Abstract
Memory T cells exhibit superior responses to pathogens and tumors compared with their naive counterparts. Memory is typically generated via an immune response to a foreign antigen, but functional memory T cells can also be produced from naive cells by homeostatic mechanisms. Using a recently developed method, we studied CD8 T cells, which are specific for model (ovalbumin) and viral (HSV, vaccinia) antigens, in unimmunized mice and found a subpopulation bearing markers of memory cells. Based on their phenotypic markers and by their presence in germ-free mice, these preexisting memory-like CD44hi CD8 T cells are likely to arise via physiological homeostatic proliferation rather than a response to environmental microbes. These antigen-inexperienced memory phenotype CD8 T cells display several functions that distinguish them from their CD44lo counterparts, including a rapid initiation of proliferation after T cell stimulation and rapid IFN-γ production after exposure to proinflammatory cytokines. Collectively, these data indicate that the unprimed antigen-specific CD8 T cell repertoire contains antigen-inexperienced cells that display phenotypic and functional traits of memory cells.
During normal primary T cell responses, naive T cells are induced to proliferate and differentiate, giving rise first to an effector pool and then to a long-lived memory population (1, 2). The size of the antigen-specific memory T cell pool is typically larger than the naive population from which they are derived, but in addition to their elevated frequency, memory T cells exhibit multiple qualitative advantages over their naive counterparts with respect to their functional versatility, speed of response, and capacity to migrate to multiple sites besides lymphoid tissues (1, 2).
Aside from this standard pathway, however, there is accumulating data that memory phenotype T cells can arise from naive T cells via homeostatic mechanisms without activation of the T cell by foreign antigen. This has been well studied in situations of extreme lymphopenia, as induced by irradiation or genetic T cell deficiency, which induces homeostatic proliferation (HP) of naive T cells (3–5). In addition, there is also evidence that HP can occur within unprimed neonatal mice (3–9). T cell HP in the lymphopenic environment is thought to be driven by reduced competition for limiting resources, including IL-7 and low-affinity TCR ligands (3–5, 10, 11), and can be further influenced by other cytokines (12–15).
HP memory cells resemble conventional memory cells in many of their phenotypic and functional traits. After HP, T cells display numerous phenotypic markers that are similar to those of true antigen-driven memory cells, such as increased CD44, LFA1, Ly6C, and CD122 expression (3–5, 16, 17). Antigen-driven memory cells display increased sensitivity to antigen stimulation leading to a more rapid proliferative response and enhanced cytotoxic and cytokine-producing effector functions (18, 19). Similarly, HP memory cells display proliferative responses, increased effector cytokine production, and an acquisition of cytotoxic functions that are significantly elevated compared with naive T cells (3–5, 16, 17). This increased functionality of HP memory cells is significant, as demonstrated by the fact that HP memory cells can provide a substantial degree of protective immunity against infectious challenge (17). Besides their enhanced functional responses, antigen-driven memory cells can traffic outside of traditional secondary lymphoid tissues (20–22) and, indeed, may even show a preference for circulating within peripheral tissues, particularly under inflammatory conditions (21, 23), although whether this also applies to HP memory cells has not been extensively studied. Finally, memory CD8 T cells also display innate-like functions, including their capacity (similar to NK cells) for production of IFN-γ in response to stimulation by IL-12 and IL-18 (24, 25).
The representation of HP memory T cells in the bulk memory T cell pool within a normal unmanipulated host is unclear. Analysis of naive mice (i.e., animals which have not been deliberately immunized) consistently shows an abundant population of memory phenotype T cells, which can amount to 15–20% of total CD8 T cells. This population is typically assumed to be the result of T cell activation and formation of memory in response to environmental antigens. However, a memory CD8 T cell population is also present in unprimed animals housed under gnotobiotic (germ free [GF]) conditions (26), which are free of what would be considered a dominant source of environmental antigens. This suggests that at least some memory phenotype cells in unprimed mice might be HP memory cells generated during physiological lymphopenia. Importantly, little is known about the antigen specificity of endogenous memory phenotype T cells or their ability to engage in primary immune responses.
Using a novel technique recently developed by ourselves (27) and others (28), we have isolated the pool of antigen-specific CD8+ T cells from unimmunized hosts and find evidence not only for naive antigen-specific cells but also for memory phenotype T cells within this pool. These cells bear cell surface markers typical of HP memory cells and are detected in GF as well as specific pathogen-free (SPF) animals. We present evidence suggesting that these preexisting memory-like CD8 T cells represent a novel additional arm of the immune repertoire capable of participating in the primary immune response.
RESULTS
Isolation of naive antigen-specific T cells from an unprimed host
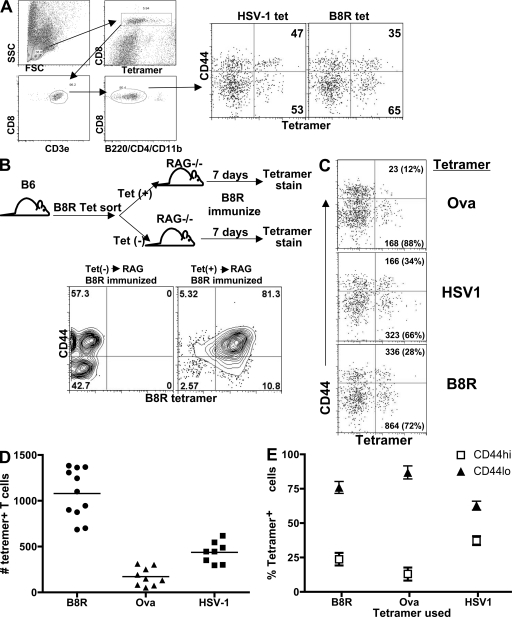
We (27) and others (28) recently devised a method for isolating antigen-specific CD4+ T cells from the normal T cell repertoire in an unprimed animal. Using a modified approach to this method, we used class I MHC tetramers loaded with various peptide antigens (OVA-Kb/SIINFEKL [references 29, 30], vaccinia virus B8R-Kb/TSYKFESV [reference 31], and HSV-1gB-Kb/SSIEFARL [reference 32]) to isolate antigen-specific CD8+ T cells, from unimmunized normal B6 mice. Spleen and/or LN cells were isolated as previously described (33) and stained with PE-labeled tetramer in the presence of azide to block internalization of the TCR. After tetramer staining, the cells were incubated with anti-PE magnetic beads. After separation on a magnetic column, the cells were stained and gated so as to most effectively capture all tetramer-stained CD8+ T cells and eliminate noise (Fig. 1 A). We confirmed that this tetramer enrichment approach was reliable at capturing antigen-specific cells by adoptively transferring either positive (column bound) or negative (flow through) fractions of B8R/Kb-stained spleen cells into RAG−/− hosts (Fig. 1 B). After immunization of these transfer recipients, B8R-specific T cells were only detectable in the RAG−/− mice transferred with the positive column fraction (Fig. 1 B), confirming that all antigen-specific T cells were contained within this fraction.
Figure 1.
Quantitation and characterization of antigen-specific CD8+ T cells from unprimed mice. (A) Gating scheme for FACS analysis of peptide/MHC tetramer binding cells isolated by magnetic bead sorting. Left dot plots show a representative gating strategy used to identify CD8+ T cells isolated by magnetic bead column after tetramer staining. After this gating strategy, the cells were then analyzed as displayed in the right dot plots and show the general phenotype of the tetramer and CD44 staining profile. Numbers in top and bottom right quadrants indicate the percentage of tetramer+ cells that are either CD44hi or CD44lo, respectively. (B) As shown in the flow diagram, B6 spleen cells stained with the B8R tetramer were subjected to magnetic bead separation. The flow through and bound fraction were then injected into separate RAG−/− hosts, which were subsequently immunized with the B8R peptide plus poly-I:C and anti-CD40. 7 d after immunization, peripheral blood (depicted) and spleen (not depicted) were analyzed by B8R tetramer staining. The results shown are representative of six mice from three independent experiments. (C) Splenocytes from unprimed B6 mice were isolated using MHC tetramers loaded with the indicated peptides. Numbers outside the parentheses indicate the total number of cells in the mouse with the phenotype in that quadrant. Numbers inside the parentheses indicate the percentage of cells, out of all tetramer+ cells, that are either CD44hi or CD44lo. (D) Scatter plot shows the distribution of total tetramer+ cell numbers per spleen as isolated by the indicated MHC tetramer, one mouse spleen per data point. Horizontal lines show the mean value of the dots displayed. (E) The frequency of CD44lo and CD44hi cells within tetramer binding CD8 T cells from unprimed mice is shown for the indicated tetramers. The results shown are representative of at least 10 independent experiments, with the staining results pooled from four independent experiments showing 11 mice for the B8R tetramer, 9 mice for the OVA tetramer, and 6 mice for the HSV-1 tetramer. Error bars show SD.
Analysis of the population of tetramer-bound T cells from unprimed animals showed several interesting properties. First, depending on the epitope, the total number of antigen-specific CD8+ T cells detected in these unprimed animals averaged ∼170 (OVA-specific T cells)–1,070 (B8R-specific T cells) cells per mouse spleen (Fig. 1, C and D), a frequency which is significantly higher than that observed for antigen-specific CD4+ T cells using a similar method (27) but is in line with recent studies of CD8 T cells using a similar method of naive T cell isolation (28). Second, in contrast to our expectations that antigen-specific cells in unimmunized animals would all be of naive phenotype, a significant percentage of these CD8+ T cells expressed high levels of CD44 (Fig. 1, A and C), a phenotype which is typically associated with antigen-experienced effector or memory CD8 T cells. The percentage of CD44hi cells varied from as little as 10% of OVA-specific CD8+ T cells to 30–40% of all HSV-1–specific CD8+ T cells (Fig. 1, C and E). Changes in cell isolation approaches, tetramer staining conditions, and gating schemes were evaluated to determine whether such CD44hi tetramer binding cells were artifacts, but the population was reliably observed under various protocols (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20081829/DC1; and see Materials and methods). Therefore, a significant percentage of antigen-inexperienced T cells in an unprimed host expressed a phenotypic marker usually associated with activation or memory.
Antigen-specific T cells from an unprimed host are both CD44hi and CD44lo
It was plausible that a population of CD44hi CD8 T cells is simply prone to nonspecific tetramer binding. As an initial test of this premise, we isolated T cells from a RAG−/−gBT-1 TCR transgenic mouse, which was specific for an HSV-1 glycoprotein B (gB) peptide (32), using Kb MHC tetramers loaded with the OVA, B8R, or HSV-1 peptides. Two observations are noteworthy from these experiments. First, the B8R- or OVA- loaded tetramers failed to isolate any cells at all from the RAG−/−gBT-1 mouse (Fig. 2 A). Second, staining with the HSVgB tetramer detected both CD44lo and hi gB-specific CD8+ T cells within the RAG−/−gBT-1 host. These CD44hi TCR+ cells are typically seen in TCR transgenic mice on a RAG-expressing background and their presence is usually attributed to the expression of endogenous α chains, whereas our data indicate that they are also detected in situations where a monoclonal TCR is used. More importantly, this CD44hi population does not bind nonspecifically to MHC tetramers, as indicated by the lack of binding to OVA or B8R tetramers. We therefore concluded that the interaction of our tetramers with either CD44lo or CD44hi T cells is antigen specific and that CD44hi T cells do not have an increased propensity to nonspecifically bind to class I MHC tetramers.
Figure 2.
Specific tetramer staining of endogenous CD44hi CD8 T cells. (A) Tetramer staining and magnetic bead sorting was performed on spleen cells from B6 and RAG−/− gBT-1 mice using the indicated tetramers. Numbers in the plots indicate percentage of cells in that quadrant after having been gated as shown in Fig. 1 A. The results shown are representative of at least three independent experiments using a total of six mice from each strain. (B) Splenocytes from unprimed B6 mice were isolated using both PE- and APC-labeled MHC tetramers loaded with the indicated peptides, as described in the Materials and methods. Dot plots show all B220−CD3+CD8+ events from either the flow through or column-bound fractions as indicated. Numbers in each quadrant indicate the percentage of total CD8+ cells in that quadrant. (C) Cells analyzed in B were further gated as indicated in the left dot plot and analyzed as shown in the right dot plots. Numbers in the top and bottom quadrants indicate the percentage of all tetramer+ events that are either CD44hi or CD44lo, respectively. The results for B and C are representative of 15 mice from at least five independent experiments.
As a further confirmation of this conclusion, we determined whether the tetramer-positive cells were staining in a peptide-selective manner by simultaneously staining the cells with two different tetramers (PE and APC labeled) loaded with either the same or different peptides. Consistent with our prediction, antigen-specific cells isolated using both B8R-PE– and B8R-APC–labeled tetramers were found to bind both tetramers, whereas cells enriched using both HSV-1-PE and B8R-APC tetramers did not demonstrate any degree of double tetramer staining (Fig. 2 B). Additionally, both CD44hi and lo cells were present within the tetramer+ fraction (Fig. 2 C). These data reinforce the conclusion that the interaction of our tetramers with either CD44lo or CD44hi T cells is indeed antigen specific.
T cells isolated by tetramer binding respond to specific antigen stimulation in vivo
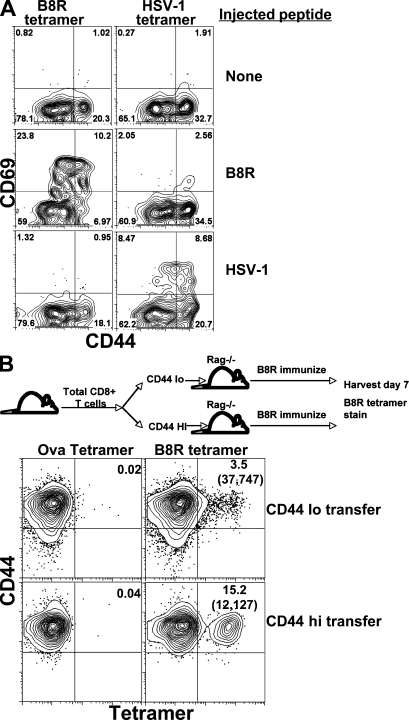
Confident in the specificity of our tetramer staining method, we next determined whether both CD44lo and CD44hi tetramer+ cells were capable of responding to peptide stimulation. B6 mice were injected i.v. with either the B8R or HSV-1 peptide. 2 h later the mice were killed, half of the spleen cells from each peptide-injected mouse were stained with the Kb/B8R tetramer, and the other half were stained with the Kb/HSVgB tetramer. The B8R- and HSV-1–specific cells were then isolated as before on the magnetic columns and the resulting cells were analyzed for CD69 expression. Both CD44hi and CD44lo B8R-specific T cells isolated from the B8R peptide-injected mice showed an increase in CD69 expression (Fig. 3 A). In contrast, cells isolated from the same B8R peptide-injected mice using the HSV-1 tetramer did not show increased CD69 expression (Fig. 3 A). The reciprocal results were true for HSV-1 peptide-injected mice. Not all tetramer-staining T cells increased expression of CD69 in response to peptide challenge (the frequency of CD69+ cells was ∼30–40% of the tetramer binding pool). This may be a result of incomplete peptide distribution and/or specific T cell activation within the 2 h stimulation or may reflect a true inability of some tetramer binding T cells to respond to the dose of antigen used. Importantly, however, CD44hi T cells were activated at least as efficiently as CD44lo cells within the appropriate tetramer binding population, suggesting that the preexisting tetramer+ CD44hi pool include bonafide antigen-reactive cells. The isolation of OVA-, HSV-1–, or B8R-specific T cells from vaccinia virus–challenged mice demonstrated a similar finding; although cells isolated with the B8R tetramer show signs of antigenic stimulation, T cells isolated from the virus challenged host with the HSV-1 or OVA tetramers show no signs of overt antigen stimulation (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20081829/DC1). Collectively, these results further support the conclusion that both CD44hi and CD44lo cells isolated with a given peptide MHC tetramer demonstrate specificity for that peptide–MHC complex.
Figure 3.
Foreign antigen-reactive CD44hi CD8 T cells found in unprimed SPF mice. (A) B6 mice were injected i.v. with 400 mg of either B8R or HSV-1 peptide. 2 h later, the mice were killed, half of the spleen cells from each peptide-injected mouse were stained with the B8R/Kb tetramer, and the other half were stained with the HSVgB/Kb tetramer. B8R- and HSV-specific T cells from either peptide-challenged mice and from control mice were then isolated by magnetic column and analyzed for CD44 and CD69 expression on all tetramer-staining cells. Numbers in the top quadrants represent the percentage of CD69+ cells of all B220−CD8+CD3+tetramer+ cells shown. The results shown are representative of six mice for each treatment group from three independent experiments. (B) As shown in the schematic, CD8+ T cells from unimmunized B6 mice were sorted based on their expression of CD44. CD44hi and CD44lo cells were transferred into separate RAG−/− mice, which were subsequently immunized with the B8R peptide in conjunction with poly-I:C and anti-CD40. 7 d after immunization, the spleen cells from each transferred and immunized host were stained with the indicated tetramers. The Kb/OVA tetramer was used as a staining control as shown. Numbers above each gate represent the percentage of tetramer-staining T cells out of total CD8+ T cells. The numbers in parentheses indicate the total number of tetramer-staining cells per mouse after immunization. The results shown are representative of eight total recipient mice from two independent experiments.
Antigen-responsive cells are present in both the CD44hi and CD44lo CD8+ T cell pools of unprimed mice
Standard models of adaptive immunity would predict that foreign antigen-specific T cells in an unprimed animal would be exclusively of naive phenotype (i.e., CD44lo). In contrast, the data discussed in the previous section indicates that antigen-specific precursors should be present in both the CD44lo and CD44hi (memory phenotype) populations of CD8+ T cells in an unprimed animal. To test this hypothesis using a method not influenced by the use of tetramer sorting, we sorted total CD8+ T cells from unprimed animals based only on their CD44 expression (Fig. 3 B). CD44lo and CD44hi populations were then transferred into separate RAG−/− hosts and the recipients immunized with B8R peptide together with adjuvants. After immunization, B8R-specific T cells were readily identified in mice transferred with either CD44lo or CD44hi precursors (Fig. 3 B), suggesting that specific precursors are present in both pools. We therefore concluded that antigen specific precursors are present in both the CD44lo and CD44hi fractions of CD8+ T cell in unprimed mice.
Antigen-specific CD44hi CD8 T cells are found in unprimed GF mice
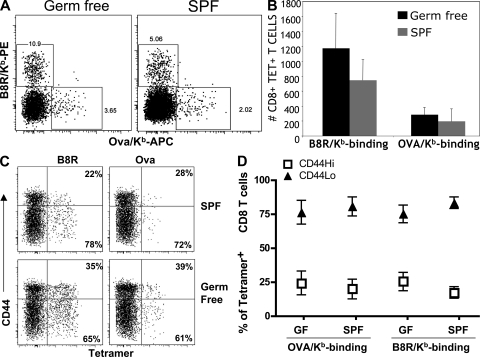
We postulated two possible explanations for the existence of antigen-specific CD44hi T cells in unimmunized animals. First, these cells may be authentic antigen-experienced memory T cells that fortuitously cross-react on the peptide–MHC complex used for tetramer-based isolation. Previous studies have shown that the memory CD8 T cell pool specific for one pathogen may contain cells reactive to an unrelated pathogen (a response called heterologous memory) (34, 35). Alternatively, the memory-like cells we detected in unimmunized animals may be antigen inexperienced, having been generated from the naive T cell pool through homeostatic mechanisms. Conversion from naive to functional memory phenotype cells occurs in response to induced lymphopenia (16, 17, 36) but has also been reported to occur in physiological circumstances of lymphopenia, such as in neonatal mice (7, 9). To begin distinguishing these possibilities, we analyzed the antigen-specific T cell pool in gnotobiotic (GF) mice. These animals are raised in sterile conditions and lack culturable gut flora. In contrast to the SPF animals, which are colonized by a diverse gut flora, the T cell pool of GF mice should be unbiased by exposure to commensal or environmental microbes.
Use of the tetramer enrichment protocol revealed a notable population of antigen-specific CD8 T cells binding B8R/Kb or OVA/Kb tetramers in unimmunized GF B6 splenocytes (Fig. 4 A), which in absolute numbers were similar to SPF B6 animals analyzed in the same experiments (Fig. 4 B). Critically, the tetramer-bound populations from GF animals also contained a population of CD44hi cells that were present at similar frequencies to those found in SPF mice (Fig. 4, C and D). These studies cannot rule out the contribution of nonmicrobial antigens in appearance of these memory phenotype CD8 T cells. However, our data do suggest that the presence of CD44hi antigen-specific pool in unprimed animals does not require exposure to environmental microbes and, hence, is unlikely to arise from the cross-reactive response to pathogens characteristic of heterologous memory (34, 35).
Figure 4.
Foreign antigen-specific CD44hi CD8 T cells are found in GF mice. Spleen and LNs were collected from B6 mice maintained in GF versus SPF conditions and subjected to tetramer enrichment using B8R/Kb and OVA/Kb tetramers. (A) Dot plots show representative tetramer staining of the column-bound pool gated on CD3+CD8+ B220/CD4− cells. (B) The total numbers of tetramer-bound CD8 T cells detected in unprimed SPF and GF mice compiled from three independent experiments (n = 9 for both SPF and GF mice). The graph shows mean and SD. (C) Representative data showing phenotype of column-bound cells. The data are gated on dump− CD3+CD8+ T cells and show CD44 versus tetramer staining for cells from SPF and GF animals. Percentages are of tetramer-bound cells. (D) Mean percentage of CD44hi and CD44lo phenotype cells detected in unprimed SPF and GF mice within the indicated tetramer+ population. Numbers reflect mean and SD, with the data compiled from nine mice in each group.
CD44hi T cells in unprimed mice bear the phenotypic signature of HP
These data lead us to speculate that the presence of the CD44hi tetramer+ T cells might be the result of HP rather than a foreign antigen-driven response. We therefore sought to more comprehensively determine whether the phenotype and function of the CD44hi tetramer+ cells was more consistent with foreign antigen experience or with HP. B8R-specific T cells were isolated from unprimed B6 mice and stained for a variety of markers known to be differentially expressed on naive and activated/memory T cells. As a control for a phenotype representative of antigen-driven activation, B8R-specific cells were simultaneously isolated from mice challenged 4 d previously with vaccinia virus. B8R-specific cells from vaccinia-challenged mice displayed a classical activation phenotype, uniformly expressing high levels of CD44, LFA1, VLA4, Ly6C, and CD122 (Fig. 5 A). A significant population of cells also expressed CD69 and CD25, which is indicative of ongoing antigenic stimulation as would be expected in mice only 4 d after vaccinia challenge. This phenotype was similar to peptide-immunized mice as well, though the peptide-challenged mice displayed a slightly advanced time course of activation compared with viral challenge (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20081829/DC1). The specific CD44hi CD8 T cells isolated from unprimed mice expressed noticeably increased levels of CD122, LFA1, and Ly6C but were low for CD69 and CD25, which is consistent with a memory (rather than effector) phenotype (16, 17, 36). The CD44hi population in GF mice also expressed elevated levels of CD122, which is similar to cells from SPF mice (Fig. S4 B). Together, these data reinforce the designation of the preexisting tetramer+ CD44hi pool as memory-like.
Figure 5.
Phenotypic analysis of CD44hi and CD44lo tetramer-bound T cells from unprimed mice. (A) B8R-specific T cells were isolated by tetramer staining and magnetic bead separation from the spleens of both unprimed and day-4 vaccinia virus–challenged mice. The cells were stained with CD44 and the indicated activation markers. The data shown is gated as in Fig. 1 A and shows all B220−/CD8+/CD3+/tetramer+ events. Numbers in each quadrant indicate the percentage of B8R tetramer+ cells within that quadrant. The results shown are representative of eight mice from four independent experiments. (B) Naive OT-I CD8 T cells were transferred into either irradiated B6 mice (to generate HP memory cells) or LM-OVA–infected mice (to generate “true” memory cells). At least 30 d later, the donor OT-I cells (identified using OVA/Kb tetramer) were assessed for their expression on α4-integrin. For comparison, α4-integrin on bulk endogenous CD8 T cells is shown, as is isotype control staining. These data are representative of at least three experiments with two to three mice per group. (C) B8R-specific CD8+ T cells isolated by magnetic bead separation, as in A, from an unprimed B6 (top contour plots) were compared with the CD8+ spleen cells from a gBT-1RAG−/− mouse (bottom contour plots) with respect to the activation markers shown. Cells were gated on all CD8+/B220−/CD3+/tetramer+ events.
Unexpectedly, we found that tetramer binding CD44hi cells in unimmunized animals expressed low levels of α4-integrin (Fig. 5 A). This protein (also called CD49d) is a component of the integrins VLA-4 and LPAM and is typically expressed at high levels on antigen-stimulated effector and memory CD8 T cells (23, 37–41) (Fig. 5 A). Interestingly, we saw similarly low expression of α4-integrin on memory CD8 T cells produced via HP. Conventional and HP memory CD8 T cells were generated from naive OT-I T cells by adoptive transfer into LM-OVA–infected or lymphopenic mice, respectively, as previously described (17). Although antigen-driven memory OT-I T cells display elevated levels of α4-integrin (compared with bulk polyclonal CD8 T cells), HP memory OT-I cells show markedly reduced α4-integrin expression (Fig. 5 B). Hence, by this marker, the tetramer binding CD44hi cells in unprimed animals resembled HP memory CD8 T cells (Fig. 5, A and B; and Figs. S4 and S5, available at http://www.jem.org/cgi/content/full/jem.20081829/DC1). This pattern of α4-integrin expression was observed on specific T cells isolated from either LN or spleen (Fig. S4 B and S5). In addition, low expression of α4-integrin was also observed on the CD44hi pool of CD8+ T cells isolated from unmanipulated RAG−/− TCR transgenic gBT mice (Fig. 5 C). Again, the T cells in these mice are all specific for the HSVgB and cannot express endogenous receptors because of the RAG deficiency and so cannot respond to environmental antigens. Therefore, the phenotype of these cells must arise via a mechanism independent of antigen/TCR stimulation. In summary, the antigen-specific pool of CD44hi T cells in unprimed WT mice, which is similar to those in TCR transgenic RAG−/− hosts, display a phenotype (CD122hi, LFA-1hi, Ly6Chi, or α4-integrinlow) consistent with their being derived from homeostatic, rather than foreign antigen-mediated, expansion.
Enhanced functional reactivity of CD44hi T cells isolated from unprimed animals
As discussed earlier, memory and naive CD8 T cells differ not only in their phenotype but also in their functional properties. Enhanced functional reactivity is a hallmark of both antigen-experienced and HP memory CD8 T cells (16, 17, 36). Hence, we sought to test the functional capacity of antigen-specific CD44hi cells from unprimed mice.
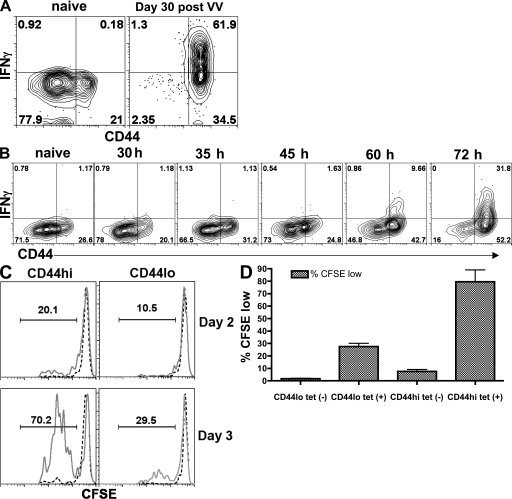
In initial experiments, we studied the response of endogenous CD44hi and CD44lo CD8 T cells after in vivo antigen stimulation. Unprimed mice were injected i.v. with the B8R peptide to provide an acute TCR stimulus for all B8R-specific T cells, which is analogous to the addition of peptide in an in vitro intracellular cytokine staining assay. This was followed 30 min later by i.v. injection of brefeldin A (BFA) to facilitate retention of intracellular cytokines (42, 43). As a positive control, this procedure was also performed on mice possessing a functional pool of memory B8R-specific T cells, having been challenged 30 d earlier with vaccinia virus. 2 h after initial peptide injection, the B8R-specific T cells were isolated by magnetic bead sorting in the continued presence of BFA and then were stained and analyzed to detect intracellular IFN-γ in the CD44hi and lo B8R-specific T cells. In keeping with published data on conventional and HP memory cells (16, 17, 36), the majority of memory B8R-specific T cells from the vaccinia immune mice readily stained positive for both tetramer and IFN-γ (Fig. 6 A). In contrast, however, the CD44hi antigen-specific T cells from unprimed mice did not. These data indicate that the lack of IFN-γ production from the B8R-specific T cells in the unprimed host was due neither to a failure of the peptide to provide an adequate TCR stimulus in vivo nor to a failure to isolate the unprimed T cells as a result of down-regulation of the TCR (44). These data suggested that antigen-specific CD44hi T cells differ from other memory CD8 T cell populations in that they do not make effector cytokines, such as IFN-γ, immediately upon TCR stimulus.
Figure 6.
During primary stimulation, antigen-specific CD44hi CD8 T cells respond more quickly than CD44lo CD8 T cells. (A) Unprimed B6 mice, or mice challenged with vaccinia virus 30 d earlier, were injected i.v. with 300 µg B8R peptide and BFA 2 h before spleen harvest as described in the Materials and methods. The B8R-specific T cells were isolated by tetramer staining and magnetic bead sorting as described in Fig. 1, except that BFA was included in all buffers. The cells were stained for surface markers and intracellular IFN-γ as previously described (52). (B) B6 mice were immunized with 100 mg B8R peptide and 50 mg of anti-CD40 antibody at the indicated times before the harvest of their spleen cells. As in A, the mice were injected with B8R peptide and BFA 2 h before being killed, and their B8R-specific T cells were isolated by tetramer staining and magnetic bead sorting. The cells were stained for surface markers and intracellular IFN-γ as previously described (52). The contour plots shown were gated on all CD8+B220− tetramer+ T cells. (C) B6 splenocytes were harvested from unprimed animals and tetramer enrichment was performed (using both B8R/Kb and HSV-1/Kb tetramers). Tetramer+ and tetramer− CD8 T cells were both sorted into CD44hi and CD44lo populations, each was CFSE-labeled, and the cells were stimulated in vitro on antigen (B8R and HSV peptide)-pulsed splenocytes. 2 or 3 d later (as indicated), the proliferation of the tetramer+ (solid lines) and tetramer− (dashed lines) pools were assessed by CFSE dye dilution. (D) Compiled data from three experiments performed as in C, showing the frequency of CFSE-diluted responder cells on day 3 after stimulation. Error bars show SD.
We next reasoned that perhaps the antigen-specific CD44hi T cells would convert to IFN-γ–producing effectors after antigen priming more rapidly that their antigen-specific CD44lo counterparts. We therefore immunized mice with B8R peptide and anti-CD40 antibody (as an adjuvant) at various time points before isolation of the specific cells by B8R/Kb tetramer sorting. Again, analogous to the addition of peptide in an in vitro intracellular cytokine staining assay, mice were injected i.v. with peptide 2 h before harvest, followed 30 min later by injection of BFA. The B8R-specific T cells were isolated and were again stained and analyzed to detect intracellular IFN-γ in the CD44hi and lo B8R-specific T cells. By 60 h after immunization, IFN-γ production could be induced selectively in the CD44hi pool (Fig. 6 B). This response was specific because IFN-γ was not produced by T cells from the same animals isolated by MHC tetramers of an unrelated antigen (unpublished data). However, by this 60-h time point, there was a substantial increase in the fraction of B8R/Kb tetramer binding CD44hi T cells, making it difficult to determine whether the responding cells were derived from the original CD44hi or CD44lo pool. In addition, although we did not observe this problem in the analysis of B8R-specific T cells from the vaccinia-immune hosts, it was formally possible that the increased degree of TCR stimulation provided by the peptide+adjuvant immunization facilitated a greater loss of antigen-responsive cells as a result of TCR down-regulation (44).
To avoid these concerns, we first isolated tetramer binding CD44lo and CD44hi CD8 T cells (from unprimed animals) and then tested their response to antigen stimulation in vitro. Specific CD8 T cells were enriched by tetramer-based isolation, and these cells were FACS sorted into tetramer+ (and tetramer−) CD44lo and CD44hi populations. These were labeled with CFSE and stimulated in vitro with peptide-pulsed APC. Both CD44lo and CD44hi populations responded to stimulation, but we consistently observed a greater fraction of CD44hi cells undergoing proliferation in these assays (Fig. 6, C and D). These data confirm that tetramer binding cells are competent to functionally respond to the specific peptide–MHC molecule and suggest that tetramer binding CD44hi cells can proliferate more efficiently than their CD44lo counterparts.
Tetramer binding CD44hi CD8 T cells from unprimed animals rapidly produce IFN-γ in response to innate immune cues
Previous studies have indicated that antigen-driven memory CD8 T cells have the capacity to display certain innate immune functions. Specifically, in the absence of TCR engagement, memory CD8 T cells efficiently produce IFN-γ in response to TLR and cytokine stimulation (24, 25, 45). Receptors for IL-12 and IL-18 are up-regulated on memory phenotype CD8 T cells and binding of these cytokines leads to production of IFN-γ (24, 25, 45). This rapid production of IFN-γ has been shown to play an important role in the early resistance to bacterial infection (24). To investigate whether antigen-specific CD44hi CD8 T cells in unprimed mice could also perform this function, we cultured splenocytes from B6 mice with IL-12, IL-18, and IL-2, conditions which have been previously shown to stimulate IFN-γ secretion from antigen-driven memory CD8 T cells (24). After the 18-h stimulation period, B8R/Kb tetramer enrichment was performed followed by immediate intracellular staining for IFN-γ. We found that when cultured with IL-2 alone, CD8 T cells produced little IFN-γ (Fig. 7 A), but with the addition of IL-12 and IL-18 we observed robust IFN-γ production by CD44hi (but not CD44lo) B8R/Kb tetramer binding CD8 T cells (Fig. 7, A and B). The same IFN-γ production pattern was observed when bulk CD8 T cells were examined, confirming that this response is not antigen specific. These findings demonstrate that antigen-specific CD44hi CD8 T cells from unimmunized animals are capable of producing the effector cytokine IFN-γ in response to innate cytokine stimulation, and such cells may therefore be able to participate in both innate and adaptive phases of the primary immune response.
Figure 7.
Antigen-specific CD44hi CD8 T cells make IFN-γ in response to innate cytokine stimulation. Spleen cells from B6 mice were cultured for 18 h in the presence of either IL-2 or a mixture of IL-2, IL-12, and IL-18. BFA was added for the last 4 h of culture. Cells were then subjected to tetramer enrichment using B8R/Kb tetramers. (A) Representative staining of either bulk CD8 T cells (top) or B8R/Kb tetramer binding CD8 T cells (bottom) for IFN-γ. (B) The percentage of IFN-γ+ cells in the CD44hi and CD44lo compartments of bulk CD8 T cells or B8R/Kb-specific CD8 T cells. Each symbol represents an independent sample (n = 5), and the data are compiled from three experiments. Horizontal lines show the mean values of each group.
DISCUSSION
Collectively, our studies indicate that the peptide–MHC-specific CD8 T cell repertoire within an unprimed host contains both the predicted naive phenotype pool and a memory phenotype population. Furthermore, our data indicate that these cells are distinct from their naive phenotype counterparts in their proliferative and cytokine production capacity.
The existence of memory-like T cells in nonimmunized animals has long been known. However, this is the first demonstration, to our knowledge, that the pool contains cells specific for (and reactive to) nominal foreign antigens. We propose the term “virtual memory” (VM) to describe this novel population of antigen-specific T cells within the unprimed T cell repertoire (in computing, VM describes a form of working memory, based on alternative utilization of existing space). Our findings differ from those of a recent publication by Obar et al. (28), who used very similar approaches to study antigen-specific CD8 T cells in the unprimed repertoire yet concluded that these cells were all of naive phenotype. Those authors did observe a range in CD44 expression among tetramer binding cells specific for VSV-N/Kb or M45/Db (the main specificities studied in that report; Fig. 1) (28), but other phenotypic markers (such as LFA-1 expression levels) did not suggest a memory phenotype. However, it is clear from our data that the frequency of VM cells varies greatly within any given specificity (Fig. 1, C–E). That being said, our preliminary studies on cells isolated from unprimed mice using VSV-N/Kb and M45/Db tetramers indicates the presence of VM CD8 T cells, which is similar to the other epitopes studied here (unpublished data). The discrepancy between our findings and those of Obar et al. (28) may either reflect the natural variation in VM frequency within the M45 and VSV-N–specific T cells or reflect differences in our methods and/or analysis of tetramer binding T cell isolation.
An important issue is how this memory phenotype pool arises in unprimed animals. By standard models, the expectation would be that this pool represents cells primed by foreign antigens encountered in the environment such as microbes or food antigens. Given such a scenario, one might anticipate that the endogenous memory CD8 T cells would be unlikely to show specificity for the nominal antigens studied in this paper (including OVA, B8R, and HSV epitopes) because their repertoire would be expected to be focused on the priming antigens. In contrast, we reliably found memory phenotype CD8 T cells in unprimed animals for all of the foreign peptide–MHC ligands studied, and such cells were present at ∼10–30% of the total tetramer-bound pool. These data suggest that the antigen specificity of VM T cells has similar diversity to the naive pool, arguing against the bias expected by reactivity to an environmental antigen.
Along the same lines, we consider it unlikely that VM cells are products of heterologous immunity, at least in the context in which this term is typically applied (34, 35). Heterologous immunity has been used to refer to the overlap in the reactivity of antigen-specific T cells responding to distinct pathogens. This response arises because the pool of T cells responding to epitopes from one pathogen will sometimes include T cells that are cross-reactive with epitopes produced by a different pathogen. Such responses can lead to improved responses to a second microbe as a result of preexisting memory against the first. However, because these responses involve fortuitous cross-reactivity between distinct epitopes, they often lead to dramatic changes in immunodominance of the immune response (34, 35). In contrast, in our studies, the precursor frequency of VM T cells responding to nominal foreign antigens was in proportion to the frequency of naive phenotype precursors, suggesting the absence of a severe bias in the repertoire of this pool. Second, VM CD8 T cells isolated by tetramer sorting from unprimed animals displayed low expression of α4-integrin, this marker being expressed at levels lower than that of bulk CD8 T cells (Fig. 5). In contrast, this marker is induced upon antigen stimulation and typically remains high on the ensuing memory population (23, 40, 41, 46) (Fig. 5). Thus, the α4-integrinlo phenotype of VM cells is inconsistent with their production by heterologous immunity, as classically defined (34, 35). It is worth noting that a substantial proportion (50–70%) of bulk CD44hi cells in unprimed mice express low levels of α4-integrin (unpublished data). This suggests that the majority of the memory phenotype cells in unprimed mice may also be generated as a result of HP. Finally, our studies using GF mice further argue against the model that the VM CD8 T pool in unprimed mice arises as a consequence of exposure to environmental microbes, although we cannot rule out the contribution of nonself antigens (e.g., components of food) in driving the generation of these cells.
The markers expressed by VM CD8 T cells (high expression of CD122, LFA-1, and Ly6C but low expression of α4-integrin) are the same as those expressed by HP memory CD8+ T cells after production in a lymphopenic host (12, 16, 17, 36) (Fig. 5). Together with the breadth of VM antigen specificities (Fig. 1), these data are most consistent with VM cells being produced by homeostatic mechanisms rather than conventional priming. In contrast, both antigen-experienced and HP memory CD8 T cells rapidly produce IFN-γ upon TCR stimulation (12, 16, 17, 36), whereas antigen-specific VM CD8 T cells appear to be unable to respond in this way (Fig. 6). At the same time, we could show that VM CD8 T cells were competent to produce IFN-γ after stimulation with the proinflammatory cytokines IL-12 and IL-18 (Fig. 7). Hence, the functional properties of VM CD8 T cells may be distinct from either conventional or HP memory CD8 T cells.
This paper focuses exclusively on CD8 T cells. A memory-like pool of CD4 T cells in unprimed animals is also found, and it will be interesting to determine their specificity and function. Previous studies using the tetramer pulldown approach (27) showed that the majority of antigen-specific CD4 T cells in the unprimed pool were of naive phenotype (CD44lo). Whether this represents a true distinction between CD4 and CD8 T cells will require more extensive study. However, it is interesting to note that Class II MHC-specific TCR transgenic RAG−/− animals typically have very few CD44hi T cells (27), whereas several Class I MHC-specific TCR transgenic RAG−/− mice (Figs. 2 and 5) contain a clearly identifiable population of CD44hi T cells. In the absence of secondary TCRs, it seems likely that the appearance of CD44hi cells in such mice arises from HP. Indeed, the fact that Class I MHC-specific H-Y TCR transgenic RAG−/− T cells do not contain a CD44hi pool (47), and that these T cells also fail to undergo HP in a lymphopenic environment (48, 49), supports that model. Although the basis for the different incidence of memory-like cells in Class I MHC– versus Class II MHC–restricted TCR transgenics is unclear, it correlates with the greater propensity of CD8 T cells to undergo HP compared with the CD4 pool (3–5, 12, 49).
Regardless of how VM CD8 T cells are generated, it is important to consider their potential significance for immune competence. Within the unprimed antigen-specific T cell pool, the majority of cells are naive phenotype, with VM T cells accounting for only 10–30% of the population. However, upon in vitro stimulation with antigen, VM CD8 T cells exhibit more robust proliferation compared with their naive counterparts (Fig. 6). In addition, VM CD8 T cells produce IFN-γ in response to the proinflammatory cytokines IL-12 and IL-18, behaving similarly to conventional CD8 memory T cells (Fig. 7). These properties suggest that VM CD8 T cells may participate in both innate and adaptive immune responses during a primary immune response. In addition, the proliferative advantage of VM T cells may give them a competitive advantage during priming. Competition among T cells has been well described (50–52), although the molecular basis for this competition is yet to be fully elucidated (52), and it is interesting to speculate that VM CD8 T cells may increase their proportional representation during an immune response. In addition, the fact that we observed variability in the frequency of VM CD8 T cells depending on the peptide–MHC ligand studied is noteworthy because the relative frequency of VM cells within an antigen-specific pool may influence aspects of the response (such as immunodominance). Finally, if VM CD8 T cells are indeed generated by homeostatic mechanisms, their functional relevance may be especially tailored to situations where the size of the naive T cell pool is limited. It is interesting to note that young mice, although lymphopenic, contain a high proportion of memory phenotype CD8 T cells compared with adult animals (6, 53). Neither the specificity of these cells, nor their similarity to VM T cells has been assessed, but it is tempting to propose that such cells may contribute to CD8 T cell–mediated immune responses in the neonate. Further studies will be required to determine the contribution of VM CD8 T cells to the effector and memory populations after priming in young and adult animals.
In summary, our data suggest that the unprimed T cell pool contains cells that bear phenotypic and functional traits of memory CD8 T cells and that appear to arise via homeostatic mechanisms. Participation of these cells will need to be considered in understanding the nature of the primary immune responses and protective immunity against pathogens.
MATERIALS AND METHODS
Mice and reagents.
6–12-wk-old female C57BL/6J mice were purchased from the National Cancer Institute. RAG−/−, RAG−/−gBT1 TCR transgenic mice, and RAG−/−OT-1 TCR transgenic mice were bred in the National Jewish Biological Resource Center or at the University of Minnesota Medical School. Spleen and LN cells from GF C57BL/6J mice were provided by S. Tonkonogy (Gnotobiotic Core of the Center for Gastrointestinal Biology and Disease, College of Veterinary Medicine, North Carolina State University, Raleigh, NC) using conditions described previously (54). GF mice were maintained in flexible film isolators under positive pressure and received autoclaved food and water. Aerobic and anaerobic cultures of freshly collected fecal samples from each isolator were performed biweekly and were found to be negative. Peptides were ordered from Global Peptide, New England Peptide, or Invitrogen. Class I MHC tetramers were produced as previously described (17, 50) or purchased from Beckman Coulter. Fluorochrome-conjugated antibodies against CD8, CD44, B220, CD4, CD11b, CD69, CD25, CD49d (α4-integrin), CD122, LFA1, and Ly6C were purchased from BD or eBioscience. Vaccinia virus (WR strain) was produced by infection of Vero cells as previously described (50). All mouse protocols were approved by the Institutional Animal Use and Care Committees at National Jewish Health or the University of Minnesota.
Tetramer staining and magnetic bead sorting.
Tissues from mice (Spleen, LN, and ovary) were removed and collagenase was digested for 45 min as previously described (50). In the absence of collagenase digestion, antigen-specific T cells are poorly isolated from antigen-challenged hosts (55). Therefore, collagenase treatment was typically used for isolating cells from both naive and antigen-stimulated mice. However, qualitatively similar recovery of naive and VM populations was observed with or without inclusion of collagenase in the isolation procedure. Cells were resuspended in 500 µl of sorting buffer (consisting of 250 µl 24G2 supernatant and 250 µl HBSS containing 2% FCS and 0.2% azide to prevent internalization of the tetramer) per spleen. Cells were stained with tetramer and anti-CD8 for 60 min at 37°C. Cells were then washed, resuspended in HBSS plus azide, and stained with anti-PE–coupled MACS MicroBeads (Miltenyi Biotec) for 30 min rotating at 4°. Cells were then washed, resuspended in HBSS, and the tetramer+ cells isolated on a magnetized MACS column (Miltenyi Biotec) according to the manufacturer's instructions. After elution, cells were centrifuged and resuspended in FACS buffer and stained for markers used to enhance gating of tetramer+ CD8+ T cells (CD8 and CD3 as a positive gate; B220 and, in some experiments, CD4 or CD11b as a dump gate), as well as with antibodies to determine activation status (CD44, α4-integrin, CD122, LFA-1, Ly6C, CD69, and CD25).
As an alternative isolation and tetramer binding protocol (used for Figs. 4, 7, S1, S4 A, and S5), tetramer binding was performed at 4°C (rather than 37°C) and the cells were stained with fluorochrome (PE or APC)-labeled tetramers at 4°C for 60 min in the absence of anti-CD8 antibodies. Cells were incubated with MACS MicroBeads coupled to anti-PE and/or anti-APC and isolated as in the previous paragraph. In these experiments, fluorescent anti-CD8 antibodies were included with other stains after enrichment of tetramer-associated cells. For dual tetramer staining experiments, spleen cells were stained for 1 h with both PE- and APC-labeled tetramers. Cells were then incubated with both anti-PE and anti-APC MicroBeads, isolated on MACS columns as in the previous paragraph, and then stained with fluorescent antibodies to CD8, CD3, and CD44 in various fluorochrome combinations. In addition, cells were staining with a cocktail of antibodies (all conjugated to Pacific Blue) specific for B220, CD11b, CD11c, F4/80, and (in some experiments) CD4. Cells positive for this “dump” stain were excluded from further analysis.
Cells were analyzed on an LSRII (BD), Cyan ADP (Dako), or FACScan (BD) retrofitted with a second laser (Cytek) to allow five-color analysis. Data were analyzed using FlowJo software according to the gating strategy described in Fig. 1.
Immunizations.
Indicated mice were injected i.v. with 50–400 µg of peptide (B8R-TSYKFESV, OVA-SIINFEKL, or HSV-1-SSIEFARL). For the experiments shown in Fig. 3, mice were challenged with peptide in the absence of any adjuvant to avoid any non–antigen-specific influence of the adjuvant on CD69 expression. In other experiments, mice were injected with peptide together with 25–50 µg of the anti-CD40 antibody FGK45 (33, 56, 57) or a combination of FGK45 and 50 µg of poly-I:C (GE Healthcare). This approach uses the capacity of CD40 stimulation paired with poly-I:C, to act as a highly potent adjuvant for T cell responses (33, 56, 58, 59). For the experiments in which we transferred enriched cells into RAG−/− hosts, we used CD40/poly-I:C as an adjuvant to give us the best opportunity at expanding the small numbers of antigen-specific cells transferred. For immunizations in which we required an adjuvant but wished to limit non–antigen-specific adjuvant effects, we used peptide in combination with CD40 alone. For viral challenge, mice were injected i.p. with 107 pfu of vaccinia virus strain WR.
In vivo and in vitro T cell activation assays.
Mice were either left as unimmunized controls or were immunized with B8R peptide and anti-CD40 at various times before being killed. 2 h before being killed, the mice were injected with 300 µg of either B8R peptide or HSV peptide. 30 min later, they were injected i.v. with 250 µg BFA in 500 µl PBS as previously described (42, 43). 1.5 h after BFA injection, the mice were killed and tetramer-based enrichment was performed as described previously (except that BFA was included in all buffers during the course of T cell staining and isolation). After magnetic column isolation, the cells were stained with the remaining surface markers, as described in Tetramer staining and magnetic bead sorting, and then fixed, permeabilized, and stained for intracellular IFN-γ as previously described (52).
For in vitro T cell activation assays, B6 splenocytes were first subjected to tetramer-based enrichment, using both B8R/Kb and HSV-1/Kb tetramers to maximize the isolation of antigen-specific T cells from the spleens. Bound cells were then sorted by flow cytometry to generate four populations: tetramer+ CD44lo, tetramer+ CD44hi, tetramer− CD44lo, and tetramer− CD44hi. The cells were labeled with CFSE and included with irradiated splenocytes (from B6.SJL animals) precoated with 1 µM HSV and B8R peptides and with poly-I:C and anti-CD40 for 1 h at 37°C. Flow cytometric analysis was performed on days 2 and 3 of the culture, with gating on live CD45.2+ cells representing the B6 responder cells.
In vitro cytokine stimulation assay.
Spleen cells from B6 mice were cultured for 18 h in the presence of 10 U/ml of recombinant human IL-2 (Biological Resources Branch, NCI-Frederick) alone or with 10 ng/ml IL-12 (a gift from Wyeth Pharmaceuticals) and 10 ng/ml IL-18 (Peprotech). During the last 4 h of culture, 2 µl/ml BFA was added. After the culture period, cells were harvested and B8R/Kb tetramer enrichment was performed as described previously. After enrichment, surface staining was performed followed by fixation, permeabilization, and intracellular staining for IFN-γ.
Preparation of HP and true memory OT-I cells.
CD44lo (naive) CD8+ OT-I T cells were isolated from OT-I mice and adoptively transferred into B6 animals to generate memory populations. For true memory cells, naive OT-I T cells were transferred into normal B6 mice which were then infected with LM-OVA and left for >30 d to allow memory generation. To generate HP memory cells, naive OT-I cells were transferred into sublethally-irradiated (∼450 rads) B6 mice and left for >30 d to allow for expansion and differentiation in response to lymphopenia. Expression of α4-integrin on these populations is indicated, as is an isotype control staining profile.
Online supplemental material.
Fig. S1 demonstrates the tetramer isolation of naive T cells using different staining methods. Fig. S2 further supports the specificity of the T cells isolated by tetramer staining and magnetic bead sorting based on their phenotype in response to viral challenge. Fig. S3 shows the phenotype of the cells isolated by tetramer staining and magnetic bead sorting from 0–4 d after peptide immunization. Fig. S4 provides additional evidence that VM cells are present within GF as well as within both LN and spleen in SPF mice. Fig. S5 shows additional data concerning the difference in VLA4 expression between memory T cells generated by antigen stimulation or by homeostatic expansion. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081829/DC1.
Acknowledgments
We gratefully thank Dr. Marc K. Jenkins from the Department of Microbiology and the Center for Immunology at the University of Minnesota, without whom our development of the tetramer staining and bead sorting methodology would not have been possible. We are also indebted to Susan Tonkonogy (Center for Gastrointestinal Biology and Disease, North Carolina State University) for providing tissues from GF mice. Thanks also to Josh Loomis and Shirley Sobus in the National Jewish Health Flow Cytometry core facility for their expertise in sorting rare and elusive populations of naive T cells.
This work was supported by National Institutes of Health grants AI06877-01 and AI066121 to R.M. Kedl and R01 AI075158 and R37 AI38903 to S.C. Jameson. Maintenance of GF mice was supported by United States Public Health Service grant P30 DK34987 to the Center for Gastrointestinal Biology and Disease at North Carolina State University.
The authors state that they have no conflicting financial interests.
Footnotes
Abbreviations used: BFA, brefeldin A; gB, glycoprotein B; GF, germ free; HP, homeostatic proliferation; SPF, specific pathogen free; VM, virtual memory.
References
- Harty J.T., Badovinac V.P. 2008. Shaping and reshaping CD8+ T-cell memory.Nat. Rev. Immunol. 8:107–119 [DOI] [PubMed] [Google Scholar]
- Seder R.A., Ahmed R. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation.Nat. Immunol. 4:835–842 [DOI] [PubMed] [Google Scholar]
- Jameson S.C. 2005. T cell homeostasis: keeping useful T cells alive and live T cells useful.Semin. Immunol. 17:231–237 [DOI] [PubMed] [Google Scholar]
- Marleau A.M., Sarvetnick N. 2005. T cell homeostasis in tolerance and immunity.J. Leukoc. Biol. 78:575–584 [DOI] [PubMed] [Google Scholar]
- Surh C.D., Sprent J. 2005. Regulation of mature T cell homeostasis.Semin. Immunol. 17:183–191 [DOI] [PubMed] [Google Scholar]
- Le Campion A., Bourgeois C., Lambolez F., Martin B., Leaument S., Dautigny N., Tanchot C., Penit C., Lucas B. 2002. Naive T cells proliferate strongly in neonatal mice in response to self-peptide/self-MHC complexes.Proc. Natl. Acad. Sci. USA. 99:4538–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B., McHugh R., Sempowski G.D., Mackall C., Foucras G., Paul W.E. 2003. Neonates support lymphopenia-induced proliferation.Immunity. 18:131–140 [DOI] [PubMed] [Google Scholar]
- Min B., Sempowski G.D., Paul W.E. 2002. Neonates support “homeostatic” proliferation.Adv. Exp. Med. Biol. 512:91–95 [PubMed] [Google Scholar]
- Schuler T., Hammerling G.J., Arnold B. 2004. Cutting edge: IL-7-dependent homeostatic proliferation of CD8+ T cells in neonatal mice allows the generation of long-lived natural memory T cells.J. Immunol. 172:15–19 [DOI] [PubMed] [Google Scholar]
- Schluns K.S., Kieper W.C., Jameson S.C., Lefrancois L. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo.Nat. Immunol. 1:426–432 [DOI] [PubMed] [Google Scholar]
- Tan J.T., Dudl E., LeRoy E., Murray R., Sprent J., Weinberg K.I., Surh C.D. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells.Proc. Natl. Acad. Sci. USA. 98:8732–8737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.H., Boyman O., Kim H.O., Hahm B., Rubinstein M.P., Ramsey C., Kim D.M., Surh C.D., Sprent J. 2007. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2.J. Exp. Med. 204:1787–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura D., Bevan M.J. 2007. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2–anti–IL-2 complex treatment in vivo.J. Exp. Med. 204:1803–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieper W.C., Prlic M., Schmidt C.S., Mescher M.F., Jameson S.C. 2001. Il-12 enhances CD8 T cell homeostatic expansion.J. Immunol. 166:5515–5521 [DOI] [PubMed] [Google Scholar]
- Sandau M.M., Winstead C.J., Jameson S.C. 2007. IL-15 is required for sustained lymphopenia-driven proliferation and accumulation of CD8 T cells.J. Immunol. 179:120–125 [DOI] [PubMed] [Google Scholar]
- Goldrath A.W., Bogatzki L.Y., Bevan M.J. 2000. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation.J. Exp. Med. 192:557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S.E., Wolkers M.C., Schoenberger S.P., Jameson S.C. 2006. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells.Nat. Immunol. 7:475–481 [DOI] [PubMed] [Google Scholar]
- Kalia V., Sarkar S., Gourley T.S., Rouse B.T., Ahmed R. 2006. Differentiation of memory B and T cells.Curr. Opin. Immunol. 18:255–264 [DOI] [PubMed] [Google Scholar]
- Masopust D., Vezys V., Wherry E.J., Ahmed R. 2007. A brief history of CD8 T cells.Eur. J. Immunol. 37:S103–S110 [DOI] [PubMed] [Google Scholar]
- Masopust D., Vezys V., Marzo A.L., Lefrancois L. 2001. Preferential localization of effector memory cells in nonlymphoid tissue.Science. 291:2413–2417 [DOI] [PubMed] [Google Scholar]
- Reinhardt R.L., Bullard D.C., Weaver C.T., Jenkins M.K. 2003. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation.J. Exp. Med. 197:751–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt R.L., Khoruts A., Merica R., Zell T., Jenkins M.K. 2001. Visualizing the generation of memory CD4 T cells in the whole body.Nature. 410:101–105 [DOI] [PubMed] [Google Scholar]
- Kedl R.M., Mescher M.F. 1997. Migration and activation of antigen-specific CD8+ T cells upon in vivo stimulation with allogeneic tumor.J. Immunol. 159:650–663 [PubMed] [Google Scholar]
- Berg R.E., Crossley E., Murray S., Forman J. 2003. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen.J. Exp. Med. 198:1583–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi T., Assarsson E., Lukacher A.E., Ljunggren H.G., Jensen P.E. 2003. Memory CD8+ T cells provide an early source of IFN-gamma.J. Immunol. 170:2399–2408 [DOI] [PubMed] [Google Scholar]
- Huang T., Wei B., Velazquez P., Borneman J., Braun J. 2005. Commensal microbiota alter the abundance and TCR responsiveness of splenic naive CD4+ T lymphocytes.Clin. Immunol. 117:221–230 [DOI] [PubMed] [Google Scholar]
- Moon J.J., Chu H.H., Pepper M., McSorley S.J., Jameson S.C., Kedl R.M., Jenkins M.K. 2007. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude.Immunity. 27:203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar J.J., Khanna K.M., Lefrancois L. 2008. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection.Immunity. 28:859–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist K.A., Jameson S.C., Heath W.R., Howard J.L., Bevan M.J., Carbone F.R. 1994. T cell receptor antagonist peptides induce positive selection.Cell. 76:17–27 [DOI] [PubMed] [Google Scholar]
- Kelly J.M., Sterry S.J., Cose S., Turner S.J., Fecondo J., Rodda S., Fink P.J., Carbone F.R. 1993. Identification of conserved T cell receptor CDR3 residues contacting known exposed peptide side chains from a major histocompatibility complex class I-bound determinant.Eur. J. Immunol. 23:3318–3326 [DOI] [PubMed] [Google Scholar]
- Tscharke D.C., Karupiah G., Zhou J., Palmore T., Irvine K.R., Haeryfar S.M., Williams S., Sidney J., Sette A., Bennink J.R., Yewdell J.W. 2005. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines.J. Exp. Med. 201:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.N., Heath W., McLain J.D., Carbone F.R., Jones C.M. 2002. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus.Immunol. Cell Biol. 80:156–163 [DOI] [PubMed] [Google Scholar]
- Sanchez P.J., McWilliams J.A., Haluszczak C., Yagita H., Kedl R.M. 2007. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo.J. Immunol. 178:1564–1572 [DOI] [PubMed] [Google Scholar]
- Selin L.K., Brehm M.A., Naumov Y.N., Cornberg M., Kim S.K., Clute S.C., Welsh R.M. 2006. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity.Immunol. Rev. 211:164–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R.M., Selin L.K. 2002. No one is naive: the significance of heterologous T-cell immunity.Nat. Rev. Immunol. 2:417–426 [DOI] [PubMed] [Google Scholar]
- Cho B.K., Rao V.P., Ge Q., Eisen H.N., Chen J. 2000. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells.J. Exp. Med. 192:549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A.C., Issekutz T.B. 2002. The role of E-selectin, P-selectin, and very late activation antigen-4 in T lymphocyte migration to dermal inflammation.J. Immunol. 168:1934–1939 [DOI] [PubMed] [Google Scholar]
- Mobley J.L., Rigby S.M., Dailey M.O. 1994. Regulation of adhesion molecule expression by CD8 T cells in vivo. II. Expression of L-selectin (CD62L) by memory cytolytic T cells responding to minor histocompatibility antigens.J. Immunol. 153:5443–5452 [PubMed] [Google Scholar]
- Mackay C.R. 1993. Homing of naive, memory and effector lymphocytes.Curr. Opin. Immunol. 5:423–427 [DOI] [PubMed] [Google Scholar]
- Andersson E.C., Christensen J.P., Marker O., Thomsen A.R. 1994. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection.J. Immunol. 152:1237–1245 [PubMed] [Google Scholar]
- Christensen J.P., Andersson E.C., Scheynius A., Marker O., Thomsen A.R. 1995. Alpha 4 integrin directs virus-activated CD8+ T cells to sites of infection.J. Immunol. 154:5293–5301 [PubMed] [Google Scholar]
- Foster B., Prussin C., Liu F., Whitmire J.K., Whitton J.L. 2007. Detection of intracellular cytokines by flow cytometry.Curr. Protoc. Immunol. 6:24. [DOI] [PubMed] [Google Scholar]
- Liu F., Whitton J.L. 2005. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections.J. Immunol. 174:5936–5940 [DOI] [PubMed] [Google Scholar]
- Valitutti S., Muller S., Cella M., Padovan E., Lanzavecchia A. 1995. Serial triggering of many T-cell receptors by a few peptide-MHC complexes.Nature. 375:148–151 [DOI] [PubMed] [Google Scholar]
- Berg R.E., Forman J. 2006. The role of CD8 T cells in innate immunity and in antigen non-specific protection.Curr. Opin. Immunol. 18:338–343 [DOI] [PubMed] [Google Scholar]
- Hamann D., Baars P.A., Rep M.H., Hooibrink B., Kerkhof-Garde S.R., Klein M.R., van Lier R.A. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells.J. Exp. Med. 186:1407–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchot C., Rocha B. 1995. The peripheral T cell repertoire: independent homeostatic regulation of virgin and activated CD8+ T cell pools.Eur. J. Immunol. 25:2127–2136 [DOI] [PubMed] [Google Scholar]
- Baldwin T.A., Sandau M.M., Jameson S.C., Hogquist K.A. 2005. The timing of TCR-α expression critically influences T cell development and selection.J. Exp. Med. 202:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst B., Lee D.S., Chang J.M., Sprent J., Surh C.D. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery.Immunity. 11:173–181 [DOI] [PubMed] [Google Scholar]
- Kedl R.M., Rees W.A., Hildeman D.A., Schaefer B., Mitchell T., Kappler J., Marrack P. 2000. T cells compete for access to antigen-bearing antigen-presenting cells.J. Exp. Med. 192:1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedl R.M., Schaefer B.C., Kappler J.W., Marrack P. 2002. T cells down-modulate peptide-MHC complexes on APCs in vivo.Nat. Immunol. 3:27–32 [DOI] [PubMed] [Google Scholar]
- Willis R.A., Kappler J.W., Marrack P.C. 2006. CD8 T cell competition for dendritic cells in vivo is an early event in activation.Proc. Natl. Acad. Sci. USA. 103:12063–12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichii H., Sakamoto A., Hatano M., Okada S., Toyama H., Taki S., Arima M., Kuroda Y., Tokuhisa T. 2002. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells.Nat. Immunol. 3:558–563 [DOI] [PubMed] [Google Scholar]
- Kim S.C., Tonkonogy S.L., Karrasch T., Jobin C., Sartor R.B. 2007. Dual-association of gnotobiotic IL-10−/− mice with 2 nonpathogenic commensal bacteria induces aggressive pancolitis.Inflamm. Bowel Dis. 13:1457–1466 [DOI] [PubMed] [Google Scholar]
- Jabbari A., Legge K.L., Harty J.T. 2006. T cell conditioning explains early disappearance of the memory CD8 T cell response to infection.J. Immunol. 177:3012–3018 [DOI] [PubMed] [Google Scholar]
- Ahonen C.L., Doxsee C.L., McGurran S.M., Riter T.R., Wade W.F., Barth R.J., Vasilakos J.P., Noelle R.J., Kedl R.M. 2004. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN.J. Exp. Med. 199:775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A., Melchers F., Andersson J. 1996. The SCID but not the RAG-2 gene product is required for S mu-S epsilon heavy chain class switching.Immunity. 5:319–330 [DOI] [PubMed] [Google Scholar]
- Ahonen C.L., Wasiuk A., Fuse S., Turk M.J., Ernstoff M.S., Suriawinata A.A., Gorham J.D., Kedl R.M., Usherwood E.J., Noelle R.J. 2008. Enhanced efficacy and reduced toxicity of multifactorial adjuvants compared with unitary adjuvants as cancer vaccines.Blood. 111:3116–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares H., Waechter H., Glaichenhaus N., Mougneau E., Yagita H., Mizenina O., Dudziak D., Nussenzweig M.C., Steinman R.M. 2007. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12–independent but CD70-dependent mechanism in vivo.J. Exp. Med. 204:1095–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]