the IL-23KO mice, but were less numerous than in IL-12KO or WT tissue (Fig. 3 D). In IL-17RAKO mice, as in IL-23KO mice, PMNs appeared to be trapped in “nests,” and there was no evidence of C. albicans phagocytosis as there was in the WT mice (compare with Fig. 1 D). Thus, IL-17 receptor signaling appears to account for much if not all of the IL-23–mediated protection, which is apparently caused in part by a failure to recruit PMNs into infected tissue.
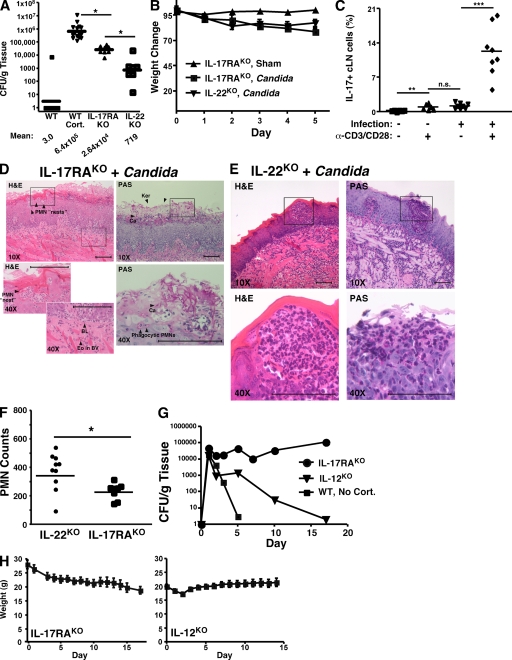
Figure 3.
IL-17 signaling is essential for host defense against OPC. (A) IL-17RAKO mice are sensitive to OPC. The indicated mouse strains (IL-17RAKO, n = 8; IL-22KO, n = 7) were infected with C. albicans and CFU/g tongue was assessed by colony enumeration. *, P < 0.05. Geometric mean is indicated by horizontal bars and below. Note, data from WT mice are the same as shown in Fig. 1 B. IL-17RAKO mice data are representative of three independent experiments, and IL-22KO data were from one experiment. (B) OPC-induced weight loss in susceptible strains. Percentage of weight change compared with day 0 is shown. (C) IL-17+ cells are induced after C. albicans infection. Cells were isolated from the draining cervical lymph nodes of infected IL-17RAKO mice. Cells were incubated ± anti-CD3 + anti-CD28 for 48 h, and percentage of cells staining positive for IL-17 was assessed by intracellular flow cytometry. **, P < 0.01. n.s. not statistically significant. (D and E) Histological evaluation of C. albicans infection in IL-17RAKO or IL-22KO mice. At day 5, sections of tongue from the indicated mice were stained with PAS or H&E and viewed at 10X-40X magnification. Data are representative of two mice per strain. Scale bars indicate 100 microns. (F) Quantitation of histological evaluation of PMNs. Six sections per mouse from 2 mice per group were evaluated for the number of PMNs per microscopic field, either in the vicinity of fungal lesions or parallel areas of sham-infected tongue. Evaluators were blinded to the identity of the samples. * P < 0.05. (G) IL-12KO but not IL-17RAKO mice can recover from oral C. albicans infection. WT (n = 4), IL-17RAKO (n = 12) and IL-12KO (n = 12) mice were infected with C. albicans. On the indicated days (ranging from 1–17), 1–3 mice per group were killed and C. albicans CFU per gram of tongue was evaluated by colony enumeration. Geometric mean for each day is shown. Time course data are from one experiment. (H) Weight loss correlates with degree of OPC susceptibility. Weight changes in IL-12KO and IL-17RAKO mice over time are shown.
We next assessed IL-17 expression in T cells from the draining cervical LN (cLN) after infection. Cortisone acetate treatment results in reduced lymphocyte numbers, so it was not surprising that WT mice treated with corticosteroids had extremely small cLNs, and not enough viable lymphocytes could be recovered to assess cytokine expression (unpublished data). However, IL-17RAKO mice (which can produce IL-17, but not respond to it) showed significantly increased numbers of IL-17+ T cells after infection compared with uninfected mice (Fig. 3 C). Even without additional stimulation of the TCR, ∼1% of the cLN lymphocytes stained positive for IL-17, comparable to reports with Toxoplasma gondii infection (26). After 2 d of in vitro restimulation of the TCR, ∼12% of T cells expressed IL-17, compared with <1% in sham-treated mice. Thus, IL-17–producing cells are activated after oral C. albicans infection. Moreover, sham-treated mice do not harbor substantial numbers of IL-17–expressing T cells before infection.
Next, we questioned whether IL-17RAKO mice could eventually clear C. albicans, or whether IL-17 signaling was necessary to recover from OPC. Accordingly, IL-17RAKO mice were monitored for weight loss and signs of infection for 2.5 wk (Fig. 3 G). Whereas WT mice showed almost no fungal burden 5 d after inoculation and fully recovered their body weight (Fig. 1 F), IL-17RAKO mice lost weight (Fig. 3 H) and maintained a mean fungal burden of 6.8 × 105 CFU/g C. albicans throughout a 17-d time course. In contrast, IL-12KO mice showed an early spike in fungal burden in the tongue, but fungal clearance and weight recovery were evident by the end of the experiment (Fig. 3, G and H). These data indicate that, whereas the oral fungal burden of the IL-17RAKO mice was lower than WT mice treated with steroids (which must be killed by day 5 for humane reasons), OPC cannot be resolved in the absence of IL-17RA signaling. In contrast, IL-12 and hence Th1 cells are not essential for host defense against oral candidiasis, although they do contribute to some extent to limiting infection.
In an attempt to determine whether IL-17 is sufficient to rescue the Th17 deficiency, we added IL-17 orally twice daily to IL-23KO mice throughout a 5-d course of infection (unpublished data). However, ectopic application of IL-17 led to a systemic reaction characterized by reddening of the skin in the face and chest, and IL-17–treated mice were noticeably sicker than controls. There was no statistically significant difference in oral fungal burden between the groups (unpublished data). Accordingly, the confounding systemic effects of IL-17 may have counteracted any beneficial effects in the oral cavity.
Th17 cells also produce IL-22, which has been shown to play a more vital role than IL-17 in host defense against certain gut and lung mucosal pathogens (27, 28). Therefore, we evaluated the effect of an IL-22-deficiency in OPC. However, IL-22KO mice showed only a mild susceptibility to OPC compared with IL-17RAKO mice, although they lost weight after infection (Fig. 3, A and B). Consistent with their ability to largely combat fungal infections, IL-22KO mice showed robust neutrophilic infiltrates into the infected lesion sites (Fig. 3 D). There was no evidence of overt fungal lesions on the tongue, either visually (not depicted) or histologically (Fig. 3 D). Therefore, in contrast to lung and gut, IL-17 signaling appears to play a more important role in mediating immunity to infection in the oral cavity than IL-22 (see Discussion).
αβ-TCR and CD8+ cells, and not γδ-TCR cells or IL-22, contribute to immunity to OPC
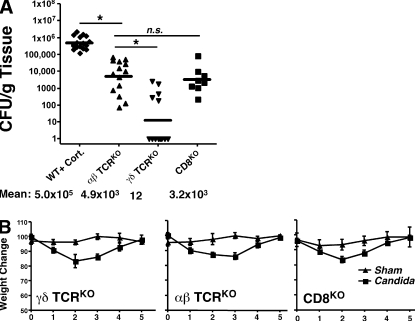
IL-17 is produced by CD4+ and CD8+ cells that express the αβ-TCR, and also by an innate population of γδ-TCR T cells in an IL-23–dependent manner (29). In Escherichia coli, Mycobacterium tuberculosis, and Klebsiella pneumonia settings, the γδ-T cell population appears to be the dominant IL-17–producing T cell subset involved in host defense (30, 31). To determine which subset was primarily responsible for immunity against OPC, αβ-T cell–deficient mice or γδ-T cell–deficient mice were infected with C. albicans. Low levels of infection were observed in γδ-TCRKO mice, whereas substantial infection occurred in αβ-TCRKO mice (Fig. 4 A). These data are consistent with the sensitivity of HIV+ individuals to OPC (3) and the finding that PBMCs from healthy human donors contain C. albicans–specific memory CD4+ Th17 cells (11, 20). CD8+ T cells and innate CD8+ NKT cells also secrete IL-17 (32, 33), and CD8KO mice showed a similar susceptibility to OPC as the αβ-TCRKO mice (Fig. 4 A). Interestingly, the fungal burden in αβ-TCRKO mice was reproducibly lower than the IL-17RAKO or IL-23KO mice (Fig. 4 A vs. Fig. 2 A and Fig. 3 A). Consistent with this observation, the αβ-TCRKO mice did not lose as much weight as IL-23KO or IL-17RAKO mice (Fig. 4 B vs. Fig. 2 B and Fig. 3 B). This is unlikely because of subtle strain differences, as we observed no quantitative or qualitative differences in susceptibility to OPC between BALB/c and C57BL/6 (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20081463/DC1). Instead, IL-17 from another source such as NKT cells (32, 33) or neutrophils (29) may contribute to defense against OPC.
Figure 4.
Host defense against oral C. albicans is mediated mainly by αβ-T cells, rather than γδ-T cells. Mice deficient in αβ-T cells (n = 14), γδ-T cells (n = 13), or CD8 (n = 8) were inoculated with C. albicans and fungal burden (CFU/g tissue) in tongue (A) and daily weight (B) were assessed. CD8KO mice were evaluated in one experiment, and the γδKO and αβKO mice were evaluated in three independent experiments.
Downstream effects of IL-17RA signaling on saliva and oral mucosal tissue
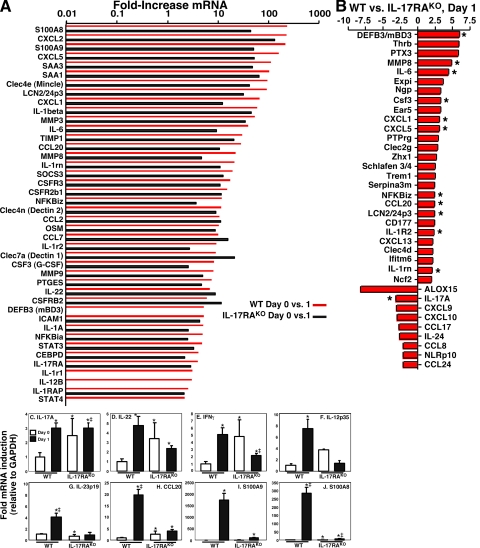
To define mechanisms by which IL-17 mediates anti–C. albicans host defenses in the oral mucosa, we performed gene profiling analysis of tongue mRNA from WT or IL-17RAKO mice at baseline (uninfected, “Day 0”) or 24 h after infection (“Day 1”). Although WT mice showed no evidence of OPC 5 d after inoculation with C. albicans (Fig. 1), many immune genes were induced in WT oral mucosa after 24 h of exposure to C. albicans. Highly represented were “Th17 signature” genes, i.e., genes associated with Th17 function or known targets of Th17 hallmark cytokines (10, 34) (Fig. 5 A; see also Fig. S3). Among the most strongly induced genes were S100A8 and S100A9 (237- and 220-fold, respectively), CXCL2 (234-fold), CXCL5 (164-fold), LCN2/24p3 (lipocalin 2; 94-fold), IL-6 (41-fold), MMP8 (23-fold), CCL20 (29.5-fold), CSF3 (G-CSF, 17.8-fold), NF-κBiz (NF-κBζ; 12.3-fold), IL-22 (7-fold), and DEFB3 (BD3; 5.3-fold). All are known targets of IL-17 signal transduction, either alone or in combination with TNF-α or IL-22. Interestingly, several C-type lectin receptors were up-regulated that have been associated with C. albicans responses, including Clec4e (Mincle; 97.3-fold), Clec4n (Dectin-2; 11.9-fold), and Clec7a (Dectin-1; 9.2-fold) (35, 36) (Fig. 5 A). In contrast, few Th1- or Th2-related genes were up-regulated (see also Fig. S3). Quantitative real-time PCR experiments demonstrated that IL-17 was enhanced by approximately threefold in WT mice after C. albicans infection (Fig. 5 C). IL-22 was also up-regulated in the microarray and in real-time RT-PCR (Fig. 5 D). Messenger RNA encoding the Th1 cytokines IFN-γ and IL-12 were induced as well (Fig. 5, E and F), consistent with the fact that Th1-deficient (IL-12KO) mice show an increased fungal burden, although no fungal lesions are manifest and almost no hyphal yeast cells are found invading the oral cavity in the IL-12KO mice (Fig. 2 C).
Figure 5.
Mechanism of IL-17–mediated host defense against OPC. (A) Oral inoculation with C. albicans induces Th17 signature genes in oral mucosal tissue, which are suppressed in IL-17RAKO mice. Tongue tissue from female WT or IL-17RAKO mice (n = 2, from independent experiments) was taken at baseline (day 0) or 24 h after oral C. albicans inoculation (day 1), and cDNA was prepared and hybridized to Affymetrix genechips. Shown are Th17-related genes found to be induced on day 1 compared with day 0 from WT mice (red bars) or IL-17RAKO mice (white bars). (B) Differentially expressed genes in WT versus IL-17RAKO mice determined by microarray analysis. Fold change of genes induced in WT mouse tongue compared with IL-17RAKO tongue on day 1 are indicated. * indicates genes previously connected to IL-17 or Th17 cells. (C–J) OPC-induced expression of selected genes. Tongue mRNA from WT or IL-17RAKO mice on day 0 or day 1 (mRNA from two mice from independent experiments) was subjected to quantitative real-time RT-PCR analysis in triplicate. Data are presented as fold induction, normalized to GAPDH. *, P < 0.05 compared with WT Day 0; ‡, P < 0.05 compared with IL-17RAKO Day 0.
In IL-17RAKO mice, up-regulation of Th17 profile genes was considerably lower than in WT (Fig. 5 A, white bars vs. red bars; and Figs. S4–S6), although many were still induced to some extent, presumably via the activity of other remaining inflammatory cytokines. IL-17 was also constitutively elevated in IL-17RAKO mice, which has been previously reported (8), as were IL-22, IFN-γ, and IL-12 (Fig. 5, D and F). When comparing differentially expressed genes in WT versus IL-17RAKO mice 24 h after infection, there was a reduction in many genes associated with neutrophil recruitment such as CXCL1, CXCL5 (both 3.1-fold), and CSF3/G-CSF (3.3-fold; Fig. 5 B), consistent with the striking decrease in neutrophilic infiltrate observed in IL-23KO and IL-17RAKO mice compared with WT mice (Fig. 2).
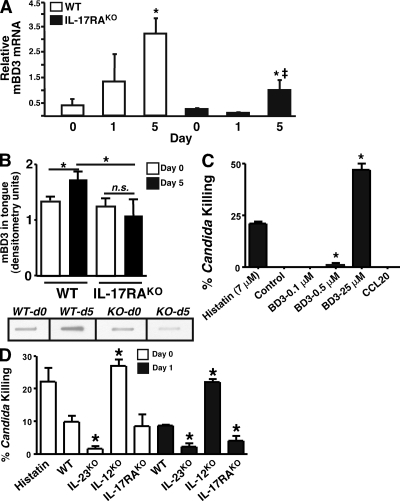
In IL-17RAKO mice compared with WT, there was a notable diminution in genes encoding antimicrobial proteins that are known to exert candidacidal activity, including BD3 (DEFB3; 6-fold) and CCL20 (2.4-fold; Fig. 5 B) (37, 38). Indeed, BD3 was the most differentially expressed gene between WT and IL-17RAKO mice based on the microarray analysis (Fig. 5 B). Differential expression of BD3 and CCL20 mRNA between WT and IL-17RAKO mice was verified by real-time RT-PCR (Fig. 5 H and Fig. 6 A). CCL20 and BD3 have both been reported to have direct killing activity against C. albicans (38). We verified that BD3 could indeed kill C. albicans cells in vitro comparable to physiological concentrations (7 µM) of a human salivary anticandidal peptide histatin-5 (37), but there was no detectable killing activity by recombinant CCL20, even at high concentrations (25 µM; Fig. 6 C).
Figure 6.
Anticandidal factors and Th17 deficiency. (A) IL-17RAKO mice show decreased expression of mBD3 mRNA. Quantitative real-time RT-PCR analysis was performed in triplicate on tongue cDNA from WT or IL-17RAKO mice (n = 2–4) at baseline (day 0) or day 1 or 5 after C. albicans infection. *, P < 0.05 compared with Day 0; ‡, P < 0.05 compared with WT sample at Day 5. (B) IL-17RAKO mice show decreased expression of mBD3 protein. Tongue tissue from WT mice or IL-17RAKO mice at day 0 or 5 (n = 4 for each condition) were applied to nitrocellulose in slot blots and probed with antibodies to mBD3. Signals were quantified by densitometry. *, P < 0.05. ns = not significant. Sample slot blot samples are shown below (note: all were taken from the same film at the same exposure level). (C) Candidacidal activity of BD3 and CCL20. Recombinant BD3 (0.1, 0.5 or 25 µM) and CCL20 (25 µM) were evaluated for fungicidal activity by incubation with 106 C. albicans cells for 1.5 h in triplicate. Human histatin-5 (7 µM) was used as a positive control (37). Yeast were diluted and spread on YPD agar for colony enumeration. Data were compared by unpaired Student's t test (*, P < 0.05 compared with WT controls). Data are representative of three independent experiments. (D) Reduced candidacidal activity in IL-17RA-deficient and Th17-deficient (IL-23KO) saliva. Saliva was collected from the indicated mice (n = 3–6) after carbachol injection and assayed for candidacidal activity in triplicate as in B. Experiments were performed twice with similar results.
Saliva contains potent antimicrobial factors, and patients with salivary gland defects caused by radiation or Sjogren's syndrome are highly susceptible to OPC (39, 40). Almost no biochemical characterization of murine saliva has been reported. As shown in Fig. 6 (A and B), WT mice express increased levels of BD3 mRNA and protein in tongue after C. albicans infection, which are markedly reduced in IL-17RAKO mice. Next, we evaluated the ability of saliva from various KO mouse strains to kill C. albicans cells in vitro (Fig. 6, C and D). As a positive control for candidacidal activity, we verified that the human salivary peptide histatin-5 at a concentration of 7 µM killed ∼22% of C. albicans, as previously reported (41) (Fig. 6, C and D). WT murine saliva was collected after carbachol injection and incubated with C. albicans cells in vitro, and yeast survival was determined by plating and colony enumeration. Saliva from WT mice killed a mean of 14.5% of C. albicans cells, and was not statistically different from saliva derived from IL-17RA–deficient mice (Fig. 6 D). However, saliva from IL-23KO mice reproducibly showed significantly reduced fungicidal activity (approximately threefold). 24 h after oral inoculation with C. albicans, saliva from both IL-23KO and IL-17RAKO mice exhibited reduced candidacidal activity compared with WT saliva. In contrast, saliva from IL-12KO mice consistently showed elevated candidacidal effect compared with WT, both at baseline and 24 h after inoculation (Fig. 6 D). Therefore, IL-23 and IL-17, but not IL-12, regulate the antimicrobial activity of mammalian saliva.
To determine whether IL-17 stimulates anticandidal activity on cells that form nonimmune tissue, we stimulated a murine stromal cell line with IL-17 and/or TNF-α and assessed conditioned media (CM) for effects on C. albicans. CM from cells treated with IL-17 or TNF-α alone did not trigger candidacidal activity, but CM from cells treated with both IL-17 and TNF-α killed ∼30% of yeast in this assay, indicating that these cytokines stimulate secretion of factors with antimicrobial activity (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20081463/DC1). Thus, in an inflammatory environment, IL-17 contributes to an antifungal state in cooperation with other inflammatory cytokines.
Th1 cells, but not Th17 cells, help limit breaching of the oral mucosal barrier
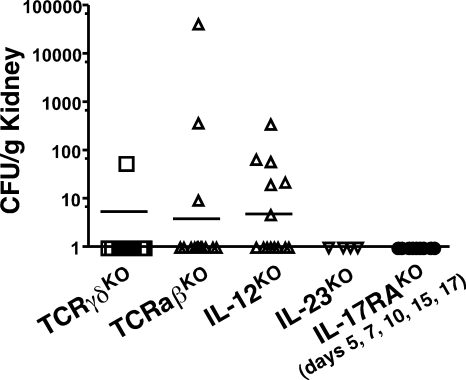
OPC generally remains contained in the oral cavity, and is not thought to breach this mucosal barrier to establish a disseminated form of disease (42). To determine whether Th1 or Th17 cells contribute to this immune barrier, we evaluated C. albicans CFU in kidney. Surprisingly, IL-12KO mice, but not IL-23KO or IL-17RAKO mice, showed some evidence of kidney infection (Fig. 7). Consistently, mice lacking αβ-TCR cells (which lack Th1 cells and other Th subsets) showed evidence of systemic infection, although CFUs were not high enough to cause lethality. Nonetheless, these data suggest that Th1 cells, although relatively less important for host defense against OPC compared with Th17 cells, may be an important component of fungal immunity by helping to limit spread beyond the oral cavity. Still, because the fungal burden was sublethal and AIDS patients with low CD4+ T cell counts do not generally contract disseminated candidiasis, additional components evidently contribute to controlling dissemination of C. albicans.
Figure 7.
IL-12, but not IL-23 or IL-17RA, limit dissemination of C. albicans from the oral cavity. The indicated KO mice (γδ-TKO, n = 13; αβ-TKO, n = 14; IL-12KO, n = 14; IL-23KO, n = 4; IL-17RAKO, n = 9) were infected orally with C. albicans as in Fig. 1, and kidneys were isolated on day of sacrifice. After homogenization, CFU/gram kidney was determined by colony enumeration in triplicate. Data were collected from all experiments in Figs. 2–4.
DISCUSSION
OPC is a debilitating disease of immunodeficiency, characterized by fungal lesions on the palate, oropharynx, tongue, and buccal mucosa. Disease can progress to esophageal candidiasis, and limit nutritional intake and threaten overall health. OPC is the most frequent opportunistic fungal infection among HIV+ individuals not receiving effective antiretroviral therapy (14), and it is also a problem in cancer patients on chemotherapy (43). Candidiasis occurs in 35% of Sjogren's syndrome patients (40), and the elderly, infants, and denture wearers are also at risk (2).
An intriguing feature of host defense against C. albicans is the anatomical compartmentalization associated with disease. For example, HIV+ individuals experience OPC but do not show enhanced incidence of vaginal or disseminated candidiasis (3). Immunity to OPC involves antifungal salivary proteins, PMNs, CD8+ cells and nonimmune cells such as oral keratinocytes, stromal cells, and epithelial cells (2, 14). Interactions between PMNs and T cells are required for maintaining host defense to OPC (2, 44). Th17 cells link CD4 cells and the PMN response by IL-17–mediated regulation of neutrophil expansion, recruitment, and migration (15, 29), and provide a satisfying explanation for why CD4+ T cells are so important in OPC. Consistently, we observed a large neutrophilic infiltrate in resistant mice (WT, IL-12KO, and IL-22KO) after C. albicans inoculation, which was greatly diminished in susceptible (IL-23KO and IL-17RAKO) mice (Figs. 2 and 3).
Gene-targeted mice permit hypothesis-driven studies to define immune components involved in candidiasis. Huang et al. (17) identified a protective role for IL-17 in disseminated candidiasis, as IL-17RAKO mice are susceptible to systemic disease caused by defects in neutrophil migration and chemokine expression. Dectin-1KO mice show defective development of Th17 cells (18, 19) and are susceptible to disseminated candidiasis, although this finding has been controversial (19, 45). Conversely, IL-23 and IL-17 drive inflammatory pathology in models of pulmonary aspergillosis and gastric candidiasis (22), and IL-17 neutralization unexpectedly protects from disseminated candidiasis in a study by this group (46). Although the relevance of the gastric candidiasis model to human disease is questionable, Th17 cells mediate pathology in airway disease, rheumatoid arthritis, and EAE (47, 48). Recent studies support the notion that the Th17 pathway, rather than the Th1 pathway, drives defense to OPC. IFN-γKO and IL-4KO mice are resistant to OPC, whereas IL-12p40KO mice are susceptible (24). Here, we demonstrate that IL-23 and IL-17RA are required for effective resistance to OPC, whereas IL-12 and IL-22 play less critical roles (Figs. 2 and 3). The propensity of proinflammatory cytokines to play opposing roles in disease is well documented (49), and IL-17 appears to be no exception. For example, in the oral cavity, the role of IL-17 in periodontal disease may be host-protective or destructive depending on disease severity and chronicity (15, 50).
Our data indicate that γδ-T cells, although a major source of IL-17 (29), are less important than αβ-T cells in preventing OPC (Fig. 4). This was surprising, given the rapid time course of disease (5 days). However, only 2% of T cells in the oral epithelium are γδ-T cells (51), and these cells apparently do not play a defensive role in vaginal candidiasis (52). γδ-T cells also produce IL-22, which we find to play a relatively less important role in OPC (Fig. 3 A). On the other hand, γδ-T cells have been suggested to enhance resistance to gastric candidiasis (53). We have noticed that some γδ-T cell–deficient mice experienced higher levels of C. albicans CFU when experiencing stress (e.g., poor recovery from anesthesia or the presence of a dead mouse in the cage; unpublished data). Although mice were eliminated from the study when such confounding factors were obvious, this may explain why a subset of γδ-T cell–deficient mice showed detectable C. albicans colonization (Fig. 4). Unidentified environmental stressors may have predisposed certain γδ-TCRKO mice to OPC, and thus the γδ T cell subset may provide an additional layer of protection against candidiasis.
Saliva plays a vital defensive role in the oral cavity (14). Saliva from IL-17RAKO and IL-23KO is defective in killing C. albicans (Fig. 6 D), which may be at least partly caused by a deficiency in antimicrobial substances (Fig. 5). BDs are produced by salivary glands but also by epithelial cells in the gingiva, buccal mucosa, and tongue (54), and they exert anticandidal activity in vitro (Fig. 6; Fig. 5 B; and Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20081463/DC1) (37, 55). IL-17 regulates BD3 in lung cells in humans and mice (56). Here, we show that BD3 mRNA and protein are up-regulated in WT mice after infection with C. albicans, and there was an impairment in BD3 expression in IL-17RAKO tongue compared with WT mice (Fig. 5 B; Fig. 6, A and B; and Figs. S3–S6). In contrast to tongue, there was little difference in BD3 levels in saliva between WT and IL-17RAKO mice (unpublished data). Thus, although the basis for the reduced salivary fungicidal activity in IL-23KO and IL-17RAKO remains unclear, BD3 likely contributes to the reduced infection on the oral mucosal surface.
Antimicrobial peptides also function as chemotactic factors for immune cells. BD2 recruits dendritic cells and T cells via CCR6 (57), and S100A8/A9 are chemotactic for neutrophils (58). Another ligand for CCR6 is CCL20, which was impaired in IL-17RAKO mice after infection (Fig. 5 H). Like BDs, CCL20 has chemotactic activity for T cells and DCs. CCL20 is regulated by IL-17 (59, 60), is expressed by Th17 cells (61) and has been linked to Th17 recruitment to inflamed sites (13). Although CCL20 has been reported to exert antimicrobial activity against C. albicans in vitro (38), we did not observe this effect, perhaps because of differences in the commercial preparations of the chemokine or the specific strain of C. albicans used (Fig. 6 C).
Our findings are similar to, yet distinct from, observations made regarding Th17 function at other mucosal sites. A microarray study of K. pneumonia–infected lung found many of the same genes, including BDs, CXC chemokines, lipocalin 2, and IL-6, although BD3 was not identified in that study (27). Microarray studies in RA synoviocytes (60) and after Salmonella enterica serovar Typhimurium infection in macaques (9) found similar IL-17 target genes induced during disease states. The K. pneumonia study reported that IL-22, another Th17 cytokine, is a critical host-protective cytokine (27), but our data suggest that IL-22 is less important than IL-17 in the oral cavity (Fig. 3 A). Humans with mutations in STAT3 (hyper-IgE syndrome [HIES]) fail to develop Th17 cells and experience recurrent oral and mucocutaneous C. albicans infections. One study indicated that HIES patients show normal levels of IL-22 in the CD4+ T cell population (20). Conversely, several other studies report reduced IL-22 levels in HIES patients (62–64). Because IL-17 and IL-22 cooperatively regulate chemokines and antimicrobial genes (65), we cannot rule out a role for IL-22 in contributing to host defense to OPC, particularly in humans.
Intriguingly, IL-12, and hence Th1 cells, may help limit transition to a disseminated form of candidiasis, as IL-12KO but not IL-23KO or IL-17RAKO exhibited detectable C. albicans infection in kidney (Fig. 7). Although OPC causes considerable morbidity, disseminated candidiasis is associated with ∼40% mortality and is the fourth most common pathogen isolated from hospitalized patients (66). It is generally thought that disseminated candidiasis is initiated when C. albicans enters the bloodstream from the gut or indwelling catheters (42, 67). This study is, to our knowledge, the first to demonstrate that oral disease can lead directly to systemic candidiasis, and moreover that Th1 cells are important in preventing a breach in the mucosal barrier. However, the kidney fungal burden in IL-12KO mice was low, and thus other mechanisms contribute to effective barrier function. This contrasts a recent study in the gut, which found that selective depletion of Th17 cells is seen after SIV infection in macaques, and thus promotes dissemination of Salmonella (9, 68). Finally, IL-12p35 was recently shown to be part of the cytokine IL-35 produced by regulatory T cells (69), which could conceivably play a role in barrier function by limiting inflammatory tissue damage during oral infection. Similarly, IL-17RA was recently reported to be a receptor subunit for the IL-25, which promotes Th2 cells (70).
Defining cytokines involved in immunity to OPC is important clinically, given increasing use of anticytokine biologics. The role of the IL-23 and IL-17 in autoimmunity has stimulated development of blocking agents for these cytokines (71). Although likely to prove useful in ameliorating inflammatory pathology, consequences to opportunistic infection need to be considered. Anti–TNF-α therapy predisposes to M. tuberculosis infection, histoplasmosis, and fungal pneumonia, but thrush is only rarely seen (72). Consistently, TNF-αKO mice only show a mild increase in susceptibility to OPC (24), but are sensitive to disseminated candidiasis (73). Our data and findings in HIES patients (20) suggest that complete IL-17 and IL-23 blockade could increase OPC susceptibility, but this will need to be evaluated in clinical settings.
In summary, immunity to C. albicans depends on multiple immune components, as well as anatomical location. This study shows unequivocally that IL-17 and Th17 cells play a key role in protecting the host against OPC, whereas Th1 cells and IL-22 play a relatively less important role.
MATERIALS AND METHODS
Mouse model of OPC.
IL-17RAKO mice were a gift from Amgen, and IL-23p19KO mice were provided by Genentech. IL-22 KO mice were produced in a collaboration between Genentech and Lexicon Pharmaceuticals to analyze the function of 500 secreted and transmembrane proteins (74). All other mice were purchased from The Jackson Laboratory. All mice were on the C57BL/6 background, and all were age and sex matched. Mice were caged individually after infection. If indicated, mice were treated with 225 mg/kg cortisone acetate (Sigma-Aldrich) and infected under anesthesia by placing a 0.0025-g cotton ball saturated with 2 × 107 CFU C. albicans (CAF2-1) sublingually for 75 min, as previously described (25). No antibiotics were administered. Saliva was collected into chilled tubes after carbachol injection (100 μl at 10 mg/ml) and used immediately in candidacidal assays. All protocols were approved by SUNY Buffalo Institutional Animal Care and Use Committee.
C. albicans culture and handling, candidacidal assays.
C. albicans was cultured in YPD by standard methods. Candidacidal assays were generally performed as previously described (41). For saliva testing, 90 μl of freshly collected murine saliva was incubated with 104 cells of C. albicans for 1 h at 37°C, plated in triplicate, and assayed for colony enumeration. BD3 and cytokines were from R&D Systems.
Cell culture, flow cytometry, and histology.
ST2 cells were stimulated with 100 ng/ml IL-17 or 2 ng/ml TNF-α for 24 h in α-MEM media supplemented with 10% FCS. Cytokines and defensins were obtained from R&D Systems. For FACS, cLNs were harvested at day of sacrifice and cells stimulated with α-CD3 and α-CD28 antibodies (BD) for 48 h. Samples were stained intracellularly with anti–IL-17-PE antibodies (BD) and analyzed on a FACSCalibur with CellQuest software. Paraffin-embedded samples were stained with hematoxylin and eosin or periodic-acid Shiff (PAS) by the University at Buffalo Histology Core. Sections were analyzed at 10–40X magnification, and all scale bars indicate 100 μm.
Microarray and real-time RT-PCR.
Whole tongues from WT or IL-17RAKO female mice, age 10–16 wk (n = 2, age-matched within infection pairs), were harvested at baseline or 24 h after C. albicans inoculation and preserved in RNALater (Ambion). Total mRNA and cDNA were prepared and hybridized on Affymetrix Mouse 430–2 Genechips. Preparation and analysis were performed by the Roswell Park Cancer Institute Gene Expression Resource Facility (Buffalo, NY) using the Partek Genomics Suite 6.4 (RMA algorithm) with a quantile normalization and probe set summarization at median Pol. Real-time RT-PCR was performed on tongue samples in triplicate using SYBR Green and normalizing to GAPDH as previously described (75). Primers were purchased from SuperArray.
BD slot blot.
Tongues from indicated mice were homogenized in lysis buffer (10 mM NaPB, 1 mM EDTA, 1 µg/ml aprotinin, leupeptin, pepstatin-A, 1 mM PMSF, and 0.1% SDS), centrifuged to remove debris, and quantified for protein content. A 1.2- µg sample was loaded per slot blot well onto nitrocellulose, and blots were probed with 1 µg/ml anti-BD3 antibodies (Santa Cruz Biotechnology). Blots were quantified by scanning densitometry on an HP Scanjet 3970 and analyzed with Quantity One software (BioRad Laboratories).
Statistics.
At least two biological replicates were performed for all experiments unless otherwise indicated. Data were compared by Mann-Whitney or unpaired Student's t test using GraphPad Prism (v. 4) or Microsoft Excel software. P values < 0.05 were considered significant.
Online supplemental material.
Fig. S1 shows that similar levels of C. albicans infection are found in IL-17RA–deficient mice on the C57BL/6 background compared with the BALB/c background. Fig. S2 shows that IL-17 and TNF-α stimulation of a murine bone marrow stromal cell line (ST2) triggers secretion of factors with significant candidacidal activity. Figs. S3–S6 show primary microarray data comparing gene expression in tongue tissue of female WT and IL-17RAKO mice either at baseline or 24 h after C. albicans inoculation. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081463/DC1.
Acknowledgments
We are grateful to Amgen for IL-17RAKO mice and Genentech for IL-23p19KO and IL-22KO mice. We thank M. Russell, L. Sucheston, J. Kolls, S. Khader, and E. Wang for helpful discussions, and R. Onishi, J. Goswami and S. Park for technical assistance.
H.R. Conti, M.J. Lindemann, and J.J. Yu were supported by a training grant to the Department. of Oral Biology at SUNY Buffalo (DE007034). A.W. Ho and J. J. Yu were supported by the Medical Scientist Training Program at SUNY Buffalo. P. Masso-Welch was supported by the American Institute for Cancer Research. The National Institutes of Health supported S.L. Gaffen (AR054389, DE018822), M. Edgerton (DE010641), and S.G. Filler (DE017088).
S.L. Gaffen received honoraria and travel reimbursements from Amgen and Wyeth. The authors have no other conflicting financial interests.
Footnotes
Abbreviations used: BD, β defensin; cLN, cervical LN; CM, conditioned media; HIES, hyper IgE syndrome; OPC, oropharyngeal candidiasis; PAS, periodic-acid Schiff.
References
- Klein R.S., Harris C.A., Small C.B., Moll B., Lesser M., Friedland G.H. 1984. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome.N. Engl. J. Med. 311:354–358 [DOI] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A., Fidel P. 2005. The host cytokine responses and protective immunity in oropharyngeal candidiasis.J. Dent. Res. 84:966–977 [DOI] [PubMed] [Google Scholar]
- Scully C., el-Kabir M., Samaranayake L.P. 1994. Candida and oral candidosis: a review.Crit. Rev. Oral Biol. Med. 5:125–157 [DOI] [PubMed] [Google Scholar]
- Fidel P.L., Sobel J.D. 1998. Protective immunity in experimental Candida vaginitis.Res. Immunol. 149:361–373 [DOI] [PubMed] [Google Scholar]
- Ghilardi N., Ouyang W. 2007. Targeting the development and effector functions of Th17 cells.Semin. Immunol. 19:383–393 [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Pflanz S., Kastelein R.A. 2003. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses.Immunity. 19:641–644 [DOI] [PubMed] [Google Scholar]
- Fantini M.C., Monteleone G., Macdonald T.T. 2007. New players in the cytokine orchestra of inflammatory bowel disease.Inflamm. Bowel Dis. 13:1419–1423 [DOI] [PubMed] [Google Scholar]
- Aujla S.J., Dubin P.J., Kolls J.K. 2007. Th17 cells and mucosal host defense.Semin. Immunol. 19:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M., Santos R.L., Verhoeven D.E., George M.D., Wilson R.P., Winter S.E., Godinez I., Sankaran S., Paixao T.A., Gordon M.A., et al. 2008. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut.Nat. Med. 14:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F., Gaffen S.L. 2008. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy.Cytokine. 41:92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells.Nat. Immunol. 8:639–646 [DOI] [PubMed] [Google Scholar]
- Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Fili L., Ferri S., Frosali F., et al. 2007. Phenotypic and functional features of human Th17 cells.J. Exp. Med. 204:1849–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K., Yoshitomi H., Hashimoto M., Maeda S., Teradaira S., Sugimoto N., Yamaguchi T., Nomura T., Ito H., Nakamura T., et al. 2007. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model.J. Exp. Med. 204:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Repentigny L., Lewandowski D., Joliceur P. 2004. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection.Clin. Microbiol. Rev. 17:729–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.J., Ruddy M., Wong G., Sfintescu C., Baker P., Smith J., Evans R., Gaffen S. 2007. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals.Blood. 109:3794–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.J., Ruddy M., Conti H., Boonanantanasarn K., Gaffen S.L. 2008. The IL-17 receptor plays a gender-dependent role in host protection against P. gingivalis-induced periodontal bone loss.Infect. Immun. 76:4206–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Na L., Fidel P.L., Schwarzenberger P. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice.J. Infect. Dis. 190:624–631 [DOI] [PubMed] [Google Scholar]
- Leibundgut-Landmann S., Gross O., Robinson M.J., Osorio F., Slack E.C., Tsoni S.V., Schweighoffer E., Tybulewicz V., Brown G.D., Ruland J., Reis E.S.C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17.Nat. Immunol. 8:630–638 [DOI] [PubMed] [Google Scholar]
- Taylor P.R., Tsoni S.V., Willment J.A., Dennehy K.M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S., Brown G.D. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection.Nat. Immunol. 8:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J.D., Brenchley J.M., Laurence A., Freeman A.F., Hill B.J., Elias K.M., Kanno Y., Spalding C., Elloumi H.Z., Paulson M.L., et al. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome.Nature. 452:773–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner X.L., Happel K., Young E., Shellito J. 2007. Interleukin-12 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection.Infect. Immun. 75:3055–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T., De Luca A., Bonifazi P., Montagnoli C., Bozza S., Moretti S., Belladonna M.L., Vacca C., Conte C., Mosci P., et al. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance.Eur. J. Immunol. 37:2695–2706 [DOI] [PubMed] [Google Scholar]
- Kolls J.K., Linden A. 2004. Interleukin-17 family members and inflammation.Immunity. 21:467–476 [DOI] [PubMed] [Google Scholar]
- Farah C.S., Hu Y., Riminton S., Ashman R. 2006. Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene targeting.Oral Microbiol. Immunol. 21:252–255 [DOI] [PubMed] [Google Scholar]
- Kamai Y., Kubota M., Kamai Y., Hosokawa T., Fukuoka T., Filler S. 2001. New model of oropharyngeal candidiasis in mice.Antimicrob. Agents Chemo. 45:3195–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer J.S., Laurence A., Wilson E.H., Huang E., Tato C.M., Johnson L.M., Villarino A.V., Huang Q., Yoshimura A., Sehy D., et al. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system.Nat. Immunol. 7:937–945 [DOI] [PubMed] [Google Scholar]
- Aujla S.J., Chan Y.R., Zheng M., Fei M., Askew D.J., Pociask D.A., Reinhart T.A., McAllister F., Edeal J., Gaus K., et al. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia.Nat. Med. 14:275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens.Nat. Med. 14:282–289 [DOI] [PubMed] [Google Scholar]
- Stark M.A., Huo Y., Burcin T.L., Morris M.A., Olson T.S., Ley K. 2005. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17.Immunity. 22:285–294 [DOI] [PubMed] [Google Scholar]
- Lockhart E., Green A.M., Flynn J. 2006. IL-17 production by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection.J. Immunol. 177:4662–4669 [DOI] [PubMed] [Google Scholar]
- Shibata K., Yamada H., Hara H., Kishihara K., Yoshikai Y. 2007. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production.J. Immunol. 178:4466–4472 [DOI] [PubMed] [Google Scholar]
- Rachitskaya A.V., Hansen A.M., Horai R., Li Z., Villasmil R., Luger D., Nussenblatt R.B., Caspi R.R. 2008. Cutting edge: NKT cells constitutively express IL-23 receptor and ROR{gamma}t and rapidly produce IL-17 upon receptor Ligation in an IL-6-independent fashion.J. Immunol. 180:5167–5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.K., Clements J.L., Gaffen S.L. 2005. Signaling through the murine T cell receptor induces IL-17 production in the absence of costimulation, IL-23 or dendritic cells.Mol. Cells. 20:329–337 [PubMed] [Google Scholar]
- Gaffen S. 2008. An overview of IL-17 receptor function and signaling.Cytokine. 42:403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willment J.A., Brown G.D. 2008. C-type lectin receptors in antifungal immunity.Trends Microbiol. 16:27–32 [DOI] [PubMed] [Google Scholar]
- Wells C.A., Salvage-Jones J.A., Li X., Hitchens K., Butcher S., Murray R.Z., Beckhouse A.G., Lo Y.L., Manzanero S., Cobbold C., et al. 2008. The macrophage-inducible C-Type lectin, Mincle, is an essential component of the innate immune response to Candida albicans.J. Immunol. 180:7404–7413 [DOI] [PubMed] [Google Scholar]
- Vylkova S., Li X.S., Berner J.C., Edgerton M. 2006. Distinct antifungal mechanisms: beta-defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-free cationic peptides.Antimicrob. Agents Chemother. 50:324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Chen Q., Hoover D., Staley P., Tucker K., Lubkowski J., Oppenheim J. 2003. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity.J. Leukoc. Biol. 74:448–455 [DOI] [PubMed] [Google Scholar]
- Leung K.C., McMillan A.S., Cheung B.P., Leung W.K. 2008. Sjogren's syndrome sufferers have increased oral yeast levels despite regular dental care.Oral Dis. 14:163–173 [DOI] [PubMed] [Google Scholar]
- Almstahl A., Kroneld U., Tarkowski A., Wikstrom M. 1999. Oral microbial flora in Sjogren's syndrome.J. Rheumatol. 26:110–114 [PubMed] [Google Scholar]
- Edgerton M., Koshlukova S.E., Araujo M.W., Patel R.C., Dong J., Bruenn J.A. 2000. Salivary histatin 5 and human neutrophil defensin 1 kill Candida albicans via shared pathways.Antimicrob. Agents Chemother. 44:3310–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F.C. 1998. Pathogenesis of candidosis. In Candida and Candidosis. F.C. Odds, editor Bailliere Tindall, 252–278 [Google Scholar]
- Raman T., Marik P. 2006. Fungal infections in bone marrow transplant recipients.Expert Opin. Pharmacother. 7:307–315 [DOI] [PubMed] [Google Scholar]
- Weindl G., Naglik J.R., Kaesler S., Biedermann T., Hube B., Korting H.C., Schaller M. 2007. Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling.J. Clin. Invest. 117:3664–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S., Fujikado N., Furuta T., Chung S.H., Kotaki H., Seki K., Sudo K., Akira S., Adachi Y., Ohno N., et al. 2007. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans.Nat. Immunol. 8:39–46 [DOI] [PubMed] [Google Scholar]
- Bozza S., Zelante T., Moretti S., Bonifazi P., DeLuca A., D'Angelo C., Giovannini G., Garlanda C., Boon L., Bistoni F., et al. 2008. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection.J. Immunol. 180:4022–4031 [DOI] [PubMed] [Google Scholar]
- Yu J.J., Gaffen S.L. 2008. Interleukin-17: A novel inflammatory cytokine that bridges innate and adaptive immunity.Front. Biosci. 13:170–177 [DOI] [PubMed] [Google Scholar]
- Linden A., Laan M., Anderson G. 2005. Neutrophils, interleukin-17A and lung disease.Eur. Respir. J. 25:159–172 [DOI] [PubMed] [Google Scholar]
- O'Shea J.J., Ma A., Lipsky P. 2002. Cytokines and autoimmunity.Nat. Rev. Immunol. 2:37–45 [DOI] [PubMed] [Google Scholar]
- Kramer J., Gaffen S. 2007. Interleukin-17: A new paradigm in inflammation, autoimmunity and therapy.J. Periodontol. 78:1083–1093 [DOI] [PubMed] [Google Scholar]
- Pepin L.F., Roger T., Morisset J., Seman M. 1993. Preferential V delta 1 expression among TcR gamma/delta-bearing T cells in human oral epithelium.Scand. J. Immunol. 37:289–294 [DOI] [PubMed] [Google Scholar]
- Wormley F.L., Jr., Steele C., Wozniak K., Fujihashi K., McGhee J.R., Fidel P.L., Jr 2001. Resistance of T-cell receptor delta-chain-deficient mice to experimental Candida albicans vaginitis.Infect. Immun. 69:7162–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Carson J., Vazquez-Torres A., van der Heyde H.C., Warner T., Wagner R.D., Balish E. 1995. Gamma delta T cell-induced nitric oxide production enhances resistance to mucosal candidiasis.Nat. Med. 1:552–557 [DOI] [PubMed] [Google Scholar]
- Mathews M., Jia H.P., Guthmiller J.M., Losh G., Graham S., Johnson G.K., Tack B.F., McCray P.B., Jr 1999. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands.Infect. Immun. 67:2740–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Jiang B., Chandra J., Ghannoum M., Nelson S., Weinberg A. 2005. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans.J. Dent. Res. 84:445–450 [DOI] [PubMed] [Google Scholar]
- Kao C.Y., Chen Y., Thai P., Wachi S., Huang F., Kim C., Harper R.W., Wu R. 2004. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways.J. Immunol. 173:3482–3491 [DOI] [PubMed] [Google Scholar]
- Yang D., Chertov O., Bykovskaia S.N., Chen Q., Buffo M.J., Shogan J., Anderson M., Schröder J.M., Wang J.M., Howard O.M.Z., Oppenheim J.J. 1999. β-Defensins: linking innate immunity and adaptive immunity through dendritic and T cell CCR6.Science. 286:525–528 [DOI] [PubMed] [Google Scholar]
- Raquil M.A., Anceriz N., Rouleau P., Tessier P.A. 2008. Blockade of antimicrobial proteins S100A8 and S100A9 inhibits phagocyte migration to the alveoli in Streptococcal pneumonia.J. Immunol. 180:3366–3374 [DOI] [PubMed] [Google Scholar]
- Kao C.-Y., Huang F., Chen Y., Thai P., Wachi S., Kim C., Tam L., Wu R. 2005. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK-NF-kappaB-dependent signaling pathway.J. Immunol. 175:6676–6685 [DOI] [PubMed] [Google Scholar]
- Zrioual S., Toh M.L., Tournadre A., Zhou Y., Cazalis M.A., Pachot A., Miossec V., Miossec P. 2008. IL-17RA and IL-17RC receptors are essential for IL-17A-induced ELR+ CXC chemokine expression in synoviocytes and are overexpressed in rheumatoid blood.J. Immunol. 180:655–663 [DOI] [PubMed] [Google Scholar]
- Wilson N.J., Boniface K., Chan J.R., McKenzie B.S., Blumenschein W.M., Mattson J.D., Basham B., Smith K., Chen T., Morel F., et al. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells.Nat. Immunol. 8:950–957 [DOI] [PubMed] [Google Scholar]
- Ma C.S., Chew G.Y., Simpson N., Priyadarshi A., Wong M., Grimbacher B., Fulcher D.A., Tangye S.G., Cook M.C. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3.J. Exp. Med. 205:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaucoudrey L., Puel A., Filipe-Santos O., Cobat A., Ghandil P., Chrabieh M., Feinberg J., von Bernuth H., Samarina A., Janniere L., et al. 2008. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells.J. Exp. Med. 205:1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich K., Foerster S., Rombold S., Seidl H.P., Behrendt H., Hofmann H., Ring J., Traidl-Hoffmann C. 2008. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22.J. Invest. Dermatol. 128:2640–2645 [DOI] [PubMed] [Google Scholar]
- Liang S.C., Tan X.Y., Luxenberg D.P., Karim R., Dunussi-Joannopoulos K., Collins M., Fouser L.A. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides.J. Exp. Med. 203:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudlaugsson O., Gillespie S., Lee K., Vande Berg J., Hu J., Messer S., Herwaldt L., Pfaller M., Diekema D. 2003. Attributable mortality of nosocomial candidemia, revisited.Clin. Infect. Dis. 37:1172–1177 [DOI] [PubMed] [Google Scholar]
- Filler S.G., Yeaman M.R., Sheppard D.C. 2005. Tumor necrosis factor inhibition and invasive fungal infections.Clin. Infect. Dis. 41(Suppl 3):S208–S212 [DOI] [PubMed] [Google Scholar]
- Cecchinato V., Trindale C., Laurence A., Heraud J., Brenchley J., Ferrari M., Zaffri L., Tryniszewska E., Tsai W., Vaccari M., et al. 2008. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques.Mucosal Immunol. 1:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison L.W., Workman C.J., Kuo T.T., Boyd K., Wang Y., Vignali K.M., Cross R., Sehy D., Blumberg R.S., Vignali D.A. 2007. The inhibitory cytokine IL-35 contributes to regulatory T-cell function.Nature. 450:566–569 [DOI] [PubMed] [Google Scholar]
- Rickel E.A., Siegel L.A., Yoon B.R., Rottman J.B., Kugler D.G., Swart D.A., Anders P.M., Tocker J.E., Comeau M.R., Budelsky A.L. 2008. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities.J. Immunol. 181:4299–4310 [DOI] [PubMed] [Google Scholar]
- Kikly K., Liu L., Na S., Sedgwick J.D. 2006. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation.Curr. Opin. Immunol. 18:670–675 [DOI] [PubMed] [Google Scholar]
- Strangfeld A., Listing J. 2006. Infection and musculoskeletal conditions: bacterial and opportunistic infections during anti-TNF therapy.Best Pract. Res. Clin. Rheumatol. 20:1181–1195 [DOI] [PubMed] [Google Scholar]
- Marino M.W., Dunn A., Grail D., Inglese M., Noguchi Y., Richards E., Jungbluth A., Wada H., Moore M., Williamson B., et al. 1997. Characterization of tumor necrosis factor-deficient mice.Proc. Natl. Acad. Sci. USA. 94:8093–8098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Danilenko D.M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., Ouyang W. 2007. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis.Nature. 445:648–651 [DOI] [PubMed] [Google Scholar]
- Shen F., Ruddy M.J., Plamondon P., Gaffen S.L. 2005. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells.J. Leukoc. Biol. 77:388–399 [DOI] [PubMed] [Google Scholar]